Abstract
Background
Separately, prenatal antibiotics and cesarian delivery have been found to be associated with increased risk of allergic diseases. It is not clear if these factors may modify the effect of each other.
Objective
Assess whether the associations between delivery types and eczema, sensitization and total IgE at age 2 years were modified by maternal use of prenatal medications.
Methods
Prenatal charts of women enrolled in the WHEALS birth cohort were reviewed for delivery mode and medications prescribed and administered throughout their entire pregnancy, including systemic antibiotics and vaginally applied antifungal medications. The associations between the delivery mode and select medications and eczema, sensitization (≥1 of 10 allergen specific IgE ≥0.35 IU/ml) and total IgE at age 2 years were assessed.
Results
There was a lower risk of eczema among vaginally versus c-section born children (relative risk adjusted for race=aRR=0.77., 95% CI 0.56, 1.05). Although not statistically significantly different, this association was stronger among the subset of children born vaginally to a mother who did not use systemic antibiotics or vaginal antifungal medications (aRR=0.69, 95% CI 0.44, 1.08) compared to those born vaginally to mothers who used systemic antibiotics or vaginal antifungals (aRR=0.81, 95% CI 0.57, 1.14). A protective association between vaginal birth and sensitization (aRR=0.86, 95% CI 0.72, 1.03) was similar for those children born vaginally to a mother who did not (aRR=0.87, 95% CI 0.69, 1.10) and who did (RR=0.85, 95% CI 0.70, 1.04) use systemic antibiotics or vaginal antifungal medications. There were no associations with total IgE.
Conclusions
Children born vaginally had lower risk of eczema and sensitization compared with those born via c-section; however, the protective association with eczema may be slightly weakened when mothers took systemic antibiotics or vaginally applied medications during pregnancy.
Keywords: antibiotics, antifungal, microbiome, c-section, eczema, IgE, prenatal
BACKGROUND
It has been reported that birth by cesarian section (c-section) has been associated with an increased risk of childhood allergies and asthma. [(1, 2)] Prenatal antibiotic use has also been found to be associated with increased risk of development of allergic diseases in the children.[(3–13)] Mechanisms for these associations have been debated and are still unknown. However, the protective effect of acquiring a healthy gut microbiome in early life which would, in turn, affect immune system development at a young age, has been hypothesized to explain these associations.[(13–19)] For example, children born vaginally are exposed to a potentially rich and diverse maternal microbial milieu during birth [(15)] but children born via c-section miss this exposure.
Antibiotic treatment of the mother can also alter the maternal microbiome and thus transform the microbiome to which children born vaginally are exposed during birth.[(20, 21)] Vaginally applied antifungal medications are often used during pregnancy. There are multiple possible relationships between the use of these medications and the bacterial components of the vaginal microbiome but the need for a topical antifungal medication suggests that the vaginal microbiome has shifted away from a normal symbiotic to a dysbiotic status. The microbes making up such a dysbiotic microbiome may not provide the proper signals to shift an infant away from developing allergic disease. Furthermore, previous studies have not simultaneously examined prenatal antibiotic use and delivery mode to assess if these factors may modify the effect of the other factor. Understanding the associations between these factors and the allergy-related outcomes could provide additional insight into potential mechanisms.
To address these knowledge gaps, delivery mode and medications prescribed and administered throughout the entire pregnancy of women enrolled in the WHEALS birth cohort in the Detroit, Michigan USA, area were examined for their associations with eczema, allergic sensitization based on allergen-specific IgE (sIgE), and total IgE in their children at age 2 years. We hypothesized that the benefits of delivering a child vaginally compared to a cesarian delivery with respect to these allergic outcomes would be attenuated in women who took systemic antibiotics or used vaginally applied antifungal medication during pregnancy compared to children born vaginally whose mothers did not use these medications.
