Abstract
Laboratory instruction of neuroscience is often limited by the lack of physical resources and supplies (e.g., brains specimens, dissection kits, physiological equipment). Online databases can serve as supplements to material labs by providing professionally collected images of brain specimens and their underlying cellular populations with resolution and quality that is extremely difficult to access for strictly pedagogical purposes. We describe a method using two online databases, the Neuromorpho.org and the Allen Brain Atlas (ABA), that freely provide access to data from working brain scientists that can be modified for laboratory instruction/exercises. Neuromorpho.org is the first neuronal morphology database that provides qualitative and quantitative data from reconstructed cells analyzed in published scientific reports. The Neuromorpho.org database contains cross species and multiple neuronal phenotype datasets which allows for comparative examinations. The ABA provides modules that allow students to study the anatomy of the rodent brain, as well as observe the different cellular phenotypes that exist using histochemical labeling. Using these tools in conjunction, advanced students can ask questions about qualitative and quantitative neuronal morphology, then examine the distribution of the same cell types across the entire brain to gain a full appreciation of the magnitude of the brain’s complexity.
Keywords: neuronal morphology; digital experiments, anatomy
INTRODUCTION
Hands-on laboratory courses are used to give students a multisensory experience of the anatomy (Grisham, 2006), vasculature (Ramos et al., 2008) and physiology (Ramos et al., 2007a) of the brain. However, the cost of acquiring, maintaining and updating the materials for these labs can be prohibitive. An alternative that would address these rising concerns could be the incorporation of more online learning (also known as E-learning) opportunities into undergraduate laboratory courses. Perhaps not surprisingly, students often choose to use the online learning method over classroom instruction because they can work at their own pace providing them with a more flexible weekly schedule. However, online learning features also have pedagogical benefits. The immediate assessment and feedback that online resources deliver allow the learner to experience immediate reinforcement that can increase a student’s motivation to meet course learning objectives more efficiently (Cox and Cordray, 2008; Nichols, 2008). Such online methods for teaching cellular diversity have been enumerated (Ramos et al., 2007b) using the Allen Brain Atlas (ABA), a free, multispecies, developmental, molecular and anatomical database managed by the Allen Brain Institute. Other online systems allow for even more interactive lab courses in neurophysiology.
A very real issue in online courses is the lack of three-dimensional manipulations that engage students. However, computer based labs can be more comprehensive in their coverage of the actual data that practicing neuroscientists gather and work with. Although the extant resources cover a large breadth of neuroscience, there is a lack of materials for student examination of neuronal morphology. To address this we have used Neuromorpho (www.Neuromorpho.org), a curated neuronal morphology database, which makes neuronal reconstructions gathered from many laboratories, freely available to all scientists (Parekh and Ascoli, 2013). Neuromorpho.org is the first neuronal morphology database that provides qualitative and quantitative data from reconstructed cells analyzed in published scientific reports. The Neuromorpho.org database contains cross species and multiple neuronal phenotype datasets which allows for comparative examinations. Thus, students will gain a full appreciation of the brain’s immense complexity using Neuromorpho’s website in conjunction with ABA’s instructive resources concerning brain structure and connectivity. We propose that these two online resources can be used to convey neuroscience related topics more effectively in the undergraduate classroom than traditional routes of using limited textbook or laboratory materials. So, how would undergraduate neuroscience faculty develop lesson plans using these rich online resources? Here we will present both ABA and Neuromorpho lesson plans that involve basic neuroscience topics discussed in any introductory undergraduate neuroscience course.
RESULTS
Neuronal morphology
Neuronal ‘structure to function’ relationships are an area of intense research that students are often unaware of due to its complexity. The clearest example of morphology affecting function is in the spinal cord, where Hennemans size principal dictates that smaller motor neurons are recruited before larger motor neurons ensuring the slow twitch, low force, fatigue resistant muscle fibers are recruited before the fast twitch high force muscles that fatigue quickly (See Mendell, [2005] for perspectives). Outside of the spinal cord, structure function relationships become trickier to discern. However, it has been reported that the size and shape of a neuron can affect its passive electronic properties, ability to integrate synaptic signals, and potentially information storage capabilities (Stiefel and Sejnowski, 2007).
