Abstract
Background
Facial emotion perception (FEP) is a critical human skill for successful social interaction, and a substantial body of literature suggests that explicit FEP is disrupted in Major Depressive Disorder (MDD). Prior research suggests that weakness in FEP may be an important phenomenon underlying patterns of emotion processing challenges in MDD and the disproportionate frequency of MDD in women.
Method
Women with (n = 24) and without (n = 22) MDD, equivalent in age and education, completed a FEP task during fMRI.
Results
The MDD group exhibited greater extents of frontal, parietal, and subcortical activation compared to the control group during FEP. Activation in inferior frontal gyrus (IFG) appeared shifted from a left > right pattern observed in healthy women to a bilateral pattern in MDD women. The ratio of left to right suprathreshold IFG voxels in healthy controls was nearly 3:1, whereas in the MDD group, there was a greater percent of suprathreshold IFG voxels bilaterally, with no leftward bias. In MDD, relatively greater activation in right IFG compared to left IFG (ratio score) was present and predicted FEP accuracy (r = .56, p < .004), with an inverse relationship observed between FEP and subgenual cingulate activation (r = −.46, p = .02).
Conclusions
This study links, for the first time, disrupted IFG activation laterality and increased subgenual cingulate activation with deficient FEP in women with MDD, providing an avenue for imaging-to-assessment translational applications in MDD.
Keywords: emotion, faces, depression, neuroimaging, laterality, women, identification
A burgeoning, yet heterogeneous area of inquiry in Major Depressive Disorder (MDD) relates to processing of emotions in faces. Individuals with MDD tend to exhibit decreased ability to recognize facial emotions (Csukly et al., 2009, Langenecker et al., 2005, Langenecker et al., 2007a, Persad and Polivy, 1993) and a negative response bias (Bouhuys et al., 1999, Elliott et al., 2002, Joormann and Gotlib, 2006, Watkins et al., 1996, Wright et al., 2009) compared to healthy controls. These deficits may be greatest among those with the most severe depressive symptomatology (Gur et al., 1992), yet also may represent a trait risk for MDD observed outside of symptomatic episodes (LeMoult et al., 2009). Using a challenging facial emotion perception (FEP) paradigm, our group observed that young women with MDD are less accurate at detecting emotions, particularly fear, than both healthy control women and depressed men, whereas young depressed men performed similarly to control men (Wright et al., 2009, Wright & Langenecker, 2008). In a recent meta-analysis, MDD performed worse than controls in all emotions, trending toward worse skills in men with MDD (and bipolar disorder Kohler et al., 2012).
There are also reports of fMRI activation abnormalities in persons diagnosed with MDD during viewing of emotional faces and related emotional stimuli (Frodl et al., 2009, Fu et al., 2004, Sheline et al., 2001). Neuroimaging studies in adults with MDD typically report heightened limbic (Sheline et al., 2001, Surguladze et al., 2005) and basal ganglia responses (Fu et al., 2004, Surguladze et al., 2005) with mixed activation differences in frontal regions (Demenescu et al., 2011, Lawrence et al., 2004, Lee et al., 2008). In MDD, there is some indication of a shift in laterality of frontal activation for emotional stimuli, from a left-greater-than-right pattern observed in healthy adults to right-greater-than-left or greater bilateral frontal activation. Specifically, in healthy individuals, left laterality in emotion labeling/identification has been associated with inferior and medial frontal activation in meta-analysis (Fusar-Poli, 2009), although others have reported greater activation in left middle frontal gyrus associated with regulation of emotion (Mak et al., 2009). A few limited studies with EEG and fMRI suggest that a right-greater-than-left or more bilateral activation pattern shift is present in MDD, but this finding has not been frequently tested (Grimm et al., 2008; Henriques & Davidson, 1991; Langenecker et al., 2009) nor is it clear whether this shift might be related to FEP accuracy.
The large majority of neuroimaging studies investigating FEP in MDD have utilized experimental tasks with oblique emotion processing paradigms, such as passive viewing of stimuli (Lee et al., 2008), presenting masked faces theoretically outside of conscious awareness (Dannlowski et al., 2008, Dannlowski et al., 2007), or gender/age discrimination tasks (Canli et al., 2005, Costafreda et al., 2009, Demenescu et al., 2011, Thomas et al., 2011). One limitation of oblique paradigms is that implicit emotion processing may still occur and vary across individuals, which limits the performance differences that might be observed as compared to explicit paradigms. A few studies have utilized tasks requiring explicit emotion judgments, although these tasks have required binary emotional classification decisions about facial expressions (e.g., emotional vs. neutral; (Almeida et al., 2010) or matching emotions presented in different faces (Frodl et al., 2009). Often, explicit studies have not capitalized upon challenging depressed subjects to the point of dysfunctional performance, which then precludes the analysis of the functional correlates of poor performance. As a result, although oblique or simple explicit matching paradigms are an excellent way to understand perturbations of emotion processing circuitry, they do not provide an exportable behavioral paradigm for ecologically valid translation to clinical settings. Moreover, FEP paradigms with low levels of challenge have precluded the direct investigation of the role that FEP deficits play in the neural responses to facial emotions and in risk for and expression of disease. In contrast, simple explicit and oblique paradigms do not have a potential for disease by performance confounds, which difficult explicit paradigms must address. It is important to evaluate the strengths and weaknesses of the different approaches, and to consider whether the goal is to focus more on internal or external validity.
The current study addressed some of the limitations of the knowledge base by investigating disrupted explicit FEP in MDD. First, to address the relationship between performance and activation directly, the experimental paradigm involved an explicit emotion classification task previously demonstrated to elicit performance deficits in individuals with MDD (Langenecker et al., 2005, Langenecker et al., 2007a). Second, in light of our recent findings suggesting female-specific vulnerability to FEP decrements in MDD (Wright et al., 2009), the current sample was comprised exclusively of women. It was hypothesized that depressed women would exhibit hyperactivity in frontal, basal ganglia and limbic regions (Fu et al., 2004, Lawrence et al., 2004, Lee et al., 2008, Sheline et al., 2001, Surguladze et al., 2005). In addition, it was expected that these areas of disrupted activation in MDD would be related to FEP performance, irrespective of the effects of medications. Finally, we hypothesized shifted laterality of activation in MDD (right greater than left), and we explored the relationship of laterality to FEP performance.
Methods
Participants
Twenty-four women with MDD and 22 healthy women controls participated. The groups did not differ with regard to age (MDD M = 37.8, SD = 14.5; HC M = 31.7, SD = 14.4; t(44) = −1.42, p = .16), education (MDD M = 16.3, SD = 2.3; HC M = 16.2, SD = 2.4, t(44) =−0.09, p = .93), or Shipley IQ (MDD M = 113.1, SD = 9.3; HC M = 115.5, SD = 7.1), t(42) = .954, p = .35). Informed consent procedures were completed per the University of Michigan Institutional Review Board guidelines and consistent with the Declaration of Helsinki. Exclusion criteria for participants included: a diagnosis of schizophrenia, bipolar disorder, brain injury, neurological condition, substance abuse in the last 2 years, or other such condition that would affect cognitive functioning. Non-depressed control participants had no personal history of any psychiatric illness, and no family history of any psychiatric disorder.
