Abstract
Background/Aims
Herpes simplex virus (HSV) type I keratitis remains a leading cause of corneal morbidity, despite the availability of effective antiviral drugs. Improved understanding of virus-host interactions at the level of the host DNA damage response (DDR), a known factor in the development of HSV-1 keratitis, may shed light on potential new therapeutic targets. This report examines the role of checkpoint kinase 2 (Chk2), a DDR mediator protein, in corneal epithelial HSV-1 infection.
Methods
A small-molecule inhibitor of Chk2 (Chk2 inhibitor II) was applied to HSV-1-infected cultured human corneal epithelial cells (hTCEpi and HCE) as well as to explanted and organotypically cultured human and rabbit corneas. Infection levels were assessed by plaque assay and real-time PCR. RNAi-mediated depletion of Chk2 was performed to confirm the effect of the inhibitor.
Results
Inhibition of the Chk2 kinase activity greatly suppresses the cytopathic effect, genome replication and infectious progeny production in vitro and ex vivo.
Conclusion
This report demonstrates the critical role of Chk2 kinase in the establishment of HSV-1 corneal epithelial infection. These data contribute to our understanding of herpesvirus-host interactions and underscore the significance of DDR activation in HSV-1 keratitis.
Keywords: Herpes simplex virus type 1, Keratitis, Checkpoint kinase 2, Corneal epithelium, Explant cornea, DNA damage response, Small-molecule inhibitor
Introduction
Ocular infections with members of the Herpesviridae family of viruses cause substantial ophthalmic impact. Among the most recognized pathogens are herpes simplex virus (HSV) types 1 and 2, varicella zoster virus, cytomegalovirus and Epstein-Barr virus [1]. The alpha-subfamily (HSV-1/2 and VZV) is notoriously problematic in the cornea, where they manifest as recurrent painful disease after periodically emerging from latency in the trigeminal ganglia. HSV-1 in particular is known to cause infection in several ocular tissues, including the cornea, conjunctiva, uvea and even the retina. It is the most common cause of both cornea-derived and infection-associated blindness in developed countries. The prevalence of herpes keratitis (HK) among the US population is approximated at 500,000, with roughly 20,000 new cases annually [2, 3] . In the majority of cases, HK is effectively treated with oral acyclovir and/or topical nucleoside analogs, such as ganciclovir, trifluridine or valaciclovir. In spite of the effectiveness of these therapies, a number of patients develop refractory disease that may have sight-threatening consequences, such as permanent scarring, thinning and opacification of the cornea [4], necessitating corneal transplantation for vision restoration. Difficult cases most commonly develop due to the breakdown of the corneal immune privilege, leading to lymphocytic involvement of the stroma [4]; however, resistance to antiviral drugs is beginning to emerge as another cause of refractory disease [5–8].
While drug-resistant HSV strains are infrequently encountered in healthy patients, the immunocompromised population is at a significantly higher risk of developing resistant infection [9] . This is primarily due to the importance of adaptive immunity in promoting ganglionic latency of the virus [10] , but is also attributed to the diminished immune response at the site of infection [11], as evidenced by the fact that immunosuppressive corticosteroids used in stromal keratitis potentiate viral replication in the cornea [12, 13]. This issue is further compounded by multidrug resistance [14], since antiviral agents currently in use for HK treatment predominantly function through the same mechanism. Most of them are delivered as prodrugs that require an activating phosphorylation by the viral thymidine kinase (TK), which enables them to directly inhibit the DNA polymerase enzyme. Since the thymidine kinase is dispensable for viral replication, mutagenesis of this gene is the ideal mechanism of developing drug resistance, accounting for approximately 95% of clinical reports [15] . Mutations in the polymerase gene itself are more likely to be deleterious, making this only a minor mechanism of resistance.
The immunocompromised/immunosuppressed population is expanding due to such major contributors as HIV/AIDS, organ transplantation and cancer, but also many milder conditions, such as rheumatoid arthritis and inflammatory bowel disease etc. In light of this trend, effective management of drug-resistant HK in this growing population necessitates the exploration of novel antiviral targets. We have previously reported the identification of ataxia telangiectasia mutated (ATM), an apical kinase in the mammalian DNA damage response (DDR), as a potential antiviral target specifically in the context of HSV-1 keratitis [16]. The DDR in general, including ATM, is manipulated by many viruses in order to optimize replication conditions [17]. HSV-1 induces rapid and potent activation of ATM, which is a critical step in establishing productive infection [16, 18]. Neither the upstream mechanisms of ATM activation nor the downstream significance of this event are known. To provide improved understanding of this mechanism, the present work aims to determine whether checkpoint kinase 2 (Chk2), a known target of ATM [19], is a relevant downstream mediator involved in HSV-1 infection of corneal epithelium.
Chk2 is a multifunctional serine/threonine kinase that was originally named for its role in cell cycle regulation [20–24], but since then has been implicated in several other critical aspects of cellular biology, including chromatin architecture and remodeling [25] , apoptosis [26], transcription [27, 28], and DNA recombination and repair [29, 30]. In this study we used a highly specific small-molecule inhibitor of Chk2, Chk2 inhibitor II [31]. We demonstrate that Chk2 activation occurs very early in the course of HSV-1 infection, and that inhibition of Chk2 kinase activity potently suppresses viral replication in human corneal epithelial cells, as well as in organotypically explanted human and rabbit corneas. Our work identifies Chk2 as a relevant factor in HSV-1 corneal infection and warrants further investigation into the mechanisms underlying its involvement. In vivo studies and toxicity evaluation are necessary to assess the therapeutic relevance of these findings.
Materials and Methods
Cells and Viruses
All cells were cultured at 37°C and 5% CO2, and supplemented with 100 U/ml of penicillin and 100 μg/ml of streptomycin. Human corneal epithelial cells immortalized with hTERT (hTCEpi [32], a kind gift from Dr. James Jester, University of California, Irvine, Calif., USA) were grown in complete Keratinocyte Growth Medium-2 (KGM-2; Lonza, Basel, Switzerland). Human corneal epithelial cells immortalized with SV40 large T antigen (HCE [33], a kind gift from Dr. Peter Reinach, SUNY College of Optometry), as well as African green monkey kidney fibroblasts (CV-1 [34], American Type Culture Collection), were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS (Cellgro, Manassas, Va., USA). The KOS strain [35] of HSV-1 (a kind gift from Dr. Stephen Jennings, Drexel University College of Medicine) was used in all infections. All viral stocks were titered on CV-1 monolayers.
