Abstract
Three commonly used murine surgical models of bone healing (closed fracture with intramedullary fixation, distraction osteogenesis (DO), and marrow ablation by reaming) are presented. Detailed surgical protocols for each model are outlined. The nature of the regenerative processes and the types of research questions that may be addressed with these models are briefly outlined. The relative strengths and weaknesses of these models are compared to a number of other surgical models that are used to address similar research questions. Refer to our companion article for more detailed overview of the underlying biology of each model.
Keywords: Orthopedic Surgery, Murine Models, Fracture, Distraction Osteogenesis, Reaming
INTRODUCTION
Three widely used surgical protocols that have been developed for studying post-natal bone repair and regeneration are presented. These three protocols are relatively easy to adapt at a moderate cost in most laboratory settings, and can be used to address a wide variety of research questions related to post-natal skeletal tissue repair. The generation of a closed simple transverse fracture is a model that is predominated by endochondral bone formation. Distraction osteogenesis is a model predominated by intramembranous bone formation that is facilitated by biomechanical signals. It also has been associated with an extensive induction during distraction of new vascular tissues that both feed the bone and the concurrently distracted muscle. Marrow ablation is strictly an endosteal model of intramembranous bone formation that is little influenced by mechanical signals since the cortical bone remains intact. Each protocol offers unique advantages to study different aspects of post-natal bone repair and regeneration as outlined below as well as in the companion review of the biology of these models.
THE FRACTURE PROTOCOL
The most common model of bone repair used in mice and rat models is produced by externally applied blunt trauma to generate a closed, simple transverse fracture. This model primarily heals through an endochondral bone formation with the development of an external callus (Salisbury-Palomares, et al., 2009; Miclau, et al., 2007; Lu, et al., 2011; Yu, et al., 2012). The most widespread application of the fracture model was first described by Bonnarens and Einhorn (1984) for the use in rats; however, it has subsequently been adapted for use in mice by numerous investigators (Hiltunen, et al., 1993; Kon, et al., 2001; Gerstenfeld, et al., 2006; Marturano, et al., 2008). The fracture is generated via a three-point bend to a long bone (usually femur or tibia). Stabilization of the fracture is achieved by inserting an intramedullary pin prior to fracture.
This model is similar in anatomical site, etiology, and fixation method to common long bone fractures seen in the clinical setting. Such fractures are typically closed injuries resulting from a traumatic event such as falls and other accidents. It is advantageous for high-throughput screening due to the simplicity, speed, and reproducibility of the procedure (Marturano, et al., 2008). This model is well suited for studies assessing basic molecular processes that affect endochondral bone formation, and can be extrapolated to embryonic development (Zhang, et al., 2002; Colnot, et al., 2003; Tsuji, et al., 2006; Jepson, et al., 2008; Grimes, et al., 2011) and post-natal epiphyseal growth of long bones (Jepson, et al., 2008; Wigner, et al., 2010).
Animals
Ten- to 18-week old mice
Individual mice used for a study should be within two weeks in age of each other
Materials
Absorbent bench underpads (for surgical bed, recovery, and x-ray station)
Non-fenestrated sterile field
Surgical gauze
Electric razor for shaving mouse fur
Small chamber for initial mouse anesthetization
Animal scale
Germinator dry bead sterilizer for instrument sterilization between surgeries
Heated pads for surgical bed and recovery
Isoflurane vaporizer (for mouse anesthesia; Surgi Vet)
Nose cone
Isoflurane for anesthesia
Betadine (10% povidone iodine solution)
Bupenorphine (Buprenex) for post-operative pain control
Enrofloxacin (Baytril) for antibiotics prophylaxis
27-gauge x ½ in TB syringes
5-0 chromic gut suture
#15 disposable scalpel blades
Curved forceps (Dumont Vessel Cannulation Forceps Inox 0.5mm)
Scalpel handle
Needle driver
Micro-dissecting scissors (Castroviejo Micro Dissecting scissors)
Small animal fracture device (Bonnarens and Einhorn, 1984)
Small animal x-ray imaging device
Stainless steel 25-gauge spinal needle stylet
Wire cutter
Preparation and Induction of Anesthesia
Ensure all surgical instruments are autoclaved prior to the procedure.
