Abstract
Epileptic encephalopathies are a phenotypically and genetically heterogeneous group of severe epilepsies accompanied by intellectual disability and other neurodevelopmental features1-6. Using next generation sequencing, we identified four different de novo mutations in KCNA2, encoding the potassium channel KV1.2, in six patients with epileptic encephalopathy (one mutation recurred three times independently). Four individuals presented with febrile and multiple afebrile, often focal seizure types, multifocal epileptiform discharges strongly activated by sleep, mild-moderate intellectual disability, delayed speech development and sometimes ataxia. Functional studies of the two mutations associated with this phenotype revealed an almost complete loss-of-function with a dominant-negative effect. Two further individuals presented with a different and more severe epileptic encephalopathy phenotype. They carried mutations inducing a drastic gain-of-function effect leading to permanently open channels. These results establish KCNA2 as a novel gene involved in human neurodevelopmental disorders by two different mechanisms, predicting either hyperexcitability or electrical silencing of KV1.2-expressing neurons.
Many of the voltage-gated potassium channels (KV1–12) are expressed in the central nervous system (CNS), playing an important role in neuronal excitability and neurotransmitter release7. Mutations in potassium channel-encoding genes cause different neurological diseases, including benign familial neonatal seizures (KCNQ2/KV7.2, KCNQ3/KV7.3)8-10, neonatal epileptic encephalopathy (KCNQ2)11,12, episodic ataxia type 1 (EA1) (KCNA1/KV1.1)13, and peripheral nerve hyperexcitability (KCNA1, KCNQ2)13-15. In addition, antibodies against KV1.1 or associated proteins like Contactin-associated protein 2 (Caspr2) or Leucine-rich, glioma-inactivated 1 protein (LGI1) cause limbic encephalitis or neuromyotonia16. Therefore, potassium channel genes represent interesting candidates for neurodevelopmental disorders.
To identify mutations in presumed genetic forms of epilepsy, we designed a targeted re-sequencing panel17 comprising 265 known and 220 candidate genes for epilepsy (Supplementary Table 1). Screening a pilot cohort of 33 patients, we identified mutations in known epilepsy genes in 16 cases17. The remaining 17 cases were evaluated for mutations in candidate genes (Supplementary note), which led to the detection of a heterozygous de novo mutation in KCNA2, c.1214C>T, p.Pro405Leu (P405L), affecting the highly-conserved pore domain of the voltage-gated potassium channel KV1.2. This mutation is not found in control databases (1000G, EVS, dbSNP138, ExAC).
The female Patient #1 carrying this mutation had unremarkable early development until epilepsy onset at 17 months old. The phenotype included febrile and afebrile alternating hemiclonic seizures and status epilepticus, reminiscent of Dravet syndrome. The electroencephalogram (EEG) showed multifocal spikes with marked activation during sleep. After seizure onset, ataxia and delay of psychomotor and language development became apparent. She had postnatal short stature, growth hormone deficiency and hypothyroidism. Seizures and ataxia responded poorly to antiepileptic drugs (topiramate, oxcarbazepine, valproic acid, bromide), including acetazolamide (known to be effective in EA1 caused by mutations in KCNA118). At last follow-up at eight years old, she had remained seizure-free for the past six months without previous change of medication.
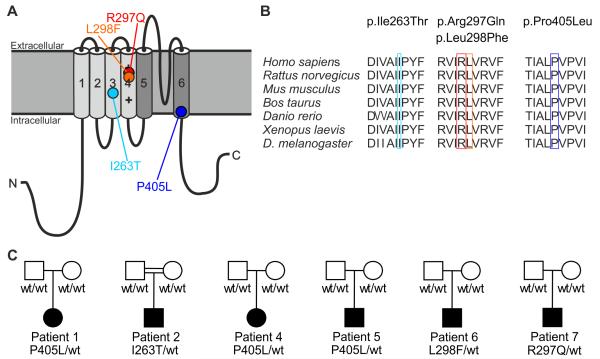
Further KCNA2 mutations were identified in several parallel studies (Supplementary Fig. 1). First, we performed whole exome sequencing (WES) in 86 parent-offspring trios with epileptic encephalopathy (31 with SCN1A-negative Dravet syndrome [DS], 39 with myoclonic-atonic epilepsy [MAE], and 16 with electrical status epilepticus in slow-wave sleep [ESES]). Second, we performed panel sequencing (Supplemental note) in 147 adult patients with a broad spectrum of epilepsy phenotypes associated with intellectual disability. Third, we performed WES in an adult cohort of 10 independent trios with severe epilepsy and intellectual disability, and WES in another cohort of 12 independent, isolated index cases with early-onset ataxia and epilepsy. We identified six additional independent cases with previously-unreported heterozygous KCNA2 variants (Table 1, Supplementary note): Patient #2 (initially classified as MAE) carried the de novo mutation c.788T>C, p.Ile263Thr (I263T). Patient #3 (intellectual disability with neonatal-onset focal epilepsy and cerebellar hypoplasia) carried the variant c.440G>A, p.Arg147Lys (R147K), of unknown inheritance. Since (i) it could not be confirmed as de novo, (ii) was predicted as benign from seven out of nine prediction tools, (iii) lysine occurs naturally at that position in drosophila and zebrafish, and (iv) did not reveal functional consequences, R147K was considered a variant of unknown significance (see Supplementary note, Supplementary Tables 1 and 2, and Supplementary Fig. 3). Patients #4 (initially classified as DS with prominent focal seizures) and #5 (intellectual disability with febrile seizures, focal seizures and status epilepticus) also carried the de novo P405L mutation (Fig. 1c and Supplementary Fig. 2). Patients #1, #2, #4 and #5 eventually became seizure-free between four and 15 years old, whereas intellectual disability and (in #1 and #4) mild to moderate ataxia remained unchanged. Recurrence of P405L in three independent cases suggests a mutational hotspot: c.1214 is located in a stretch of cytosines and guanines and the C>T mutation likely occurs due to a methylated CpG sequence, possibly bypassing the DNA repair system and so becoming prone to this pyrimidine-pyrimidine substitution.
