Abstract
Background
Cocaine addicted patients with positive cocaine urine status at treatment entry are far less likely to have a successful treatment outcome. This work aims to identify brain substrates that can distinguish this group of patients from their cocaine-negative counterparts in order to better understand this clinical phenotype. Going a step beyond conventional functional connectivity, we used wavelet transform coherence (WTC) to determine in which ways the temporal pattern of fMRI cerebral blood flow (CBF) signals during attempted inhibition of cue-induced cocaine craving may differ between these two groups.
Methods
Using a critical node in motivational circuitry, amygdala, as a seed, whole brain correlations for the entire sample revealed a functional connection with the dorsal cingulate. Next, WTC maps of CBF were constructed for each individual, characterizing the temporal patterns between these two regions during craving inhibition.
Results
As revealed by WTC, during attempted craving inhibition, the cocaine-negative subjects had significantly stronger and longer negative coherence between the amygdala and the dorsal cingulate, as compared to the cocaine-positive subjects. This relationship was neither evident in the resting state nor between two regions unrelated to inhibition processes.
Conclusions
The duration and strength of negative coherence calculated from wavelet-transformed CBF provide an objective and well-defined way to characterize brain responses during attempted inhibition of cue-induced craving, at the level of the individual. The stronger and sustained negative coherence in CBF between motivational (amygdala) and modulatory (dorsal cingulate) regions in cocaine-negative subjects may be a critical brain strength that fosters improved craving inhibition and thus, better clinical outcome.
Keywords: Cerebral blood flow (CBF), Wavelet transform coherence (WTC), Arterial spin labeling functional magnetic, resonance imaging (ASL fMRI), Cocaine, Attempted cue-induced craving inhibition, Treatment intake cocaine urine status
1. Introduction
There is striking heterogeneity in clinical outcome among cocaine-dependent (CD) patients seeking treatment. Some patients have long periods of abstinence (become “drug free”), whereas others never become abstinent, or do so only briefly and relapse rapidly (McLellan et al., 1996). One of the most robust predictors of clinical outcome is urine status at treatment entry. Patients having cocaine-negative (CocNeg) urine tests usually have less subsequent drug use and less frequent relapse, whereas cocainepositive (CocPos) urine subjects have more drug use and rapid relapse (Alterman et al., 1997; Ehrman et al., 2001; Sofuoglu et al., 2003). This difference in treatment outcome between these two groups is often attributed to factors such as “readiness for treatment”, “motivation for abstinence”, and “commitment to recovery”. Advances in brain imaging tools allow us to test another possible reason for outcome variability between these groups: CocPos and CocNeg patients may have measureable differences in the functioning of relapse-relevant brain circuits. In the current paper, we compare these two clinically distinct phenotypes on temporal variation in the connectivity of brain circuits critical for resisting craving.
In our view, the brain vulnerabilities associated with drug use and risk of rapid relapse may lie in the poor connection between two brain systems: (1) the limbic motivational (“GO!”) circuitry, activated by natural rewards (e.g., food, sex) and rewarding drugs of abuse, and their signals (i.e. cues), and (2) the frontal modulatory (“STOP!”) circuitry, which inhibits the downstream motivational systems (Childress, 2006). Several studies have noted brain differences between CD patients and comparison groups in these brain networks (Childress et al., 1999; Ersche et al., 2012; Franklin et al., 2002; Volkow et al., 2010; Wilson et al., 2004); some of these differences may help explain addicted patients’ struggles to inhibit craving, and their high rates of relapse. We and other investigators have begun to examine the functional connections between limbic and frontal networks in substance abuse populations using neuroimaging (Gu et al., 2010; Jiang et al., 2011; Ma et al., 2010), and to link brain differences to clinical prognostic phenotypes/clinical outcome (D’Sa et al., 2011). Specifically, one recent finding indicated that frontal regions maintain significantly stronger amygdala functional connectivity during modulatory attempts in patients who submitted a cocaine-negative urine at treatment entry than those with a cocaine-positive urine (Suh et al., 2009).
Functional magnetic resonance imaging (fMRI) signals from coactivated regions oscillate in patterns across time (Baria et al., 2011; Varela et al., 2001), some of which are negatively correlated (“anticorrelated”): while activity from one region goes up, activity in an anticorrelated region goes down (Fox et al., 2005). Conventional connectivity analysis collapses all time points to yield a correlation coefficient (Biswal et al., 1995) expressing the overall relationship between two regions for an entire time period. Unfortunately, this conventional approach cannot reveal dynamic variations (time-frequency changes) within a given time period. As time-frequency relationships may carry important, clinically relevant information (Broyd et al., 2009; Salomon et al., 2012), we used a time-frequency analysis technique, wavelet transform coherence (WTC), to investigate the connectivity between the “STOP!” and “GO!” circuitry for the two above-described cocaine subgroups (CocNeg and CocPos) from their fMRI signals. In this study, we hypothesized that the CocNeg group would exhibit a stronger anticorrelation (inverse functional relationship) between frontal and downstream motivational regions than the CocPos group, consistent with inhibition of cocaine craving. Further, we tested whether the temporal characteristics of the signal (e.g., sustained vs. brief anticorrelation) would be associated with urinalysis results.
Here, treatment-seeking CD subjects were asked to inhibit their craving induced by cocaine-related videos while inside a magnetic resonance imaging (MRI) scanner. Cerebral blood flow (CBF) variation was measured throughout the whole session of a craving inhibition task, using arterial spin labeling (ASL) perfusion fMRI (Detre et al., 1992; Wang et al., 2003b). Comparing with blood-oxygen-level-dependent (BOLD) measurement, ASL perfusion fMRI allows functional connectivity analysis based on a single quantitative physiological parameter, CBF, and had shown good within-session reproducibility for the conventional seedbased functional connectivity analysis (Chuang et al., 2008).