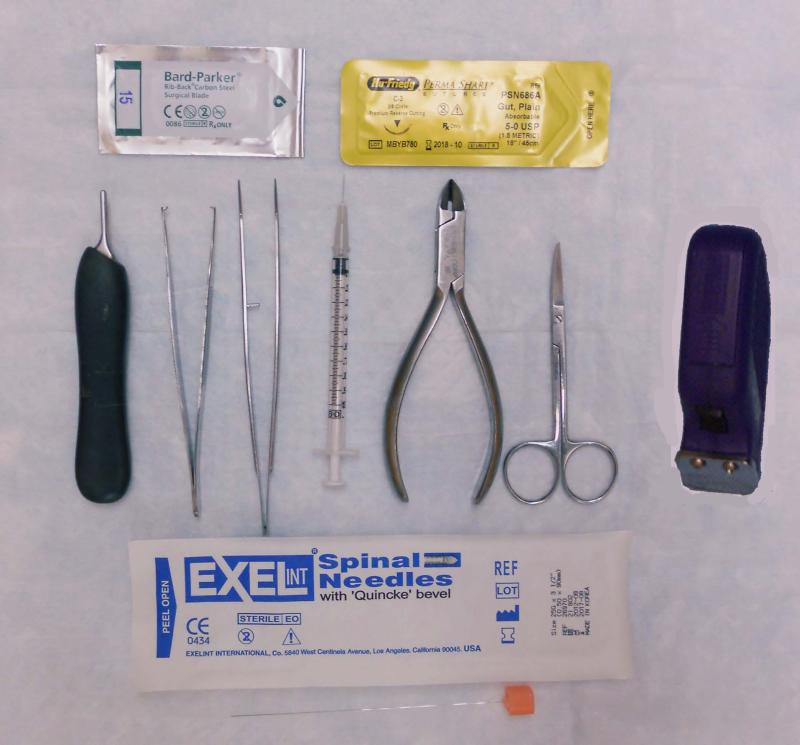
Figure 1 demonstrates basic instruments required for the procedure.
Set up the surgical bed with the heating pad covered by a non-fenestrated sterile field drape.
Set up recovery bed with heating pad covered by absorbent bench underpad
The mouse is weighed prior to the procedure.
The mouse is induced in a closed chamber with a 4% isoflurane/oxygen mixture. Once induced, the animal is maintained on a 2% isoflurane/oxygen mixture with the use of a nose cone.
Fur is removed from the surgical area with a small animal electric razor.
The surgical site is wiped down with surgical gauze that has been dipped in betadine (10% povidone-iodine) solution.
Prior to incision, a dose of Buprenex (0.01mg/kg) is given for immediate postoperative pain management as well as a dose of Baytril (0.01 mL) for infection prophylaxis.
Figure 1.
Surgical Instruments
Surgery
The mouse is placed on its back and the operative leg is maximally flexed at the knee. The leg is held with the non-dominant hand between the thumb and index finger throughout the procedure.
Using a #15 scalpel blade, an anterior longitudinal midline incision is made centered over the knee joint.
A subsequent incision is made just medial to the patella and extensor mechanism. It is important to identify the extensor mechanism consisting of the quadriceps, patella tendon, and patella to avoid disrupting this mechanism in order to allow for immediate ambulation of the mouse following surgery.
The extensor mechanism is elevated and displaced in a lateral fashion using forceps.
After the extensor mechanism is subluxated laterally, the distal end of the femur as well as proximal end of the tibia will be exposed. From this exposure, an entry portal is created using a 27-gauge x ½ in TB syringe in the center of the trochlea groove of the femur for pin insertion.
The tip of the TB syringe is buried (this is approximately the length of an average mouse femur) and removed. The stylet of a 25-gauge spinal needle is then inserted down the length of the medullary canal in a retrograde manner. The depth of insertion can be manually felt with resistance upon meeting cortical bone of the greater trochanter.
The pin is cut flush with the distal end of the femur with wire cutters then buried under the surface of the condyle using forceps to apply gentle downward force. Care must be taken to not dislocate the knee posteriorly and disrupt the cruciate ligaments with this maneuver.
The extensor mechanism is pulled back to its anatomic location using forceps.
The incision is then closed with 5-0 absorbable chromic gut suture.
With the mouse still under anesthesia, a fracture is generated by dropping a weight onto the operative extremity using a small animal fracture device. The weight is set at a defined initial height that will generate a large enough bending moment upon impact to fracture the bone. The combination of weight and initial height should be empirically determined for the specific strain, age, and sex of the mice.
Immediately after fracture and before the animal wakes up from anesthesia, an xray should be taken (we use a mobile dental x-ray unit) to ensure the placement of the intramedullary pin is adequate and that the fracture is mid-diaphyseal without comminution.
