Abstract
Deep white matter hyperintensity (DWMH) and periventricular white matter lesion volumes (PV) are associated with age and subsequent stroke. We studied age differences in these volumes accounting for collinearity and risk factors. Subjects were 563 healthy family members of early-onset coronary artery disease (CAD) patients. Using 3T MRI, lesions were classified as DWMH or PV. Age association with lesion classification was analyzed using random effects Tobit regression, adjusting for intracranial volume (ICV), and risk factors. Subjects were 60% women, 36% African-American, mean age 51 ± 11 years. In multivariable analysis adjusted for PV and ICV, DWMH was associated with age (p<0.001), and female sex (p = 0.003) . PV, adjusted for DWMH and ICV, was age associated (p<0.001). For each age decade, DWMH showed 0.07 log units/decade greater volume (95% CI = 0.04-0.11); PV was 0.18 log units/decade greater (95% CI 0.14 – 0.23); slope differences (p < 0.001). In people with a family history of CAD, PV and DWMH are independently and differentially associated with age controlling for traditional risk factors.
Keywords: white matter disease, women and minorities, coronary, imaging, risk factors
1. INTRODUCTION
White matter hyperintensities (WMH) on cranial magnetic resonance imaging (MRI) are thought to represent lesions caused largely by ischemic small vessel disease in the brain, although other pathophysiologic possibilities exist. WMH lesions increase in volume and number with age and are associated with an increased risk for subsequent stroke and dementia.(Vermeer, et al., 2003,Young, et al., 2008) Two lesion subtypes have been proposed based on their proximity to the ventricles (1) periventricular lesions (PV), which are contiguous with the ventricles, and (2) deep WMH (DWMH) lesions, which are distinct and separate from the ventricles. (Enzinger, et al., 2006,Fazekas, et al., 1993,Rostrup, et al., 2012,Spilt, et al., 2006) Extreme DWMH lesion volumes have been associated with African American (AA) race, and in women in the oldest age ranges. (Nyquist, et al., 2014,van den Heuvel, et al., 2004) Additionally, hypertension causing aortic pulse wave variability, and pulse wave encephalopathy may affect PV and DWMH lesions differently.(Henry-Feugeas and Koskas, 2012,Inatomi, et al., 2008,Ishimitsu, et al., 2008) Both types are strongly associated with age.(The, et al., 2011) These lesion classifications have been supported by previous pathologic and radiologic studies. Specifically, DWMHs have been associated with endothelial activation and different magnetization transfer and diffusion tensor imaging characteristics.(Enzinger, et al., 2006,Fernando, et al., 2006,Jack, et al., 2001,Rostrup, et al., 2012,Spilt, et al., 2006,Young, et al., 2008) Nonetheless, physiologic differences and the robustness of these categorizations remain uncertain. Cross-sectional studies have shown the two subtypes to be highly collinear, with similar progression patterns in lesion size and total WMH burden.(DeCarli, et al., 2005,Sachdev and Wen, 2005) Distinctions in the total burden of PV and DWMH lesions across different age groups may help to establish a physiologic substrate for each lesion type. We hypothesized that DWMH volume and PV volume would demonstrate different patterns of association with age even when accounting for the correlation between the two types of lesions, intracranial volume (ICV), and risk factors.
We have reported a markedly higher prevalence of vascular disease risk factors in healthy family members of patients with early-onset coronary artery disease (CAD). (Becker, et al., 1998, Nyquist, et al., 2009) This population is enriched for vascular disease of all types and may represent a group at increased risk for ischemic white matter disease at younger ages. To date, almost all studies of PV and DWMH have been carried out in older populations or in the context of dementia, or other neurologic disease where WML volumes of both subtypes are so great that discrimination is not possible. We thus examined distinct DWMH and PV lesion volumes in relation to age and risk factors in a cross-sectional study of generally middle-aged healthy asymptomatic subjects at an increased risk of vascular disease in order to determine the extent to which the different regions represent possible distinct and different pathophysiologic subprocesses.
2. METHODS
2.1 Sample and Recruitment
Using a cross-sectional study design, subjects were randomly selected from among 2573 participants in a long-term prospective study of predictors of incident cardiovascular and cerebrovascular disease in families of index patients with documented early-onset CAD (The GeneSTAR Study: Genetic Study of Atherosclerosis Risk).(Vaidya, et al., 2007) The study sample consisted of 563 healthy asymptomatic individuals from 315 families with an early-onset CAD index case (one per family); on average, 1.8 ± 1.2 relatives per family (range 1– 8). Early-onset CAD, defined as any acute coronary syndrome before the age of 60 years, was used as a marker for increased risk of vascular disease in healthy relatives. CAD index patients and study subjects were 36% African American. Index cases were 33.6% female. The original index cases, not included in this MRI study, were identified during hospitalization for an acute myocardial infarction or an acute coronary syndrome with angiographic evidence of a flow-limiting stenosis of >50% diameter in at least one coronary artery. Apparently healthy asymptomatic siblings, their offspring, and the offspring of the index cases were eligible for this MRI study if they were 30 to 75 years of age and had no known history of CAD, stroke, or transient ischemic attacks, no history of atrial fibrillation, heart disease of any kind, life-threatening diseases such as active AIDS, renal failure, or cancer; no neurologic diseases that would preclude accurate MRI interpretation; and no implanted metals that were contraindications to performing cranial MRI.
