Abstract
Biliverdin reductase (BVR) isozymes, BVRA and BVRB, are cell surface membrane receptors with pleiotropic functions. This review compares, for the first time, the structural and functional differences of the isozymes. They reduce biliverdin, a byproduct of heme catabolism, to bilirubin, display kinase activity and BVRA, but not BVRB, can act as a transcription factor. The binding motifs present in the BVR isozymes allow for a wide range of interactions with components of metabolically important signaling pathways, such as with the insulin receptor kinase cascades, protein kinase, and inflammatory mediators. In addition, serum bilirubin levels have been negatively associated with abdominal obesity and hypertriglyceridemia. We will discuss the roles of the BVR isozymes in metabolism, and their potential as therapeutic targets.
Keywords: Biliverdin Reductase, BVRB, BVRA, Bilirubin, Heme Oxygenase, Obesity, Diabetes
Introduction
In obese individuals, adipocyte expansion leads to increased inflammation as a result of elevated proinflammatory molecule secretion from adipose tissue, which leads to an exacerbated oxidative state that contributes to adipocyte hypertrophy (1). Antioxidants may prove useful for reducing reactive oxygen species (ROS), preventing adipocyte expansion and chronic inflammation. Several studies have shown that activation of the heme oxgenase (HO) system reduces ROS, body weight, and blood glucose levels (2–7). HO catabolizes heme to produce carbon monoxide (CO), iron, and the tetrapyrrolic bile pigment biliverdin, which is further reduced to the antioxidant bilirubin, by the enzyme biliverdin reductase (BVR). Recent studies have demonstrated that BVR, through production of bilirubin as well as direct signaling, may interact with the insulin receptor and thus affect insulin signaling (8), whereas serum total bilirubin levels have been negatively associated with hypertriglyceridemia and abdominal obesity (9). However, the pathways by which BVR and its end product bilirubin signal in tissues like adipose and liver to help prevent insulin resistance and obesity are not well understood. Herein, we will discuss interactions and signaling pathways of the BVR system and its novel role as a metabolic regulator.
BVR isozymes; the basics
Structure and function
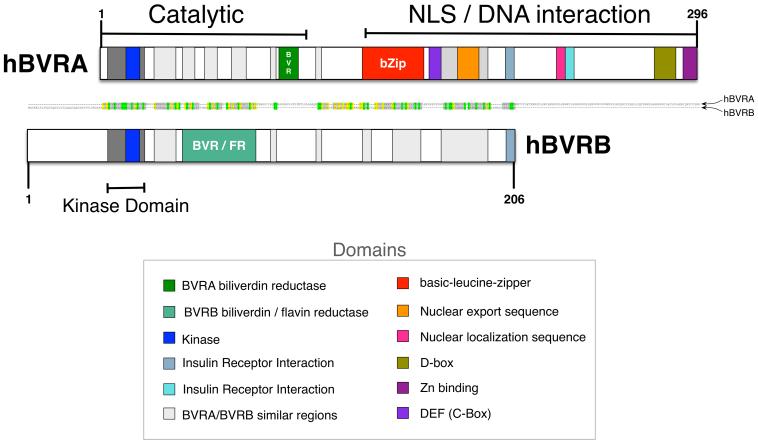
Specific structural sites of BVRA allow for the modulation of a diverse set of signaling pathways (Figure 1). The structure of BVRA can be divided into two major regions, the catalytic and the regulatory/DNA interaction domains. The N-terminus of BVRB and BVRA contains the catalytic domain and houses a binding motif for NADP(H) and NAD(H) cofactors (10). The C-terminus of BVRA encompasses the domain structures for the basic-leucine-zipper (bZiP) to function as a transcription factor, as well as the nuclear location sequence (NLS) and nuclear export sequences (NES) that are required for translocation into the nucleus and binding to regulatory regions of DNA (11, 12). However, these structures are absent in BVRB suggesting that this protein may not function in the same capacity as BVRA. The bZip site on BVRA has been shown to bind activating transcription factor-2 (ATF-2), which is known to regulate cAMP response elements (CRE) to increase the expression of a variety of genes, including c-jun, (13–17). The NLS and NES are also necessary for BVRA-facilitated movement of various signaling components, such as interaction with ERK (18), as well as binding to transcription regulators (14, 19). The SH2 binding motif on the C-terminus is associated with the insulin receptor kinase (IRK) signaling, allowing it to modulate insulin dependent pathways (20), discussed in detail below. The C-Box and D-Box domains serve as binding sites for various kinases in the mitogen activated protein kinase (MAPK) pathway and are important in the BVRA regulation of MEK (MAPK-ERK kinase)-ERK-ELK (eukaryotic-like protein kinase) signaling (18).
Figure 1. Domain structures of human biliverdin reductase A and B isozymes.
Domain structural comparisons of BVRA and BVRB indicate that there is a similar kinase and catalytic domain for both isozymes. The major difference is in the C-terminus, where BVRA contains a bZip DNA binding domain, nuclear localization signaling, and nuclear export signal that are not located within BVRB.
BVRA has the ability to bind DNA sequences such as the antioxidant response element (ARE) and hypoxia response elements (HRE) (11), which are bound by the bZip transcription factors, including NF-E2-related factor (Nrf2) (21, 22). Nrf2 proteins are known to be involved in combating oxidative stress and increasing the expression of HO-1 (23). It has also been shown that abrogation of the Nrf-2/ARE pathway leads to increased oxidative damage, which attenuates the ability to recover from hypoxia and apoptosis in mice (22, 24). Whether the BVRB isoform can also bind directly to DNA and activate similar response elements in genes is not know. However, given the structural differences between the BVRA and BVRB proteins, it is unlikely that BVRB acts as a transcription factor.
Developmental profile
BVRB can be identified beginning at 14–15 weeks of human development, whereas BVRA expression increases closer to 20 weeks in gestation (25). BVRB remains present in all tissues into adulthood; however, its functional role is not known. BVRA is ubiquitously expressed in adult tissue with expression levels higher in brain and lungs as compared to tissues such as liver and placenta (26). The functional differences between BVR isozymes begin with their reduction specificity. BVRA reduces biliverdin IXα to bilirubin IXα, whereas BVRB reduces biliverdin IXβ to bilirubin IXβ (25, 27). HO cleaves fetal heme at the β-meso position producing biliverdin IXβ, whereas adult heme is predominately constructed to biliverdin IXα. An important advantage of bilirubin IXβ is its higher solubility that allows for its excretion in the absence of any type of conjugation, which is required for excretion of bilirubin IXα (28). During the early stages of development, fetal heme is the primary heme present within circulation. The use of BVRB in early maturation allows for the removal of biliverdin metabolites that would otherwise accumulate in high concentrations leading to toxic conditions in the developing fetus (27). To make bilirubin IXα soluble requires conjugation with glucuronic acid, which is driven by UDP-glucuronyltransferase (UGT1A1), an enzyme that is only present after the first week of birth (25). Bilirubin IXα is the major metabolite that is correlated with a reduction in obesity and increased during weight loss. The signaling involvement of the different bilirubin isotypes are unknown, but known structural similarities and differences in BVRA and BVRB (Box 1) may suggest that they have diverse functions.
