Abstract
Bone fractures and non-union defects often require surgical intervention where biomaterials are used to correct the defect, and approximately 10% of these procedures are compromised by bacterial infection. Currently, treatment options are limited to sustained, high doses of antibiotics and surgical debridement of affected tissue, leaving a significant, unmet need for the development of therapies to combat device-associated biofilm and infections. Engineering implants to prevent infection is a desirable material characteristic. Tissue engineered scaffolds for bone repair provide a means to both regenerate bone and serve as a base for adding antimicrobial agents. Incorporating anti-infection properties into regenerative medicine therapies could improve clinical outcomes and reduce the morbidity and mortality associated with biomaterial implant-associated infections. This review focuses on current animal models and technologies available to assess bone repair in the context of infection, antimicrobial agents to fight infection, the current state of antimicrobial scaffolds, and future directions in the field.
Key Terms: Biomaterial, Scaffold, Bone Repair, Regeneration, Infection
Implant-associated infection is a significant clinical problem23. Bacterial colonization of implants is associated with surgical sites, central line access points, ventilators, surgical drains and shunts, urinary and central venous catheters, and others. Current strategies used to prevent such infections include, but are not limited to, antibiotic therapy, healthcare-provider hygiene, environmental controls such as isolation or negative pressure rooms, surface coatings and modifications, sterilization, and the use of sterile technique during procedures. Nearly all types of bacteria and fungi are capable of infecting implanted devices23. Some of the most common pathogens include Staphylococcus aureus, Staphylococcus epidermis, Pseudomonas aeruginosa, Propionibacterium acnes, beta hemolytic Streptococcus, Proteus mirabilis, and Escherichia coli37,77. The development of biomaterials with antimicrobial properties to prevent device-associated infection is a rapidly expanding field.
DEVICE-ASSOCIATED INFECTIONS IN BONE RECONSTRUCTION
In the field of orthopedics alone, 2–5% of all procedures involving implants are complicated by infection23. This number can be as high as 30% when open fractures are present93. Significant morbidity and even death are associated with implant-related infections, with outcomes often leading to complete implant removal, surgical debridement of the affected tissue, and long-term antibiotic therapy55,56. Device-associated infections not only occur from direct implantation of bacteria, but also develop post-operatively following hematogenous bacteremia, or direct spreading from a nearby infection site55,56. Further complicating treatment is the emergence of antibiotic-resistant bacteria34. Choosing the correct antibiotic for initial treatment is directly correlated with successful infection management and becomes more difficult in the case of nosocomial infections, due to the inherent resistance that these organisms possess75. The above circumstances motivate the development of implantable materials with antibacterial properties to significantly improve surgical outcomes and reduced patient morbidity and mortality. Engineered scaffolds for regenerative medicine applications provide a framework for tissue repair as well as a substrate for the inclusion of antimicrobial properties.
BIOFILM AND NONUNION DEFECTS
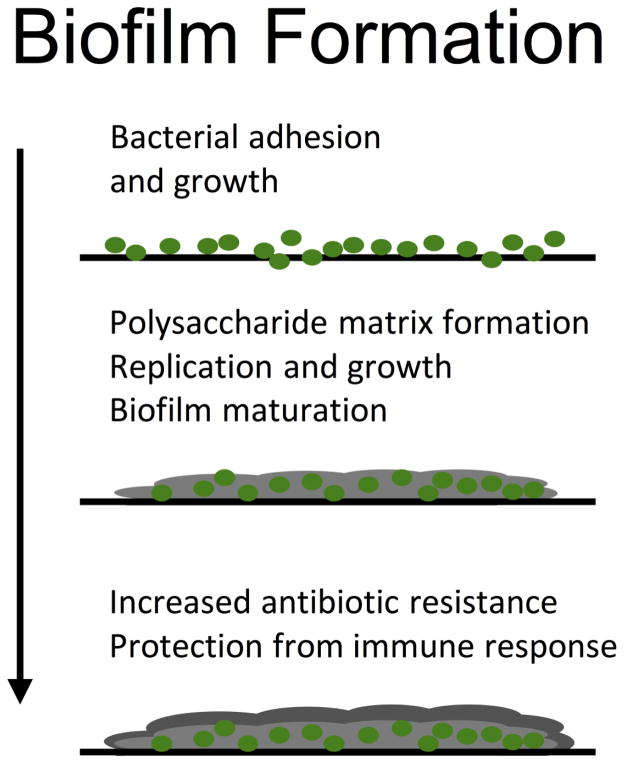
Device-associated infection is characterized by bacterial adhesion, colonization, and biofilm development, which is outlined in Figure 19,94. The most common organisms associated with orthopedic implant infections include the gram positive strains Staphylococcus epidermis, Staphylococcus aureus, and Propionibacterium acnes, as well as the gram negative Pseudomonas aeruginosa94. Osteomyelitis is inflammation of the bone, which can be due to biofilm formation, causing increased bone resorption and reactive bone formation55,56. These biofilms are composed of secreted bacterial components, such as protein, lipid, lipopolysaccharide, and DNA9, forming a matrix around the bacteria that provides protection from antibiotic therapy and immune defenses21,57. Bacteria in a biofilm have higher mutation rates30, and can display increased virulence73 than if growing planktonically, and when exposed to antibiotics, mutation rates increase further, allowing for accelerated development of a drug-resistant phenotype64. Moreover, incomplete resolution following therapy leads to highly resistant cells, or persistors, that then replenish the biofilm58. These characteristics paired with availability of only semi-effective treatment options leave a significant, unmet need for the development of therapies to combat device-associated biofilm and infection9,63.
Figure 1. Bacterial adhesion and biofilm development.
Biofilm formation begins by bacteria adhering and growing on a surface. As the pathogen continues to replicate a polysaccharide matrix is deposited. This matrix protects the pathogen from the host immune system and increases the development of antibiotic resistance.
Non-union bone defects are fracture injuries that cannot heal without intervention. Currently, standard medical therapies include the use of bone auto- and allo-grafts, or delivering high doses of therapeutic protein, such as bone morphogenetic protein 2 (BMP-2), to facilitate healing. However, there are unacceptably high failure and complication rates associated with these interventions25, which are significantly increased when an infection develops47,60,66. Advances in biomaterials and regenerative therapies have led to the development of engineered scaffolds capable of correcting non-union defects without the need for bone grafting procedures27. These strategies for bone repair often rely on biomaterial-based scaffolds to bridge the defect. This provides a convenient framework to introduce antibacterial agents to prevent and treat infection after surgical intervention.
Engineering regenerative medicine implants to overcome bacterial contamination is a critical and emerging area of biomaterials research. These technologies require rigorous in vitro and in vivo evaluation, bringing together the fields of microbiology and biomaterials engineering. Significant progress has been made in the design of infection-resistant surfaces, as recently reviewed by Campoccia et al.14,15. Therefore this review will focus on relevant animal models and techniques to assess antimicrobial tissue scaffolds in the context of bone repair, potential therapeutic additives to fight infection, and the current and future of scaffolds with infection resistant properties to promote bone repair.
ANIMAL MODELS TO ASSESS INFECTION AND BONE REPAIR
Successful evaluation of antimicrobial scaffolds for bone regeneration requires the development of reliable and robust infection models. This proves to be a very challenging task, as pathogenic bacteria are required to induce the infection without causing overly adverse harm to the host. Furthermore, the model should provide a sustained infection over a prolonged period of time to have increased relevance to human health. Several animal models to assess fracture therapy exist, and appropriate model selection was discussed in depth by Mills and Simpson69. Bone regeneration is most frequently evaluated using critical-sized, segmental defect models. Critical-sized segmental defects are bone injuries that do not spontaneously heal, allowing for assessment of bone regeneration due to the therapy, such as implantation of a scaffold.
Few validated models exist to evaluate bone regeneration in infected defects. These models introduce infection to bone repair models. Table 1 summarizes validated models developed to characterize the efficacy of antimicrobial bone repair scaffolds. The most common repair model extends the rat femoral segmental defect102 to include pathogenic bacteria17–20,103. Femoral segmental defects have been widely used in regenerative medicine studies, and allow for the evaluation of a long, weight-bearing bone that will not spontaneously heal. This procedure requires bone fixation hardware and effectively tests the reparative capacity of regenerative scaffolds. To introduce infection, two distinct inoculation techniques exist. In one, a segment of the femur is excised, the bone is stabilized, bacteria is introduced, and the surgical site is closed. Once enough time has elapsed for the infection to become established, reoperation occurs and the infected tissue is debrided and a sterile regenerative scaffold is placed17–20. This method has been used to evaluate osteogenic protein-117 as well as systemic antibiotic therapy paired with recombinant human BMP-218 and recombinant human osteogenic protein-119 in the presence of infection. This technique provides clinical relevance, as it mimics how implant infections are treated, but two surgical procedures may increase variability and add more stress to the animal. The second approach requires a single procedure, where the defect is created and stabilized and the implant is placed. Following implant placement, the pathogen is injected into the implant (or the implant is inoculated prior to implantation), simulating intraoperative contamination103. This technique is advantageous because it only requires a single procedure, which may reduce variability associated with surgery. This model was later adapted to realize a 50% overall infection rate in order to reduce the chances of observing false-negative (type II error) in vivo results74. An infected femoral segmental defect model in the rabbit has also been reported where infection was induced 48 hours after bone excision and defect stabilization by a percutaneous injection of a bacterial suspension88. These models provide an economical way to assess bone-healing strategies, but are complicated by requiring defect fixation with plates and wires. Stabilization can pose a problem when assessing the antimicrobial abilities of regenerative scaffolds if the stabilization pins become infected and cause failure17.
