Abstract
When a comprehensive report on BPA was published in 2008, few data were available to assess the extent to which known poor glucuronidation capacity impacts BPA internal dose in infants and young children. In this paper, evidence that has emerged since the 2008 report is summarized, including: 1) human biomarker studies in children aged 0–5 years; 2) animal studies of neonatal toxicokinetics; and 3) physically based pharmacokinetic (PBPK) models. To address limitations in these studies, we recommend more human biomonitoring studies in children aged 0–5 years in which unmetabolized (free) BPA and BPA metabolites are separately quantified and detailed quality-control data are reported, investigation of metabolic differences between humans and animal species used for the study of BPA metabolism, and enzyme ontogeny studies, which along with biomonitoring studies would reduce uncertainty in PBPK models of early-life BPA metabolism.
Keywords: Early life, Metabolism, Bisphenol A, BPA, Chemicals, Toxicity, Toxicokinetics, Exposure
Introduction
Bisphenol A (BPA) is a high-production-volume chemical used in the synthesis of polycarbonate plastics and epoxy resins; materials with widespread commercial applications that include drink and food containers, soda, and food can liners; electronics and car parts; and dental sealants [1, 2]. BPA is also present as a thin coating on the surface of thermal printing papers, where it serves as an ink-developing agent, and as a result is widely found on cash register receipts and paper with a high-recycled-paper content [3].
Widespread BPA exposure in the general population has been documented in several countries and regions of the world, with detection in human urine exceeding 90% in representative samples of the population [4–7]. Exposure is thought to occur mainly through the diet due to the presence of BPA in food packaging. Human health concerns arise from its estrogenic properties, which can potentially confer or modify a range of health effects across the lifespan, including developmental and reproductive effects, diabetes and metabolic diseases, obesity, cancer of the reproductive organs, and cardiovascular disease [8–11].
Early-life exposures to endocrine disruptors such as BPA are of particular concern due to their potential to impact key stages of rapid growth and development [12]. Although much research has focused on prenatal exposure to BPA, infancy and childhood may present exposure scenarios with health implications that are unique to those life stages, such as disruption of estrogen- and testosterone-driven synaptogenesis, which peaks in early childhood [13].
Urinary biomarker data from the U.S. population over the age of 5 years demonstrate that BPA body burden decreases with age and intake rates are higher in children and adolescents than they are in adults [14]. Limited biomarker data exist in children under the age of 6 years, but estimates of weight-normalized intake rates in young children based on source aggregation suggest that the highest levels of exposure may occur in infants [15] and that diet is the main known contributor to BPA exposure [16]. BPA may be present in infant formula and baby food due to leaching from packaging or bottles or can be indirectly passed by the mother through breast milk.
Upon ingestion of BPA in adults, the biologically active parent molecule undergoes rapid first-pass metabolism to BPA glucuronide, which is biologically inert and rapidly cleared in the urine [17]. The formation of BPA glucuronide is driven by uridine diphosphate glucuronosyltransferases (UGTs) in the liver and gut. Enzyme activity for hepatic UGTs is lower at birth compared to the adult, a condition that can manifest clinically as jaundice in the newborn. UGT enzyme activity increases with age until it reaches adult levels, which occurs between the ages of 3–6 months and 10 years, depending on the UGT isoform [18]. Since glucuronidation is the main detoxication pathway for BPA in humans and other species, understanding the impact of low infant hepatic UGT activity on BPA metabolism is key to evaluating the potential health risks from early-life exposure to BPA, especially in the neonatal period and infancy.
In this paper, we have systematically reviewed epidemiologic, experimental, and modeling studies of BPA metabolism in early life published in the peer-reviewed literature since the release in 2008 of a comprehensive monograph by the National Toxicology Program Committee for the Evaluation of Risks to Human Reproduction (NTP-CERHR) [1]. We present conclusions based on evidence available since the report and identify current research gaps.
Methods
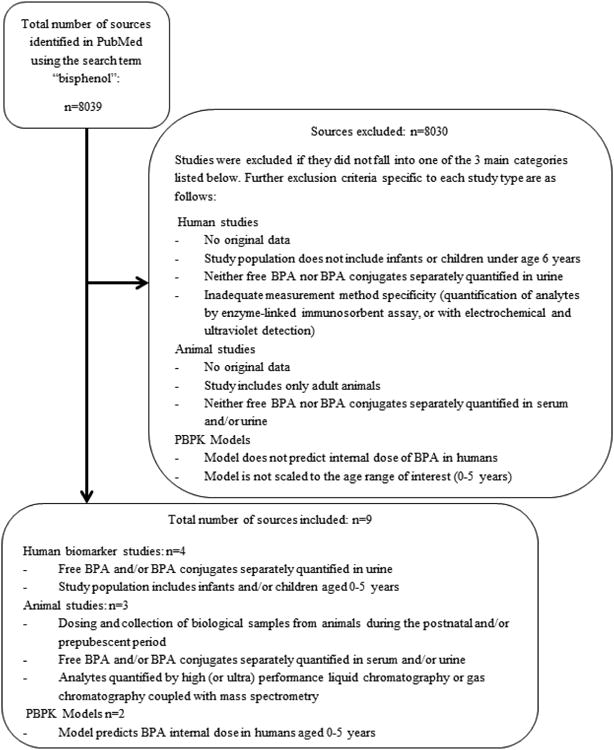
Articles published between June 2008 and August 2013 and catalogued in PubMed were evaluated independently by two investigators (RMN and JCH). The NTP-CERHR monograph provides a thorough review of the literature through mid-2008 on human toxicokinetics of BPA. Literature relevant to postnatal and early-life toxicokinetics published since 2008 was abstracted in the course of conducting a larger, broader literature review for which a search on the term “Bisphenol” was performed. Titles, abstracts and, when necessary, the text of the article were reviewed to identify publications of original research falling into three categories (Fig. 1): 1) studies reporting both free BPA and either conjugated BPA or total BPA in biologic fluids from children aged 0–5 years; 2) studies of neonatal toxicokinetics using animal models; and 3) physically based pharmacokinetic (PBPK) models of internal BPA dose in infants and young children. Publications reporting measurements of BPA in human biological samples were included even if the studies were not designed specifically to investigate toxicokinetics. An upper age limit of 5 years was chosen because enzyme activity develops rapidly and usually reaches adult levels by this age [10]. In order to ensure adequate laboratory method specificity, only human biomarker and animal studies were considered in which analytes were quantified by high- (or ultra-) performance liquid chromatography or gas chromatography coupled with mass spectrometry; studies in which measurements made using enzyme-linked immunosorbent assay or electrochemical and ultraviolet detection were excluded because they are not adequately specific and may overestimate concentrations of BPA in samples [20]. In addition, human studies were limited to those in which BPA was measured in urine, since serum BPA concentrations in children in the general population are generally too low to be detected [21]. Studies of cord blood, amniotic fluid, or fluids collected from mothers during pregnancy reflecting prenatal exposure and metabolism were not included in this review. All reported or modeled concentrations were converted to the units μg/L for comparability across studies.