MDD diagnosis was from Structured Clinical Interview for DSM-IV (First, 1996) criteria by a licensed psychologist with mean age of onset of 24.4 years (SD = 13.7). Severity of depressive symptomatology was evaluated with the 17 item Hamilton Depression Rating Scale (HDRS-17; (Hamilton, 1960), MDD M = 15.8, SD = 7.2) and Beck Depression Inventory-II (M = 21.2, SD = 10.3). Ten of the MDD participants were unmedicated. Of the medicated group, 11 were taking antidepressants only (e.g., SSRIs, MAO-Is, TCAs, and buproprion), and 3 were taking antidepressants plus/or medications with reported cognitive side effects (one prescribed Seroquel, Neurontin, and Lamictal; one prescribed Ativan, Cymbalta, and Trazodone, and one prescribed Xanax PRN, Ambien, Celexa, and Wellbutrin). The study was a cross-sectional, naturalistic design; therefore, it was not possible to control for dose, severity, or effects of expectation (placebo) on medicated volunteers.
Affect Classification Paradigm
The Facial Emotion Perception Test (FEPT; (Langenecker et al., 2005, Langenecker et al., 2007a, Rapport et al., 2002) was used to assess the accuracy and speed of identification of facial expressions. There are parallel computer (facial stimuli from (Ekman and Friesen, 1976) and imaging (MacBrain, color photographs of faces from the NimStim stimuli; (Tottenham et al., 2009) versions. Participants were required to categorize faces into one of four emotion categories (happy, sad, angry, or fearful). Each presentation (total of 21 blocks, 147 stimuli) began with an orienting cross in the center of the screen (500 ms), followed by a facial emotion stimulus (300 ms), then a visual mask to prevent visual afterburn (100 ms), followed by a response period (2600 ms). As a control task, blocks requiring participants to identify and categorize pictured animals (8 blocks, 56 total stimuli) were interspersed amongst facial emotion recognition blocks. These animal blocks were used as a method of controlling for activation related to visual processing and praxis, and for response selection and execution. The task consisted of five, 3.5-minute runs with counterbalanced animal and face blocks, each run ending with a rest block. Each presentation in the imaging experiment was a novel presentation of an emotional stimulus per actor/actress, although individual actors/actresses did repeat.
Prior to entering the fMRI scanner, all participants completed a computer-only version of the FEPT. The computer test utilized the Ekman faces (Ekman and Friesen, 1976) and was presented on a standard computer monitor. The practice version was one 6-minute run and consisted of 12 animal trials and 54 face trials and had parameters for presentation and response times identical to the fMRI version.
Performance on the FEPT task was captured by accuracy and reaction time for correct responses for each emotional expression, in addition to animal classification.
Scanning Procedures
MRI Acquisition
Whole brain imaging was performed using a GE Signa 3 T scanner (release VH3). fMRI series consisted of 30 contiguous oblique-axial 4 mm sections acquired using a forward-reverse spiral sequence, which provides excellent fMRI sensitivity. The image matrix was 64 × 64 over a 24cm field of view for a 3.75 × 3.75 × 4mm voxel. The 30-slice volume was acquired serially at 1750 ms temporal resolution for a total of 590 time points for FEPT. One hundred six high-resolution fast SPGR IR axial anatomic images [TE (echo time) = 3.4 ms; TR (repetition time) = 10.5 ms, 27 degree flip angle, NEX (number of excitations) = 1, slice thickness = 1.5 mm, field of view = 24 cm, matrix size = 256 × 256] were obtained for each participant for co-registration purposes.
MRI Processing
Pre-processing of all fMRI data was conducted in SPM2, similar to our previously reported work (Langenecker et al., 2007b, Langenecker et al., 2012). This process included realignment, slice timing correction, co-registration of anatomical and functional images, normalization to Montreal Neurological Institute (MNI) space, and smoothing with a full width at half maximum (FWHM) filter of 5 mm. Contrast images were created using the subtraction method, such that the Blood Oxygenation Level Dependent (BOLD) signal for animal processing blocks was subtracted from that of faces processing blocks, in order to examine the BOLD response for the stimuli specifically related to facial emotion processing (Faces - Animals for all imaging contrasts). First-level individual and second-level group models were completed in SPM5. Coordinates for activation foci were transformed to the Talairach system.
Statistical Analyses
Prior to analyses, data were screened for assumptions associated with the statistical models employed according to guidelines recommended by (Tabachnick & Fidell, 2007). Analyses of the neuroimaging data began with one-sample t tests of the Faces minus Animals contrast for the Healthy Control (HC) and MDD groups individually. Next, to examine whether this sample resembled those observed in prior research (i.e., a pattern of poorer performance among women with MDD), FEPT performance within the scanner was compared between MDD and HC groups using one-tailed t tests and effect sizes in Cohen’s d. Analysis of covariance (ANCOVA) was then performed with group as the independent variable, and overall faces accuracy and age as covariates, as prior research shows that these are pertinent features for which to control in understanding group differences (Gunning-Dixon et al., 2003, Wright et al., 2009). Age was moderately correlated with accuracy (r = −.45, p = .002). Finally, to evaluate the role of performance in task activation, a separate regression model with only MDD subjects was conducted, with task activation as the criterion and overall faces accuracy as the predictor. The index analysis between the MDD and HC groups was FDR corrected at p < .05 at whole brain resolution and extent threshold of 160 mm3, and that threshold was used for all analyses. For the Animals-only contrast, HC and MDD groups differed only in lateral, posterior globus pallidus bilaterally (MDD > HC, +/− 20, +/−19, −8/−2, Zs 3.79/3.82), indicating that any between-group differences in activation for the Faces minus Animals contrast outside these small regions are more likely to be related to social/emotional facial processing involved in the Faces condition.
Results
Individual Group Tests for Facial Emotion Processing
Individual group maps for the Faces minus Animals contrast for MDD group alone and HC group alone are illustrated in Figure 1a and 1b (MDD in blue, HC in green). The areas of overlapping activation for both groups (purple) included bilateral inferior and middle frontal gyrus, insula, inferior parietal lobule, precuneus, posterior putamen, and medial dorsal nucleus of the thalamus. The HC group exhibited significant activation that was not present in the MDD group in primarily posterior subcortical areas, such as bilateral parahippocampal gyrus, right caudate, right pulvinar, and left pons/raphe. The MDD group exhibited activation that was not present in the HC group in right precentral and postcentral gyrus, left anterior cingulate, as well as bilateral superior temporal gyrus, left parahippocampal gyrus/amygdala, substantia nigra, and left cerebellum. Independent activation foci for HC and MDD groups are included in Supplemental Tables 1 and 2 for the interested reader.