METHODS
Study Population
The study population for these analyses is comprised of the offspring of the participants in “WHEALS” – a cohort study that enrolled pregnant women receiving care at Henry Ford Health System (HFHS) obstetrics clinics in urban and suburban Detroit, Michigan. Recruitment began in September 2003 and the last child was born in December 2007. These analyses combine information from the interviews, a clinic visit with a study physician and results from biological and environmental samples. Details of the cohort’s creation have been published.[(22, 23)]
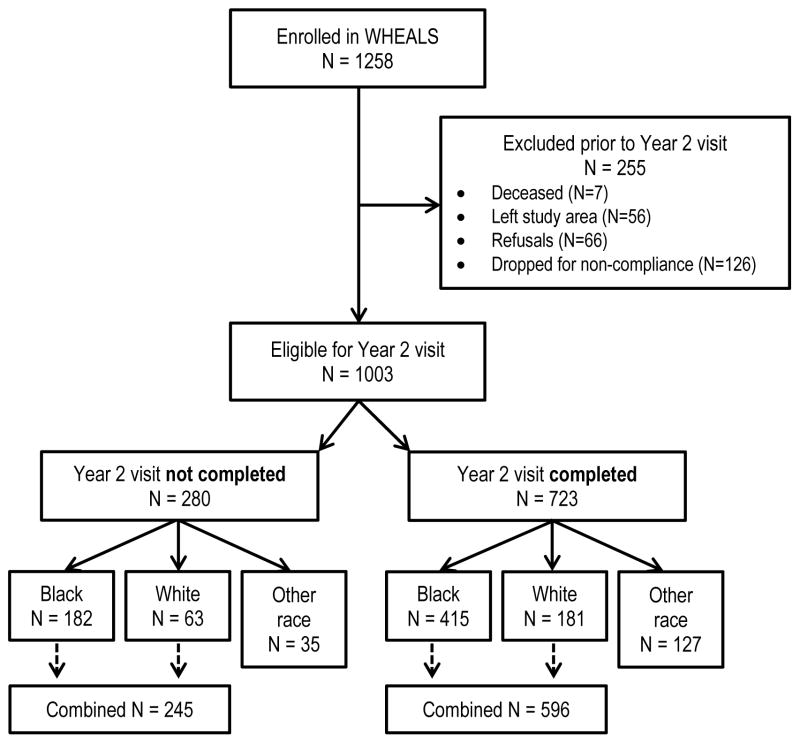
Figure 1 provides details on participant exclusion from enrollment through the current analyses. WHEALS recruited 1258 women, of which 1003 were eligible for the clinic visit at age 2 years. Of the 1003, 723 completed the clinic visit and 415 of those children were Black and 181 were White. The child’s race was based on maternal report for her child. The subgroups of children who are Asian, Hispanic or Middle Eastern may have different risks for the outcomes and were too few in number to analyze as subgroups, so they were excluded from these analyses. Participants who were included and not included in the analyses were compared to assess differences (Table 1). The children included in the present analyses have information on at least one outcome at age 2 years (evaluation of eczema/atopic dermatitis [AD], allergen specific IgE [sIgE] and total IgE).
Figure 1.
Enrollment and participation details of the current analyses.
Table 1.
Comparison of women from WHEALS whose children were included or excluded from the current analyses.*
| Women with a Child Included in the Analyses | Women with a Child Excluded from the Analyses | p value | |
|---|---|---|---|
| N | 596 | 245 | |
| Ever breastfed the child (n(%) yes) | 319 (53.5%) | 111 (45.5%) | 0.035 |
| Mother had an indoor dog during pregnancy | 167 (28.0%) | 57 (23.3%) | 0.16 |
| Child lived with an indoor dog in the first year of life | 118 (22.5%) | 26 (15.0%) | 0.035 |
| Mother sensitized to allergens | 336 (58.4%) | 137 (57.6%) | 0.82 |
| Child’s gender | |||
| Male | 306 (51.3%) | 111 (45.3%) | 0.11 |
| Female | 290 (48.7%) | 134 (54.7%) | |
| Race | |||
| Black | 415 (69.6%) | 181 (74.2%) | 0.18 |
| White | 181 (30.4%) | 63 (25.8%) | |
| Delivery type | |||
| Vaginal | 376 (63.1%) | 156 (64.2%) | 0.76 |
| C-section | 220 (36.9%) | 87 (35.8%) | |
| Child was firstborn | 132 (22.1%) | 57 (23.3%) | 0.72 |
There are some missing data. P-values are for a comparison of those included and those excluded.
Data Collection
At the time of recruitment during pregnancy, a blood sample was collected and interviews were conducted to collect information on maternal demographics and health history. Additional interviews were conducted with the mothers at 1, 6 and 12 month home visits about the health of their children, breastfeeding and exposure to animals as part of the WHEALS protocol. Medical chart review was performed for all mothers to collect extensive information on the entire course of their prenatal care and the details of their delivery. Charts were reviewed by credentialed Registered Health Information Technicians. The data included all medications prescribed and administered during the entire pregnancy, as well as route of medication and delivery mode. The dates that the medications were prescribed or administered were also recorded. Information was also collected on whether the woman was positive for Group B Streptococcus (GBS+) based on standard screening during routine prenatal care.
At age 2 years, children were invited for a clinical evaluation by a physician using a protocol standardized for the study. Using a patient history and physical examination, the physician was then asked to record a response to the following question: “By your clinical evaluation do you believe that this child has or has had atopic dermatitis or eczema?” The average age at completion of the 2 year clinic visit was 2.2 years (standard deviation = SD = 0.2 years).
Measurement of total IgE and allergen-specific IgE (sIgE) was performed according to the manufacturer’s standard protocols using the Pharmacia UniCAP system (Pharmacia-Upjohn Diagnostic Division, Kalamazoo, Michigan, USA). Sensitization was defined as having at least one sIgE ≥0.35 IU/ml. Samples collected from the children during the clinic visit were assessed for the following sIgEs: dust mite (Der f), dog, cat, timothy grass, ragweed, Alternaria, egg, peanut, milk and German cockroach. sIgE levels in mothers blood samples were assessed for a subset of the allergens (Alternaria, cat, cockroach, dog, Der f, short ragweed, timothy grass, and egg) using the same methods.