Figure 1. Diagram of the article selection process.
Human Biomarker Studies
We identified 13 studies reporting urinary BPA concentrations in infants and children aged birth to 5 years, of which four studies met the inclusion criteria because concentrations of both free BPA and either total BPA or BPA glucuronide were reported (Table 1). Studies were conducted in Boston, Massachusetts (USA); Munich, Germany; and Baltimore, Maryland (USA). Sample sizes ranged from 12 to 47 individuals. Three of the 4 studies included both male and female subjects. Information on sex was not reported by Volkel et al., but participants were recruited randomly during pregnancy, so likely both sexes were represented in the study population. The ages of the study populations ranged from 7 days to 15 months. Of note, study population ages ranged from 2 to 5 years in the 9 studies that were excluded because only total BPA was reported [9, 22–30].
Table 1. Measurements of Urinary Free BPA, BPA Glucuronide, and Total BPA in Infants and Young Children.
| Authors and publication ear | Country | Population age | N | Laboratory method | LOD | Free BPA*(μg/L) | BPA-glucuronide* (μg/L) | Total BPA* (μg/L) | Detection Frequency: (Free/Total) |
|---|---|---|---|---|---|---|---|---|---|
| Volkel et al. 201134 | Germany (Munich) | 1 – 5 months | 47 | LC/MS-MS | 0.15 μg/L (LOQ = 0.45 μg/L) | All but 3 sample concentrations < LOQ | Not measured | Median < LOD | 9%/66% (per LOD); 3%/42% (per LOQ) |
| Calafat et al. 200931 | U.S. (Boston) | Premature, ≤ 44 weeks corrected gestational age | 41 | LC/MS-MS | 0.4 μg/L | GM: 1.8, Median: 1.7 | Not measured | GM: 30.3, Median: 28.6 | 92%/100% |
| Mendonca et al. 201235 | U.S. (Boston) | 2 – 15 months | 29 | LC/MS-MS | 0.4 μg/L | Mean: 0.5, GM < LOD, Median < LOD | Not measured | Mean: 6.0, GM: 2.3, Median: 1.8 | 28%/93% |
| Nachman et al. 201336 | U.S. (Baltimore) | 1 – 6 weeks | 12 | LC/MS-MS | LOQ = 0.1 μg/L | < LOQ | Mean: 0.87, Median: 0.66 | Not meas. | 0%/100% |
Mean, geometric mean (GM) and median if reported. LOD = limit of detection; LOQ = limit of quantification.
Three of the 4 reviewed studies reported concentrations of free BPA and total BPA. Total BPA is equal to the sum of free BPA and BPA glucuronide and is quantified by measuring free BPA in a sample that has been treated with β-glucuronidase, which cleaves the glucuronide group from the metabolite via hydrolysis. Nachman et al. reported free BPA and BPA glucuronide concentrations, measured directly, without enzymatic hydrolysis. Detection frequencies of total BPA or BPA glucuronide ranged from 66% to 100%, demonstrating widespread exposure in all the study populations. By comparison, detection frequencies for free BPA were much more variable, ranging from 0% to 92%.
The first urinary BPA concentrations measured in infants were reported in 2009 by Calafat et al. This study is distinct in that the 41 participating infants were premature and were staying in a neonatal intensive care unit (NICU), both of which are factors that could potentially influence BPA exposure and metabolism [31]. Urine was collected from cotton gauze and cotton diaper filling. Medical equipment such as polyvinyl chloride bags and tubing used in NICU settings are known sources of BPA exposure [31], and urinary total BPA concentrations in the study population were higher than any concentrations since reported for infants in the general population, who would not be expected to be exposed to BPA via these sources. The reported median concentration of 28.6 μg/L is an order of magnitude higher than the median in the U.S. general population aged 6 years and greater [32]. The reported maximum total BPA concentration of 946 μg/L exceeded urinary concentrations measured in an occupationally exposed population [33]. The detection frequencies for both free BPA and total BPA were 92% and 100%, respectively, but free BPA concentrations were much lower compared with total BPA concentrations (median = 1.7 μg/L), demonstrating that the infants were capable of a significant level of BPA conjugation. The exact ages of infants in the study were not available, but investigators conducted a visual age assessment, and only infants less than or equal to 44 weeks corrected gestational age (gestational age + weeks since birth) were included in the study. The high correlation between free and total BPA concentrations in the NICU study (r=0.86) may indicate that a consistent percent of free BPA went unconjugated such that the infants with the highest total BPA concentrations also had the highest free BPA concentrations. However, this correlation may also be the result of BPA contamination in that the total BPA concentrations are high because of contamination with BPA.