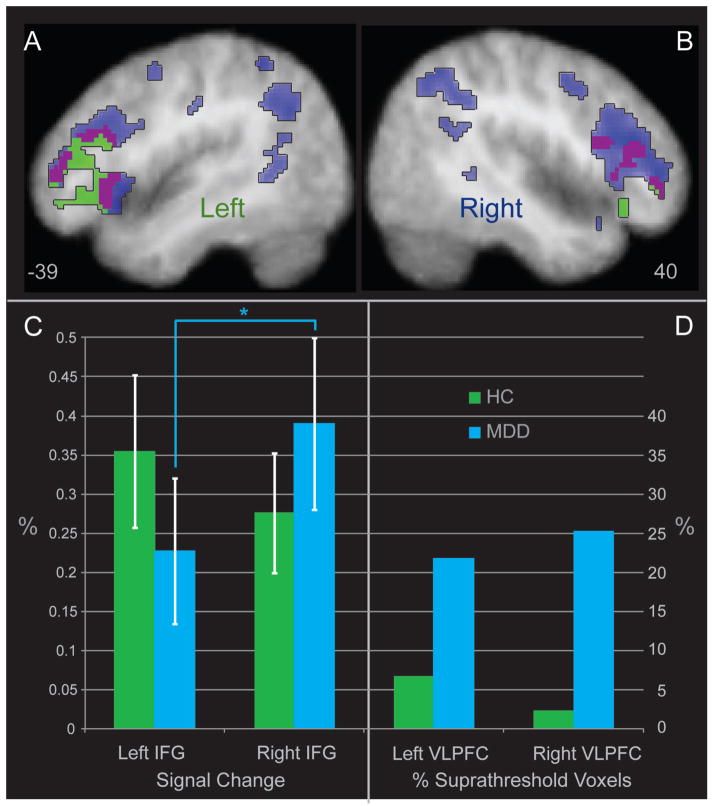
Figure 1.
Lateral Activation in the Healthy Control Group, the Major Depressive Disorder Group, including Evidence for Laterality Shifts by Extent and Degree of Activation. The figure illustrates significant areas of activation in the MDD and Healthy Control groups alone in blue and green, respectively. Areas of overlapping activation are displayed in purple. Coordinates are x axis in the Talairach system for Panels 1A and 1B. Panel 1C illustrates increased right signal in MDD relative to the left in the ventral and dorsal IFG clusters, with no significant difference between left and right signal in HC. Panel 1D depicts the percentage of significant suprathreshold voxels in each group including the ventro-lateral prefrontal cortex (VLPFC), corresponding to Brodmann areas 9, 10, 11, 44, 45, 46, and 47.
Laterality Tests by Group – Height of Activation
Figure 1a and 1b illustrate the evidence of left frontal laterality in the HC group, and an absence of this effect in MDD. Thus, two sets of post hoc analyses tested whether the reduced left laterality of activation in MDD was significant and meaningful in magnitude. First, the HC left inferior frontal cluster shown in Figure 1 was used as a center of mass for a 5mm ROI from which to extract mean BOLD signal for each subject using MarsBaR (Brett et al., 2002). This cluster in the ventral inferior frontal gyrus (VIFG; −45, 30, −1) is similar in coordinates to activation foci in healthy subjects reported for angry and fearful facial expressions in a meta-analysis of facial emotion processing by Fusar-Poli et al. (2009; −46, 33, 4 for fear, and −44, 26, 2 for anger). A right-hemisphere homologue cluster was created by inverting the x coordinate (x = 45). In addition, a right-hemisphere cluster in the dorsal aspect of the inferior frontal gyrus of significant activation in the MDD subjects (DIFG; 40, 26, 17) that was not observed in the HC group was used to create a similar sphere with a 5mm radius ROI and matching bilateral homologue. Mean BOLD activation for each subject was extracted for each subject for these two ROIs and contralateral homologue ROIs and then subjected to a 2 (Group) x 2 (Hemisphere) x 2 (Location [dorsal, ventral]) analysis of variance (ANOVA). In the IFG, there was an interaction between group and hemisphere (F(1,44) = 5.11, p = .03), with greater right than left activation in the MDD group (t(23) = −2.12, p = .045, d = −0.38), and non-significantly greater left than right IFG activation in the HC group (p = .30). There were no other significant effects or interactions (displayed in Figure 1c, labeled left and right IFG). The mean extracted BOLD signal from these four MARSBAR ROIs were used to compute a laterality index (R>L) using the formula (RVIFG+RDIFG)/ (LVIFG+LDIFG) for each subject and this index was used in subsequent posthoc analyses (see Matsuo, Chen & Tseng, 2012, for a description of some approaches to laterality indexing).
Laterality Tests by Group – Extent of Activation
As a second, novel post hoc strategy for evaluating shifted inferior frontal laterality of MDD, chi-square analysis tested the number of significant voxels activated in the MDD group relative to the HC group, primarily within the inferior and middle frontal gyri (Brodmann areas 9, 10, 11, 44, 45, 46, 47 using WFU Pickatlas (Maldjian et al., 2003, dilation 2 mm). The HC group exhibited a strong left laterality for activation during identification of emotions in faces, with a ratio of 2.8:1 (left to right) activated clusters. In contrast, the MDD group exhibited two effects of interest. There were substantially more activated frontal voxels in both the left VLPFC (X2(1, N = 46) = 2238.45, p < .0001) and the right VLPFC (X2(1, N = 46) = 13440.01, p < .0001) for the MDD group as compared to the HC group. More specifically, the pattern of left-specific frontal activation in the HC group for this contrast was absent in the MDD subjects. In the MDD group, the ratio was 0.93:1 (left to right) activated clusters, revealing an inverted frontal laterality pattern in the MDD group for identification of emotions in faces. The percent of significant voxels activated for each group in the entire middle and inferior frontal BA masks are presented in Figure 1d (labeled left and right PFC).
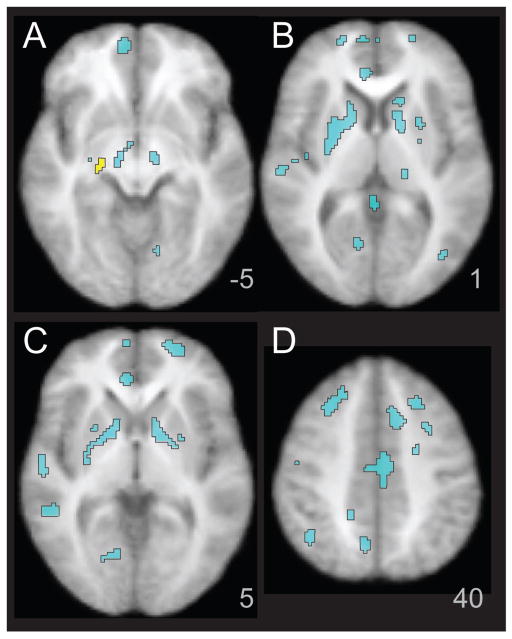
Between-Group Differences in fMRI Activation for Facial Emotion Processing
Group comparisons were conducted using ANCOVA in SPM5. Results are reported in Table 1 and displayed in Figure 2A–D, with HC > MDD in yellow and MDD > HC in cyan (p < .05, FDR, whole brain corrected). The HC group exhibited greater activation relative to the MDD group in a right parahippocampal cluster in the Faces-Animals contrast. In contrast, the MDD group had greater activation relative to the HC group in a large number of cortical and subcortical areas bilaterally, including bilateral superior frontal, middle frontal and precentral gyri, anterior, dorsal, and posterior cingulate, lingual gyrus, inferior parietal lobule, superior and middle temporal gyrus, middle occipital gyrus, cuneus, putamen, pulvinar, and substantia nigra. To investigate group effects within the amygdala (Dannlowski et al., 2007, Sheline et al., 2001), MarsBaR pick-atlas ROI was created, with a dilation of 1.5 and a threshold of p <.05, uncorrected. The MDD group exhibited bilateral greater amygdala activation than HC (left: t = 3.41, p = .0007, k = 29; right: t = 2.15, p = .019, k = 10).