2.2 Participant Screening
Subjects underwent a physical examination, medical history, and comprehensive screening for traditional Framingham stroke and cardiovascular risk factors, including blood hypertension, total cholesterol, diabetes, obesity and smoking.(Wang, et al., 2003) We measured height (in inches) and weight (in pounds) in light clothing with no shoes. Body mass index (BMI) was calculated as weight in kilograms/ (height in meters) 2. Obesity was defined as a BMI ≥ 30 kg/m2 in accordance with national guidelines.(Expert-Panel, 1998) Current cigarette smoking was assessed by self-report of any smoking within the past month and/or two expired carbon monoxide levels of ≥8 ppm. Blood pressure was measured at three standard times over the course of an eight hour day. The average systolic and diastolic blood pressures were each recorded and used to characterize blood pressure. Hypertension was defined as an average blood pressure ≥140 mmHg systolic or ≥90 mmHg diastolic, and/or use of any antihypertensive medication. After subjects had fasted for 9-12 hours overnight, blood was taken for measurement of total cholesterol and glucose levels. Type 2 diabetes was defined as a fasting glucose level ≥126 mg/dL (≥ 7 mmol/L), and/or use of antidiabetic medications. Total cholesterol was measured according to the United States Centers for Disease Control standardized methods. (Myers, et al., 2000)
2.3 Magnetic Resonance Imaging
All subjects underwent magnetic resonance imaging (MRI) with a Philips 3T scanner according to a standard protocol. The series included the following imaging sequences. 1) Axial T1-weighted MPRAGE (magnetization prepared rapid gradient echo): TR (repetition time) 10 ms, TE (time to echo) 6 ms, TI (inversion time) 983 ms, voxel size 0.75 × 0.75 × 1.0 mm3, contiguous slices, with field of view imaging (FOV) 240 mm, matrix 256×256×160 mm. 2) Axial turbo spin echo FLAIR (fluid attenuated inversion recovery): TR 11000 ms, TI 2800 ms, TE 68 ms, voxel size 0.47 × 0.47 × 3.0 mm3, contiguous slices, FOV 240 mm, matrix 256 X 256 mm. All images were reviewed by a study neuroradiologist for clinical pathology, and confirmed by a clinical neuroradiologist, and then stored on the in-house reading system for processing. Final image processing and volumetric analyses were completed by study biomedical engineers, neuroradiologists, radiologists, and their technical staff.
2.4 Volumetric Assessment
MPRAGE images were skull-stripped and co-registered to FLAIR images. Spatial normalization of the co-registered MPRAGE and FLAIR images into MNI space was performed via affine transformation. A trained neuroimaging rater manually delineated the WMHs on the normalized FLAIR images (with reference to the MPRAGE images for verification of pathology) using Medical Image Processing, Analysis, and Visualization (MIPAV) software.(Bazin, et al., 2007) We segmented the brain in native MPRAGE space using an automated probabilistic methodology that employs a topology-preserving algorithm and mapped the resulting tissue mask to MNI space.(Shiee, et al., 2010) We measured total brain, intracranial, cortical grey matter, and white matter volumes in native MPRAGE space, and WMH volumes in MNI space (taking into account the transformation of volume between the two spaces). Total brain volume (in cubic millimeters) was identified as the sum of white matter, WMH, and grey matter volume from the vertex of the brain to the foramen magnum. Intracranial volume was defined (in cubic millimeters) as the sum of all meningeal material, soft tissue, and sulcal and ventricular cerebrospinal volumes inferior to bone from the vertex to the foramen magnum.(Carass, et al., 2011)
We characterized the WMHs spatially using in-house software designed by biomedical engineers to determine location in relation to the ventricles and subcortical region (also called the deep white matter region) in three-dimensional space. Connected components of WMHs were determined by using digital 26 connectivity.