BVR and Bilirubin in Metabolic Disorders
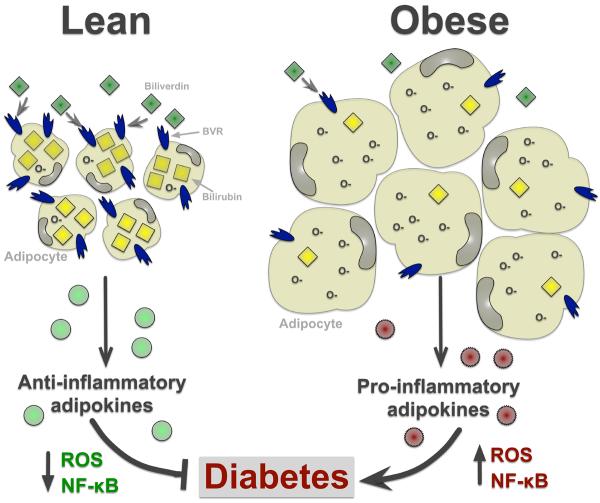
Along with being a potent antioxidant, bilirubin may also play a key role in obesity. Bilirubin can easily diffuse into the lipid environment (29), and moderately elevated plasma levels of bilirubin are associated with decreased abdominal obesity and lowered risk of metabolic syndrome (Figure 2) (30). This correlated with the observation that obese patients with elevated insulin and visceral adiposity have decreased levels of plasma total bilirubin (31). Patients with elevated plasma bilirubin also had increased adiponectin levels in blood, an anti-inflammatory cytokine directly secreted from adipose tissue (32). Andersson et al. investigated short-term weight loss in obese high-risk cardiovascular patients and found that plasma total bilirubin concentration increases as body weight decreases (33). It was recently shown that cobalt-protoporphyrin (CoPP) induction of HO and increased production of bilirubin in obese mice resulted in the elevation of peroxisome proliferator-activated receptor alpha (PPARα) expression and gene-regulatory activity, thus increasing fatty acid oxidation and amelioration of fatty liver development, and reduced body weight and blood glucose (2). In addition, CoPP and bilirubin treatments have been recently reported to increase insulin sensitivity and glucose tolerance in leptin-receptor deficient and in diet-induced obese mice through suppression of endoplasmic reticulum stress (34). However, it must be noted that whether CoPP acts solely through induction of HO-1 or through indirect effects of protoporphyrins on other heme containing proteins is not clear (35). There is also the possibility that increased HO activity may increase signaling through biliverdin to the BVR enzyme to increase production of bilirubin which has in its own right the capacity to prevent diabetes and obesity. This hypothesis is supported by a recent study that demonstrated that BVR protects against glucose intolerance and diabetes by affecting the pancreatic islet. Treatment of rat pancreata with mesobiliverdin IXκ, a biliverdin IXκ analog that acts as a substrate for BVR, increased the yield of functional pancreatic islets; and transplantation of these islets into diabetic rats lowered blood glucose levels and reversed insulin dysfunction in diabetic rats (36). This study suggests an important role for intercellular generation of bilirubin in protection against diabetes and highlights the clinical potential of biliverdin analogs in protecting islets during allograft transplantation; however, specific studies in which intracellular bilirubin generation is decreased via knockout of the BVR isozymes are needed to fully elucidate the protective role of this pathway in metabolic disorders such as diabetes.
Figure 2. BVR and bilirubin levels and their association with obesity.
Increasing plasma levels of bilirubin are associated with decreased adipocyte size by scavenging free radicals (O-) and increasing production of anti-inflammatory adipokines (e.g. adiponectin), thus lowering the risk of type II diabetes. Obese patients have decreased levels of plasma total bilirubin (31), which results in increased free radicals and ROS, and in the enhancement of NF-kB mediated inflammation. This leads to adipocyte hypertrophy and increased production of pro-inflammatory adipokines (e.g. TNFα) that contribute to diabetes.
Biliverdin Reductase and Insulin Signaling Pathways
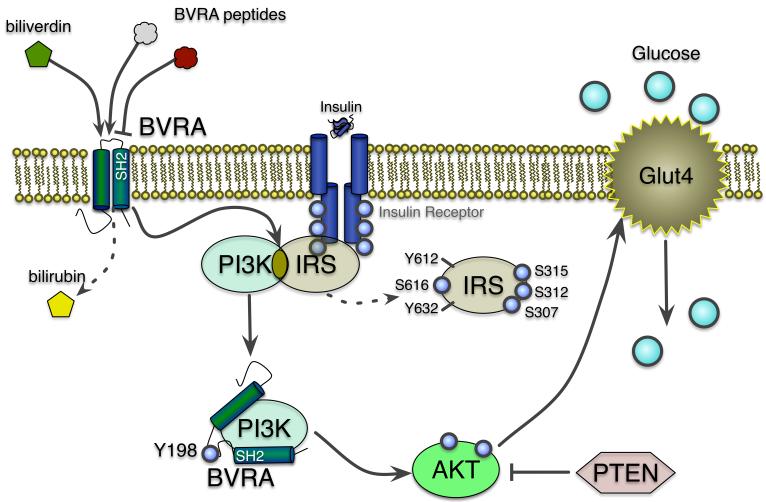
The most important mediator of metabolic processes is insulin, which is secreted after feeding from the pancreatic beta islet cells to initiate signal transduction pathways that increase glucose and fatty acid uptake by insulin responsive tissues. In non-insulin dependent diabetic patients, these processes become resistant in peripheral organs and result in insulin and glucose intolerance. Insulin causes autophosphorylation of tyrosine residues in the cytoplasmic portion of the transmembrane insulin receptor that phosphorylates insulin receptor substrate 1 (IRS-1) in tyrosine residues and activates IRK signaling pathways. The insulin signaling cascade diverges into two major arms, the PI3-kinase/Akt, and the IRK/IRS/PI3-kinase/MAPK pathway. BVRA but not BVRB is known to interact with components in both pathways, and thus may have a role in the regulation of metabolic processes (Figure 3) (8, 18).