Table 1.
Infection-based segmental defect models
| Ref. | Animal | Procedure | Advantages | Disadvantages |
|---|---|---|---|---|
| 17–20,103 | Rat | Femoral segmental defect, debridement following 2 weeks later | Evaluates long weight bearing bone, widely validated, not self healing | Failure if infection extends to fixation pins, two surgical procedures required |
| 74,103 | Rat | Femoral segmental defect, contaminated scaffold | Evaluates long weight bearing bone, widely validated, not self healing, single operation | Failure if infection extends to fixation pins, complicated procedure |
| 88 | Rabbit | Femoral segmental defect | Evaluates long weight bearing bone, not self healing | Failure if infection extends to fixation pins |
| 8 | Rabbit | Radial segmental defect | No fixation device required, evaluates long bone, not self healing, single procedure, simulates intraoperative contamination | Non-weight bearing bone |
Self-stabilizing segmental defects could be a means to avoid complications associated with infected stabilization hardware. Self-stabilization is achieved by removing a segment of a non-weight bearing bone, such as the radius. This allows for the study of regenerative implants in critically sized defects of long bones that will not self-heal, but may not be as clinically relevant since many orthopedic procedures require fixation of long bones. Bi et al. developed a lapine radial segmental defect infection model to assess localized antibiotic release compared to systemic therapy8. In this model, a defect was created and a bacterial suspension was placed in the wound. After 30 minutes, the area was washed, the implant was placed and the wound was closed. This model only requires a single procedure and also simulates intraoperative contamination. Although several different animal models have been developed to assess bone repair, to our knowledge a validated murine model has not yet been published, even though murine models have been used extensively throughout the osteomyelitis literature78.
The advent of in vivo imaging systems has significantly improved the analysis of biomaterial-associated infections87. Genetic engineering of bioluminescence genes into clinically relevant bacterial strains allows for in vivo monitoring of infection. Commercially available gram positive (Xen29 S. aureus) and gram negative (Xen5 P. aeruginosa) strains contain a stable luminescence reporter, and can be tracked over time in vivo, providing the assessment of infection progression48, and treatment efficacy49. However, limitations do exist. For example, the luminescence signal detected is not a direct marker of the number of bacteria, but of the metabolic activity of the colony32,48,49. The population of bacteria making up a biofilm is composed of both rapidly dividing and quiescent cells. This heterogeneity may be a possible explanation for the large variability between bacterial counts and bioluminescent signal. The use of bioluminescent bacteria has been successfully established in vivo in the context of osteomyelitis36,42, suggesting that this technology could be adaptable to monitoring scaffold-associated infections in bone repair. Nevertheless, genetic modification of bacteria through bioluminescent gene insertion could reduce the virulence of the clinically isolated strains, which could complicate the evaluation of infection resistant materials.
In addition to bioluminescent bacteria, several in vivo probes utilizing fluorescent, magnetic, and radioactive tracers have been developed. Near infrared (near-IR) imaging probes that specifically identify bacteria have received heightened interest as a viable alternative to luminescent bacteria. Discrimination between infection and inflammation is the key challenge associated with their development31. Eggleston and Panizzi provide an extensive review on this topic31. Our lab has recently developed near-IR probes that specifically discriminate between infection and inflammation through targeting the products produced by the inflammatory response91. Reactive oxygen species (ROS) are characteristic of the body’s response to biomaterials implants, whereas large quantities of nitric oxide (NO) are produced by macrophages and neutrophils in a direct response to bacteria. Dual administration of ROS- and NO-selective probes allows for the simultaneous in vivo observation of infection and inflammation with high specificity91. Furthermore, we have shown these fluorescent probes exhibit increased sensitivity compared to bioluminescent strains. Fluorescent probes also have a dose dependent response to the number of bacteria regardless of metabolic activity, in a strain independent manner28. Other strategies to achieve specificity include utilizing antimicrobial peptides that have been labeled with radioactive isotopes and paired with clinically available imaging systems, such as SPECT (single photon emission computed tomography)12, and labeling the antibiotic vancomycin with a near-IR fluorophore to identify gram positive infections96. The technologies described above provide real-time, in vivo means to monitor infection initiation, progression, and resolution, and could provide an indispensable tool in the development of infection-resistant scaffolds.
Although significant effort has been made to develop finely tuned animal models for the assessment of a materials antibacterial properties as described above, ethical concerns do exist surrounding these methods. This is especially relevant when evaluating infection resistant properties of scaffolds after a sterile implantation, which is the most clinically realistic scenario. These types of studies require large animal numbers to adequately power the analysis due to the relatively low rates of spontaneous infection developing (less than 7%) and that both the control and treatment groups will require large animal numbers to resolve a difference54. Concerns also exist surrounding animal welfare. Many infection models are highly variable and it can be challenging defining a sub-lethal bacterial dose that does not cause animal suffering. This is particularly difficult, as simply increasing the bacterial dose could result in sepsis and termination before the desired experimental end point.
ANTIMICROBIAL AGENTS TO FIGHT INFECTION
Several different strategies exist to combat bacterial infection. Table 2 provides a list of major antimicrobial strategies 2,24,39,40,67. Brief overviews of the major antibacterial classes, including the advantages and limitations of each follow.
Table 2.
Review articles detailing various antimicrobial strategies.
| Ref. | Antimicrobial | Topics Covered |
|---|---|---|
| 24 | Antibiotics | Mechanisms of action, and how resistance has emerged |
| 67 | Silver | Antimicrobial properties of silver nanomaterials and effects on human health and the environment |
| 2 | Host Defense Peptides | Host defense peptides as therapeutics for antibiotic resistant infections |
| 40 | Host Defense Peptides | Immunomodulatory aspects of host defense peptides |
| 39 | Bacteriophage | Bacteriophages and how they can be used to treat infection |
Clinically, antibiotics are the most common agent used to clear bacterial infections. They are widely used throughout clinical medicine as treatment and prophylaxis. However, over the past decades, the emergence of antibiotic-resistant bacteria, such as methicillin resistant Staphylococcus aureus (MRSA), have become more common24. Sub-inhibitory aminoglycoside antibiotic treatment can induce biofilm formation41. The development of biofilm can potentiate the emergence of resistant cells, further complicating the infection89. Biofilm requires higher doses and longer trials of therapy to eradicate infection, thereby prolonging the patient’s exposure to drug side effects. Moreover, it has been shown that bactericidal antibiotics are toxic to mammalian cells, causing mitochondrial dysfunction50. However, the benefits of treatment far outweigh the risks, and until viable alternatives are available, antibiotics will remain the standard of care. For a comprehensive review of antibiotic therapy including drug mechanisms, specificities, and the development of resistance, refer to Davies and Davies24.
Silver is a broad-spectrum antimicrobial agent used in research and clinically. Silver exerts bactericidal activity on both gram positives and gram negatives through several mechanisms. Silver ions enter the bacterium and generate ROS capable of damaging DNA, they interact with membrane proteins affecting their function, and alter membrane permeability leading to cell death67. It is believed that silver resistance is widespread, but not realized since it is not widely tested for. A Chicago hospital revealed that over 10% of enteric bacteria exhibit silver resistance86, and overuse could potentiate the problem. Furthermore, the bactericidal mechanisms of silver ions are not specific to bacterial cells, and also disrupt mammalian cell function placing significant concern on toxicity5,10. However, it has been reported that silver can be effective against antibiotic-resistant bacterial strains, and even induce susceptibility towards antibiotics that were ineffective in the absence of silver71. Silver can also be adapted to reduce bacterial adherence to orthopedic implants by killing adherent pathogens95. For a more detailed discussion, the reader is referred to Marambio-Jones and Hoek67. Clinically, silver has translated to several applications, including wound dressings, creams, urinary catheters and endotracheal tubes. However, little if any data has demonstrated efficacy. An analysis of 2066 patients enrolled in several clinical trials failed to show any benefits to silver-doped wound dressings90. Silver-coated endotracheal tubes53 have exhibited modest efficacy in preventing bacterial colonization, whereas silver-coated urinary catheters have shown mixed results6.
Host defense peptides or antimicrobial peptides (AMPs) have activity against bacteria, viruses, and fungi45. Defensins, cathelicidins and histatins are AMPs produced by many mammalian cells26. AMPs are amphiphilic peptides characterized by a several cationic and hydrophobic residues and exhibit broad-spectrum activity against both gram positive and gram negative bacteria26,40,45. The cationic residues associate with the negatively charged bacterial membrane. The hydrophobic and hydrophilic residues cause membrane penetration, leading to instability, pore formation, osmotic changes, and bacterial lysis45. As with all antimicrobial strategies, the development of resistance is a concern. This could be especially problematic since AMPs are part of the natural host response to pathogens and resistance could make simple infections dangerous2,72. Another drawback is the observation that AMPs are not stable over long periods of time in an in vivo environment. However, AMPs are easily engineered, and several synthetic peptides have been developed in an attempt to overcome these shortcomings11. It has been well documented that AMPs possess immunomodulatory activity in addition to being antipathogenic26,40,45. AMPs modulate both the innate and adaptive immune responses to control infection and stimulate regenerative processes40. These attributes make AMPs an enticing candidate for antimicrobial regenerative scaffolds. However, there are no reports of human safety or efficacy trials for AMPs.