Neuromorpho allows you to observe different cellular phenotypes identified morphologically in a three-dimensional brain module supported by the ABA. The brain model can be rotated, made translucent, and selected brain regions or cell types can be visualized or hidden. Anatomical identification of specific brain regions can even be done using the coronal or sagittal section viewer.
Our goal is to provide guidance for students to understand how neuronal morphology looks, how it is analyzed, and what that may mean in the context of the existing research. To achieve this goal we have developed supplemental background, instructions on using Neuromorpho.org and the ABA (PowerPoint slides, Chu et al., 2015a), and exercises (five exercises in supplemental material) with the ultimate goal of preparing students to understand, articulate and present primary research material on the topic of neuronal morphology.
Advantages to digital neuronal morphology
One key benefit to using Neuromorpho is the ability to observe qualitative (2-D and 3-D) and quantitative difference between neurons from different brain regions and species under different experimental conditions. Even under control conditions, it is clear that many cell types have drastically different morphologies that may correlate with their function (Figure 1).
Figure 1.

Example of motor and sensory neuron morphologies. A, motor neuron from a 10 day old mouse (neuromorpho.org ID: NMO_00909), this neuron resides in the spinal cord and controls muscle contractions. B, ganglion neuron from the 60 day old mouse retina (NMO_10769), this neuron sends axons from the retina to the brain. White, Soma; Green, dendrites; gray, axon.
Providing students the opportunity to work with published scientific data has a key advantage over looking at pictures on the web or even working with real data specimens. Since the work has been published, we can assume that the data are ‘good’ and yield a significant result. We have provided exercises that allow students to ‘follow the footsteps’ of scientists who have submitted their data into Neuromorpho by analyzing their cells. The advantage of this is that students can not only see the raw data, but also get to read about what these data mean in the context of the field.
Quantitative analysis is also possible using Neuromorpho and is particularly useful for understanding how real research is conducted. Many published articles provide >10 cells to Neuromorpho and so may be somewhat amenable to statistical analysis (as long as the students understand the basic caveats associated with this type of sample).
Even if students do not find the results the authors did, it may be instructional to note the differences in the type of analysis conducted by the authors versus what data is available, the limitations of the research, and the importance of operational definitions.
Methods
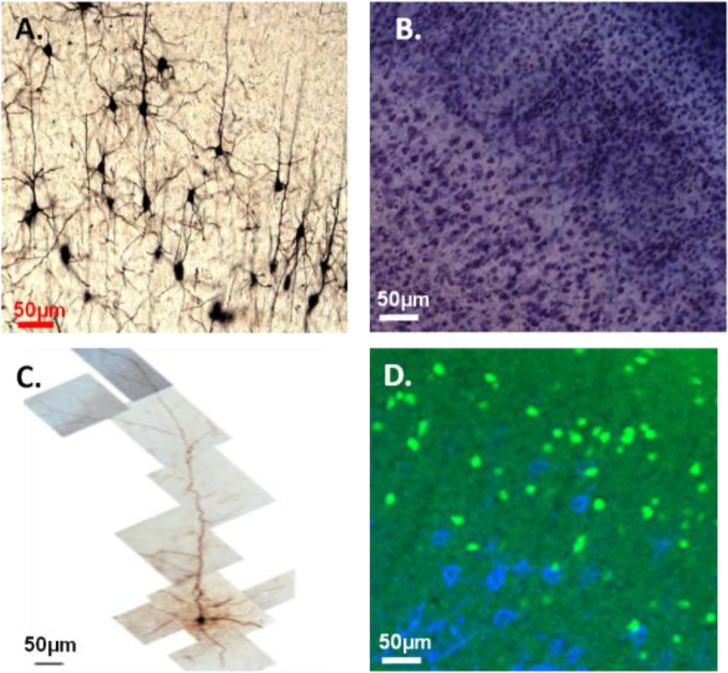
We included a section reviewing different methodologies for visualizing neuronal morphology (Figure 2). It is not intended to be comprehensive and avoids using jargon as much as possible. For example Biotin Dextran Amine retrograde labeling can reveal neuronal morphology as well, but is not mentioned in that section. The hope was that students would become familiar with some of the common techniques to analyze neurons which can then provide them some foundation for the exercises based on research articles (Exercises 2 and 3, Chu et al. 2015b).