Time- and frequency-localized correlations from regional cerebral blood flow (rCBF) signals within specific regions-of-interest (ROIs) in the “STOP!” and “GO!” regions were computed using wavelet transform coherence (Grinsted et al., 2004) for each CocNeg and CocPos subject. Bootstrapping was applied to test the statistical significance of individuals’ WTC results, after which the strength and duration of coherence were derived. Self-reported craving in response to drug cues was also recorded.
2. Materials and methods
2.1. Subjects and study design
For this exploratory study with WTC, we made use of a data set (see Section 2.2) from a cohort of cocaine-dependent subjects characterized for the two phenotypes of clinical interest (CocPos, CocNeg) on the basis of objective urine samples at treatment entry. Nineteen male, treatment-seeking cocaine-dependent subjects participated; the project was conducted at the University of Pennsylvania Addiction Treatment Research Center. The University of Pennsylvania Institutional Review Board approved the study, and all subjects signed a written informed consent prior to participation. Sixteen subjects were African American, two were Caucasian and one was Hispanic. Prior to the scanning session, all subjects were admitted to a controlled therapeutic residential setting for 8–10 days to ensure a stable, cocainefree state. In the week prior to scanning, subjects were introduced to a manualized Coping with Craving Program (Childress et al., 1991), and were given an individualized training session in which they attempted to inhibit cue-induced craving by considering the possible negative consequences of their cocaine use (Childress et al., 2005).
Urine samples were obtained on their first day of admission. Eleven subjects had cocaine-positive urine tests (CocPos group, ≥300ng/ml of benzoylecognine, the metabolite of cocaine) and eight subjects had cocaine-negative urine tests (CocNeg group) at treatment entry. There was no statistical significant difference (all p> 0.3) between the two groups in age, years of education, years of cocaine and alcohol use, number of days since last cocaine use (Table 1). Tetrahydrocannabinol, indicating marijuana usage, was found in the urine from four and three subjects in CocPos and CocNeg group, respectively.
Table 1.
Age, education and drug use data at treatment entry.
| CocPos group (N = 11)
|
CocNeg group (N = 8)
|
|||
|---|---|---|---|---|
| Range | Mean (S.D.) | Range | Mean (S.D.) | |
| Age/years | 30–49 | 40.2 (6.0) | 31–44 | 37.5 (5.4) |
| Years of education | 12–14 | 12.6 (1.1) | 12–18 | 13(2.1) |
| Years of cocaine use | 5–25 | 14.2 (6.6) | 8–25 | 15.6(5.7) |
| Years of alcohol use | 0–40 | 20.3 (11.1) | 4–25 | 16.6(6.4) |
| Days since last cocaine use | 1–12 | 4.5 (4.2) | 2–10 | 5.5 (3.8) |
| Number of subjects had other drugs at admission | 4THCa | – | 3 THC | – |
THC: tetrahydrocannabinol.
On days 7–10 of the residential stay, subjects were transported to the Hospital of the University of Pennsylvania for the fMRI session, which consisted of several 5-min acquisitions. A 5-min resting state fMRI session was acquired first. The subjects were asked to stay comfortably still inside the MRI scanner with their eyes open. In the attempted craving inhibition condition, the subjects were instructed to try to reduce (inhibit) their cocaine craving by considering the negative consequences of their cocaine use while they were watching a 5-min cocaine-related video. The subjects also rated their cocaine craving (on a scale of 0–9; Childress et al., 2005) before and after the video. Results from WTC analysis of both states were compared.
2.2. MRI acquisition
All scans were conducted on a Siemens 3 Tesla Trio whole-body scanner (Siemens AG, Erlangen, Germany), using a standard 8-channel receive head coil. fMRI CBF time series data were obtained with two custom variants of the ASL fMRI method (Kim, 1995; Wang et al., 2002). ASL MRI is a non-invasive technique measuring CBF with magnetically labeled arterial blood as endogenous tracer (Detre et al., 1992). It has the capability for capturing dynamic brain activity as well (Detre et al., 2009). Although ASL fMRI has lower temporal resolution as compared to BOLD fMRI, it has the potential to provide better localization of functionally connected regions than BOLD (Duong et al., 2001). Of note, our signal analysis techniques are by no means specific to ASL, and could be used with BOLD data as well (Chang and Glover, 2010; Curtis et al., 2005; Sun et al., 2005). We conducted statistical significance tests on both functional connectivity results obtained from conventional correlation (p value from cross-correlation) and WTC (bootstrapping, please see Section 2.6) to ensure the validity of the temporal relationship between the two CBF time series. Calculation of functional connectivity using conventional correlation and WTC from CBF data was performed within each subject after which the resulting connectivity results were compared among the subjects. fMRI parameters of individual subjects are presented in Table S1.1 High-resolution T1-weighted structural images were acquired using a 3D MPRAGE sequence using the same coil with parameters of resolution = 1 mm3, TR/TE = 1620/3 ms, matrix size = 192 × 256 × 160, flip angle = 9°.
2.3. ASL CBF signal preprocessing
ASL CBF signal preprocessing including motion correction, filtering, spatial smoothing, CBF quantification, registration to the Montreal Neurological Institute (MNI) standard brain via structural image was performed using a number of SPM-based (Wellcome Department of Imaging Neuroscience, Institute of Neurology, London, UK) batch scripts provided in ASLtbx (Wang et al., 2008). CBF values were calculated with a simplified two-compartment model with sinc-interpolated subtraction method which tends to whiten the low frequency noise typically observed in fMRI experiments and provides relatively flat power spectra in the frequency range below 0.10 Hz (Wang et al., 2003a). No further regression or normalization with any global or physiological signal was done to avoid potential risk of inducing artificial antiphasic relationships.