Table 1.
Main phenotypic characteristics of patients carrying a disease-causing de novo KCNA2 mutation.
| Patient 1 | Patient 2 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | |
|---|---|---|---|---|---|---|
| Cohort | 1st epilepsy panel (n=33) | MAE (n=39) | DS (n=31) | adult EE I (n=147) | adult EE II (n=10) | Ataxia & epilepsy (n=12) |
| Mutation | c.1214C>T, p.Pro405Leu de novo | c.788T>C, p.Ile263Thr de novo | c.1214C>T, p.Pro405Leu de novo | c.1214C>T, p.Pro405Leu de novo | c.894G>T, p.Leu298Phe de novo | c.890G>A, p.Arg297Gln de novo |
| Functional consequence | loss of function | gain of function | ||||
| Gender/Age | F/8y | M/7y | F/5y | M/19y | M/36y | M/26y |
| Development prior to seizure onset | normal | |||||
| Age at seizure onset | 17m | 11m | 10m | 8m | 6m | 5m |
| Seizure type at onset | FS, hemiclonic seizures | MC | FS, FDS | Febrile SE | GTCS | Febrile SE |
| Other seizure types | FS, MC, FDS, focal motor seizures, secondary GTCS | MC, MA | FS, FDS, focal motor seizures, possible extension spasms | FS, focal motor seizures, secondary GTCS | MC, atypical absences | GTCS, absences |
| Seizure outcome | Seizure free since age 7 ½y old | Seizure free since age 4y old | Seizure free since age 4y old | Seizure free since age 15y old | GTCS bimonthly on polytherapy | GTCS once a year on lamotrigine |
| EEG at onset | Focal sharp waves | Focal sharp waves and spikes | normal | Sharp waves, bilateral centro-temporo-frontal spikes | n.a. | n.a. |
| Course of EEG | Multifocal sharp waves and sharp slow waves, accentuated over the left frontocentral region with significant increase during sleep | Multifocal sharp waves and polyspikes. Since age 6y: normal |
Focal sharp waves. From age 2y: sharp waves, spike-waves and polyspike-waves over both centro-temporal regions, independently or bilaterally synchronous (left more than right); increase during sleep |
At age 4y: multifocal epileptiform discharges activated by sleep Since age 17y: normal |
At age 22y: frequent generalized spike wave discharges in a diffusely slow background | At age 6y: generalized spike waves and polyspike-waves |
| Neurological examination | Mild-moderate ataxia, constant myoclonus | normal | Mild ataxia, myoclonus at rest in hand and fingers | normal | Moderate ataxia, occasional myoclonus at rest | Moderate-severe ataxia, hyperreflexia |
| Development at last follow up | Mild-moderate ID, delayed speech development | Mild-moderate ID | Learning disability, delayed speech development | Moderate ID, delayed speech development | Severe ID, no speech, requires help with all aspects of daily activities | Moderate ID |
| MRI | normal | |||||
| Additional features | GH deficiency, IGF-1: −0.7 SDS (1y2m), −8.5 SDS (3y5m) subclinical hypothyroidism | Severe scoliosis | Facial dysmorphism (broad forehead, bulbous nasal tip, deep set eyes, synophris, full lips) | |||
Abbreviations: F: female; FDS: focal dyscognitive seizures; FS: febrile seizures; GH: growth hormone; GTCS: generalized tonic-clonic seizures; ID: intellectual disability; HC: head circumference; m: months; M: male; MA: myoclonic-atonic seizures; MC: myoclonic seizures; n.a.: not available; SE: status epilepticus; y: years
Figure 1.
Mutations in the KV1.2 channel. (A) Structure of the voltage-gated potassium channel KV1.2 with transmembrane segments S1–S4 forming the voltage sensor domain (light gray) and the pore region S5-S6 (in dark gray) with its pore-forming loop. Mutations are localized in highly-conserved regions in the S3 segment (I263T, light blue), the S4 segment constituting the voltage sensor (R297Q, red; L298F, orange) and the S6 segment (P405L, dark blue). (B) I263, R297, L298 and P405 and the respective surrounding amino acids show evolutionary conservation. (C) Pedigrees of patients #1, #2 and #4–7.
Patient #6 carried the de novo mutation c.894G>T, p.Leu298Phe (L298F). His phenotype was different and much more severe, presenting with severe intellectual disability with gradual loss of language and motor skills, pharmacoresistant generalized tonic-clonic, atypical absence and myoclonic seizures, facial dysmorphism, generalized epileptic discharges and moderate ataxia (Table 1 and Supplementary note). Similarly, patient #7 carrying the de novo mutation c.890G>A, p.Arg297Gln (R297Q) presented with a more severe phenotype consisting of moderate intellectual disability, moderate to severe ataxia and pharmacoresistant seizures.
We subsequently screened a follow-up cohort of 99 patients, comprising 47 individuals with unresolved epileptic encephalopathy, short stature and/or ataxia as well as 52 individuals with intellectual disability and idiopathic severe GH deficiency without detecting additional sequence alterations by Sanger sequencing. We excluded copy number variations affecting KCNA2 in all 99 follow-up cases as well as 86 trio-WES cases using an in-house-developed multiplex amplicon quantification technique (Online Methods and Supplementary Fig. 1).