Table 1.
Differences between Healthy Control (HC) and Major Depressive Disorder (MDD) Groups in Facial Emotion Processing
| Contrast/lobe | BA | Talairach Coordinates
|
Z | mm3 | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| HC greater than MDD | ||||||
| Parahippocampal | 28/35 | 20 | −21 | −7 | 3.43 | 200 |
|
| ||||||
| MDD greater than HC | ||||||
| Frontal | ||||||
| Superior Frontal | 9 | −24 | 43 | 28 | 3.17 | 160 |
| 10 | −18 | 53 | 1 | 3.89 | 864 | |
| 10/24 | 8 | 51 | −1 | 3.87 | 2016 | |
| Middle Frontal | 6 | −31 | 3 | 39 | 3.50 | 400 |
| 8 | −22 | 20 | 43 | 4.20 | 1128 | |
| 8/10 | 19 | 32 | 42 | 4.53 | 2472 | |
| Inferior Frontal | 47 | 31 | 14 | −14 | 3.25 | 176 |
| Precentral | 4 | −15 | −23 | 60 | 3.61 | 328 |
| 4 | 34 | −17 | 48 | 3.86 | 376 | |
| Anterior Cingulate | 24 | −6 | 35 | 0 | 3.56 | 824 |
| Dorsal Cingulate | 24 | −20 | −7 | 45 | 3.34 | 168 |
| 24/32 | −10 | 10 | 44 | 4.29 | 2088 | |
| Subcallosal | 34 | 24 | 8 | −10 | 3.41 | 160 |
| Parietal | ||||||
| Postcentral | 2/3 | −40 | −23 | 30 | 3.99 | 1072 |
| 43 | 54 | −13 | 17 | 3.55 | 288 | |
| Paracentral | 2/3/5 | 10 | −38 | 63 | 4.83 | 2208 |
| Inferior Parietal | 39/40 | 41 | −50 | 31 | 3.50 | 424 |
| −41 | −62 | 26 | 3.63 | 392 | ||
| 39 | 36 | −60 | 41 | 3.77 | 688 | |
| Posterior Cingulate | 31 | −11 | −52 | 27 | 3.82 | 384 |
| Posterior Cingulate/Precuneus | 13 | −52 | 31 | 5.01 | 20608 | |
| Temporal | ||||||
| Superior Temporal | 22 | 50 | −21 | 2 | 3.73 | 648 |
| Middle Temporal | 21 | 43 | −42 | −1 | 3.23 | 440 |
| 19/39 | −43 | −73 | 15 | 3.36 | 192 | |
| Insula | 13 | 38 | −17 | 8 | 3.41 | 408 |
| Occipital | ||||||
| Lingual | 18 | 10 | −69 | 0 | 3.83 | 464 |
| Lingual/Declive | −18 | −69 | −16 | 4.36 | 648 | |
| Middle Occipital | 18 | −24 | −91 | 18 | 3.26 | 176 |
| 19 | −40 | −69 | 7 | 3.9 | 392 | |
| Cuneus | 18 | −1 | −87 | 11 | 3.33 | 256 |
| Subcortical | ||||||
| Putamen | −24 | 3 | 9 | 3.41 | 368 | |
| Lateral Globus Pallidus | 17 | 1 | 3 | 4.81 | 3128 | |
| Putamen/Globus Pallidus | −11 | 1 | 2 | 3.89 | 1256 | |
| Substantia Nigra | 10 | −17 | −6 | 4.04 | 240 | |
| −10 | −15 | −8 | 4.16 | 296 | ||
| Pulvinar | −18 | −29 | 9 | 3.88 | 520 | |
Note. BA = Brodmann’s area; x, y, z = Talairach coordinates of significant effects.
Figure 2.
Greater Activation in MDD for Facial Emotion Identification compared to Healthy Control Subjects. The figure illustrates areas of significantly greater activation in healthy control subjects relative to MDD subjects in yellow. Clusters with greater activation in MDD relative to healthy control subjects are shown in cyan. Listed as z coordinates in the Talairach system.
Explicit Emotion Identification in MDD and Evaluation of Medication Effects
Prior to investigating relationships between performance and activation, one-tailed t tests and effect sizes in Cohen’s d were used to examine the groups on FEPT performance. For overall accuracy, MDD performed more poorly than did HC (t(44) = 1.86, p = .04, d = .75). Of note, although power for this comparison is low due to sample size, the pattern of effect sizes observed closely parallels prior studies that compared FEP in depressed and non-depressed adults (Langenecker et al., 2007). Moderate effect sizes indicating lower accuracy in MDD versus HC were observed for identifying happy (t(44) = 2.07, p = .02, d = .90) and fearful faces (t(44) = 1.57, p = .06, d = 0.55). Performance did not differ between the medicated and unmedicated MDD groups on any FEPT variables. Supplemental Table 3 displays accuracy and reaction time data and statistical results for within-scanner performance. Thus, performance-activation analyses could proceed on the supported premise that this sample performed similarly to those in prior studies.
Correlates of Intact and Disrupted Emotion Perception Performance in MDD
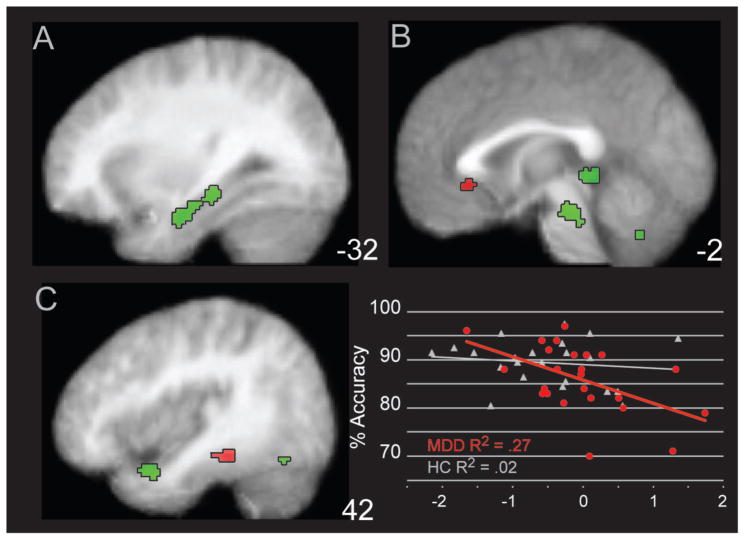
To investigate the role of performance on activation within the MDD group, a regression analysis was conducted with overall faces accuracy regressed upon FEP activation in the MDD group only. Results from this analysis are reported in Table 2, with some foci illustrated in Figure 3. A number of foci were positively related to task performance, including right amygdala, bilateral hippocampal and parahippocampal foci, left fusiform gyrus, in addition to cerebellum and habenula. In contrast, regions inversely associated with accuracy included left IFG and insula, right inferior parietal and parahippocampal foci, plus bilateral sgAC.