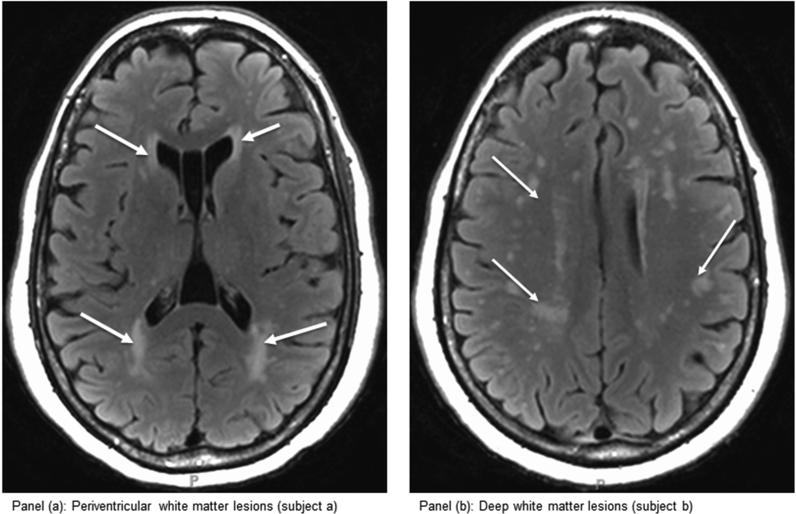
Periventricular lesions were defined as contiguous with a lesion voxel that was within 4 mm of a ventricle. DWMH lesions were those that were not contiguous with the ventricles (For reference, total WMH was the sum of the DWMH and PV lesion volumes in cubic millimeters). Figures 1 (a) and (b) respectively represent concentrated PV lesions and DWMH lesions.
Figure 1.
(a) and (b). Axial magnetic resonance imaging showing the position of nearly exclusive perventricular white matter lesions (a) and deep white matter lesions (b)
While certain approaches for separating WMH voxels into PV and DWMH are based on thresholding the distances from the ventricle, meaning that part of a contiguous lesion might be classified as both PV and DWMH (DeCarli 2005, Sachdev 2004, Sachdev 2007), others assign a single class to a contiguous lesion (Ramirez 2011). These contiguous models require direct connectivity with the ventricles to classify a lesion as PV and designate the rest as DWMH.
We followed a modified version of the latter contiguous approach and required that a PV lesion be in contact with the 4 mm-thick strip around the ventricles, as defined in three dimensional space. This 4mm threshold modification was motivated by the observation that the region around the ventricles is affected by cerebrospinal fluid leakage, suggesting an alternative periventricular etiology for lesions that are close enough to, but not immediately in contact with the ventricles (Sachdev 2008). Our choice of 4 mm was supported by a multivariate Gaussian mixture model analysis performed on the lesion volume and ventricular distance data. We found that the upper threshold for the ventricular distance for the periventricular class was approximately 4 mm. The 4 mm strip was defined in all 3 dimensions using our method incorporating 26 voxel connectivity.
2.5 Statistical Analysis
Demographic and cardiovascular risk factor variables were tabulated for the full study sample, and also by the presence of detectable DWMH or PV. Distributions were presented either as means and standard deviations for continuous variables, or as percentages for categorical variables and group differences were tested using t-tests or χ2 tests, respectively. The distributions of DWMH and PV were skewed with a large number of zeros, likely representing very small volumes below the level of detection. We thus added 0.5* (minimum detected volume of DWMH or PV) before log-transforming them prior to graphing, and these points were placed with a slight random offset to differentiate overlaid symbols. We examined the association of DWMH and PV separately with demographic and risk factor variables using random effects Tobit regression that corrected for intrafamilial correlations, and zero volumes were flagged as censored low values. The regression coefficients are presented as differences in logarithmically transformed volume of comparison groups vs. reference groups as described in the results. Risk factors in the models included female sex, AA race, diabetes, smoking, hypertension, total cholesterol, obesity, ICV, and age decade. The differences between the regression coefficients for PV and DWMH for specific independent variables were tested using the standard error of the difference of the estimates.
3. RESULTS
The sample had more women, more European Americans, and a higher prevalence of all risk factors than found in healthy reference populations. (Table 1) This is congruent with our other studies in this population with a family history of early coronary disease.(Becker, et al., 1998) Nearly half were obese and nearly half had hypertension. No symptomatic lacunar infarcts were observed and less than 1% of our population had cystic lesions consistent with silent lacunes. Most subjects had white matter lesions: 89.9% had DWMH lesions and 73.7% had PV lesions. The median (interquartile range) PV was 475 (range = 0-1741) mm3, and median DWMH volume was 306.5 (range = 104-724.5) mm3. PV lesion volume was greater than DWMH lesion volume in 55% of individuals, DWMH volume was greater than PV in 37% of individuals, and in the remaining 8%, both PV and DWMH volume were zero (nonparametric sign rank test for paired difference between PV and DWMH volumes, p<0.001). The demographic characteristics of the zero and non-zero values for DWMH and PV with associated p values can be seen in table 2. Persons with non-zero lesion volumes of either type were older and more likely to be hypertensive (both p < 0.001)
Table 1.
Sample demographic and risk factor characteristics (n = 563)
| Characteristic | Mean (SD) or %a |
|---|---|
| Age (years) | 51 (11) |
| Female sex | 60% |
| African-American race | 36% |
| Diabetes | 14% |
| Current smoking | 18% |
| Hypertension | 43% |
| Obesity (BMI ≥ 30 kg/m2) | 44% |
| Total cholesterol (mmol/L) | 5.02 (1.06) |
BMI, body mass index.