Bacteriophage therapy has gained renewed interest with the increased prevalence of antibiotic resistance62. Bacteriophages are viruses that specifically infect bacteria. The phage binds to a membrane receptor, introducing phage DNA into the cell. This DNA is replicated and translated by the host bacterium, leading to phage replication, progeny assembly, bacterial lysis, release of progeny, and phage propagation to surviving bacteria. Following eradication of the infecting organism, phage replication ceases, allowing for resolution of the affected tissue. Bacteriophage DNA can also code for lysins, lytic enzymes that destroy the bacterial cell wall84, as well as polysaccharide depolymerases, enzymes that break down the biofilm matrix created by bacteria44,61. This allows bacteriophages to disperse biofilm as well as eradicate infection. In addition, synergism between phage therapy and antibiotics has been demonstrated81. Host bacterial strains can develop resistance to phage infection, which can be reduced using several different phages at once35. There are also concerns surrounding the immunogenicity of in vivo phage administration, even though adverse events have not been reported in the literature4,70. Currently, the safety of bacteriophage therapy administered orally13 and cutaneously79 has been evaluated in humans in phase I clinical trials. Preliminary results of the first controlled trial to evaluate bacteriophage efficacy in chronic otitis to treat antibiotic-resistant P. aeruginosa have been positive, demonstrating therapeutic value in humans99.
ANTIMICROBIAL SCAFFOLDS FOR BONE REPAIR
Recently, tissue engineered scaffolds for bone repair have started to include antimicrobial agents to prevent or fight infection. These scaffolds provide a substrate for sustained, localized drug release, tunable degradation properties to promote tissue integration, and support for cell delivery. Rigorous evaluation of the antimicrobial efficacy exhibited by these scaffolds has proven difficult, and requires expertise in both microbiology techniques as well as biomaterials engineering. Antimicrobial scaffolds are required to be toxic to bacterial cells, while promoting local tissue regeneration and minimizing the adverse inflammatory events. Figure 2 is a schematic diagram illustrating how engineering antimicrobial properties into scaffolds for bone repair can improve outcomes associated with bacterial infection. Bacterial contamination is introduced into a model used to evaluate regenerative implants. If the contaminate is not cleared by the immune system or an infection-resistant scaffold, the infection becomes established, which may lead to the development of osteomyelitis. Infection fighting scaffolds can be implanted into defects with ongoing infection or osteomyelitis to remove the existing pathogen and facilitate repair. Further development of these technologies will allow for bone repair to occur in both sterile and contaminated conditions.
Figure 2. Critically-sized non-union bone defects are used to assess the therapeutic efficacy of regenerative scaffolds.
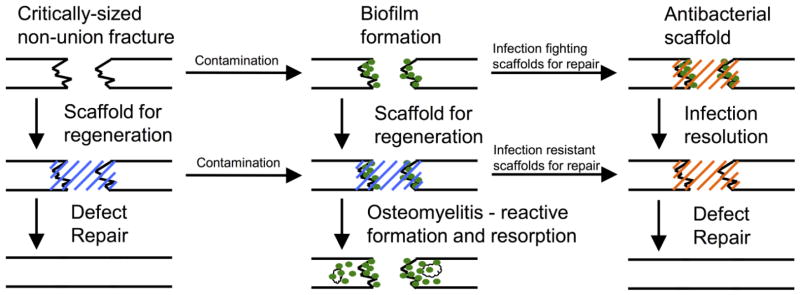
Contamination of these defects can be introduced before or after the scaffold is placed to establish the infection. Absence of antimicrobial agents will lead to the development of osteomyelitis, which is characterized by bone resorption and reactive bone formation. Infection resistant scaffolds are designed to prevent initial bacterial colonization whereas infection fighting scaffolds can be used to resolve an established biofilm and promote defect repair.
Antibiotic-releasing scaffolds
Antibiotic delivery scaffolds are the most well developed area in the literature. In orthopedics, antibiotic-loaded fillers and bone cements have been used clinically for a number of years. Zilberman and Elsner published an extensive review on antibiotic-releasing materials104. We will focus on advances in tissue engineering scaffolds that incorporate antibiotics.
Scaffolds provide an ideal substrate to deliver long-term bactericidal doses of antibiotics to the injury site. This is accomplished by modifying drug release characteristics through encapsulation within degradable matrices. Antibiotic releasing matrices have been used as coatings for orthopedic implants prone to infection. Sol-gel thin films have been engineered to provide sustained release of vancomycin and protect against implant associated infection of titanium rods. The addition of the thin film minimized bacterial adherence to the implant, and protected against the development of osteomyelitis in vivo1. A similar has also been applied to stainless steel K-wires7.
Antimicrobial activity can also be engineered into scaffolds for tissue regeneration. Poly (L-lactic acid) (PLLA) nanofiber scaffolds were synthesized with poly (lactic-co-glycolic acid) (PLGA) nanospheres to provide extended release of the antibiotic doxycycline33. These scaffolds provided sustained antimicrobial activity against S. aureus and E. coli over 42 days in bacterial culture, demonstrating an approach to provide extended, localized antibiotic release, which would reduce the systemic side effects associated with antibiotic therapy. This is especially important when treating osteomyelitis, which typically involves extended courses of high dose antibiotics. Poly(caprolactone) (PCL) scaffolds were synthesized by electrospinning PCL using 10% and 20% (w/w) rifampin80. These scaffolds exhibited extended rifampin release over eight hours and were bactericidal towards S. epidermis and P. aeruginosa in vitro. Shi et al. demonstrated the addition of lecithin can increase the encapsulation efficiency of gentamicin and protein into PLGA microsphere-based scaffolds85. After an initial burst release, gentamicin release occurred for 60 days, and protein was released for 18 days. The material was active against E. coli while still supporting mesenchymal stem cell (MSC) viability, proliferation, and mineralization. These observations suggest that this scaffold is a viable candidate for delivering protein therapeutics as well as antibiotics, and supporting bone formation for the treatment of infected bone defects. The encapsulation of growth factors has also been paired with antibiotic encapsulation. Calcium sulfate scaffolds with chitosan microspheres containing vancomycin, recombinant human BMP-2 (rhBMP-2), or both were developed and assessed in vitro for bactericidal activity and regenerative properties29. It was shown that these scaffolds are bactericidal against S. aureus for 18 days and release rhBMP-2 over 6 weeks, causing increased alkaline phosphatase (a marker of osteoblast differentiation) expression. These investigators found that an optimal balance between antibiotic and growth factor release is required for optimal osteoblastic differentiation, as high antibiotic concentrations can lead to inhibition of osteoblastic differentiation. However, these techniques have not yet been extended to in vivo models.
As mentioned above, the current standard of care for critically sized bone defects is bone grafting. Infection is one of the most significant side effects associated with grafting procedures. Bi et al. engineered bone xenografts (grafts from a different animal species) composed of antigen-free calf cancellous bone combined with calf cortical bone extract and bovine BMP impregnated with clindamycin to treat critically sized defects contaminated with S. aureus8. This scaffold was evaluated in vivo within a rabbit radial segmental defect. After the graft was implanted, 1×106 colony forming units (CFU) of S. aureus were administered to the injury. All animals in the clindamycin-impregnated graft group healed completely. Defect repair was observed in the clindamycin-graft group including recanalization of the medullary cavity. Systemic clindamycin therapy resulted in either non-union, or delayed union after the 16 week period, whereas the non-treatment control developed osteomyelitis characterized by reactive bone formation and resorption. This study shows that local, sustained delivery of antibiotics can overcome an infection, while still providing regenerative properties.
The bone graft substitute calcium sulfate has also been combined with antibiotics and assessed in vivo. The antibiotic moxifloxacin has been evaluated with commercially available Stimulan®, a synthetic semihydrate form of calcium sulfate51. In this study, osteomyelitis was induced in a rabbit tibia by injection of 2×107 CFU of a clinical osteomyelitis isolate of MRSA into the intramedullary cavity. After the infection was allowed to develop for three weeks, the rabbits underwent surgical debridement of all necrotic bone tissue and implant placement. The results showed a significant reduction in viable bacteria throughout the six week observation period. In vitro assessment of the delivery system showed sustained moxifloxacin release over 35 days. However, this study did not evaluate whether the regenerative properties of the moxifoxacin doped Stimulan® are still intact in the presence of sustained antibiotic release. Xie et al. compared bioactive borate glass to the clinically used calcium sulfate as a carrier for vacomycin to treat MRSA-induced osteomyelitis in rabbits101. Bioactive borate glass provided sustained vancomycin release over 28 days in vitro and improved mechanical properties compared to calcium sulfate. The scaffold was assessed in vivo using a rabbit model for osteomyelitis. After three weeks of infection, surgical debridement was performed and scaffolds were placed within the defect. Both the vancomycin-loaded calcium sulfate and vancomycin-loaded borate glass significantly reduced the number of bacteria, improved the radiographic score and improved the histopathologic score at the end of the eight-week observation period. This study further illustrates that scaffolds serve as an effective mechanism to provide sustained antibiotic therapy to eradicate osteomyelitis.
