Abstract
H-Y antigens are a group of minor histocompatibility antigens encoded on the Y-chromosome with homologous H-X antigens on the X-chromosome. The disparate regions of the H-Y antigens are highly immunogenic and play an important role in understanding human alloimmunity. In this review, we investigate the history of H-Y antigen discovery along with their critical contributions in transplantation and pregnancy. In hematopoietic cell transplantation, male recipients with female donors who become seropositive for B-cell responses as H-Y antibodies following transplantation have increased rates of chronic graft-versus-host disease and decreased rates of relapse. Conversely, female patients who receive male kidney allografts are more likely than other gender combinations to develop H-Y antibodies and reject their allografts. Finally, in the setting of pregnancy, mothers who initially gave birth to boys are more likely to have subsequent pregnancy complications, including miscarriages, in association with H-Y antibody development. H-Y antigens continue to serve as a model for alloimmunity in new clinical scenarios. Our development of more sensitive antibody detection and next-generation DNA sequencing promises to further advance our understanding and better predict the clinical consequences of alloimmunity.
Keywords: H-Y antigen, Graft-versus-host disease, Graft rejection, Pregnancy complications, Alloimmunity, Kidney transplantation
Introduction
Human alloimmunity has significant consequences in a variety of transplantation settings. For human leukocyte antigen (HLA)-matched transplants, minor histocompatibility antigens (mHAs) are important targets for alloimmunity. mHAs are peptides which, when presented in HLA class I and class II proteins, are able to elicit an adaptive immune response [1]. H-Y antigens are a class of well-characterized mHAs encoded on the Y-chromosome. H-Y proteins tend to be highly expressed throughout the body and show a great degree of similarity to the homologous H-X proteins located on the X-chromosome, but with distinct regions of disparity which are generally immunogenic [2]. H-Y antigens provide an important model for alloimmunity because they serve as significant immunogenic targets with clinical consequences in either the donor graft or the recipient in sex-mismatched transplantation.
In hematopoietic cell transplantation (HCT), for example, grafts from female donors to male recipients (F → M) lead to increased rates of graft-versus-host disease (GVHD), a common complication of HCT which affects the skin, GI tract, liver and other organs. In sex-mismatched transplantation, GVHD is associated with alloimmunity, which occurs when naïve donor lymphocytes target mHAs such as H-Y antigens on normal host tissues in order to produce a combined humoral and cellular responses leading to significant morbidity and mortality [3–5].
Conversely, in kidney transplantation, kidney grafts from male donors to female recipients (M → F) experience increased rates of graft rejection [6]. The rationale behind this increase in graft rejection is that the recipient's lymphocytes develop an alloimmune response against the H-Y antigens present on the donor graft [7]. Finally, pregnant women with male fetuses may develop alloimmune response against these H-Y antigens. This is particularly important in the context of secondary recurrent miscarriage (SRM), defined as having three or more recurrent miscarriages after a successful birth [8].
In this review, we aim to explore the historical discovery of H-Y antigens as T- and B-cell alloimmune targets. We elucidate the clinical impact of H-Y alloimmunity in sex-mismatched HCT, organ transplantation and pregnancy.
H-Y alloimmunity in hematopoietic cell transplantation (HCT)
The first biological model of sex-mismatched transplantation tested skin graft rejection in mice. In the 1950s, Eichwald et al. first described that skin grafts from male donors to female recipients (M → F) had the highest rate of skin graft rejection among all gender combinations [9–12]. Eichwald et al. predicted that the female mice became sensitized to antigens encoded on the Y-chromosome, thus leading to graft rejection [12]. Further studies by Billingham showed that this effect could be prevented by tolerizing the females with injections of male donor cells into newborn females [13, 14]. These studies were the first to identify the importance of sex-mismatched transplants and led to the coining of the term “H-Y factor.”
Clinical studies of patients following HCT have found that F → M patients are between 1.5 and 4 times as likely to develop chronic graft-versus-host disease (cGVHD) in comparison to male recipients with male donors (M → M) [15–20]. Additionally, further studies have shown that male patients who receive allografts from female donors with high parity (more than two pregnancies) are more likely to develop cGVHD than male patients who receive allografts from nulliparous female donors (Table 1) [21].
Table 1. Clinical findings in sex-mismatch hematopoietic cell transplantation (HCT).
| Reference | Significant results | Conclusions |
|---|---|---|
| Carlens et al. [4] n = 451 (75 F → M) |
cGVHD developed in 61 % of F → M patients compared to 40 % in all others (RR 1.70, p = 0.006) | cGVHD increased in F → M patients |
| Kollman et al. [20] n = 6,978 (1,543 F → M) |
Registry study showing cGVHD in 54 % of patients with multiparous female donors versus 44 % in male donors (HR 1.40, p < 0.0001) |
Donor parity increases the risk of cGVHD |
| Randolph et al. [19] n = 3,238 (858 F → M) |
Increased cGVHD in F → M HCT (RR 1.56, p < 0.0001) Decreased relapse in F → M HCT (RR 0.70, p < 0.0003) compared to M → M patients CML-restricted analysis showed F → M benefit in relapse (RR 0.68, p = 0.04) |
Large study showing cGVHD risk is specific to F → M HCT GVL benefit confirmed through the use of single-disease analysis (CML) |
| Loren et al. [21] n = 2,626 (449 F → M) |
Donor parity increases cGVHD HR1.00 M → M HR 1.44 Nulliparous F → M HR 1.56 Parous F → M Donor parity decreases relapse: HR 1.00 M → M HR 0.75 Parous F → M |
Female donor sensitization by prior pregnancies leads to increased adoptive alloimmunity |
cGVHD chronic graft-versus-host disease, F → M graft from female donor to male recipient, RR relative risk, HR hazard ratio, GVL graft-versus-leukemia benefit, CML chronic myelogenous leukemia
Consistent with the mouse models above, the rationale is that lymphocytes from the female donor graft recognize several mHAs found on the Y-chromosome as foreign and mount an adaptive immune response against these targets. H-Y antigens present on normal host tissue are attacked leading to detrimental GVHD.
However, H-Y antigens present on malignant cells are also attacked, leading to a graft-versus-leukemia (GVL) benefit, which explains why high H-Y alloimmunity is associated with both high levels of GVHD and low levels of relapse.