Continuous variables are presented as mean (SD); categorical variables are presented as percent.
Table 2.
Sample demographic and risk factor characteristics for DWMH and PV lesions with Tobit “zero” and “non-zero” volumes with P values
| DWMH “zero” | DWMH “non-zero | PV “zero” | PV “non-zero” | |||
|---|---|---|---|---|---|---|
| Characteristics | Mean (SD) | or %a | P | Mean (SD) | or %a | P |
| Age (years) | 43 (9.12) | 52 (10) | <0.001 | 44 (8) | 54 (10) | <0.001 |
| Female Sex | 56 | 60 | 0.565 | 58 | 60 | 0.569 |
| African American | 39 | 36 | 0.696 | 32 | 38 | 0.234 |
| Diabetes | 89 | 13 | 0.488 | 9 | 15 | 0.047 |
| Current Smoker | 14 | 18 | 0.437 | 20 | 17 | 0.377 |
| Hypertensive | 14 | 46 | <0.001 | 28 | 48 | <0.001 |
| Obese (BMI ≥ 30 kg/m2) | 46 | 43 | 0.802 | 48 | 43 | 0.302 |
| Total Cholesterol (mmol/L) | 191 (42) | 194 (40) | 0.517 | 191 (39) | 195 (41) | 0.381 |
BMI, body mass index.
Continuous variables are presented as mean (SD); categorical variables are presented as percent.
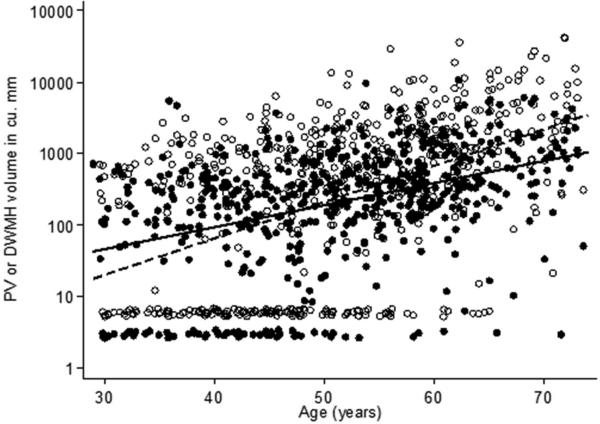
Figure 2 is a scatter plot of the relationship between PV and DWMH and age. In the supplementary material (online appendix), the prevalence of non-zero volumes of DWMH are presented by decade of age (Appendix Figure 1). Also presented in the supplementary material are a multivariate relative prevalence regression analysis for detectable DWMH and detectable PV (Appendix table 1), and a second stage regression analysis of lesion volumes restricted to non-zero DWMH and non-zero PV (Appendix Table 2). In each analysis the slope of the association of DWMH versus age is steeper than that of PV versus age, showing that our results are robust to alternative models of analysis.
Figure 2. Scatter plot of DWMH and PV vs. age.
Deep white matter hyperintensity volumes (filled circles and solid line) and periventricular hyperintensity volumes (hollow symbols and dotted line) on the logarithmic scale vs. age and best fit unadjusted linear fit. Volumes below the level of detection have been plotted at the level of half the minimum detectable volume. Small random numbers have been added to the plotted points so that overlaid symbols can be seen separately. The unadjusted slope for DWMH (7% higher volume per year) was lower than that for PV (12% higher volume per year, p < 0.001)
Table 3 shows the relationship of DWMH lesion volumes to risk factors and other brain volumes using multivariable random effects Tobit regression. Female sex and age were significantly associated when adjusted for PV lesion volumes and ICV which were also significant. Smoking, obesity, diabetes, and total cholesterol level were not significant. Table 4 shows the relationship of PV lesions to risk factors and other brain volumes, adjusted for DWMH lesions and ICV. Only age and the other brain volumes were significantly associated, with no risk factor or gender effect significantly associated.
Table 3.
Multivariate regression analysis of DWMH (Random effects Tobit regression)
| Characteristic | Regression coefficient (natural log units) | 95% CI | p |
|---|---|---|---|
| Female sex | 0.08 | 0.02 to 0.15 | 0.011 |
| African-American race | 0.02 | −0.05 to 0.08 | 0.57 |
| Diabetes | −0.05 | −0.13 to 0.03 | 0.22 |
| Current smoking | 0.07 | 0.00 to 0.14 | 0.068 |
| Hypertension | 0.05 | −0.01 to 0.12 | 0.080 |
| Obesity (BMI ≥ 30 kg/m2) | −0.02 | −0.08 to 0.04 | 0.48 |
| Periventricular lesion volume (log scale) | 0.34 | 0.27 to 0.40 | <0.001 |
| Intracranial volume (log scale) | 0.48 | 0.10 to 0.87 | <0.001 |
| Age (per decade) | 0.07 | 0.04 to 0.11 | <0.001 |
BMI, body mass index; CI, confidence interval; DWMH, deep white matter hyperintensity; PV, periventricular lesion volume.
