Abstract
Neuroimaging has demonstrated anatomical overlap between covert and overt attention systems, although behavioral and electrophysiological studies have suggested that the two systems do not rely on entirely identical circuits or mechanisms. In a parallel line of research, topographically-specific modulations of alpha-band power (~8-14Hz) have been consistently correlated with anticipatory states during tasks requiring covert attention shifts. These tasks, however, typically employ cue-target-interval paradigms where attentional processes are examined across relatively protracted periods of time and not at the rapid timescales implicated during overt attention tasks. The anti-saccade task, where one must first covertly attend for a peripheral target, before executing a rapid overt attention shift (i.e. a saccade) to the opposite side of space, is particularly well-suited for examining the rapid dynamics of overt attentional deployments. Here, we asked whether alpha-band oscillatory mechanisms would also be associated with these very rapid overt shifts, potentially representing a common neural mechanism across overt and covert attention systems. High-density electroencephalography in conjunction with infra-red eye-tracking was recorded while participants engaged in both pro- and anti- saccade task blocks. Alpha power, time-locked to saccade onset, showed three distinct phases of significantly lateralized topographic shifts, all occurring within a period of less than one second, closely reflecting the temporal dynamics of anti-saccade performance. Only two such phases were observed during the pro-saccade task. These data point to substantially more rapid temporal dynamics of alpha-band suppressive mechanisms than previously established, and implicate oscillatory alpha-band activity as a common mechanism across both overt and covert attentional deployments.
Keywords: Vision, Oscillations, Attention, Cueing, EEG, Electrophysiology, High-density electrical mapping
INTRODUCTION
Effective interaction with a complex environment often involves rapid focusing of limited cognitive resources on locations, objects or other information sources of interest. Humans accomplish this using both ‘overt’ and ‘covert’ attention systems (Helmholtz Hv 1910/1924). Overt attention refers to explicit engagement of a motor system to bring an object of interest into attentional focus, such as pointing, head-turning or eye movements, whereas covert attention, as its name implies, refers to the surreptitious deployment of the focus of spatial attention without a change in gaze direction. Despite the lack of motor engagement, covert attention has often been referred to as a form of ‘movement’ (Posner M et al. 1982), and it has been theorized that both ‘overt’ and ‘covert’ attention systems rely on the same underlying neural mechanisms, a thesis known as the “premotor theory of attention” (Rizzolatti G et al. 1987). Certainly, behavioral evidence has pointed to considerable overlap between covert and overt attention mechanisms (Hoffman JE and B Subramaniam 1995; Kowler E et al. 1995; Deubel H and WX Schneider 1996), although studies have also shown dissociation is possible (Montagnini A and E Castet 2007). Premotor theory also receives considerable support from functional neuroimaging studies where significant anatomical overlap between the covert and overt systems has been shown (Corbetta M 1998; Corbetta M et al. 1998). In turn, electrophysiological studies have shown both common and dissociable processes (Eimer M et al. 2007; Van Der Werf J et al. 2008; Kelly SP et al. 2010). Thus, there is good evidence for both shared and dissociable mechanisms, but what precisely these shared mechanisms are remains a matter of considerable research interest.
A very well-studied class of overt attention shifts is the saccadic eye movements used to scan the visual world and bring objects of interest into focus on the highly innervated foveal region of the retina. This rapid scanning of visual space involves complex spatial transformations and motor planning. Saccade-targeting also seems to parallel the spatial cuing enhancements observed during covert attention tasks such as increased discrimination of difficult to detect inputs. For example, increased discrimination at saccade target locations is seen prior to the actual movement itself (Deubel H and WX Schneider 1996), and enhanced detection at saccade target locations is seen even when the movement is aborted prior to action (Hoffman JE and B Subramaniam 1995). However, by separating the saccadic target from a covertly attended location with probabilistic cues (i.e. 25-75% valid), it was found that covert attention and saccade targeting could in fact be separated when the covertly attended target was likely to be in a different place than the saccadic target (Montagnini A and E Castet 2007). Interestingly, this dissociation waned as the moment of the overt movement approached, such that attention targets that were not saccade targets did not receive enhancement when presented in close temporal proximity to saccade onset. Like covert attention, which can be both rapidly drawn to features in the world (i.e. exogenous attention), and also deployed intentionally and voluntarily to features or locations (i.e. endogenous attention), the saccadic system allows for rapid overt shifts that can be captured exogenously or deployed endogenously (i.e. voluntarily). It certainly seems a reasonable proposition that these two systems might rely on at least partially shared mechanisms.
Covert visual attention shifts have also been very well-studied and have been correlated with substantial modulation of sensory processing as indexed by visual evoked potentials (Mangun GR and SA Hillyard 1991; Foxe JJ et al. 2003; Foxe JJ and GV Simpson 2005; Gould IC et al. 2011), as well as strong modulations of oscillatory neural activity (Womelsdorf T and P Fries 2006; Chalk M et al. 2010). Of particular interest to the present investigation, changes in alpha-band power (~8-14Hz) have been consistently shown to correlate with preparatory states in tasks that require covert shifting of attention (Foxe JJ and AC Snyder 2011). It has been demonstrated that increases in alpha power are associated with active suppression of distracters in unattended spatial locations (Worden MS et al. 2000; Kelly S et al. 2006; Thut G et al. 2006; Banerjee S et al. 2011), as well as suppression of ignored visual features (Snyder AC and JJ Foxe 2010) and irrelevant sensory modalities (Foxe JJ et al. 1998; Fu KM et al. 2001; Jones SR et al. 2010; Gomez-Ramirez M et al. 2011). Moreover, anticipatory alpha power has been directly linked to behavioral outcomes (O'Connell RG et al. 2009; Haegens S, BF Handel, et al. 2011) and the disruption of alpha synchrony via trans-cranial magnetic stimulation affects performance in a target discrimination task (Capotosto P et al. 2009). Similarly, it has been shown that spatially-specific measures of anticipatory alpha-power over visual cortex are predictive of reaction times (Gould IC et al. 2011) and subsequent discrimination performance during covert spatial deployments (Thut G et al. 2006; Kelly SP et al. 2009; Handel BF et al. 2011). In the current study, we set out to establish whether alpha-band oscillatory suppression mechanisms were also invoked during the rapid overt attentional shifts that are characteristic of saccadic targeting. If true, this finding would dramatically reduce the timescale at which alpha might be understood to operate, and further expand the role of alpha rhythms as a mechanism of overt attention.
To date, studies of alpha activity in covert attention have typically employed so-called cue-target-interval paradigms where the preparatory attentional processes are examined across relatively protracted periods of time. In these tasks, participants receive an instructional cue (e.g. an arrow) that tells them to direct their attention to a particular location or to a particular stimulus feature. Then after a delay period, typically on the order of 1-2 seconds, and sometimes longer, a potential target stimulus is presented upon which the attentional task is then performed. The tradition has been to examine oscillatory activity during the relatively prolonged anticipatory intervals between the cue and the target stimulus. As such, these studies have not examined alpha-band processes at the more rapid timescales that are implicated during overt attention tasks. Here, we reasoned that if alpha oscillatory suppression mechanisms also play a role during deployments of the overt attention system, one should observe topographically specific alpha power shifts that follow the rapid dynamics of the saccadic system. That is, since saccades can be executed with latencies well below 200 msec (Fischer B and R Boch 1983), up to 3-to-4 times per second, similarly rapid redeployments of alpha-suppression should be observable.
To address this issue, we examined the temporal dynamics of alpha-band oscillatory activity using a combination of pro- and anti-saccade tasks. During pro-saccades, participants make rapid eye movements to stimuli appearing to the left or right of fixation, whereas during anti-saccade performance, they are required to move their eyes to a position equi-opposite to the location at which the stimulus appeared (i.e. in the opposite hemifield). We made particular use of the anti-saccade task because of its peculiar attentional demands relative to standard saccadic targeting. Successful anti-saccades involve first deploying covert attention to the hemifield of cue appearance, which must then be suppressed to execute an overt shift of attention to the opposite hemifield. It is precisely this sort of rapid attentional shifting that was of primary interest to us here. Since lateralization of alpha-band power has been previously shown to be correlated with retinotopic attention shifts(Worden MS et al. 2000; Thut G et al. 2006), we asked whether this same mechanism might also be flexible and rapid enough in the deployments of attention during anti-saccade performance. Since saccades naturally occur at intervals that are much faster than those studied in most previous alpha-band investigations (Gutteling TP et al. 2010), it would stand to reason that if alpha power dynamics represent a shared mechanism for covert and overt attention, such rapid dynamics should be revealed as a shift in power across hemifields from the cue-side to saccade-side, during the anti-saccade task.
MATERIALS AND METHODS
Participants
Sixteen healthy volunteers (2 left-handed, 8 female, mean age= 29.4 with a standard deviation =7.5 years) participated in this study, the procedures for which were approved by the Institutional Review Boards of the City College of New York and the Albert Einstein College of Medicine. All experimental procedures were carried out in accordance with the ethical standards codified by the World Medical Association in the Declaration of Helsinki (World Medical Organization 1996). All subjects gave written informed consent to participate in this study and received a modest fee ($12/hour). All reported normal or corrected-to-normal vision. Exclusion criteria included any history of psychiatric illness, severe head injury, including any previous loss of consciousness, or current/recent psychotropic medication use.