A tetracycline-inducible Chk2 knockdown cell line was derived by lentivirally transducing HCE cells with a construct harboring shRNA sequence against the Chk2 transcript. The construct was generated as previously described [36, 37] . The Chk2 shRNA sequence (NM_007194.2–1299s1c1; Sigma-Aldrich, St. Louis, Mo., USA) targets the following region: 5′-CGCCGTCCTTTGAATA-ACAAT-′. Lentiviral particles were produced in 293T packaging cells using a previously described method [38]. HCE cells were selected with neomycin after transduction, and knockdown induction was verified by Western blot. Chk2 was optimally knocked down after a 72-hour treatment with doxycycline (0.25 μg/ml).
Infection and Treatments of Cultured Cells
Cells were grown in 6-well plates and used in experiments at ~80% confluence. Drug treatments were administered 45 min prior to infection and continued for the entire duration of each experiment. Unless indicated otherwise, Chk2 inhibitor II (>98% purity by HPLC) was used at a 10-μM final concentration, and phosphonoacetic acid (PAA) at 400 μg/ml (both from Sigma-Aldrich). Chk2 inhibitor II was dissolved in dimethyl sulfoxide (DMSO) such that the final concentration of DMSO in both Chk2 inhibitor II and mock treatment was 0.1%. Infections with the KOS strain of HSV-1 were carried out in 6-well plates in a 200-μl inoculum volume at 37°C for 1 h with intermittent rocking. The cells were then thoroughly rinsed and overlaid with fresh medium.
Corneal Explant Model
Human corneas were obtained from the Lions Eye Bank of Delaware Valley. Rabbit corneas were excised from intact fresh eyeballs of young (8–12 weeks) albino rabbits (Pel-Freez Biologicals, Rogers, Ark., USA). The protocol established by Alekseev et al. [39] for ex vivo corneal culture and infection was followed, and treatment was administered immediately after infection. Briefly, corneoscleral buttons were excised and rinsed in PBS containing 200 U/ml of penicillin and 200 μg/ml of streptomycin. The endothelial concavity was filled with culture medium containing 1% low melting temperature agarose. The corneas were cultured epithelial side up in minimum essential medium supplemented with nonessential amino acids (1×), 2 mM of L -glutamate, 200 U/ml of penicillin and 200 μg/ml of streptomycin (Cellgro). The next day, they were infected with 1 × 10 4 PFU/cornea of strain KOS HSV-1 for 1 h, rinsed, and overlaid with fresh medium. Drug treatments were administered at the same concentrations as for cultured cells. The epithelial cell layer was collected by scraping the corneas for isolation of total DNA. For immunohistochemistry studies, corneas were flash-frozen in Tissue-Tek optimal cutting temperature compound (Sakura Finetek, Tokyo, Japan), sectioned, and immunostained using standard protocols.
Viral Replication
Total DNA from infected cells and corneas was isolated using the DNeasy Blood and Tissue Kit (QIAGEN, Hilden, Germany). Real-time quantitative PCR was performed with SYBR Green (Bio-Rad, Hercules, Calif., USA). Target primers for UL30 (DNA polymerase catalytic subunit) and reference primers for GAPDH were used to measure the viral genome abundance. Primer sequences were based on the KOS genome (accession No. JQ673480.1) and had been published previously. UL30 primers [40] (Fwd: AGAGGGACATCCAGGACTTTGT; Rev: CAGGCGCTTGTTGGTGTAC) produce a 74-bp amplicon, and GAPDH primers [41] (Fwd: GCTTGCCCTGTCCAGTTAAT; Rev: TAGCTCAGCTGCACCCTTTA) produce a 101-bp amplicon. All real-time PCR data were processed using the Pfaffl method [42], which yields relative template levels via the following equation:
Primer efficiencies (E) were calculated for both primer pairs. Melt peaks were examined for every reaction in every experiment, and reactions with aberrant melt peaks were excluded from calculations.
Immunohistochemistry
Corneas were flash-frozen in optimal cutting temperature compound, sectioned at a 10-μm thickness, dried, fixed in 3% paraformaldehyde/2% sucrose solution for 10 min, and permeabilized with 0.5% Triton X-100 (Sigma-Aldrich) for 5 min. Indirect immunofluorescence was performed with primary antibodies against cleaved caspase-3 (rabbit polyclonal; Cell Signaling, Danvers, Mass., USA). Nuclei were counterstained with 10 mg/ml Höchst 33258 (Sigma-Aldrich).
Western Blot
Standard protocol was followed for Western blot analysis. Cell lysates were collected in 200 μl of Laemmli buffer, vortexed, and boiled at 95°C for 5 min. Protein concentrations were measured by reducing agent-compatible BCA assay. SDS-PAGE was followed by transfer onto a PVDF membrane, which was then blocked in 5% BSA. Primary antibodies against the following proteins were used: nucleolin (mouse monoclonal; Santa Cruz Biotechnology, Santa Cruz, Calif., USA), ATM and pATM S1981 (rabbit polyclonal and mouse monoclonal, respectively; Rockland, Gilbertsville, Pa., USA), Chk2 and pChk2 T68 (rabbit polyclonal and mouse monoclonal, respectively; Cell Signaling). Blots were stained with secondary antibodies and visualized with the Odyssey near-infrared system (LI-COR, Lincoln, Nebr., USA).
Statistical Analysis
Statistical significance was determined using two-tailed unpaired Student’s t test and is indicated with: no significance (n.s.), *p < 0.05, **p < 0.01, or ***p < 0.001.