Table 2.
Regions Positively and Negatively Associated with Facial Emotion Identification Accuracy within the MDD Group
|
Accuracy Positive
|
BA | Talairach Coordinates
|
Z | mm3 | ||
|---|---|---|---|---|---|---|
| Contrast/Lobe | x | y | z | |||
| Temporal | ||||||
| Uncus/Superior Temporal | 38 | 38 | 6 | −19 | 3.53 | 600 |
| Middle Temporal | 21 | 48 | −5 | −15 | 4.13 | 160 |
| Fusiform | 37 | −38 | −60 | −12 | 3.38 | 880 |
| Parahippocampal/Hippocampal | 13 | −27 | −17 | 3.18 | 464 | |
| 35 | −25 | −27 | −10 | 3.58 | 2000 | |
| Amygdala | 27 | −7 | −8 | 3.14 | 160 | |
| Subcortical | ||||||
| Habenula | −1 | −36 | 0 | 3.87 | 576 | |
| Cerebellum, Vermis | −3 | −63 | −32 | 3.67 | 184 | |
| Cerebellum, Declive | 34 | −63 | −18 | 3.32 | 248 | |
| Pons | −3 | −27 | −21 | 4.02 | 2648 | |
|
Accuracy Negative
|
BA | Talairach Coordinates
|
Z | mm3 | ||
|---|---|---|---|---|---|---|
| Contrast/Lobe | x | y | z | |||
| Frontal | ||||||
| Inferior Frontal | 47 | −25 | 24 | −13 | 3.14 | 208 |
| Subgenual Anterior Cingulate | 25 | −1 | 26 | −2 | 3.26 | 176 |
| Parietal | ||||||
| Inferior Parietal | 40 | 54 | −21 | 36 | 4.07 | 448 |
| Temporal | ||||||
| Insula | 13 | −45 | −17 | 16 | 3.16 | 248 |
| Inferior Temporal | 37/20 | 54 | −44 | −13 | 4.22 | 352 |
| Parahippocampal | 36/37 | 33 | −34 | −13 | 3.52 | 448 |
Note. Post. = Posterior; BA = Brodmann’s area; x, y, z = Talairach coordinates of significant effects.
Figure 3.
Regions of Positive and Negative Association in Activation for MDD subjects during Facial Emotion Identification. The figure illustrates areas of activation that are positively associated with facial emotion accuracy classification in green, as well as areas of activation negatively correlated with activation in red. The activation for the subgenual anterior cingulate is displayed in the scatterplot relative to performance accuracy in MDD in red. The activation for healthy controls (HC) was extracted for comparative purposes and is displayed in white. Panels are listed as z coordinates in the Talairach system
Relationship of Shifted Laterality Extent of Activation with Emotion Perception Performance
To investigate the relationship between laterality and FEPT performance, a right/left ratio was created from the MARSBAR ROIs extracted in the “height” of activation, posthoc analysis (Figure 1). The sum of the two right hemisphere IFG clusters was divided by the sum of the two left hemisphere IFG clusters. Correlations were conducted examining the relationship of ratio scores with task performance. Right-to-left ratio scores were positively associated with accuracy in the MDD group and showed a substantial effect (r = .56, p = .004, Fisher’s Exact z = .63), whereas this relationship showed a small and nonsignificant effect size in the HC group (r = .21, p = .34, Fisher’s exact Z = .21). The HC group showed a trend inverse relationship between performance and right IFG activation (r = −.41, p = .06), but not in left IFG (r = −.08, p = .74). In the MDD group, an opposite pattern was observed: a positive relationship between performance and left IFG (r = .31, p = .14) and same direction but not as strong for right IFG (r = .15, p = .47, Supplemental Figure 1). The Fisher’s test of significance for one-tailed comparisons of correlations between MDD and HC for comparisons of the correlations of left IFG, right IFG, and right-to-left ratio scores exceeded the p < .05 threshold difference score of .263 in all three sets of correlations. These effects were similar when computing a partial correlation correcting for depression severity (HDRS), including the correlation of right-to-left IFG ratio score (r = .57, p =.005) and sgAC (r = −.41, p = .05) with FEPT accuracy.
This laterality-performance relationship was explored further to determine whether it was primarily driven by activation or deactivation on the right or left in the MDD group. Among the 19 MDD participants who showed increased right hemisphere activation (i.e., right+/left+ or right+/left−), there was a strong relationship between the laterality ratio to FEPT performance, r2 = .39, p = .004); in contrast, among participants who did not show right hemisphere activation, laterality ratio was unrelated to FEPT performance, r2 = .08, p = .65. Taken together, these results indicate that less activation of left relative to right (i.e., left < right IFG, or deactivation of the left IFG combined with right IFG activation) is associated with poor performance in women with MDD.
Evaluation of Possible Medication Effects related to Hyperactivation within the MDD Group
To determine whether aberrant activation in the MDD group was associated with psychotropic medication status, extracted (MarsBaR) activation values from the Faces minus Animals contrast in which group differences were observed (see Table 1) were evaluated. There were no significant effects of medication status using an uncorrected threshold of p < .05.
Discussion
During facial emotion processing, women with Major Depressive Disorder (MDD) showed hyperactivation in a large bilateral network of cortical and subcortical regions. The hyperactivity observed in women with MDD was characterized by shifted inferior frontal laterality compared to healthy controls. Whereas non-depressed healthy women exhibited a bilateral frontal network in response to the challenge of identifying facial emotions, women with MDD exhibited right-greater-than-left hyperactivation in inferior frontal gyrus (IFG), combined with extensive bilateral IFG activation. Furthermore, right-greater-than-left IFG laterality was associated with preserved performance in women with MDD. In contrast, left-greater than-right IFG and increased subgenual cingulate activation were associated with poor performance in women with MDD. Overall, these findings help to illustrate neural correlates of the relative impairments in facial emotion identification in women with MDD, whereby some effects were associated with diagnosis and others directly linked to diminished performance.