2.4. Selection of a priori regions-of-interest (ROIs) and control experiments
We chose the amygdala as the anatomical seed region-of-interest (ROI) for the preliminary correlational analyses. The amygdala is a critical node in the limbic motivational (“GO!”) circuitry (Everitt and Robbins, 2005): it is triggered by cues for both natural (e.g., food (Morris and Dolan, 2001), sex (Cahill et al., 2004)) and drug (Franklin et al., 2007; See et al., 2003; Wilson et al., 2004) rewards, including appetitive cues occurring completely outside awareness (Childress et al., 2008). Using the amygdala as a seed ROI enabled us to empirically determine which frontal regions were in anticorrelation (inverse relationship) with the amygdala.
For each side of the brain, the region with over 25% probability of being an amygdala defined in Harvard-Oxford subcortical structural atlas was chosen as an ROI. The mean rCBF time series within the amygdala ROI was extracted and used as the regressor in a subsequent whole brain regression analysis. The correlation coefficient between the regressor (amygdala time series) and the rest of the brain was represented as a functional connectivity map for each individual patient. The same analysis was performed for the left and right amygdala separately. A group-level analysis was then performed on the individual level regression results using a one-sample t-test implemented in SPM.
The current analysis focused on the negative correlations with the amygdala, our a priori seed region; these were found only in the prefrontal region. The most negatively correlated clusters were in the dorsal cingulate cortex (dCC) with maximum at (left, 8, −12, 45) and (right, 4, −10, 48), respectively (MNI coordinates; t=2.07 and 2.36; p< 0.05; see Fig. S1, see footnote1). For each dCC cluster, a sphere of 3 mm radius was drawn around the peak location, and a mean rCBF time series was extracted for use in the subsequent WTC analysis. (Positive correlations with the amygdala were intra-limbic, and are detailed in a separate manuscript using standard correlation analysis, Suh, personal communication.)
Two additional control examinations were conducted to verify the validity of the “active” attempted inhibition state and the dCC-amygdala connectivity in the WTC analysis. (1) For a non-inhibition comparison condition, we examine the resting state rCBF time signals extracted from the same amygdala and dCC ROIs described above were analyzed with WTC. (2) For specificity comparison, we examine WTC for two regions with no a priori hypothesis related to inhibition (left premotor cortex and anterior cerebellum). The left premotor cortex and anterior cerebellum ROIs were identified using the Wakeforest University PickAtlas (http://fmri.wfubmc.edu/software/PickAtlas) integrated in SPM8.
2.5. Time–frequency analysis and wavelet transform coherence
The above-described seed-region-based functional connectivity analysis provides a way to check signal coherence for the whole spectrum. Although widely used, the conventional correlation analysis cannot provide information about which frequency components, at what time range, contribute most to the observed correlation. Wavelet transform (Mallat, 1989) is a versatile tool to characterize the signal at different time-frequency resolution by representing the original signal (the mean rCBF signal here) with a series of scaled and time-translated versions of a mother wavelet function via convolution. The wavelet transform coherence (WTC) coefficient R2 between two rCBF signals, which is analogous to time and frequency localized correlation coefficient, ranges between 0 and 1. Their wavelet-coherency phase difference ϕ, which is also localized in time and frequency, ranges between −π and π.
Due to the low frequency nature of the rCBF signals, WTC results are presented in terms of period (T=1/frequency) to avoid long decimal numbers. The coherency phase difference between two rCBF signals at each period and time is presented with an overlaid phasor diagram in which the arrow pointing horizontally right and left indicating the two signals are completely in phase (ϕ = 0) and out of phase (ϕ = π), respectively. Implementation of WTC was based on the Matlab wavelet coherence package from Grinsted et al. (2004). All other implementations and analysis were done in Matlab as well. Mathematical details of WTC are illustrated in supplementary materials, see footnote1.
2.6. Statistical testing of WTC variability and magnitude with bootstrapping
The statistical significance of the temporal pattern variation and magnitude of the WTC between two rCBF signals was tested using a Monte Carlo simulation method based on time-series bootstrapping using the detailed procedures described in Chang and Glover (2010). rCBF time series of individual patients were fitted into a stationary vector autoregressive (VAR) model after which a large number of simulated rCBF series were generated based on the model. R2 and ϕ were calculated for each simulated series. The R2 at any period was considered to be non-significant if the variance from real data fell outside the confidence interval of the null distribution from the simulation. As the VAR model is independent of the sampling rate (i.e. TR), this bootstrapping test actually guarantees the statistical significance of WTC results for each individual regardless of its TR. The periods and times that passed the test were bounded with thick black contours (Fig. 1). More details can be found from supplementary materials, see footnote1.
Fig. 1.
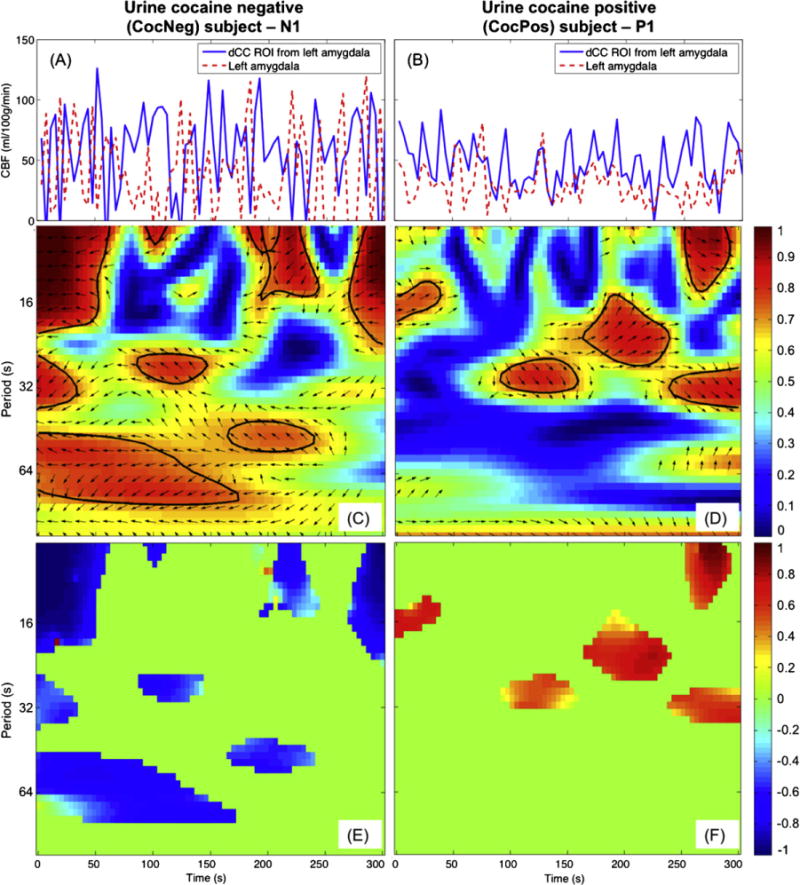
CocNeg subject (left column) showed consistent antiphase between the left amygdala and dCC rCBF signals but antiphase was not evident in the CocPos subject (right column). rCBF time signals series (A and B), WTC maps with phasor arrow overlaid (C and D) and characteristic coherence K maps (Eand F) of the left amygdala and dCC ROI.