Polyurethane scaffolds have also received interest as a substrate to deliver antibiotics and growth factors for bone repair. Polyurethane scaffolds designed for prolonged release of vancomycin were compared to the clinically used vancomycin-loaded PMMA- beads59. Extended vancomycin release from polyurethane scaffolds could be controlled by changing the solubility of vancomycin. In vivo evaluation of the vancomycin-loaded polyurethane scaffolds in a contaminated rat femoral segmental defect reduced viable bacterial counts as well as the clinical standard of vancomycin-loaded PMMA beads. Importantly, nearly 10 times less antibiotic was loaded to the polyurethane scaffolds. Dual delivery of vancomycin and the growth factor BMP-2 to a S. aureus infected rat femoral segmental defect using a biodegradable polyurethane scaffold demonstrated increased bone formation as determined by microCT and histological analysis38. The addition of vancomycin to the scaffold reduced the clinical signs of infection while not affecting bone regeneration. Together, these studies illustrate that extended release of vancomycin can eradicate infection and the addition of BMP-2 can enhance regeneration in contaminated defects.
Clearly there has been significant progress in towards the development of antibiotic releasing materials for bone repair. However, as mentioned before, biofilm can offer protection to the microorganisms against antibiotic therapy, leading to the development of resistance. In a study evaluating the efficacy of gentamicin-loaded bone cement against the well-known biofilm former S. epidermis in rats, it was shown that even though the number of bacteria was reduced, there was a significant increase in the number of gentamicin-resistant bacteria92. In another study evaluating vancomycin-releasing polyurethane scaffolds in an infected rat segmental defect, significantly fewer bacteria were recovered at two weeks99. Nonetheless, this was only a roughly three-fold reduction, leaving over 1×105 CFU/gram of bone tissue, demonstrating a significant limitation in effectively treating infections.
Silver-presenting scaffolds
Silver can be easily incorporated into materials through various manufacturing techniques such as reduction or the addition of silver nanoparticles. This ease of incorporation combined with silver’s broad spectrum antimicrobial activity has led to the development of several silver-containing antimicrobial scaffolds. Several designs have demonstrated in vitro efficacy, but success in vivo has been limited.
Naturally derived tissue engineered scaffolds have been used for a multitude of applications, include bone repair. These materials can be modified to present silver and exhibit infection resistance. Collagen scaffolds were fabricated to include silver nanoparticles coated with poly(ethylene glycol) (PEG) and Triton X-100 65. The scaffolds had increased elasticity and antimicrobial effects against both gram positives (B. cereus and S. aureus) and gram negatives (E. coli and P. mirabilis). Silver nanoparticles have also been incorporated into type I collagen scaffolds synthesized using UV initiation of a non-toxic, water-soluble benzoin to facilitate polymerization3. The collagen scaffolds served to stabilize the nanoparticles and supported fibroblast and keratinocyte viability at silver concentrations less than or equal to 100 μM. Bactericidal activity (E. coli, B. megaterium and S. epidermis) was determined using a modified minimum inhibitory concentration assay. These studies show that collagen-based scaffolds that include silver nanoparticles can prevent bacterial growth in vitro, while also supporting mammalian cell viability. Further development of these technologies and evaluation in in vivo models is necessary to establish the feasibility of silver nanoparticle-containing collagen scaffolds for infection prevention and bone repair. Bioactive glass containing silver has been incorporated into extracellular matrix-derived hydrogels to exhibit sustained antimicrobial effects and bone regenerative properites16. These materials show sustained silver ion release over 25 days and is bactericidal against E. coli and E. faecalis. The composite hydrogels support dental pulp cell viability, making them a plausible candidate for tooth or bone regeneration. Silver ions have been added to composite chitosan/nano-hydroxyapatite scaffolds to add antimicrobial properties83. The chitosan/nano-hydroxyapatite scaffolds were immersed in silver nitrate, allowing for an ion-exchange and reduction to occur between the scaffold and silver. The scaffolds support osteoprogenitor and osteosarcoma cell viability and demonstrate antimicrobial effects against both gram positive and gram negative bacteria (S. aureus and E. coli).
PLGA has been of particular interest for bone repair due to its biocompatibility, degradable properties, and being used in FDA-approved devices. Silver was incorporated into tricalcium phosphate (TCP) nanocomposite, mixed with PLGA and then electrospun to form a fibrous scaffold. These scaffolds provided sustained silver release at bactericidal levels in vitro against E. coli, a frequent contaminator of dental implants. The scaffolds were equally as effective as the clinical standard of tetracycline-soaked cotton swabs. However, upon media exchange in the assay, the silver scaffolds maintained antimicrobial ability due to sustained release characteristics. This study demonstrates the importance of sustained antimicrobial release, and that scaffolds for tissue engineering provide a convenient avenue to accomplish this. Zheng et al. reported a promising antimicrobial regenerative scaffold103. In this study, microporous PLGA scaffolds were fabricated to contain silver nanoparticles. Interestingly, 1.0% silver containing grafts supported increased osteoblastic differentiation and increased alkaline phosphatase activity compared to the 2.0% silver grafts in vitro. These scaffolds were evaluated using a rat femoral segmental defect. After implantation, 1×108 CFUs of vancomycin-resistant MRSA was injected into the implant. Radiographic and histological analysis showed that the 2.0% silver implants completely eliminated infection and supported defect bridging, whereas the 1.0% silver implants only reduced the number of bacteria present, but supported some bone regeneration. Control scaffolds that did not contain silver were grossly infected, demonstrating bone resorption and reactive bone formation, indicative of osteomyelitis. The in vitro analysis paired with the in vivo data show that although high concentrations of silver can inhibit osteoblast differentiation, it is more important to eliminate the contaminating bacteria to facilitate bone formation. This is a clear demonstration that developing implants capable of resisting infection while providing functional cues to facilitate bone repair is possible.
As an alternative to silver, copper ions loaded into microporous bioactive glass scaffolds reduce bacterial growth and support MSC viability and differentiation100. These scaffolds significantly reduced E. coli growth, and promoted human MSC differentiation towards osteoblasts in a dose dependent manner. Vascular endothelial growth factor levels were also elevated, suggesting the scaffold could promote vascularization.
Antimicrobial Peptides, Bacteriophage, and Other Antimicrobial Strategies
Interest has been building surrounding technologies that take advantage of alternative antimicrobial therapies. These alternatives to silver and antibiotics could expand the arsenal against infection, while also reducing the chances of bacteria developing resistance to our most efficacious treatments. Scaffolds provide a means to extend the activity of these agents by providing sustained release characteristics. Antimicrobial peptides have been introduced into scaffolds designed for orthopedic regeneration. Poly(caprolactone) (PCL)-chitosan nanofiber scaffolds were synthesized and PEG-microgels containing the cationic antimicrobial peptide L5 were electrostatically associated with the nanofibers98. These novel scaffolds demonstrated antimicrobial activity against S. epidermis, and maintained L5 stability and activity. The scaffolds supported osteoblast adhesion, spreading, and proliferation.
Stainless steel K wires used in orthopedic procedures coated with a hydroxypropylmethlycellulose (HPMC) hydrogel containing bacteriophage, the antibiotic linezolid, or both were developed to prevent MRSA infection52. The coated wires showed sustained phage and linezolid release over several days, as well as inhibiting MRSA adherence in a dose dependent manner. The bacteriophage and linezolid group exhibited the greatest efficacy toward inhibiting MRSA attachment and growth, suggesting synergism exists between the co-delivery of antibiotics and bacteriophage. This claim was further supported by analysis of recovered MRSA after treatment showing reduced mutation rates in the dual treatment group suggesting lower drug resistance. This in vitro evaluation of scaffolds presenting bacteriophage and antibiotics suggests the treatment could be extended to an in vivo environment to prevent infection associated with stainless steel implants. Bacteriophage has also been evaluated in a regenerative context. In one study, the E. coli bacteriophage λ was loaded into microporous hydroxyapatite or beta-tricalcium phosphate scaffolds with various porosities by passive adsorption68. Bactericidal activity against E. coli K12 was observed in vitro, demonstrating the prophylactic potential bacteriophage loaded materials could provide.
Polyelectrolyte scaffolds assembled by electrostatic interactions of chitosan gamma-polyglutamic acid and carboxy-methylcellulose were developed for treating dental bone defects. These scaffolds supported pre-osteoblast cell adhesion and viability in vitro, and antimicrobial activity against S. aureus and E. coli. Scaffold biocompatibility was assessed by extracting the second pre-molars of beagle dogs and replacing them with the material. The scaffolds were explanted after 10 weeks and histology revealed no adverse foreign body reaction.