Figure 3. Involvement of BVRA in the insulin receptor signaling cascade.
Activation of BVRA increases insulin receptor and PI3K signaling (39, 41), which enhances phosphorylation of Akt (8, 39, 41) and Glut4-mediated glucose uptake. BVRA inhibits the IRS-1 and PI3K interaction by phosphorylation of IRS-1. The serine 315 phosphorylation of IRS-1 uncouples it from the insulin receptor and inhibiting function (55). The SH2 domains in BVRA bind directly to PI3K to enhance pAkt signaling and glucose uptake (39–41). Biliverdin activation of BVRA increases pAkt and glucose uptake, while the BVRA peptides have inhibitory and stimulatory roles. The BVRA peptides that target the SH2-binding domain inhibits glucose uptake.
PI3-kinase/Akt
Insulin stimulation of the PI3K pathway leads to downstream phosphorylation events that include the insulin kinase cascade (IRK), and Akt signaling pathways, known regulators of glucose uptake and energy storage (37). Phosphorylation of Akt (pAkt) modulates downstream targets that enhance glucose uptake via increased expression and translocation of glucose transporters (e.g. Glut4) to the cellular membrane, and positively effect glucose uptake through this pathway (Figure 3) (38). For these reasons, the PI3K/Akt axis has been a major target for drug development for insulin resistant and type II diabetes. BVRA has been shown to interact with several components of the IRK signaling cascade which make it a potential target for the treatment of type II diabetes. BVRB does contain a similar insulin receptor-interaction domain as BVRA, but whether BVRB can influence insulin signaling is not currently known. Most of what is known about the kinase activity function of BVRA and its involvement in insulin signaling has been pioneered by the Maines laboratory (8, 20), but the interaction between BVRA and PI3K has also been reported by other independent laboratories (39, 40). The effect of BVRA on insulin signaling remains controversial. There have been conflicting reports on BVRA and insulin signaling. Initial studies showed that siRNA suppression of BVRA enhanced glucose uptake in 293A embryonal human kidney cells by decreasing serine phosphorylation of IRS-1(20). However, later investigations showed that siRNA suppression of BVRA increased pAkt in HK-2 proximal tubule epithelial human cells (41), human macrophages (40) and rat heart H9c2 cells (39), suggesting that BVRA may positively affect glucose uptake. The duality of BVRA on insulin signaling was further highlighted in a recent study using different peptide sequences of BVRA (8). The terminal 290KYCCSRK peptide was found to increase glucose uptake and potentiate insulin, while a peptide (194KEDQYMKMTV) corresponding to BVRA's SH2-binding domain was a potent inhibitor of glucose uptake and insulin signaling (8). This highlights the importance of the BVRA-PI3K interaction, and recommends that inhibition is detrimental to glucose uptake and possibly translocation of Glut4 to the cellular membrane (Figure 3). It appears that differences in response between the two peptides is due to where each of them targets the IRK signaling cascade (8). The differential effects exhibited by these BVRA peptides make them potential candidates for drugs targeting disorders of glucose metabolism especially type II diabetes. It is now apparent that not only is BVRA mediating bilirubin production, a potential therapeutic target for diabetes, but BVRA peptides could also be novel targets for the treatment of diabetes (Figure 3).
The PI3K pathway plays an essential role in protection from oxidative damage and serves as a cytoprotectant (42). Several mechanistic connections exist between the PI3K pathway and BVR, as it has been shown that BVR can link and activate the PI3K as well as the MAPK (Box 2) pathway arms of the IRK signaling pathway (20, 50). In addition, the human enzyme may function as an ERK nuclear transporter and ERK activator (50).
BVR can also protect from oxidative damage by regulation of HO-1 expression, as well as generation of bilirubin, a powerful antioxidant. Several studies have shown that reduction of BVRA protein via siRNAs leads to an increase in ROS levels (11, 43–46). BVRA has also been shown to increase HO-2 levels in cardiomyocytes, most likely via a decrease in HO-2 turnover, which allows for increased cardiac protection and decreased cardiomyocyte apoptosis, possibly through decreases in levels of ROS (47), and by activation of PI3K.
The PI3K pathway is inhibited by phosphatase and tensin homolog (PTEN), that catalyzes the dephosporylation of 3-phosphoinositide (PIP3) to 2-phosphoinositide (PIP2) (48) resulting in inhibition of the Akt signaling pathway. PTEN has been shown to be important for suppression of growth (49), and a reduction in PTEN leads to exacerbated Akt guided proliferation (37). Furthermore, inhibition of PTEN expression increases Akt phosphorylation, reversing insulin and glucose intolerance (50). It will be of interest to investigate if BVR isozymes can interact with the PTEN protein and/or promoter and if this interaction leads to increased PI3K signaling and attenuation of oxidative damage.
IRK/IRS/PI3-kinase
Insulin receptor activation leads to phosphorylation tyrosines and activation of IRS-1. However, interaction with c-Jun N-terminal kinase (JNK) or PKC results in phosphorylation of serines that is inhibitory to IRS-1 and IRK (20, 51–53). It has been shown that both the tyrosine and the serine/threonine kinase activities of BVR may contribute to insulin action and glucose uptake. BVRA was identified to contain a Y198MKM sequence that functions as the binding site for proteins with Src homology 2 (SH-2) domains, such as in PI3K (20). The four acidic residues YMXM in the N-terminus of BVR are also found in IRS, a feature frequently associated with tyrosine residues that are known substrates for protein tyrosine kinases (PTK) (20). BVRA has been shown to be a substrate for the insulin receptor, and a protein kinase for IRS-1 (Figure 3) (20). Three of the six-tyrosine residues (Y198, Y228, and Y291) in BVRA have been identified as targets for IRK-mediated phosphorylation, whereas tyrosines 72 and 83 are autophosphorylated (20). Tyrosine 198 in BVRA is the major target of IRK phosphorylation, and a mutation at this site enhanced glucose uptake, in 293A cells (20). The N-terminal serine/threonine kinase domain in BVRA contains an ATP-binding site at the G17 position that was shown to be responsible for phosphorylation of IRS-1 on serine residues (20). IRS-1 phosphorylation of proximal serines inhibits function and promotes insulin resistance (54, 55) and IRS-1 phosphorylation mutant analysis has revealed that serines 307, 312, 315, and 616 are all possible kinase targets of BVRA, implicating the enzyme in the control of IRS-1 function. Oxidative stress caused by tert-butylhydroperoxide (tBHP) robustly induced IRS-1 S616 phosphorylation in chondrocytes (56), suggesting that BVRA may enhance phosphorylation of S616 site in response to oxidative stress. However, this is yet to be shown. The BVRA-PI3K interaction may lead to the enhancement of IRS-1 S616 phosphorylation due to the direct effect of BVRA kinase activation on PI3K. As most of these findings are based on in vitro experiments, it is still unclear what is the involvement of the BVR isozymes in insulin signaling in relevant tissues in vivo, or their precise role in insulin resistance and diabetes, in the context of the whole organism. The generation of rodent model where the expression of the BVR isozymes is manipulated will help determine their function in insulin responsive tissues,.