Stimuli and Task
Subjects were seated 80 cm from a 50.8 cm (20in) CRT monitor (NEC MultiSync FE2111) running at 60 Hz horizontal refresh rate. Fixation was maintained on a 0.45 degree bold white cross in the center of the screen. Cues were maximum contrast, 3 cycles/degree circular checkerboards subtending one degree of visual angle (DVA) in diameter (40 pixels) presented 10 DVA to the left or right of the fixation cross. These cues appeared for 17ms (one refresh of the monitor) in one of two locations represented as ‘holes’ in the background composed of a circle extending 0.75 DVA (30 pixels), in a slightly lighter halftone (8-bit monitor red-green-blue [RGB] values: 155,155,155) than the overall background (RGB: 127,127,127; Figure 1). Note that both target locations and the fixation were visible continually throughout the experiment, whether a cue appeared or not. Conditions were presented in a blocked design: pro- and anti-saccade (each 5x100 trials), or a ‘no saccade’ condition in which the stimulus was presented exactly as in the active conditions, but there were no eye movements (5x100 trials); participants were required to maintain fixation throughout the block. In the pro-saccade condition, participants were asked to move their eyes as quickly as possible TOWARDS the cue randomly presented at the left or right position. In the anti-saccade condition, participants were asked to move their eyes to the side AWAY FROM, or opposite to the side that the cue randomly appeared. Randomization approximated 50% of cues presented left or right. After each saccade to one of the target locations, the subject was required to return gaze to central fixation to begin the next trial. New trials were presented with a random inter-trial interval (ITI) of 1000-2000 msec in one msec steps in a square distribution from the time the subject returned to fixation so as to avoid any effects of anticipation. Blocks of pro-, anti-, and ‘no saccade’ were presented pseudo-randomly with periodic breaks.
Fig 1. Stimulus paradigm.
In blocks of 100 trials, participants were asked to move their eyes as quickly as possible either “Towards” (A. Pro-) or “Away From” (B. Anti-) the checkerboards appearing randomly left or right. These were presented for 17ms. After each saccade, participants returned to fixation to begin the next trial. Control blocks were also interleaved in which the stimuli presentation was identical, but no saccade was made. Panel (C.) shows a typical anti-saccade trial in which a checkerboard appears (in green) 10 degrees from central fixation in the right hemifield, the eyes move to the left after some reaction time, before returning to 0 degrees. Note that target is artificially offset from time zero (x-axis), and that the eyes would actually start at degree =‘0’ (y-axis). Panel (D.) shows a sample distribution of saccades amplitude throughout a trial. ITI= Inter-trial interval; SRT=Saccade Reaction Time.
EEG and Saccade recording
EEG was continuously recorded from 168 scalp electrodes using an ActiveTwo (BioSemi, Amsterdam, The Netherlands) system sampled at 512 Hz, 100Hz low-pass filtered, while simultaneously tracking the participant's eyes with an EyeLink 2K system (resolution 0.01°; SR Research, Kanata, Ontario, Canada)recording at 500 Hz. The EEG and eyetracking recording systems were synced on each trial using trigger signals. With the BioSemi system, every electrode or combination of electrodes can be assigned as a reference, which is done purely in software after acquisition. BioSemi replaces the ground electrodes that are used in conventional systems with two separate electrodes: Common Mode Sense (CMS) and Driven Right Leg (DRL) passive electrodes. These two electrodes create a feedback loop, thus rendering them as references. Signals are recorded as the voltage between the individual electrode and the CMS. Active electrodes integrate the first amplification stage into the Ag/AgCl electrodes, reducing the effects of electronic noise. The output impedance is thus considered to be <1Ω. EEG data were processed using the FieldTrip toolbox (Oostenveld R et al. 2011) (see http:///www.ru.nl/neuroimaging/fieldtrip) and custom scripts for MATLAB (MathWorks, Natick, MA).
Analysis Strategy
Eye-tracking analysis
During offline analysis, saccades were detected automatically using a velocity threshold of 10°/s and a minimum latency criterion of 100 msec to exclude anticipatory movements. All saccade traces were previewed in MATLAB using a custom-designed graphic interface. Trials with blinks at any point prior to 500ms post-saccade were not considered. Paired t-tests were used to compare amplitude and reaction time for each condition. Return saccades were considered as the second saccade that was also in the direction of the fixation.
EEG analysis
Data were re-referenced to the average activity offline. Separate three-second epochs were defined from 2 seconds preceding to 1second following saccade onset. This epoch definition emphasizes the time period of particular relevance to this study, namely the time between the cue stimulus and the execution of the saccade. Only trials on which saccade reaction time was less than 1000 msec were considered. Since most trials had eye movements, and thus occulomotor artifacts, each trial was visually inspected to distinguish a typical eye movement EOG from blinks or other artifacts. Channels with errant signals were interpolated using the proximity-weighted value of the surrounding eight electrodes. Trials with more than seven bad channels (out of 168), or with blinks or other artifacts were discarded. On average these criteria resulted in 277 ±111 trials in the Against condition and 275±113 trials in the Towards condition.
Alpha lateralization
Our hypothesis regarded the pace of alpha dynamics in an overt attention task that would be distinguished in the anti-saccade task in which one must first suppress a lateralized cue in one hemifield, and rapidly switch attention to opposite hemifield in order to make a successful saccade. In order to quantify these hemispheric dynamics of alpha synchrony, the data were transformed in a two-stage process following (Thut G et al. 2006) and (Kerlin JR et al. 2010)
Stage 1: Alpha Power Analysis
After epoching and artifact rejection, instantaneous amplitude in the alpha band was derived by applying the Hilbert-transform on data band-pass filtered between 8 and 14 Hz using a 4th order zero phase-shift digital Butterworth filter. The transformed signals were then averaged across trials for each participant and grand averaged across participants. One electrode of interest (EOI) was selected from the parietal-occipital area over each hemisphere (see Figure 2). Based on the well-characterized topographic patterns of alpha lateralization showing symmetric responses across hemiscalp as a function of lateral attentional deployment (Worden MS et al. 2000), we first collapsed across right and left cue presentations over each hemisphere. For the pro-saccade trials, we define alpha lateralization (AL) values by subtracting the transformed alpha power of trials in which the stimulus was presented on the right from trials in which the stimulus was on the left. This can be written as:
For the anti-saccade trials, we define alpha lateralization (AL) values in reverse by subtracting the trials in which the stimulus was presented on the left from trials in which the stimulus was on the right:
This transformation normalizes the effect of the cue to give a hemispheric index of the effects of ipsilateral versus contralateral cues since they will be aligned after this transformation. Such a procedure is also beneficial since the visual evoked response to the cue is expected to have power in the alpha band, and this procedure subtracts this contribution.
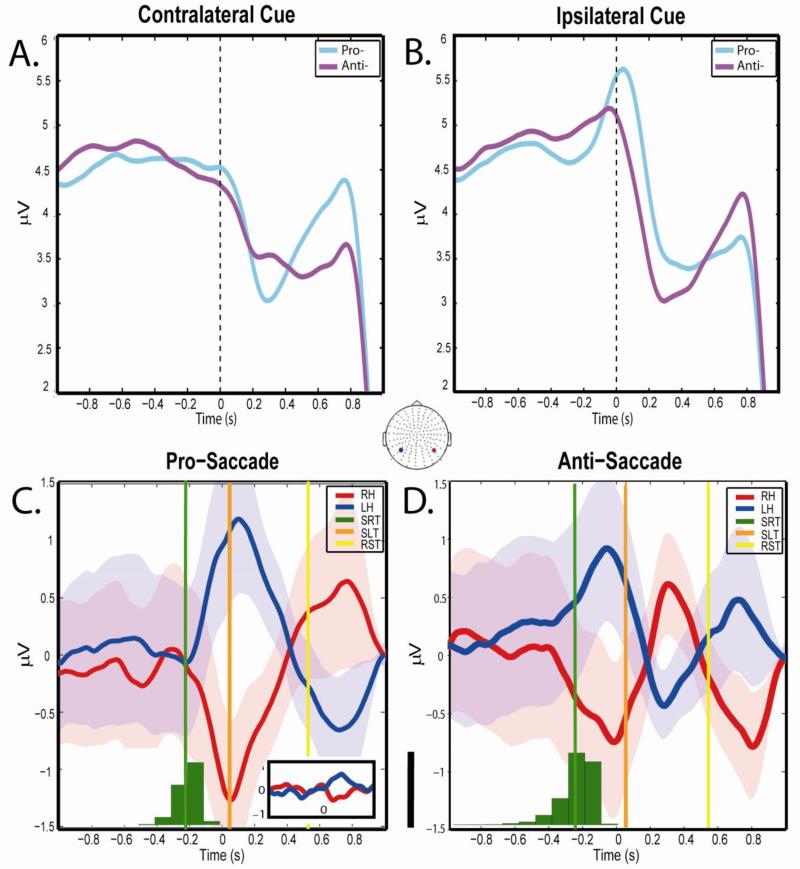
Fig 2. Alpha lateralization by condition.
