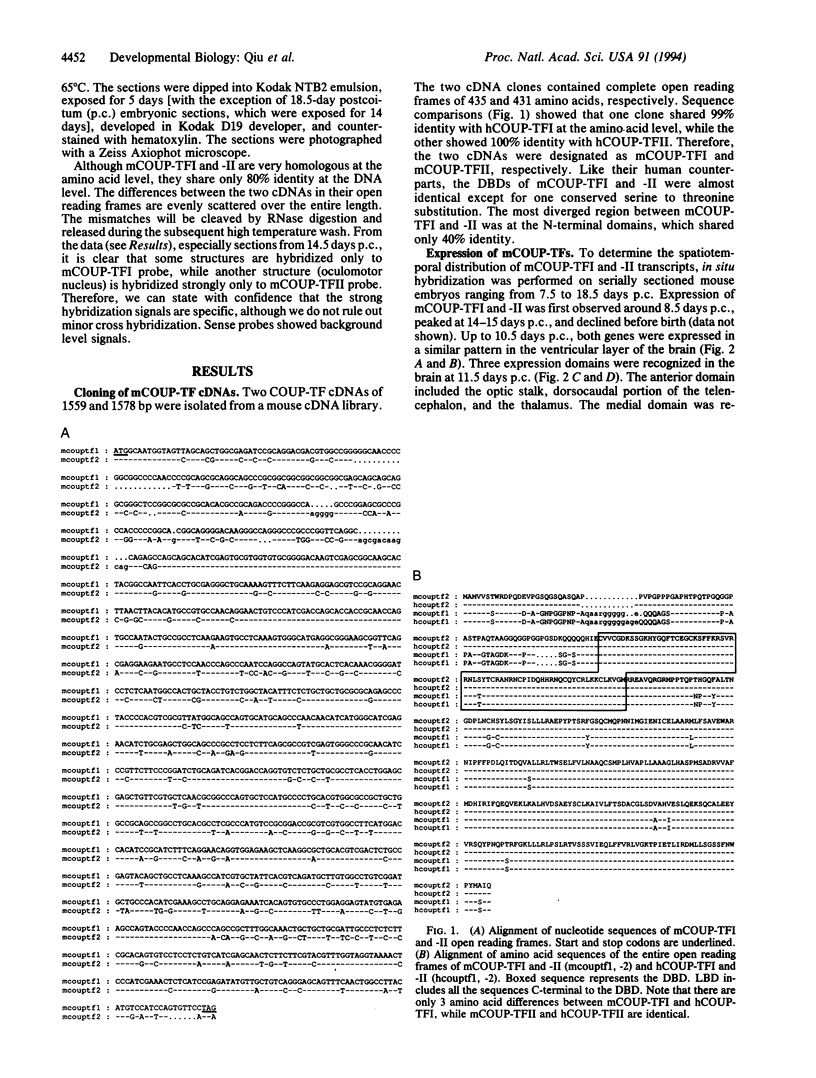
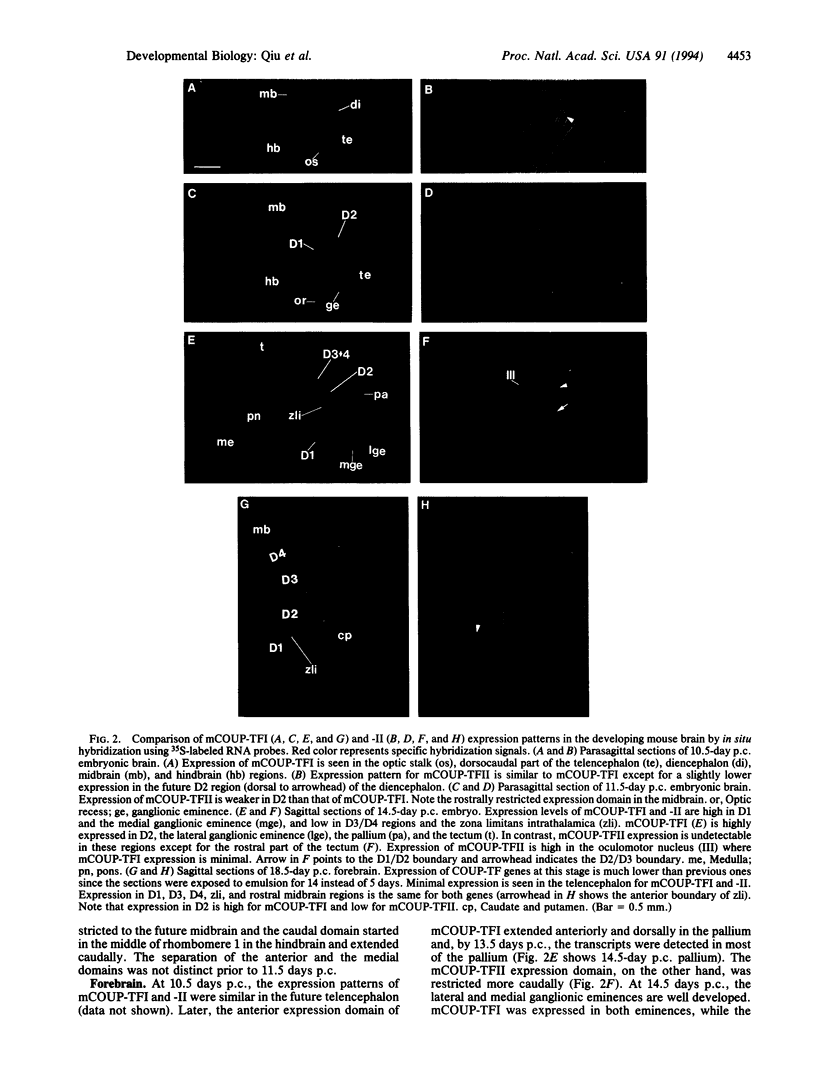
Abstract
Chicken ovalbumin upstream promoter-transcription factor (COUP-TF) genes encode transcription factors belonging to the orphan subfamily of the steroid/thyroid hormone receptor superfamily. Two COUP-TF counterparts have been cloned from mouse. In an attempt to study the function of these genes in the developing central nervous system (CNS), the spatiotemporal expression patterns of the two mouse genes have been examined by in situ hybridization. Both genes are widely expressed in the developing CNS, with patterns that are overlapping yet distinct from each other. The differential expression of murine COUP-TFI and -II in the diencephalon is striking in that high levels of expression from each gene are confined to specific segmental compartments--the neuromeres. Our results suggest that murine COUP-TFs may play important roles in the development and differentiation of the CNS, including the specification of diencephalic neuromeres.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bocquel M. T., Kumar V., Stricker C., Chambon P., Gronemeyer H. The contribution of the N- and C-terminal regions of steroid receptors to activation of transcription is both receptor and cell-specific. Nucleic Acids Res. 1989 Apr 11;17(7):2581–2595. doi: 10.1093/nar/17.7.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulfone A., Kim H. J., Puelles L., Porteus M. H., Grippo J. F., Rubenstein J. L. The mouse Dlx-2 (Tes-1) gene is expressed in spatially restricted domains of the forebrain, face and limbs in midgestation mouse embryos. Mech Dev. 1993 Mar;40(3):129–140. doi: 10.1016/0925-4773(93)90071-5. [DOI] [PubMed] [Google Scholar]
- Chan S. M., Xu N., Niemeyer C. C., Bone J. R., Flytzanis C. N. SpCOUP-TF: a sea urchin member of the steroid/thyroid hormone receptor family. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10568–10572. doi: 10.1073/pnas.89.22.10568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney A. J., Leng X., Tsai S. Y., O'Malley B. W., Tsai M. J. Multiple mechanisms of chicken ovalbumin upstream promoter transcription factor-dependent repression of transactivation by the vitamin D, thyroid hormone, and retinoic acid receptors. J Biol Chem. 1993 Feb 25;268(6):4152–4160. [PubMed] [Google Scholar]