Biliverdin Reductase Regulation of Protein Kinase Family
The protein kinase (PK) family regulates metabolic processes that include insulin actions on carbohydrate and fat metabolism, in muscle and adipose tissues, and lipid synthesis in liver. The PKA isoforms are important for lipolytic actions in adipose and inhibition of insulin signaling whereas the PKC class of serine/threonine kinases have diverse functions. BVRA is both a substrate and activator of PKCβII, via phosphorylation and protein-protein interactions (57). PKCβII may regulate insulin resistance through interaction with IRS-1 and the insulin-signaling pathway (58). PKCβII expression and activation, but not PKCα, are elevated in the fat tissues of diabetic ob/ob mice and in high-fat diet-fed mice, compared to lean animals (58). BVRA enhances PKCβII localization at the cellular membrane and is subsequently phosphorylated (57). Palmitate-induction of the super-oxide generating enzyme, Nox2, which forms ROS, was dependent on the activation of classical PKCs, specifically PKCβII (59). The BVRA and PKCβII interaction may be a sensor for palmitic acid induced ROS that occurs in fat accumulation in obesity. Also, BVRA may potentially inhibit PKCβII actions in adipocytes of obese patients to prevent the accumulation of ROS and activation of Nox2. Investigating the expression, localization and action of BVRA in the obese in the future is warranted.
BVRA also modulates PKCδ, a novel PKC involved in the regulation of cellular growth, survival, antigen presentation, and various cancers (60). PKCδ and BVRA share a number of common activators including ROS, IGF-1, and insulin. Multiple BVRA sites have been examined including the D (δ)-Box like motif, which most likely physically interacts to increase the potency of the PKCδ catalytic domain (60). Suppression of BVRA expression has been shown to attenuate PKCδ mediated activation of NF-κB and Elk1, a discovery that could lend to inhibition of PKCδ mediated cancer proliferation (60). The ability of PKCδ to modulate BVRA in such a manner allows for its causal significance in many downstream effects linked to BVRA activity (45). The BVR substrate biliverdin is also a known inhibitor of PKCδ. Therefore, the reduction of biliverdin by BVR may alter PKCδ activity (60). It is unlikely that BVRB directly regulates PKC since it lacks the D (δ)-Box like motif; however, the regulation of PKC by BVRB has yet to be examined.
BVRA also interacts with PKCζ, an atypical PKC linked to TNF-α and NF-κB activity, effecting cellular growth and differentiation (61). BVRA and PKCζ share common activators and substrates; both have modulatory effects on the insulin/PI3K pathway, and have the ability to phosphorylate IRS-1, allowing for negative feedback (61). BVRA potentiates the activation of PKCζ by TNFα, a process likely involving sulfide bonds between the cysteine rich carboxyl terminal of BVR and cysteine rich regions of PKCζ (61). Studies have shown that BVRA peptide fragments have the ability to decrease the activity of PKC, a process that could be useful for the treatment involving excessive cellular growth and cancer proliferation (60, 61), as well as metabolic disorders. PKCθ also regulates IRS-1 (62); however, the BVRA-PKCθ interaction is yet to be investigated.
Biliverdin Reductase Regulation of Inflammatory Pathways
The autophosphorylation of specific serine/threonine residues stimulates the reductase activity of BVRA (20). Phosphorylated BVRA regulates the expression of oxidative-stress-responsive genes such as HO-1 or inducible nitric oxide synthase (iNOS) and interacts with members of the MAPK kinase family, including ERK1/2 (11, 63, 64). These effects highlight BVRA as one of the early events in adaptive response to stress, which has the capability to reduce ROS-induced damage and reduce inflammation. ROS enhances activity of the major mediator of inflammation, NF-kB, which then increases expression of proinflammatory genes, such as TNFα, and enhances the proliferation of immune cells. TNFα, which is increased in obese individuals, promotes infiltration of immune cells in adipose and liver leading to a chronic inflammatory state and insulin resistance (65). BVR is expressed on the surface of macrophages. In the context of a lipopolysaccharide (known activator of TNFα (66)) induced inflammatory response, BVR surface expression and phosphorylation is increasing, and through its kinase activity, BVR initiates an inflammatory response via the Akt/PI3K pathway and enhanced production of the anti-inflammatory cytokine interleukin-10 (IL-10) (40).
The interaction of pro- or anti- inflammatory molecules with the BVR isozymes and their signaling potential has yet to be specifically investigated. It is possible that differential signaling pathways could regulate the levels of BVRA and BVRB proteins and that each of the isozymes could have differential effects on inflammatory cytokine signaling. For example, BVRA recruitment to the cell surface in obese individuals may inhibit TNFα induced inflammation, reducing chronic inflammation and the accumulation of fat. Overall, the biliverdin IXα/BVRA axis inhibits ROS, preventing the increase of inflammatory pathways, which may play a major role against pathways promoting insulin resistance and inflammation seen in obesity.