This is the first fMRI study to demonstrate that women with MDD show a shifted frontal laterality of activation during classification of emotional faces relative to healthy women controls. To our knowledge, the only related study demonstrating shifted laterality in response to emotion processing in women with MDD was conducted with EEG (Henriques & Davidson, 1991). The present findings support this prior work and further demonstrate that the laterality shift has two components, characterized by a greater magnitude of activation in the right compared to left inferior gyrus, in addition to greater bilateral spatial extent of activation in response to the challenge of processing facial emotion.
Although the pattern of shifted laterality in women for FEP during fMRI has not previously been reported, there is some support for right hyperactivity in individuals with MDD during tasks involving viewing emotional words and pictures (Langenecker et al., 2009, Rotenberg, 2004). Evidence for shifted laterality has also been demonstrated in other types of emotion processing paradigms. For example, Johnstone and colleagues (2007) reported a bilateral pattern of inferior frontal gyrus (IFG) activation among MDD patients during an affect regulation task. IFG activation among HC was left-lateralized, however this effect was not quantified. Furthermore, Mathersul et al. (2008) reported left-lateralized frontal alpha EEG activity in healthy young adults, accompanied by symmetrical frontal activity among adults with subclinical MDD. In contrast, two studies (Davidson et al., 2003, Grimm et al., 2008) reported reduced left lateral prefrontal activation combined with equivalent right hemisphere activation among adults with MDD compared to HC while viewing emotionally salient pictorial stimuli. These prior fMRI studies used generally qualitative interpretations of laterality shifts, unlike the quantitative approach utilized by EEG studies (Henriques and Davidson, 1991); Mathersul et al., 2008) and the present study. In a complementary study, Almeida and colleagues (2009) demonstrated reduced top-down control in effective connectivity from orbital medial frontal seed to amygdala in MDD and BD patients relative to HC for happy stimuli, and at the trend level for sad stimuli. Deficient medial and orbital frontal “automatic” top-down control of emotional responses has been carefully considered, although the relationship with lateral more “effortful” control region remains tenuous (Price & Drevets, 2012, Phillips, Ladouceur, & Drevets, 2008, Egner et al., 2006).
In women with MDD, poor emotion identification performance was associated with lower left relative to right IFG activation, suggesting a link between shifted laterality and emotion processing skill. Reduced activation of left, but not right IFG, was associated with poor performance. Intriguingly, a different pattern of results was observed in non-depressed women, such that those with greater right IFG activation performed worse on the task, and no relationship was observed between performance and left IFG or right: left IFG ratio. A speculative interpretation of these findings is that in a subset of women with MDD, the left IFG may not effectively engage during emotion processing, and successful recruitment of the right IFG in this context may serve a compensatory function. In non-depressed women, the meaning of increased right IFG activation is unclear. It might reflect an effort to compensate for less efficient emotion processing skill, to regulate emotional response to stimuli that interfere with performance, or reflect unexpressed illness in the presence of risk.
To our knowledge, the present study is also the first to show that increased activation in sgAC is related to poor FEP performance in women with MDD. The cluster bridges an important overlapping region between anterior Brodmann area 25 and ventral Brodmann area 24. This is similar to the rostral/subgenual cingulate region that is positively associated with treatment response after deep brain stimulation in treatment-resistant MDD (Lozano et al., 2008). A recent study (van Wingen et al., 2011), reported that dorso-rostral cingulate and dorsal cingulate regions were more active in current MDD than recovered MDD for emotion matching, with an inverse pattern for emotion labeling. The active MDD group also performed more poorly in labeling (but not the matching) emotions relative to the recovered MDD group, making interpretation of the results difficult in light of the relationships observed in the current study. Hyperactivity of the sgAC in response to facial expressions has been observed among individuals with MDD (Gotlib et al., 2005) and has been linked to greater disease severity (Keedwell et al., 2009), regulation in emotional but not cognitive interference tasks (Egner et al., 2006, Etkin, Egner, & Kalisch, 2011) as well as prediction of positive response to pharmacological treatment (Keedwell et al., 2010; Pizzagalli et al., 2011).
The general finding of hyperactivation in MDD in response to emotion processing is consistent with several prior reports (Demenescu et al., 2011, Frodl et al., 2009, Keedwell et al., 2009), but not uniformly so (Lawrence et al., 2004, Lee et al., 2008). Hyperactivation findings suggest that individuals who are depressed may require substantially greater cortical and subcortical circuitry across a broad network to perform emotional-cognitive tasks. The present study suggests that there exists a subset of depressed women with facial emotion identification deficits who may 1) engage relatively inefficient neural systems to support emotion processing, 2) use additional neural resources in attempt to compensate for a weaker system, 3) engage in self-referential processing irrelevant to the task at hand, or 4) exhibit hyperactivation as a result of attempts at emotion regulation. In contrast, activation of the right parahippocampal gyrus, important in emotion processing and regulation in healthy adults (Phillips et al., 2003), was reduced in the MDD group compared to healthy controls. One prior study also reported less hippocampal activation in individuals with MDD as compared to HC when viewing sad faces (Lee et al., 2008). This decreased activation may pertain directly to why women with MDD have difficulty in accurately identifying emotions in faces, especially given the extensive cluster in the hippocampus being positively associated with performance in MDD.
There are several limitations of the present study. First, there was some variability in severity of depressive symptoms within this sample, although the modal participant was in the moderately depressed range. Second, participants varied with regard to medication status; however, there were not activation differences based upon psychotropic medication status. Medication status likewise did not affect performance on the task. Thus, the network of hyperactive regions observed in the depressed group appears specific to disease process, rather than to medication artifacts, consistent with a recent meta-analysis of antidepressant effects on emotion processing in MDD (Delaveau et al., 2011). Due to the relatively modest sample size and the lack of placebo controlled medication trial, we can only state that medications do not appear to play a meaningful role in the results for this experiment; we cannot comment on the broader medication effect literature. Also due to sample size and power constraints we did not investigate whether individual emotion (e.g., happiness) by disease interactions are driving the hyperactivation in MDD (e.g., Eugene et al., 2010). Future studies with focus on event-related designs can better elaborate potential emotion by disease interactions. In addition, the use of ROI strategies for post hoc analyses of laterality is inherently explanatory in nature, and we must rely on replication in a separate sample to substantiate the pattern of shifted laterality in women with MDD. Finally, the present study focused exclusively on women, which may be viewed as both a limitation and a strength. This study was designed to be performed in women with MDD to minimize disease heterogeneity and capitalize on our previous findings, but this may also limit generalizability. Future research could test whether these phenomena are only present in females, as would be suggested by previous non-imaging studies from our group (Wright et al., 2009, Wright & Langenecker, 2008) or are also present in similar males diagnosed with MDD.
In summary, the present study provides novel links between activation abnormalities in MDD and performance on an explicit facial emotion identification task with known performance decrements in MDD (Langenecker et al., 2005). The study also extends existing findings of disease-specific abnormalities in emotion processing networks in MDD. Facial emotion processing decrements in women with MDD were associated with a unique pattern of disrupted laterality in brain activation and enhanced subgenual cingulate activation. The role of this laterality shift in the genesis and maintenance of MDD remains unclear, but is an exciting lead toward better definition of homogeneous subtypes in MDD, with potential downstream benefits for treatment tailoring and evaluation of the efficacy of existing treatments.