- Cooney A. J., Tsai S. Y., O'Malley B. W., Tsai M. J. Chicken ovalbumin upstream promoter transcription factor (COUP-TF) dimers bind to different GGTCA response elements, allowing COUP-TF to repress hormonal induction of the vitamin D3, thyroid hormone, and retinoic acid receptors. Mol Cell Biol. 1992 Sep;12(9):4153–4163. doi: 10.1128/mcb.12.9.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easter S. S., Jr, Ross L. S., Frankfurter A. Initial tract formation in the mouse brain. J Neurosci. 1993 Jan;13(1):285–299. doi: 10.1523/JNEUROSCI.13-01-00285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichele G. Retinoids and vertebrate limb pattern formation. Trends Genet. 1989 Aug;5(8):246–251. doi: 10.1016/0168-9525(89)90096-6. [DOI] [PubMed] [Google Scholar]
- Figdor M. C., Stern C. D. Segmental organization of embryonic diencephalon. Nature. 1993 Jun 17;363(6430):630–634. doi: 10.1038/363630a0. [DOI] [PubMed] [Google Scholar]
- Fjose A., Nornes S., Weber U., Mlodzik M. Functional conservation of vertebrate seven-up related genes in neurogenesis and eye development. EMBO J. 1993 Apr;12(4):1403–1414. doi: 10.1002/j.1460-2075.1993.tb05784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkers G. E., van der Leede B. J., van der Saag P. T. The retinoic acid receptor-beta 2 contains two separate cell-specific transactivation domains, at the N-terminus and in the ligand-binding domain. Mol Endocrinol. 1993 Apr;7(4):616–627. doi: 10.1210/mend.7.4.8389001. [DOI] [PubMed] [Google Scholar]
- Goulding M. D., Chalepakis G., Deutsch U., Erselius J. R., Gruss P. Pax-3, a novel murine DNA binding protein expressed during early neurogenesis. EMBO J. 1991 May;10(5):1135–1147. doi: 10.1002/j.1460-2075.1991.tb08054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel M., Gruss P. Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell. 1991 Oct 4;67(1):89–104. doi: 10.1016/0092-8674(91)90574-i. [DOI] [PubMed] [Google Scholar]
- Kessel M. Respecification of vertebral identities by retinoic acid. Development. 1992 Jun;115(2):487–501. doi: 10.1242/dev.115.2.487. [DOI] [PubMed] [Google Scholar]
- Keyser A. The development of the diencephalon of the Chinese hamster. An investigation of the validity of the criteria of subdivision of the brain. Acta Anat Suppl (Basel) 1972;59:1–178. [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Heyman R. A., Mangelsdorf D. J., Dyck J. A., Evans R. M. Retinoid X receptor-COUP-TF interactions modulate retinoic acid signaling. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1448–1452. doi: 10.1073/pnas.89.4.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladias J. A., Karathanasis S. K. Regulation of the apolipoprotein AI gene by ARP-1, a novel member of the steroid receptor superfamily. Science. 1991 Feb 1;251(4993):561–565. doi: 10.1126/science.1899293. [DOI] [PubMed] [Google Scholar]