2.7. Parameters for characterization of CD subject brain status
For each statistically significant R2 value, the characteristic coherence was calculated as K=R2 × cos ϕ to summarize the strength and direction of coherence. The quantity K, ranging from −1 to 1, characterizes two signals that are completely and strongly antiphase and in phase, respectively. This is also analogous to conventional correlation in that −1 and 1 implicate two signals that are completely anticorrelated and correlated, respectively, in a time- and frequency-localized fashion. The mean of significant characteristic coherence in each s was calculated and the duration of coherence D for each s was calculated as the coherence time spanned overs. Independent samples t-test and ANOVA were used to test the statistical significance of these parameters between the CocNeg and CocPos groups and self-reported craving results. Periods common to all subjects were used for current analysis (15s<T< 107 s).
3. Results
3.1. Illustration of WTC: example from one CocNeg and one CocPos subject
The neuronal activities of the dCC and amygdala during attempted inhibition of cue-induced craving were reflected in illustrative subjects’ rCBF signals (Fig. 1A and B). From the CocNeg subject, several antiphase temporal rCBF patterns were found between the dCC and amygdala (Fig. 1A), indicating primarily inverse rCBF couplings (anticorrelation) between these two regions. In contrast, the CocPos subject, mainly in-phase rCBF relationships were observed between the dCC and amygdala (Fig. 1B).
The nature of the antiphase relationship of the CocNeg subject N1 was fully revealed in a WTC map overlaid with black left-pointing phasor arrows, as shown in Fig. 1C. The combined coherence strength and phase direction at the periods and time where R2 was statistically significant (bounded with black contours) are shown in the characteristic coherence K map (Fig. 1E). Negative K relationships (blue patches in Fig. 1E) were found centered around T=8, 32 and 60s, which spanned over from one-sixth to one-half of the inhibitory task. In contrast, for the CocPos subject, P1, there were only in phase (right-pointing phasor arrows in Fig. 1D) and positive K (red patches in Fig. 1F) relationships.
3.2. Group-level analysis results
Characteristic coherence K provides a summarized description of coherence in terms of phase and magnitude. As there was no significant difference for ϕ = π/2 and 3π/4 (Fig. S2, see footnote1), we further reduced the data by binning the values of characteristic coherence Kinto negative (−1 ≤ K< 0) and positive (0 < K ≤ 1) coherence with −1 and1 representing completely and strongly antiphase and in phase, respectively.
Comparing the K values between the CocNeg and CocPos group during attempted inhibition state, CocPos group had higher mean K values than CocNeg group for all T>24 s in both sides (both p<9 × 10−10, ANOVA, Fig. 2A, please refer to Fig. S3A (see footnote1) for the right amygdala vs. dCC), while the values were similar for T ≤ 24. This demonstrated that the rCBF between amygdala and dCC in CocNeg was primarily more negatively correlated compared to the CocPos group. There was no significant difference in K among the periods within the groups (all p > 0.7, ANOVA).
Fig. 2.
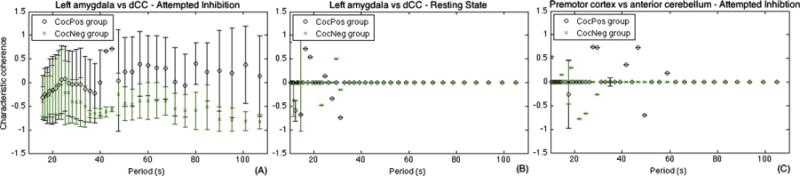
The CocNeg group demonstrated more negative characteristic coherence K than the CocPos group between left amygdala and dCC ROI (A). In contrast, this relationship is absent during the resting state (B). Finally, the relationship was also absented when examining two non a priori regions, left premotor cortex and anterior cerebellum (C). Each longitudinal point (circle/cross) represents the mean K across subjects in the 2 groups, and error bar represents standard error. Please referto supplementary materials Fig. S3 (see footnote1) for right amygdala vs. its corresponding dCC which show similar trends.
On the other hand, for both the resting state between amygdala and dCC (Figs. 2B and S3B (see footnote1) for the right side), and attempted inhibition state between premotor cortex and anterior cerebellum (Fig. 2C), no generalizable patterns were found for the K values between the two groups. Furthermore, there were no statistical significant differences between the two groups in all cases as well (p > 0.20, ANOVA).
No significant correlation was found between the self-reported craving score and the longest total duration as well as K values over all periods for both sides of the amygdalae (all p>0.6, t-test). Furthermore, there was no significant difference in the craving score between the two groups as well (p = 0.53, t-test).