Neutrophils and macrophages produce peroxide and other free radicals to kill invading pathogens. This mechanism was extended to electrospun polycaprolactone (PCL) scaffolds with different concentrations of calcium peroxide to exhibit antimicrobial activity by releasing a significant initial burst of peroxide97. This short-term antimicrobial response was effective in controlling E. coli and S. epidermis in vitro, illustrating broad applicability. The nanowires supported osteoblast viability for four days of culture despite the cells being exposed to toxic peroxide levels for the first 24 hours. This novel method of direct peroxide generation from a PLC scaffold shows that burst release from materials can be toxic to bacteria but still provide a means to promote bone growth.
Berberine is a natural antimicrobial agent that exhibits activity against several different organisms and is non-toxic to mammalian cells. For these reasons, Huang et al. incorporated it into a chitosan coating on a nano-hydroxyapatite/polyamide66 scaffold developed for bone regeneration43. These scaffolds provided a continuous release of berberine over 150 hours and were bactericidal to S. aureus. Furthermore, the scaffolds supported MG63 cell adhesion, proliferation, and spreading, supporting that berberine is nontoxic. However, this material has not been evaluated in vivo. These data provided preliminary evidence that berberine may be suitable for in vivo evaluation to provide antimicrobial and regenerative properties in a bone repair setting.
Preventing biofilm formation may be another way to protect against chronic osteomyelitis. Sanchez et al. demonstrated biofilm dispersal agents reduce infection in vivo82. A polyurethane scaffold containing D-amino acids was contaminated with S. aureus and implanted into a rat femoral segmental defect. The treated scaffolds significantly reduced the number of contaminating bacteria, showing that preventing biofilm formation can improve post-operative outcomes, by preventing the biofilm from shielding the bacteria from endogenous antimicrobial defenses.
CONCLUSIONS AND OUTLOOK
Preventing infection in the presence of biomaterials implants is a major unmet need and will significantly improve patient outcomes. Currently, implant infection leads to removal, and significant medical costs from reoperations and extended antibiotic therapy. Moreover, after an initial infection, patients are at a much higher risk for relapse, further complicating management and causing increased patient morbidity. As medicine advances, we have become more and more reliant on implantable devices to more effectively correct patient problems, which increase the risk of implant-associated infections23. Demand exists for the prevention of orthopedic implant infections due to the frequency of their occurrence, as well the challenges associated with combating osteomyelitis. Despite improvements in intraoperative techniques and the invention of antibiotic-doped cements and fillers, infection continues to be a significant issue associated with non-union defects. Furthermore, the increased prevalence of antibiotic resistant bacteria raises concern over widespread use of antibiotic presenting materials. This suggests alternative antimicrobials such as silver, antimicrobial peptides or bacteriophage could help to preserve the efficacy of our most potent weapons against infection. These alternative strategies to fight infection offer exciting opportunities to introduce new properties into scaffolds. For example, the rapid expansion, but self-limiting characteristics of a bacteriophage infection provide a way to engineer materials that respond only when a pathogen is present. Scaffolds can shield the phage from the host response, while providing activity only in the presence of offending bacteria. Antimicrobial peptides can enhance the body’s defenses against pathogens, and even promote wound healing. Scaffolds can serve a means to extend the stability of these peptides and enhance their utility.
In addition to extending the stability of antimicrobial agents, scaffolds provide a highly controlled means to release therapeutics. Modulation of scaffold degradation typically correlates with therapeutic drug release. Traditionally, bone repair is driven by a scaffold degradation leading to therapeutic release. The drug release recruits cells and further promotes scaffold degradation, leading to tissue healing. This process is outlined in Figure 3. Degradable scaffolds are also advantageous from an in vivo infection resolution point of view. Implanted biomaterials are prone to infection after implantation by transient bacteremia causing colonization and direct bacterial spreading from infection sites55,56. Degradable scaffolds provide the benefit of controlled therapeutic release while facilitating integration into the native tissue.
Figure 3. Scaffold based drug delivery for tissue repair.
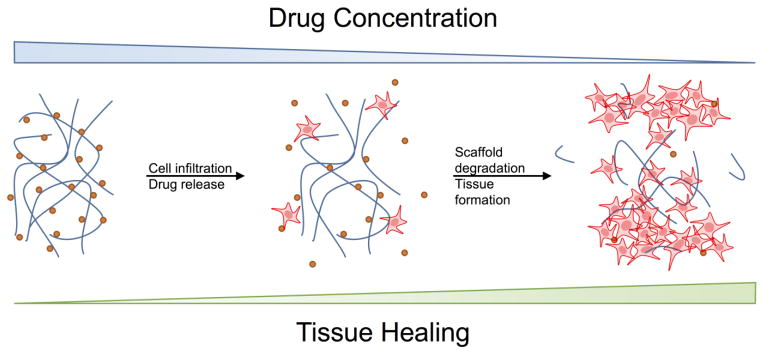
Current regenerative medicine strategies focus on delivering therapeutics to drive cell recruitment and tissue repair. As cells are recruited the scaffold degrades, releasing therapeutics, and promoting integration. Next generation biomaterials will include abilities to prevent or eliminate pathogens and provide regenerative cues.
Degradable scaffolds to treat infection and regenerate bone have been primarily investigated in the bioactive glass literature76. These studies are mostly centered on extending the release of antibiotics to provide continuous antimicrobial activity22,46,101. However, a significant gap exists in understanding how the degradation properties of scaffolds influence antimicrobial efficacy in vivo. Future studies could focus on optimizing scaffold degradation properties to efficiently eliminate pathogens and guide the bone repair process. These studies can then be extended to characterize and understand how engineering extended release of antimicrobial therapies affects drug activity though drug scaffold interactions. Modifications of scaffolds to provide continuous release may negatively impact the efficacy of the loaded therapeutic.
The next generation of antimicrobial scaffolds for bone repair will optimally balance antimicrobial delivery with regenerative therapeutics. This could be achieved by tuning material properties such as porosity, charge, degradation speed, density, antimicrobial agent, growth factors, and the bulk material. Understanding how material design choices prevent bacterial contamination, biofilm development, eradication of existing osteomyelitis, while simultaneously regenerating bone, will lead to optimized scaffold designs.
In order for these new technologies to translate into the clinic, several challenges need to be overcome. The development of robust, controlled, and reproducible animal models of infected scaffolds is a critical need for the success of this fast emerging field. Animal models that utilize bioluminescent bacteria allow for real time monitoring of infection progression without animal sacrifice, which addresses some of the ethical concerns of biomaterial infection research. Reproducible, controlled infections that accurately simulate clinical scenarios are required to effectively evaluate experimental materials to prevent infection and facilitate bone regeneration.
Scaffolds provide an ideal substrate for designing regenerative therapies due to the exquisite engineering control we have over them. They provide a platform for controlled drug release, a substrate for therapeutic cell delivery, tunable degradation characteristics that facilitate replacement by regenerating tissue, reduced immunogenicity, and response to the surrounding environment. It is clear that progress is being made towards the development of infection-resistant bone repair implants. However, the in vivo validation of these technologies is still in its infancy. The advancement of in vivo imaging techniques, paired with robust bone repair models will facilitate the translation from the bench to the bedside.
Acknowledgments
The authors gratefully acknowledge funding from the National Institutes of Health (R01 AR062920, R01 AR062368).