To validate our findings statistically and corroborate KCNA2 as a new disease-predisposing gene for epileptic encephalopathy, we calculated the probability for recurring KCNA2 mutations occurring by chance in our cohorts. Comparing the allele frequency of six (two times P405L) KCNA2 non-synonymous variants in our validation cohorts (6/(354×2), excluding the first P405L mutation detected in the discovery cohort of 33 patients) with those missense and nonsense variants reported in the largest available control database (ExAC, 144/122828), revealed a significant enrichment of KCNA2 variants in our patient cohorts using Fisher’s exact test (p=2.6×10−4). Further statistical evidence is provided in the Supplementary Note. KCNA2 had not been associated with a human disease so far. However, during the review process of this manuscript, a single case report was published describing a 7-year-old boy with the KCNA2 de novo mutation R297Q presenting with ataxia and myoclonic epilepsy, similar to our patient #7.19 In addition, the Pingu mouse presenting with ataxia and growth retardation carries a Kcna2 loss-of-function mutation, p.Ile402Thr, in close proximity to P405L; Kcna2 knock-out mice present with severe seizures and premature death20,21.
KV1.2 belongs to the KV1 family (KV1.1–8), all members of which are expressed in the CNS. These channels consist of four subunits with six transmembrane segments (S1-S6). S4 forms the voltage-sensor and S5-S6 the pore region containing a selectivity filter and gating ion flow22 (Fig. 1a). All four KCNA2 sequence alterations detected in patients #1–7 (except the one in #3) are localized in highly-conserved and functionally-important protein regions (Fig. 1b), and were predicted as pathogenic. P405L disrupts the highly-conserved, KV-specific PVP motif in S6, which is thought to link the gate to the voltage-sensor23,24. A PVP>AVP mutation in KV1.5 leads to a non-functional channel25. I263T in S3 may disrupt a hydrophobic segment proposed to focus the electric field across the cell membrane, thus enabling the S4 gating charges to translocate over a smaller distance rather than the entire depth of the membrane bilayer26. Furthermore, I263T in KV1.2 corresponds to I262T in KV1.1 causing EA1 with distal weakness.27 Finally, R297Q and L298F directly affect the S4 voltage sensor, and R297Q has been described before to induce a negative shift of the activation curve.27,28
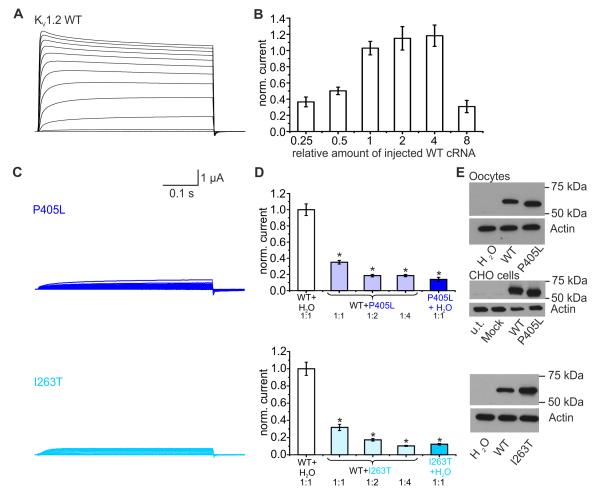
Functional effects of all detected sequence alterations were examined using an automated two-microelectrode voltage-clamp oocyte testing system. We found a sigmoidal relationship between the amount of injected wildtype (WT) cRNA and potassium current amplitude, with a strong decrease in amplitude for the 8-fold cRNA amount (Fig. 2b, Supplementary Fig. 5). This quantitative titration of protein levels by varying the amounts of injected RNA was used to determine the amount of injected cRNA for further experiments. For P405L and I263T, we found a dramatic reduction of current amplitudes and thus a clear loss of channel function (Figs. 2c). When either of the two mutations were co-expressed with WT KV1.2 in a 1:1, 1:2 or 1:4 ratio, with constant amount of injected WT cRNA, current amplitudes significantly decreased (Figs. 2d) compared with similar amounts of WT alone (Fig. 2b). Hence, both P405L and I263T exert a clear dominant-negative effect on WT KV1.2 channels. Furthermore, I263T caused a depolarizing shift of voltage-dependent activation, and slight changes in inactivation were found for P405L (Supplementary Fig. 4).
Figure 2.
Functional effects of the KCNA2 mutations P405L and I263T. (A) Representative current traces of KV1.2 wildtype (WT) channels recorded in a Xenopus laevis oocyte during voltage steps (from −80 mV to +70 mV). (B) Effect of increasing amounts of injected WT-KCNA2 cRNA on current amplitude (0.25: n=13; 0.5: n=18; 1: n=22; 2: n=17; 4: n=20; 8: n=19). Shown are means ± SEM. (C) Current traces derived from KV1.2-P405L (top) and KV1.2-I263T (bottom) channels recorded as described in (A). (D) K+-currents were reduced for mutants P405L (top) and I263T (bottom) compared to WT-cRNA (top: P405L: n=10; WT: n=44; bottom: I263T: n=10; WT: n=34). A dominant-negative effect of P405L and I263T mutants on KV1.2-WT channels was shown when a constant amount of WT cRNA (amount 1 in (B)) was injected with either H2O or increasing amounts of mutant cRNA (top: P405L: ratio 1:1: n=47; ratio 1:2: n=40; ratio 1:4: n=36; bottom: I263T: ratio 1:1: n=34; ratio 1:2: n=42; ratio 1:4: n=38). Co-expression of P405L or I263T and the WT led to a significant reduction of the current amplitude compared to the WT alone. Groups were statistically different (One-way ANOVA (p<0.001), posthoc Dunn’s method (p<0.05)). Shown are means ± SEM. (E) Western blot analysis from lysates of Xenopus laevis oocytes injected with equal amounts of KV1.2-WT or mutant cRNA (P405L: top; I263T: bottom) or from lysates of CHO cells transiently transfected with KV1.2-WT and P405L cDNAs (middle). For P405L-mutant channels there was a shift from 57 kDa to ~58.5 kDa (n=3). KV1.2-WT or I263T (n=3) mutant channels revealed similar bands (57 kDa).