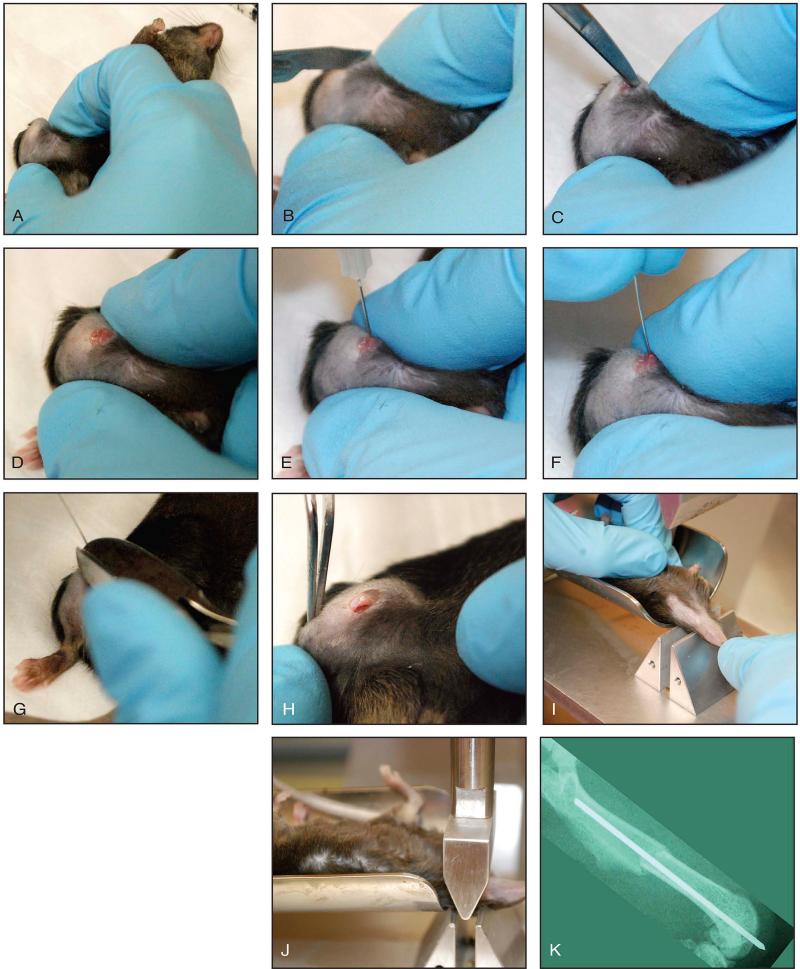
Figure 2 outlines the critical steps of this procedure.
Figure 2. Fracture procedure.
A: The knee is maximally flexed and help using the thumb and index finger of the non-dominant hand. B: A 2-3 mm longitudinal incision is made centered over the knee. C: A subsequent incision is made medial to the patella, which extends into the quadriceps muscle and along the patella tendon to release the tissue. The patella is subluxated laterally. D: Exposure of the distal femur. E: Entry portal to distal femur created using 27-gauge needle in the center of the femoral groove. F: Insertion of femoral pin down the length of the medullary canal. G: This is cut flush with the distal femur. H: The extensor mechanism is placed back to its anatomic location. I, J: Demonstrates creation of closed mid-shaft femur fracture. K: Example of completed fracture.
Post-operative Management
Animals are monitored on the heated recovery bed until they awaken from surgery before going back to their cage.
Analgesia is maintained with Buprenex for 48 hours post-operatively at 24-hour increments. Use of non-steroidal anti-inflammatory drugs (NSAIDs) should be avoided since they have been shown to impair bone healing after surgery (Simon, et al., 2002; Gerstenfeld, et al., 2007). Animals should be able to regain free mobility within 48-hours. If not, they should be euthanized.
Infection is a rare occurrence following fracture. Post-operative prophylactic antibiotics are generally not necessary. Signs of infection would include a swollen thigh and general lethargy noticed from the mouse. Occasionally there may be purulent drainage. The risk of infection generally is of most concern during the first week following surgery. If infection is noticed, the animal must be euthanized as this could affect bone healing.
Pin slippage is common following surgery. If the pin is not fully seated in the femur at the knee, it can become dislodged and may be visualized outside the skin. If this occurs early in the post-operative course, the fracture may displace. When visualized outside the skin, the pin should be removed using a needle driver. This is typically well tolerated by the mouse, and the fracture usually goes onto uneventful union.
THE DISTRACTION OSTEOGENESIS PROTOCOL
Distraction osteogenesis (DO) is a bone-regenerative method by which an osteotomy followed by gradual distraction leads to two vascularized bone surfaces from which new bone is formed (Al-Aql, et al., 2008). This method was first described by Codivilla in 1905 for the treatment of limb length discrepancies; however, it was not until the work of Ilizarov in the 1980's that DO gained wide acceptance clinically as a method for enhancing bone regeneration in orthopaedic surgery (Codivilla, 1905; Ilizarov, 1989).