Next, the total duration of positive and negative K are investigated. While the CocNeg group subjects were attempting to inhibition their craving, the mean durations of negative K were much longer than those of the positive for all periods and for both sides of the amygdalae (both p<4 × 10−18, ANOVA, Figs. 3A and S4A (see footnote1) for right amygdala), whereas in the CocPos group, the mean durations of negative and positive K were of similar levels (p = 0.29, ANOVA, Fig. 3D) and longer mean durations were found in positive K comparing to the negative (p = 0.01, ANOVA, Fig. 3D). These results indicated that the rCBF signals between amygdala and dCC in CocNeg group were in an anticorrelated relationship, which was not found from CocPos group. The durations of both negative and positive K in the CocNeg group were significantly longer and shorter, respectively, than those of the CocPos group in both sides (all p<2 × 10−7, ANOVA). There was also no significant difference in duration among the periods within the groups (all p > 0.8, ANOVA).
Fig. 3.
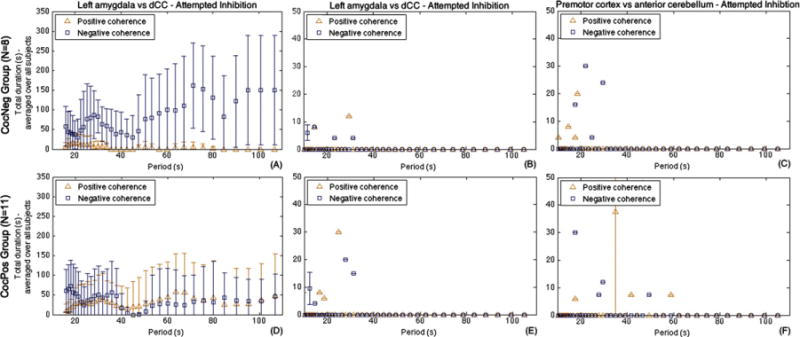
The CocNeg group (top row) had longer duration of negative coherence (blue squares) between left amygdala and corresponding dCC ROI as compared to the CocPos group (A and D). This relationship was absent during the resting state (B and E). Additionally, the relationship was absent when examining two non a priori regions, left premotor cortex and anterior cerebellum, during attempted inhibition (C and F). Each longitudinal point (triangle/square) represents the mean total duration of the corresponding characteristic coherence K across the subjects in the 2 groups, and error bar represents standard error. Please refer to supplementary materials Fig. S4 (see footnote1) for right amygdala vs. its corresponding dCC.
For both CocNeg and CocPos group, there was no identifiable pattern in D found from the resting state between amygdala and dCC (Figs. 3B, E, and S4B, D, see footnote1 for the right amygdala) as well as attempted inhibition state between premotor cortex and anterior cerebellum (Fig. 3C and F). There were no statistically significant differences in all cases as well (all p > 0.22, t-test).
3.3. Characterization of coherence for individual subjects during attempted inhibition of cocaine craving
As WTC may offer an individual biomarker for inhibitory ability, we examined the strength and the duration (more sustained, or more brief) of negative coherence for each participant in the attempted inhibition state. All but one CocNeg subject had negative K values (KN2-left = 0.75) with their longest total durations. Two of the CocNeg subjects (N4 and N6) sustained negative K (KN4-left= −0.75, KN6-left= −0.61) for the whole video challenge on the left side, while this was only found from N4 (KN4-right = −0.79) on the right side. N6 had negative K for more than half of the video. For all CocNeg subjects, their longest total duration of coherence ranged from 53 s to 164 s with K ranged from −0.65 to −0.85 for the left side, and duration from 92 s to 173 s with K from −0.35 to −0.80 for the right side.
Interestingly, the K values of CocPos spread over almost the whole range of K (from −0.80 to 0.87) with relatively shorter total durations for CocPos individuals having a negative K. There were three CocPos subjects who were consistently negative for both sides (P5, P6 and P10). Their total durations (left/right) were 148/68, 90/42 and 105/128, and their K (left/right) were −0.80/−0.18, −0.72/−0.74 and −0.82/−0.71, respectively. The total duration of coherence and the corresponding K value of all individuals are presented in Fig. 4.
Fig. 4.
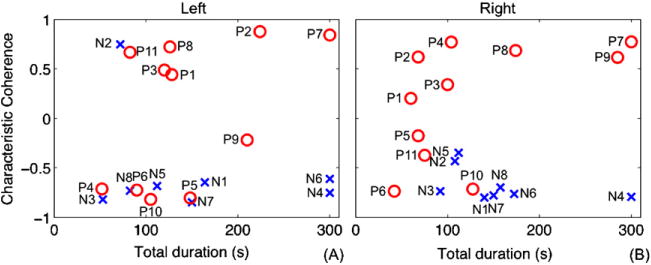
Longest total duration of coherence overall periods of individual subjects and their corresponding characteristic coherence K of (A) left amygdala and its dCC ROI and (B) right amygdala and its dCC ROI. Red circle (O) and blue cross (X) represent CocPos and CocNeg subjects, respectively.
The group mean correlation coefficients between the rCBF signals for amygdala and dCC were (left/right) −0.25/−0.17 and −0.04/0.02 for the CocNeg and CocPos group, respectively. The medians between two groups were very close. For both sides, the CocPos group had a wider range of values than the CocNeg group (Fig. S5, see footnote1). This result of this correlation agrees with those obtained with WTC although statistical significance was lacking (i.e. p> 0.05) for some of the individuals (left/right: 6/13).
4. Discussion
This study demonstrates the feasibility of using sustained coherence of fMRI signals with wavelet transform analysis to characterize individual differences among CD patients during attempted inhibition of cue-induced craving. Physiological meaningful parameters, the strength and duration of antiphase relationship between the inhibitory and motivation regions, were derived. These parameters could be used to reveal brain differences across CocNeg and CocPos individuals, as a critical first step to a broader understanding of the brain vulnerabilities in cocaine relapse.
fMRI scans during the attempted inhibition of cue-induced craving provide objective measurements of the brain regions involved. Our broad hypothesis is that fronto-limbic connections are critical for modulating drug motivation. The prefrontal cortex is of course a large domain–we thus took an empirical approach, using amygdala as a seed, to determining which frontal regions are participating in this particular “attempted inhibition of craving” task. The data revealed the dorsal cingulate, and we were guided by these data for our secondary analysis with WTC. Interestingly, a meta-analysis of the functional neuroimaging results from twenty tasks of various cognitive demands reported bilateral activations in one or more of the mid-dorsolateral, mid-ventrolateral and dorsal (anterior) cingulate cortex regions but not in other regions (Duncan and Owen, 2000). As the literature evolves, it will be interesting to see how these subdomains of the frontal cortex participate in modulation of drug motivation, and this may be expected to vary according to task demands.