A. and B. plot the average alpha power of left and right electrodes of interest (highlighted by red and blue circles on electrode map) for trials in which the cue was presented contralateral (i.e. right electrode when cue left, and left electrode when cue right) and ipsilateral (i.e. right electrode when cue right, and left electrode when cue left) aligned from saccade onset (time ‘0'). In these figures, a clear pattern emerges describing an increase in alpha power beginning ipsilateral to cue well before the saccade across both conditions. Panels C. and D. aligning the cue presentation side as described in Stage 1 lateralization (see Methods), where positive values indicate increased lateralization left, and negative values indicate increased lateralization right. This transformation reveals an additional inflection in the Anti-Saccade condition, regardless of stimulus side implying attention rapidly shifted from cue-side to saccade-side. The histograms depicted along the x-axis show distributions of saccade reaction time thereby showing the times of stimulus presentation with respect to saccade onset. Standard error of the mean are shown for each trace. Inset shows alpha power for the same electrodes in stimulus-only condition. Note that time ‘0’ in the inset is stimulus onset since there are no saccades. Black scale bar between C. and D. represents 2,000 saccades. SRT=Mean saccade reaction time; SLT=Mean saccade landing time; RST=Mean return saccade time.
Stage 2: Alpha Lateralization Index (ALI)
Our main hypothesis predicted that differential lateralization in the anti-saccade condition will reflect the rapid shifts in alpha-suppression necessary to deploy overt and covert attention in opposing hemifields when compared to the pro-saccade condition in which the eyes are moving in the same direction as covert attention. To compare these dynamics across conditions, a second subtraction of the lateralized time courses from Stage 1 was made to determine an Alpha Lateralization Index (ALI). This transformation collapses across hemisphere and has the effect of highlighting overall changes in lateralization (i.e. ipsilateral versus contralateral, regardless of left-right eye movements). The formula for both pro- and anti-saccade conditions is ALIR/L= EOIL(EyesLeft - EyesRight) - EOIR (EyesLeft - EyesRight). This transformation is used in in the statistics below, but only shown in Figure 5.
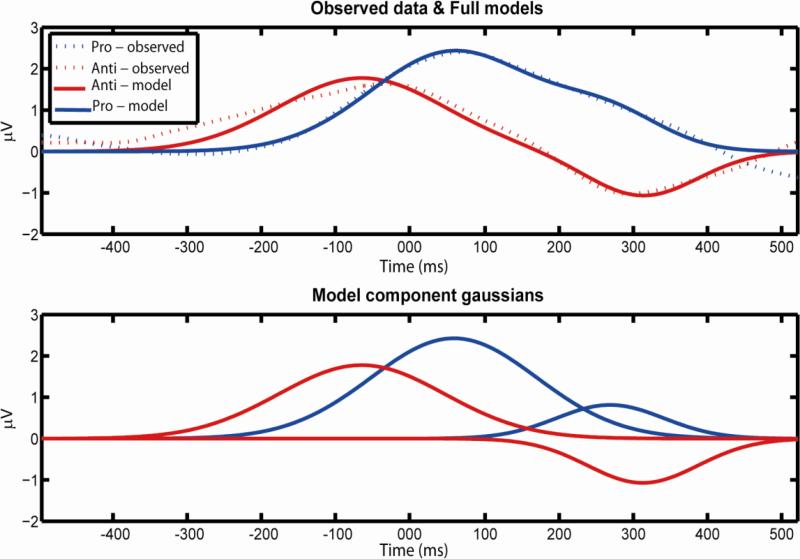
Figure 5. Model fit.
A model-of-best-fit summing two Gaussian curves (solid line, combined above, separated below) is proposed to best fit the recorded pattern of results (dashed line, above). In this interpretation, the first curve represents the alpha response to the stimulus onset, the second curve would represent the movement-related component. Note the inversion in the anti-saccade condition.
For Alpha Lateralization analyses, only the periods of interest from stimulus onset until gaze returned to fixation were analyzed. These periods of activity, as detailed further in the results, were down-sampled to 20% (effective sampling rate 100Hz).This down-sampling can be thought of as a Fourier transform in which the high-frequency dynamics resulting from grand averaging are disregarded to analyze only the temporal scale of interest. The pattern of results was then submitted to a non-linear regression analysis to identify the curve of best fit, starting with a linear function and adding terms until additional terms no longer accounted for significantly reduced variance as compared to lower-ordered functions. If the anti-saccade condition required additional lateralized shifts of alpha-power, this should be reflected in a higher-order function; that is, a function with additional inflections.
Statistical Cluster plots (SCP)
In order to fully analyze the wealth of information captured in the high-density electrophysiological dataset, additional exploratory analyses were performed. So-called statistical cluster plots were created for each normalized condition APPRO and APANTI. These maps were created using paired-sample, two-tailed t-tests between the alpha power indices for each condition, for each time point. This technique allows assessment of the all significantly different activations on the scalp, across time. Since the potential for Type 1 error is high due to multiple comparisons, our analysis was constrained to accept only those tests that reach a significant alpha of .05 for 15 consecutive samples (~30ms at our initial 500Hz sampling rate), and are adjacent to at least one other electrode that is also significant for the same time period (Guthrie D and JS Buchwald 1991; Molholm S et al. 2002). The rationale for this corrective procedure is that unlike true effects, Type 1 errors due to chance are unlikely to occur at consecutive independent time points. Although adjacent time points in the EEG are not completely independent, previous work has shown this criterion is quite conservative in protecting against Type I error inflation (Guthrie D and JS Buchwald 1991).
Bi-Phasic Alpha Dynamics
In addition to examining the speed of alpha-power dynamics in an overt attention task, our hypothesis also concerned the possibility of the dual role that alpha-power dynamics might play across overt and covert attentional tasks. If changes in alpha-band power reflect a mechanism that is utilized both in the covert suppression of distracters (the cue location in the anti-saccade condition), and also in the overt shifting of eye movements, this would predict additional activity in the anti-saccade task, since unlike during pro-saccades the participants must shift first towards the side containing the cue, and after inverting the direction, shift to the opposite hemifield. This pattern of activity might be described as “biphasic” in that an initial shift of alpha-power ought to be observed ipsilateral to cue location in the Worden Effect (Worden MS et al. 2000) to suppress a contralateral distracter. Alpha-power dynamics would next reflect a secondary shift to account for the overt shift of attention. In the pro-saccade task, these shifts should be ipsilateral, that is the eyes move to the same hemifield as the stimulus. However, to perform the anti-saccade task, the second, overt shift should reverse hemifields from the side of distracter to the side of the eventual eye movement. These dynamics can be evaluated by testing the transformed sign of the Alpha Lateralization Index described above such that midline values equal zero, or no lateralization, positive values denote ipsilateral lateralization, and negative values contralateral. A Wilcoxon signed-ranks test can thus provide an additional test of lateralization in the pre- and post-saccade periods across condition.
RESULTS
Overall, as hypothesized the lateralization of alpha power was indeed found to shift rapidly across hemisphere during performance of overt attention tasks and was particularly evident in the anti-saccade condition. We will first describe the observed pattern of results, followed by a formal statistical analysis as described in the Methods.
Behavioral performance
Mean saccade reaction time was significantly greater in the anti-saccade condition (Pro Mean= 211msec, sd= 34; Anti Mean= 257msec, sd= 59; t(15)= 5.17 p<.001). Overall the saccade amplitude showed that participants made accurate, and similar saccades in both conditions (Pro Mean= 10.7 DVA, sd 0.6; Anti Mean= 10.4, sd0.9; t(15)= -1.87,p>0.05). The reaction time of the saccade returning to fixation (second saccade reaction time minus the first saccade reaction time) was nearly identical (Anti Mean= 537msec, sd= 173; Pro Mean= 519msec, sd= 172, t(15)= -0.96 p= 0.35) for both conditions. Owing to the pre-potent response in the anti-saccade condition, participants made inadvertent pro-saccades on 3.9% (sd= 4.1) of trials. Less than 1% of pro-saccade trails were made in error. Only correct trials were included in the analyses. Since previous research has shown correlations between alpha and reaction times e.g. (Haegens S, BF Handel, et al. 2011; Premereur E et al. 2012), we tested whether a linear model describe the relationship between saccadic reaction times and the time that it took to reach maximum alpha power across trials. No significant correlations in either anti- (R2=0.02, p=.416) or pro-saccade conditions (R2=0.10 , p=.08) were observed.
Alpha Power Analysis
Figure 2 shows that the alpha power at representative parietal EOI, averaged across subjects and aligned to saccade onset (time ‘0’), increased in the 300 msec before the saccade in the ipsilateral, but not contralateral cues (Fig 2 A,B). This was the case for both pro- and anti- saccade conditions. Collapsing across cue presentation by subtracting cue-right trials, from those in which the cue was on the left (see Methods: Stage 1 lateralization analysis) highlighted the lateralization for each condition (Fig 2 C,D). Here, positive values indicate greater left lateralization, and negative values represent right lateralization as measured over each hemisphere. A similar pattern of results can be seen across both conditions in the period pre-saccade onset such that alpha power in the left hemisphere increased when the stimulus was presented on the left, and alpha power increased over the right hemisphere when the stimulus was presented to the right. Alpha power thus reacted in a similar way to the same attended stimulus. Specifically it appears that alpha power increased ipsilateral to the side of cue presentation in full accordance with the previous literature (Worden MS et al. 2000; Kelly S et al. 2006), suggesting the suppression of visual space opposite the stimulus while participants’ covert attention is drawn towards it.