Classification of Medications
Medications were classified in two ways: by their therapeutic goal and by the route of their administration. All medications were classified for their therapeutic goal as being: 1) antibiotic; 2) antifungal; or, 3) neither. This was done as some antibiotics were prescribed for fungal skin infections (e.g., Nystatin is described as an antibiotic antifungal). Route of administration was classified in the following mutually exclusive ways: 1) systemic (ingestion, intravenous, intramuscular); 2) vaginal insert; or 3) topical (cream, gel, or drops on skin or in eye, nose or ears). Children were classified into one of the three mutually exclusive groups: 1) vaginal birth and mother took a systemic antibiotic and/or used a vaginal insert antifungal medication during pregnancy; 2) vaginal birth and mother did not take a systemic antibiotic and did not use a vaginally applied antifungal medication during pregnancy; or, 3) birth by c-section. All classifications were mutually exclusive.
Statistical Analyses
Log-binomial models (eczema, sensitized) and linear regression models (total IgE) were used to assess the associations between medication/delivery mode groups and allergic outcomes. Interaction terms were used to assess whether any of the following variables were effect modifiers of the associations between delivery/medication group and the allergic outcomes: child race (Black or White), maternal sensitization based on sIgE, child gender, whether the mother lived with a dog during pregnancy, whether the child lived with a dog during the first year of their life, whether the child was first born, and whether the child was ever breastfed. The variables were chosen a priori for the analyses as they are commonly investigated by our team and related to the prenatal and early life period. Stratum-specific estimates are provided in cases where the interaction term was p<0.05 for at least one of the delivery/medication groups.
We evaluated a list of potential confounders to see if any were associated with the exposure and the outcome. Factors evaluated were: race, maternal sensitization (defined as yes or no whether mother had elevated sIgE to any of the selected allergens, child gender, breastfed, child lived with dog in 1st year and mom lived with a dog during pregnancy. Race was associated with the “exposure” (delivery type and antimicrobial medication usage) and each outcome and the results are adjusted for race.
Relative risks (RR) and differences with 95% confidence intervals (CI) were calculated to describe associations. C-section delivery served as the referent group. Use of c-section delivery as the referent was appropriate given our hypothesis relates to the vaginal microbiome which is not encountered during c-section delivery.
RESULTS
Information on participants who were included in and excluded from the analyses was compared to assess differences (Table 1). There were 596 children included in the analyses as they had information on prenatal care and attended the clinic visit at age 2 years. Rates of ever having been breastfed and living with a dog in the 1st year of life were higher in those who were retained in the study. These and other variables were assessed for potential effect modification in the analyses. Most of the children included in the analyses were Black (69.6%) and 58.4% had a mother who was sensitized, 28.0% had a mother who lived with an indoor dog during pregnancy and 22.5% lived with an indoor dog in the first year of life (Table 1). Most of the children were born vaginally (376, 63.1%) of which 243 (64.6%) had a mother who used systemic antibiotics or vaginal antifungals during pregnancy (Table 2). Other information about the population included in the analyses appears in Table 2.
Table 2.
Rates of prenatal medication use, eczema and sensitization (sIgE ≥0.35 IU/ml) at age 2 years in the WHEALS birth cohort.
| All Children N=596 | Children born Vaginally N=376 | Children born via C-section N=220 | p value‡ | |
|---|---|---|---|---|
| Administered or prescribed any antibiotic* in pregnancy | 341 (57.2%) | 223 (59.3%) | 118 (53.6%) | 0.18 |
| Administered or prescribed any antibiotic* in pregnancy that was: | ||||
| - Systemic | 328 (55.0%) | 213 (56.7%) | 115 (52.3%) | 0.30 |
| - Topical | 15 (2.5%) | 11 (2.9%) | 4 (1.8%) | 0.40 |
| - Vaginal insert | 25 (4.2%) | 15 (4.0%) | 10 (4.5%) | 0.74 |
| Administered or prescribed any antifungal* in pregnancy | 116 (19.5%) | 67 (17.8%) | 49 (22.3%) | 0.19 |
| Administered or prescribed any antifungal* in pregnancy that was: | ||||
| - Systemic | 55 (9.2%) | 32 (8.5%) | 23 (10.4%) | 0.43 |
| - Topical | 4 (0.7%) | 3 (0.8%) | 1 (0.4%) | 0.62 |
| - Vaginal insert | 71 (11.9%) | 39 (10.4%) | 32 (14.5%) | 0.13 |
| Administered either an antibiotic or antifungal medication in pregnancy | 377 (63.3%) | 243 (64.6%) | 134 (60.9%) | 0.36 |
| Eczema at age 2 years N=59 missing eczema data |
118 (22.0%) | 66 (19.3%) | 52 (26.7%) | 0.047 |
| Sensitized to at least 1 allergen at age 2 years N=146 missing sensitization data |
232 (51.6%) | 138 (48.2%) | 94 (57.3%) | 0.064 |
| Mom was Group B Strep positive† | 222 (37.2%) | 132 (35.1%) | 90 (40.9%) | 0.16 |
| Mom was Group B Strep positive N=63 missing data |
159 (29.8%) | 104 (29.9%) | 55 (29.7%) | 0.97 |
| Geometric mean (95% CI) of total IgE at 2 years of age | 22.0 (19.4, 25.0) | 21.7 (18.4, 25.6) | 22.6 (18.6, 27.5) | 0.71 |
By medication type based on therapeutic goal
Assumes those with missing status are treated the same as those who are positive.
p-value is for the comparison of those born vaginally versus those born by c-section.