While ASL perfusion fMRI has demonstrated its efficacy in capturing resting state connectivity using conventional cross-correlation (Fernandez-Seara et al, 2011), the experimental conditions with the current target subject group were even more challenging; CD patients often have micro-infarcts and patchy brain blood flow which further compromise SNR (Kaufman et al., 1998). Despite these challenges, WTC made it possible to extract clinically relevant biologic information at the level of the individual.
The characteristic coherence K, and duration of coherence D quantify (respectively) the magnitude and length of the coherence relationship sustained between the dCC and amygdala ROIs. In turn, this relationship reflects executive cognitive inhibition and activation by cocaine-related cues (Childress et al., 1999; Posner and DiGirolamo, 1998; Posner et al., 1988). Our experimental task (attempted craving inhibition during a video cue challenge), data acquisition (ASL, rather than BOLD) and analysis methods (WTC, instead of conventional GLM methods) distinguish the current study from previous fMRI studies linking brain responses to clinical outcomes (Brewer et al., 2008; Janes et al., 2010; Jia et al., 2011) and phenotypes (Cole et al., 2010; Kaufman et al., 2003; Tomasi et al., 2010) in cocaine and other addicted populations.
Cocaine videos imitate environmental cues that the subjects encounter in their daily lives. While trying to inhibit their cue-induced craving, the CocPos group (vs. the CocNeg group) had less negative coherence between dCC and amygdala, as well as shorter negative coherence duration. A consistent negative coherence relationship in the rCBF signals between dCC and amygdala over the course of the video suggests that the neurons in these two regions are firing in an alternating manner, when one region is active, the other is suppressed, and vice versa. Greater magnitude and longer duration of negative coherence/anticorrelation indicate that the subjects could maintain a better coupling between dCC and amygdala, which may be important for inhibition (Padmala and Pessoa, 2010). On the other hand, shorter and weaker negative coherence in the CocPos group may reflect a deficiency. Interestingly, three CocPos subjects demonstrated some negative coherence. The negative coherence relationship was neither evident in the resting state, nor between two anatomical regions unrelated to inhibition processes (premotor cortex and anterior cerebellum). Overall, this suggests that the negative coherence pattern obtained from our WTC analysis is specific to attempted inhibition.
Self-reported craving scores before and after the video challenge did not differentiate the subjects in this study. The failure of subjective report to distinguish clinical subgroups and/or predict outcome is not uncommon (Ferguson and Shiffman, 2009; Heinz et al., 2009; Langleben et al., 2008; Ooteman et al., 2006), and may be due to a variety of factors, including individual idiosyncrasies in the labeling and scaling of craving, and the impact of embarrassment or other “social demand” effects on self-report (e.g., patients often minimize reports of craving, as they are worried that admitting craving is sometimes unfortunately construed by the treatment providers as indicating lack of motivation or commitment to treatment, etc.). These difficulties with self-report strongly encourage the use of objective measures, as represented by neuroimaging (as in this study) or other biological markers (e.g., genetics).
General technical limitations of WTC have been addressed previously (Chang and Glover, 2010). There are a number of challenges that are specific to the current study. First, a faster fMRI technique would enhance the temporal resolution for WTC analysis. Although the current results were drawn from coherence signals that passed the bootstrapping test of statistical significance to validate the temporal stability, a faster fMRI technique could measure the neuronal processes that are more transient. Second, the process of cue-induced craving and inhibition alter physiological signals (e.g., heart rate, respiratory signal) to some extent (Li and Sinha, 2008). Monitoring these signals would provide additional information on physiological status. Thirdly, a larger sample size would have allowed us to investigate the large variation in period of coherence among the subjects in the current study. Further, we of course cannot determine from these results whether the evidenced presence/absence of anticorrelations reflects differences in the patients’ “motivation to inhibit” vs. “ability to inhibit”, etc. This distinction could be addressed in future studies, e.g., using designs that incentivize patients for attempted inhibition. Since there was no statistically significant difference in clinical characteristics other than cocaine urine status at treatment entry in our subjects, demographic and clinical characteristics are unable to explain the observed brain differences with WTC. Finally, the current cross-sectional design (CocPos-CocNeg split at treatment entry) offers a “proxy” for clinical outcome; these studies will stimulate future longitudinal studies with long-term outcome.
Despite these limitations, the brain signatures found in this study may have direct clinical implications. An important feature of the current analysis is the ability to characterize each individual, which is essential for a prognostic tool. Furthermore, since the current method provides a well-defined measure of the patient’s inhibition, it could be used to monitor treatment response. Importantly, while urine testing can predict outcome, it provides no guidance on treatment targets that may help change outcome. Thus, though a preliminary study, the current approach exemplifies the potential power of brain imaging to reveal treatment targets and to guide future tailored interventions: e.g., medications or behavioral strategies to improve the anticorrelation between limbic and frontal regulatory regions.
Supplementary Material
Acknowledgments
We would like to thank Dr. Henry Kranzler for his constructive comments, Robert Fabianski and Kathleen Marquez for organizing the data.
Role of funding source
This study was funded by NIH/NIDA T32 DA028874, R01 DA025906, R33 DA026114, P60 DA05186, P50 DA12756, VA VISN4 MIRECC, and Commonwealth of Pennsylvania Department of Health: CURE Addiction Center of Excellence: Brain Mechanisms of Relapse and Recovery. The funding organizations played no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.drugalcdep.2012.08.018.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org. Please see Appendix A for more information.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org. Please see Appendix A for more information.