In the other 3 human biomarker studies reviewed, the study populations included infants and young children carried to term who had no known exposures beyond those expected in the general population [34–36]. Reported concentrations of total BPA and BPA glucuronide were lower in these studies compared to Calafat et al., likely because infants in these studies were not exposed to the same sources of BPA as the infants in the NICU (Table 1). In Germany, free BPA was detected in 8 of 91 samples from 47 healthy full-term infants aged 1–5 months [34]. In the same study, total BPA was detected in 66% of the infants, demonstrating exposure was prevalent in the study population. Median concentrations of both analytes were below the limit of quantification (LOQ). Urine was collected using polyethylene urine collection bags, which would not be expected to contain BPA. Given the proximity of total BPA concentrations to the method LOQ, unless glucuronidation were severely impaired, free BPA concentrations, being a fraction of the total, would be expected to be below the LOQ.
In Boston, Mendonca et al. measured free and total BPA in the urine of 29 healthy full-term infants aged 2–15 months [35]. The median urinary total BPA concentration was 1.8 μg/L. The detection frequency for free BPA was notably high (28%), although the median urinary free BPA concentration was below the limit of detection (0.4 μg/L). The authors reported that the small sample size and low free BPA detection frequency prevented the evaluation of free BPA as an indicator of metabolic capacity of the study population and that the wood pulp and cotton diapers used for sample collection are possible but unconfirmed sources of BPA contamination. Free BPA detected in urine from one infant with a high urinary total BPA concentration of 89 μg/L may indicate incomplete glucuronidation of BPA in that subject, although it may have been random contamination. A similar situation was reported by Volkel et al. in which a free BPA concentration of 16 μg/L was quantified in urine from an infant with an unusually high urinary total BPA concentration of 17.35 μg/L in the study by Volkel et al [34].
Nachman et al. measured free BPA and BPA glucuronide in urine collected from 12 healthy full-term infants aged 1–6 weeks in Baltimore, Maryland [36]. BPA glucuronide was detected in all samples (median = 0.66 μg/L), confirming exposure to BPA in all 12 infants. In contrast to the other studies, free BPA was undetected in all but one sample, for which the replicate was a nondetect. The lack of free BPA detection despite confirmation of BPA exposure in all infants suggests efficient conjugation of BPA in infants, specifically by the glucuronidation pathway, at a very young age. Given the small sample size, these results should be interpreted with caution, as there may be variability in the capacity to conjugate BPA in this age group, and the study population may have inadvertently included only those infants with higher glucuronidation enzyme activities. Unique features of the laboratory analysis method may have contributed to the low incidence of sample contamination including direct measurement of BPA glucuronide and derivatization of both analytes with dansyl chloride. The study did not report total BPA, and thus any BPA sulfate that might have been present in the urine was not accounted for.
A significant degree of glucuronidation was found to occur in infants in all studies. High detection frequency of free BPA and correlation of free BPA concentrations with total BPA concentrations in the urine of premature infants in a NICU demonstrated that, although BPA conjugation occurs in this sensitive subpopulation, it may be less efficient than it is in full-term or older infants. The youngest infant of known age studied was 1 week old; thus the results of these studies may not apply in the first days of life, which may be a critical period in terms of both impaired metabolic capacity and developmental susceptibility. When total BPA concentrations are close to the LOQ, free BPA may not be detectable, even under conditions of impaired BPA glucuronidation.
Animal Studies
We identified 4 studies evaluating neonatal toxicokinetics in animal models. One study was excluded from this review because serum concentrations were measured in only one age group [37]. The remaining three studies were all performed in the same laboratory by Doerge and colleagues (Table 2). Both oral and subcutaneous routes of exposure were examined through the administration of 100 μg/kg doses of d6-BPA in three different animal models: CD-1 mouse, Sprague Dawley (SD) rat, and rhesus monkey [38–40]. The dose of 100 μg/kg was considered by the authors to be close enough to the range of human exposure to be relevant to the general population and to be high enough to be detectable in serum enabling the determination of toxicokinetic parameters. HPLC-MS/MS with 13C-BPA as an internal standard was used to identify and quantify both free and total d6-BPA. The use of d6 BPA for dosing solutions eliminated any possible influence of background contamination on the measurements.
Table 2. Studies of Postnatal BPA Metabolism in Animal Models.
| Authors and publication year | Animal model | N | PND age at dosing | Dose (μg/kg) | Dosing Route | Biological fluids analyzed | Laboratory Analysis method | Analytes | Glucuronidation increase with age | % free BPA* |
|---|---|---|---|---|---|---|---|---|---|---|
| Doerge et al. 2011.38 | CD-1 mouse | 504 pups (12 per age/route group); 96 adults | PND 3, 10, and 21 | 100 | Oral and SC | serum | LC-MS/MS | Free and total d6-BPA | Yes (significant) | 23% (per Cmax), 2.2% (per AUC) |
| Doerge et al. 201039 | SD rat | 144 pups (4 per age/route group); 12 adults | PND 3, 10, and 21 | 100 | Oral and SC | serum | LC-MS/MS | Free and total d6-BPA | Yes (significant) | 6.6% (per Cmax), 1.4% (per AUC) |
| Doerge et al. 201040 | Rhesus monkey | 4 adult female, 6 neonatal (3 female, 3 male) | PND 5,‡ 35, 70 (oral); PND 77 (IV) | 100 | Oral and IV | serum, urine, feces | LC-MS/MS | Free and total d6-BPA | No | <1% (per Cmax and AUC) |
Denominator: total BPA. Data for lowest age group, orally dosed is presented. PND=post natal day; LC-MS/MS = liquid chromatography with tandem mass spectrometry; SC = subcutaneous; IV = intravenous; Cmax = peak serum concentration; AUC = area under the curve
Doerge et al. reported a significant effect of age on peak serum free BPA concentrations (i.e., the Cmax or maximum concentration) in neonatal CD-1 mice and SD rats, evidence that early-life impairment of BPA conjugation impacts internal BPA dose in rodents [38, 39]. In both mice and rats, free BPA concentrations decreased with age and were not statistically different from those observed in adult animals by postnatal-day (PND) 21. In primates – specifically rhesus monkeys – considered to be a more appropriate model for human BPA toxicokinetics than rodents, age-related differences in BPA conjugation were not statistically significant at PND 5, 35, 70, or adult [40]. At all postnatal ages, free BPA concentrations in primates (based on the Cmax and area under the curve) accounted for less than 1% of total BPA concentrations in serum. Interestingly, the percent serum BPA concentrations at the Cmax in 4 adult female rhesus monkeys (29 ± 19% free BPA) were higher than they were in the younger age groups, suggesting that interindividual variability in BPA glucuronidation may exist independent of age.