While endochondral ossification via callus formation is predominant in the fracture model, this is not the case for DO. Although endochondral ossification does take place during the early stages of DO, intramembranous bone formation is the primary mechanism of ossification. DO is typically divided into latency, distraction, and consolidation phases. The latency phase occurs after the osteotomy until the onset of active distraction. This allows for initial inflammatory response to trauma, which is analogous to early stages of fracture repair. The distraction phase then follows this latency period during which time tensile forces are applied to the callus. With steady distraction, fibroblasts and chondrocyte-like cells form in the gap, which ultimately undergo mineralization by differentiating osteoblasts (Aronson, 1994; Aronson, 1990; Vauhkonen, et al., 1990; Sato, 1998). Consolidation is the final phase that occurs after distraction ceases during which time bone and osteoid undergo mineralization and remodeling.
The DO model is most applicable to studies involving the biological processes that occur in response to mechanical strain applied to the callus during healing. Furthermore, DO is well suited for the study of the cellular and molecular regulators of bone regeneration. This has clinical translation to the treatment of limb length discrepancies and large bone defects.
Animals
Ten- to 18-week old mice
Individual mice used for a study should be within two weeks in age of each other
Materials
Absorbent bench underpads (for surgical bed, recovery, and x-ray station)
Non-fenestrated sterile field
Surgical gauze
Electric razor for shaving mouse fur
Small chamber for initial mouse anesthetization
Animal scale
Germinator dry bead sterilizer for instrument sterilization between surgeries
Heated pads for surgical bed and recovery
Isoflurane vaporizer (for mouse anesthesia; Surgi Vet)
Nose cone
Isoflurane for anesthesia
Betadine (10% povidone iodine solution)
Bupenorphine (Buprenex) for post-op pain control
Enrofloxacin (Baytril) for antibiotic prophylaxis
27-gauge x ½ in TB syringes
5-0 & 6-0 absorbable chromic gut suture
#15 disposable scalpel blades
Curved forceps (Dumont Vessel Cannulation Forceps Inox 0.5mm)
Scalpel handle
Needle driver
Micro-dissecting scissors (Castroviejo Micro Dissecting scissors)
Distraction device
Thin stainless steel wire (Standard Kobayashi Hooks 0.012”)
Wire cutters
Small soft tissue elevator
Foot-powered circular saw and diamond disc
18-gauge needle pre-bent for inserting wire around the bone
Distraction tool
Preparation and Induction of Anesthesia
This is the same as previously described for the fracture protocol.
Surgery
The mouse is placed on its side with the operative extremity up.
Using a #15 blade scalpel, a longitudinal incision is carried out in line with the femur from the greater trochanter to the knee.
The muscle fascia layer between the anterior and posterior muscle compartments is opened and split with micro-dissecting scissors to expose the femur.
Muscle fibers attached to the femur are bluntly dissected off using a tissue elevator.
Once the femur is exposed, a path is bluntly dissected posteriorly using the tissue elevator at the level of the distal femur and greater trochanter. The pre-bent 18-gauge needle is then passed posteriorly around the femur to facilitate placement of wire.
Wire is passed through the 18-gauge needle and placed at the distal femur and greater trochanter respectively.
Prior to placement of the DO device, it is maximally opened and closed to ensure proper function. From the closed position, the device is then opened 9-10 turns for placement.
The leg is then held with the non-dominant hand using the long and small fingers to hold the foot down while the DO device is applied to the femur by grasping it between the thumb and index fingers.
The DO device is then applied to the femur by threading the wires through the device. This is secured to the femur through tension banding technique. A needle driver is used to grasp the ends of the wire, and the wire is twisted counterclockwise until an appropriate tension is generated such that the device is secured to the femur. Using forceps, the device can be gently pulled to ensure it is secured tight enough to the bone. Care must be taken to ensure over-tensioning of the wires does not occur. This will cause the wires to break.
Next, a second set of wires is placed at the distal femur and greater trochanter. Again, the tissue elevator is used to create a path posterior to the femur to insert the 18-gauge needle wire guide.
Wires are then passed through the guide and placed in such a manner to cross the already placed wires at the distal femur and greater trochanter respectively.
Once proper placement is verified, wires are tightened using a needle driver and tension band technique as previously described.
At this point, all wires are bent and cut flush to the device using wire cutters.
A transverse osteotomy of the femoral shaft is generated using a small foot-powered circular saw. Care must be taken to ensure surrounding tissues are protected from the saw blade.
The muscle fascia is then closed with 6-0 absorbable suture followed by closure of skin with 5-0 absorbable suture around the device.