Eczema
Children born vaginally were less likely to have had eczema than children born via c-section. (RR=0.72, 95% CI 0.53, 0.99; adjusted for race, RR=0.77., 95% CI 0.56, 1.05). Although not statistically significantly different, this association was stronger among the subset of children born vaginally to a mother who did not use systemic antibiotics or vaginal antifungal medications as they were even less likely to have had eczema by age 2 compared to children born via c-section (RR=0.60, 95% CI 0.38, 0.94; adjusted for race RR=0.69, 95% CI 0.44, 1.08) (Table 3). However, the decreased risk compared to c-section was attenuated for those born vaginally to mothers who used systemic antibiotics or vaginal antifungals (RR=0.81, 95% CI 0.57, 1.15; adjusted for race, RR=0.81, 95% CI 0.57, 1.14).
Table 3.
Relative risks and 95% confidence intervals for the associations between medications and eczema by age 2 years adjusted for race. Referent group is c-section.
| Vaginal delivery plus systemic antibiotic/ vaginal antifungal medication during pregnancy | P for interaction term | Vaginal delivery without systemic antibiotics/ vaginal antifungal medication during pregnancy | P for interaction term | |
|---|---|---|---|---|
| All children | 0.81 (0.57, 1.14) | N/A | 0.69 (0.44, 1.08) | N/A |
| Maternal sensitization | 0.049 | 0.84 | ||
| - Mom not sensitized | 1.31 (0.74, 2.30) | 0.66 (0.27, 1.57) | ||
| - Mom is sensitized | 0.63 (0.40, 1.00) | 0.73 (0.43, 1.23) | ||
| Lived with a dog during pregnancy | 0.027 | 0.52 | ||
| - No | 0.99 (0.69, 1.43) | 0.73 (0.45, 1.20) | ||
| - Yes | 0.28 (0.10, 0.80) | 0.51 (0.18, 1.48) | ||
| Lived with a dog in the first year of life | 0.038 | 0.29 | ||
| - No | 0.86 (0.58, 1.27) | 0.72 (0.43, 1.19) | ||
| - Yes | 0.10 (0.01, 0.79) | 0.29 (0.07, 1.25) |
We assessed effect modification by examining the associations in various subgroups to assess if the risks were similar across those subgroups. Associations between medication use, delivery mode and eczema varied by maternal sensitization status and whether the mother or child lived with a dog (Table 3). Among the children born to a mother who was sensitized, those whose mothers took the selected medications during pregnancy were less likely to have eczema compared to children born via c-section (RR=0.60, 95% CI 0.38, 0.96; adjusted for race, RR=0.63, 95% CI 0.40, 1.00). Among children who lived with a dog in the first year of life or whose mothers lived with a dog during pregnancy, those born to mothers who took the selected medications were less likely to have eczema compared to children born via c-section (dog in pregnancy: RR=0.31, 95%CI 0.11, 0.90; dog in first year: RR=0.11, 95% CI 0.015, 0.84; adjusted for race, RR=0.28, 95% CI 0.10, 0.80 and RR=0.10, 95% CI 0.01, 0.79, respectively).
Sensitization by Age 2 years
When compared to eczema, the protective association between a vaginal birth and being sensitized at age 2 years was somewhat weaker (RR=0.84, 95% CI 0.70, 1.01 for vaginal versus c-section birth; adjusted for race, RR=0.86, 95% CI 0.72, 1.03). Children born vaginally to a mother who did not use systemic antibiotics or vaginal antifungal medications had lower risk of of sensitization by age 2 compared to children born via c-section (RR=0.83, 95% CI 0.65, 1.04; adjusted for race RR=0.87, 95% CI 0.69, 1.10) (Table 4). This was also true for children born vaginally to a mother who did use systemic antibiotics or vaginal antifungal medications during pregnancy (RR=0.85, 95% CI 0.70, 1.04; adjusted for race RR=0.85, 95% CI 0.70, 1.04), with a single exception. Among children whose mothers lived with a dog during pregnancy, children born vaginally to a mother who did use systemic antibiotics or vaginal antifungal medications were less likely than children born via c-section to be sensitized by age 2 years (RR=0.50, 95% CI 0.32, 0.78; adjusted for race RR=0.49, 95% CI 0.32, 0.75).
Table 4.