Just prior to saccade onset, hemispheric alpha power began to differ by condition. In the prosaccade, alpha power continued to increase ipsilateral to cue location, and later saccade target location, suggesting that the eyes were essentially following covert attention. In the anti-saccade condition, however, the power across the hemispheres made a sharp reversal in direction, ultimately crossing the baseline to peak over the opposite hemisphere. This is interpreted as a shift in service of the eye movements in the opposite direction of the initial cue. This effect holds regardless of which hemifield contained the cue.
Visual inspection of the overall scalp topographies (Fig 4.) suggested a potential locus of alpha activation over the temporal lobe around the time of the saccade. Although this appears as a third region of interest in the head map, it would not be explicitly captured in our arrangement of quadrants on the weather map. Previous literature (and a reviewer) had suggested that in contrast to the inhibitory role of alpha in visual cortices, alpha synchrony in inferotemporal (IT) areas might facilitate visual processing (Mo J et al. 2011). We thus conducted additional post-hoc statistical analyses comparing lateralized alpha activity from electrodes over these regions, but found no significant differences.
Figure 4. Combined timeline of Alpha dynamics.
Showing the evolution of scalp topography of alpha lateralization between conditions. Red lines give relative position of gaze following the cue presentation along with key time points across the averaged epoch. Figure 2 has been reproduced for reference. Scale shows relative levels of alpha-lateralization transformed in Stage 1 analysis (Stimulus Right - Stimulus Left), see Methods. Time ‘0’ is saccade onset. Note the distinguishable activation from the eye movements such that frontal and occipital activations are ipsilateral in the pro- condition, but contralateral in the anti- condition.
Statistical cluster plots (SCPs)
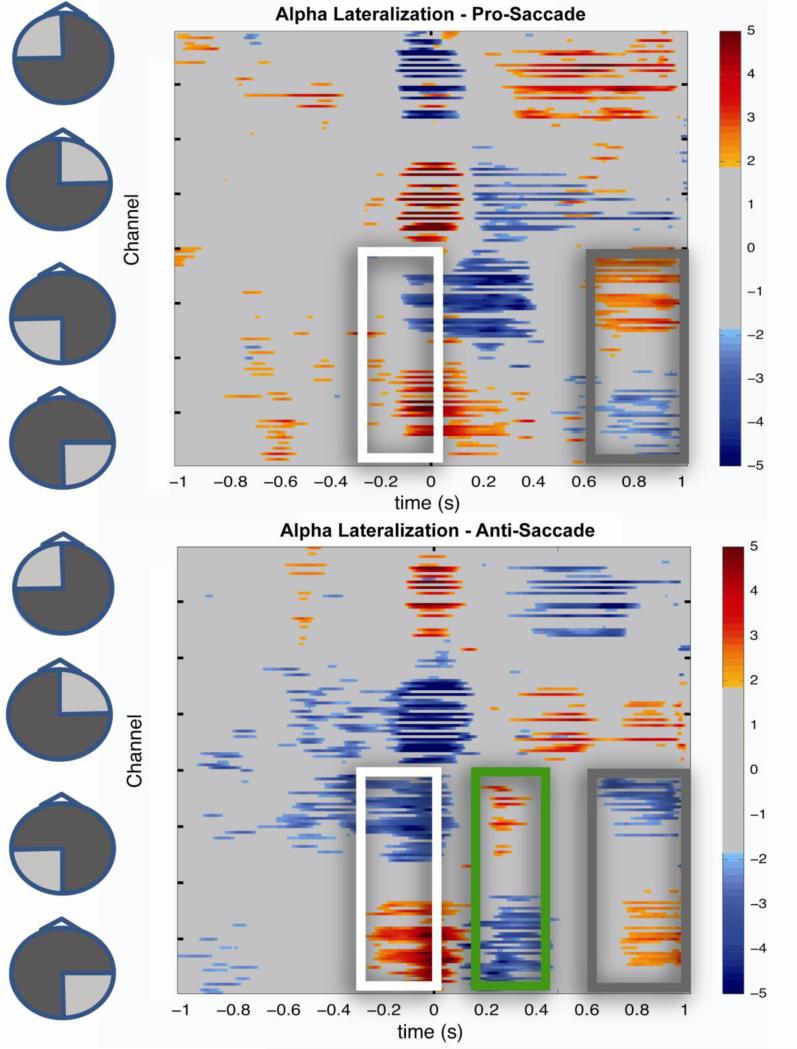
The SCP (Fig. 3) examined every channel over time highlighting all significant paired sample t-tests comparing hemispheric alpha lateralization for pro- and anti-saccade conditions. This figure shows clear differences in alpha lateralization (t-values significant at p<.05, corrected for multiple comparisons as described in Methods) in both conditions. Both pro- and anti-saccade conditions show similar patterns of increased alpha power ipsilateral, and decreased power contralateral, to stimulus presentation, over occipital-parietal electrodes in the period just before saccade onset (outlined in white). Notably, however, this pattern of activation switched its hemispheric lateralization in the anti-saccade condition only, around the time of saccade onset. This highlighted switch in lateralization is clearly seen as an additional reversal from negative (blue) to positive (red), of significant t-values over occipital-parietal electrodes, only in the anti-saccade condition (outlined in green rectangle).
Fig 3. Significant T-values in Pro- and Anti-Saccade Conditions.
T-tests comparing Alpha-lateralization (left-right) for every channel (y-axis) at every time point (x-axis). Scale represents t-values and only those found to be significant at the p<.05 (corrected) level are shown. Time ‘0’ is saccade onset. Highlighted regions shows area of interests: White= Stimulus-related covert attention; Green= additional lateralization in Anti-saccade; Grey= presumed Inhibition of Return.
Large periods of significance are also seen bilaterally across anterior electrodes, clearly highlighting the occulomotor potentials in these channels. These periods matched nearly exactly for both the pro- and anti- saccade conditions but are presented with the eyes moving in the opposite direction around time zero (saccade onset). At around 500msec, coinciding with the reaction time of the saccade returning to fixation, both pro- and anti-saccade conditions once again showed a similar lateralization, though reversed in sign, over these same anterior electrodes, attributable to the eye movement back to fixation. It is notable, however, that although these artifacts permeate a relatively large group of electrodes, they do not correlate with the activity across the posterior sites of interest. This is evident by comparing the pattern of positive and negative values, from anterior to posterior sites as seen in the time-course shown in Figure 4. As transformed above, the stimulus presentation was to the same hemifield for both conditions, while the eyes were moving to opposite sides, resulting in relative increases over the right posterior region in both conditions.
Finally, a late period of significant activity was seen in both conditions over occipital-parietal electrodes beginning approximately 700ms after the onset of the initial saccade until the end of the epoch. This activity was not explicitly predicted but considering the nearly identical dynamics in both conditions in this period, and that in both cases this activity lateralized opposite the initial saccade, it is possible that this represents an automatic suppression of recently attended space as in so-called inhibition of return (Posner M and Y Cohen 1984).
Alpha lateralization index
Collapsing across hemisphere (Stage 2 lateralization from Methods), anon-linear regression performed on the transformed data indicated that in the pro- condition, no significant additional variance was accounted for by employing the cubic coefficient (Standardized Beta = .496, p=.534). However, in the anti- condition, a cubic model accounted for significantly greater variance (Standardized Beta = 9.17, p<.001). These results confirm that, in line with the main hypothesis, the curve fitting the pro-saccade trace described a single phase that reversed direction once, whereas the function describing the alpha lateralization in the anti-saccade case reversed twice, reflecting an additional phase, or deployment of alpha lateralization. This result is clearly evident in the single EOI analysis shown in Figure 2c,d, and provides evidence for the hypothesized dynamics of alpha lateralization whereby alpha power synchronized first to attend covertly to stimulus onset, and secondly to the overt shift of overt attention.
Bi-phasic alpha dynamics
Mean saccade reaction times, and visual inspection of results as seen in Figure 3, indicated that the period of greatest lateralized activity began 300msec pre-saccade in the anti-saccade condition, and continued until approximately 300msec post-saccade onset. These two periods (-300 – 0msec; 0 – +300msec) were transformed as described in the Methods and submitted to additional statistical analysis. Quantification of the alpha lateralization showed that in the pre-saccade period (-300 – 0 msec), alpha power was not significantly different across conditions (Wilcoxon Signed Ranks test p= .535) suggesting a similar reaction to stimulus onset in both conditions. However, in the period post-saccade (0 – 300ms), the lateralization across conditions was significantly different (Wilcoxon Signed Ranks p = .015) further confirming that the sign of the lateralization was inverted in the anti-saccade condition compared to the pro-saccade.