Table 4.
Multivariate regression analysis of PV (Random effects Tobit regression)
| Characteristic | Regression coefficient (natural log units) | 95% CI | p |
|---|---|---|---|
| Female sex | 0.08 | −0.03 to 0.18 | 0.14 |
| African-American race | 0.04 | −0.06 to 0.14 | 0.41 |
| Diabetes | 0.06 | −0.06 to 0.18 | 0.34 |
| Current smoking | 0.03 | −0.08 to 0.15 | 0.56 |
| Hypertension | 0.03 | −0.06 to 0.13 | 0.50 |
| Obesity (BMI ≥ 30 kg/m2) | −0.07 | −0.16 to 0.02 | 0.13 |
| DWMH lesion volume (log scale) | 0.74 | 0.60 to 0.88 | <0.001 |
| Intracranial volume (log scale) | 0.79 | 0.20 to 1.38 | 0.009 |
| Age (per decade) | 0.18 | 0.14 to 0.23 | <0.001 |
BMI, body mass index; CI, confidence interval; DWMH, deep white matter hyperintensity; PV, periventricular lesion volume.
Comparing the strength of association of correlates of DWMH and PV lesion volumes, every decade of age was associated with 0.07 log units higher DWMH lesion volumes (95% CI = 0.04-0.10), and PV lesion volumes were 0.18 log units higher per decade (95% CI 0.13 – 0.22) with a statistically significant difference between slopes (p < 0.001).
4. DISCUSSION
In our cross-sectional study of asymptomatic healthy generally middle-aged family members of index cases with early-onset CAD we observed that PV and DWMH lesion volumes were associated with age and each other. Additionally, female sex was significantly associated with DWMH, but not with PV lesion volumes. Current smoking and hypertension showed a trend towards association (p<0.10) with DWMH, but not with PV. DWMH lesion volumes were greater in women after adjustment for age, race, PV and ICV. The sex effect is not a novel finding. Van den Heuvel et al, similarly observed this in a prospective study in elderly cohorts with repeated measures where DWMH lesion volumes increased at a greater rate in women.(van den Heuvel, et al., 2004) The reason for this sex difference remains unknown. It may be biological, or it may also result from an epidemiological “artifact” such as strong survival bias in women, as men who were higher risk a priori may have had higher mortality rates at younger ages and not been in the cohorts studied.
White matter lesions in these two different regions have been associated with different neurologic sequelae including differences in declining motor function and cognition possibly related to different regional tract involvement, although specific and consistent patterns in the literature are difficult to discern.(Benson, et al., 2002,Bolandzadeh, et al., 2012,Duering, et al., 2014,The, et al., 2011) Varying neurological findings related to motor and cognitive function may be attributed to underlying effects of separate pathophysiological processes or simply the effects of ischemic white matter disease on different functional tracks located in different anatomic regions, PV versus DWMH.(Duering, et al., 2014) It has been suggested that PV lesions may be related to several processes associated with aging while DWMH lesions may more likely differentially represent greater ischemic small vessel disease, although this is controversial. DWMH lesion volumes have been reported to be subject to the influences of small vessel atherosclerosis and endothelial activation associated with increased inflammation and atherosclerotic risk factors.(Fazekas, et al., 1993, Fernando, et al., 2006, Hassan, et al., 2003) Our own data show that even adjusted for age, hypertension had a trend toward associatation with DWMH lesions. PV lesions may be more strongly affected by additional factors related primarily to aging including hypotension, hypoperfusion, and atrophy. (Wang, et al., 2003, Wen and Sachdev, 2004, Wen, et al., 2009) Definitive evidence of specific causal variation, however, does not yet exist.
In prior studies, identification of distinct PV and DWMH lesion volumes has been limited by inadequate measurement methods and population extremes of age and clinical pathology. Our healthy study population is unique, with a wide age distribution, from 30 to 75 years. Our observations were applied to all age decades, not just the elderly. This enabled us to detect associations that are often not observable in much older or diseased cohorts where WMH burden is so great the two regions coalesce and cannot be discriminated well.(Appelman, et al., 2009) With 3T MRI, we directly measured lesion volumes using advanced white matter segmentation algorithms and a combination of standardized and replicable semi-automated and manual lesion delineation. We were able to directly measure PV lesion and DWMH volumes and more robustly determine their association with age. Our 3T volumetric analysis likely has better sensitivity for the detection of WMH than MRI techniques with smaller field strengths. It provides improved signal-to-noise ratios (Jack, et al., 2001,Zwanenburg, et al., 2010), which allow for more accurate assessment of lesion volumes as well. In general, the prevalence and volumes measured for DWMH and PV lesions are significantly higher than those observed with 1.5T methods. (Wen and Sachdev, 2004, Wen, et al., 2009) We also used replicable in-house advanced computational methods to separate, localize, and measure WMH volumes as compared to previous studies where methods generally utilized in part more subjective visualization. (Bazin, et al., 2007).