Discovery of H-Y antigens as T-cell targets
Considering that F → M patients were more likely to develop GVHD, scientists predicted that there were proteins encoded by genes on the Y-chromosome which triggered an immune response from the female-derived allograft. However, it was not until the 1970s when sex-mismatched models began to be applied to human HCT. In 1977, Goulmy et al. [22, 23] reported a case in which blood from an HLA-matched F → M patient who had experienced acute GVHD possessed HLA-A2-restricted cytotoxic T lymphocytes (CTLs) which only attacked male cells. Therefore, she demonstrated that GVHD occurred in sex-mismatched HLA-matched patients and discovered HLA-restricted CTLs against an unidentified mHA presumed to be encoded on the Y-chromosome [24, 25]. Furthermore, she isolated T-cell clones specific to five autosomal mHAs and used these to score for the presence and absence of mHA expression in HCT patients and donors. Her first clinical application showed that GVHD associates with mHA disparity, meaning that the mHA is expressed in the recipient, but was absent in the donor. Therefore, naïve donor T lymphocytes would react to mHA expression following HCT. As such, Goulmy was the first to demonstrate mHA alloimmunity in humans in association with GVHD [26, 27]. In 1995, the first H-Y antigen was biochemically identified from the protein lysine (K)-specific demethylase 5D (SMCY) [28]. The identification of this H-Y peptide sequence facilitated subsequent soluble presentation of the mHA in HLA, called tetramers. These fluorescently conjugated tetramers stain mHA-specific T cells, thereby quantifying them and facilitating their isolation. Clinical studies utilizing tetramers showed H-Y-specific CTLs develop following sex-mismatched transplantation in association with GVHD [29].
In the late 1990s, the completion of the human genome project provided the complete Y-chromosome sequence and identified nine mHA candidate genes that Page postulated may play a role in graft rejection, paving the way for the molecular characterization of H-Y antigens [30–32]. Thus far, six of these H-Y candidate genes have been shown to encode T-cell-detected antigens and six encode B-cell antigens (Table 2) [5, 28, 33–46].
Table 2. Selection of H-Y antigens discovered as T-cell targets.
| H-Y antigen | HLA restriction | mHA sequence | Reference |
|---|---|---|---|
| DDX3Y (DBY) | DQB1*05 | HIENFSDIDMGE | Vogt [38] |
| B*2705 | SRDSRGKPGY | Rosinski [40] | |
| DRB1*1501 | SKGRYIPPHLR | Porcheray [47] | |
| USP9Y (DFFRY) | A*0101 | IVDCLTEMY | Pierce [39] |
| Vogt [36] | |||
| KDM5D (SMCY) | B*0702 | SPSVDKARAEL | Wang [28] |
| A*0201 | FIDSYICQV | Ofran [43] | |
| RPS4Y | DRB3*0301 | VIKVNDTVQI | Spierings [41] |
| B*5201 | TIRYPDPVI | Ivanov [44] | |
| DRB1*07 | TGKIINFIKFDTGNL | Eljaafari [33] | |
| TMSB4Y UTY | A*3303 | EVLLRPGLHFR | Torikai [42] |
| B*60 | RESEEESVSL | Vogt [37] | |
| B*8 | LPHNHTDL | Warren [34] | |
| A*2402 | YYNAFHWAI | Mortensen [45] |
mHA minor histocompatibility antigen
Detection of H-Y antibodies and H-Y-specific allogeneic B cells
While H-Y antigens were first described as T-cell-specific targets, humoral H-Y immune responses have developed as a clinically useful measurement of alloimmunity. In fact, H-Y antigens have been shown to elicit a coordinated B-cell and T-cell response [5, 47, 48]. The utility of H-Y antibodies was first established in sex-mismatched HCT.
Alloantibodies were first detected by Miklos et al. studying F → M HCT targeting H-Y antigen DEAD box helicase 3, Y-linked (DBY) using enzyme-linked immunosorbent assay (ELISA). His study of 150 patients using H-Y ELISA found that H-Y antibodies are ten times more frequent in F → M patients compared to M → M patients (50 % of F → M patients as opposed to only 5 % in M → M patients) [2].
Subsequently, clinical studies have shown that H-Y antibodies detected a year post-HCT associate with cGVHD development and long-term disease remission (Table 1) [49–51]. Characterizing 75 F → M patients, seropositivity against any of five H-Y antigens was significantly associated with cGVHD development (OR 56.5, p < 0.0001). Furthermore, none of the H-Y seropositive patients relapsed compared to 48 % relapse in H-Y seronegative patients [46].
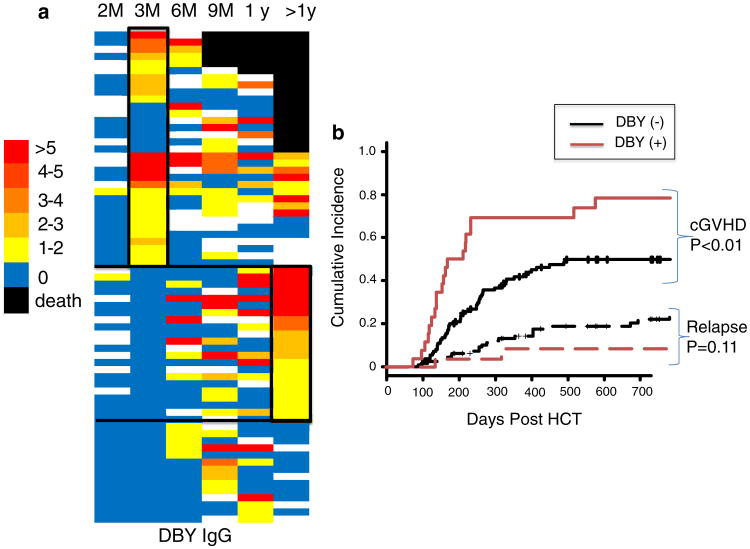
Although ELISA could effectively characterize H-Y antibody responses, more sensitive technologies have been developed. Protein microarray technology, for example, facilitates high-throughput, ultra-sensitive, multiplexed antibody detection which is considered to be more sensitive than ELISA [52, 53]. As an example of serologic utility, Fig. 1 shows H-Y microarray detected DBY antibodies in 136 F → M HCT patients measured prospectively over 3 years. In this study, 51 % of F → M patients are DBY seropositive at some point post-HCT (Fig. 1a). DBY antibody is absent 2 months post-HCT and detected in 19 % of F → M patients at 3 months post-HCT and further develops in association with cGVHD onset (Fig. 1b) (Nakasone Unpublished Data).
Fig. 1.
DBY seropositivity measured 3 months post-HCT predicts cGVHD development. a Heatmap representation of IgG specific for H-Y antigen DBY in 69 seropositive F → M patients. 67 seronegative patients are not shown. Overall, DBY seropositivity at 3 months was 19 and 51 % at any time point following HCT. Each row represents the results of a separate patient with the time of serum collection shown on the x-axis. The threshold for seropositivity was determined measuring 60 normal male donors. The heat reflects relative DBY antibody level relative to this threshold. Importantly, patient death is denoted by black, and missing samples are white. b The competing incidence of cGVHD development is greater in DBY seropositive patients than in DBY seronegative patients (p < 0.01)
Microarray technology has also led to the discovery of immune-dominant epitopes through the multiplexed testing of “tiled” overlapping peptides across multiple H-Y antigens. This led to the discovery of the 18 amino acid DBY-2 peptide as an immune-dominant epitope. Using fluorescence-activated cell sorting (FACS), this epitope was used to identify H-Y-specific B cells. Identifying H-Y-specific B cells should provide earlier alloimmunity detection relative to H-Y antibodies. For example, a prospective analysis of DBY-2-specific B cells in a study of 28 F → M patients confirmed that allogeneic B cells preceded cGVHD development [54].