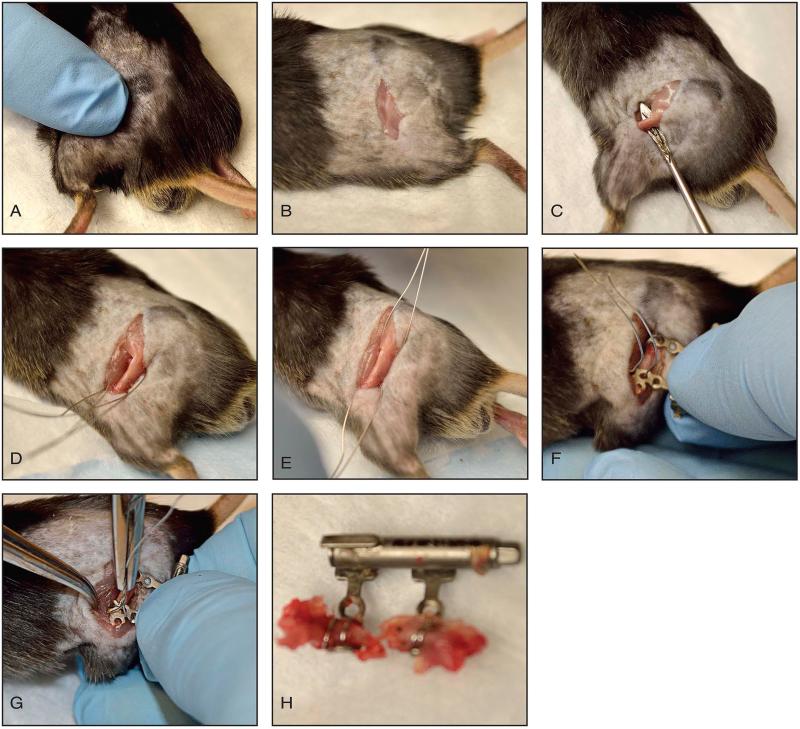
Figure 3 outlines the critical steps of this procedure.
Figure 3. Distraction osteogenesis procedure.
A: Palpation of the femur. B: Incision centered at femoral shaft. C: Tissue elevator used to strip muscles and expose femur. D, E: Wires passed around femur and centered at distal femur and greater trochanter. F: DO device passed through wires and tightened to bone. Wires tensioned to secure device to femur. G: A second set of wires are passed around the femur and centered at the distal femur and greater trochanter so they cross the previously placed wires. Wires tensioned using a needle driver. H: Demonstrates the DO device secure to bone with osteotomy present.
Post-operative Management
Animals are monitored on the heated recovery bed until they awaken from surgery before going back to their cage.
Analgesia is maintained with Buprenex for 48 hours post-operatively at 24-hour increments. Use of non-steroidal anti-inflammatory drugs (NSAIDs) should be avoided since they have been shown to impair bone healing after surgery (Simon, et al., 2002; Gerstenfeld, et al., 2007).
Animals should be able to regain free mobility within 48-hours. If not, they should be euthanized.
Infection is a fairly uncommon complication following DO surgery. Postoperative prophylactic antibiotics are given post-operative day one. Signs of infection would include general lethargy noticed from the mouse with a swollen leg. Occasionally there may be purulent drainage. The risk of infection generally is of most concern during the first week following surgery. If infection is noticed, the animal must be euthanized as this could affect bone healing.
Wound dehiscence is very common. Sutures need to be placed around the arms of the distraction device, which occasionally break and result in the skin opening back up. When this occurs, the wound is re-sutured using 5-0 chromic with the mouse under anesthesia as described previously. The wound may continue to open up despite attempts at re-suturing. If this occurs, the wound may be left to heal by secondary intention with daily wound care using antibiotic ointment. The wound is monitored for signs of infection until the time of harvest.
COMMERCIALLY AVAILABLE DEVICES FOR OPEN OSTEOTOMY AND DISTRACTION OSTEOGENESIS
Both the pharmaceutical and biotech industry has shown increased interest in recent years to develop new products to study and promote bone healing in mice and rats. Several companies affiliated with the AO Foundation including RISystem have developed a series of standardized commercially available devices for use in distraction osteogenesis and bone healing studies.
Open osteotomy has been proposed for the study of bone healing. This procedure allows for greater uniformity over the injury site as compared to fracture generated by blunt trauma. Matthys and Perren (2009) reported the use of an internal fixator, which consists of a plate that is secured to the bone with locking screws. This device allows for controlled variability of the rigidity of the construct with compression or splinting fixation (Matthys and Perren, 2009). Controlling rigidity of the fracture is important for the uniform study of bone healing. A rigid construct will heal by intramembranous ossification whereas a more flexible construct will heal by endochondral ossification with callus formation.