References
- 1.Adams CS, Antoci V, Jr, Harrison G, Patal P, Freeman TA, Shapiro IM, Parvizi J, Hickok NJ, Radin S, Ducheyne P. Controlled release of vancomycin from thin sol-gel films on implant surfaces successfully controls osteomyelitis. J Orthop Res. 2009;27:701–709. doi: 10.1002/jor.20815. [DOI] [PubMed] [Google Scholar]
- 2.Afacan NJ, Yeung AT, Pena OM, Hancock RE. Therapeutic potential of host defense peptides in antibiotic-resistant infections. Curr Pharm Des. 2012;18:807–819. doi: 10.2174/138161212799277617. [DOI] [PubMed] [Google Scholar]
- 3.Alarcon EI, Udekwu K, Skog M, Pacioni NL, Stamplecoskie KG, Gonzalez-Bejar M, Polisetti N, Wickham A, Richter-Dahlfors A, Griffith M, Scaiano JC. The biocompatibility and antibacterial properties of collagen-stabilized, photochemically prepared silver nanoparticles. Biomaterials. 2012;33:4947–4956. doi: 10.1016/j.biomaterials.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 4.Alemayehu D, Casey PG, McAuliffe O, Guinane CM, Martin JG, Shanahan F, Coffey A, Ross RP, Hill C. Bacteriophages phimr299-2 and phinh-4 can eliminate pseudomonas aeruginosa in the murine lung and on cystic fibrosis lung airway cells. MBio. 2012;3:e00029–00012. doi: 10.1128/mBio.00029-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.AshaRani PV, Low Kah Mun G, Hande MP, Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009;3:279–290. doi: 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]
- 6.Beattie M, Taylor J. Silver alloy vs. Uncoated urinary catheters: A systematic review of the literature. J Clin Nurs. 2011;20:2098–2108. doi: 10.1111/j.1365-2702.2010.03561.x. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharyya S, Agrawal A, Knabe C, Ducheyne P. Sol-gel silica controlled release thin films for the inhibition of methicillin-resistant staphylococcus aureus. Biomaterials. 2014;35:509–517. doi: 10.1016/j.biomaterials.2013.09.073. [DOI] [PubMed] [Google Scholar]
- 8.Bi L, Hu Y, Fan H, Meng G, Liu J, Li D, Lv R. Treatment of contaminated bone defects with clindamycin-reconstituted bone xenograft-composites. J Biomed Mater Res B Appl Biomater. 2007;82:418–427. doi: 10.1002/jbm.b.30747. [DOI] [PubMed] [Google Scholar]
- 9.Bjarnsholt T, Ciofu O, Molin S, Givskov M, Hoiby N. Applying insights from biofilm biology to drug development - can a new approach be developed? Nat Rev Drug Discov. 2013;12:791–808. doi: 10.1038/nrd4000. [DOI] [PubMed] [Google Scholar]
- 10.Braydich-Stolle L, Hussain S, Schlager JJ, Hofmann MC. In vitro cytotoxicity of nanoparticles in mammalian germline stem cells. Toxicol Sci. 2005;88:412–419. doi: 10.1093/toxsci/kfi256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brogden NK, Brogden KA. Will new generations of modified antimicrobial peptides improve their potential as pharmaceuticals? Int J Antimicrob Agents. 2011;38:217–225. doi: 10.1016/j.ijantimicag.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brouwer CP, Sarda-Mantel L, Meulemans A, Le Guludec D, Welling MM. The use of technetium-99m radiolabeled human antimicrobial peptides for infection specific imaging. Mini Rev Med Chem. 2008;8:1039–1052. doi: 10.2174/138955708785740670. [DOI] [PubMed] [Google Scholar]
- 13.Bruttin A, Brussow H. Human volunteers receiving escherichia coli phage t4 orally: A safety test of phage therapy. Antimicrob Agents Chemother. 2005;49:2874–2878. doi: 10.1128/AAC.49.7.2874-2878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campoccia D, Montanaro L, Arciola CR. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials. 2013;34:8533–8554. doi: 10.1016/j.biomaterials.2013.07.089. [DOI] [PubMed] [Google Scholar]
- 15.Campoccia D, Montanaro L, Arciola CR. A review of the clinical implications of anti-infective biomaterials and infection-resistant surfaces. Biomaterials. 2013;34:8018–8029. doi: 10.1016/j.biomaterials.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 16.Chatzistavrou X, Fenno JC, Faulk D, Badylak S, Kasuga T, Boccaccini AR, Papagerakis P. Fabrication and characterization of bioactive and antibacterial composites for dental applications. Acta Biomater. 2014 doi: 10.1016/j.actbio.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Kidder LS, Lew WD. Osteogenic protein-1 induced bone formation in an infected segmental defect in the rat femur. J Orthop Res. 2002;20:142–150. doi: 10.1016/S0736-0266(01)00060-2. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Schmidt AH, Mahjouri S, Polly DW, Jr, Lew WD. Union of a chronically infected internally stabilized segmental defect in the rat femur after debridement and application of rhbmp-2 and systemic antibiotic. J Orthop Trauma. 2007;21:693–700. doi: 10.1097/BOT.0b013e31815a7e91. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Schmidt AH, Tsukayama DT, Bourgeault CA, Lew WD. Recombinant human osteogenic protein-1 induces bone formation in a chronically infected, internally stabilized segmental defect in the rat femur. J Bone Joint Surg Am. 2006;88:1510–1523. doi: 10.2106/JBJS.E.01136. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Tsukayama DT, Kidder LS, Bourgeault CA, Schmidt AH, Lew WD. Characterization of a chronic infection in an internally-stabilized segmental defect in the rat femur. J Orthop Res. 2005;23:816–823. doi: 10.1016/j.orthres.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 22.Cui X, Zhao C, Gu Y, Li L, Wang H, Huang W, Zhou N, Wang D, Zhu Y, Xu J, Luo S, Zhang C, Rahaman MN. A novel injectable borate bioactive glass cement for local delivery of vancomycin to cure osteomyelitis and regenerate bone. J Mater Sci Mater Med. 2014;25:733–745. doi: 10.1007/s10856-013-5122-z. [DOI] [PubMed] [Google Scholar]
- 23.Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350:1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 24.Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74:417–433. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Long WG, Jr, Einhorn TA, Koval K, McKee M, Smith W, Sanders R, Watson T. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. J Bone Joint Surg Am. 2007;89:649–658. doi: 10.2106/JBJS.F.00465. [DOI] [PubMed] [Google Scholar]
- 26.De Smet K, Contreras R. Human antimicrobial peptides: Defensins, cathelicidins and histatins. Biotechnol Lett. 2005;27:1337–1347. doi: 10.1007/s10529-005-0936-5. [DOI] [PubMed] [Google Scholar]
- 27.Dimitriou R, Jones E, McGonagle D, Giannoudis PV. Bone regeneration: Current concepts and future directions. BMC Med. 2011;9:66. doi: 10.1186/1741-7015-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dinjaski N, Suri S, Valle J, Lehman SM, Lasa I, Prieto MA, Garcia AJ. Near-infrared fluorescence imaging as an alternative to bioluminescent bacteria to monitor biomaterial-associated infections. Acta Biomater. 2014;10:2935–2944. doi: 10.1016/j.actbio.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doty HA, Leedy MR, Courtney HS, Haggard WO, Bumgardner JD. Composite chitosan and calcium sulfate scaffold for dual delivery of vancomycin and recombinant human bone morphogenetic protein-2. J Mater Sci Mater Med. 2014;25:1449–1459. doi: 10.1007/s10856-014-5167-7. [DOI] [PubMed] [Google Scholar]
- 30.Driffield K, Miller K, Bostock JM, O’Neill AJ, Chopra I. Increased mutability of pseudomonas aeruginosa in biofilms. J Antimicrob Chemother. 2008;61:1053–1056. doi: 10.1093/jac/dkn044. [DOI] [PubMed] [Google Scholar]
- 31.Eggleston H, Panizzi P. Molecular imaging of bacterial infections in vivo: The discrimination between infection and inflammation. Informatics. 2014;1:72–99. doi: 10.3390/informatics1010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engelsman AF, van der Mei HC, Francis KP, Busscher HJ, Ploeg RJ, van Dam GM. Real time noninvasive monitoring of contaminating bacteria in a soft tissue implant infection model. J Biomed Mater Res B Appl Biomater. 2009;88:123–129. doi: 10.1002/jbm.b.31158. [DOI] [PubMed] [Google Scholar]
- 33.Feng K, Sun H, Bradley MA, Dupler EJ, Giannobile WV, Ma PX. Novel antibacterial nanofibrous plla scaffolds. J Control Release. 2010;146:363–369. doi: 10.1016/j.jconrel.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fridkin SK. Increasing prevalence of antimicrobial resistance in intensive care units. Crit Care Med. 2001;29:N64–68. doi: 10.1097/00003246-200104001-00002. [DOI] [PubMed] [Google Scholar]
- 35.Fu W, Forster T, Mayer O, Curtin JJ, Lehman SM, Donlan RM. Bacteriophage cocktail for the prevention of biofilm formation by pseudomonas aeruginosa on catheters in an in vitro model system. Antimicrob Agents Chemother. 2010;54:397–404. doi: 10.1128/AAC.00669-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Funao H, Ishii K, Nagai S, Sasaki A, Hoshikawa T, Aizawa M, Okada Y, Chiba K, Koyasu S, Toyama Y, Matsumoto M. Establishment of a real-time, quantitative, and reproducible mouse model of staphylococcus osteomyelitis using bioluminescence imaging. Infect Immun. 2012;80:733–741. doi: 10.1128/IAI.06166-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gristina AG. Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science. 1987;237:1588–1595. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- 38.Guelcher SA, Brown KV, Li B, Guda T, Lee BH, Wenke JC. Dual-purpose bone grafts improve healing and reduce infection. J Orthop Trauma. 2011;25:477–482. doi: 10.1097/BOT.0b013e31821f624c. [DOI] [PubMed] [Google Scholar]