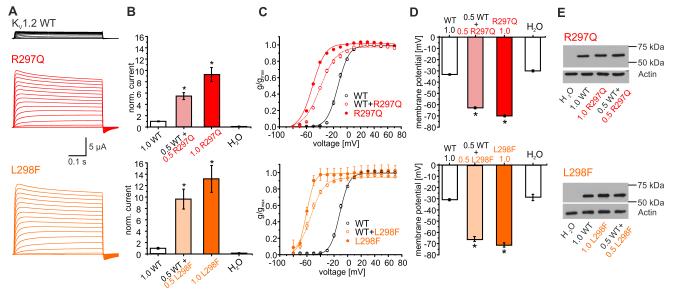
In contrast to P405L and I263T, both R297Q and L298F induced strong gain-of-function effects. Neutralization of the second arginine in the voltage sensor in KV1.2-R297Q increased current amplitudes by 9-fold and shifted the voltage dependence of steady-state activation by −40 mV compared with WT (Fig. 3a–c), The gain-of-function of the L298F mutation was even more pronounced with a 13-fold increase in current amplitudes and a −50 mV shift of the activation curve (Fig. 3a–c). As a consequence of the permanently open mutant channels, resting membrane potentials of oocytes expressing R297Q or L298F channels were about 40 mV more negative than of those expressing WT (Fig. 3d). Both mutations exerted a dominant effect on the WT, since co-injection of either R297Q or L298F with WT in a 0.5:0.5 ratio revealed very similar alterations as with one of the mutations (1.0) alone (Fig. 3b–d).
Figure 3.
Functional effects of the KV1.2 mutations R297Q and L298F. (A) Representative current traces derived from KV1.2-WT (top), R297Q (middle) or L298F mutant channels (bottom) recorded as described in Fig. 2A. (B) Mean current amplitudes of top: KV1.2-WT (1.0, n=23), WT + R297Q (0.5:0.5, n=37), R297Q (1.0, n=35) and H2O injection (n=25); bottom: KV1.2-WT (1.0, n=13), WT + L298F (0.5:0.5, n=26), L298F (1.0, n=14), and H2O injection (n=10). Shown are means±SEM. There was a statistical significant difference between WT and tested groups (ANOVA on ranks; p<0.001) with posthoc Dunn’s Method (p<0.05)). (C) Mean voltage dependence of KV1.2 channel activation for WT, R297Q (red, top) or L298F channels (orange, bottom). Shown are means ± SEM. Lines represent Boltzmann functions fit to data points. Activation curves of mutant channels were significantly shifted to more hyperpolarized potentials (p<0.05). For details see Supplementary notes. (D) Resting membrane potentials of oocytes injected with: top: WT (1.0, n=44), WT+R297Q (0.5:0.5, n=42), R297Q (1.0; n=38) or H2O (n=24); bottom: WT (1.0, n=30), WT+L298F (0.5:0.5, n=34), L298F (1.0; n=28) or H2O (n=13). Shown are means ± SEM. Statistically significant differences between WT and tested groups was verified by ANOVA on ranks (p<0.001) with posthoc Dunn’s Method (p<0.05). (E) Western blot analysis from lysates of Xenopus oocytes injected with KV1.2-WT (1.0), KV1.2-WT (0.5) + R297Q (0.5, top), mutant R297Q (1.0, top), KV1.2-WT (0.5) + L298F (0.5, bottom) or mutant L298F (1.0, bottom) cRNA (n=3). All channels revealed similar bands (57 kDa).
To examine protein production and stability, we performed SDS-page analysis of total cell lysates using a monoclonal anti-KV1.2 antibody. Representative Western blots show that all mutations generate a protein expression level similar to the 57-kD band of the WT (Figs. 2e and 3e). A slight but reproducible shift was found for the band of P405L in both oocytes and mammalian cells (Fig. 2e, top, middle). Steric properties of proline can disrupt secondary structure elements, which could be important for the function of the conserved PVP motif. A leucine in this position (LVP) could induce a structural change resulting in altered gel migration28.
KV1.2 belongs to the delayed rectifier class of potassium channels enabling efficient neuronal repolarization following an action potential. Loss-of-function mutations predict hyperexcitable neuronal membranes and repetitive neuronal firing due to impaired repolarization. This hypothesis is corroborated by the epileptic phenotype of the Kcna2 knock-out mouse21. In stark contrast, R297Q and L298F predict permanently open channels at physiological membrane potentials, and electrical silencing by membrane hyperpolarization (as observed in oocytes). It is difficult to speculate about the pathophysiological consequences of a KV1.2 loss- or gain-of-function beyond the level of single neurons, particularly since this channel has been detected in a broad range of both excitatory and inhibitory neurons29,30. Further experiments in gene-targeted mouse models could answer these questions.
In summary, we identified de novo mutations in KCNA2 causing mild to severe epileptic encephalopathy in roughly 1.7% of cases across our different cohorts. The phenotype associated with dominant-negative loss-of-function mutations comprised infantile/early-childhood seizure onset, frequent febrile and afebrile focal motor and dyscognitive seizures with overlap to DS (#1, #4, #5) and MAE (#2). However, focal seizures are uncommon in these syndromes and in particular the observed multifocal epileptiform discharges with marked activation during sleep are not described either in DS or MAE. All four patients became seizure-free between four and 15 years old with no apparent association to a recent change of medication. Thus, this improvement might either be due to a cumulative treatment response or simply represent a spontaneous resolution (Table 1, Supplementary note). Initially normal psychomotor development slowed after seizure onset, resulting in mild-moderate intellectual disability associated with mild-moderate ataxia and continuous myoclonus in some cases. By contrast, the phenotypes of patients #6 and #7, carrying mutations with dominant gain-of-function, were more severe in terms of epilepsy, ataxia and intellectual disability, and also differed electrographically, with generalized epileptic discharges. This may suggest that different pathomechanisms underlie distinctive clinical symptoms. Clinical-genetic studies and correlation with functional investigations from additional patients with further mutations are needed to confirm this genotype-phenotype relationship.