Results
Inhibition of Chk2 Suppresses HSV-1 Replication in Human Corneal Epithelial Cells
As previously reported by us [16] and others [18], the activating autophosphorylation of ATM (Ser 1981) and the subsequent activating phosphorylation of Chk2 (Thr 68) are detected within the first hour of HSV-1 infection (fig. 1a). In a control experiment, UV-inactivated virus failed to induce Chk2 phosphorylation (online suppl. fig. 1; see www.karger.com/doi/10.1159/000366228 for all online suppl. material), indicating that this is not an experimental artifact due to a contaminant present in the inoculum, but rather a direct consequence of HSV-1 infection. Li et al. [43] have previously shown that human colorectal carcinoma cells (HCT116) deficient in Chk2 expression are impaired in their ability to support productive HSV-1 infection compared to Chk2-expressing controls. In order to address this phenotype in nontumorigenic cells, we used two human corneal epithelial cell lines – hTCEpi and HCE – which are known to be contact-inhibited and are derived from healthy corneas. These cell lines were also chosen based on their different immortalization methods (hTERT and SV40 large T antigen, respectively) to exclude the possibility of immortalization-specific results. We infected subconfluent cells with HSV-1 at a relatively low multiplicity of infection (MOI 0.1) to imitate the physiological condition, and used a highly specific small-molecule inhibitor of Chk2, Chk2 inhibitor II, to assess the significance of this kinase during infection. We first performed dose optimization in hTCEpi cells, which confirmed the 10-μM concentration that is accepted in the literature (online suppl. fig. 2a). Treatment with this inhibitor almost completely eliminated the cytopathic effect (CPE) associated with HSV-1. CPE reduction was pronounced even past 20 hpi (hours postinfection; fig. 1b), a time point at which these cells undergo at least three rounds of reinfection. HSV-1-associated CPE in hTCEpi cells typically manifests as pronounced elongation of the cell body, which is later followed by rounding and detachment from the tissue culture plate.
Fig. 1.
Chk2 inhibition suppresses HSV-1 CPE in human corneal epithelial cells. a hTCEpi cells were infected with HSV-1 at MOI 5.0. Lysates were collected at the indicated time points and analyzed by Western blot with antibodies specific to the indicated proteins. pATM antibody detects autophosphorylation of ATM on Ser 1981, and pChk2 antibody detects its activation by phosphorylation on Thr 68 by ATM. Nucleolin is a loading control. b hTCEpi cells were infected at MOI 0.1 in the presence of Chk2 inhibitor II (10 μM ). Control cells were neither infected nor treated. Mock treatment (DMSO) and viral polymerase inhibitor (PAA, 400 μg/ml) were used as negative and positive treatment controls, respectively. Phase contrast images were taken at 20 hpi. A representative field is shown for each treatment. n = at least 5 independent experiments.
Fig. 2.
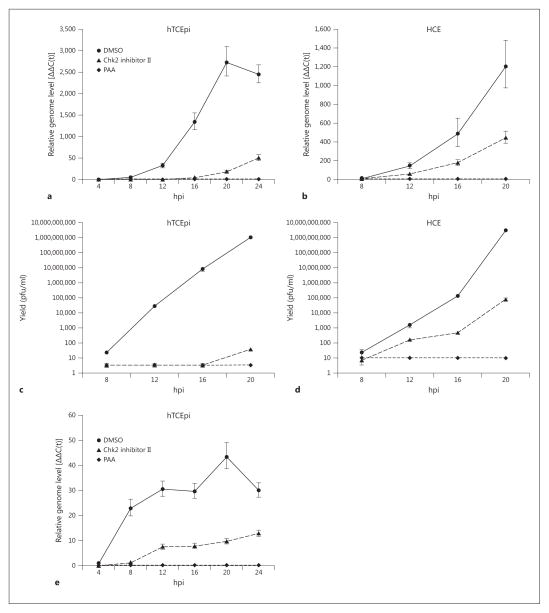
Chk2 inhibition suppresses HSV-1 infection in vitro. hTC-Epi and HCE cells were infected at MOI 0.1 in the presence of Chk2 inhibitor II (10 μM). Mock treatment (DMSO) and viral polymerase inhibitor (PAA, 400 μg/ml) were used as negative and positive treatment controls, respectively. Total DNA and medium supernatants were collected for analysis by qPCR with primers for the viral genome (a, b ) and plaque assay (c, d ). hTCEpi cells were infected at MOI 5.0 and treated in the same manner. Total DNA was analyzed by qPCR with primers for the viral genome (e). Bars represent average values ± SEM. n = 3 experimental replicates for all.
To obtain a quantitative measure of the antiviral effect of Chk2 inhibitor II, we used a qPCR assay to detect viral genomes in the treated monolayers. Inhibition of Chk2 profoundly reduced viral replication in both cell types (fig. 2a, b). Accordingly, this inhibitory effect was paralleled by a reduction in the generation of infectious viral particles in treated cells compared to controls, as measured by plaque assay (fig. 2c, d). To test the antiviral potency of Chk2 inhibitor II in a setting of heavy HSV-1 infection, we treated hTCEpi cells that had been infected at MOI 5, a viral load 50-fold higher than that used earlier. qPCR measurement of viral genome levels revealed a reduced yet still substantial decrease in replication associated with Chk2 inhibition (fig. 2e).