It would appear the use of alpha-rhythms to suppress to-be-ignored stimuli may also be recruited as a tool similarly during a saccade. Since these results indicate a pattern of multiple lateralized phases of alpha rhythm dynamics involved in an eye movement task, we were interested in further characterizing these phases. To describe these dynamics, a minimally-constrained model of best fit was produced. As seen in Figure 5, this model describes a series of Gaussian curves, representing first the reaction to covert shifts of attention to the stimulus, and secondly the effect of eye movements. In the anti-saccade condition this second function is inverted in sign from the first. While we cannot be certain of the cognitive processes that caused this pattern of alpha power recorded on the scalp, since the principal difference in the anti-saccade condition is the inversion of the saccade, it is striking that a simple inversion of directional sign might adequately model the results. This model further supports a role for alpha rhythms in overt attention tasks, at least producing similar changes in alpha power at the scalp.
Stimulus only condition
As a control of stimulus-related effects on alpha-power in these conditions, an additional condition was also recorded wherein participants were instructed to not move their eyes, but maintain fixation while the same lateralized stimulus was presented. Although there was an initial lateralized increase in alpha-power ipsilateral to stimulus onset, there was no discernable lateralization of alpha-power after approximately 250msec (see inset, Figure 2). These results lend further evidence to the dual-role of alpha power in spatial attentional deployments in both covert and overt attention.
DISCUSSION
A growing body of research on the ongoing oscillatory activity of the brain points to a prominent role for the ~8-14Hz alpha rhythm in attentional processes (Dockree PM et al. 2007; Foxe JJ and AC Snyder 2011; Romei V et al. 2012). Previous work has clearly demonstrated that increases in alpha power correlate with active suppression of unattended space under both visuospatial (Worden MS et al. 2000; Kelly S et al. 2006; Rihs TA et al. 2007) and audiospatial (Kerlin JR et al. 2010; Banerjee S et al. 2011) task conditions. To date, however, explorations of the suppressive role of alpha rhythms have been mainly limited to tasks involving top-down, covert attention. These paradigms, in which a delay is inserted between the instructive cue and the imperative target, have reported relatively slowly changing alpha dynamics. By measuring alpha rhythms during rapid eye movement tasks, the current investigation showed that retinotopic shifts in alpha power are also involved during overt attentional deployments, and that these shifts can occur at the more rapid timescale required for the reactive shifts of attention that are crucial during saccade task performance.
The Timecourse of Alpha Lateralization
An important theoretical issue in attention research has centered on determining just how rapidly the focus of attention can be effectively switched between two potential information sources. The rapid topographic switches of alpha reported here suggest a temporal profile of attentional switching that is somewhat at variance with previous estimates(see supplementary material for video of continuous scalp recordings depicting these rapid topographic transitions). Previous literature on the role of alpha-band activity in attentional deployment has tended to focus on the type of cued, covert tasks that have been conducted for well over 100 years (Donders FC 1868/1969). Given the experimental set-up, these experiments by their nature, have detailed only relatively slow alpha-band shifts across space. Nonetheless, work that aimed at explicitly addressing this issue of the speed of attentional switching has also pointed to relatively slow dynamics. For example, Ward and colleagues (Ward R et al. 1996) conducted a series of psychophysical experiments in which they presented two objects in succession and asked how long attentional processing of the first object would be seen to affect subsequent processing of the second object. They found that interference persisted for up to and beyond 500 msec, concluding “visual attention is not a high speed switching mechanism”. Similarly, studies from the task-switching literature also suggest that attentional switches are not as effective below 400-500 msec (Wylie GR et al. 2004; Wylie GR et al. 2009).
Previous electrophysiological reports on the time course of attentional switching have also pointed to relatively sluggish dynamics. For example, earlier work attempted to measure the lower bounds of attentional switching by recording an ongoing electrophysiological measure of sensory processing, the steady-state visual-evoked potential (SSVEP) during a target detection task (Müller MM et al. 1998). It is well-established that the SSVEP, like components of the visual evoked potential, can be modulated by spatial attention (Morgan ST et al. 1996; Senkowski D et al. 2008). In Müller and colleagues experiment, a central cue instructed participants to attend to one of two lateralized rows of LEDs, each continuously flickering at a different rate, where they were to ascertain a particular target pattern that appeared randomly every 400-700msec. The authors reported a gradual increase in the amplitude of the SSVEP to the attended stream becoming significant around 450- 600msec after subjects had made a switch from one stream to the other. These results were interpreted to confirm Ward et al. (Ward R et al. 1996), as another demonstration of the relatively slow pace of visual attention switching. However, participants in this study were actually able to ascertain almost 50% of targets as early as 288msec in some conditions. This demonstration of more rapid shifts of attention, is thus compatible with the results reported here when attention must shift prior to the saccade, suggesting a more rapid deployment is possible.
Specific investigations of alpha oscillatory activity during saccade tasks have likewise followed traditional covert attention task designs using relatively protracted cue-target intervals. Brignani and colleagues (2007),for example, also reported modulation of alpha rhythms in a saccade task where a two second interval occurred between the instructive cue and the saccade target (Brignani D et al. 2007). Their results showed increased alpha synchrony for about one second around the time of the stimulus, and later for about 500 msec prior to the saccade. Although the authors did not compare lateralized components, instead averaging across leftward and rightward saccades, these results are quite compatible with the data reported here in their demonstration of increased alpha power to both cue and saccade. However, our use of a saccade task with no delay interval, that required not only very rapid shifts of attention, but also lateralized shifting that differed between the pro- and anti-saccade conditions, compressed the timeframe in which alpha modulations must be employed, and was better able to distinguish the differing modulations due to cue and saccade targeting.
Previous work has attempted to force more rapid shifting of overt attention through use of the so-called “gap paradigm” in which a brief temporal gap is added between cue and target, thereby allowing a brief saccade planning period. The gap paradigm is known to cause an abundance of very fast “express” saccades with reaction times around 100 msec. Hamm et al. (2010) and Skrandies and Anagnosou (1999) both examined alpha rhythms during express saccade tasks and found increases in alpha power within the gap period before an express saccade was executed, compared to normal targeting saccades(Skrandies W and E Anagnostou 1999; Hamm JP et al. 2010). Since the gap was only about 200 msec, both groups provide concurrent support for the suggestion that alpha may in fact modulate attention at rapid timescales. Hamm and colleagues went on to speculate that these differences in power might be related to phase-reset of the ongoing oscillations that occur to the onset of the gap. Since phase-specific effects of alpha synchrony were recently reported to influence saccadic reaction times (Drewes J and R VanRullen 2011), and target detection rates (Mathewson KE et al. 2011), these results highlight a potential functional mechanism through which alpha rhythms may work.
Overt vs. Covert Attention
For decades now, researchers have debated the relationship between overt and covert attention. One of the critical findings of Posner and colleagues’ early seminal research was that attention shifts could precede eye movements by approximately 200msec. By measuring reaction times to detect targets either at the fovea, or at a location in the periphery, they detected faster reaction times to the attended location before the eye movements began (Posner 1980). They thus concluded that “attention movements are not an inevitable consequence of eye movements” (emphasis added) providing early evidence for the dissociability of covert and overt attention (Posner M et al. 1982). Conversely, even in a task that did not require eye movements, Rizzolatti et al (1987) advanced the ‘pre-motor theory of attention’ in order to explain why subjects had added difficulty in covertly monitoring both sides of the vertical meridian (Rizzolatti G et al. 1987). They proposed that covert attention might actually be the preparation of an eye movement, even if the action did not ultimately occur.
Previous electrophysiological experiments have similarly reported evidence for overlapping mechanisms of overt and covert attention. Eimer and colleagues (2007) as well as Kelly and colleagues (2010) examined several event-related potential (ERP) measures of attention, the late directing attention positivity (LDAP) and anterior directing attention negativity (ADAN) during a saccade task (Eimer M et al. 2007; Kelly SP et al. 2010).The ADAN had previously been associated with frontal attentional control (Nobre AC et al. 2000) and the LDAP, like alpha amplitude, had been associated with anticipatory modulation preceding an upcoming target (Hopf JM and GR Mangun 2000; Simpson GV et al. 2006; Dale CL et al. 2008). By separating the cue for an initial attention shift, from a later Go/NoGo cue to make a saccade, Eimer and colleagues found that the ADAN was reliably invoked whether or not the second cue signaled the overt shift of attention. The LDAP, however, was attenuated when the overt shift was required. Eimer and colleagues results thus suggested a shared, but distinguishable attentional mechanism between overt and covert attention. Kelly and colleagues (2010) also utilized a cued saccade task, and likewise reported differences in the amplitude of the LDAP but not the ADAN depending on the occurrence of distracters in the hemifield opposite the location of the planned saccade. Notably, they also found that expectation of distracters in the unattended hemifield correlated with differences in alpha modulation, but were not dependent on the occurrence of a saccade (Kelly SPet al. 2010). That is, as in the present results, alpha band suppressive mechanisms appeared to subserve both covert and overt tasks.