Concluding remarks and future perspectives
The diversity of functions associated with the BVR isozymes allows for their modulation of many downstream target molecules via protein-protein interaction, phosphorylation, and transcriptional regulation, which makes them potential targets for the development of new drugs in several areas including cardiovascular, metabolism and cancer. With regards to metabolism disorders, development of BVRA peptide agonists (8) could provide novel therapeutics for the treatment of type II diabetes. Also strategies to increase the levels of BVRA protein itself could be beneficial through increases in intracellular bilirubin generation as well as direct signaling through cytoprotective pathways such as IRK/IRS/PI3-kinase and PKC. In cancer, inhibition of the BVR isozymes could provide novel opportunities for the development of new chemotherapeutics as well as increase the efficacy of existing drugs. For example, it was shown that overexpression of BVRA was associated with increased protection from cisplatin and doxorubicin treatment in cultured cells (67). These results would indicate that specific targeting of BVRA could be a novel approach for the development of a whole new class of cancer chemotherapeutics. BVRB allows for the proper function of heme breakdown and metabolite removal in the early stages of fetal development. Apart from its role early in fetal life, functions for BVRB in adulthood are not known. Recent studies have shown BVRB levels to be significantly increased in both hepatocellular carcinoma and prostate cancer (68, 69), suggesting that inhibitors of BVRB could also be a new class of chemotherapeutic compounds. However, much research into the underlying mechanism(s) by which BVRB promotes cancer growth is needed. Future insights into the complex physiological roles for BVRA and BVRB will be derived mainly from studies in which the levels of each of these isoforms are altered using genetic approaches via infusion of siRNAs or creation of tissue-specific knockouts for each isozyme. It is possible as a result of this work that new and effective drugs for a wide-variety of disease could be based upon alterations in BVRA/BVRB signaling.
BOX 1. Comparison of the BVR isozymes.
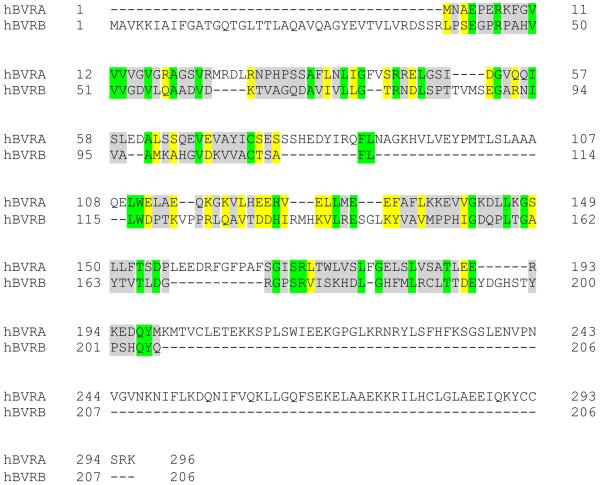
The reduction of biliverdin to bilirubin was first characterized in 1936 by Lemberg and Wyndam (70). Later, Singleton and Laster purified the reducing enzyme, BVR, and demonstrated that the enzymatic reaction was specific for biliverdin (71). Today, BVR is known to be present as two major isozymes, BVRA and BVRB (72). The BVRA gene is on chromosome 19 at the q13.1–13.2 region, and BVRB is located on chromosome 7 at the p14 region (73, 74). Unique chromosomal locations suggest that BVRA and BVRB may have major differences in their structure and function; comparison of human BVRA to BVRB shows only an 11.3% identity (20.4% similarity) (Figure I). There is an 81.8% identity (91.9% similarity) between the human and mouse BVRA, and 93.2% identity (96.6% similarity) between the human and mouse BVRB, indicating that BVR function is conserved across species. However, their roles in reducing biliverdin vary throughout development.
BOX 2. BVRA and MAPK signaling crosstalk.
The MAPK proteins are a group of kinases that consist of various subfamilies including ERK1/2, p38, and JNK. These kinases take part in one branch of the insulin-signaling pathway. ERK1/2 is activated by MEK1/2, which is regulated by BVRA, possibly by interaction with the PKC family (18, 75). BVRA contains two sites that are able to bind to ERK, one being the D-box docking site and the other being the FXFP site (18, 76). Potentially, this interaction is mediated by the C-terminal α-helix of BVRA, which is similar to the D-(δ) box/domain found in ERK1/2 and JNK substrates (18). BVRA allows for communication between cytosolic and downstream nuclear proteins, along with subsequent molecular and morphological changes dependent on these pathways. BVRA forms a ternary complex with MEK and ERK and helps to position ERK in the MEK-ERK complex, allowing for the activation of ERK by MEK (18) (Figure I). Once BVRA complexes with MEK and ERK, it transports the activated ERK into the nucleus, and the two proteins complex with Elk for further downstream activation and regulation of gene expression (18).
Highlights.
Biliverdin reductase isozymes are major regulators of metabolic processes.
The BVR isozymes have structural differences that lead to variances in signaling.
BVRA directly regulates insulin receptor signaling.
Bilirubin treatments may function to prevent obesity and diabetes.
Figure I, Box 1. Alignment of human biliverdin reductase A and B isozymes.
The alignment of BVRA and BVRB was done using the EMBOSS Pairwise Sequence Alignment (PROTEIN) software (https://www.ebi.ac.uk/Tools/psa/emboss_needle/). Identical regions are highlighted in green, similar regions are in yellow, and gray indicates no similarity between amino acid alignments.
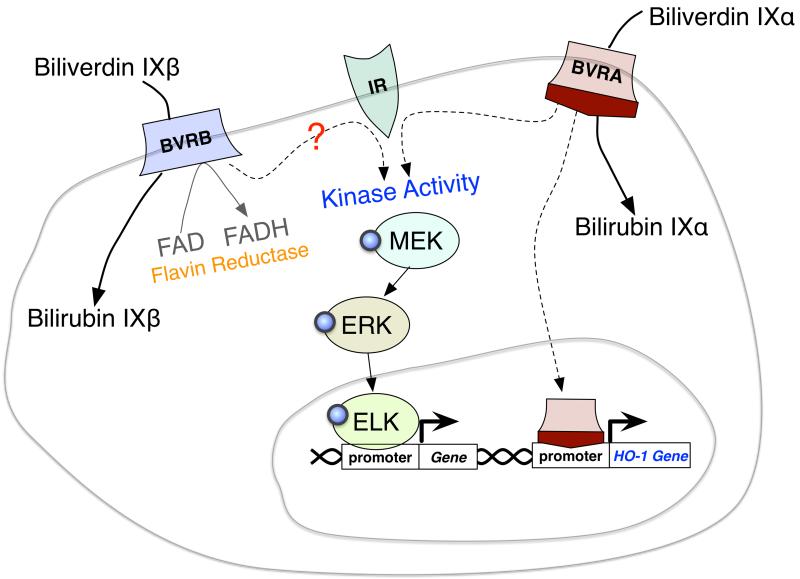
Figure II, Box 2. Diagram of the differences in biliverdin reductase A and B signaling and enzymatic activity.