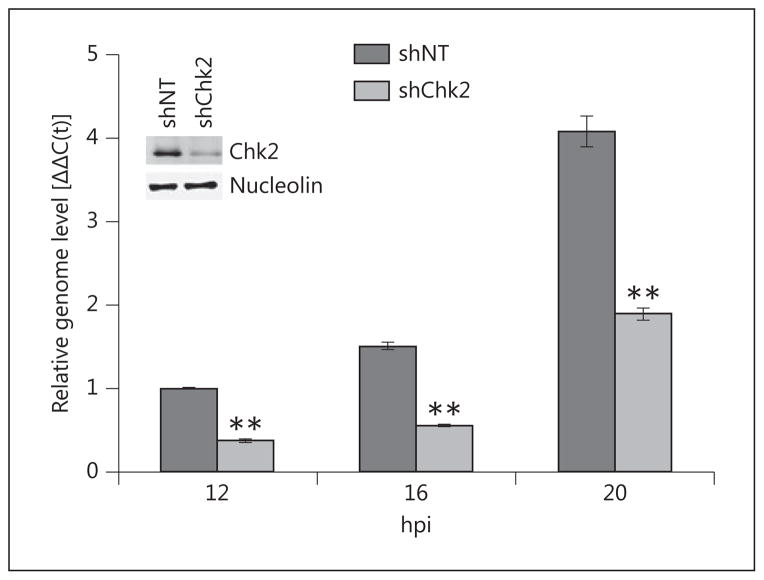
In order to confirm the antiviral effect of the inhibitor, we interfered with Chk2 activity by RNAi-mediated gene knockdown. Stable depletion of Chk2 in normal corneal epithelial cells was not possible due to its toxic consequences. To circumvent this, we used HCE cells to derive stable cell lines harboring tetracycline-inducible shRNA against Chk2 or nontargeting shRNA control. Chk2 knockdown was induced with doxycycline for 72 h prior to infection with HSV-1, and genome replication was measured by qPCR. Chk2 protein levels were assessed by Western blot using lysates collected at the time of infection (fig. 3, inset). Chk2 knockdown had an inhibitory effect on viral infection in HCE cells (fig. 3), albeit not as pronounced as the effect of Chk2 inhibitor II. This discrepancy is most likely due to the residual Chk2 kinase that could not be eliminated in our system, since densitometry measurements show incomplete knock-down (81.7%); however, we cannot rule out that the inhibitor may exert off-target effects that contribute to reduced viral replication. Nevertheless, this result agrees with our inhibitor data and confirms that the antiviral activity of Chk2 inhibitor II, at least to a large extent, is achieved through specific inhibition of the Chk2 kinase.
Fig. 3.
Chk2 knockdown reduces HSV-1 replication in vitro. HCE cells harboring tetracycline-inducible expression of shRNA against Chk2 or nontargeting control were cultured in the presence of doxycycline (0.25 μg/ml) for 72 h to induce Chk2 knockdown. Following the induction, cells were infected with HSV-1 at MOI 0.1, and total DNA was collected at the indicated time points for analysis by qPCR with primers for the viral genome and GAPDH. Doxycycline was present in the medium for the entire duration of infection. Protein lysates were collected at the time of infection to verify knockdown by Western blot (inset). Nucleolin is a loading control. Values represent average Δ ΔC(t) ± SEM for a representative of two independent experiments. n = 2 reaction replicates.
Inhibition of Chk2 Suppresses HSV-1 Replication in Explanted Human and Rabbit Corneas
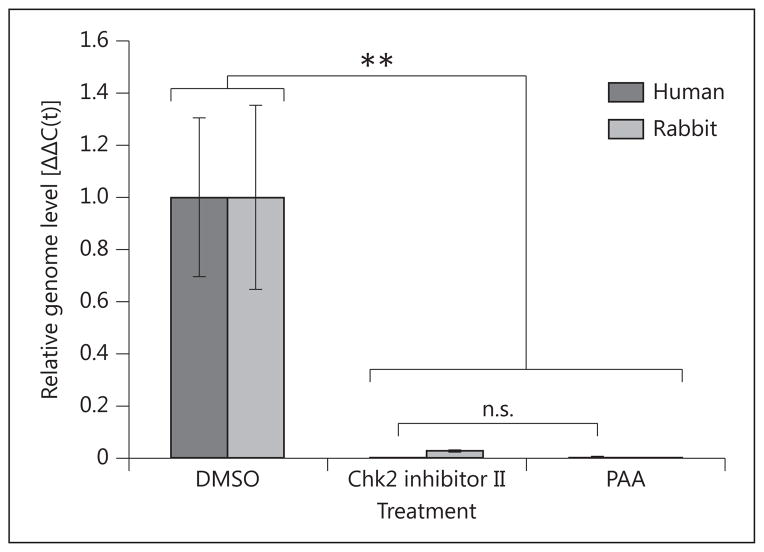
In order to extend our in vitro findings to a more physiologically relevant model, we followed the method of Alekseev et al. [39] for ex vivo corneal HSV-1 infection. Human and rabbit corneoscleral buttons were maintained in organotypic tissue culture and infected with HSV-1 in the presence of Chk2 inhibitor II. A 10-μM drug concentration was used based on additional dose optimization carried out in explanted human corneas (online suppl. fig. 2b). qPCR measurement of viral genome levels at 48 hpi demonstrated that corneas treated with the inhibitor did not support productive infection, as compared to mock-treated controls (fig. 4). There was no statistical significance between viral genome levels in the Chk2 inhibitor II-treated human corneas and positive controls treated with PAA. HSV-1 inhibition was slightly less potent in rabbit than in human corneas, which may be explained by the specificity of the inhibitor for the human enzyme.
Fig. 4.
Chk2 inhibition suppresses HSV-1 replication in explanted human and rabbit corneas. Human and rabbit corneas were infected with 1 × 10 4 PFU/cornea. At 1 hpi, they were treated with Chk2 inhibitor II (10 μM). Mock treatment (DMSO) and PAA (400 μg/ml) were included as negative and positive controls, respectively. DNA was isolated from the epithelial layers at 48 hpi and analyzed by qPCR with primers for the viral genome and GAPDH. Bars represent average Δ ΔC(t) values ± SEM. n = 6 corneas per treatment.