- 39.Hanlon GW. Bacteriophages: An appraisal of their role in the treatment of bacterial infections. Int J Antimicrob Agents. 2007;30:118–128. doi: 10.1016/j.ijantimicag.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Hilchie AL, Wuerth K, Hancock RE. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat Chem Biol. 2013;9:761–768. doi: 10.1038/nchembio.1393. [DOI] [PubMed] [Google Scholar]
- 41.Hoffman LR, D’Argenio DA, MacCoss MJ, Zhang Z, Jones RA, Miller SI. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature. 2005;436:1171–1175. doi: 10.1038/nature03912. [DOI] [PubMed] [Google Scholar]
- 42.Horst SA, Hoerr V, Beineke A, Kreis C, Tuchscherr L, Kalinka J, Lehne S, Schleicher I, Kohler G, Fuchs T, Raschke MJ, Rohde M, Peters G, Faber C, Loffler B, Medina E. A novel mouse model of staphylococcus aureus chronic osteomyelitis that closely mimics the human infection: An integrated view of disease pathogenesis. Am J Pathol. 2012;181:1206–1214. doi: 10.1016/j.ajpath.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Huang D, Zuo Y, Zou Q, Zhang L, Li J, Cheng L, Shen J, Li Y. Antibacterial chitosan coating on nano-hydroxyapatite/polyamide66 porous bone scaffold for drug delivery. J Biomater Sci Polym Ed. 2011;22:931–944. doi: 10.1163/092050610X496576. [DOI] [PubMed] [Google Scholar]
- 44.Hughes KA, Sutherland IW, Jones MV. Biofilm susceptibility to bacteriophage attack: The role of phage-borne polysaccharide depolymerase. Microbiology. 1998;144(Pt 11):3039–3047. doi: 10.1099/00221287-144-11-3039. [DOI] [PubMed] [Google Scholar]
- 45.Izadpanah A, Gallo RL. Antimicrobial peptides. J Am Acad Dermatol. 2005;52:381–390. doi: 10.1016/j.jaad.2004.08.026. quiz 391–382. [DOI] [PubMed] [Google Scholar]
- 46.Jia WT, Zhang X, Zhang CQ, Liu X, Huang WH, Rahaman MN, Day DE. Elution characteristics of teicoplanin-loaded biodegradable borate glass/chitosan composite. Int J Pharm. 2010;387:184–186. doi: 10.1016/j.ijpharm.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 47.Journeaux SF, Johnson N, Bryce SL, Friedman SJ, Sommerville SM, Morgan DA. Bacterial contamination rates during bone allograft retrieval. J Arthroplasty. 1999;14:677–681. doi: 10.1016/s0883-5403(99)90222-x. [DOI] [PubMed] [Google Scholar]
- 48.Kadurugamuwa JL, Sin L, Albert E, Yu J, Francis K, DeBoer M, Rubin M, Bellinger-Kawahara C, Parr TR, Contag PR. Direct continuous method for monitoring biofilm infection in a mouse model. Infection and Immunity. 2003;71:882–890. doi: 10.1128/IAI.71.2.882-890.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kadurugamuwa JL, Sin LV, Yu J, Francis KP, Kimura R, Purchio T, Contag PR. Rapid direct method for monitoring antibiotics in a mouse model of bacterial biofilm infection. Antimicrobial Agents and Chemotherapy. 2003;47:3130–3137. doi: 10.1128/AAC.47.10.3130-3137.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalghatgi S, Spina CS, Costello JC, Liesa M, Morones-Ramirez JR, Slomovic S, Molina A, Shirihai OS, Collins JJ. Bactericidal antibiotics induce mitochondrial dysfunction and oxidative damage in mammalian cells. Sci Transl Med. 2013;5:192ra185. doi: 10.1126/scitranslmed.3006055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanellakopoulou K, Galanopoulos I, Soranoglou V, Tsaganos T, Tziortzioti V, Maris I, Papalois A, Giamarellou H, Giamarellos-Bourboulis EJ. Treatment of experimental osteomyelitis caused by methicillin-resistant staphylococcus aureus with a synthetic carrier of calcium sulphate (stimulan) releasing moxifloxacin. Int J Antimicrob Agents. 2009;33:354–359. doi: 10.1016/j.ijantimicag.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 52.Kaur S, Harjai K, Chhibber S. Bacteriophage mediated killing of staphylococcus aureus in vitro on orthopaedic k wires in presence of linezolid prevents implant colonization. PLoS One. 2014;9:e90411. doi: 10.1371/journal.pone.0090411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kollef MH, Afessa B, Anzueto A, Veremakis C, Kerr KM, Margolis BD, Craven DE, Roberts PR, Arroliga AC, Hubmayr RD, Restrepo MI, Auger WR, Schinner R, Group NI. Silver-coated endotracheal tubes and incidence of ventilator-associated pneumonia: The nascent randomized trial. JAMA. 2008;300:805–813. doi: 10.1001/jama.300.7.805. [DOI] [PubMed] [Google Scholar]
- 54.Kuijer R, Jansen EJ, Emans PJ, Bulstra SK, Riesle J, Pieper J, Grainger DW, Busscher HJ. Assessing infection risk in implanted tissue-engineered devices. Biomaterials. 2007;28:5148–5154. doi: 10.1016/j.biomaterials.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 55.Lazzarini L, Mader JT, Calhoun JH. Osteomyelitis in long bones. J Bone Joint Surg Am. 2004;86-A:2305–2318. doi: 10.2106/00004623-200410000-00028. [DOI] [PubMed] [Google Scholar]
- 56.Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364:369–379. doi: 10.1016/S0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- 57.Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother. 2001;45:999–1007. doi: 10.1128/AAC.45.4.999-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lewis K. Persister cells. Annu Rev Microbiol. 2010;64:357–372. doi: 10.1146/annurev.micro.112408.134306. [DOI] [PubMed] [Google Scholar]
- 59.Li B, Brown KV, Wenke JC, Guelcher SA. Sustained release of vancomycin from polyurethane scaffolds inhibits infection of bone wounds in a rat femoral segmental defect model. J Control Release. 2010;145:221–230. doi: 10.1016/j.jconrel.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 60.Lord CF, Gebhardt MC, Tomford WW, Mankin HJ. Infection in bone allografts Incidence, nature, and treatment. J Bone Joint Surg Am. 1988;70:369–376. [PubMed] [Google Scholar]
- 61.Lu TK, Collins JJ. Dispersing biofilms with engineered enzymatic bacteriophage. Proc Natl Acad Sci U S A. 2007;104:11197–11202. doi: 10.1073/pnas.0704624104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu TK, Koeris MS. The next generation of bacteriophage therapy. Curr Opin Microbiol. 2011;14:524–531. doi: 10.1016/j.mib.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 63.Ma H, Darmawan ET, Zhang M, Zhang L, Bryers JD. Development of a poly(ether urethane) system for the controlled release of two novel anti-biofilm agents based on gallium or zinc and its efficacy to prevent bacterial biofilm formation. J Control Release. 2013;172:1035–1044. doi: 10.1016/j.jconrel.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Macia MD, Perez JL, Molin S, Oliver A. Dynamics of mutator and antibiotic-resistant populations in a pharmacokinetic/pharmacodynamic model of pseudomonas aeruginosa biofilm treatment. Antimicrob Agents Chemother. 2011;55:5230–5237. doi: 10.1128/AAC.00617-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mandal A, Meda V, Zhang WJ, Farhan KM, Gnanamani A. Synthesis, characterization and comparison of antimicrobial activity of peg/tritonx-100 capped silver nanoparticles on collagen scaffold. Colloids Surf B Biointerfaces. 2012;90:191–196. doi: 10.1016/j.colsurfb.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 66.Mankin HJ, Hornicek FJ, Raskin KA. Infection in massive bone allografts. Clin Orthop Relat Res. 2005:210–216. doi: 10.1097/01.blo.0000150371.77314.52. [DOI] [PubMed] [Google Scholar]
- 67.Marambio-Jones C, Hoek EMV. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. Journal of Nanoparticle Research. 2010;12:1531–1551. [Google Scholar]
- 68.Meurice E, Rguiti E, Brutel A, Hornez JC, Leriche A, Descamps M, Bouchart F. New antibacterial microporous cap materials loaded with phages for prophylactic treatment in bone surgery. J Mater Sci Mater Med. 2012;23:2445–2452. doi: 10.1007/s10856-012-4711-6. [DOI] [PubMed] [Google Scholar]
- 69.Mills LA, Simpson AH. In vivo models of bone repair. J Bone Joint Surg Br. 2012;94:865–874. doi: 10.1302/0301-620X.94B7.27370. [DOI] [PubMed] [Google Scholar]