Figure 2.
Different methods for visualizing neurons. The Golgi method only stains about 5% of neurons, but provides a detailed picture of the neurons morphology (micrograph generously provided by Adesh Bajnath), A. Pan-cellular Nissl stain labels all cells excluding red blood cells. Blue spots are cell bodies in the neocortex. Note the dark and light bands indicating denser and sparser regions of cell distribution, B. A single cell filled pyramidal neuron imaged using a high magnification lens and stitched following electrophysiological recording experiments (Yang et al., 2014), C. Using immunohistochemistry to label extracellular matrix molecules (blue) and GFP to identify the 5HT3a serotonin receptor expressing inhibitory interneurons (green) simultaneously demonstrates non-overlapping populations of cells showcasing the advantage to fluorescence, D.
Integration of primary literature
The neuronal morphology data collected from different labs and the actual publications that those data helped to inform are available on Neuromorpho. Students can be instructed to read the articles associated with the specific cells they included in their analysis. This allows students to look at the same data that the authors did, and then read about how these were analyzed and how the data was interpreted. Students often do not get to look at the complexity and complications of real data outside of volunteering in a laboratory or during post graduate work, but digital experiments and websites enable this to occur freely and easily.
Qualitative exercises
We have provided background material into neuronal morphology and function relationships at the cellular level in our supplemental materials (Exercise 1, Chu et al., 2015b). A prime example is the Purkinje cell, which receives up to 200,000 synapses from parallel fibers and could not function without its distinctly flat and fanned-out dendritic morphology (Figure 3).
Figure 3.
Purkinje cell from the Guinea-pig cerebellum, (NMO_00610). Notice the highly arborized dendrites (green), A. The Purkinje dendritic tree forms a nearly flat 2-D layer when rotated on its axis, B. This can be viewed by searching the Neurmorpho ID on Neuromorpho.org and viewing the animation, which rotates the cell in 3-D. Alternatively, the cell can be viewed using the 3D neuron viewer, and rotated manually by holding right click and dragging the mouse to rotate. The purkinje cell axons, not shown here, are inhibitory, and provide the entire output of the cerebellar cortex.
We also provide materials describing some general morphological differences between inhibitory interneurons and excitatory neurons. Inhibitory interneurons come in a diverse set of morphologies that distinctly correlate with their function. For example, Chandelier cells (morphological descriptor) make cartridge synapses that wind around post synaptic axons. This is sometimes visible even from morphological reconstructions of the chandelier cells axons in 2-D and becomes even more apparent in 3-D.
The animation option and 3-D neuron viewer (requires updated Java) provide the neophyte an entirely new perspective and appreciation of the beauty of neuronal structure.
One exercise that we have included asks students to navigate the 3-D rat brain in Neuromorpho and choose pyramidal neurons in different functional brain regions, asks the students to sketch the neurons based on the morphological reconstructions provided, and then describe them. We also provide some nomenclature commonly used to describe neuronal morphology (e.g., dendritic arbor, bifurcation) with the goal of eventually providing the students with enough experience to read and present primary literature articles.
Quantitative exercises
Using data from two primary source articles that were generously provided to Neuromorpho (Jacobs et al., 2001; Kole et al., 2004), we provide an opportunity for students to follow in the footsteps of the authors using the published data (Exercise 2 and 3, Chu et al., 2015b). Students are asked to read through the paper and search for basic questions that researchers use to understand the important points of an article (Exercise 2, Chu et al., 2015b). Data is also collected from the Neuromorpho database by searching for the first authors last name in the metadata--> archive section. Students then collect a small sample in the table provided and determine whether there is a different or not. Statistical analysis is not required from our exercise, but can be useful as a supplement with an instructors guidance. We attempted to find papers that the students may find interesting and generally received positive responses. For the Jacobs and colleagues (2001) article, students compare the basilar dendritic morphology across regions of the human brain that vary in complexity (Figure 4).