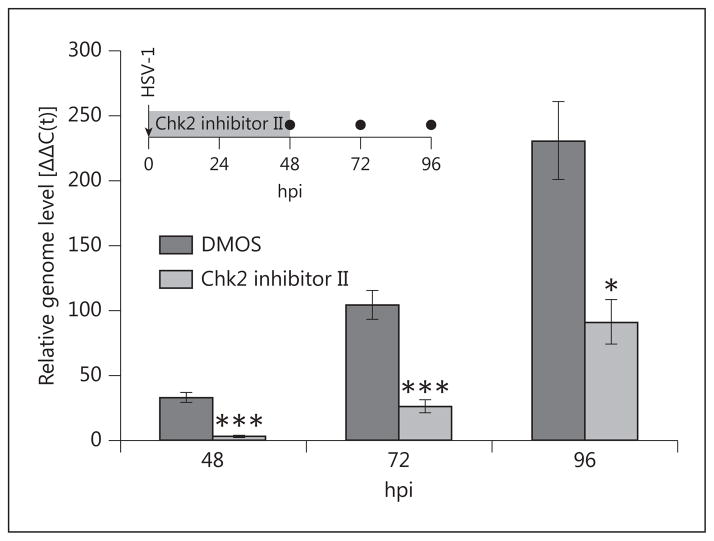
In light of these findings, we sought to investigate the long-term effects of Chk2 inhibition in the explant model. To this end, we infected rabbit corneas and maintained them in culture with uninterrupted treatment with Chk2 inhibitor II for 2 days. At this point, the drug was removed from the medium, and all corneas were cultured in inhibitor-free medium for 2 more days, during which time epithelial DNA samples were collected (fig. 5, inset). qPCR analysis revealed a lasting effect of Chk2 inhibition that was maintained as late as 96 hpi (the latest time point tested; fig. 5). HSV-1 seemed to resume normal growth following the removal of inhibitor, indicating that Chk2 inhibition suppresses viral replication, but does not eliminate the infected cells.
Fig. 5.
Prolonged effect of Chk2 inhibition on HSV-1 replication in explanted corneas. Rabbit corneas were infected with 1 × 10 4 PFU/cornea and treated with Chk2 inhibitor II (10 μM) or mock treatment (DMSO) for 48 h. Corneas were rinsed and cultured in fresh inhibitor-free medium for an additional 48 h (inset), during which time total DNA was isolated from the epithelial layers at the indicated time points (●) and analyzed by qPCR with primers for the viral genome and GAPDH. Samples were collected from independent groups of corneoscleral buttons (6 corneas per time point and treatment), rather than by sequential scraping of the same group. Bars represent average Δ ΔC(t) values ± SEM. n = 6 corneas per each time point and treatment.
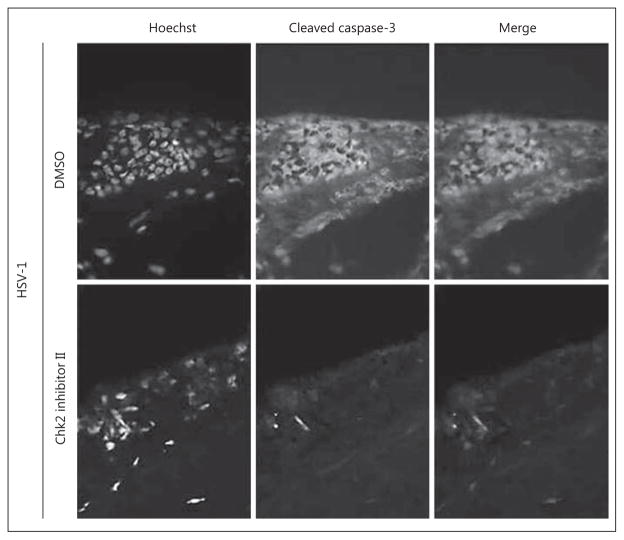
Finally, we assessed the effect of Chk2 inhibition on overall corneal health during infection. Explanted human corneas were infected with HSV-1 and treated with Chk2 inhibitor II or mock (DMSO), as in the previous experiments (fig. 4). At 48 hpi, corneas were analyzed by immunohistochemistry with antibodies against cleaved caspase-3, a common marker of apoptosis. Mock-treated corneas developed notable limbal apoptosis in response to HSV-1 infection; however, this was abrogated in corneas treated with Chk2 inhibitor II (fig. 6).
Fig. 6.
Chk2 inhibition reduces HSV-1- associated apoptosis in explanted corneas. Ex vivo human corneas were infected with 1× 10 4 PFU/cornea and treated with Chk2 inhibitor II (10 μM) or mock treatment (DMSO). Corneas were flash-frozen at 48 h and processed for indirect immunofluorescence staining with antibodies against cleaved caspase-3. The counterstain is Hoechst 33258. A representative limbal field for each treatment is shown. n = 2 corneas per treatment.
Discussion
Our prior work had highlighted the critical role of the ATM kinase in the development of HSV-1 keratitis and demonstrated that inhibition of ATM may be a potential therapeutic strategy in combatting HK [16]. ATM is an apical DDR protein known to have numerous cellular roles and downstream targets [44] . The mechanism whereby ATM activation facilitates HSV-1 replication in the cornea is unknown. Since Chk2 kinase is a widely recognized signaling target of ATM that has been implicated in viral infections in general and HSV-1 in particular, this report examines the significance of Chk2 activity in corneal epithelial HSV-1 infection. We show that blocking Chk2 kinase activity with a small-molecule inhibitor produces pronounced inhibition of infection in two different human corneal epithelial cell lines. This inhibition is detectable by monitoring viral genome levels and the production of infectious viral particles, and visually by observing the CPE of the virus. In addition, we were able to extend these in vitro findings into the ex vivo model of corneal epithelial keratitis, where Chk2 inhibition blocked viral replication in human and rabbit corneas. These findings expand our knowledge on the role of the DDR in the pathogenesis of HK, and establish Chk2 kinase as a significant factor that mediates the pro-viral effect of ATM activation in corneal epithelial HSV-1 infection.