Online Methods
Whole exome and panel sequencing analysis
High throughput sequencing has been performed as described previously by our group for whole exome analysis31 and panel analysis17.
The panel used to screen the pilot cohort of 33 patients (including the index patient) comprised 485 known and putative epilepsy genes. (Supplementary Table 1) The candidates comprised genes that were suggestive for being involved in epileptogenesis due to several reasons, e.g. genes belong to neurotransmitter receptor families or other ion channels, genes were discussed by different research groups as putatively involved in epilepsy, genes are associated with seizures in animals or associated with human neurodevelopmental phenotypes, etc. The gene panel used to screen the second cohort of 147 patients was an updated version of the initial panel. To improve sequence coverage and adapting the panel for purely diagnostic purposes, we excluded a few metabolic and mitochondrial genes as well as most candidate genes and added all recently published novel epileptic encephalopathy genes. This panel finally contained 280 genes including 20 candidates for research settings (Supplementary Table 2).
Sanger sequencing analysis and CNV analysis
We performed bidirectional Sanger sequencing of all three exons of KCNA2 (ENST00000485317, NM_004974) and its intron-exon boundaries using the BigDye Terminator v3.1 Cycle Sequencing kit on an ABI3730XL DNA Analyzer (Applied Biosystems, Foster City, CA; primers available upon request) in 47 patients with epileptic encephalopathy and ataxia and/or short stature as well as 52 patients with intellectual disability and severe growth hormone deficiency.
Additionally, the genomic region containing KCNA2 was screened for CNVs by use of an in-house-developed technique for multiplex amplicon quantification (MAQ). With this MAQ technique, we screened all 99 individuals of the Sanger sequencing cohort as well as all 86 individuals of the WES cohort (Supplementary Fig. 1). This assay comprises a multiplex PCR amplification of fluorescently-labeled target and reference amplicons, followed by fragment analysis on the ABI3730 DNA Analyzer32. The comparison of normalized peak areas between the test individual and the average of seven control individuals results in the target amplicon doses indicating the copy number of the target amplicon (using the in-house developed Multiplex Amplicon Quantification Software. The multiplex PCR reaction consists of three test amplicons located in the genomic region of KCNA2 and three reference amplicons located on different chromosomes (primer mix is available upon request).
Pathogenicity prediction
For the prediction of the pathogenicity of nonsynonymous variants we used the ANNOVAR33 table_annovar.pl script together with the LJB23 database (dbNSFP)34 from June 2013 comprising prediction scores from SIFT, Polyphen2 (HDIV and HVAR), LRT, MutationTaster, MutationAssessor, FATHMM, MetaSVM and MetaLR scores. Scores were used as given on the ANNOVAR webpage. Additional three conservation scores (GERP+, PhyloP, SiPhy) were used to determine the conservation of a genomic position (More details in Supplementary Table 2).
Testing the enrichment of pathogenic variants
To test the enrichment of probably damaging nonsynonymous KCNA2 variants in our data, we used the Exome Aggregation Consortium (ExAC) database as a control dataset. It comprises data from 61,486 individuals coming from various exome sequencing projects including control cohorts data but also data from studies on neurological disorders like schizophrenia and bipolar disorder. We extracted all 64 nonsynonymous (missense and nonsense) variants for KCNA2 from ExAC [11/2014]. Some of them occurred in more than one individual yielding altogether 144 alleles with variation in KCNA2 out of a total number of 122828 alleles in the ExAC database. Significant enrichment of nonsynonymous variants was then tested determining the difference of allele counts in our data and the ExAC dataset using Fisher’s exact test.
Probability assessment of de novo mutation events
We first obtained an estimate for the single-nucleotide mutation rate in the KCNA2 gene. This rate equals the product of the average de novo mutation rate in humans of 1.2×10−8 per nucleotide per generation35 and the length of the largest coding sequence of KCNA2 (coding ID in CCDS database: 827.1) of 1,500 base pairs, yielding 1.8×10−5 per generation. The probability of observing a de novo mutation in KCNA2 in k out of n parent-offspring trios then simply follows a binomial distribution with a success probability equaling the gene-based mutation rate, Bin(n, k, 1.8×10−5).
Functional investigations
Mutagenesis and RNA preparation
Site-directed mutagenesis was performed to engineer the mutations into the human KCNA2 cDNA using Quickchange™ (Agilent Technologies, USA; primers are available upon request). The mutant cDNA was fully resequenced before being used in experiments to confirm the introduced mutation and exclude any additional sequence alterations. cRNA was prepared using the SP6 mMessage kit from Ambion. The human KV1.2 in the pcDNA3.1 vector was kindly provided by Stephan Grissmer (Institute of Applied Physiology, Ulm University).
Electrophysiology
Xenopus laevis oocytes were obtained from the Institute of Physiology I, Tübingen. Preparation of the oocytes was performed as described previously12. Oocytes were treated with collagenase (1 mg/ml of type CLS II collagenase, Biochrom KG) in OR-2 solution (in mM: 82.5 NaCl, 2.5 KCl, 1 MgCl2 and 5 Hepes, pH 7.5) followed by three washing steps and storage at 16°C in Barth solution (in mM: 88 NaCl, 2.4 NaHCO3, 1 KCl, 0.33 Ca(NO3)2, 0.41 CaCl2, 0.82 MgSO4 and 5 Tris/HCl, pH 7.4 with NaOH) supplemented with 50 μg/ml gentamicin (Biochrm KG). 50 nl of cRNA encoding wildtype (WT) or mutated KV1.2 subunits (1μg/μl) was injected into oocytes using the Roboocyte2 (Multi Channel Systems, Reutlingen, Germany) and stored for two days (at 17°C) prior to the experiment. Amplitudes of currents of WT and mutant channels recorded on the same day were normalized to the mean value of the 1.0 KV1.2 WT on that day to pool the normalized data from different experiments together.