BVRA and BVRB reduce biliverdin to bilirubin via their BVR domain. BVRB reduces only biliverdin IXβ, and BVRA has only enzymatic activity on biliverdin IXα. BVRB also has the ability to reduce flavins (FAD to FADH). BVRA has been shown to interact directly with the insulin receptor (IR) and proteins involved in the IR signaling cascade (MEK, ERK, and ELK). BVRA can also directly bind to the promoter of genes, such as HO-1 and regulate transcription. BVRB cannot bind directly to DNA, but does contain the domain that BVRA has been shown to interact with IR, suggesting a potential role in insulin signaling.
Acknowledgements
This work was supported by the National Institutes of Health PRIDE grant [HL106365] (T.D.H.) and by grants from the National Heart, Lung and Blood Institute [K01HL-125445] (T.D.H.) and (PO1HL-051971), [HL088421] (D.E.S.) and 1T32HL105324 (P.A.H) and the National Institute of General Medical Sciences (P20GM-104357).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT: The authors have nothing to disclose.
References
- 1.Nakamura K, Fuster JJ, Walsh K. Adipokines: a link between obesity and cardiovascular disease. Journal of cardiology. 2014;63:250–259. doi: 10.1016/j.jjcc.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hinds TD, Jr., Sodhi K, Meadows C, Fedorova L, Puri N, Kim DH, Peterson SJ, Shapiro J, Abraham NG, Kappas A. Increased HO-1 levels ameliorate fatty liver development through a reduction of heme and recruitment of FGF21. Obesity. 2013 doi: 10.1002/oby.20559. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Vanella L, Sodhi K, Kim DH, Puri N, Maheshwari M, Hinds TD, Jr., Bellner L, Goldstein D, Peterson SJ, Shapiro JI, Abraham NG. Increased heme-oxygenase 1 expression in mesenchymal stem cell-derived adipocytes decreases differentiation and lipid accumulation via upregulation of the canonical Wnt signaling cascade. Stem cell research & therapy. 2013;4:28. doi: 10.1186/scrt176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li M, Peterson S, Husney D, Inaba M, Guo K, Terada E, Morita T, Patil K, Kappas A, Ikehara S, Abraham NG. Interdiction of the diabetic state in NOD mice by sustained induction of heme oxygenase: possible role of carbon monoxide and bilirubin. Antioxidants & redox signaling. 2007;9:855–863. doi: 10.1089/ars.2007.1568. [DOI] [PubMed] [Google Scholar]
- 5.Nicolai A, Li M, Kim DH, Peterson SJ, Vanella L, Positano V, Gastaldelli A, Rezzani R, Rodella LF, Drummond G, Kusmic C, L'Abbate A, Kappas A, Abraham NG. Heme oxygenase-1 induction remodels adipose tissue and improves insulin sensitivity in obesity-induced diabetic rats. Hypertension. 2009;53:508–515. doi: 10.1161/HYPERTENSIONAHA.108.124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ndisang JF. The heme oxygenase system selectively modulates proteins implicated in metabolism, oxidative stress and inflammation in spontaneously hypertensive rats. Current pharmaceutical design. 2014;20:1318–1327. doi: 10.2174/13816128113199990551. [DOI] [PubMed] [Google Scholar]
- 7.Ndisang JF, Jadhav A, Mishra M. The heme oxygenase system suppresses perirenal visceral adiposity, abates renal inflammation and ameliorates diabetic nephropathy in Zucker diabetic fatty rats. PloS one. 2014;9:e87936. doi: 10.1371/journal.pone.0087936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibbs PE, Lerner-Marmarosh N, Poulin A, Farah E, Maines MD. Human biliverdin reductase-based peptides activate and inhibit glucose uptake through direct interaction with the kinase domain of insulin receptor. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014;28:2478–2491. doi: 10.1096/fj.13-247015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Y, Li M, Xu M, Bi Y, Li X, Chen Y, Ning G, Wang W. Low serum total bilirubin concentrations are associated with increased prevalence of metabolic syndrome in Chinese. Journal of diabetes. 2011;3:217–224. doi: 10.1111/j.1753-0407.2011.00138.x. [DOI] [PubMed] [Google Scholar]
- 10.Fu G, Liu H, Doerksen RJ. Molecular modeling to provide insight into the substrate binding and catalytic mechanism of human biliverdin-IXalpha reductase. The journal of physical chemistry. B. 2012;116:9580–9594. doi: 10.1021/jp301456j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tudor C, Lerner-Marmarosh N, Engelborghs Y, Gibbs PE, Maines MD. Biliverdin reductase is a transporter of haem into the nucleus and is essential for regulation of HO-1 gene expression by haematin. The Biochemical journal. 2008;413:405–416. doi: 10.1042/BJ20080018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmad Z, Salim M, Maines MD. Human biliverdin reductase is a leucine zipper-like DNA-binding protein and functions in transcriptional activation of heme oxygenase-1 by oxidative stress. The Journal of biological chemistry. 2002;277:9226–9232. doi: 10.1074/jbc.M108239200. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs SY, Ronai Z. Ubiquitination and degradation of ATF2 are dimerization dependent. Molecular and cellular biology. 1999;19:3289–3298. doi: 10.1128/mcb.19.5.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kravets A, Hu Z, Miralem T, Torno MD, Maines MD. Biliverdin reductase, a novel regulator for induction of activating transcription factor-2 and heme oxygenase-1. The Journal of biological chemistry. 2004;279:19916–19923. doi: 10.1074/jbc.M314251200. [DOI] [PubMed] [Google Scholar]
- 15.van Dam H, Wilhelm D, Herr I, Steffen A, Herrlich P, Angel P. ATF-2 is preferentially activated by stress-activated protein kinases to mediate c-jun induction in response to genotoxic agents. The EMBO journal. 1995;14:1798–1811. doi: 10.1002/j.1460-2075.1995.tb07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srebrow A, Muro AF, Werbajh S, Sharp PA, Kornblihtt AR. The CRE-binding factor ATF-2 facilitates the occupation of the CCAAT box in the fibronectin gene promoter. FEBS letters. 1993;327:25–28. doi: 10.1016/0014-5793(93)81031-t. [DOI] [PubMed] [Google Scholar]