Relative risks and 95% confidence intervals for the associations between medications and sensitization by age 2 years adjusted for race. Reference group is c-section.
| Vaginal delivery plus systemic antibiotic/ vaginal antifungal medication during pregnancy | P for interaction term | Vaginal delivery without systemic antibiotics/ vaginal antifungal medication during pregnancy | P for interaction term | |
|---|---|---|---|---|
| All children | 0.85 (0.70, 1.04) | 0.87 (0.69, 1.10) | ||
| Lived with a dog during pregnancy | 0.003 | 0.76 | ||
| - No | 1.03 (0.82, 1.30) | 0.88 (0.66, 1.17) | ||
| - Yes | 0.49 (0.32, 0.75) | 0.89 (0.60, 1.32) |
Total IgE
There were no associations between the exposure groups and the level of total IgE at age 2 years (Vaginal/no medication versus c-section: difference in ln(total IgE)=0.01, 95% CI −0.32, 0.33; adjusted for race 0.09, 95% CI −0.24, 0.41; vaginal with medication versus c-section: difference in ln(total IgE)=−0.08, 95% CI −0.37, 0.22; adjusted for race −0.09, 95% CI −0.38, 0.21) overall or for any subgroup.
Group B Streptococcus
Results were unchanged after women who were either GBS+ or had an unknown GBS status were removed from the analyses.
DISCUSSION
The results extend previous observations that delivery type and antibiotic use during pregnancy are associated with allergic diseases in the offspring by using epidemiological data to investigate the potential role of the vaginal microbiome in disease development. Overall, children born vaginally had lower risk of both eczema and sensitization at age 2 years. A non-statistically significant trend was noted however, that among children born vaginally, children born to mothers who did not use antimicrobial medications potentially associated with an altered vaginal microbiome tended to have less eczema compared to those whose mothers took those medications. Overall, there was a modest association in which vaginal delivery was associated with a decreased risk of sensitization compared to c-section delivery. Delivery type combined with medication use was not associated with total IgE at age 2 years. This suggests that the vaginal microbiome hypothesis may not be supported with respect to total IgE and that the associations with sensitization may be weaker than those observed with eczema.
There is growing evidence of differences in the gut microbiomes of children with and without eczema and other allergic diseases. Wang et al. reported reduced diversity in the early fecal microbiota of infants with atopic eczema at age 18 months in a small group of 35 infants.[(18)] There is also evidence that suggests that eczema was not associated with any particular culturable intestinal commensal bacteria in a cohort of 324 children in London and Europe.[(19)] A case-control study of infants 3–6 months with (n=37) and without (n=24) eczema found evidence that presence of B pseudocatenultaum in feces was associated with eczema; however, the fecal samples were not obtained prior to disease development.[(17)] Recently, a more diverse intestinal microbiota in the first week of life was associated with a reduced risk of eczema in the first year of life in a cohort of 98 children born to women with allergic disease histories.[(16)] In a randomized, placebo controlled trial for the prevention of atopic dermatitis, colonization with clostridia at 5 weeks and 13 weeks was associated with an increased risk of developing atopic dermatitis.[(24)]
Studies of a link between the gut microbiome and atopic sensitization have resulted in mixed findings. In a study of 19 atopic and 12 non-atopic children ages 4–14 years, the intestinal microbiota of atopic children showed significantly lower levels of members of the Clostridium cluster IV, Faecelibacterium prausnitzii, Akkermansia muciniphila and an increase in the abundance of Enterobacteriaceae.[(25)] Children who had a stool sample at one month of age colonized with Clostridium difficile, were more likely than children without the colonization to be sensitized as defined by their sIgE levels (adjusted OR=1.54, 95% CI 1.02, 2.31).[(26)] In the study of 98 infants at high risk for allergic disease, there was no difference in the gut microbial diversity of children who did and did not have a positive skin prick test at age one year.[(16)] The differences in results could be due to the timing of the microbiome sample – some samples were collected prior to and some concurrent with the outcome.
The vaginal microbiome helps colonize the child’s gut microbiome during vaginal delivery. The child’s gut microbiome is then believed to affect the child’s developing immune system, which in turn, would affect the child’s allergic disease risk. Thus, anything disturbing the mother’s vaginal microbiome could affect a child’s allergic disease risk. Separately, children born via c-section would be expected to have a different gut microbiome and subsequent allergic disease risks than either children born vaginally with mothers who did not use vaginal microbiome altering medications or children with mothers who did use the medications.