- Lutz B., Kuratani S., Cooney A. J., Wawersik S., Tsai S. Y., Eichele G., Tsai M. J. Developmental regulation of the orphan receptor COUP-TF II gene in spinal motor neurons. Development. 1994 Jan;120(1):25–36. doi: 10.1242/dev.120.1.25. [DOI] [PubMed] [Google Scholar]
- Matharu P. J., Sweeney G. E. Cloning and sequencing of a COUP transcription factor gene expressed in Xenopus embryos. Biochim Biophys Acta. 1992 Feb 11;1129(3):331–334. doi: 10.1016/0167-4781(92)90512-x. [DOI] [PubMed] [Google Scholar]
- McMahon A. P., Joyner A. L., Bradley A., McMahon J. A. The midbrain-hindbrain phenotype of Wnt-1-/Wnt-1- mice results from stepwise deletion of engrailed-expressing cells by 9.5 days postcoitum. Cell. 1992 May 15;69(4):581–595. doi: 10.1016/0092-8674(92)90222-x. [DOI] [PubMed] [Google Scholar]
- Miyajima N., Kadowaki Y., Fukushige S., Shimizu S., Semba K., Yamanashi Y., Matsubara K., Toyoshima K., Yamamoto T. Identification of two novel members of erbA superfamily by molecular cloning: the gene products of the two are highly related to each other. Nucleic Acids Res. 1988 Dec 9;16(23):11057–11074. doi: 10.1093/nar/16.23.11057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlodzik M., Hiromi Y., Weber U., Goodman C. S., Rubin G. M. The Drosophila seven-up gene, a member of the steroid receptor gene superfamily, controls photoreceptor cell fates. Cell. 1990 Jan 26;60(2):211–224. doi: 10.1016/0092-8674(90)90737-y. [DOI] [PubMed] [Google Scholar]
- NIIMI K., HARADA I., KUSAKA Y., KISHI S. The ontogenetic development of the diencephalon of the mouse. Tokushima J Exp Med. 1961 Nov;8:203–238. [PubMed] [Google Scholar]
- Porteus M. H., Bulfone A., Ciaranello R. D., Rubenstein J. L. Isolation and characterization of a novel cDNA clone encoding a homeodomain that is developmentally regulated in the ventral forebrain. Neuron. 1991 Aug;7(2):221–229. doi: 10.1016/0896-6273(91)90260-7. [DOI] [PubMed] [Google Scholar]
- Price M., Lemaistre M., Pischetola M., Di Lauro R., Duboule D. A mouse gene related to Distal-less shows a restricted expression in the developing forebrain. Nature. 1991 Jun 27;351(6329):748–751. doi: 10.1038/351748a0. [DOI] [PubMed] [Google Scholar]
- Puelles L., Amat J. A., Martinez-de-la-Torre M. Segment-related, mosaic neurogenetic pattern in the forebrain and mesencephalon of early chick embryos: I. Topography of AChE-positive neuroblasts up to stage HH18. J Comp Neurol. 1987 Dec 8;266(2):247–268. doi: 10.1002/cne.902660210. [DOI] [PubMed] [Google Scholar]