Automated two-electrode voltage-clamp
Potassium currents in oocytes were recorded at room temperature (20-22°C) using Roboocyte2 (Multi channel Systems, Reutlingen, Germany). For two-electrode voltage-clamp (TEVC) recordings, oocytes were impaled with two glass electrodes with a resistance of 0.4 – 1 MΩ containing 1 M KCl/ 1.5 M KAc and clamped at a holding potential of −80 mV. Oocytes were perfused with a ND96 bath solution containing (in mM): 93.5 NaCl, 2 KCl, 1.8 CaCl2, 2 MgCl2, 5 HEPES (pH 7.6). Currents were sampled at 5 kHz.
Voltage clamp protocols and data analysis
The membrane was depolarized to various test potentials from a holding potential of −80 mV to record potassium currents. The activation curve (conductance–voltage relationship) was derived from the current–voltage relationship that was obtained by measuring the peak current at various step depolarizations from the holding potential of −80 mV (10 mV increment, depolarized to +70 mV). The following Boltzmann function was fitted to the obtained data points:
with g (V) = I/(V-Vrev) being the conductance, I the recorded current amplitude at test potential V, Vrev the potassium reversal potential, gmax the maximal conductance, V1/2 the voltage of half-maximal activation and kV a slope factor. Voltage-dependent inactivation of WT and mutated KV1.2 channels were analyzed using 25-s conditioning pulses at potentials ranging −60 mV to 0 mV (increment 10 mV) from a holding of −80 mV, the test pulse was 30 mV. A standard Boltzmann function was fitted to the inactivation curves:
with I being the recorded current amplitude at the conditioning potential V, Imax being the maximal current amplitude, V1/2 the voltage of half-maximal inactivation, and kV a slope factor.
Western Blot Analysis
For Western blot, injected Xenopus oocytes were lysed in a buffer containing (in mM) 20 Tris, 100 NaCl, 1 ethylenediaminetetraacid, 0.5% Triton X-100 and 10% glycerol with protease inhibitor cOmplete (Roche, Basel, Switzerland). In addition, for the P405L mutation CHO cells were transfected with 10 μg/μl DNA using Mirus “TransIT®-LT1” reagent. CHO cells were lysed in a buffer containing (in M): 2 Tris (pH 7.5), 3 NaCl, 0.2 EDTA, 0.2 EGTA, 0.25 Napyrophosphate, 0.1 β-glycerolphosphate, 0.1 sodium-orthovanadate, 1 DTT, 0.1 1% Triton and 25x cOmplete solution (Roche). For measuring protein concentrations (BCA systems, Thermo Fisher Scientific) 15 – 20 μg of protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS Page) on 8% polyacrylamide gels. The proteins were transferred onto polyvinylidene fluoride (PVDF) membranes (PALL Corporation, Port Washington, NY), and Western blotting was performed using a mouse-Anti-KV1.2 antibody (NeuroMab clone K14/16). Water-injected oocytes, untransfected (u.t.) and water transfected (Mock) CHO cells were used as controls.
Data and statistical analysis
Sample size was estimated by using GraphPad StatMate Software. TEVC recordings were analyzed using Roboocyte 2+ (Multi Channel Systems, Germany) and Clampfit (pClamp, Axon Instruments), Origin 6.1 (Origin-Lab Corp., Northampton, USA), and Excel (Microsoft, USA) software. Data were tested for normal distribution using SigmaPlot12 (Systat Software). For statistical evaluation one-way ANOVA with Dunnett’s posthoc test (normally distributed data) or one-way ANOVA on ranks with Dunn’s posthoc test (not-normally distributed data) was used for comparing multiple groups, with one-way ANOVA testing the overall difference between groups and posthoc tests telling the difference between specific groups. For unpaired data sets Student’s t-test (normally distributed unpaired data sets) or Mann-Whitney rank-sum (not-normally distributed) were used. All data are shown as mean ± SEM. For all statistical tests, significance with respect to control is indicated in the figures using the following symbols: *p<0.05, **p<0.01, ***p<0.001.
Supplementary Material
Acknowledgements
We thank all patients and family members for their participation in this study, Dr. S. Grissmer for providing the human cDNA clone of KCNA2, and Dr. F. Lang and his colleagues from the Institute of Physiology I, University of Tuebingen, for providing Xenopus laevis oocytes. J.R.L. (32EP30_136042 / 1), J.M.S. (EUI-EURC2011-4325), H.Le. (DFG Le1030/11-1), P.D.J. (G.A.136.11.N, FWO/ESF-ECRP), and I.H. (DFG HE 5415 3-1) received financial support within the EuroEPINOMICS-RES and - CoGIE networks (www.euroepinomics.org), a Eurocores project of the European Science Foundation. R.S. received funding from the European Union (E-Rare JTC grant 01GM1408B and PIOF-GA-2012-326681). J.M.S. received further support from the Ministerio de Economía y Competitividad (SAF2010-18586). H.Le., S.B., and S.Ma. received further support from the Federal Ministry for Education and Research (BMBF, program on rare diseases, IonNeurONet: 01GM1105A). S.Z. received support from the NIH (R01NS072248). S.M.S. received support from the Wellcome Trust (084730), NIHR UCLH Biomedical Research Centre and Epilepsy Society, UK. M.Sy. received support by the Interdisciplinary Center for Clinical Research IZKF Tübingen (2191-0-0). A.S. received funding for a postdoctoral fellowship by the Fonds Wetenschappelijk Onderzoek. T.D. is a PhD fellow of the Institute of Science and Technology (IWT).