Figure 4.

Example of morphological parameters provided by Neuromorpho for one human pyramidal neuron (NMO_03481) from Jacobs et al. (2001). For exercise 2, students are asked to collect a sample of cells from different brain regions to compare their basilar dendrite morphology using quantitative measures derived from these measurements.
For the Kole et al. (2004) work, the students are asked to compare hippocampal CA3 pyramidal neuron dendrites between control animals and those that were stressed. Although the interpretations of the results can potentially be complex, reading the authors own words gives a better understanding of the reasoning process used by scientists. For the Jacobs et al (2001) work, the take home message is that more complex dendrites are associated with more complex brain regions. For Kole et al. (2004), stress alters dendritic spines which may impact memory formation.
Supplementing digital morphology
Navigating through brain anatomy can be a challenging task and is usually only emphasized at the graduate level and during medical school training. However, using the ABA it is possible to teach undergraduate students important anatomical concepts, such as lamination patterns, and areal segregation of the cortex (Ramos et al., 2007b; Figure 1, Tutorial available on http://www.brain-map.org/tutorials/index.html). Students can browse through labeled sections, choose a region that looks interesting to them and pull up the anatomical reference side by side to learn what the region is. Going one step further, recent additions to the ABA have allowed for specific protein localization in mouse sections. The Mouse connectivity tab and the reference data module allows for the selection of various fluorescently labeled proteins in high resolution to look at the gross and microscopic distribution of different molecularly identified cell types in the brain.
We have included one exercise (4) using the ABA to supplement our digital morphology exercises. The purpose of using the ABA along with Neuromorpho is to provide a fuller understanding of not just morphology, but different cell types. For example, we point out that inhibitory interneurons in the neocortex come in many flavors, with different properties that can be used to classify them. Chandelier cells synapse onto the axons of post-synaptic excitatory neurons (morphological type), using the inhibitory neurotransmitter GABA (neurotransmitter type), fire high frequency action potentials (physiological type) and express the calcium binding protein Parvalbumin (PV, protein expressing class). Although Neuromorpho allows us to visualize the 3-D structure of neurons and quantitatively analyze their structure, we can only do this one neuron at a time. The ABA contains reference data for many types of cells using in situ hybridization, including PV. Using the reference data, as well the gene search functions, students can see for themselves how different cell types they have identified morphologically are distributed throughout the brain.
Looking at the distribution of PV neurons shows that they are generally expressed throughout the cerebellum and less so in other brain regions. Students are asked to fill in the table describing the function of the brain regions and level of expression of PV and to think about whether there may be a correlation between cell type, structure and function. Then students are asked to look at the ABA quantitative analysis of the Pvalb RNA to see how their observations matched up with the analysis. For more advanced students, a reading of the primary literature or review provides a deeper understanding of what these different distributions may mean for behavior (see Lewis et al. 2005).
Caveats
One major difficulty is that the data available on Neuromorpho.org is limited, and setting sufficiently controlled parameters limits the number of available neurons even further. However, finding relatively well controlled sets of neurons to compare is possible.
For example, setting relatively strict criteria in the metadata search still allowed for the comparison of two mouse pyramidal layer 3 cells in the visual cortex and five pyramidal layer 3 cells in the monkey neocortex (Supplemental materials, Chu et al., 2015b). These data show that at least under these criteria, the mouse neurons are larger than the monkey, a relatively counterintuitive discovery given that the monkey brain is more than two orders of magnitude larger than the mouse (Herculano-Houzel, 2009). These results suggest higher packing density and thus smaller cells which may be more beneficial for improved visual processing in monkeys versus mouse.