The identification of allogeneic B-cell responses in association with cGVHD supported the use of in vivo B-cell depletion for cGVHD prevention and treatment. In vivo B-cell depletion has been safely accomplished with rituximab, a humanized monoclonal antibody against B-surface antigen CD20. Clinical trials using rituximab have confirmed that B-cell depletion therapy is both effective cGVHD therapy and prophylaxis [55–59].
In the field of HCT, measured alloimmune response to H-Y antigens has been an important biomarker associated with significant clinical outcomes including cGVHD and disease relapse. While there are a variety of autosomal mHAs which have been shown to be targeted by lymphocytes, their low disparity rates limit their clinical usefulness [60, 61].
From T cells to antibodies and now B cells, the targets of H-Y alloimmunity have been molecularly identified allowing for their disease impact assessment (Table 3). Therefore, H-Y antigens remain the most powerful model to better understand the effect of alloimmunity in HCT.
Table 3. Significant studies regarding discovery of T-cell and B-cell response to H-Y antigens.
| Significant result | Conclusion | References |
|---|---|---|
| H-Y factor | ||
| Skin graft rejection found only in M → F mice | Hypothesized “H-Y” factor on Y-chromosome that led to female sensitization | Eichwald et al. [9] |
| T-cell response | ||
| HLA-matched F → M patient who had experienced acute GVHD possessed HLA A2-restricted CTLs against unknown mHA | First demonstration of sex-mismatched CTL against unknown H-Y antigen | Goulmy et al. [22] |
| Isolated and sequenced the first H-Y antigen from SMCY using CTL identification | Biochemical identification of first H-Y antigen | Wang et al. [28] |
| Quantification of HLA-A2-specific T cells in patient with GVHD using SMCY-restricted tetramers | Tetramer complex allowed visualization of H-Y-specific CTL and showed association between H-Y-specific CTL and GVHD | Mutis et al. [29] |
| B-cell response | ||
| 50 % of F → M patients found to be seropositive for the H-Y antigen DBY | F → M patients develop H-Y-specific antibodies post-HCT | Miklos et al. [2] |
| F → M patient with cGVHD found to have both CTL and antibodies specific for DBY | First demonstration of a coordinated B-cell and T-cell response against H-Y antigens in the context of cGVHD | Zorn et al. [5] |
| H-Y seropositivity associated with increased cGVHD in a clinical study of 75 F → M patients. | H-Y antibodies in F → M patients demonstrate both increased cGVHD and decreased relapse | Miklos et al. [46] |
| Quantification and isolation of H-Y-specific B cells using immune-dominant epitope DBY-2 | DBY-2-specific B cells are detected in 50 % of F → M patients and nearly all develop cGVHD | Sahaf et al. [54] |
M → F graft from male donor to female recipient, F → M graft from female donor to male recipient, GVHD graft-versus-host disease, mHA minor histocompatibility antigen, CTL cytotoxic T lymphocyte, HCT hematopoietic cell transplantation
Parity in HCT donors
Mothers with previous male births may become sensitized to the male fetus and develop H-Y alloimmunity leading to subsequent miscarriages. Studies have shown the persistence of H-Y-specific T cells in parous female donors who had given birth to boys many years previously [62, 63]. For example, a study found H-Y-specific CTLs in 50 % of female donors with multiple male pregnancies [64]. These studies support the notion that multiparous females are likely to induce an alloimmune H-Y response with an impact on pregnancy and transplantation. Thus, female donors with high parity may put male recipients at higher risk of GVHD due to H-Y alloimmunity (Table 1).
Consistent with the H-Y hypothesis, studies have shown that F → M patients with multiparous female donors have a higher rate of cGVHD and a lower rate of relapse relative to nulliparous female donors [20, 21]. However, it is important to note that there is still a much larger difference in outcomes between F → M patients with nulliparous female donors and M → M patients than F → M patients with nulliparous female donors and F → M patients with multiparous female donors, thus suggesting that the large naïve repertoire due to gender disparity plays a larger role in GVHD development than H-Y sensitization from pregnancy. Ultimately, studies measuring H-Y antibodies and T cells found within multiparous female donors are necessary to characterize adoptive H-Y alloimmunity and its clinical impact.
H-Y alloimmunity in kidney transplantation
HLA-matched kidney transplants survive longer on average than mismatched ones [65]. Studies have shown that donor-specific antibodies (DSA) including anti-HLA antibodies associate with both acute and chronic kidney rejection [66–70]. When HLA-matched organs are transplanted, significant levels of kidney graft rejection still occur [71]. For example, in an analysis of the UNOS Renal Transplant Registry, Terasaki estimated that 38 % of the kidney failures in the transplant registry were due to non-HLA immunogenic factors [72, 73]. This analysis supports the conclusion that kidney rejections in the presence of HLA-matched donors most likely represented other immunologic factors, including mHAs. Furthermore, biopsies of kidneys undergoing rejection show high levels of C4d, a component of the complement cascade, thus implicating pathogenic B cells and antibodies in kidney graft rejection [74–76]. Not surprisingly, H-Y alloimmunity is believed to contribute to kidney graft rejection in HLA-matched, sex-mismatched patients.
Sex-mismatch kidney transplantation and kidney graft rejection
Although the mechanism for increased graft rejection is not fully understood, the H-Y model suggests that host lymphocytes from the female recognize several mHAs such as H-Y proteins on the male graft as foreign, thus leading to decreased engraftment and increased rates of kidney graft rejection. As opposed to F → M HCT in which a functional immune system from the female donor is transferred into the male recipient, M → F solid organ transplantation involves the transfer of an immune system target (the kidney graft) from the male host to the female recipient, which explains why M → F patients were found to have higher rates of kidney graft rejection.
Unlike HCT where most studies agree that F → M transplants result in more cGVHD, the impact of sex mismatch in organ transplantation is less clear. While some studies have shown that M → F patients have increased rates of both acute and chronic graft failure [6], others have only shown significantly increased rates of acute graft failure [77–79] or no difference at all [80]. Although there are many risk factors responsible for kidney failure, these studies suggest that alloimmunity against mHAs such as H-Y antigens may be a major cause of rejection.
H-Y antibodies and acute kidney rejection
Some researchers have investigated H-Y antigens as a potential risk factor for the development of acute kidney failure post-transplant. In a study of 26 M → F kidney transplant patients, it was shown that 54 % of these patients developed H-Y antibodies, higher than any other gender combination (p < 0.001). Furthermore, 92 % of these M → F patients developed acute rejection compared to 21 % of those who were H-Y seronegative. The study also showed that those who developed H-Y antibodies were found to have higher amount of plasma cell infiltrates on renal biopsy, thus further implicating B-cell pathogenesis [7].