Further elucidation of the events downstream of Chk2 is necessary to gain detailed understanding of this mechanism in order to propose target-specific therapies. This mechanism is currently being investigated. Chk2 has several known phosphorylation substrates, one or more of which could be potentially relevant in HSV-1 infection. These include the DNA repair and recombination proteins BRCA1 [29] and XRCC1 [45]; cell cycle regulators Cdc25A/C [20–24], Rb [46], Mdm4 [47] and p53 [48]; transcription factors E2F1/3 [49, 50] and FoxM1 [28] , and chromatin packaging regulators PML [51] and histone 1 [52] . All of the processes in which these proteins are involved have been implicated in HSV-1 infection. DNA repair and recombination are important for the resolution of complex branched structures that arise during the replication of the viral genome [53] ; however, the virus also protects its genome by inhibiting repair processes. Lilley et al. [54] have shown that HSV-1-mediated disruption of BRCA1 recruitment prevents the formation of stable ionizing radiation-induced foci. Since BRCA1 is a Chk2 substrate, phosphorylation by Chk2 may play a role in controlling its recruitment to ionizing radiation-induced foci. The cell cycle is manipulated in order to prime the cellular environment for maximal viral genome replication. The virus pushes quiescent cells into the G1 phase and prevents their exit by inducing arrest at the G1/S and G2/M checkpoints [55] . Activation of Chk2 is known to arrest the cell cycle at both of these checkpoints, and Li et al. [43] have demonstrated the importance of Chk2-mediated G2/M stalling for HSV-1 infection in colorectal carcinoma cells. HSV-1 transcriptional dynamics are complex, tightly regulated, and involve combinations of cellular and viral factors [56] . Transcription factors phosphorylated by Chk2 may contribute to the enhanced or focused transcription necessary to maximize viral replication.
Finally, manipulation of chromatin structure is necessary for the virus to overcome the propensity of the cell to suppress the viral genome. Phosphorylation of the H1 linker histone is known to occur in HSV-1 infection and leads to the mobilization of linker histones from the DNA [57]. Since the accessibility of the viral genome to the transcriptional machinery and the DNA polymerase is necessary for productive infection, Chk2 phosphorylation of H1 linker histone may also be a contributing event. Chromatin disruption is accomplished, at least in part, by the dispersal of promyelocytic leukemia (PML) nuclear domains, the intrinsic antiviral defense mechanism of the cell, which rapidly assemble on the incoming genome to suppress expression and replication [58]. Thus, Chk2-mediated PML phosphorylation may be a regulatory step in PML nuclear domain dispersal. Of note is the fact that Chk2 is normally found in PML nuclear domains, structures that serve as depots for many DDR proteins [59]. PML phosphorylation by Chk2 leads to the release of additional Chk2 molecules into the nucleoplasm, where they are subsequently autophosphorylated by activated Chk2 [51, 60]. Overall, this creates a feed-forward signal amplification mechanism that could be used by HSV-1 to maximize the potential benefits of DDR activation.
In summary, this study establishes Chk2 kinase activity as a critical factor in the interaction between HSV-1 and the host DDR, and expands our understanding of the role of ATM signaling in the molecular pathology of HK. Animal experimentation is necessary to determine if reduction of epithelial keratitis through Chk2 inhibition translates into amelioration of the more severe stromal keratitis, and whether the corneal toxicity profile of Chk2 inhibitors is compatible with a potential therapeutic use.
Acknowledgments
The authors thank the Lions Eye Bank of Delaware Valley for providing human corneas, and also Dr. Peter Laibson, Dr. Stephen Jennings and members of the Clifford laboratory for their intellectual input. This work was supported by Drexel University College of Medicine as well as an NRSA training fellowship to O.A. (1F30DK094612-O1A1).
Footnotes
Disclosure Statement
None of the authors have any conflicts of interest to declare.
References
- 1.Farooq AV, Shah A, Shukla D. The role of herpesviruses in ocular infections. Virus Adaptation Treat. 2010;2:115–123. [Google Scholar]
- 2.Toma HS, Murina AT, Areaux RG, Jr, Neumann DM, Bhattacharjee PS, Foster TP, Kaufman HE, Hill JM. Ocular HSV-1 latency, reactivation and recurrent disease. Semin Ophthalmol. 2008;23:249–273. doi: 10.1080/08820530802111085. [DOI] [PubMed] [Google Scholar]
- 3.Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol. 2012;57:448–462. doi: 10.1016/j.survophthal.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rowe AM, St Leger AJ, Jeon S, Dhaliwal DK, Knickelbein JE, Hendricks RL. Herpes keratitis. Prog Retin Eye Res. 2013;32:88–101. doi: 10.1016/j.preteyeres.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choong K, Walker NJ, Apel AJ, Whitby M. Aciclovir-resistant herpes keratitis. Clin Experiment Ophthalmol. 2010;38:309–313. doi: 10.1111/j.1442-9071.2010.02209.x. [DOI] [PubMed] [Google Scholar]
- 6.Laibson PR. Resistant herpes simplex keratitis. Clin Experiment Ophthalmol. 2010;38:227–228. doi: 10.1111/j.1442-9071.2010.02250.x. [DOI] [PubMed] [Google Scholar]
- 7.Duan R, de Vries RD, Osterhaus AD, Remeijer L, Verjans GM. Acyclovir-resistant corneal HSV-1 isolates from patients with herpetic keratitis. J Infect Dis. 2008;198:659–663. doi: 10.1086/590668. [DOI] [PubMed] [Google Scholar]