- 70.Morello E, Saussereau E, Maura D, Huerre M, Touqui L, Debarbieux L. Pulmonary bacteriophage therapy on pseudomonas aeruginosa cystic fibrosis strains: First steps towards treatment and prevention. PLoS One. 2011;6:e16963. doi: 10.1371/journal.pone.0016963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morones-Ramirez JR, Winkler JA, Spina CS, Collins JJ. Silver enhances antibiotic activity against gram-negative bacteria. Sci Transl Med. 2013;5:190ra181. doi: 10.1126/scitranslmed.3006276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nijnik A, Hancock R. Host defence peptides: Antimicrobial and immunomodulatory activity and potential applications for tackling antibiotic-resistant infections. Emerg Health Threats J. 2009;2:e1. doi: 10.3134/ehtj.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.O’Loughlin CT, Miller LC, Siryaporn A, Drescher K, Semmelhack MF, Bassler BL. A quorum-sensing inhibitor blocks pseudomonas aeruginosa virulence and biofilm formation. Proc Natl Acad Sci U S A. 2013;110:17981–17986. doi: 10.1073/pnas.1316981110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Penn-Barwell JG, Rand BC, Brown KV, Wenke JC. A versatile model of open-fracture infection : A contaminated segmental rat femur defect. Bone Joint Res. 2014;3:187–192. doi: 10.1302/2046-3758.36.2000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pierce GE. Pseudomonas aeruginosa, candida albicans, and device-related nosocomial infections: Implications, trends, and potential approaches for control. J Ind Microbiol Biotechnol. 2005;32:309–318. doi: 10.1007/s10295-005-0225-2. [DOI] [PubMed] [Google Scholar]
- 76.Rahaman MN, Bal BS, Huang W. Review: Emerging developments in the use of bioactive glasses for treating infected prosthetic joints. Mater Sci Eng C Mater Biol Appl. 2014;41:224–231. doi: 10.1016/j.msec.2014.04.055. [DOI] [PubMed] [Google Scholar]
- 77.Ramage G, Tunney MM, Patrick S, Gorman SP, Nixon JR. Formation of propionibacterium acnes biofilms on orthopaedic biomaterials and their susceptibility to antimicrobials. Biomaterials. 2003;24:3221–3227. doi: 10.1016/s0142-9612(03)00173-x. [DOI] [PubMed] [Google Scholar]
- 78.Reizner W, Hunter JG, O’Malley NT, Southgate RD, Schwarz EM, Kates SL. A systematic review of animal models for staphylococcus aureus osteomyelitis. Eur Cell Mater. 2014;27:196–212. doi: 10.22203/ecm.v027a15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rhoads DD, Wolcott RD, Kuskowski MA, Wolcott BM, Ward LS, Sulakvelidze A. Bacteriophage therapy of venous leg ulcers in humans: Results of a phase i safety trial. J Wound Care. 2009;18:237–238. 240–233. doi: 10.12968/jowc.2009.18.6.42801. [DOI] [PubMed] [Google Scholar]
- 80.Ruckh TT, Oldinski RA, Carroll DA, Mikhova K, Bryers JD, Popat KC. Antimicrobial effects of nanofiber poly(caprolactone) tissue scaffolds releasing rifampicin. J Mater Sci Mater Med. 2012;23:1411–1420. doi: 10.1007/s10856-012-4609-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ryan EM, Alkawareek MY, Donnelly RF, Gilmore BF. Synergistic phage-antibiotic combinations for the control of escherichia coli biofilms in vitro. FEMS Immunol Med Microbiol. 2012;65:395–398. doi: 10.1111/j.1574-695X.2012.00977.x. [DOI] [PubMed] [Google Scholar]
- 82.Sanchez CJ, Jr, Prieto EM, Krueger CA, Zienkiewicz KJ, Romano DR, Ward CL, Akers KS, Guelcher SA, Wenke JC. Effects of local delivery of d-amino acids from biofilm-dispersive scaffolds on infection in contaminated rat segmental defects. Biomaterials. 2013;34:7533–7543. doi: 10.1016/j.biomaterials.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 83.Saravanan S, Nethala S, Pattnaik S, Tripathi A, Moorthi A, Selvamurugan N. Preparation, characterization and antimicrobial activity of a bio-composite scaffold containing chitosan/nano-hydroxyapatite/nano-silver for bone tissue engineering. Int J Biol Macromol. 2011;49:188–193. doi: 10.1016/j.ijbiomac.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 84.Schuch R, Lee HM, Schneider BC, Sauve KL, Law C, Khan BK, Rotolo JA, Horiuchi Y, Couto DE, Raz A, Fischetti VA, Huang DB, Nowinski RC, Wittekind M. Combination therapy with lysin cf-301 and antibiotic is superior to antibiotic alone for treating methicillin-resistant staphylococcus aureus-induced murine bacteremia. J Infect Dis. 2014;209:1469–1478. doi: 10.1093/infdis/jit637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shi X, Wang Y, Ren L, Huang W, Wang DA. A protein/antibiotic releasing poly(lactic-co-glycolic acid)/lecithin scaffold for bone repair applications. Int J Pharm. 2009;373:85–92. doi: 10.1016/j.ijpharm.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 86.Silver S. Bacterial silver resistance: Molecular biology and uses and misuses of silver compounds. FEMS Microbiol Rev. 2003;27:341–353. doi: 10.1016/S0168-6445(03)00047-0. [DOI] [PubMed] [Google Scholar]
- 87.Sjollema J, Sharma PK, Dijkstra RJ, van Dam GM, van der Mei HC, Engelsman AF, Busscher HJ. The potential for bio-optical imaging of biomaterial-associated infection in vivo. Biomaterials. 2010;31:1984–1995. doi: 10.1016/j.biomaterials.2009.11.068. [DOI] [PubMed] [Google Scholar]
- 88.Southwood LL, Frisbie DD, Kawcak CE, Ghivizzani SC, Evans CH, McIlwraith CW. Evaluation of ad-bmp-2 for enhancing fracture healing in an infected defect fracture rabbit model. J Orthop Res. 2004;22:66–72. doi: 10.1016/S0736-0266(03)00129-3. [DOI] [PubMed] [Google Scholar]
- 89.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–138. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 90.Storm-Versloot MN, Vos CG, Ubbink DT, Vermeulen H. Topical silver for preventing wound infection. Cochrane Database Syst Rev. 2010:CD006478. doi: 10.1002/14651858.CD006478.pub2. [DOI] [PubMed] [Google Scholar]
- 91.Suri S, Lehman SM, Selvam S, Reddie K, Maity S, Murthy N, Garcia AJ. In vivo fluorescence imaging of biomaterial-associated inflammation and infection in a minimally invasive manner. J Biomed Mater Res A. 2014 doi: 10.1002/jbm.a.35162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thomes B, Murray P, Bouchier-Hayes D. Development of resistant strains of staphylococcus epidermidis on gentamicin-loaded bone cement in vivo. J Bone Joint Surg Br. 2002;84:758–760. doi: 10.1302/0301-620x.84b5.11907. [DOI] [PubMed] [Google Scholar]
- 93.Trampuz A, Widmer AF. Infections associated with orthopedic implants. Curr Opin Infect Dis. 2006;19:349–356. doi: 10.1097/01.qco.0000235161.85925.e8. [DOI] [PubMed] [Google Scholar]
- 94.Trampuz A, Zimmerli W. Diagnosis and treatment of infections associated with fracture-fixation devices. Injury. 2006;37(Suppl 2):S59–66. doi: 10.1016/j.injury.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 95.Trujillo NA, Oldinski RA, Ma HY, Bryers JD, Williams JD, Popat KC. Antibacterial effects of silver-doped hydroxyapatite thin films sputter deposited on titanium. Materials Science & Engineering C-Materials for Biological Applications. 2012;32:2135–2144. [Google Scholar]
- 96.van Oosten M, Schafer T, Gazendam JA, Ohlsen K, Tsompanidou E, de Goffau MC, Harmsen HJ, Crane LM, Lim E, Francis KP, Cheung L, Olive M, Ntziachristos V, van Dijl JM, van Dam GM. Real-time in vivo imaging of invasive- and biomaterial-associated bacterial infections using fluorescently labelled vancomycin. Nat Commun. 2013;4:2584. doi: 10.1038/ncomms3584. [DOI] [PubMed] [Google Scholar]
- 97.Wang J, Zhu Y, Bawa HK, Ng G, Wu Y, Libera M, van der Mei HC, Busscher HJ, Yu X. Oxygen-generating nanofiber cell scaffolds with antimicrobial properties. ACS Appl Mater Interfaces. 2011;3:67–73. doi: 10.1021/am100862h. [DOI] [PubMed] [Google Scholar]
- 98.Wang Q, Yu X, Libera M. Reducing bacterial colonization of 3-d nanofiber cell scaffolds by hierarchical assembly of microgels and an antimicrobial peptide. Adv Healthc Mater. 2013;2:687–691. doi: 10.1002/adhm.201200306. [DOI] [PubMed] [Google Scholar]
- 99.Wright A, Hawkins CH, Anggard EE, Harper DR. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant pseudomonas aeruginosa; a preliminary report of efficacy. Clin Otolaryngol. 2009;34:349–357. doi: 10.1111/j.1749-4486.2009.01973.x. [DOI] [PubMed] [Google Scholar]
- 100.Wu C, Zhou Y, Xu M, Han P, Chen L, Chang J, Xiao Y. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials. 2013;34:422–433. doi: 10.1016/j.biomaterials.2012.09.066. [DOI] [PubMed] [Google Scholar]
- 101.Xie Z, Liu X, Jia W, Zhang C, Huang W, Wang J. Treatment of osteomyelitis and repair of bone defect by degradable bioactive borate glass releasing vancomycin. J Control Release. 2009;139:118–126. doi: 10.1016/j.jconrel.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 102.Yasko AW, Lane JM, Fellinger EJ, Rosen V, Wozney JM, Wang EA. The healing of segmental bone defects, induced by recombinant human bone morphogenetic protein (rhbmp-2). A radiographic, histological, and biomechanical study in rats. J Bone Joint Surg Am. 1992;74:659–670. [PubMed] [Google Scholar]
- 103.Zheng Z, Yin W, Zara JN, Li W, Kwak J, Mamidi R, Lee M, Siu RK, Ngo R, Wang J, Carpenter D, Zhang X, Wu B, Ting K, Soo C. The use of bmp-2 coupled - nanosilver-plga composite grafts to induce bone repair in grossly infected segmental defects. Biomaterials. 2010;31:9293–9300. doi: 10.1016/j.biomaterials.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zilberman M, Elsner JJ. Antibiotic-eluting medical devices for various applications. J Control Release. 2008;130:202–215. doi: 10.1016/j.jconrel.2008.05.020. [DOI] [PubMed] [Google Scholar]