In the 3 studies by Doerge et al. described above, reduced metabolic capacity in the neonatal period was observed to impact internal dose of BPA in rodents but not primates. The 100 μg/kg dose in these experiments corresponds to approximately the same dose administered to adult human volunteers studied by Volkel et al. [17], and is 5 times the estimated 95th percentile daily intake for 6–11-year-olds based on urinary concentrations in the United States (assuming a body weight of 40 kg) [14] and twice the reference dose of 50 μg/kg for BPA [41]. The null finding in neonatal rhesus monkeys at this dose suggests that, contrary to previous thinking, differences between neonatal and adult metabolism may not significantly impact internal dosimetry of BPA following oral exposure. While data from rhesus monkeys would be expected to be the most relevant to humans of the three animal models explored, the possibility of species differences between humans and rhesus monkeys cannot be dismissed.
PBPK Studies
Two PBPK models have been published estimating serum free BPA concentrations at early ages (Table 3). Mielke et al. reported estimated steady-state as well as peak blood concentrations for free BPA for several age groups ranging from birth to 4.5 years [42]. Steady-state concentration, a measure of internal dose, represents the average serum free BPA concentration over time. The peak concentration, on the other hand, is analogous to the Cmax in a controlled toxicokinetic study and is an appropriate metric for BPA internal dose, as most BPA exposure is thought to occur episodically with food intake, resulting in blood concentrations that fluctuate with time. Intake rates from two European Union reports [43, 44] were applied as inputs in the model, along with a number of age-appropriate physiologic and toxicokinetic parameters. Mielke et al. estimated that the highest peak serum concentration would be 0.34 μg/L in newborns and infants up to 3 months of age who were fed from polycarbonate bottles. For infants fed from non-polycarbonate bottles, a lower estimated peak of 0.071 μg/L was reported. Steady-state concentrations reported by Mielke et al. are presented in Table 3. This model is unique in its inclusion of both sulfation and glucuronidation as presystemic metabolic pathways [45].
Table 3. PBPK Models of Infant Internal Dose of BPA.
| Authors and publication year | Published Sources of Model Inputs* | Age groups modeled | Free BPA Steady State Serum Concentration (μg/L) | Free BPA Peak Serum Concentration (μg/L) | Bioavailability* |
|---|---|---|---|---|---|
| Mielke and Gundert-Remy. 200942 | Intake Rates: EFSA 200643; EU 200844 TK Parameters: Kuester and Sipes 200745 TK Scaling factors: Edginton and Ritter 200652 |
Newborn 3 months 6 months 6-12 months, 1.5 years 1.5-4.5 years Adult |
0.096 (pc bottle) 0.0008 (breast), 0.020 (non-pc bottle) 0.096 (pc bottle) 0.030 0.0085 0.010 0.012 o.oo4 |
0.34 (pc bottle) 0.0033 (breast), 0.071 (non-pc bottle) 0.34 (pc bottle) 0.17 0.049 0.059 0.069 0.023 |
Not reported |
| Edginton and Ritter 200946 | Intake Rates: EFSA 200643; Ye et al. 200847 TK Parameters: Volket et al. 200217 TK Scaling factors: Edginton and Ritter 200652 |
Newborn 3 months 6 months 1.5 years adult |
Presented in graph. Exact values not reported. | Not modeled. | 88% 48% 32% 23% 18% |
TK Parameters=toxicokinetic parameters for adults, TK scaling factors=fraction of adult enzyme activity based on age of infant or child
Fraction absorbed orally compared with IV absorption
EFSA=European Food Safety parameters
In a second PBPK study, Edginton and Ritter estimated steady-state serum concentrations of free BPA and BPA glucuronide with a model that reflects reduced glucuronidation capacity in the 0–3-month age group, followed by rapid maturation of this metabolic pathway [46]. When age-appropriate external exposure estimates were used as inputs in the model, the resulting steady-state serum free BPA concentrations (presented only in graph form) were similar to those reported by Mielke et al., except for in the newborn age group, for which Edginton and Ritter assumed a lower external exposure compared with that chosen by Mielke et al [47]. Peak serum concentrations of free or conjugated BPA were not estimated by Edginton and Ritter.
The PBPK models presented here produced quantitative data on serum free and conjugated BPA levels in humans under the age of 5 years that would not otherwise be available for this age group. A number of age-specific human physiologic parameters were included in the models. Significant uncertainty in both models arises from the use of toxicokinetic parameters derived from clinically obtained data on early-life metabolism of morphine, which is primarily glucuronidated by the isoform UGT2B7. UGT2B15 is known to play a much larger role in glucuronidation of BPA than UGT2B7 [48]; however, comparable age-specific toxicokinetic data for UGT2B15 are not available.
Discussion
Summary of the Findings
Although not a large body of literature, the publication of 4 human biomarker studies in infant populations, 3 animal studies in neonatal mice, rats, and rhesus monkeys, and 2 PBPK models of age-dependent internal BPA dosimetry represent significant progress and increased data availability relating to neonatal BPA metabolism within the last 5 years. In 2008, only 2 studies of neonatal metabolism in rats – one in vitro [49] and the other in vivo [50] represented the entirety of the literature on this topic. In their list of 10 Critical Data Needs for the evaluation of BPA toxicity, the NTP-CERHR specifically identified the need for more measurements of free and conjugated BPA in neonatal biological samples as well as the need for PBPK models of early-life internal dose [1]. Three types of studies were considered in this review, and the findings differed by study type.