EuroEPINOMICS RES Consortium:
Rudi Balling19, Nina Barisic43, Stéphanie Baulac44-46, Hande S Caglayan14, Dana C. Craiu26,27, Peter De Jonghe6,7,36, Christel Depienne44,46,47, Padhraig Gormley34, Renzo Guerrini48, Ingo Helbig37,38, Helle Hjalgrim8, Dorota Hoffman-Zacharska28, Johanna Jähn37, Karl Martin Klein49, Bobby P.C. Koeleman50, Vladimir Komarek25, Roland Krause19, Eric LeGuern44-46,51, Anna-Elina Lehesjoki22,23,24, Johannes R. Lemke1,4,40, Holger Lerche2, Carla Marini48, Patrick May19, Rikke S. Møller8,9, Hiltrud Muhle37, Aarno Palotie33,34,35, Deb Pal52, Felix Rosenow49, Kaja Selmer53,54, José M. Serratosa16,17, Sanjay M. Sisodiya10,11, Ulrich Stephani37, Katalin Sterbova25, Pasquale Striano55, Arvid Suls6,7, Tiina Talvik56,57, Sarah von Spiczak37, Yvonne Weber2, Sarah Weckhuysen6,7 & Federico Zara58
43Department of Paediatrics, University of Zagreb, Medical School, University Hospital Centre Zagreb, Zagreb, Croatia.
44INSERM UMR 975, Institut du Cerveau et de la Moelle Epinière, Hôpital Pitié-Salpêtrière, Paris, France.
45CNRS 7225, Hôpital Pitié-Salpêtrière, Paris, France.
46Université Pierre et Marie Curie–Paris 6 (UPMC), UMRS 975, Paris, France.
47Institüt für Humangenetik, Universität Würzburg, Würzburg, Germany.
48Pediatric Neurology Unit and Laboratories, Children’s Hospital A. Meyer, University of Florence, Florence, Italy.
49Epilepsy Center Hessen, Department of Neurology, University Hospitals Marburg and Philipps, University Marburg, Marburg, Germany.
50Department of Medical Genetics, University Medical Center Utrecht, Utrecht, The Netherlands.
51Assistance Publique–Hôpitaux de Paris (AP-HP), Hôpital Pitié-Salpêtrière, Département de Génétique et de Cytogénétique, Unité Fonctionnelle de Neurogénétique Moléculaire et Cellulaire, Paris, France.
52Department of Clinical Neuroscience, Institute of Psychiatry, King’s College London, London, UK.
53Department of Medical Genetics, Oslo University Hospital, Oslo, Norway.
54Institute of Medical Genetics, University of Oslo, Oslo, Norway.
55Pediatric Neurology and Muscular Diseases Unit, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health, ‘G Gaslini Institute’, Genova, Italy.
56Department of Pediatrics, University of Tartu, Tartu, Estonia.
57Department of Neurology and Neurorehabilitation, Children’s Clinic, Tartu University Hospital, Tartu, Estonia.
58Laboratory of Neurogenetics, Department of Neurosciences, Gaslini Institute, Genova, Italy.
URLs.
dbSNP Build 138, http://www.ncbi.nlm.nih.gov/projects/SNP/; 1000 Genomes Project database, http://www.1000genomes.org/; Exome Variant Server, http://evs.gs.washington.edu/EVS/; ExAC, http://exac.broadinstitute.org/; PolyPhen-2, http://genetics.bwh.harvard.edu/pph2/; MutationTaster, http://www.mutationtaster.org/; Multiplex Amplicon Quantification, http://www.multiplicom.com/multiplex-amplicon-quantification-maq; Multiplex Amplicon Quantification Software, http://www.multiplicom.com/maq-s; ANNOVAR, http://www.openbioinformatics.org/annovar/annovar_filter.html#ljb23; GEM.app browser, https://genomics.med.miami.edu/.
Footnotes
Competing financial interests The authors declare no competing financial interests.
Accession codes Data of the panel sequencing cohort is accessible on the GEM.app browser as “EuroEPINOMICS CH/DK cohort”. Data of trio exome sequencing cohorts is accessible on the GEM.app browser as “RES EE trio sequencing” cohort.
References
- 1.Capovilla G, Wolf P, Beccaria F, Avanzini G. The history of the concept of epileptic encephalopathy. Epilepsia. 2013;54(Suppl 8):2–5. doi: 10.1111/epi.12416. [DOI] [PubMed] [Google Scholar]
- 2.Guerrini R, Pellock JM. Age-related epileptic encephalopathies. Handb Clin Neurol. 2012;107:179–93. doi: 10.1016/B978-0-444-52898-8.00011-2. [DOI] [PubMed] [Google Scholar]
- 3.Claes L, et al. De novo mutations in the sodium-channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am J Hum Genet. 2001;68:1327–32. doi: 10.1086/320609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nava C, et al. De novo mutations in HCN1 cause early infantile epileptic encephalopathy. Nat Genet. 2014;46:640–5. doi: 10.1038/ng.2952. [DOI] [PubMed] [Google Scholar]
- 5.Epi KC, et al. De novo mutations in epileptic encephalopathies. Nature. 2013;501:217–21. doi: 10.1038/nature12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lerche H, et al. Ion channels in genetic and acquired forms of epilepsy. J Physiol. 2013;591:753–64. doi: 10.1113/jphysiol.2012.240606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nature Reviews Neuroscience. 2006;7:548–562. doi: 10.1038/nrn1938. [DOI] [PubMed] [Google Scholar]
- 8.Biervert C, et al. A potassium channel mutation in neonatal human epilepsy. Science. 1998;279:403–6. doi: 10.1126/science.279.5349.403. [DOI] [PubMed] [Google Scholar]
- 9.Charlier C, et al. A pore mutation in a novel KQT-like potassium channel gene in an idiopathic epilepsy family. Nat Genet. 1998;18:53–5. doi: 10.1038/ng0198-53. [DOI] [PubMed] [Google Scholar]
- 10.Singh NA, et al. A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nat Genet. 1998;18:25–9. doi: 10.1038/ng0198-25. [DOI] [PubMed] [Google Scholar]
- 11.Weckhuysen S, et al. KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol. 2012;71:15–25. doi: 10.1002/ana.22644. [DOI] [PubMed] [Google Scholar]