Similar to studies in kidney transplantation, sex mismatch in other solid organ transplantation has shown mixed clinical outcomes. However, there does tend to be an increased risk of graft rejection in cardiac transplantation in particular [81–84] and limited evidence for increases in chronic liver rejection in sex-mismatched patients [85].
H-Y alloimmunity in secondary recurrent miscarriage
Pregnancy is a natural phenomenon which has very interesting qualities in regard to immunology. Considering that a fetus is only half identical to the mother, it is reasonable to interpret pregnancy as a time when a haploidentical fetus resides in the mother's body [86, 87]. Consequentially, pregnancy has become an intriguing field for the study of H-Y alloimmunity. In particular, H-Y alloimmunity has provided one potential explanation for secondary recurrent miscarriage (SRM).
SRM is defined as three or more recurrent miscarriages after a successful birth. It is important to contrast SRM from primary recurrent miscarriage (PRM), in which three or more miscarriages occur without a preceding successful birth. PRMs are believed to be caused primarily by chromosomal abnormalities along with problems with proper implantation of the embryo, while SRMs are more likely to be the result of immunological causes [8].
Interestingly, most SRM patients initially give birth to a boy prior to the recurrent miscarriages [88]. Furthermore, mothers who initially gave birth to boys are also more likely to experience infertility and future miscarriages [89]. In fact, studies have shown that the sex ratio [defined as the ratio of male/female (M/F) births] in SRM patients is shifted toward a significantly higher M/F sex ratio prior to SRM and then shifted to a lower M/F sex ratio for successful births subsequent to SRM [90]. For example, in a 20-year cohort study of unexplained SRMs, the sex ratio for SRM was 1.49 previous to miscarriages and 0.76 in births subsequent to miscarriages [91].
The H-Y hypothesis suggests that, during pregnancy with a male fetus, the mother's immune system develops an alloimmune response against these H-Y antigens, which would harm future male fetuses and potentially contribute to future miscarriages [8, 92, 93].
H-Y antibodies, along with the presence of H-Y restricted HLA alleles, have been associated with the development of SRM along with other pregnancy complications [90, 94–96]. One study of 84 SRM patients found that H-Y-specific antibodies were present in a significantly higher percentage of SRM patients (46 %) compared to female controls (19 %). This study also found that H-Y seropositive patients only delivered boys 12 % of the time compared to 44 % of the time in H-Y seronegative patients [97]. Thus, measured H-Y seropositivity may be preventing implantation or successful gestation of the male fetus.
Consistent with the idea that H-Y antibodies might be responsible for SRM, intravenous immunoglobulin (IVIG) infusion, a treatment used normally to neutralize circulating antibodies, has been shown to increase the birth rate in SRM patients [98–100] while others have failed to show a significant difference [101]. Nevertheless, immunosuppressive therapies continue to be investigated in this patient population.
Many investigations aimed to understand why only some mothers with previous male births develop H-Y antibodies and others do not [93]. Clinical trials implicate both regulatory T cells and CTLs to be involved in developing tolerance to mHA [62, 64, 102–104]. Murine studies have shown that depletion of H-Y-specific regulatory T cells in pregnant mice resulted in rejection of male fetuses [104].
Future of H-Y alloimmunity
Although H-Y antigens were first discovered in the 1970s, ongoing studies continue to identify clinically important T-and B-cell epitopes. A contemporary challenge in understanding H-Y alloimmunity is determining the progression and coordination of T- and B-cell alloimmunity. These adaptive immune responses are now being directly measured using next-generation high-throughput sequencing of the B- and T-cell receptors.
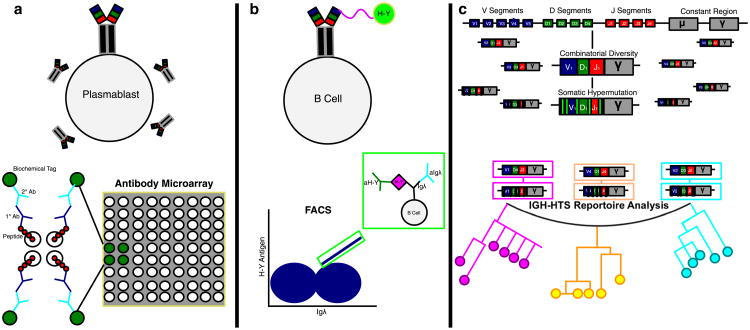
The recent identification of immune-dominant B-cell epitopes now makes it possible to use FACS to sort H-Y antigen-specific B cells for functional studies. This is analogous to the use of mHA tetramers to isolate and characterize H-Y-specific T cells in the late 1990s. The combination of antigen-specific cell sorting and immune receptor high-throughput sequencing is going to allow us to detail the evolution of adaptive alloimmunity (Fig. 2).
Fig. 2.
Methods for studying H-Y humoral alloimmunity. a H-Y-specific alloantibodies are quantified by protein microarray. Their immune-dominant epitopes are identified using overlapping peptides from H-Y proteins. b These immune-dominant peptides facilitate the identification of H-Y-specific B cells by fluorescence-activated cell sorting (FACS). c The B-cell receptor-binding specificity can be further elucidated by high-throughput sequencing of the immunoglobulin chain which can be used to characterize both heavy- and light-chain repertoire. The evolution of the changes in immunoglobin clonotypes over time is represented by the phylogeny tree
With the advent of these new technologies, there is potential to advance our understanding of alloimmunity and improve our ability to predict alloimmune clinical phenomenon in transplantation and pregnancy, thus allowing for more effective immune modulation therapies. Furthermore, these same technologies hold the promise to help us understand more about the various other mHAs implicated in alloimmunity in both sex-matched and sex-mismatched transplantation.
Since the study of murine skin graft rejection in the 1950s, H-Y antigens have provided an essential model for studying alloimmunity in a variety of clinical settings. As discussed in this review, our understanding of H-Y alloimmunity has progressed significantly from their initial discovery as T-cell-specific targets. Furthermore, H-Y alloimmunity has expanded beyond HCT to solid organ transplantation and pregnancy and will continue to be the driving model of clinical alloimmunity.