Human Biomarker Studies
In human studies, BPA glucuronide accounted for most or all of the BPA measured in urine from 4 different infant populations, which both confirmed exposure in this population and demonstrated substantial capacity to metabolize BPA at the levels at which the infants were exposed.
Inconsistency of findings among biomarker studies may be due to differences in exposure levels or developmental stages of the study populations. In the case of the study of premature infants in a NICU, urinary concentrations in some cases were 2–3 orders of magnitude higher than measurements reported in other infant studies, reflecting higher exposures in a NICU setting. It is possible that BPA is more likely to be detected in a high-exposure scenario and that it could reflect low conjugation capacity that is undetectable in lower-exposure scenarios. Also, impaired glucuronidation capacity may be more pronounced or more widespread in premature infants compared with those carried to term. Low sample sizes and different population demographics may also contribute to the inconsistencies among the 3 studies of healthy full-term infants, although infrequent or no detection of free BPA in 2 of the 3 studies, one including neonates as young as 7 days of age, suggests that very efficient BPA glucuronidation may be possible in infants with no mitigating factors. On the other hand, detection of free BPA in 92% of urine samples in a population of premature infants in a NICU indicates that prematurity and/or high exposure may be factors that mediate the impact of neonatal toxicokinetics on internal BPA dose.
Inadvertent contamination of samples by BPA during handling is always a consideration when interpreting free BPA concentrations in any biological samples and may have accounted for the detection of free BPA in healthy infants. The ubiquitous presence of BPA in both field and laboratory settings significantly complicates the determination of metabolic profiles for BPA in human biological fluids and tissues [51]. BPA contamination from background sources is indistinguishable from free BPA eliminated in the urine following absorption, metabolism, and distribution in the body. Background concentrations also tend to be highly variable, preventing the quantification of a mean background level with which to blank-correct samples. BPA glucuronide would not be expected to be found outside the body, and thus contamination should not affect levels of BPA glucuronide in biological samples. An experimental study of BPA toxicokinetics in humans using d16-BPA, a stable isotope that behaves like BPA in the body, has been conducted in adults [17] but, for ethical reasons, is not possible in children. Nonetheless, measurements of free BPA and BPA conjugates in urine of infants and young children who are exposed through everyday activities can be useful in exploring changes in the BPA metabolic profile that occur at an early age.
Animal Studies
Although age was significantly associated with BPA glucuronidation in CD-1 mice and SD rats in studies by Doerge et al., no significant age-related differences in BPA glucuronidation were found in a study of neonatal and adult rhesus monkeys conducted by the same investigators using similar methods. Serum free BPA levels were <1% of total BPA in neonatal monkeys at postnatal day 5, even at peak levels (Cmax), following an oral dose of 100 μg/kg. On the other hand, 29 ± 19% free BPA accounted for total BPA concentrations in adult monkeys in the same study, raising the possibility of interindividual variability that was not captured by the small sample size. Because rhesus monkeys are expected to be a better model of human BPA metabolism than rodents such as the CD-1 mouse and SD rats, despite the observed effect of age on BPA metabolism in rodents, the lack of evidence of reduced BPA conjugation in rhesus monkeys suggests that neonatal conjugation capacity may not significantly alter BPA internal dose in human neonates.
The use of d6-BPA by Doerge et al. for the dosing of animals in all 3 studies included in this review adds considerable validity to the reported findings, as any contamination of samples with BPA during handling does not impact measured concentrations of free d6-BPA. The ability of the investigator to circumvent issues such as sample contamination and inadequate measurement method sensitivity by administering controlled doses of d6-BPA is one advantage of studying neonatal BPA toxicokinetics in animals. However, the relevance of animal study data to human populations is uncertain due to possible interspecies differences, such as UGT isoform sequences or UGT ontogeny, such that BPA conjugation occurs at a lower rate or develops at a later age in humans than in rhesus monkeys.
PBPK Models
Both PBPK models predicted that reduced glucuronidation capacity would increase the bioavailability of free BPA in neonates and infants under the age of 3 months. Consistency among PBPK models and with toxicokinetic data from an intentionally exposed group of adult volunteers supports the validity of these models. Given the same weight-normalized intake of BPA, steady-state serum free BPA concentrations were predicted to be higher in infants aged 0–3 months compared to adults by a factor of 3 in the model by Mielke el al., and by a factor of 11 in the model by Edginton and Ritter.
PBPK models of internal dose in infants and children combine age-dependent toxicokinetic parameters, external exposure data, and allometric scaling of other physiologic features to calculate serum concentrations of the substance of interest. One influential input in a PBPK model estimating early-life internal free BPA concentrations is the age-dependent rate at which BPA undergoes glucuronidation in the liver. Given estimates that the oral route accounts for as much as 99% of BPA exposure [16], first-pass metabolism of BPA plays an important role in determining whether BPA enters the blood compartment as either the active (unconjugated) or inactive (conjugated) form. Toxicokinetic parameters for glucuronidation vary greatly by substance and by age. By way of example, morphine and acetaminophen, the metabolism of which are well studied in infants and young children in clinical settings, both undergo glucuronidation, but because they have different affinities for different UGT isoforms, and those isoforms in turn have different developmental timelines, hepatic metabolism of morphine is estimated to occur at adult levels by the age of 1 year, compared with 10 years of age for acetaminophen [52].
It should be noted that both models cited in this review relied on studies of UGT2B7 ontogeny, not UGT2B15, for inputs of toxicokinetic parameters. While UGT2B7 may contribute to BPA conjugation, UGT2B15 enzyme activity is about 3 times higher than UGT2B7 activity in an in vitro model testing several recombinant UGT isoforms [48]. Also, a significant limitation of PBPK models is the scarcity of urine biomarker data in infants from which to estimate exposures. Exposure estimates in the model were based on source aggregations, which are much less certain than estimates from urine biomarkers [41, 50, 52].