It has been shown that the vaginal microbiome can become altered with the use of antibiotic medications during pregnancy. In a study of 50 pregnant women, administration of antibiotics during the intrapartum period was associated with a decrease in transmission rates of vaginal Lactobacillus-dominant mixed flora to the newborn.[(21)] In the Danish cohort, COPSAC, women who received oral antibiotics at any time during pregnancy had an increased rate of vaginal colonization by Staphylococcus species compared to pregnant women who did not take antibiotics.[(20)]
Delivery mode has been shown to affect the postnatal colonization patterns of infants. Children who had a c-section birth had lower total microbial diversity and delayed colonization of the Bacteroidetes phylum throughout the first two years of life.[(27)] Other studies have supported the conclusion that method of delivery is associated with differential intestinal microbiota in early life and have generally found similar results.[(2, 24, 28–32)]
There were some associations in subgroups that may provide additional evidence supporting the role of the microbiome in allergy-related diseases. For vaginally born children whose mothers took the medications, those children who did not have dog exposure (prenatal or first year of life) or were not born to a sensitized mother had little difference in the risk of having eczema compared to children born via c-section. In accordance with the microbiome hypothesis, we think that living with a dog provides exposure to a home microbiome that is “anti-allergic” and that the modifying effects of a dog in the home could explain why these subgroups still show a strong beneficial effect of vaginal delivery compared to c-section even with potentially gut and vaginal microbiome-altering medication use. Our previous work has indicated differences in the microbiome of dust from homes with and without pets.[(33)] Stokholm et al. reported that in a cohort of 668 pregnant Danish women (COPSAC), those who had a cat or dog in the home during pregnancy had increased Escherichia coli colonization in their vaginal flora compared with women with neither animal.[(34)] Further, having a dog in the home during pregnancy is highly correlated with having a dog in the home during the child’s few first years of life. Any deficiencies in the child’s acquired microbiome due to a maternal vaginal or gut microbiome that has been altered by medication, may be subsequently and rapidly overcome by this very early exposure to a dog in the home environment.
For children born to mothers who were not sensitized, the risk of eczema was no different for those born via c-section and those born vaginally to mothers who took the medications. However, in the subgroup of children born to sensitized mothers, c-section delivery is associated with greater risk than vaginal delivery regardless of maternal medication use. Children who have a sensitized mother may have an increased underlying risk of eczema compared to those who do not have a sensitized mother and this risk may be significantly increased by c-section delivery compared with risk increased by prenatal maternal medicine use in children born vaginally.
The novel part of this work is the simultaneous consideration of medication use and delivery type, as well as the inclusion of antifungal medications in our analyses. Previous work focused on antibiotics likely due to the suspicion that their mechanism was related to infection and the immune system. However, with the development of the gut microbiome hypothesis in allergic disease development and the importance of the maternal microbiomes on the colonization of the neonate’s gut, the role of vaginal antifungal medication warrants similar consideration. Importantly, what these results suggest is that any study of the role of the vaginal microbiome in allergy-related outcomes will need to address multiple subgroups. The study design, sample size and analytical plan will need to simultaneously take into consideration delivery mode and medication use, as well as possibly the presence of pets in the home and the sensitization status of the mothers. Failure to do so could result in the obscuring of important and significant relationships.
There are limitations to this study. Medications were administered in a clinic or hospital setting or were prescribed. Prescribed medications may not have been obtained or used. However, if it is not medication use but rather the reason for the medication that is causing the associations, then the results would be unchanged by whether the prescription was either obtained or used. Microbiota “recovery” after medication use may vary in its time and level of recovery. However, there is little literature that describes the recovery periods for various medications on the vaginal tract.[(35)]
Our sample size may have impacted our work in several ways. First, the difference between born vaginally to mothers who did and did not use medications was not statistically significant. A larger sample size would have improved the precision of the relative risk estimates and allowed us to make stronger conclusions about the observed trend. Furthermore, we did not have a sample size sufficient enough to permit study of specific time periods during which medication use might be the most likely to affect outcomes. Some medications taken early in pregnancy may provide sufficient time for the vaginal microbiome to recover prior to delivery. The degree to which each medication could reasonably affect the vaginal microbiome is also unknown. Due to sample size limitations, we were not able to analyze whether any particular medication or set of medications may have been driving the observed associations. This also means that we were not able to examine the specific roles of antibiotics or vaginally applied antifungal medications to determine if either group in particular was associated with the outcomes. However, the data presented here may also benefit the design of future studies by providing rates of overlap of medication use that will inform sample size estimates. Finally, the observed results could be due to some unmeasured confounder; however, the analyses have attempted to consider the role of many well-measured variables in our analyses.
While prioritization of research areas is needed due to limited resources, the epidemiological evidence presented here suggests that the vaginal microbiome hypothesis demands ongoing investigation in allergic disease development, especially eczema. Important to such an investigation is the identification of a microbial composition that is optimal for allergic disease prevention and understanding of the factors that may cause a deviation from this optimal microbial composition. The consideration of prenatal medication use and delivery mode is important in the design of the study, the selection of the participants and the analyses of data.