- 8.Burrel S, Aime C, Hermet L, Ait-Arkoub Z, Agut H, Boutolleau D. Surveillance of herpes simplex virus resistance to antivirals: a 4-year survey. Antiviral Res. 2013;100:365–372. doi: 10.1016/j.antiviral.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2011;55:459–472. doi: 10.1128/AAC.00615-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. CD8 + T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med. 2000;191:1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaye S, Choudhary A. Herpes simplex keratitis. Prog Retin Eye Res. 2006;25:355–380. doi: 10.1016/j.preteyeres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Thygeson P. Controversies in Ophthalmology. Philadelphia: WB Saunders; 1977. pp. 450–469. [Google Scholar]
- 13.Jaanus SD, Cheetham JK, Lesher GA. Anti-inflammatory drugs. In: Bartlett JD, Jaanus SD, editors. Clinical Ocular Pharmacology. Boston: Butterworth; 2001. pp. 273–276. [Google Scholar]
- 14.Malvy D, Treilhaud M, Bouee S, Crochard A, Vallee D, El Hasnaoui A, Aymard M. A retrospective, case-control study of acyclovir resistance in herpes simplex virus. Clin Infect Dis. 2005;41:320–326. doi: 10.1086/431585. [DOI] [PubMed] [Google Scholar]
- 15.Gaudreau A, Hill E, Balfour HH, Jr, Erice A, Boivin G. Phenotypic and genotypic characterization of acyclovir-resistant herpes simplex viruses from immunocompromised patients. J Infect Dis. 1998;178:297–303. doi: 10.1086/515626. [DOI] [PubMed] [Google Scholar]
- 16.Alekseev O, Donovan K, Azizkhan-Clifford J. Inhibition of ataxia telangiectasia mutated (ATM) kinase suppresses herpes simplex virus type 1 (HSV-1) keratitis. Invest Ophthalmol Vis Sci. 2014;55:706–715. doi: 10.1167/iovs.13-13461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lilley CE, Schwartz RA, Weitzman MD. Using or abusing: viruses and the cellular DNA damage response. Trends Microbiol. 2007;15:119–126. doi: 10.1016/j.tim.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Lilley CE, Carson CT, Muotri AR, Gage FH, Weitzman MD. DNA repair proteins affect the lifecycle of herpes simplex virus 1. Proc Natl Acad Sci USA. 2005;102:5844–5849. doi: 10.1073/pnas.0501916102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuoka S, Rotman G, Ogawa A, Shiloh Y, Tamai K, Elledge SJ. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc Natl Acad Sci USA. 2000;97:10389–10394. doi: 10.1073/pnas.190030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartek J, Falck J, Lukas J. Chk2 kinase – a busy messenger. Nat Rev Mol Cell Biol. 2001;2:877–886. doi: 10.1038/35103059. [DOI] [PubMed] [Google Scholar]
- 21.Bartek J, Lukas C, Lukas J. Checking on DNA damage in S phase. Nat Rev Mol Cell Biol. 2004;5:792–804. doi: 10.1038/nrm1493. [DOI] [PubMed] [Google Scholar]
- 22.Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003;3:421–429. doi: 10.1016/s1535-6108(03)00110-7. [DOI] [PubMed] [Google Scholar]
- 23.Falck J, Mailand N, Syljuasen RG, Bartek J, Lukas J. The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature. 2001;410:842–847. doi: 10.1038/35071124. [DOI] [PubMed] [Google Scholar]
- 24.Sorensen CS, Syljuasen RG, Falck J, Schroeder T, Ronnstrand L, Khanna KK, Zhou BB, Bartek J, Lukas J. Chk1 regulates the S phase checkpoint by coupling the physiological turnover and ionizing radiation-induced accelerated proteolysis of Cdc25A. Cancer Cell. 2003;3:247–258. doi: 10.1016/s1535-6108(03)00048-5. [DOI] [PubMed] [Google Scholar]
- 25.Pommier Y, Weinstein JN, Aladjem MI, Kohn KW. Chk2 molecular interaction map and rationale for Chk2 inhibitors. Clin Cancer Res. 2006;12:2657–2661. doi: 10.1158/1078-0432.CCR-06-0743. [DOI] [PubMed] [Google Scholar]
- 26.Hirao A, Cheung A, Duncan G, Girard PM, Elia AJ, Wakeham A, Okada H, Sarkissian T, Wong JA, Sakai T, De Stanchina E, Bristow RG, Suda T, Lowe SW, Jeggo PA, Elledge SJ, Mak TW. Chk2 is a tumor suppressor that regulates apoptosis in both an ataxia telangiectasia mutated (ATM)-dependent and an ATM-independent manner. Mol Cell Biol. 2002;22:6521–6532. doi: 10.1128/MCB.22.18.6521-6532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takai H, Naka K, Okada Y, Watanabe M, Harada N, Saito S, Anderson CW, Appella E, Nakanishi M, Suzuki H, Nagashima K, Sawa H, Ikeda K, Motoyama N. Chk2-deficient mice exhibit radioresistance and defective p53-mediated transcription. EMBO J. 2002;21:5195–5205. doi: 10.1093/emboj/cdf506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan Y, Raychaudhuri P, Costa RH. Chk2 mediates stabilization of the FoxM1 transcription factor to stimulate expression of DNA repair genes. Mol Cell Biol. 2007;27:1007–1016. doi: 10.1128/MCB.01068-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Willers H, Feng Z, Ghosh JC, Kim S, Weaver DT, Chung JH, Powell SN, Xia F. Chk2 phosphorylation of BRCA1 regulates DNA double-strand break repair. Mol Cell Biol. 2004;24:708–718. doi: 10.1128/MCB.24.2.708-718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bahassi EM, Ovesen JL, Riesenberg AL, Bernstein WZ, Hasty PE, Stambrook PJ. The checkpoint kinases Chk1 and Chk2 regulate the functional associations between hBRCA2 and Rad51 in response to DNA damage. Oncogene. 2008;27:3977–3985. doi: 10.1038/onc.2008.17. [DOI] [PubMed] [Google Scholar]
- 31.Arienti KL, Brunmark A, Axe FU, McClure K, Lee A, Blevitt J, Neff DK, Huang L, Crawford S, Pandit CR, Karlsson L, Breitenbucher JG. Checkpoint kinase inhibitors: SAR and radio-protective properties of a series of 2-arylbenz-imidazoles. J Med Chem. 2005;48:1873–1885. doi: 10.1021/jm0495935. [DOI] [PubMed] [Google Scholar]
- 32.Robertson DM, Li L, Fisher S, Pearce VP, Shay JW, Wright WE, Cavanagh HD, Jester JV. Characterization of growth and differentiation in a telomerase-immortalized human corneal epithelial cell line. Invest Ophthalmol Vis Sci. 2005;46:470–478. doi: 10.1167/iovs.04-0528. [DOI] [PubMed] [Google Scholar]