Conclusion and Future Research Directions
The inconsistencies across the 3 types of studies need to be interpreted cautiously, as each study type has a unique set of limitations, some of which can be addressed in future research. We make the following recommendations to address data gaps and reduce uncertainty in estimates of internal dose of BPA in infants and young children.
Better reporting of quality-control methods and data is needed to assist readers in evaluating the influence of free BPA from field and lab sources on the results.
More measurements are needed of urinary free BPA and total BPA from larger and more diverse study populations that include children aged 5 years and younger, especially neonates and young infants. The contribution of these measurements is twofold. First, back-calculation of intake rates from urinary total BPA concentrations would provide better estimates of exposure in this age group. The two PBPK studies reviewed here demonstrate the strong influence of external exposure estimates on modeled internal dose. Secondly, separate quantification of free BPA in urine is an important indicator of presystemic metabolism in infant populations under real life conditions. With proper quality-control measures to ensure that samples are not contaminated during collection, handling, storage, and/or analysis, data on free BPA have the potential to demonstrate age-dependent variations in the capacity for BPA conjugation.
Separate quantification of BPA glucuronide and BPA sulfate concentrations in early-life urine samples is needed to explore the potential role of sulfation in early-life BPA metabolism. BPA sulfate can undergo enzymatic hydrolysis in the presence of β-glucuronidase such that measurements of total BPA by enzymatic hydrolysis potentially reflect the sum of not only free BPA and BPA glucuronide, but BPA sulfate as well. Sulfation of BPA has been demonstrated in vitro and is known to be more developed in the neonate than glucuronidation [19].
More study of UGT isoforms responsible for BPA metabolism in humans and in animal models is needed to better gauge the validity of extrapolation of BPA toxicokinetic data from nonhuman species to humans.
In vitro studies are needed that better characterize the ontogeny and interindividual variability of glucuronidation by the UGT2B15 isoform. These would improve scaling of toxicokinetic parameters in PBPK models of internal dose in infants and young children.
The internal dose of BPA in neonates, infants, and young children remains an important factor in estimates of health risk from BPA exposure early in life. The quality of internal dose estimates obtained via PBPK models depends on the quality of pharmacokinetic data available to be used as inputs in the model. Human biomarker studies and animal toxicokinetic studies provide context in which to consider the validity of PBPK models for predicting age-dependent internal BPA dose. Conjugation of BPA via glucuronidation (and potentially sulfation) is an important detoxication pathway in humans that may be impaired at birth and for a period of time in early human development. Understanding the timing and extent of impairment will improve assessments of human health risk in this vulnerable subpopulation. However, it should be noted that even given rapid and efficient first-pass metabolism of BPA, biologically active BPA may still reach target tissues either via bypassing presystemic conjugation as plasma-bound BPA or through deconjugation at target tissues. In addition, although BPA exposures have recently decreased in the general population [53], this trend is likely due in part to the introduction of replacements, some of which are similar in structure to BPA and may act by a similar mode of action, raising the possibility of other environmental exposures of concern in the general population and/or possible cumulative effects of exposure to several related phenolic compounds, including BPA.
In this paper we have reviewed the literature on a topic that has generated debate among researchers in the field. The study of BPA toxicokinetics cannot independently provide an answer regarding the health risks associated with BPA exposure, but it is a crucial component of any evaluation of the toxicity of this ubiquitous compound. To assess whether known poor glucuronidation capacity impacts the internal dose of BPA in infants and young children exposed to BPA, we examined published results of human biomarker studies, animal studies, and PBPK models. Inconsistent findings among studies may be explained by the limitations of each study design. By addressing issues such as contamination of human biological samples with BPA from field and laboratory sources, gaps in our knowledge of interspecies differences in BPA metabolism, and the need for better data to inform exposure and toxicologic parameters in PBPK models, we can gain a better understanding of age-dependent differences in BPA metabolism.
Acknowledgments
This research was supported in part with grants from The Johns Hopkins Center for a Livable Future, the Wendy Klag Memorial Fund, and the NIH (P01 ES006052, P30 ES003819, and training grant T32 ES007141).
Footnotes
Compliance with Ethics Guidelines: Conflict of Interest: Rebecca M. Nachman, Jennifer C. Hartle, Peter S. J. Lees, and John D. Groopman declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
•Of importance
••Of major importance
- 1.National Toxicology Program (Center for the Evaluation of Risks to Human Reproduction) NTP-CERHR monograph on the potential human reproductive and developmental effects of bisphenol A. 2008 NIH Publication No. 08 – 5994. [Google Scholar]
- 2.European Commission Joint Research Center (Institute for Health and Consumer Protection) European Union risk assessment report: bisphenol-A. Luxembourg: Office for Official Publications of the European Communities; 2003. EUR 20843 EN. [Google Scholar]
- 3.Liao C, Kannan K. Widespread occurrence of bisphenol A in paper and paper products: implications for human exposure. Environ Sci Technol. 2011;45:9372–9379. doi: 10.1021/es202507f. [DOI] [PubMed] [Google Scholar]