Distraction osteogenesis systems have been manufactured as well. One system commercialized by RISystem, secures the device to the femur through bicortical screw placement. This allows for increased rigidity for controlled distraction and easy application.
THE MARROW ABLATION PROTOCOL
Marrow ablation is a procedure in which the intramedullary cavity of a long bone is reamed (typically tibia) to generate an injury to the bone marrow. This model was initially described by Suva, et al (1993) in rat tibias; however, it was adapted for use in mice by Gerstenfeld, et al (2001). Marrow ablation primarily heals through an endosteal bone formation with a phase of osteogenesis replacing the initial blood clot after injury. Subsequently, this newly formed bone is resorbed by osteoclasts to restore normal bone marrow (Matthys and Perren, 2009).
Marrow ablation can be correlated to reaming for long bone intramedullary nail (IMN) fixation seen in the clinical setting. Long bone fractures, such as femur and tibia fractures, are commonly stabilized via IMN fixation in which the medullary cavity is reamed producing an injury to the bone marrow and stimulation of endosteal bone healing. This model is well suited for studies assessing basic molecular processes that affect endosteal bone formation as well as post-natal regulators of osteogenesis that affect the mesenchymal stem cell populations of cells found in the marrow space (Gerstenfeld, et al., 2001; Bais, et al., 2009; Bais, et al., 2012).
Animals
Ten- to 18-week old mice
Individual mice used for a study should be within two weeks in age of each other
Materials
Absorbent bench underpads (for surgical bed, recovery, and x-ray station)
Non-fenestrated sterile field
Surgical gauze
Electric razor for shaving mouse fur
Small chamber for initial mouse anesthetization
Animal scale
Germinator dry bead sterilizer for instrument sterilization between surgeries
Heated pads for surgical bed and recovery
Isoflurane vaporizer (for mouse anesthesia; Surgi Vet)
Nose cone
Isoflurane for anesthesia
Betadine (10% povidone iodine solution)
Bupenorphine (Buprenex) for post-op pain control
Enrofloxacin (Baytril) for antibiotic prophylaxis
27-gauge x ½ in TB syringes
5-0 chromic gut suture
#15 disposable scalpel blades
Curved forceps (Dumont Vessel Cannulation Forceps Inox 0.5mm)
Scalpel handle
Needle driver
Micro-dissecting scissors (Castroviejo Micro Dissecting scissors)
Stainless steel 25-gauge and 23-gauge needles
Sterile saline
Preparation and Induction of Anesthesia
This is the same as previously described for the fracture protocol
Surgery
The mouse is placed on its back and the operative leg is maximally flexed. The leg is held with the non-dominant hand between the thumb and index finger throughout the procedure.
Using a #15 scalpel blade, an approximate 2-3 mm anterior longitudinal midline incision is made centered over the knee joint.
A subsequent incision is made just medial to the patella and extensor mechanism. It is important to identify the extensor mechanism consisting of the quadriceps, patella tendon, and patella to avoid disrupting this mechanism in order to allow for immediate ambulation of the mouse following surgery.
The extensor mechanism is elevated and displaced in a lateral fashion using forceps.
After the extensor mechanism is subluxated laterally, the distal end of the femur as well as proximal end of the tibia will be exposed. From this exposure, an entry portal to the tibial medullary canal is created using a 27-gauge x ½ in TB syringe in the center of the proximal tibia.
The syringe is inserted until resistance is felt at the distal tibial growth plate, and then removed. Care must be taken to not force the needle further once resistance is felt; this can result in an iatrogenic tibia fracture.
The stylet of a 25-gauge spinal needle is inserted in an antegrade fashion through the medullary canal down to the distal growth plate. The marrow is ablated by inserting and rotating spinal needles of increasing diameter from the 25-gauge to the 23-gauge spinal needle.
After final canal reaming, the bone marrow cavity is flushed with sterile saline to remove loose marrow elements.
The extensor mechanism is pulled back to its anatomic location using forceps.
The incision is then closed with 5-0 absorbable chromic gut suture.