Supplementary Material
Acknowledgments
This project was supported by a KL2 Career Development Award (NIMH RR024987, SAL); K23 Award (NIMH074459, SAL); Psychiatry Research Committee (SAL); National Alliance for Research in Schizophrenia and Depression Young Investigator Award (SAL), General Clinical Research Center pilot grant to MNS and SAL (for some control fMRI scans, from # MO1 RR00042); Rachel Upjohn Clinical Scholars Awards (SAL, SLW); and some fMRI scans from the University of Michigan functional MRI lab (SAL, SLW); as well as the Phil F. Jenkins Foundation (JKZ). Allison M. Kade, Michael L. Brinkman, Leslie Guidotti-Breting, Benjamin D. Long, Lawrence S. Own, Thomas M. Hooven, and Karandeep D. Singh are thanked for their assistance in data collection and analysis for this work. The staff and faculty at the University of Michigan fMRI lab are gratefully acknowledged for their assistance and support in completing this work.
Abbreviations
- MDD
Major Depressive Disorder
- FEP
facial emotion perception
- FEPT
Facial Emotion Perception Test
- HC
Healthy Control
- DIFG
dorsal inferior frontal gyrus
- sgAC
subgenual anterior cingulate gyrus
- VIFG
ventral inferior frontal gyrus
- VLPFC
ventro-lateral prefrontal cortex
References
- Almeida JRC, Versace A, Hassel S, Kupfer DJ, Phillips ML. Elevated amygdala activity to sad facial expressions: A state marker of bipolar but not unipolar depression. Biological Psychiatry. 2010;67:414–421. doi: 10.1016/j.biopsych.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida JRC, Versace A, Mechelli A, Hassel S, Quevedo K, Kupfer DJ, Phillips ML. Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biological Psychiatry. 66:451–459. doi: 10.1016/j.biopsych.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhuys AL, Geerts E, Gordijn MCM. Depressed patients’ perceptions of facial emotions in depressed and remitted states are associated with relapse - A longitudinal study. Journal of Nervous and Mental Disease. 1999;187:595–602. doi: 10.1097/00005053-199910000-00002. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabreque R, Poline J-B. Region of interest analysis using an SPM toolbox. 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2002. [Google Scholar]
- Canli T, Cooney RE, Goldin P, Shah M, Sivers H, Thomason ME, Whitfield-Gabrieli S, Gabrieli JDE, Gotlib IH. Amygdala reactivity to emotional faces predicts improvement in major depression. Neuroreport. 2005;16:1267–1270. doi: 10.1097/01.wnr.0000174407.09515.cc. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Khanna A, Mourao-Miranda J, Fu CHY. Neural correlates of sad faces predict clinical remission to cognitive behavioural therapy in depression. Neuroreport. 2009;20:637–641. doi: 10.1097/WNR.0b013e3283294159. [DOI] [PubMed] [Google Scholar]
- Csukly G, Czobor P, Szily E, Takacs B, Simon L. Facial expression recognition in depressed subjects: The impact of intensity level and arousal dimension. Journal of Nervous and Mental Disease. 2009;197:98–103. doi: 10.1097/NMD.0b013e3181923f82. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Deckert J, Hohoff C, Kugel H, Arolt V, Heindel W, Kersting A, Baune BT, Suslow T. 5-HTTLPR biases amygdala activity in response to masked facial expressions in major depression. Neuropsychopharmacology. 2008;33:418–424. doi: 10.1038/sj.npp.1301411. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Kugel H, Arolt V, Heindel W, Kersting A. Amygdala reactivity to masked negative faces is associated with automatic judgmental bias in major depression: a 3 T fMR1 study. Journal of Psychiatry & Neuroscience. 2007;32:423–429. [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W, Anderle MJ, Kalin NH. The neural substrates of affective processing in depressed patients treated with venlafaxine. American Journal of Psychiatry. 2003;160:64–75. doi: 10.1176/appi.ajp.160.1.64. [DOI] [PubMed] [Google Scholar]
- Delaveau P, Jabourian M, Lemogne C, Guionnet S, Bergouignan L, Fossati P. Brain effects of antidepressants in major depression: A meta-analysis of emotional processing studies. Journal of Affective Disorders. 2011;130:66–74. doi: 10.1016/j.jad.2010.09.032. [DOI] [PubMed] [Google Scholar]
- Demenescu LR, Renken R, Kortekaas R, van Tol MJ, Marsman JBC, van Buchem MA, van der Wee NJA, Veltman DJ, den Boer A, Aleman A. Neural correlates of perception of emotional facial expressions in out-patients with mild-to-moderate depression and anxiety. A multicenter fMRI study. Psychological Medicine. 2011;41:2253–2264. doi: 10.1017/S0033291711000596. [DOI] [PubMed] [Google Scholar]
- Egner T, Etkin A, Gale S, Hirsch J. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cerebral Cortex. 2008;18:1475–1484. doi: 10.1093/cercor/bhm179. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect. Consulting Psychologists Press; Palo Alto, CA: 1976. [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. The neural basis of mood-congruent processing biases in depression. Archives of General Psychiatry. 2002;59:597– 604. doi: 10.1001/archpsyc.59.7.597. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM–IV Axis I disorders. American Psychiatric Press; Washington, DC: 1996. [Google Scholar]
- Frodl T, Scheuerecker J, Albrecht J, Kleemann AM, Muller-Schunk S, Koutsouleris N, Moller HJ, Bruckmann H, Wiesmann M, Meisenzahl E. Neuronal correlates of emotional processing in patients with major depression. World Journal of Biological Psychiatry. 2009;10:202–208. doi: 10.1080/15622970701624603. [DOI] [PubMed] [Google Scholar]
- Fu CHY, Williams SCR, Cleare AJ, Brammer MJ, Walsh ND, Kim J, Andrew CM, Pich EM, Williams PM, Reed LJ, Mitterschiffthaler MT, Suckling J, Bullmore ET. Attenuation of the neural response to sad faces in major depression by antidepressant treatment - A prospective, event-related functional magnetic resonance imaging study. Archives of General Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry & Neuroscience. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Beck J, Schuepbach D, Hell D, Boesiger P, Bermpohl F, Niehaus L, Boeker H, Northoff G. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: An fMRI study in severe major depressive disorder. Biological Psychiatry. 2008;63:369–376. doi: 10.1016/j.biopsych.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Gur RC, Perkins AC, Schroeder L, Turner T, Turetsky BI, Chan RM, Loughead JW, Alsop DC, Maldjian J, Gur RE. Age-related differences in brain activation during emotional face processing. Neurobiology of Aging. 2003;24:285–295. doi: 10.1016/s0197-4580(02)00099-4. [DOI] [PubMed] [Google Scholar]
- Gur RC, Erwin RJ, Gur RE, Zwil AS, Heimberg C, Kraemer HC. Facial emotion discrimination. 2. Behavioral findings in depression. Psychiatry Research. 1992;42:241– 251. doi: 10.1016/0165-1781(92)90116-k. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. Journal of Abnormal Psychology. 1991;100:535–545. doi: 10.1037//0021-843x.100.4.535. [DOI] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: Counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. Journal of Neuroscience. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Is this happiness I see? Biases in the identification of emotional facial expressions in depression and social phobia. Journal of Abnormal Psychology. 2006;115:705–714. doi: 10.1037/0021-843X.115.4.705. [DOI] [PubMed] [Google Scholar]