- 17.Kietzmann T, Samoylenko A, Immenschuh S. Transcriptional regulation of heme oxygenase-1 gene expression by MAP kinases of the JNK and p38 pathways in primary cultures of rat hepatocytes. The Journal of biological chemistry. 2003;278:17927–17936. doi: 10.1074/jbc.M203929200. [DOI] [PubMed] [Google Scholar]
- 18.Lerner-Marmarosh N, Miralem T, Gibbs PE, Maines MD. Human biliverdin reductase is an ERK activator; hBVR is an ERK nuclear transporter and is required for MAPK signaling. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6870–6875. doi: 10.1073/pnas.0800750105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibbs PE, Maines MD. Biliverdin inhibits activation of NF-kappaB: reversal of inhibition by human biliverdin reductase. International journal of cancer. Journal international du cancer. 2007;121:2567–2574. doi: 10.1002/ijc.22978. [DOI] [PubMed] [Google Scholar]
- 20.Lerner-Marmarosh N, Shen J, Torno MD, Kravets A, Hu Z, Maines MD. Human biliverdin reductase: a member of the insulin receptor substrate family with serine/threonine/tyrosine kinase activity. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:7109–7114. doi: 10.1073/pnas.0502173102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li W, Khor TO, Xu C, Shen G, Jeong WS, Yu S, Kong AN. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochemical pharmacology. 2008;76:1485–1489. doi: 10.1016/j.bcp.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Surh YJ, Kundu JK, Na HK. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta medica. 2008;74:1526–1539. doi: 10.1055/s-0028-1088302. [DOI] [PubMed] [Google Scholar]
- 23.Hamada N, Tanaka A, Fujita Y, Itoh T, Ono Y, Kitagawa Y, Tomimori N, Kiso Y, Akao Y, Nozawa Y, Ito M. Involvement of heme oxygenase-1 induction via Nrf2/ARE activation in protection against H2O2-induced PC12 cell death by a metabolite of sesamin contained in sesame seeds. Bioorganic & medicinal chemistry. 2011;19:1959–1965. doi: 10.1016/j.bmc.2011.01.059. [DOI] [PubMed] [Google Scholar]
- 24.Reddy NM, Kleeberger SR, Kensler TW, Yamamoto M, Hassoun PM, Reddy SP. Disruption of Nrf2 impairs the resolution of hyperoxia-induced acute lung injury and inflammation in mice. Journal of immunology. 2009;182:7264–7271. doi: 10.4049/jimmunol.0804248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blumenthal SG, Stucker T, Rasmussen RD, Ikeda RM, Ruebner BH, Bergstrom DE, Hanson FW. Changes in bilirubins in human prenatal development. The Biochemical journal. 1980;186:693–700. doi: 10.1042/bj1860693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komuro A, Tobe T, Nakano Y, Yamaguchi T, Tomita M. Cloning and characterization of the cDNA encoding human biliverdin-IX alpha reductase. Biochimica et biophysica acta. 1996;1309:89–99. doi: 10.1016/s0167-4781(96)00099-1. [DOI] [PubMed] [Google Scholar]
- 27.Pereira PJ, Macedo-Ribeiro S, Parraga A, Perez-Luque R, Cunningham O, Darcy K, Mantle TJ, Coll M. Structure of human biliverdin IXbeta reductase, an early fetal bilirubin IXbeta producing enzyme. Nature structural biology. 2001;8:215–220. doi: 10.1038/84948. [DOI] [PubMed] [Google Scholar]
- 28.Cunningham O, Gore MG, Mantle TJ. Initial-rate kinetics of the flavin reductase reaction catalysed by human biliverdin-IXbeta reductase (BVR-B) The Biochemical journal. 2000;345(Pt 2):393–399. [PMC free article] [PubMed] [Google Scholar]
- 29.Zucker SD, Goessling W, Hoppin AG. Unconjugated bilirubin exhibits spontaneous diffusion through model lipid bilayers and native hepatocyte membranes. The Journal of biological chemistry. 1999;274:10852–10862. doi: 10.1074/jbc.274.16.10852. [DOI] [PubMed] [Google Scholar]
- 30.Choi SH, Yun KE, Choi HJ. Relationships between serum total bilirubin levels and metabolic syndrome in Korean adults. Nutrition, metabolism, and cardiovascular diseases: NMCD. 2011 doi: 10.1016/j.numecd.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Torgerson JS, Lindroos AK, Sjostrom CD, Olsson R, Lissner L, Sjostrom L. Are elevated aminotransferases and decreased bilirubin additional characteristics of the metabolic syndrome? Obesity research. 1997;5:105–114. doi: 10.1002/j.1550-8528.1997.tb00650.x. [DOI] [PubMed] [Google Scholar]
- 32.Cure E, Cicek Y, Cumhur Cure M, Yuce S, Kirbas A, Yilmaz A. The evaluation of relationship between adiponectin levels and epicardial adipose tissue thickness with low cardiac risk in Gilbert's syndrome: an observational study. Anadolu kardiyoloji dergisi: AKD = the Anatolian journal of cardiology. 2013;13:791–796. doi: 10.5152/akd.2013.266. [DOI] [PubMed] [Google Scholar]
- 33.Andersson C, Weeke P, Fosbol EL, Brendorp B, Kober L, Coutinho W, Sharma AM, Van Gaal L, Finer N, James WP, Caterson ID, Rode RA, Torp-Pedersen C, Committee, S. E. S., and investigators, S. Acute effect of weight loss on levels of total bilirubin in obese, cardiovascular high-risk patients: an analysis from the lead-in period of the Sibutramine Cardiovascular Outcome trial. Metabolism: clinical and experimental. 2009;58:1109–1115. doi: 10.1016/j.metabol.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Dong H, Huang H, Yun X, Kim DS, Yue Y, Wu H, Sutter A, Chavin KD, Otterbein LE, Adams DB, Kim YB, Wang H. Bilirubin increases insulin sensitivity in leptin-receptor deficient and diet-induced obese mice through suppression of ER stress and chronic inflammation. Endocrinology. 2014;155:818–828. doi: 10.1210/en.2013-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jais A, Einwallner E, Sharif O, Gossens K, Lu TT, Soyal SM, Medgyesi D, Neureiter D, Paier-Pourani J, Dalgaard K, Duvigneau JC, Lindroos-Christensen J, Zapf TC, Amann S, Saluzzo S, Jantscher F, Stiedl P, Todoric J, Martins R, Oberkofler H, Muller S, Hauser-Kronberger C, Kenner L, Casanova E, Sutterluty-Fall H, Bilban M, Miller K, Kozlov AV, Krempler F, Knapp S, Lumeng CN, Patsch W, Wagner O, Pospisilik JA, Esterbauer H. Heme oxygenase-1 drives metaflammation and insulin resistance in mouse and man. Cell. 2014;158:25–40. doi: 10.1016/j.cell.2014.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ito T, Chen D, Chang CW, Kenmochi T, Saito T, Suzuki S, Takemoto JY. Mesobiliverdin IXalpha Enhances Rat Pancreatic Islet Yield and Function. Frontiers in pharmacology. 2013;4:50. doi: 10.3389/fphar.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stechschulte LA, Wuescher L, Marino JS, Hill JW, Eng C, Hinds TD., Jr. Glucocorticoid Receptor beta Stimulates Akt1 Growth Pathway by Attenuation of PTEN. The Journal of biological chemistry. 2014 doi: 10.1074/jbc.M113.544072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kandel ES, Hay N. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Experimental cell research. 1999;253:210–229. doi: 10.1006/excr.1999.4690. [DOI] [PubMed] [Google Scholar]