Endogenous vs. Exogenous attention
One of the unique features of the results revealed here using the anti-saccade task is the striking similarity of alpha power dynamics in both the time period immediately following the cue, driven in a bottom-up ‘exogenous’ fashion, and in the immediately following period of rapid alpha shifts reflecting the ‘endogenous’ top-down eye-movement. It has been suggested that lower frequencies like theta and alpha, might only be representative of ‘top-down’ processes, while fast gamma indicates ‘bottom-up’ capture (von Stein et al., 2000). These data seem to suggest alpha may be fast enough to work at an exogenous level. However, it is possible our emphasis on alpha neglects interaction with other frequencies. For instance, Engel and Fries (2010) in a review of ~15-30Hz beta rhythms in the motor system, theorized that the reciprocal relationship of gamma and beta often found in motor studies, represents a mechanism by which the sensorimotor system can both switch between (through brief periods of gamma), and maintain (through beta entrainment) particular tasks (Engel & Fries, 2010). Whether the fast modulation of alpha oscillations seen here should be considered a mechanism of ongoing attention, or instead result from a switching or disengagement process, will require further investigation.
One previous study that specifically investigated the modulation of alpha power in shifting versus maintaining attention during a task that varied the cue-target interval, found somewhat conflicting results to those reported here (Rihs TA et al. 2009). The authors reported that lateralized alpha power initially decreased contralateral, but later increased ipsilateral to attended space as the participants maintained lateralized attention through the longer interval, nicely confirming the suppressive role of alpha (Worden MS et al. 2000). The authors ultimately concluded that the initial shift did not represent an exogenous shift to the cue, but an endogenous disengagement of attention in order to prepare for the upcoming task. Since Rihs and colleagues were interested in comparing the shifting versus maintaining roles of alpha power across the delay period, they ultimately focused on the 200 msec prior to target onset (500, or 1700 msec after cue). In our paradigm, participants were not pre-cued in advance to either the timing or hemifield of the impending cue, and were required to attend at least partially to both sides of space. Examination of pre-cue alpha power (not presented here) confirmed no significant lateralization at the time of the cue. Thus, it remains possible that the results reported by Rihs and colleagues, of anticipatory decreases to the expected saccade targets were either not necessary, or not possible, because of the short timescale in our task. However, the findings by Rihs and colleagues otherwise provide complementary evidence of an expanded role for alpha rhythms during overt attention and suggest that alpha may act similarly to beta in maintaining resources on task (Engel AK and P Fries 2010).
Oscillatory Effects in Other Bands
Our method of analyzing alpha power by using the absolute value of the Hilbert transformed 8-14Hz filtered signal only allows us to report on power dynamics in this range. However, alpha should not be assumed to exist without interactions among many other ongoing oscillations. If, as previously proposed, alpha activity results from activity in cortico-thalamo-cortical loops (Lopes da Silva F 1991), this circuit could be altered not only by other neural circuits among similar regions (Banerjee S et al. 2011), but also other circuits, both cortical and subcortical (Leventhal DK et al. 2012). A growing body of work concerning a variety of oscillatory mechanisms across various frequency bands such as 1-4 Hz ‘delta’, 4-7 Hz ‘theta’, 15-30 Hz ‘beta’ and 30-100Hz ‘gamma’ bands, confirms that alpha is only one constituent of a more complex oscillatory system (Schroeder CE and P Lakatos 2009; Fiebelkorn I et al. 2012).
For example, in addition to alpha, gamma band (>30Hz) modulations have also been reported in saccadic tasks (Van Der Werf J et al. 2008; Nagasawa T et al. 2011). Van Der Werf et al. (2008) utilized magnetoencepalography (MEG), in a memory-guided anti-saccade task in which the position of a briefly flashed peripheral target position had to be maintained in memory until a secondary cue initiated saccade onset, either to the same, or equal position in the opposite hemifield. The authors found distinguishable oscillatory effects across both alpha and gamma frequencies that modulated to the peripheral stimulus and saccade target (Van Der Werf J et al. 2008). The authors primarily reported an increase in gamma band activity that seemed to encode impending saccade targets, which, like here, was particularly distinguishable during the anti-saccade condition. The authors also reported varying alpha-dynamics throughout the trials but concluded that alpha decreases across contralateral sensors, were attributable only to the stimulus, not the saccade target. Similar to the Brignani et al. study (Brignani D et al. 2007), however, the authors also incorporated a 1.5 sec cue-task interval. Likewise, although they did not report sustained alpha lateralization that may be attributable to upcoming saccade goal, the authors did in fact report significant contralateral decreases in both the period around the stimulus and just prior to the saccade, thus also providing complimentary findings to those reported here.
A general framework in which to understand these interactions would suggest the phases of rhythmic activity represent peak and off-peak periods that perform a type of ‘gating’ that facilitates, or suppresses incoming activity (Schroeder CE and P Lakatos 2009; Gomez-Ramirez M et al. 2011). Under this view, slower rhythms (e.g. ~1-4 Hz delta) might carry faster rhythms through periods of higher and lower relevance. Lakatos and colleagues (2005) went even further to describe a ‘hierarchy’ of oscillations such that the phase of lower frequencies modulated amplitude in the next higher frequency band i.e. delta modulates theta which in turn modulates gamma. Since overt saccadic attention shifts produce pulsed excitatory input to the visual system at about 3-4Hz, it is possible that this slow delta-band rhythm might in turn rhythmically modulate downstream cortices through cross-frequency coupling mechanisms (Lakatos P et al. 2005).
Issues of Phase
While the current study focuses on alpha-power, the specific phase of ongoing oscillations both within and across trials has emerged as another potentially informative dependent measure. In fact, a growing body of research has correlated phase in the alpha band with reaction time (Drewes J and R VanRullen 2011) and target detection (Busch NA et al. 2009; Mathewson KE et al. 2009). Converging evidence has also provided a potential cellular mechanism for how alpha phase could influence behavior. Haegens and colleagues (2011) recorded local field potentials (LFPs) and individual neuronal activity in somatosensory, premotor and motor cortices of monkeys who were performing a tactile discrimination task. The authors reported an inverse relationship between alpha phase and firing rates, such that higher firing rates were recorded during the trough of the alpha cycle (Haegens S, V Nacher, et al. 2011). In turn, overall alpha decreases were correlated with spiking increases and predicted better discrimination performance. Similarly, Bartlett and colleagues (2011) recording from the upper superior temporal sulcus in monkeys, an area generally associated with ventral stream object processing, detected phase concentration across alpha, beta and gamma bands when the animals fixated. The spiking activity of single neurons was also modulated by the phase of all three frequency bands (Bartlett AM et al. 2011). The consideration of how specific phase of alpha might relate to active, pulsed inhibition at a cellular and network level (Klimesch W et al. 2007) has been recently suggested in a model by Jensen and colleagues (2012) and awaits empirical verification (Jensen O et al. 2012).
Study Limitations
While these results suggest a much more rapid dynamic for alpha than previously described, it is worth considering the lower-limits of our measurements. Our use of a recursive, Butterworth filter to extract power information in this frequency, creates temporal smearing that is an artifactual remnant of the filter itself. However, while we cannot be confident of the millisecond precision that is typical of electrophysiology, the results presented here have still revealed that reversal of hemispheric lateralization must occur about 257msec after the initial cue (i.e. the mean anti-saccade reaction time) to accommodate the eye movement. As seen in the distribution of reaction times (Figure 2), many trials actually had reaction times below this mean. Furthermore, our alpha lateralization measure described three hemispheric lateralizations occurring in about one second. While oscillatory measures have the potential to describe shifts at the level of phase (Drewes J and R VanRullen 2011), our use of the anti-saccade task has at least demonstrated a more rapid dynamic for alpha power than had been previously shown.
Since any interpretation must remain limited to the temporal resolution of the dependent measure, the methods here do not allow us to assay whether the reported shifts of alpha power are necessary to make an overt response. A reversal of alpha lateralization does appear prior to saccade onset, and certainly prior to the saccade landing time in the anti-saccade condition. However, other interpretations are possible. It might be that in the anti-saccade condition, one must actively suppress the cue when it appears. As the eyes find their target opposite the stimulus, the suppression shifts in a retinotopic fashion over the fixation. The relief of this suppression necessary to shift the gaze back to fixation might thus account for the reversal seen only in the anti-saccade. This interpretation, however, does not call into question the pace of the alpha dynamics reported here, only their underlying cause.
In the traditional anti-saccade paradigm, participants are asked to identify a target position, and then invert the motor plan to produce a movement internally calculated to reflect a mirrored position. Since the stimulus presentation here includes target ‘holes’ in the background, it could be argued that this is not a true anti-saccade task since one does not need to make such calculations. As such, the checkerboard presentation acts merely as a left-right directional cue. Although this task does not reveal any activity presumed to be related to such a calculated conversion, the results clearly show alpha-lateralization shifting dramatically across hemispheres in concert with presumed shifts of attention from cue presentation side, to the side of saccade targeting as would be necessary to perform the anti-saccade. Further research might attempt to better clarify the retinotopic extension of the target (e.g. 10 DVA vs. 20 DVA) with the degree of alpha lateralization to disentangle hemifield from true retinotopic shifts.