Acknowledgments
We would like to thank Fang Wu and John Coller, Director of the Stanford Functional Genomics Facility, for their technical assistance, along with Carl Grumet and Joanne Otani for their helpful comments and suggestions. This work was supported by National Heart Lung and Blood Institute Grant R21 HL084318, National Cancer Institute Grant P01 CA049605 and Stanford University Cancer Institute Support Grant 1P030CA124435-01.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Brickner AG. Mechanisms of minor histocompatibility antigen immunogenicity: the role of infinitesimal versus structurally profound polymorphisms. Immunol Res. 2006;36(1–3):33–41. doi: 10.1385/IR:36:1:33. [DOI] [PubMed] [Google Scholar]
- 2.Miklos DB, Kim HT, Zorn E, et al. Antibody response to DBY minor histocompatibility antigen is induced after allogeneic stem cell transplantation and in healthy female donors. Blood. 2004;103(1):353–9. doi: 10.1182/blood-2003-03-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arai S, Jagasia M, Storer B, et al. Global and organ-specific chronic graft-versus-host disease severity according to the 2005 NIH Consensus Criteria. Blood. 2011;118(15):4242–9. doi: 10.1182/blood-2011-03-344390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlens S, Ringdén O, Remberger M, et al. Risk factors for chronic graft-versus-host disease after bone marrow transplantation: a retrospective single centre analysis. Bone Marrow Transplant. 1998;22(8):755–61. doi: 10.1038/sj.bmt.1701423. [DOI] [PubMed] [Google Scholar]
- 5.Zorn E, Miklos DB, Floyd BH, et al. Minor histocompatibility antigen DBY elicits a coordinated B and T cell response after allogeneic stem cell transplantation. J Exp Med. 2004;199(8):1133–42. doi: 10.1084/jem.20031560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gratwohl A, Döhler B, Stern M, Opelz G. H-Y as a minor histocompatibility antigen in kidney transplantation: a retrospective cohort study. Lancet. 2008;372(9632):49–53. doi: 10.1016/S0140-6736(08)60992-7. [DOI] [PubMed] [Google Scholar]
- 7.Tan JC, Wadia PP, Coram M, et al. H-Y antibody development associates with acute rejection in female patients with male kidney transplants. Transplantation. 2008;86(1):75–81. doi: 10.1097/TP.0b013e31817352b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nielsen HS. Secondary recurrent miscarriage and H-Y immunity. Hum Reprod Update. 2011;17(4):558–74. doi: 10.1093/humupd/dmr005. [DOI] [PubMed] [Google Scholar]
- 9.Eichwald EJ, Silmser CR. Skin. Transplant Bull. 1955;2:148–9. [PubMed] [Google Scholar]
- 10.Eichwald EJ, Silmser CR, Wheeler N. The genetics of skin grafting. Ann NY Acad Sci. 1957;64(5):737–40. doi: 10.1111/j.1749-6632.1957.tb52469.x. [DOI] [PubMed] [Google Scholar]
- 11.Lustgraaf EC, Fuson RB, Eichwald EJ. Sex tolerance and split tolerance. Transplant Bull. 1960;26:145–50. doi: 10.1097/00006534-196007000-00050. [DOI] [PubMed] [Google Scholar]
- 12.Eichwald EJ, Lustgraaf EC, Weissman I, Strainer M. Attempts to demonstrate sex-linked histocompatibility genes. Transplant Bull. 1958;5(4):387–8. doi: 10.1097/00006534-195810000-00036. [DOI] [PubMed] [Google Scholar]
- 13.Billingham RE, Silvers WK. Induction of tolerance of skin isografts from male donors in female mice. Science. 1958;128(3327):780–1. doi: 10.1126/science.128.3327.780. [DOI] [PubMed] [Google Scholar]
- 14.Billingham RE, Silvers WK. Studies on tolerance of the Y chromosome antigen in mice. J Immunol. 1960;85:14–26. [PubMed] [Google Scholar]
- 15.Carlens S, Ringdén O, Remberger M, et al. Factors affecting risk of relapse and leukemia-free survival in HLA-identical sibling marrow transplant recipients with leukemia. Transplant Proc. 1997;29(7):3147–9. doi: 10.1016/s0041-1345(97)81731-9. [DOI] [PubMed] [Google Scholar]
- 16.Remberger M, Kumlien G, Aschan J, et al. Risk factors for moderate-to-severe chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2002;8(12):674–82. doi: 10.1053/bbmt.2002.v8.abbmt080674. [DOI] [PubMed] [Google Scholar]
- 17.Remberger M, Mattsson J, Hassan Z, et al. Risk factors for acute graft-versus-host disease grades II–IV after reduced intensity conditioning allogeneic stem cell transplantation with unrelated donors: a single centre study. Bone Marrow Transplant. 2008;41(4):399–405. doi: 10.1038/sj.bmt.1705913. [DOI] [PubMed] [Google Scholar]
- 18.Spierings E, Kim YH, Hendriks M, et al. Multicenter analyses demonstrate significant clinical effects of minor histocompatibility antigens on GvHD and GvL after HLA-matched related and unrelated hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(8):1244–53. doi: 10.1016/j.bbmt.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Randolph SS, Gooley TA, Warren EH, Appelbaum FR, Riddell SR. Female donors contribute to a selective graft-versus-leukemia effect in male recipients of HLA-matched, related hematopoietic stem cell transplants. Blood. 2004;103(1):347–52. doi: 10.1182/blood-2003-07-2603. [DOI] [PubMed] [Google Scholar]
- 20.Kollman C, Howe CW, Anasetti C, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98(7):2043–51. doi: 10.1182/blood.v98.7.2043. [DOI] [PubMed] [Google Scholar]
- 21.Loren AW, Bunin GR, Boudreau C, et al. Impact of donor and recipient sex and parity on outcomes of HLA-identical sibling allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12(7):758–69. doi: 10.1016/j.bbmt.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Goulmy E, Termijtelen A, Bradley BA, van Rood JJ. Y-antigen killing by T cells of women is restricted by HLA. Nature. 1977;266(5602):544–5. doi: 10.1038/266544a0. [DOI] [PubMed] [Google Scholar]
- 23.Goulmy E, Bradley BA, Lansbergen Q, van Rood JJ. The importance of H-Y incompatibility in human organ transplantation. Transplantation. 1978;25(6):315–9. doi: 10.1097/00007890-197806000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Goulmy E, Gratama JW, Blokland E, Zwaan FE, van Rood JJ. A minor transplantation antigen detected by MHC-restricted cytotoxic T lymphocytes during graft-versus-host disease. Nature. 1983;302(5904):159–61. doi: 10.1038/302159a0. [DOI] [PubMed] [Google Scholar]
- 25.Goulmy E, van Leeuwen A, Blokland E, Sachs ES, Geraedts JP. The recognition of abnormal sex chromosome constitution by HLA-restricted anti-H-Y cytotoxic T cells and antibody. Immunogenetics. 1983;17(5):523–31. doi: 10.1007/BF00696875. [DOI] [PubMed] [Google Scholar]