5.1 Study Considerations
We have documented an age association of two different white matter lesions areas of the brain and shown different patterns of risk factor and demographic relationships in our population of healthy subjects with a family history of early-onset CAD. (Becker, et al., 1998) This group may represent a population with unique properties that predispose them to subclinical vascular disease, resulting in increased WMH lesion burden. Possible reasons include a potential genetic susceptibility in addition to exposure to shared environmental factors and related inherited comorbidities that are proatherogenic, such as diabetes and hypertension.(Becker, et al., 1998)
Our study population is thus generally enriched for traditional atherosclerotic risk factors. These factors may have contributed to our observation of increased DWMH lesions and their association with hypertension in younger age groups. Thus, these findings may not apply to a broad population with a lower propensity to vascular disease.
Because our study was cross-sectional, it cannot demonstrate lesion progression over time and with incremental age in the same persons. Other longitudinal studies have reported increased rates of WMH lesions with aging, including increased rates of DWMH enlargement as compared to those of PV and total WMH. (Sachdev, et al., 2007) These prior studies used different methods of lesion volume measurement, such as assigning subjective values according to ordinal scales based on visual inspection of MRIs that had smaller field strengths. (Sachdev, et al., 2007, Schmidt, et al., 1999) Our definition of PV lesions required a voxel-by-voxel analysis of the connectivity of all voxels to a voxel within 4 mm of the ventricles. It is also possible that in individuals with particularly large lesion sizes, some lesions that were originally DWMHs may have been misclassified because of the development of confluence with periventricular lesions.
6. Conclusion
In our sample of healthy family members of persons with early-onset CAD, periventricular and deep white matter lesion volumes were each associated with age and different patterns of risk factors adjusting for one another and intracranial volume. These findings support the hypothesis that PV and DWMH lesions are distinct lesion subtypes with different patterns of associations with age, sex and risk factors. By improved identification of the two distinct areas over the age range, these results support further studies that promote a better understanding of pathophysiologic processes. Prospective studies starting at younger ages than heretofore studied may ultimately promote early detection and novel therapeutic modalities to prevent clinical sequelae of white matter lesions that increase with age in the brain.
Supplementary Material
Highlights.
We imaged 563 participants with 3T imaging identifying PV and DWMH volumes.
We analyzed DWMH and PV to determine independence of age and other risk factors.
PV and DWMH are independently related to age and DWMH is associated with female sex.
Acknowledgement
The authors wish to acknowledge their staff and the participants and their family for their dedication to the project. Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number R01NS062059. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors report no actual or potential conflicts of interest.
References
- Appelman AP, Exalto LG, van der Graaf Y, Biessels GJ, Mali WP, Geerlings MI. White matter lesions and brain atrophy: more than shared risk factors? A systematic review. Cerebrovasc Dis. 2009;28(3):227–42. doi: 10.1159/000226774. doi:10.1159/000226774. [DOI] [PubMed] [Google Scholar]
- Bazin PL, Cuzzocreo JL, Yassa MA, Gandler W, McAuliffe MJ, Bassett SS, Pham DL. Volumetric neuroimage analysis extensions for the MIPAV software package. J Neurosci Methods. 2007;165(1):111–21. doi: 10.1016/j.jneumeth.2007.05.024. doi:10.1016/j.jneumeth.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker DM, Yook RM, Moy TF, Blumenthal RS, Becker LC. Markedly high prevalence of coronary risk factors in apparently healthy African-American and white siblings of persons with premature coronary heart disease. Am J Cardiol. 1998;82(9):1046–51. doi: 10.1016/s0002-9149(98)00553-0. [DOI] [PubMed] [Google Scholar]
- Benson RR, Guttmann CR, Wei X, Warfield SK, Hall C, Schmidt JA, Kikinis R, Wolfson LI. Older people with impaired mobility have specific loci of periventricular abnormality on MRI. Neurology. 2002;58(1):48–55. doi: 10.1212/wnl.58.1.48. [DOI] [PubMed] [Google Scholar]