In addition to increased target discrimination, anticipatory alpha has at times also been correlated with reaction times e.g. (Haegens S, BF Handel, et al. 2011; Premereur E et al. 2012). Our data, however, revealed no such relationship. This could be for one of several reasons: 1) as with many papers describing behavioral effects related to alpha, the above-mentioned articles included a cue-target interval in which anticipatory alpha is modulated. However in our paradigm, saccadic reaction time is already so short (averaging around 250 ms, and often shorter), that variation within this range may not have been detectable; 2) despite the long-tailed distribution, the majority of saccadic reaction times fall within a relatively narrow range; 3) there is little variability in pre-cue alpha shifts as participants seemed to follow instructions and attend equally to both hemifields; and 4) in this paradigm, alpha must shift within only a cycle or two, and we do not have the temporal resolution to differentiate this, even when considered as a function of slowest versus fastest quartiles.
Conclusions
The data presented here suggest that rapidly shifting alpha-power is a shared neural mechanism during both covert and overt attention tasks. These results not only expand the conception of an active role of 8-14Hz alpha rhythms to overt attention tasks, but they also suggest a more rapid timescale for the deployment of spatial attention by capitalizing on the very rapid shifts of attention across hemifield necessary in the anti-saccade task.
Supplementary Material
ACKNOWLEDGMENTS
This work was primarily supported by a National Science Foundation (NSF) grant to J.J.F. (BCS0642584). Additional support was derived from a National Institute of Mental Health(NIMH) grant (MH085322 to J.J.F.). Support for the work of J.W. and M.R.H. was derived from NSF grant BCS0842464 and a National Eye Institute grant (EY019508 to JW and MRH). Dr. Snyder was supported by a Ruth L. Kirschstein National Research Service Award (NRSA) pre-doctoral fellowship from the NIMH (MH087077).
Dr. Snyder's new address is: Visual Neuroscience Laboratory, Eye and Ear Institute, Rm. 914, University of Pittsburgh, 203 Lothrop St., Pittsburgh, PA 15213. The authors would also like to acknowledge our gratitude for the helpful comments of two anonymous reviewers and Dr. Sophie Molholm who provided comments on an earlier draft of this manuscript.
During final preparation of this manuscript, our dear friend and close colleague, Josh Wallman, passed away. We would like to acknowledge his extraordinary intellectual leadership, his profound passion for his science and his selfless mentorship. Josh, you are so very badly missed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Banerjee S, Snyder AC, Molholm S, Foxe J, Molholm S. Oscillatory Alpha-Band Mechanisms and the Deployment of Spatial Attention to Anticipated Auditory and Visual Target Locations: Supramodal or Sensory- Specific Mechanisms? The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:9923–9923. doi: 10.1523/JNEUROSCI.4660-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett AM, Ovaysikia S, Logothetis NK, Hoffman KL. Saccades during object viewing modulate oscillatory phase in the superior temporal sulcus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:18423–18432. doi: 10.1523/JNEUROSCI.4102-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignani D, Maioli C, Maria Rossini P, Miniussi C. Event-related power modulations of brain activity preceding visually guided saccades. Brain Res. 2007;1136:122–131. doi: 10.1016/j.brainres.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Busch NA, Dubois J, VanRullen R. The phase of ongoing EEG oscillations predicts visual perception. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:7869–7876. doi: 10.1523/JNEUROSCI.0113-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capotosto P, Babiloni C, Romani GL, Corbetta M. Frontoparietal cortex controls spatial attention through modulation of anticipatory alpha rhythms. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:5863–5872. doi: 10.1523/JNEUROSCI.0539-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalk M, Herrero JL, Gieselmann MA, Delicato LS, Gotthardt S, Thiele A. Attention reduces stimulus- driven gamma frequency oscillations and spike field coherence in V1. Neuron. 2010;66:114–125. doi: 10.1016/j.neuron.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: identical, independent, or overlapping neural systems? Proc Nat Acad Sci USA. 1998;95:831–838. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Deubel H, Schneider WX. Saccade target selection and object recognition: evidence for a common attentional mechanism. Vision Res. 1996;36:1827–1837. doi: 10.1016/0042-6989(95)00294-4. [DOI] [PubMed] [Google Scholar]
- Dockree PM, Kelly SP, Foxe JJ, Reilly RB, Robertson IH. Optimal sustained attention is linked to the spectral content of background EEG activity: greater ongoing tonic alpha (approximately 10 Hz) power supports successful phasic goal activation. Eur J Neurosci. 2007;25:900–907. doi: 10.1111/j.1460-9568.2007.05324.x. [DOI] [PubMed] [Google Scholar]
- Donders FC, Koster WG. Over de snelheid van psycholische processen/ On the speed of mental processes. In: Koster WG, editor. Acta Psychol : Attention and Performance II 1969 ed. North-Holland Publishing Company p; Amsterdam: 1868/1969. pp. 412–431. [DOI] [PubMed] [Google Scholar]
- Drewes J, VanRullen R. This is the rhythm of your eyes: the phase of ongoing electroencephalogram oscillations modulates saccadic reaction time. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:4698–4708. doi: 10.1523/JNEUROSCI.4795-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M, Van Velzen J, Gherri E, Press C. ERP correlates of shared control mechanisms involved in saccade preparation and in covert attention. Brain research. 2007;1135:154–166. doi: 10.1016/j.brainres.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations--signalling the status quo? Current opinion in neurobiology. 2010;20:156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Fiebelkorn I, Snyder A, Mercier M, Butler J, Molholm S, Foxe J. Cortical Cross-frequency Coupling Drives Perceptual Outcomes. Review. tbd:tbd. 2012 doi: 10.1016/j.neuroimage.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B, Boch R. Saccadic Eye Movements After Extremely Short Reaction Times in the Monkey Target. Brain Res. 1983;260:21–26. doi: 10.1016/0006-8993(83)90760-6. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, McCourt ME, Javitt DC. Right hemisphere control of visuospatial attention: line-bisection judgments evaluated with high-density electrical mapping and source analysis. NeuroImage. 2003;19:710–726. doi: 10.1016/s1053-8119(03)00057-0. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV. Biasing the brain's attentional set: II. effects of selective intersensory attentional deployments on subsequent sensory processing. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 2005;166:393–401. doi: 10.1007/s00221-005-2379-6. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV, Ahlfors SP. Parieto-occipital approximately 10 Hz activity reflects anticipatory state of visual attention mechanisms. Neuroreport. 1998;9:3929–3933. doi: 10.1097/00001756-199812010-00030. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Snyder AC. The Role of Alpha-Band Brain Oscillations as a Sensory Suppression Mechanism during Selective Attention. Front Psychology. 2011;2:1–13. doi: 10.3389/fpsyg.2011.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu KM, Foxe JJ, Murray MM, Higgins BA, Javitt DC, Schroeder CE. Attention-dependent suppression of distracter visual input can be cross-modally cued as indexed by anticipatory parieto-occipital alpha-band oscillations. Brain Res Cogn Brain Res. 2001;12:145–152. doi: 10.1016/s0926-6410(01)00034-9. [DOI] [PubMed] [Google Scholar]
- Gomez-Ramirez M, Kelly SP, Molholm S, Sehatpour P, Schwartz TH, Foxe JJ. Oscillatory sensory selection mechanisms during intersensory attention to rhythmic auditory and visual inputs: a human electrocorticographic investigation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:18556–18567. doi: 10.1523/JNEUROSCI.2164-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould IC, Rushworth MF, Nobre AC. Indexing the graded allocation of visuospatial attention using anticipatory alpha oscillations. J Neurophysiol. 2011;105:1318–1326. doi: 10.1152/jn.00653.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie D, Buchwald JS. Significance testing of difference potentials. Psychophysiology. 1991;28:240–244. doi: 10.1111/j.1469-8986.1991.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Gutteling TP, van Ettinger-Veenstra HM, Kenemans JL, Neggers SF. Lateralized Frontal Eye Field Activity Precedes Occipital Activity Shortly before Saccades: Evidence for Cortico-cortical Feedback as a Mechanism Underlying Covert Attention Shifts. J Cogn Neurosci. 2010;22:1931–1943. doi: 10.1162/jocn.2009.21342. [DOI] [PubMed] [Google Scholar]
- Haegens S, Handel BF, Jensen O. Top-down controlled alpha band activity in somatosensory areas determines behavioral performance in a discrimination task. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:5197–5204. doi: 10.1523/JNEUROSCI.5199-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S, Nacher V, Luna R, Romo R, Jensen O. alpha-Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:19377–19382. doi: 10.1073/pnas.1117190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm JP, Dyckman KA, Ethridge LE, McDowell JE, Clementz BA. Preparatory activations across a distributed cortical network determine production of express saccades in humans. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:7350–7357. doi: 10.1523/JNEUROSCI.0785-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel BF, Haarmeier T, Jensen O. Alpha oscillations correlate with the successful inhibition of unattended stimuli. J Cogn Neurosci. 2011;23:2494–2502. doi: 10.1162/jocn.2010.21557. [DOI] [PubMed] [Google Scholar]