- 26.Goulmy E, Schipper R, Pool J, et al. Mismatches of minor histocompatibility antigens between HLA-identical donors and recipients and the development of graft-versus-host disease after bone marrow transplantation. N Engl J Med. 1996;334(5):281–5. doi: 10.1056/NEJM199602013340501. [DOI] [PubMed] [Google Scholar]
- 27.Goulmy E. Human minor histocompatibility antigens. Curr Opin Immunol. 1996;8(1):75–81. doi: 10.1016/s0952-7915(96)80108-7. [DOI] [PubMed] [Google Scholar]
- 28.Wang W, Meadows LR, den Haan JM, et al. Human H-Y: a male-specific histocompatibility antigen derived from the SMCY protein. Science. 1995;269(5230):1588–90. doi: 10.1126/science.7667640. [DOI] [PubMed] [Google Scholar]
- 29.Mutis T, Gillespie G, Schrama E, Falkenburg JH, Moss P, Goulmy E. Tetrameric HLA class I-minor histocompatibility antigen peptide complexes demonstrate minor histocompatibility antigen-specific cytotoxic T lymphocytes in patients with graft-versus-host disease. Nat Med. 1999;5(7):839–42. doi: 10.1038/10563. [DOI] [PubMed] [Google Scholar]
- 30.Tilford CA, Kuroda-Kawaguchi T, Skaletsky H, et al. A physical map of the human Y chromosome. Nature. 2001;409(6822):943–5. doi: 10.1038/35057170. [DOI] [PubMed] [Google Scholar]
- 31.Skaletsky H, Kuroda-Kawaguchi T, Minx PJ, et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature. 2003;423(6942):825–37. doi: 10.1038/nature01722. [DOI] [PubMed] [Google Scholar]
- 32.Lahn BT, Page DC. Functional coherence of the human Y chromosome. Science. 1997;278(5338):675–80. doi: 10.1126/science.278.5338.675. [DOI] [PubMed] [Google Scholar]
- 33.Eljaafari A, Yuruker O, Ferrand C, et al. Isolation of human CD4/CD8 double-positive, graft-versus-host disease-protective, minor histocompatibility antigen-specific regulatory T cells and of a novel HLA-DR7-restricted HY-specific CD4 clone. J Immunol. 2013;190(1):184–94. doi: 10.4049/jimmunol.1201163. [DOI] [PubMed] [Google Scholar]
- 34.Warren EH, Gavin MA, Simpson E, et al. The human UTY gene encodes a novel HLA-B8-restricted H-Y antigen. J Immunol. 2000;164(5):2807–14. doi: 10.4049/jimmunol.164.5.2807. [DOI] [PubMed] [Google Scholar]
- 35.Pennisi E. Long-sought H-Y antigen found. Science. 1995;269(5230):1515–6. doi: 10.1126/science.7667633. [DOI] [PubMed] [Google Scholar]
- 36.Vogt MH, de Paus RA, Voogt PJ, Willemze R, Falkenburg JH. DFFRY codes for a new human male-specific minor transplantation antigen involved in bone marrow graft rejection. Blood. 2000;95(3):1100–5. [PubMed] [Google Scholar]
- 37.Vogt MH, Goulmy E, Kloosterboer FM, et al. UTY gene codes for an HLA-B60-restricted human male-specific minor histocompatibility antigen involved in stem cell graft rejection: characterization of the critical polymorphic amino acid residues for T-cell recognition. Blood. 2000;96(9):3126–32. [PubMed] [Google Scholar]
- 38.Vogt MH, van den Muijsenberg JW, Goulmy E, et al. The DBY gene codes for an HLA-DQ5-restricted human male-specific minor histocompatibility antigen involved in graft-versus-host disease. Blood. 2002;99(8):3027–32. doi: 10.1182/blood.v99.8.3027. [DOI] [PubMed] [Google Scholar]
- 39.Pierce RA, Field ED, den Haan JM, et al. Cutting edge: the HLA-A*0101-restricted HY minor histocompatibility antigen originates from DFFRY and contains a cysteinylated cysteine residue as identified by a novel mass spectrometric technique. J Immunol. 1999;163(12):6360–4. [PubMed] [Google Scholar]
- 40.Rosinski KV, Fujii N, Mito JK, et al. DDX3Y encodes a class I MHC-restricted H-Y antigen that is expressed in leukemic stem cells. Blood. 2008;111(9):4817–26. doi: 10.1182/blood-2007-06-096313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spierings E, Vermeulen CJ, Vogt MH, et al. Identification of HLA class II-restricted H-Y-specific T-helper epitope evoking CD4+ T-helper cells in H-Y-mismatched transplantation. Lancet. 2003;362(9384):610–5. doi: 10.1016/S0140-6736(03)14191-8. [DOI] [PubMed] [Google Scholar]
- 42.Torikai H, Akatsuka Y, Miyazaki M, et al. A novel HLA-A*3303-restricted minor histocompatibility antigen encoded by an unconventional open reading frame of human TMSB4Y gene. J Immunol. 2004;173(11):7046–54. doi: 10.4049/jimmunol.173.11.7046. [DOI] [PubMed] [Google Scholar]
- 43.Ofran Y, Kim HT, Brusic V, et al. Diverse patterns of T-cell response against multiple newly identified human Y chromosome–encoded minor histocompatibility epitopes. Clin Cancer Res. 2010;16(5):1642–51. doi: 10.1158/1078-0432.CCR-09-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ivanov R, Aarts T, Hol S, et al. Identification of a 40S ribosomal protein S4-derived H-Y epitope able to elicit a lymphoblast-specific cytotoxic T lymphocyte response. Clin Cancer Res. 2005;11(5):1694–703. doi: 10.1158/1078-0432.CCR-04-1772. [DOI] [PubMed] [Google Scholar]
- 45.Mortensen BK, Rasmussen AH, Larsen ME, et al. Identification of a novel UTY-encoded minor histocompatibility antigen. Scand J Immunol. 2012;76(2):141–50. doi: 10.1111/j.1365-3083.2012.02708.x. [DOI] [PubMed] [Google Scholar]
- 46.Miklos DB, Kim HT, Miller KH, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105(7):2973–8. doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Porcheray F, Miklos DB, Floyd BH, et al. Combined CD4 T-cell and antibody response to human minor histocompatibility antigen DBY after allogeneic stem-cell transplantation. Transplantation. 2011;92(3):359–65. doi: 10.1097/TP.0b013e3182244cc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Veerapathran A, Pidala J, Beato F, et al. Human regulatory T cells against minor histocompatibility antigens: ex vivo expansion for prevention of graft-versus-host disease. Blood. 2013;122(13):2251–61. doi: 10.1182/blood-2013-03-492397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stern M, Brand R, de Witte T, et al. Female-versus-male alloreactivity as a model for minor histocompatibility antigens in hematopoietic stem cell transplantation. Am J Transplant. 2008;8(10):2149–57. doi: 10.1111/j.1600-6143.2008.02374.x. [DOI] [PubMed] [Google Scholar]
- 50.Markiewicz M, Siekiera U, Karolczyk A, et al. Immunogenic disparities of 11 minor histocompatibility antigens (mHAs) in HLA-matched unrelated allogeneic hematopoietic SCT. Bone Marrow Transplant. 2009;43(4):293–300. doi: 10.1038/bmt.2008.326. [DOI] [PubMed] [Google Scholar]