- 33.Araki-Sasaki K, Ohashi Y, Sasabe T, Hayashi K, Watanabe H, Tano Y, Handa H. An sv40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci. 1995;36:614–621. [PubMed] [Google Scholar]
- 34.Jensen FC, Girardi AJ, Gilden RV, Koprowski H. Infection of human and simian tissue cultures with Rous sarcoma virus. Proc Natl Acad Sci USA. 1964;52:53–59. doi: 10.1073/pnas.52.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith KO. Relationship between the envelope and the infectivity of herpes simplex virus. Proc Soc Exp Biol Med. 1964;115:814–816. doi: 10.3181/00379727-115-29045. [DOI] [PubMed] [Google Scholar]
- 36.Wee S, Wiederschain D, Maira SM, et al. PTEN-deficient cancers depend on PIK3CB. Proc Natl Acad Sci USA. 2008;105:13057–13062. doi: 10.1073/pnas.0802655105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiederschain D, Wee S, Chen L, et al. Single-vector inducible lentiviral RNAi system for oncology target validation. Cell Cycle. 2009;8:498–504. doi: 10.4161/cc.8.3.7701. [DOI] [PubMed] [Google Scholar]
- 38.Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alekseev O, Tran AH, Azizkhan-Clifford J. Ex vivo organotypic corneal model of acute epithelial herpes simplex virus type I infection. J Vis Exp. 2012:e3631. doi: 10.3791/3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim YH, Seo SK, Choi BK, Kang WJ, Kim CH, Lee SK, Kwon BS. 4-1BB costimulation enhances HSV-1-specific CD8 + T cell responses by the induction of CD11c +CD8+ T cells. Cell Immunol. 2005;238:76–86. doi: 10.1016/j.cellimm.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Berkovich E, Monnat RJ, Jr, Kastan MB. Roles of ATM and NBS1 in chromatin structure modulation and DNA double-strand break repair. Nat Cell Biol. 2007;9:683–690. doi: 10.1038/ncb1599. [DOI] [PubMed] [Google Scholar]
- 42.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li H, Baskaran R, Krisky DM, Bein K, Grandi P, Cohen JB, Glorioso JC. Chk2 is required for HSV-1 ICP0-mediated G2/M arrest and enhancement of virus growth. Virology. 2008;375:13–23. doi: 10.1016/j.virol.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shiloh Y, Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Biol. 2013;14:197–210. [PubMed] [Google Scholar]
- 45.Chou WC, Wang HC, Wong FH, Ding SL, Wu PE, Shieh SY, Shen CY. Chk2-dependent phosphorylation of XRCC1 in the DNA damage response promotes base excision repair. EMBO J. 2008;27:3140–3150. doi: 10.1038/emboj.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inoue Y, Kitagawa M, Taya Y. Phosphorylation of pRB at Ser612 by Chk1/2 leads to a complex between pRB and E2F-1 after DNA damage. EMBO J. 2007;26:2083–2093. doi: 10.1038/sj.emboj.7601652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen L, Gilkes DM, Pan Y, Lane WS, Chen J. ATM and Chk2-dependent phosphorylation of MDMX contribute to p53 activation after DNA damage. EMBO J. 2005;24:3411–3422. doi: 10.1038/sj.emboj.7600812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shieh SY, Ahn J, Tamai K, Taya Y, Prives C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 2000;14:289–300. [PMC free article] [PubMed] [Google Scholar]
- 49.Martinez LA, Goluszko E, Chen HZ, Leone G, Post S, Lozano G, Chen Z, Chauchereau A. E2F3 is a mediator of DNA damage-induced apoptosis. Mol Cell Biol. 2010;30:524–536. doi: 10.1128/MCB.00938-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stevens C, Smith L, La Thangue NB. Chk2 activates E2F-1 in response to DNA damage. Nat Cell Biol. 2003;5:401–409. doi: 10.1038/ncb974. [DOI] [PubMed] [Google Scholar]
- 51.Yang S, Kuo C, Bisi JE, Kim MK. PML-dependent apoptosis after DNA damage is regulated by the checkpoint kinase hCds1/Chk2. Nat Cell Biol. 2002;4:865–870. doi: 10.1038/ncb869. [DOI] [PubMed] [Google Scholar]
- 52.Zhou M, Meng Z, Jobson AG, Pommier Y, Veenstra TD. Detection of in vitro kinase generated protein phosphorylation sites using γ [18O4]-ATP and mass spectrometry. Anal Chem. 2007;79:7603–7610. doi: 10.1021/ac071584r. [DOI] [PubMed] [Google Scholar]
- 53.Ward SA, Weller SK. HSV-1 DNA replication. In: Weller SK, editor. Alphaherpesviruses: Molecular Virology. Wymondham: Caister Academic Press; 2011. pp. 89–112. [Google Scholar]
- 54.Lilley CE, Chaurushiya MS, Boutell C, Landry S, Suh J, Panier S, Everett RD, Stewart GS, Durocher D, Weitzman MD. A viral E3 ligase targets RNF8 and RNF168 to control histone ubiquitination and DNA damage responses. EMBO J. 2010;29:943–955. doi: 10.1038/emboj.2009.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chaurushiya MS, Weitzman MD. Viral manipulation of DNA repair and cell cycle checkpoints. DNA Repair. 2009;8:1166–1176. doi: 10.1016/j.dnarep.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roizman B, Knipe DM, Whitley RJ. Herpes simplex viruses. In: Knipe DM, Howley PM, editors. Fields Virology. Vol. 2. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 2501–2602. [Google Scholar]
- 57.Conn KL, Hendzel MJ, Schang LM. Linker histones are mobilized during infection with herpes simplex virus type 1. J Virol. 2008;82:8629–8646. doi: 10.1128/JVI.00616-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cuchet D, Sykes A, Nicolas A, Orr A, Murray J, Sirma H, Heeren J, Bartelt A, Everett RD. PML isoforms I and II participate in PML-dependent restriction of HSV-1 replication. J Cell Sci. 2011;124:280–291. doi: 10.1242/jcs.075390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lallemand-Breitenbach V, de Thé H. PML nuclear bodies. Cold Spring Harb Perspect Biol. 2010;2:a000661. doi: 10.1101/cshperspect.a000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Venere M, Mochan TA, Halazonetis TD. Chk2 leaves the PML depot. Nat Cell Biol. 2002;4:E255–E256. doi: 10.1038/ncb1102-e255. [DOI] [PubMed] [Google Scholar]