- Helmholtz Hv., Southall JPC. Handbuchder Physiologischen Optik/ Treatise on Physiological Optics. 3rd ed. Dover; New York: 1910/1924. [Google Scholar]
- Hoffman JE, Subramaniam B. The role of visual attention in saccadic eye movements. Perception & psychophysics. 1995;57:787–795. doi: 10.3758/bf03206794. [DOI] [PubMed] [Google Scholar]
- Jensen O, Bonnefond M, VanRullen R. An oscillatory mechanism for prioritizing salient unattended stimuli. Trends in cognitive sciences. 2012;16:200–206. doi: 10.1016/j.tics.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Jones SR, Kerr CE, Wan Q, Pritchett DL, Hämäläinen M, Moore CI. Cued spatial attention drives functionally relevant modulation of the mu rhythm in primary somatosensory cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:13760–13765. doi: 10.1523/JNEUROSCI.2969-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S, Lalor E, Reilly R, Foxe J. Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuospatial attention. J Neurophysiol. 2006;95:3844–3844. doi: 10.1152/jn.01234.2005. [DOI] [PubMed] [Google Scholar]
- Kelly SP, Foxe JJ, Newman G, Edelman J. Prepare for conflict: EEG correlates of the anticipation of target competition during overt and covert shifts of visual attention. Eur J Neurosci. 2010:1690–1700. doi: 10.1111/j.1460-9568.2010.07219.x. [DOI] [PubMed] [Google Scholar]
- Kelly SP, Gomez-Ramirez M, Foxe JJ. The strength of anticipatory spatial biasing predicts target discrimination at attended locations: a high-density EEG study. Eur J Neurosci. 2009;30:2224–2234. doi: 10.1111/j.1460-9568.2009.06980.x. [DOI] [PubMed] [Google Scholar]
- Kerlin JR, Shahin AJ, Miller LM, Shahin AJ. Attentional gain control of ongoing cortical speech representations in a “cocktail party”. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:620–628. doi: 10.1523/JNEUROSCI.3631-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain research reviews. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Kowler E, Anderson E, Dosher B, Blaser E. The role of attention in the programming of saccades. Vision Res. 1995;35:1897–1916. doi: 10.1016/0042-6989(94)00279-u. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Shah AS, Knuth KH, Ulbert I, Karmos G, Schroeder CE. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J Neurophysiol. 2005;94:1904–1911. doi: 10.1152/jn.00263.2005. [DOI] [PubMed] [Google Scholar]
- Leventhal DK, Gage GJ, Schmidt R, Pettibone JR, Case AC, Berke JD. Basal Ganglia Beta Oscillations Accompany Cue Utilization. Neuron. 2012;73:523–536. doi: 10.1016/j.neuron.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes da Silva F. Neural mechanisms underlying brain waves: from neural membranes to networks. Electroencephalography and clinical neurophysiology. 1991;79:81–93. doi: 10.1016/0013-4694(91)90044-5. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hillyard SA. Modulations of sensory-evoked brain potentials indicate changes in perceptual processing during visual-spatial priming. Journal of experimental psychology Human perception and performance. 1991;17:1057–1074. doi: 10.1037//0096-1523.17.4.1057. [DOI] [PubMed] [Google Scholar]
- Mathewson KE, Gratton G, Fabiani M, Beck DM, Ro T. To see or not to see: prestimulus alpha phase predicts visual awareness. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:2725–2732. doi: 10.1523/JNEUROSCI.3963-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson KE, Lleras A, Beck DM, Fabiani M, Ro T, Gratton G. Pulsed out of awareness: EEG alpha oscillations represent a pulsed-inhibition of ongoing cortical processing. Front Psychol. 2011;2:99. doi: 10.3389/fpsyg.2011.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo J, Schroeder CE, Ding M. Attentional modulation of alpha oscillations in macaque inferotemporal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:878–882. doi: 10.1523/JNEUROSCI.5295-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molholm S, Ritter W, Murray MM, Javitt DC, Schroeder CE, Foxe JJ. Multisensory auditory-visual interactions during early sensory processing in humans: a high-density electrical mapping study. Brain Res Cogn Brain Res. 2002;14:115–128. doi: 10.1016/s0926-6410(02)00066-6. [DOI] [PubMed] [Google Scholar]
- Montagnini A, Castet E. Spatiotemporal dynamics of visual attention during saccade preparation: Independence and coupling between attention and movement planning. Journal of vision. 2007;78:1–16. doi: 10.1167/7.14.8. [DOI] [PubMed] [Google Scholar]
- Morgan ST, Hansen JC, Hillyard SA. Selective attention to stimulus location modulates the steady-state visual evoked potential. Proc Natl Acad Sci U S A. 1996;93:4770–4774. doi: 10.1073/pnas.93.10.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MM, Teder-Sälejärvi W, Hillyard SA. The time course of cortical facilitation during cued shifts of spatial attention. Nat Neurosci. 1998;1:631–634. doi: 10.1038/2865. [DOI] [PubMed] [Google Scholar]
- Nagasawa T, Matsuzaki N, Juhasz C, Hanazawa A, Shah A, Mittal S, Sood S, Asano E. Occipital gamma-oscillations modulated during eye movement tasks: simultaneous eye tracking and electrocorticography recording in epileptic patients. NeuroImage. 2011;58:1101–1109. doi: 10.1016/j.neuroimage.2011.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell RG, Dockree PM, Robertson IH, Bellgrove MA, Foxe JJ, Kelly SP. Uncovering the neural signature of lapsing attention: electrophysiological signals predict errors up to 20 s before they occur. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:8604–8611. doi: 10.1523/JNEUROSCI.5967-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational intelligence and neuroscience. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M, Cohen Y. BHaB D, editor. Components of visual orienting. Attention and performance X: Control of language processes. 1984:531–556. [Google Scholar]
- Posner M, Cohen Y, Rafal R. Neural systems control of spatial orienting. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 1982;298:187–198. doi: 10.1098/rstb.1982.0081. [DOI] [PubMed] [Google Scholar]
- Premereur E, Vanduffel W, Janssen P. Local field potential activity associated with temporal expectations in the macaque lateral intraparietal area. J Cogn Neurosci. 2012;24:1314–1330. doi: 10.1162/jocn_a_00221. [DOI] [PubMed] [Google Scholar]
- Rihs TA, Michel CM, Thut G. Mechanisms of selective inhibition in visual spatial attention are indexed by alpha-band EEG synchronization. Eur J Neurosci. 2007;25:603–610. doi: 10.1111/j.1460-9568.2007.05278.x. [DOI] [PubMed] [Google Scholar]
- Rihs TA, Michel CM, Thut G. A bias for posterior alpha-band power suppression versus enhancement during shifting versus maintenance of spatial attention. NeuroImage. 2009;44:190–199. doi: 10.1016/j.neuroimage.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Riggio L, Dascola I, Umiltá C. Reorienting attention across the horizontal and vertical meridians: evidence in favor of a premotor theory of attention. Neuropsychologia. 1987;25:31–40. doi: 10.1016/0028-3932(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Romei V, Thut G, Mok RM, Schyns PG, Driver J. Causal implication by rhythmic transcranial magnetic stimulation of alpha frequency in feature-based local vs. global attention. Eur J Neurosci. 2012;35:968–974. doi: 10.1111/j.1460-9568.2012.08020.x. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Lakatos P. The gamma oscillation: master or slave? Brain topography. 2009;22:24–26. doi: 10.1007/s10548-009-0080-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkowski D, Saint-Amour D, Gruber T, Foxe JJ. Look who's talking: the deployment of visuo-spatial attention during multisensory speech processing under noisy environmental conditions. NeuroImage. 2008;43:379–387. doi: 10.1016/j.neuroimage.2008.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrandies W, Anagnostou E. Electroencephalographic cortical oscillations and saccadic eye movements in humans. Neuroscience letters. 1999;261:57–60. doi: 10.1016/s0304-3940(98)01014-3. [DOI] [PubMed] [Google Scholar]
- Snyder AC, Foxe JJ. Anticipatory attentional suppression of visual features indexed by oscillatory alpha- band power increases: a high-density electrical mapping study. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:4024–4032. doi: 10.1523/JNEUROSCI.5684-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brand SA, Pascual-Leone A. {alpha}-Band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Werf J, Jensen O, Fries P, Medendorp WP. Gamma-band activity in human posterior parietal cortex encodes the motor goal during delayed prosaccades and antisaccades. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:8397–8405. doi: 10.1523/JNEUROSCI.0630-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R, Duncan J, Shapiro K. The Slow Time-Course of Visual Attention. Cogn Psychol. 1996;30:79–109. doi: 10.1006/cogp.1996.0003. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Fries P. Neuronal coherence during selective attentional processing and sensory-motor integration. Journal of physiology, Paris. 2006;100:182–193. doi: 10.1016/j.jphysparis.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Worden MS, Foxe JJ, Wang N, Simpson GV. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha-band electroencephalography increases over occipital cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:RC63–RC63. doi: 10.1523/JNEUROSCI.20-06-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Medical Organization Declaration of Helsinki. British Medical Journal. 1996;313:1448–1449. [Google Scholar]
- Wylie GR, Javitt DC, Foxe JJ. The role of response requirements in task switching: dissolving the residue. Neuroreport. 2004;15:1079–1087. doi: 10.1097/00001756-200404290-00030. [DOI] [PubMed] [Google Scholar]
- Wylie GR, Murray MM, Javitt DC, Foxe JJ. Distinct neurophysiological mechanisms mediate mixing costs and switch costs. J Cog Neurosci. 2009;21:105–118. doi: 10.1162/jocn.2009.21009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.