- Bolandzadeh N, Davis JC, Tam R, Handy TC, Liu-Ambrose T. The association between cognitive function and white matter lesion location in older adults: a systematic review. BMC Neurol. 2012;12:126. doi: 10.1186/1471-2377-12-126. doi:10.1186/1471-2377-12-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carass A, Cuzzocreo J, Wheeler MB, Bazin PL, Resnick SM, Prince JL. Simple paradigm for extra-cerebral tissue removal: algorithm and analysis. Neuroimage. 2011;56(4):1982–92. doi: 10.1016/j.neuroimage.2011.03.045. doi:10.1016/j.neuroimage.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical mapping of white matter hyperintensities (WMH): exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke. 2005;36(1):50–5. doi: 10.1161/01.STR.0000150668.58689.f2. doi:10.1161/01.STR.0000150668.58689.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duering M, Gesierich B, Seiler S, Pirpamer L, Gonik M, Hofer E, Jouvent E, Duchesnay E, Chabriat H, Ropele S, Schmidt R, Dichgans M. Strategic white matter tracts for processing speed deficits in age-related small vessel disease. Neurology. 2014;82(22):1946–50. doi: 10.1212/WNL.0000000000000475. doi:10.1212/WNL.0000000000000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzinger C, Smith S, Fazekas F, Drevin G, Ropele S, Nichols T, Behrens T, Schmidt R, Matthews PM. Lesion probability maps of white matter hyperintensities in elderly individuals: results of the Austrian stroke prevention study. J Neurol. 2006;253(8):1064–70. doi: 10.1007/s00415-006-0164-5. doi:10.1007/s00415-006-0164-5. [DOI] [PubMed] [Google Scholar]
- Expert-Panel Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Am J Clin Nutr. 1998;68(4):899–917. doi: 10.1093/ajcn/68.4.899. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, Radner H, Lechner H. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43(9):1683–9. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- Fernando MS, Simpson JE, Matthews F, Brayne C, Lewis CE, Barber R, Kalaria RN, Forster G, Esteves F, Wharton SB, Shaw PJ, O'Brien JT, Ince PG, Function MRCC, Ageing Neuropathology Study G. White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006;37(6):1391–8. doi: 10.1161/01.STR.0000221308.94473.14. doi:10.1161/01.STR.0000221308.94473.14. [DOI] [PubMed] [Google Scholar]
- Hassan A, Hunt BJ, O'Sullivan M, Parmar K, Bamford JM, Briley D, Brown MM, Thomas DJ, Markus HS. Markers of endothelial dysfunction in lacunar infarction and ischaemic leukoaraiosis. Brain. 2003;126(Pt 2):424–32. doi: 10.1093/brain/awg040. [DOI] [PubMed] [Google Scholar]
- Henry-Feugeas MC, Koskas P. Cerebral vascular aging: extending the concept of pulse wave encephalopathy through capillaries to the cerebral veins. Curr Aging Sci. 2012;5(2):157–67. doi: 10.2174/1874609811205020157. [DOI] [PubMed] [Google Scholar]
- Inatomi Y, Yonehara T, Hashimoto Y, Hirano T, Uchino M. Correlation between ventricular enlargement and white matter changes. J Neurol Sci. 2008;269(1-2):12–7. doi: 10.1016/j.jns.2007.12.007. doi:10.1016/j.jns.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Ishimitsu T, Nakano N, Sudo Y, Akashiba A, Takahashi T, Ohta S, Minami J, Matsuoka H. Predictive significance of blood pressure values for the incidence of cardiovascular events in chronic hemodialysis patients. Hypertens Res. 2008;31(9):1703–9. doi: 10.1291/hypres.31.1703. doi:10.1291/hypres.31.1703. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr., O'Brien PC, Rettman DW, Shiung MM, Xu Y, Muthupillai R, Manduca A, Avula R, Erickson BJ. FLAIR histogram segmentation for measurement of leukoaraiosis volume. J Magn Reson Imaging. 2001;14(6):668–76. doi: 10.1002/jmri.10011. doi:10.1002/jmri.10011 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers GL, Kimberly MM, Waymack PP, Smith SJ, Cooper GR, Sampson EJ. A reference method laboratory network for cholesterol: a model for standardization and improvement of clinical laboratory measurements. Clin Chem. 2000;46(11):1762–72. [PubMed] [Google Scholar]