- Ritchie H. H., Wang L. H., Tsai S., O'Malley B. W., Tsai M. J. COUP-TF gene: a structure unique for the steroid/thyroid receptor superfamily. Nucleic Acids Res. 1990 Dec 11;18(23):6857–6862. doi: 10.1093/nar/18.23.6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson G. W., Wray S., Mahon K. A. Spatially restricted expression of a member of a new family of murine Distal-less homeobox genes in the developing forebrain. New Biol. 1991 Dec;3(12):1183–1194. [PubMed] [Google Scholar]
- Roelink H., Nusse R. Expression of two members of the Wnt family during mouse development--restricted temporal and spatial patterns in the developing neural tube. Genes Dev. 1991 Mar;5(3):381–388. doi: 10.1101/gad.5.3.381. [DOI] [PubMed] [Google Scholar]
- Simeone A., Acampora D., Gulisano M., Stornaiuolo A., Boncinelli E. Nested expression domains of four homeobox genes in developing rostral brain. Nature. 1992 Aug 20;358(6388):687–690. doi: 10.1038/358687a0. [DOI] [PubMed] [Google Scholar]
- Sundin O. H., Eichele G. A homeo domain protein reveals the metameric nature of the developing chick hindbrain. Genes Dev. 1990 Aug;4(8):1267–1276. doi: 10.1101/gad.4.8.1267. [DOI] [PubMed] [Google Scholar]
- Tata J. R. Gene expression during metamorphosis: an ideal model for post-embryonic development. Bioessays. 1993 Apr;15(4):239–248. doi: 10.1002/bies.950150404. [DOI] [PubMed] [Google Scholar]
- Tora L., White J., Brou C., Tasset D., Webster N., Scheer E., Chambon P. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell. 1989 Nov 3;59(3):477–487. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- Tran P., Zhang X. K., Salbert G., Hermann T., Lehmann J. M., Pfahl M. COUP orphan receptors are negative regulators of retinoic acid response pathways. Mol Cell Biol. 1992 Oct;12(10):4666–4676. doi: 10.1128/mcb.12.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther C., Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991 Dec;113(4):1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Ing N. H., Tsai S. Y., O'Malley B. W., Tsai M. J. The COUP-TFs compose a family of functionally related transcription factors. Gene Expr. 1991;1(3):207–216. [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Tsai S. Y., Cook R. G., Beattie W. G., Tsai M. J., O'Malley B. W. COUP transcription factor is a member of the steroid receptor superfamily. Nature. 1989 Jul 13;340(6229):163–166. doi: 10.1038/340163a0. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bailes J. A., Champion J. E., McMahon A. P. A molecular analysis of mouse development from 8 to 10 days post coitum detects changes only in embryonic globin expression. Development. 1987 Apr;99(4):493–500. doi: 10.1242/dev.99.4.493. [DOI] [PubMed] [Google Scholar]