- 4.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bushnik T, Haines D, Levallois P, Levesque J, Van Oostdam J, Viau C. Lead and bisphenol A concentrations in the Canadian population. Health Rep. 2010;21:7–18. [PubMed] [Google Scholar]
- 6.Koch HM, Kolossa-Gehring M, Schroter-Kermani C, Angerer J, Bruning T. Bisphenol A in 24 h urine and plasma samples of the German Environmental Specimen Bank from 1995 to 2009: a retrospective exposure evaluation. J Expo Sci Environ Epidemiol. 2012;22:610–616. doi: 10.1038/jes.2012.39. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z, Alomirah H, Cho HS, et al. Urinary bisphenol A concentrations and their implications for human exposure in several Asian countries. Environ Sci Technol. 2011;45:7044–7050. doi: 10.1021/es200976k. [DOI] [PubMed] [Google Scholar]
- 8.Lang IA, Galloway TS, Scarlett A, et al. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300:1303–1310. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- 9•.Braun JM, Kalkbrenner AE, Calafat AM, et al. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics. 2011;128:873–882. doi: 10.1542/peds.2011-1335. This paper presents results of an epidemiologic study in which maternal urinary BPA concentrations during pregnancy were associated with behavior changes in 3 year old children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trasande L, Attina TM, Blustein J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. JAMA. 2012;308:1113–1121. doi: 10.1001/2012.jama.11461. [DOI] [PubMed] [Google Scholar]
- 11.Acevedo N, Davis B, Schaeberle CM, Sonnenschein C, Soto AM. Perinatally Administered Bisphenol A Acts as a Mammary Gland Carcinogen in Rats. Environ Health Perspect. 2013 doi: 10.1289/ehp.1306734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selevan SG, Kimmel CA, Mendola P. Identifying critical windows of exposure for children's health. Environ Health Perspect. 2000;108(Suppl 3):451–455. doi: 10.1289/ehp.00108s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hajszan T, Leranth C. Bisphenol A interferes with synaptic remodeling. Front Neuroendocrinol. 2010;31:519–530. doi: 10.1016/j.yfrne.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lakind JS, Naiman DQ. Bisphenol A (BPA) daily intakes in the United States: estimates from the 2003-2004 NHANES urinary BPA data. J Expo Sci Environ Epidemiol. 2008;18:608–615. doi: 10.1038/jes.2008.20. [DOI] [PubMed] [Google Scholar]
- 15.European Commission Joint Research Center (Institute for Health and Consumer Protection) European Union risk assessment report: 4-4′ isopropylidenediphenol (Bisphenol A), Part 2 Human Health. Luxembourg: Publications Office of the European Union; 2008. EINECS No. 201-245-8. [Google Scholar]
- 16.Wilson NK, Chuang JC, Morgan MK, Lordo RA, Sheldon LS. An observational study of the potential exposures of preschool children to pentachlorophenol, bisphenol-A, and nonylphenol at home and daycare. Environ Res. 2007;103:9–20. doi: 10.1016/j.envres.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Volkel W, Colnot T, Csanady GA, Filser JG, Dekant W. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem Res Toxicol. 2002;15:1281–1287. doi: 10.1021/tx025548t. [DOI] [PubMed] [Google Scholar]
- 18.McCarver DG, Hines RN. The ontogeny of human drug-metabolizing enzymes: phase II conjugation enzymes and regulatory mechanisms. J Pharmacol Exp Ther. 2002;300:361–366. doi: 10.1124/jpet.300.2.361. [DOI] [PubMed] [Google Scholar]
- 19.Alcorn J, McNamara PJ. Pharmacokinetics in the newborn. Adv Drug Deliv Rev. 2003;55:667–686. doi: 10.1016/s0169-409x(03)00030-9. [DOI] [PubMed] [Google Scholar]
- 20.Dekant W, Volkel W. Human exposure to bisphenol A by biomonitoring: methods, results and assessment of environmental exposures. Toxicol Appl Pharmacol. 2008;228:114–134. doi: 10.1016/j.taap.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 21•.Ye X, Zhou X, Wong LY, Calafat AM. Concentrations of bisphenol A and seven other phenols in pooled sera from 3-11 year old children: 2001-2002 National Health and Nutrition Examination Survey. Environ Sci Technol. 2012;46:12664–12671. doi: 10.1021/es303109c. This paper demonstrates that urine, not blood, is the appropriate matrix for BPA biomonitoring in the general population. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perera F, Vishnevetsky J, Herbstman JB, et al. Prenatal bisphenol a exposure and child behavior in an inner-city cohort. Environ Health Perspect. 2012;120:1190–1194. doi: 10.1289/ehp.1104492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgan MK, Jones PA, Calafat AM, et al. Assessing the quantitative relationships between preschool children's exposures to bisphenol A by route and urinary biomonitoring. Environ Sci Technol. 2011;45:5309–5316. doi: 10.1021/es200537u. [DOI] [PubMed] [Google Scholar]
- 24.Sathyanarayana S, Alcedo G, Saelens BE, et al. Unexpected results in a randomized dietary trial to reduce phthalate and bisphenol A exposures. J Expo Sci Environ Epidemiol. 2013;23:378–384. doi: 10.1038/jes.2013.9. [DOI] [PubMed] [Google Scholar]
- 25.Casas M, Valvi D, Luque N, et al. Dietary and sociodemographic determinants of bisphenol A urine concentrations in pregnant women and children. Environ Int. 2013;56:10–18. doi: 10.1016/j.envint.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Hoepner LA, Whyatt RM, Just AC, Calafat AM, Perera FP, Rundle AG. Urinary concentrations of bisphenol A in an urban minority birth cohort in New York City, prenatal through age 7 years. Environ Res. 2013;122:38–44. doi: 10.1016/j.envres.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harley KG, Aguilar Schall R, Chevrier J, et al. Prenatal and postnatal bisphenol A exposure and body mass index in childhood in the CHAMACOS cohort. Environ Health Perspect. 2013;121:514–20. 520e1–6. doi: 10.1289/ehp.1205548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harley KG, Gunier RB, Kogut K, et al. Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environ Res. 2013;126:43–50. doi: 10.1016/j.envres.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Health Canada. Second Report on Human Biomonitoring of Environmental Chemicals in Canada. 2013 This report includes biomonitoring data for environmental chemicals (including BPA) for children as young as age 3 years from a nationally representative sample of Canadians; the previous report and the analysis report for the U.S. population includes children aged 6 and up. [Google Scholar]