- 51.Markiewicz M, Siekiera U, Dzierzak-Mietla M, Zielinska P, Kyrcz-Krzemien S. The impact of H-Y mismatches on results of HLA-matched unrelated allogeneic hematopoietic stem cell transplantation. Transplant Proc. 2010;42(8):3297–300. doi: 10.1016/j.transproceed.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 52.Li L, Wadia P, Chen R, et al. Identifying compartment-specific non-HLA targets after renal transplantation by integrating transcriptome and “antibodyome” measures. Proc Natl Acad Sci USA. 2009;106(11):4148–53. doi: 10.1073/pnas.0900563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wadia PP, Sahaf B, Miklos DB. Recombinant antigen micro-arrays for serum/plasma antibody detection. Methods Mol Biol. 2011;723:81–104. doi: 10.1007/978-1-61779-043-0_7. [DOI] [PubMed] [Google Scholar]
- 54.Sahaf B, Yang Y, Arai S, Herzenberg LA, Miklos DB. H-Y antigen-binding B cells develop in male recipients of female hematopoietic cells and associate with chronic graft vs. host disease. Proc Natl Acad Sci USA. 2013;110(8):3005–10. doi: 10.1073/pnas.1222900110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cutler C, Miklos D, Kim HT, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108(2):756–62. doi: 10.1182/blood-2006-01-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zaja F, Bacigalupo A, Patriarca F, et al. Treatment of refractory chronic GVHD with rituximab: a GITMO study. Bone Marrow Transplant. 2007;40(3):273–7. doi: 10.1038/sj.bmt.1705725. [DOI] [PubMed] [Google Scholar]
- 57.Kharfan-Dabaja MA, Mhaskar AR, Djulbegovic B, Cutler C, Mohty M, Kumar A. Efficacy of rituximab in the setting of steroid-refractory chronic graft-versus-host disease: a systematic review and meta-analysis. Biol Blood Marrow Transplant. 2009;15(9):1005–13. doi: 10.1016/j.bbmt.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 58.Arai S, Sahaf B, Narasimhan B, et al. Prophylactic rituximab after allogeneic transplantation decreases B-cell alloimmunity with low chronic GVHD incidence. Blood. 2012;119(25):6145–54. doi: 10.1182/blood-2011-12-395970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cutler C, Kim HT, Bindra B, et al. Rituximab prophylaxis prevents corticosteroid-requiring chronic GVHD after allogeneic peripheral blood stem cell transplantation: results of a phase 2 trial. Blood. 2013;122(8):1510–7. doi: 10.1182/blood-2013-04-495895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dzierzak-Mietla M, Markiewicz M, Siekiera U, et al. Occurrence and impact of minor histocompatibility antigens' disparities on outcomes of hematopoietic stem cell transplantation from HLA-matched sibling donors. Bone Marrow Res. 2012;2012:257086. doi: 10.1155/2012/257086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hobo W, Broen K, van der Velden WJ, et al. Association of disparities in known minor histocompatibility antigens with relapse-free survival and graft-versus-host disease after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(2):274–82. doi: 10.1016/j.bbmt.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verdijk RM, Kloosterman A, Pool J, et al. Pregnancy induces minor histocompatibility antigen-specific cytotoxic T cells: implications for stem cell transplantation and immunotherapy. Blood. 2004;103(5):1961–4. doi: 10.1182/blood-2003-05-1625. [DOI] [PubMed] [Google Scholar]
- 63.James E, Chai JG, Dewchand H, Macchiarulo E, Dazzi F, Simpson E. Multiparity induces priming to male-specific minor histocompatibility antigen, HY, in mice and humans. Blood. 2003;102(1):388–93. doi: 10.1182/blood-2002-10-3170. [DOI] [PubMed] [Google Scholar]
- 64.Piper KP, McLarnon A, Arrazi J, et al. Functional HY-specific CD8+ T cells are found in a high proportion of women following pregnancy with a male fetus. Biol Reprod. 2007;76(1):96–101. doi: 10.1095/biolreprod.106.055426. [DOI] [PubMed] [Google Scholar]
- 65.Takemoto SK, Terasaki PI, Gjertson DW, Cecka JM. Twelve years' experience with national sharing of HLA-matched cadaveric kidneys for transplantation. N Engl J Med. 2000;343(15):1078–84. doi: 10.1056/NEJM200010123431504. [DOI] [PubMed] [Google Scholar]
- 66.Cecka JM, Zhang Q, Reed EF. Preformed cytotoxic antibodies in potential allograft recipients: recent data. Hum Immunol. 2005;66(4):343–9. doi: 10.1016/j.humimm.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 67.Clatworthy MR. Targeting B cells and antibody in transplantation. Am J Transplant. 2011;11(7):1359–67. doi: 10.1111/j.1600-6143.2011.03554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jordan SC, Kahwaji J, Toyoda M, Vo A. B-cell immunotherapeutics: emerging roles in solid organ transplantation. Curr Opin Organ Transplant. 2011;16(4):416–24. doi: 10.1097/MOT.0b013e32834874f7. [DOI] [PubMed] [Google Scholar]
- 69.Platt JL, Cascalho M. Donor specific antibodies after transplantation. Pediatr Transplant. 2011;15(7):686–90. doi: 10.1111/j.1399-3046.2010.01436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Rood JJ, van Leeuwen A, Persijn GG, et al. Role of the HLA system in transplantation. HLA compatibility in clinical transplantation. Transplant Proc. 1977;9(1):459–67. [PubMed] [Google Scholar]
- 71.Bestard O, Cruzado JM, la Franquesa M, Grinyó JM. Bio-markers in renal transplantation. Curr Opin Organ Transplant. 2010;15(4):467–73. doi: 10.1097/MOT.0b013e32833b9ccb. [DOI] [PubMed] [Google Scholar]
- 72.Terasaki PI. Deduction of the fraction of immunologic and non-immunologic failure in cadaver donor transplants. Clin Transpl. 2003:449–452. [PubMed] [Google Scholar]
- 73.Cecka JM. The OPTN/UNOS Renal Transplant Registry. Clin Transpl. 2005:1–16. [PubMed] [Google Scholar]
- 74.Stegall MD, Chedid MF, Cornell LD. The role of complement in antibody-mediated rejection in kidney transplantation. Nat Rev Nephrol. 2012;8(11):670–8. doi: 10.1038/nrneph.2012.212. [DOI] [PubMed] [Google Scholar]
- 75.Cornell LD. Renal allograft pathology in the sensitized patient. Curr Opin Organ Transplant. 2013;18(3):327–36. doi: 10.1097/MOT.0b013e3283614c5a. [DOI] [PubMed] [Google Scholar]
- 76.Haas M. Pathology of C4d-negative antibody-mediated rejection in renal allografts. Curr Opin Organ Transplant. 2013;18(3):319–26. doi: 10.1097/MOT.0b013e32835d4daf. [DOI] [PubMed] [Google Scholar]
- 77.Kim SJ, Gill JS. H-Y incompatibility predicts short-term outcomes for kidney transplant recipients. J Am Soc Nephrol. 2009;20(9):2025–33. doi: 10.1681/ASN.2008101110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wagner S. H-Y antigen in kidney transplant: Does gender matter? Gend Med. 2012;9(5):387–8. doi: 10.1016/j.genm.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 79.Pfeffer PF, Thorsby E. HLA-restricted cytotoxicity against male-specific (H-Y) antigen after acute rejection of an HLA-identical sibling kidney: clonal distribution of the cytotoxic cells. Transplantation. 1982;33(1):52–6. doi: 10.1097/00007890-198201000-00011. [DOI] [PubMed] [Google Scholar]