- Nyquist PA, Bilgel MS, Gottesman R, Yanek LR, Moy TF, Becker LC, Cuzzocreo J, Prince J, Yousem DM, Becker DM, Kral BG, Vaidya D. Extreme deep white matter hyperintensity volumes are associated with African American race. Cerebrovasc Dis. 2014;37(4):244–50. doi: 10.1159/000358117. doi:10.1159/000358117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyquist PA, Wityk R, Yanek LR, Vaidya D, Yousem DM, Becker LC, Becker DM. Silent small-vessel cerebrovascular disease and silent myocardial ischemia in families with premature coronary disease. Neuroepidemiology. 2009;33(1):66–7. doi: 10.1159/000215831. doi:10.1159/000215831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostrup E, Gouw AA, Vrenken H, van Straaten EC, Ropele S, Pantoni L, Inzitari D, Barkhof F, Waldemar G, group L.s. The spatial distribution of age-related white matter changes as a function of vascular risk factors--results from the LADIS study. Neuroimage. 2012;60(3):1597–607. doi: 10.1016/j.neuroimage.2012.01.106. doi:10.1016/j.neuroimage.2012.01.106. [DOI] [PubMed] [Google Scholar]
- Sachdev P, Wen W. Should we distinguish between periventricular and deep white matter hyperintensities? Stroke. 2005;36(11):2342–3. doi: 10.1161/01.STR.0000185694.52347.6e. author reply 3-4. doi:10.1161/01.STR.0000185694.52347.6e. [DOI] [PubMed] [Google Scholar]
- Sachdev P, Wen W, Chen X, Brodaty H. Progression of white matter hyperintensities in elderly individuals over 3 years. Neurology. 2007;68(3):214–22. doi: 10.1212/01.wnl.0000251302.55202.73. doi:10.1212/01.wnl.0000251302.55202.73. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Fazekas F, Kapeller P, Schmidt H, Hartung HP. MRI white matter hyperintensities: three-year follow-up of the Austrian Stroke Prevention Study. Neurology. 1999;53(1):132–9. doi: 10.1212/wnl.53.1.132. [DOI] [PubMed] [Google Scholar]
- Shiee N, Bazin PL, Ozturk A, Reich DS, Calabresi PA, Pham DL. A topology-preserving approach to the segmentation of brain images with multiple sclerosis lesions. Neuroimage. 2010;49(2):1524–35. doi: 10.1016/j.neuroimage.2009.09.005. doi:10.1016/j.neuroimage.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilt A, Goekoop R, Westendorp RG, Blauw GJ, de Craen AJ, van Buchem MA. Not all age-related white matter hyperintensities are the same: a magnetization transfer imaging study. AJNR Am J Neuroradiol. 2006;27(9):1964–8. [PMC free article] [PubMed] [Google Scholar]
- The LSG, Poggesi A, Pantoni L, Inzitari D, Fazekas F, Ferro J, O'Brien J, Hennerici M, Scheltens P, Erkinjuntti T, Visser M, Langhorne P, Chabriat H, Waldemar G, Wallin A, Wahlund A. 2001-2011: A Decade of the LADIS (Leukoaraiosis And DISability) Study: What Have We Learned about White Matter Changes and Small-Vessel Disease? Cerebrovasc Dis. 2011;32(6):577–88. doi: 10.1159/000334498. doi:10.1159/000334498. [DOI] [PubMed] [Google Scholar]
- Vaidya D, Yanek LR, Moy TF, Pearson TA, Becker LC, Becker DM. Incidence of coronary artery disease in siblings of patients with premature coronary artery disease: 10 years of follow-up. Am J Cardiol. 2007;100(9):1410–5. doi: 10.1016/j.amjcard.2007.06.031. doi:10.1016/j.amjcard.2007.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel DM, Admiraal-Behloul F, ten Dam VH, Olofsen H, Bollen EL, Murray HM, Blauw GJ, Westendorp RG, de Craen AJ, van Buchem MA, Group PS. Different progression rates for deep white matter hyperintensities in elderly men and women. Neurology. 2004;63(9):1699–701. doi: 10.1212/01.wnl.0000143058.40388.44. [DOI] [PubMed] [Google Scholar]
- Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34(5):1126–9. doi: 10.1161/01.STR.0000068408.82115.D2. doi:10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- Wang TJ, Massaro JM, Levy D, Vasan RS, Wolf PA, D'Agostino RB, Larson MG, Kannel WB, Benjamin EJ. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. JAMA. 2003;290(8):1049–56. doi: 10.1001/jama.290.8.1049. doi:10.1001/jama.290.8.1049. [DOI] [PubMed] [Google Scholar]
- Wen W, Sachdev P. The topography of white matter hyperintensities on brain MRI in healthy 60-to 64-year-old individuals. Neuroimage. 2004;22(1):144–54. doi: 10.1016/j.neuroimage.2003.12.027. doi:10.1016/j.neuroimage.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Wen W, Sachdev PS, Li JJ, Chen X, Anstey KJ. White matter hyperintensities in the forties: their prevalence and topography in an epidemiological sample aged 44-48. Hum Brain Mapp. 2009;30(4):1155–67. doi: 10.1002/hbm.20586. doi:10.1002/hbm.20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young VG, Halliday GM, Kril JJ. Neuropathologic correlates of white matter hyperintensities. Neurology. 2008;71(11):804–11. doi: 10.1212/01.wnl.0000319691.50117.54. doi:01.wnl.0000319691.50117.54. [DOI] [PubMed] [Google Scholar]
- Zwanenburg JJ, Hendrikse J, Visser F, Takahara T, Luijten PR. Fluid attenuated inversion recovery (FLAIR) MRI at 7.0 Tesla: comparison with 1.5 and 3.0 Tesla. Eur Radiol. 2010;20(4):915–22. doi: 10.1007/s00330-009-1620-2. doi:10.1007/s00330-009-1620-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.