- 12.Orhan G, et al. Dominant-negative effects of KCNQ2 mutations are associated with epileptic encephalopathy. Ann Neurol. 2014;75:382–94. doi: 10.1002/ana.24080. [DOI] [PubMed] [Google Scholar]
- 13.Browne DL, et al. Episodic ataxia/myokymia syndrome is associated with point mutations in the human potassium channel gene, KCNA1. Nat Genet. 1994;8:136–40. doi: 10.1038/ng1094-136. [DOI] [PubMed] [Google Scholar]
- 14.Wuttke TV, et al. Peripheral nerve hyperexcitability due to dominant-negative KCNQ2 mutations. Neurology. 2007;69:2045–53. doi: 10.1212/01.wnl.0000275523.95103.36. [DOI] [PubMed] [Google Scholar]
- 15.Dedek K, et al. Myokymia and neonatal epilepsy caused by a mutation in the voltage sensor of the KCNQ2 K+ channel. Proc Natl Acad Sci U S A. 2001;98:12272–7. doi: 10.1073/pnas.211431298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irani SR, et al. Antibodies to KV1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain. 2010;133:2734–48. doi: 10.1093/brain/awq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemke JR, et al. Targeted next generation sequencing as a diagnostic tool in epileptic disorders. Epilepsia. 2012;53:1387–98. doi: 10.1111/j.1528-1167.2012.03516.x. [DOI] [PubMed] [Google Scholar]
- 18.Lubbers WJ, et al. Hereditary myokymia and paroxysmal ataxia linked to chromosome 12 is responsive to acetazolamide. J Neurol Neurosurg Psychiatry. 1995;59:400–5. doi: 10.1136/jnnp.59.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pena SD, Coimbra RL. Ataxia and myoclonic epilepsy due to a heterozygous new mutation in KCNA2: proposal for a new channelopathy. Clin Genet. 2015;87:e1–3. doi: 10.1111/cge.12542. [DOI] [PubMed] [Google Scholar]
- 20.Xie G, et al. A new KV1.2 channelopathy underlying cerebellar ataxia. J Biol Chem. 2010;285:32160–73. doi: 10.1074/jbc.M110.153676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brew HM, et al. Seizures and reduced life span in mice lacking the potassium channel subunit KV1.2, but hypoexcitability and enlarged KV1 currents in auditory neurons. J Neurophysiol. 2007;98:1501–25. doi: 10.1152/jn.00640.2006. [DOI] [PubMed] [Google Scholar]
- 22.Jan LY, Jan YN. Voltage-gated potassium channels and the diversity of electrical signalling. J Physiol. 2012;590:2591–9. doi: 10.1113/jphysiol.2011.224212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmgren M, Shin KS, Yellen G. The activation gate of a voltage-gated K+ channel can be trapped in the open state by an intersubunit metal bridge. Neuron. 1998;21:617–21. doi: 10.1016/s0896-6273(00)80571-1. [DOI] [PubMed] [Google Scholar]
- 24.Long SB, Campbell EB, Mackinnon R. Voltage sensor of KV1.2: structural basis of electromechanical coupling. Science. 2005;309:903–8. doi: 10.1126/science.1116270. [DOI] [PubMed] [Google Scholar]
- 25.Labro AJ, Raes AL, Bellens I, Ottschytsch N, Snyders DJ. Gating of shaker-type channels requires the flexibility of S6 caused by prolines. J Biol Chem. 2003;278:50724–31. doi: 10.1074/jbc.M306097200. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Wang Q, Ni F, Ma J. Structure of the full-length Shaker potassium channel KV1.2 by normal-mode-based X-ray crystallographic refinement. Proc Natl Acad Sci U S A. 2010;107:11352–7. doi: 10.1073/pnas.1000142107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein A, Boltshauser E, Jen J, Baloh RW. Episodic ataxia type 1 with distal weakness: a novel manifestation of a potassium channelopathy. Neuropediatrics. 2004;35:147–9. doi: 10.1055/s-2004-817921. [DOI] [PubMed] [Google Scholar]
- 28.Nybo K. Molecular biology techniques Q&A. Western blot: protein migration. Biotechniques. 2012;53:23–4. doi: 10.2144/000113887. [DOI] [PubMed] [Google Scholar]
- 29.Lorincz A, Nusser Z. Cell-type-dependent molecular composition of the axon initial segment. J Neurosci. 2008;28:14329–40. doi: 10.1523/JNEUROSCI.4833-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, Kunkel DD, Schwartzkroin PA, Tempel BL. Localization of Kv1.1 and Kv1.2, two K channel proteins, to synaptic terminals, somata, and dendrites in the mouse brain. J Neurosci. 1994;14:4588–99. doi: 10.1523/JNEUROSCI.14-08-04588.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suls A, et al. De novo loss-of-function mutations in CHD2 cause a fever-sensitive myoclonic epileptic encephalopathy sharing features with Dravet syndrome. Am J Hum Genet. 2013;93:967–75. doi: 10.1016/j.ajhg.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suls A, et al. Microdeletions involving the SCN1A gene may be common in SCN1A-mutation-negative SMEI patients. Hum Mutat. 2006;27:914–20. doi: 10.1002/humu.20350. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Kunkel DD, Martin TM, Schwartzkroin PA, Tempel BL. Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature. 1993;365:75–9. doi: 10.1038/365075a0. [DOI] [PubMed] [Google Scholar]
- 34.Liu X, Jian X, Boerwinkle E. dbNSFP v2.0: a database of human non-synonymous SNVs and their functional predictions and annotations. Hum Mutat. 2013;34:E2393–402. doi: 10.1002/humu.22376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kong A, et al. Rate of de novo mutations and the importance of father’s age to disease risk. Nature. 2012;488:471–5. doi: 10.1038/nature11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.