Evaluation
Students are more invested in learning about anatomy, cellular phenotypes and systems if they are given a choice and allowed to explore it themselves over several class sessions. Questions are also provided in exercises 1–4 (Supplemental materials, Chu et al., 2015b) that can be used for instructor evaluation. In class presentations requires students to read the primary literature articles and promote critical thinking and analysis. From our experience, several presentations provide the opportunity for critical questioning, feedback, and progressive improvement for students. Exercise 5, (Chu et al., 2015b) in our supplemental materials provides a basic format for students to present a primary literature article, using the knowledge and experience they have gained from Exercises 1–4 (Chu et al., 2015b).
DISCUSSION
From the student viewpoint, frequent assessment and feedback from online systems that allow for interactive lab courses in neuroscience (e.g., Neuromorpho) can provide concept reinforcement that leads to more efficient learning in this evolving discipline. Further, online learning features provide students with a more flexible schedule, a self-paced learning style, and personal motivation that seems highly desirable. For example, here are some anecdotal comments from our students about using the online exercises (presented here) when compared to traditional learning methods that they encountered in their neuroscience classroom. An undergraduate at the State University of New York at Cortland said, “The online exercises provided me a more intuitive feel when learning about the structure and function of the brain’s cells. My behavioral neuroscience course didn’t use online resources and most of the instruction was either from the textbook or power points. I think I learned more about the structure and variations of different neurons in this two hour exercise than I did throughout a whole semester.” Another student, from the same University states, “This would have been very helpful when I was studying for my neuroscience exams. The fact that I would have had access to these websites to study at home would have helped me a lot in learning the very complex material. Not to mention, it’s a very interactive way to learn about neuroscience.”
Anecdotal evidence from an instructor of a Neurobiology laboratory course who has used similar exercises have also been positive: “Students reported that the exercise increased their knowledge and appreciation of both gross and cellular anatomical organization within the brain. Specifically, students reported that understanding how to initially navigate the Neuromorpho software helped them to appreciate the organization of the gross and micro-neuroanatomical structures, serving as a primer for more in depth anatomical exercises”. Given that these are just a few accounts of the effectiveness of these online resources, future research should assess group neuroscience exam scores with and without the online experiences in a proper experimental design.
Excitingly, these resources that are likely ideal for undergraduate neuroscience faculty to use as part of their instructive methods in the classroom will only continue to grow in content and sophistication. In addition, online resources would aid in lowering the costs of acquiring, maintaining and updating the materials necessary for teaching in undergraduate laboratories. In fact, the ABA and Neuromorpho are both freely available data sets that require no software installation or special equipment and remind students that neuroscience is multidisciplinary and requires true multilevel analysis. They both provide data and visualizations from expert scientists and are in fact data that have been used for research in neuroscience (Oh et al., 2014; Van Dine et al., 2013). Although the lack of multisensory integration on the part of the user makes digital labs less compelling, they can be a powerful supplement to existing material based courses. Additionally, it is possible to garner student interest through multimedia introductions that they can relate to. For example, the ABA can be introduced using the Tedx talk by Allan Jones which is available on YouTube (https://www.youtube.com/watch?v=XBcPFhg0BC8) and an introduction into neuronal morphology can be supplemented with a talk given by Terry Sejnowski at the ABA Brain Science Symposium in 2011, which is also available on YouTube (https://www.youtube.com/watch?v=AUl0plhOXHs).
The format of these exercises can easily be expanded to examine developmental differences in the expression of various cellular markers and genes from the ABA and compare them to the morphologies of cells at those periods. Further, in silico methods such as the ABA, have been used as instructive tools to teach about neurons as one of the fundamental units of the brain that can easily be forgotten when focusing on gross anatomy or when looking at functional imaging studies of the brain. In addition, other anatomical connectivity databases also exist (www.mouseconnectome.com, www.brainarchitecture.org) which each have their cost benefit and can potentially be interchangeable with the ABA for these exercises. Thus, by using these tools, the beauty and complexity of cells can become apparent to students at all levels.