- Keedwell P, Drapier D, Surguladze S, Giampietro V, Brammer M, Phillips M. Neural markers of symptomatic improvement during antidepressant therapy in severe depression: subgenual cingulate and visual cortical responses to sad, but not happy, facial stimuli are correlated with changes in symptom score. Journal of Psychopharmacology. 2009;23:775–788. doi: 10.1177/0269881108093589. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Drapier D, Surguladze S, Giampietro V, Brammer M, Phillips M. Subgenual cingulate and visual cortex responses to sad faces predict clinical outcome during antidepressant treatment for depression. Journal of Affective Disorders. 2010;120:120–125. doi: 10.1016/j.jad.2009.04.031. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Hoffman LJ, Eastman LB, Healey K, Moberg PJ. Facial emotion perception in depression and bipolar disorder: A quantitative review. Psychiatry Research. 2011;188:303–309. doi: 10.1016/j.psychres.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Bieliauskas LA, Rapport LJ, Zubieta JK, Wilde EA, Berent S. Face emotion perception and executive functioning deficits in depression. Journal of Clinical and Experimental Neuropsychology. 2005;27:320–333. doi: 10.1080/13803390490490515720. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Caveney AF, Giordani B, Young EA, Nielson KA, Rapport LJ, Biellauskas LA, Mordhorst MJ, Marcus S, Yodkovik N, Kerber K, Berent S, Zubieta JK. The sensitivity and psychometric properties of a brief computer-based cognitive screening battery in a depression clinic. Psychiatry Research. 2007a;152:143–154. doi: 10.1016/j.psychres.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Kennedy SE, Guidotti LM, Briceno EM, Own LS, Hooven T, Young EA, Akil H, Noll DC, Zubieta JK. Frontal and limbic activation during inhibitory control predicts treatment response in major depressive disorder. Biological Psychiatry. 2007b;62:1272–1280. doi: 10.1016/j.biopsych.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenecker SA, Lee HJ, Bieliauskas LA. Neuropsychology of depression and related mood disorders. In: Grant I, Adams KM, editors. Neuropsychological Assessment of Neuropsychiatric Disorders. Oxford University Press; 2009. [Google Scholar]
- Langenecker SA, Weisenbach SL, Giordani B, Briceno EM, Guidotti-Breting LM, Schallmo MP, Leon HM, Noll DC, Zubieta JK, Schteingart DE, Starkman MN. Impact of chronic hypercortisolemia on affective processing. Neuropharmacology. 2012;62:217–225. doi: 10.1016/j.neuropharm.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, Frangou S, Ecker C, Phillips ML. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biological Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Lee BT, Seok JH, Lee BC, Cho SW, Yoon BJ, Lee KU, Chae JH, Choi IG, Ham BJ. Neural correlates of affective processing in response to sad and angry facial stimuli in patients with major depressive disorder. Progress in Neuro- Psychopharmacology & Biological Psychiatry. 2008;32:778–785. doi: 10.1016/j.pnpbp.2007.12.009. [DOI] [PubMed] [Google Scholar]
- LeMoult J, Joormann J, Sherdell L, Wright Y, Gotlib IH. Identification of emotional facial expressions following recovery from depression. Journal of Abnormal Psychology. 2009;118:828–833. doi: 10.1037/a0016944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biological Psychiatry. 2008;64:461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- Mak A, Hu Z, Zhang J, Xiao Z, Lee T. Neural correlates of regulation of positive and negative emotions: An fMRI study. Neuroscience Letters. 2009;457:101–106. doi: 10.1016/j.neulet.2009.03.094. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitechtonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Chen SHA, Tseng WYI. AveLI: A robust lateralization index in functional magnetic resonance imaging using unbiased threshold-free computation. Journal of Neuroscience Methods. 2012;205:119–129. doi: 10.1016/j.jneumeth.2011.12.020. [DOI] [PubMed] [Google Scholar]
- Persad SM, Polivy J. Differences between depressed and nondepressed individuals in the recognition of and response to facial emotional cues. Journal of Abnormal Psychology. 1993;102:358–368. doi: 10.1037//0021-843x.102.3.358. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry. 2008;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biological Psychiatry. 2003;54:504– 514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA. Frontocingulate dysfunction in depression: Toward biomarkers of treatment response. Neuropsychopharmacology. 2010;36:83–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends in Cognitive Sciences. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Rapport LJ, Friedman SL, Tzelepis A, Van Voorhis A. Experienced emotion and affect recognition in adult attention-deficit hyperactivity disorder. Neuropsychology. 2002;16:102–110. doi: 10.1037//0894-4105.16.1.102. [DOI] [PubMed] [Google Scholar]
- Rotenberg VS. The peculiarity of the right-hemisphere function in depression: solving the paradoxes. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2004;28:1–13. doi: 10.1016/S0278-5846(03)00163-5. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biological Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, Williams SCR, Phillips ML. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biological Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. Pearson; Boston: 2007. [Google Scholar]
- Thomas EJ, Elliott R, McKie S, Arnone D, Downey D, Juhasz G, Deakin JFW, Anderson IM. Interaction between a history of depression and rumination on neural response to emotional faces. Psychological Medicine. 2011;41:1845–1855. doi: 10.1017/S0033291711000043. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wingen GA, van Eijndhoven P, Tendolkar I, Buitelaar J, Verkes RJ, Fernandez G. Neural basis of emotion recognition deficits in first-episode major depression. Psychological Medicine. 2011;41:1397–1405. doi: 10.1017/S0033291710002084. [DOI] [PubMed] [Google Scholar]
- Watkins PC, Vache K, Verney SP, Muller S, Mathews A. Unconscious mood-congruent memory bias in depression. Journal of Abnormal Psychology. 1996;105:34–41. doi: 10.1037//0021-843x.105.1.34. [DOI] [PubMed] [Google Scholar]
- Wright SL, Langenecker SA, Deldin PJ, Rapport LJ, Nielson KA, Kade AM, Own LS, Akil H, Young EA, Zubieta JK. Gender-specific disruptions in emotion processing in younger adults with depression. Depression and Anxiety. 2009;26:182–189. doi: 10.1002/da.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SL, Langenecker SA. Differential risk for emotion processing difficulties by gender and age in Major Depresive Disorder. In: Hernandez P, Alonso S, editors. Women and Depression. Nova Science Publishers, Inc; 2008. pp. 1–33. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.