- 80.Keith DS, Patrie JT. H-Y antigen incompatibility not associated with adverse immunologic graft outcomes: deceased donor pair analysis of the OPTN database. J Transplant. 2011;2011:148457. doi: 10.1155/2011/148457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khush KK, Kubo JT, Desai M. Influence of donor and recipient sex mismatch on heart transplant outcomes: analysis of the International Society for Heart and Lung Transplantation Registry. J Heart Lung Transplant. 2012;31(5):459–66. doi: 10.1016/j.healun.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kittleson MM, Patel JK, Moriguchi JD, et al. Heart transplant recipients supported with extracorporeal membrane oxygenation: outcomes from a single-center experience. J Heart Lung Transplant. 2011;30(11):1250–6. doi: 10.1016/j.healun.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 83.Kfoury AG, Hammond ME, Snow GL, et al. Cardiovascular mortality among heart transplant recipients with asymptomatic antibody-mediated or stable mixed cellular and antibody-mediated rejection. J Heart Lung Transplant. 2009;28(8):781–4. doi: 10.1016/j.healun.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 84.Welp H, Spieker T, Erren M, Scheld HH, Baba HA, Stypmann J. Sex mismatch in heart transplantation is associated with increased number of severe rejection episodes and shorter long-term survival. Transplant Proc. 2009;41(6):2579–84. doi: 10.1016/j.transproceed.2009.06.098. [DOI] [PubMed] [Google Scholar]
- 85.Candinas D, Gunson BK, Nightingale P, Hubscher S, McMaster P, Neuberger JM. Sex mismatch as a risk factor for chronic rejection of liver allografts. Lancet. 1995;346(8983):1117–21. doi: 10.1016/s0140-6736(95)91797-7. [DOI] [PubMed] [Google Scholar]
- 86.Thellin O, Heinen E. Pregnancy and the immune system: between tolerance and rejection. Toxicology. 2003;185(3):179–84. doi: 10.1016/s0300-483x(02)00607-8. [DOI] [PubMed] [Google Scholar]
- 87.Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann NY Acad Sci. 2011;1221:80–7. doi: 10.1111/j.1749-6632.2010.05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Christiansen OB, Steffensen R, Nielsen HS. The impact of anti-HY responses on outcome in current and subsequent pregnancies of patients with recurrent pregnancy losses. J Reprod Immunol. 2010;85(1):9–14. doi: 10.1016/j.jri.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 89.Christiansen OB, Pedersen B, Nielsen HS, Nybo Andersen AM. Impact of the sex of first child on the prognosis in secondary recurrent miscarriage. Hum Reprod. 2004;19(12):2946–51. doi: 10.1093/humrep/deh516. [DOI] [PubMed] [Google Scholar]
- 90.Christiansen OB, Steffensen R, Nielsen HS. Anti-HY responses in pregnancy disorders. Am J Reprod Immunol. 2011;66(Suppl 1):93–100. doi: 10.1111/j.1600-0897.2011.01038.x. [DOI] [PubMed] [Google Scholar]
- 91.Nielsen HS, Steffensen R, Lund M, et al. Frequency and impact of obstetric complications prior and subsequent to unexplained secondary recurrent miscarriage. Hum Reprod. 2010;25(6):1543–52. doi: 10.1093/humrep/deq091. [DOI] [PubMed] [Google Scholar]
- 92.Christiansen OB. Reproductive immunology. Mol Immunol. 2013;55(1):8–15. doi: 10.1016/j.molimm.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 93.Khosrotehrani K, Bianchi DW. Multi-lineage potential of fetal cells in maternal tissue: a legacy in reverse. J Cell Sci. 2005;118(Pt 8):1559–63. doi: 10.1242/jcs.02332. [DOI] [PubMed] [Google Scholar]
- 94.Linscheid C, Petroff MG. Minor histocompatibility antigens and the maternal immune response to the fetus during pregnancy. Am J Reprod Immunol. 2013;69(4):304–14. doi: 10.1111/aji.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nielsen HS, Mogensen M, Steffensen R, Kruse C, Christiansen OB. Indications of anti-HY immunity in recurrent placental abruption. J Reprod Immunol. 2007;75(1):63–9. doi: 10.1016/j.jri.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 96.Nielsen HS, Steffensen R, Varming K, et al. Association of HY-restricting HLA class II alleles with pregnancy outcome in patients with recurrent miscarriage subsequent to a firstborn boy. Hum Mol Genet. 2009;18(9):1684–91. doi: 10.1093/hmg/ddp077. [DOI] [PubMed] [Google Scholar]
- 97.Nielsen HS, Wu F, Aghai Z, et al. H-Y antibody titers are increased in unexplained secondary recurrent miscarriage patients and associated with low male: female ratio in subsequent live births. Hum Reprod. 2010;25(11):2745–52. doi: 10.1093/humrep/deq242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Christiansen OB, Pedersen B, Rosgaard A, Husth M. A randomized, double-blind, placebo-controlled trial of intravenous immunoglobulin in the prevention of recurrent miscarriage: evidence for a therapeutic effect in women with secondary recurrent miscarriage. Hum Reprod. 2002;17(3):809–16. doi: 10.1093/humrep/17.3.809. [DOI] [PubMed] [Google Scholar]
- 99.Christiansen OB, Nielsen HS, Pedersen B. Active or passive immunization in unexplained recurrent miscarriage. J Reprod Immunol. 2004;62(1–2):41–52. doi: 10.1016/j.jri.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 100.Hutton B, Sharma R, Fergusson D, et al. Use of intravenous immunoglobulin for treatment of recurrent miscarriage: a systematic review. BJOG. 2007;114(2):134–42. doi: 10.1111/j.1471-0528.2006.01201.x. [DOI] [PubMed] [Google Scholar]
- 101.Stephenson MD, Kutteh WH, Purkiss S, et al. Intravenous immunoglobulin and idiopathic secondary recurrent miscarriage: a multicentered randomized placebo-controlled trial. Hum Reprod. 2010;25(9):2203–9. doi: 10.1093/humrep/deq179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112(1):38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van Halteren AG, Jankowska-Gan E, Joosten A, et al. Naturally acquired tolerance and sensitization to minor histocompatibility antigens in healthy family members. Blood. 2009;114(11):2263–72. doi: 10.1182/blood-2009-01-200410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kahn DA, Baltimore D. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proc Natl Acad Sci USA. 2010;107(20):9299–304. doi: 10.1073/pnas.1003909107. [DOI] [PMC free article] [PubMed] [Google Scholar]