- 30.Becker K, Göen T, Seiwert M, Conrad A, Pick-Fuss H, Müller J, Wittassek M, Schulz C, Kolossa-Gehring M. GerES IV: phthalate metabolites and bisphenol A in urine of German children. Int J Hyg Environ Health. 2009 Nov;212(6):685–92. doi: 10.1016/j.ijheh.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 31•.Calafat AM, Weuve J, Ye X, et al. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ Health Perspect. 2009;117:639–644. doi: 10.1289/ehp.0800265. This paper presents the first BPA biomonitoring data in infants and demonstrates that medical equipment in a NICU is an important potential source of BPA exposure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals, updated tables. 2012 Feb;2012 [Google Scholar]
- 33.Li D, Zhou Z, Qing D, et al. Occupational exposure to bisphenol-A (BPA) and the risk of self-reported male sexual dysfunction. Hum Reprod. 2010;25:519–527. doi: 10.1093/humrep/dep381. [DOI] [PubMed] [Google Scholar]
- 34.Volkel W, Kiranoglu M, Fromme H. Determination of free and total bisphenol A in urine of infants. Environ Res. 2011;111:143–148. doi: 10.1016/j.envres.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Mendonca K, Hauser R, Calafat AM, Arbuckle TE, Duty SM. Bisphenol A concentrations in maternal breast milk and infant urine. Int Arch Occup Environ Health. 2012 doi: 10.1007/s00420-012-0834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nachman RM, Fox SD, Golden WC, et al. Urinary free bisphenol A and bisphenol A-glucuronide concentrations in newborns. J Pediatr. 2013;162:870–872. doi: 10.1016/j.jpeds.2012.11.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prins GS, Ye SH, Birch L, Ho SM, Kannan K. Serum bisphenol A pharmacokinetics and prostate neoplastic responses following oral and subcutaneous exposures in neonatal Sprague-Dawley rats. Reprod Toxicol. 2011 Jan;31(1):1–9. doi: 10.1016/j.reprotox.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doerge DR, Twaddle NC, Vanlandingham M, Fisher JW. Pharmacokinetics of bisphenol A in neonatal and adult CD-1 mice: inter-species comparisons with Sprague-Dawley rats and rhesus monkeys. Toxicol Lett. 2011;207:298–305. doi: 10.1016/j.toxlet.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 39.Doerge DR, Twaddle NC, Vanlandingham M, Fisher JW. Pharmacokinetics of bisphenol A in neonatal and adult Sprague-Dawley rats. Toxicol Appl Pharmacol. 2010;247:158–165. doi: 10.1016/j.taap.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Doerge DR, Twaddle NC, Woodling KA, Fisher JW. Pharmacokinetics of bisphenol A in neonatal and adult rhesus monkeys. Toxicol Appl Pharmacol. 2010;248:1–11. doi: 10.1016/j.taap.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 41.U.S. Environmental Protection Agency. IRIS Database. [PubMed] [Google Scholar]
- 42.Mielke H, Gundert-Remy U. Bisphenol A levels in blood depend on age and exposure. Toxicol Lett. 2009;190:32–40. doi: 10.1016/j.toxlet.2009.06.861. [DOI] [PubMed] [Google Scholar]
- 43.European Food Safety Authority. Opinion of the scientific panel on food additives, flavourings, food processing aids, and materials in contact with food (AFC) The EFSA Journal. 2006;759:1–10. doi: 10.2903/j.efsa.2008.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.European Commission. European Union Updated Risk Assessment of 4, 4′-isopropylidenediphenol (Bisphenol-A) (CAS Number: 80-05-7, EINECS Number: 201-245-8).
- 45.Kuester RK, Sipes IG. Prediction of metabolic clearance of bisphenol A (4,4′-dihydroxy-2,2-diphenylpropane) using cryopreserved human hepatocytes. Drug Metab Dispos. 2007;35:1910–1915. doi: 10.1124/dmd.107.014787. [DOI] [PubMed] [Google Scholar]
- 46.Edginton AN, Ritter L. Predicting plasma concentrations of bisphenol A in children younger than 2 years of age after typical feeding schedules, using a physiologically based toxicokinetic model. Environ Health Perspect. 2009;117:645–652. doi: 10.1289/ehp.0800073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ye X, Bishop AM, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for measuring parabens, triclosan, and other environmental phenols in human milk. Anal Chim Acta. 2008;622:150–156. doi: 10.1016/j.aca.2008.05.068. [DOI] [PubMed] [Google Scholar]
- 48.Hanioka N, Naito T, Narimatsu S. Human UDP-glucuronosyltransferase isoforms involved in bisphenol A glucuronidation. Chemosphere. 2008;74:33–36. doi: 10.1016/j.chemosphere.2008.09.053. [DOI] [PubMed] [Google Scholar]
- 49.Matsumoto J, Yokota H, Yuasa A. Developmental increases in rat hepatic microsomal UDP-glucuronosyltransferase activities toward xenoestrogens and decreases during pregnancy. Environ Health Perspect. 2002;110:193–196. doi: 10.1289/ehp.02110193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Domoradzki JY, Thornton CM, Pottenger LH, et al. Age and dose dependency of the pharmacokinetics and metabolism of bisphenol A in neonatal sprague-dawley rats following oral administration. Toxicol Sci. 2004;77:230–242. doi: 10.1093/toxsci/kfh054. [DOI] [PubMed] [Google Scholar]
- 51.Ye X, Zhou X, Hennings R, Kramer J, Calafat AM. Potential external contamination with bisphenol A and other ubiquitous organic environmental chemicals during biomonitoring analysis: an elusive laboratory challenge. Environ Health Perspect. 2013;121:283–286. doi: 10.1289/ehp.1206093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edginton AN, Schmitt W, Voith B, Willmann S. A mechanistic approach for the scaling of clearance in children. Clin Pharmacokinet. 2006;45:683–704. doi: 10.2165/00003088-200645070-00004. [DOI] [PubMed] [Google Scholar]
- 53.Wells J, Koontz Decline in urinary bisphenol A concentrations in the U.S. Epidemiology. 2013 doi: 10.1097/EDE.0b013e31827849b4. [DOI] [PubMed] [Google Scholar]