Acknowledgments
This work was funded by the National Institutes of Health (HL113010, AI051598, AI089473), USA
Abbreviations
- WHEALS
Wayne County Health, Environment, Allergy and Asthma Longitudinal Study
- sIgE
allergen specific IgE
- GBS
Group B Streptococcus
References
- 1.Bager P, Wohlfahrt J, Westergaard T. Caesarean delivery and risk of atopy and allergic disease: meta-analyses. Clin Exp Allergy. 2008 Apr;38(4):634–42. doi: 10.1111/j.1365-2222.2008.02939.x. [DOI] [PubMed] [Google Scholar]
- 2.van Nimwegen FA, Penders J, Stobberingh EE, Postma DS, Koppelman GH, Kerkhof M, et al. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J Allergy Clin Immunol. 2011 Nov;128(5):948–55. e1-3. doi: 10.1016/j.jaci.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 3.Bisgaard H, Halkjaer LB, Hinge R, Giwercman C, Palmer C, Silveira L, et al. Risk analysis of early childhood eczema. J Allergy Clin Immunol. 2009 Jun;123(6):1355–60. e5. doi: 10.1016/j.jaci.2009.03.046. [DOI] [PubMed] [Google Scholar]
- 4.Dietert RR, Zelikoff JT. Early-life environment, developmental immunotoxicology, and the risk of pediatric allergic disease including asthma. Birth Defects Res B Dev Reprod Toxicol. 2008 Dec;83(6):547–60. doi: 10.1002/bdrb.20170. [DOI] [PubMed] [Google Scholar]
- 5.Dom S, Droste JH, Sariachvili MA, Hagendorens MM, Oostveen E, Bridts CH, et al. Pre- and post-natal exposure to antibiotics and the development of eczema, recurrent wheezing and atopic sensitization in children up to the age of 4 years. Clin Exp Allergy. 2010 Sep;40(9):1378–87. doi: 10.1111/j.1365-2222.2010.03538.x. [DOI] [PubMed] [Google Scholar]
- 6.Murk W, Risnes KR, Bracken MB. Prenatal or early-life exposure to antibiotics and risk of childhood asthma: a systematic review. Pediatrics. 2011 Jun;127(6):1125–38. doi: 10.1542/peds.2010-2092. [DOI] [PubMed] [Google Scholar]
- 7.Jedrychowski W, Galas A, Whyatt R, Perera F. The prenatal use of antibiotics and the development of allergic disease in one year old infants. A preliminary study. Int J Occup Med Environ Health. 2006;19(1):70–6. doi: 10.2478/v10001-006-0010-0. [DOI] [PubMed] [Google Scholar]
- 8.Tsakok T, McKeever TM, Yeo L, Flohr C. Does early life exposure to antibiotics increase the risk of eczema? A systematic review. Br J Dermatol. 2013 Nov;169(5):983–91. doi: 10.1111/bjd.12476. [DOI] [PubMed] [Google Scholar]
- 9.Stensballe LG, Simonsen J, Jensen SM, Bonnelykke K, Bisgaard H. Use of antibiotics during pregnancy increases the risk of asthma in early childhood. J Pediatr. 2013 Apr;162(4):832–8e3. doi: 10.1016/j.jpeds.2012.09.049. [DOI] [PubMed] [Google Scholar]
- 10.Sariachvili M, Droste J, Dom S, Wieringa M, Vellinga A, Hagendorens M, et al. Is breast feeding a risk factor for eczema during the first year of life? Pediatr Allergy Immunol. 2007 Aug;18(5):410–7. doi: 10.1111/j.1399-3038.2007.00543.x. [DOI] [PubMed] [Google Scholar]
- 11.McKeever TM, Lewis SA, Smith C, Hubbard R. The importance of prenatal exposures on the development of allergic disease: a birth cohort study using the West Midlands General Practice Database. Am J Respir Crit Care Med. 2002 Sep 15;166(6):827–32. doi: 10.1164/rccm.200202-158OC. [DOI] [PubMed] [Google Scholar]
- 12.Kozyrskyj AL, Bahreinian S, Azad MB. Early life exposures: impact on asthma and allergic disease. Curr Opin Allergy Clin Immunol. 2011 Oct;11(5):400–6. doi: 10.1097/ACI.0b013e328349b166. [DOI] [PubMed] [Google Scholar]
- 13.Kalliomaki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol. 2001 Jan;107(1):129–34. doi: 10.1067/mai.2001.111237. [DOI] [PubMed] [Google Scholar]
- 14.Bjorksten B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol. 2001 Oct;108(4):516–20. doi: 10.1067/mai.2001.118130. [DOI] [PubMed] [Google Scholar]
- 15.Benn CS, Thorsen P, Jensen JS, Kjaer BB, Bisgaard H, Andersen M, et al. Maternal vaginal microflora during pregnancy and the risk of asthma hospitalization and use of antiasthma medication in early childhood. J Allergy Clin Immunol. 2002 Jul;110(1):72–7. doi: 10.1067/mai.2002.125833. [DOI] [PubMed] [Google Scholar]
- 16.Ismail IH, Oppedisano F, Joseph SJ, Boyle RJ, Licciardi PV, Robins-Browne RM, et al. Reduced gut microbial diversity in early life is associated with later development of eczema but not atopy in high-risk infants. Pediatr Allergy Immunol. 2012 Nov;23(7):674–81. doi: 10.1111/j.1399-3038.2012.01328.x. [DOI] [PubMed] [Google Scholar]