Contributors
SCBL, COB and ARC conceived the idea; SCBL, ZW and YL implemented the software. SCBL conducted analyses with substantial input from JFM and TF. ARC collected the data. SCBL and ARC drafted the manuscript. All authors approved the final manuscript.
Conflict of interest
No conflict declared.
References
- Alterman AI, Kampman K, Boardman CR, Cacciola JS, Rutherford MJ, McKay JR, Maany I. A cocaine-positive baseline urine predicts outpatient treatment attrition and failure to attain initial abstinence. Drug Alcohol Depend. 1997;46:79–85. doi: 10.1016/s0376-8716(97)00049-5. [DOI] [PubMed] [Google Scholar]
- Baria AT, Baliki MN, Parrish T, Apkarian AV. Anatomical and functional assemblies of brain BOLD oscillations. J Neurosci. 2011;31:7910–7919. doi: 10.1523/JNEUROSCI.1296-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biol Psychiatry. 2008;64:998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Cahill L, Uncapher M, Kilpatrick L, Alkire MT, Turner J. Sex-related hemispheric lateralization of amygdala function in emotionally influenced memory: an FMRI investigation. Learn Mem. 2004;11:261–266. doi: 10.1101/lm.70504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Glover GH. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage. 2010;50:81–98. doi: 10.1016/j.neuroimage.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR. What can human brain imaging tell us about vulnerability to addiction and to relapse? In: Miller WR, Carroll KM, editors. Rethinking Substance Abuse. The Guilford Press; New York: 2006. pp. 46–60. [Google Scholar]
- Childress AR, Ehrman RN, Wang Z, Li Y, Sciortino N, Hakun J, Jens W, Suh J, Listerud J, Marquez K, Franklin T, Langleben D, Detre J, O’Brien CP. Prelude to passion: limbic activation by “unseen” drug and sexual cues. PLoS One. 2008;3:e1506. doi: 10.1371/journal.pone.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Hole AV, DePhilippis D. The Coping with Craving Program. University of Pennsylvania, School of Medicine and The Philadelphia VA Medical Center; Philadelphia: 1991. [Google Scholar]
- Childress AR, Hole AV, DePhilippis D, editors. The Coping with Craving Program: A Manual of Active Tools for Reducing the Craving/Arousal to Drug-Related Cues. The Addiction Treatment Research Center of the University of Pennsylvania School of Medicine and the Philadelphia VA Medical Center; Philadelphia, PA: 2005. [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang KH, van Gelderen P, Merkle H, Bodurka J, Ikonomidou VN, Koretsky AP, Duyn JH, Talagala SL. Mapping resting-state functional connectivity using perfusion MRI. Neuroimage. 2008;40:1595–1605. doi: 10.1016/j.neuroimage.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Beckmann CF, Long CJ, Matthews PM, Durcan MJ, Beaver JD. Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. Neuroimage. 2010;52:590–599. doi: 10.1016/j.neuroimage.2010.04.251. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Sun FT, Miller LM, D’Esposito M. Coherence between fMRI time-series distinguishes two spatial working memory networks. Neuroimage. 2005;26:177–183. doi: 10.1016/j.neuroimage.2005.01.040. [DOI] [PubMed] [Google Scholar]
- D’Sa C, Fox HC, Hong AK, Dileone RJ, Sinha R. Increased serum brain-derived neurotrophic factor is predictive of cocaine relapse outcomes: a prospective study. Biol Psychiatry. 2011;70:706–711. doi: 10.1016/j.biopsych.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med. 1992;23:37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- Detre JA, Wang J, Wang Z, Rao H. Arterial spin-labeled perfusion MRI in basic and clinical neuroscience. Curr Opin Neurol. 2009;22:348–355. doi: 10.1097/WCO.0b013e32832d9505. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Duong TQ, Kim DS, Ugurbil K, Kim SG. Localized cerebral blood flow response at submillimeter columnar resolution. Proc Natl Acad Sci USA. 2001;98:10904–10909. doi: 10.1073/pnas.191101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Cornish JW. Results of a baseline urine test predict levels of cocaine use during treatment. Drug Alcohol Depend. 2001;62:1–7. doi: 10.1016/s0376-8716(00)00137-x. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335:601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. J Subst Abuse Treat. 2009;36:235–243. doi: 10.1016/j.jsat.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Fernandez-Seara MA, Aznarez-Sanado M, Mengual E, Irigoyen J, Heukamp F, Pastor MA. Effects on resting cerebral blood flow and functional connectivity induced by metoclopramide: a perfusion MRI study in healthy volunteers. Br J Pharmacol. 2011;163:1639–1652. doi: 10.1111/j.1476-5381.2010.01161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O’Brien CP, Detre JA, Childress AR. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Grinsted A, Moore JC, Jevrejeva S. Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Process Geophys. 2004;11:561–566. [Google Scholar]
- Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Beck A, Grusser SM, Grace AA, Wrase J. Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addict Biol. 2009;14:108–118. doi: 10.1111/j.1369-1600.2008.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, de BFB, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, Worhunsky PD, Carroll KM, Rounsaville BJ, Stevens MC, Pearlson GD, Potenza MN. An initial study of neural responses to monetary incentives as related to treatment outcome in cocaine dependence. Biol Psychiatry. 2011;70:553–560. doi: 10.1016/j.biopsych.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang GH, Qiu YW, Zhang XL, Han LJ, Lv XF, Li LM, Lin CL, Zhuo FZ, Hu SY, Tian JZ. Amplitude low-frequency oscillation abnormalities in the heroin users: a resting state fMRI study. Neuroimage. 2011;57:149–154. doi: 10.1016/j.neuroimage.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J Neurosci. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MJ, Levin JM, Ross MH, Lange N, Rose SL, Kukes TJ, Mendelson JH, Lukas SE, Cohen BM, Renshaw PF. Cocaine-induced cerebral vasoconstriction detected in humans with magnetic resonance angiography. JAMA. 1998;279:376–380. [PubMed] [Google Scholar]