REFERENCES
- Chu PC, Peck J, Brumberg JC. 2015a. Supplemental PowerPoint slides associated with this article can be found at: http://qcpages.qc.cuny.edu/Psychology/people/faculty/brumberg/Publications/chuetalsupp.pdf.
- Chu PC, Peck J, Brumberg JC. 2015b. Supplemental Laboratory exercises associated with this article can be found at: http://qcpages.qc.cuny.edu/Psychology/people/faculty/brumberg/Publications/chuetallabex.pdf.
- Cox MF, Cordray DS. Assessing pedagogy in bioengineering classrooms: quantifying elements of the “how people learn” model using the VaNTH Observation System (VOS) School of Engineering Education Faculty Publications. 2008 Paper 1. http://docs.lib.purdue.edu/enepubs/1. [Google Scholar]
- Grisham W. Resources for teaching Mammalian neuroanatomy using sheep brains: a review. J Undergrad Neurosci Educ. 2006;5:R1–R6. [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S. The human brain in numbers: a linearly scaled-up primate brain. Front Hum Neurosci. 2009;3:31. doi: 10.3389/neuro.09.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs B, Schall M, Prather M, Kapler E, Driscoll L, et al. Regional dendritic and spine variation in human cerebral cortex: a quantitative golgi study. Cereb Cortex. 2001;11:558–571. doi: 10.1093/cercor/11.6.558. [DOI] [PubMed] [Google Scholar]
- Kole MHP, Costoli T, Koolhaas JM, Fuchs E. Bidirectional shift in the cornu ammonis 3 pyramidal dendritic organization following brief stress. Neuroscience. 2004;125:337–347. doi: 10.1016/j.neuroscience.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Mendell LM. The size principle: a rule describing the recruitment of motoneurons. J Neurophysiol. 2005;93:3024–3026. doi: 10.1152/classicessays.00025.2005. [DOI] [PubMed] [Google Scholar]
- Nichols M. Institutional perspectives: the challenges of e-learning diffusion. Br J Educ Technol. 2008;39:598–609. [Google Scholar]
- Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, et al. A mesoscale connectome of the mouse brain. Nature. 2014;508:207–214. doi: 10.1038/nature13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh R, Ascoli GA. Neuronal morphology goes digital: a research hub for cellular and system neuroscience. Neuron. 2013;77:1017–1038. doi: 10.1016/j.neuron.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos RL, Moiseff A, Brumberg JC. Utility and versatility of extracellular recordings from the cockroach for neurophysiological instruction and demonstration. J Undergrad Neurosci Educ. 2007a;5:A28–A34. [PMC free article] [PubMed] [Google Scholar]
- Ramos RL, Smith PT, Brumberg JC. Novel in silico Method for Teaching Cytoarchitecture, Cellular Diversity, and Gene Expression in the Mammalian Brain. J Undergrad Neurosci Educ. 2007b;6:A8–A13. [PMC free article] [PubMed] [Google Scholar]
- Ramos RL, Smith PT, Croll SD, Brumberg JC. Demonstrating cerebral vascular networks: a comparison of methods for the teaching laboratory. J Undergrad Neurosci Educ. 2008;6:A53–A59. [PMC free article] [PubMed] [Google Scholar]
- Stiefel KM, Sejnowski TJ. Mapping function onto neuronal morphology. J Neurophysiol. 2007;98:513–526. doi: 10.1152/jn.00865.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dine SE, Salem E, George E, Siu NY, Dotzler T, Ramos RL. Cellular and axonal diversity in molecular layer heterotopia of the rat cerebellar vermis. BioMed Res Int. 2013;2013:805467. doi: 10.1155/2013/805467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Chen CC, Ramos RL, Katz E, Keller A, Brumberg JC. Intrinsic properties of thalamocortical inputs onto identified corticothalamic-VPM neurons. Somatosens Mot Res. 2014;31:78–93. doi: 10.3109/08990220.2013.869495. [DOI] [PMC free article] [PubMed] [Google Scholar]