- 17.Gore C, Munro K, Lay C, Bibiloni R, Morris J, Woodcock A, et al. Bifidobacterium pseudocatenulatum is associated with atopic eczema: a nested case-control study investigating the fecal microbiota of infants. J Allergy Clin Immunol. 2008 Jan;121(1):135–40. doi: 10.1016/j.jaci.2007.07.061. [DOI] [PubMed] [Google Scholar]
- 18.Wang M, Karlsson C, Olsson C, Adlerberth I, Wold AE, Strachan DP, et al. Reduced diversity in the early fecal microbiota of infants with atopic eczema. J Allergy Clin Immunol. 2008 Jan;121(1):129–34. doi: 10.1016/j.jaci.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Adlerberth I, Strachan DP, Matricardi PM, Ahrne S, Orfei L, Aberg N, et al. Gut microbiota and development of atopic eczema in 3 European birth cohorts. J Allergy Clin Immunol. 2007 Aug;120(2):343–50. doi: 10.1016/j.jaci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Stokholm J, Schjorring S, Eskildsen CE, Pedersen L, Bischoff AL, Folsgaard N, et al. Antibiotic use during pregnancy alters the commensal vaginal microbiota. Clin Microbiol Infect. 2013 Oct 1; doi: 10.1111/1469-0691.12411. [DOI] [PubMed] [Google Scholar]
- 21.Keski-Nisula L, Kyynarainen HR, Karkkainen U, Karhukorpi J, Heinonen S, Pekkanen J. Maternal intrapartum antibiotics and decreased vertical transmission of Lactobacillus to neonates during birth. Acta Paediatr. 2013 May;102(5):480–5. doi: 10.1111/apa.12186. [DOI] [PubMed] [Google Scholar]
- 22.Wegienka G, Havstad S, Zoratti EM, Woodcroft KJ, Bobbitt KR, Ownby DR, et al. Regulatory T cells in prenatal blood samples: variability with pet exposure and sensitization. J Reprod Immunol. 2009 Jul;81(1):74–81. doi: 10.1016/j.jri.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wegienka G, Havstad S, Bobbitt KR, Woodcroft KJ, Zoratti EM, Ownby DR, et al. Within-woman change in regulatory T cells from pregnancy to the postpartum period. J Reprod Immunol. 2011 Jan;88(1):58–65. doi: 10.1016/j.jri.2010.06.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Penders J, Gerhold K, Stobberingh EE, Thijs C, Zimmermann K, Lau S, et al. Establishment of the intestinal microbiota and its role for atopic dermatitis in early childhood. J Allergy Clin Immunol. 2013 Sep;132(3):601–7 e8. doi: 10.1016/j.jaci.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 25.Candela M, Rampelli S, Turroni S, Severgnini M, Consolandi C, De Bellis G, et al. Unbalance of intestinal microbiota in atopic children. BMC Microbiol. 2012;12:95. doi: 10.1186/1471-2180-12-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, et al. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut. 2007 May;56(5):661–7. doi: 10.1136/gut.2006.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 2014 Apr;63(4):559–66. doi: 10.1136/gutjnl-2012-303249. [DOI] [PubMed] [Google Scholar]
- 28.Gronlund MM, Lehtonen OP, Eerola E, Kero P. Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J Pediatr Gastroenterol Nutr. 1999 Jan;28(1):19–25. doi: 10.1097/00005176-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006 Aug;118(2):511–21. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 30.Fallani M, Young D, Scott J, Norin E, Amarri S, Adam R, et al. Intestinal microbiota of 6-week-old infants across Europe: geographic influence beyond delivery mode, breast-feeding, and antibiotics. J Pediatr Gastroenterol Nutr. 2010 Jul;51(1):77–84. doi: 10.1097/MPG.0b013e3181d1b11e. [DOI] [PubMed] [Google Scholar]
- 31.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010 Jun 29;107(26):11971–5. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biasucci G, Rubini M, Riboni S, Morelli L, Bessi E, Retetangos C. Mode of delivery affects the bacterial community in the newborn gut. Early Hum Dev. 2010 Jul;86( Suppl 1):13–5. doi: 10.1016/j.earlhumdev.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Fujimura KE, Johnson CC, Ownby DR, Cox MJ, Brodie EL, Havstad SL, et al. Man's best friend? The effect of pet ownership on house dust microbial communities. J Allergy Clin Immunol. 2010 Aug;126(2):410–2. 2 e1–3. doi: 10.1016/j.jaci.2010.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stokholm J, Schjorring S, Pedersen L, Bischoff AL, Folsgaard N, Carson CG, et al. Living with cat and dog increases vaginal colonization with E. coli in pregnant women. PLoS One. 2012;7(9):e46226. doi: 10.1371/journal.pone.0046226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ling Z, Liu X, Chen W, Luo Y, Yuan L, Xia Y, et al. The restoration of the vaginal microbiota after treatment for bacterial vaginosis with metronidazole or probiotics. Microb Ecol. 2013 Apr;65(3):773–80. doi: 10.1007/s00248-012-0154-3. [DOI] [PubMed] [Google Scholar]