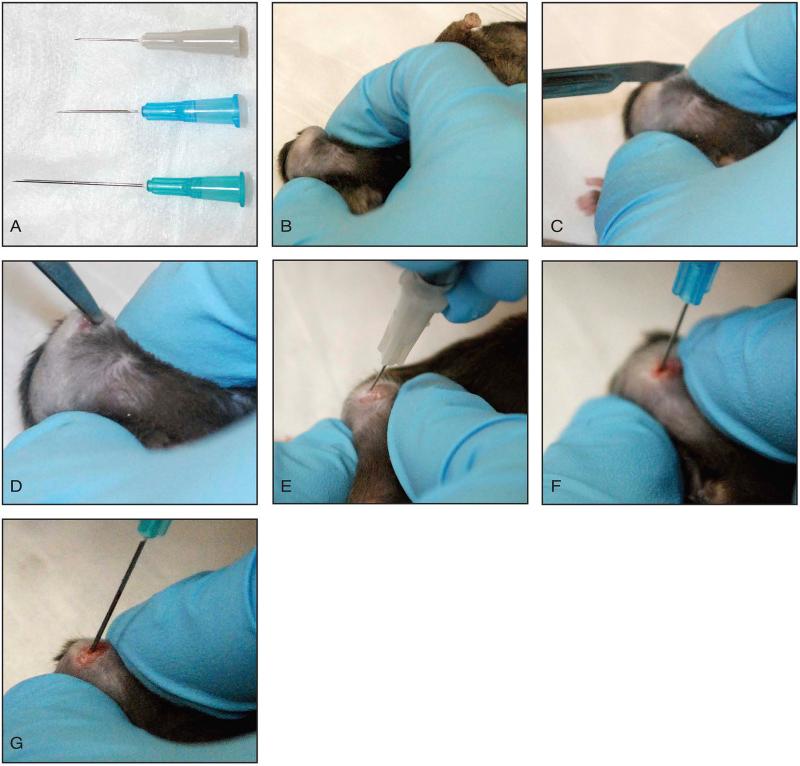
Figure 4 outlines the critical steps of this procedure.
Figure 4. Marrow ablation procedure.
A: Three different needle sizes required for progressive ablation of the tibia. B: The knee is maximally flexed. C: A 2-3 mm longitudinal incision is made centered over the knee. D: An incision is made medial to the patella, which extends into the quadriceps muscle and along the patella tendon to release the tissue. The patella is subluxated laterally. E, F, G: The proximal tibia is instrumented and progressively larger needles are used to ablate the medullary canal.
Post-operative Management
Animals are monitored on the heated recovery bed until they awaken from surgery before going back to their cage.
Analgesia is maintained with Buprenex for 48 hours post-operatively at 24-hour increments. Use of non-steroidal anti-inflammatory drugs (NSAIDs) should be avoided since they have been shown to impair bone healing after surgery (Simon, et al., 2002; Gerstenfeld, et al., 2007). Animals should be able to regain free mobility within 48-hours. If not, they should be euthanized.
Infection is a rare occurrence following the marrow ablation procedure. Postoperative prophylactic antibiotics are generally not necessary. Signs of infection would include a swollen leg and general lethargy noticed from the mouse. Occasionally there may be purulent drainage. The risk of infection generally is of most concern during the first week following surgery. If infection is noted, the animal must be euthanized as this could affect bone healing.
During the procedure, there is a risk of iatrogenic tibia fracture while instrumenting the medullary canal. Fracture is typically immediately evident during the procedure; however this can occasionally go unnoticed. Postoperatively, the mouse should be observed for inability to bear weight on the operative extremity or deformity, which would indicate the possibility of a tibia fracture. The animal should be euthanized if this were to occur.
Acknowledgements
This work was supported by funding from NIH/NIAMS to LCG; R01AR056637 and R01AR059741.
REFERENCE
- Al-Aql ZS, et al. Molecular Mechanisms Controlling Bone Formation during Fracture Healing and Distraction Osteogenesis. J Dent Res. 2008;87(2):107–118. doi: 10.1177/154405910808700215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson J, Good B, Stewart C, Harrison B, Harp J. Preliminary studies of mineralization during distraction osteogenesis. Clin Orthop Relat Res. 1990;250:43–49. [PubMed] [Google Scholar]
- Aronson J. Experimental and clinical experience with distraction osteogenesis. Cleft Palate Craniofac J. 1994;31:473–482. doi: 10.1597/1545-1569_1994_031_0473_eacewd_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Bais MV, et al. BMP2 is essential for post natal osteogenesis but not for recruitment of osteogenic stem cells. Bone. 2009;45:254–266. doi: 10.1016/j.bone.2009.04.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bais MV, et al. Role of Nanog in the maintenance of marrow stromal stem cells during post natal bone regeneration. Biochemical and Biophysical Research Communications. 2012;417:211–216. doi: 10.1016/j.bbrc.2011.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnarens F, Einhorn T. Production of a standard closed fracture in laboratory animal bone. J Ortho Res. 1984;2(1):97–101. doi: 10.1002/jor.1100020115. [DOI] [PubMed] [Google Scholar]
- Codivilla A. On the means of lengthening in the lower limbs. Am J Orthop Surg. 1905;2:353–369. [Google Scholar]
- Colnot C, et al. Altered fracture repair in the absence of MMP9. Development. 2003;130(17):4123–33. doi: 10.1242/dev.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstenfeld LC, et al. Impaired intramembranous bone formation during bone repair in the absence of tumor necrosis factor-alpha signaling. Cells Tissues Organs. 2001;169:285–94. doi: 10.1159/000047893. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld LC, et al. Three Dimensional Reconstruction of Fracture Callus Morphogenesis Demonstrates Asymmetry in Callus Development. J Histochem Cytochem. 2006;54(11):1215–1228. doi: 10.1369/jhc.6A6959.2006. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld LC, et al. Selective and nonselective cyclooxygenase-2 inhibitors and experimental fracture-healing: Reversibility of effects after short-term treatment. J Bone Joint Surg Am. 2007;89(1):114–25. doi: 10.2106/JBJS.F.00495. [DOI] [PubMed] [Google Scholar]