- Kim SG. Quantification of relative cerebral blood flow change by flowsensitive alternating inversion recovery (FAIR) technique: application to functional mapping. Magn Reson Med. 1995;34:293–301. doi: 10.1002/mrm.1910340303. [DOI] [PubMed] [Google Scholar]
- Langleben DD, Ruparel K, Elman I, Busch-Winokur S, Pratiwadi R, Loughead J, O’Brien CP, Childress AR. Acute effect of methadone maintenance dose on brain FMRI response to heroin-related cues. Am J Psychiatry. 2008;165:390–394. doi: 10.1176/appi.ajp.2007.07010070. [DOI] [PubMed] [Google Scholar]
- Li CS, Sinha R. Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neurosci Biobehav Rev. 2008;32:581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Liu Y, Li N, Wang CX, Zhang H, Jiang XF, Xu HS, Fu XM, Hu X, Zhang DR. Addiction related alteration in resting-state brain connectivity. Neuroimage. 2010;49:738–744. doi: 10.1016/j.neuroimage.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat SG. A theory for multiresolution signal decomposition: the wavelet representation. IEEE Trans Pattern Anal Mach Intell. 1989;11:674–693. [Google Scholar]
- McLellan AT, Woody GE, Metzger D, McKay J, Durrell J, Alterman AI, O’Brien CP. Evaluating the effectiveness of addiction treatments: reasonable expectations, appropriate comparisons. Milbank Q. 1996;74:51–85. [PubMed] [Google Scholar]
- Morris JS, Dolan RJ. Involvement of human amygdala and orbitofrontal cortex in hunger-enhanced memory for food stimuli. J Neurosci. 2001;21:5304–5310. doi: 10.1523/JNEUROSCI.21-14-05304.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooteman W, Koeter MW, Vserheul R, Schippers GM, van den Brink W. Measuring craving: an attempt to connect subjective craving with cue reactivity. Alcohol Clin Exp Res. 2006;30:57–69. doi: 10.1111/j.1530-0277.2006.00019.x. [DOI] [PubMed] [Google Scholar]
- Padmala S, Pessoa L. Moment-to-moment fluctuations in fMRI amplitude and interregion coupling are predictive of inhibitory performance. Cogn Affect Behav Neurosci. 2010;10:279–297. doi: 10.3758/CABN.10.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, DiGirolamo GJ. Executive attention: conflict, target detection and cognitive control. In: Parasuraman R, editor. The Attentive Brain. MIT Press; Cambridge, MA: 1998. pp. 401–423. [Google Scholar]
- Posner MI, Petersen SE, Fox PT, Raichle ME. Localization of cognitive operations in the human brain. Science. 1988;240:1627–1631. doi: 10.1126/science.3289116. [DOI] [PubMed] [Google Scholar]
- Salomon RM, Karageorgiou J, Dietrich MS, McLellan JY, Charboneau EJ, Blackford JU, Cowan RL. MDMA (Ecstasy) association with impaired fMRI BOLD thalamic coherence and functional connectivity. Drug Alcohol Depend. 2012;120:41–47. doi: 10.1016/j.drugalcdep.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Fuchs RA, Ledford CC, McLaughlin J. Drug addiction, relapse, and the amygdala. Ann N Y Acad Sci. 2003;985:294–307. doi: 10.1111/j.1749-6632.2003.tb07089.x. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Gonzalez G, Poling J, Kosten TR. Prediction of treatment outcome by baseline urine cocaine results and self-reported cocaine use for cocaine and opioid dependence. Am J Drug Alcohol Abuse. 2003;29:713–727. doi: 10.1081/ada-120026256. [DOI] [PubMed] [Google Scholar]
- Suh JJ, Ehrman R, Li Y, Wang Z, Jens W, Franklin T, Hole A, Goldman M, O’Brien CP, Childress AR. Poor fronto-limbic connectivity: a brain endophenotype for rapid relapse to cocaine?. The 71st Annual Scientific Meeting of the College on Problems of Drug Dependence; Reno, NV. 2009. [Google Scholar]
- Sun FT, Miller LM, D’Esposito M. Measuring temporal dynamics of functional networks using phase spectrum of fMRI data. Neuroimage. 2005;28:227–237. doi: 10.1016/j.neuroimage.2005.05.043. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, Wang R, Carrillo JH, Maloney T, Alia-Klein N, Woicik PA, Telang F, Goldstein RZ. Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS One. 2010;5:e10815. doi: 10.1371/journal.pone.0010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Jayne M, Ma Y, Pradhan K, Wong C, Swanson JM. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage. 2010;49:2536–2543. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Aguirre GK, Kimberg DY, Detre JA. Empirical analyses of null-hypothesis perfusion FMRI data at 1.5 and 4T. Neuroimage. 2003a;19:1449–1462. doi: 10.1016/s1053-8119(03)00255-6. [DOI] [PubMed] [Google Scholar]
- Wang J, Aguirre GK, Kimberg DY, Roc AC, Li L, Detre JA. Arterial spin labeling perfusion fMRI with very low task frequency. Magn Reson Med. 2003b;49:796–802. doi: 10.1002/mrm.10437. [DOI] [PubMed] [Google Scholar]
- Wang J, Alsop DC, Li L, Listerud J, Gonzalez-At JB, Schnall MD, Detre JA. Comparison of quantitative perfusion imaging using arterial spin labeling at 1.5 and 4.0 Tesla. Magn Reson Med. 2002;48:242–254. doi: 10.1002/mrm.10211. [DOI] [PubMed] [Google Scholar]
- Wang Z, Aguirre GK, Rao H, Wang J, Fernandez-Seara MA, Childress AR, Detre JA. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging. 2008;26:261–269. doi: 10.1016/j.mri.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci. 2004;7:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