- Grimes R, et al. The Transcriptome of Fracture Healing Defines Mechanisms of Coordination of Skeletal and Vascular Development during Endochondral Bone Formation. J Bone Miner Res. 2011;26(11):2597–609. doi: 10.1002/jbmr.486. [DOI] [PubMed] [Google Scholar]
- Hiltunen A, Vuorio E, Aro H. A standardized experimental fracture in the mouse tibia. J Ortho Res. 1993;11(2):305–12. doi: 10.1002/jor.1100110219. [DOI] [PubMed] [Google Scholar]
- Ilizarov GA. The tension-stress effect on the genesis and growth of tissues. Part I. The influence of stability of fixation and soft-tissue preservation. Clin Orthop Relat Res. 1989;238:249–281. [PubMed] [Google Scholar]
- Jepsen KJ, et al. Genetic variation in the patterns of skeletal progenitor cell differentiation and progression during endochondral bone formation affects the rate of fracture healing. J Bone Miner Res. 2008;23(8):1204–16. doi: 10.1359/JBMR.080317. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon T, et al. Expression of osteoprotegerin, receptor activator of NF-kappaB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J Bone Miner Res. 2001;16(6):1004–14. doi: 10.1359/jbmr.2001.16.6.1004. [DOI] [PubMed] [Google Scholar]
- Lu C, et al. Mechanical stability affects angiogenesis during early fracture healing. J Orthop Trauma. 2011;25(8):494–9. doi: 10.1097/BOT.0b013e31822511e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marturano JE, et al. An improved murine femur fracture device for bone healing studies. J Biomech. 2008;41(6):1222–8. doi: 10.1016/j.jbiomech.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Matthys R, Perren SM. Internal fixator for use in the mouse. Injury. 2009;40:S103–S109. doi: 10.1016/j.injury.2009.10.044. [DOI] [PubMed] [Google Scholar]
- Miclau T, et al. Effects of delayed stabilization on fracture healing. J Orthop Res. 2007;25(12):1552–8. doi: 10.1002/jor.20435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury-Palomares KT, et al. Mechanical stimulation alters tissue Differentiation and molecular expression during bone healing. J. Orthop. Res. 2009;27:1123–1132. doi: 10.1002/jor.20863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, et al. Expression of bone matrix proteins mRNA during distraction osteogenesis. J Bone Miner Res. 1998;13:1221–1231. doi: 10.1359/jbmr.1998.13.8.1221. [DOI] [PubMed] [Google Scholar]
- Simon AM, Manigrasso MB, O'Connor JP. Cyclo-oxygenase 2 function is essential for bone fracture healing. J Bone Miner Res. 2002;17(6):963–76. doi: 10.1359/jbmr.2002.17.6.963. [DOI] [PubMed] [Google Scholar]
- Suva LJ, et al. Pattern of gene expression following rat tibial marrow ablation. J Bone Miner Res. 1993;8:379–88. doi: 10.1002/jbmr.5650080315. [DOI] [PubMed] [Google Scholar]
- Tsuji K, et al. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38(12):1424–9. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- Vauhkonen M, et al. Collagen synthesis and mineralization in the early phase of distraction bone healing. Bone Miner. 1990;10:171–181. doi: 10.1016/0169-6009(90)90260-m. [DOI] [PubMed] [Google Scholar]
- Wigner NA, et al. Acute phosphate restriction leads to impaired fracture healing and resistance to BMP-2. J Bone Miner Res. 2010;25(4):724–33. doi: 10.1359/jbmr.091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YY, et al. Creating rigidly stabilized fractures for assessing intramembranous ossification, distraction osteogenesis, or healing of critical sized defects. J Vis Exp. 2012;11:62. doi: 10.3791/3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, et al. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin Invest. 2002;109(11):1405–15. doi: 10.1172/JCI15681. [DOI] [PMC free article] [PubMed] [Google Scholar]