- 39.Pachori AS, Smith A, McDonald P, Zhang L, Dzau VJ, Melo LG. Heme-oxygenase-1-induced protection against hypoxia/reoxygenation is dependent on biliverdin reductase and its interaction with PI3K/Akt pathway. Journal of molecular and cellular cardiology. 2007;43:580–592. doi: 10.1016/j.yjmcc.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wegiel B, Baty CJ, Gallo D, Csizmadia E, Scott JR, Akhavan A, Chin BY, Kaczmarek E, Alam J, Bach FH, Zuckerbraun BS, Otterbein LE. Cell surface biliverdin reductase mediates biliverdin-induced anti-inflammatory effects via phosphatidylinositol 3-kinase and Akt. The Journal of biological chemistry. 2009;284:21369–21378. doi: 10.1074/jbc.M109.027433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeng R, Yao Y, Han M, Zhao X, Liu XC, Wei J, Luo Y, Zhang J, Zhou J, Wang S, Ma D, Xu G. Biliverdin reductase mediates hypoxia-induced EMT via PI3-kinase and Akt. Journal of the American Society of Nephrology: JASN. 2008;19:380–387. doi: 10.1681/ASN.2006111194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mo L, Yang C, Gu M, Zheng D, Lin L, Wang X, Lan A, Hu F, Feng J. PI3K/Akt signaling pathway-induced heme oxygenase-1 upregulation mediates the adaptive cytoprotection of hydrogen peroxide preconditioning against oxidative injury in PC12 cells. International journal of molecular medicine. 2012;30:314–320. doi: 10.3892/ijmm.2012.1002. [DOI] [PubMed] [Google Scholar]
- 43.Young SC, Storm MV, Speed JS, Kelsen S, Tiller CV, Vera T, Drummond HA, Stec DE. Inhibition of biliverdin reductase increases ANG II-dependent superoxide levels in cultured renal tubular epithelial cells. American journal of physiology. Regulatory, integrative and comparative physiology. 2009;297:R1546–1553. doi: 10.1152/ajpregu.90933.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aktan F. iNOS-mediated nitric oxide production and its regulation. Life sciences. 2004;75:639–653. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 45.Gibbs PE, Miralem T, Lerner-Marmarosh N, Tudor C, Maines MD. Formation of ternary complex of human biliverdin reductase-protein kinase Cdelta-ERK2 protein is essential for ERK2-mediated activation of Elk1 protein, nuclear factor-kappaB, and inducible nitric-oxidase synthase (iNOS) The Journal of biological chemistry. 2012;287:1066–1079. doi: 10.1074/jbc.M111.279612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wegiel B, Gallo D, Csizmadia E, Roger T, Kaczmarek E, Harris C, Zuckerbraun BS, Otterbein LE. Biliverdin inhibits Toll-like receptor-4 (TLR4) expression through nitric oxide-dependent nuclear translocation of biliverdin reductase. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:18849–18854. doi: 10.1073/pnas.1108571108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding B, Gibbs PE, Brookes PS, Maines MD. The coordinated increased expression of biliverdin reductase and heme oxygenase-2 promotes cardiomyocyte survival: a reductase-based peptide counters beta-adrenergic receptor ligand-mediated cardiac dysfunction. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2011;25:301–313. doi: 10.1096/fj.10-166454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hers I, Vincent EE, Tavare JM. Akt signalling in health and disease. Cellular signalling. 2011;23:1515–1527. doi: 10.1016/j.cellsig.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 49.Goswami A, Ranganathan P, Rangnekar VM. The phosphoinositide 3-kinase/Akt1/Par-4 axis: a cancer-selective therapeutic target. Cancer research. 2006;66:2889–2892. doi: 10.1158/0008-5472.CAN-05-4458. [DOI] [PubMed] [Google Scholar]
- 50.Butler M, McKay RA, Popoff IJ, Gaarde WA, Witchell D, Murray SF, Dean NM, Bhanot S, Monia BP. Specific inhibition of PTEN expression reverses hyperglycemia in diabetic mice. Diabetes. 2002;51:1028–1034. doi: 10.2337/diabetes.51.4.1028. [DOI] [PubMed] [Google Scholar]
- 51.De Fea K, Roth RA. Modulation of insulin receptor substrate-1 tyrosine phosphorylation and function by mitogen-activated protein kinase. The Journal of biological chemistry. 1997;272:31400–31406. doi: 10.1074/jbc.272.50.31400. [DOI] [PubMed] [Google Scholar]
- 52.Lee YH, Giraud J, Davis RJ, White MF. c-Jun N-terminal kinase (JNK) mediates feedback inhibition of the insulin signaling cascade. The Journal of biological chemistry. 2003;278:2896–2902. doi: 10.1074/jbc.M208359200. [DOI] [PubMed] [Google Scholar]
- 53.Backer JM, Myers MG, Jr., Shoelson SE, Chin DJ, Sun XJ, Miralpeix M, Hu P, Margolis B, Skolnik EY, Schlessinger J, et al. Phosphatidylinositol 3'-kinase is activated by association with IRS-1 during insulin stimulation. The EMBO journal. 1992;11:3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu YF, Herschkovitz A, Boura-Halfon S, Ronen D, Paz K, Leroith D, Zick Y. Serine phosphorylation proximal to its phosphotyrosine binding domain inhibits insulin receptor substrate 1 function and promotes insulin resistance. Molecular and cellular biology. 2004;24:9668–9681. doi: 10.1128/MCB.24.21.9668-9681.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gual P, Le Marchand-Brustel Y, Tanti JF. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie. 2005;87:99–109. doi: 10.1016/j.biochi.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 56.Yin W, Park JI, Loeser RF. Oxidative stress inhibits insulin-like growth factor-I induction of chondrocyte proteoglycan synthesis through differential regulation of phosphatidylinositol 3-Kinase-Akt and MEK-ERK MAPK signaling pathways. The Journal of biological chemistry. 2009;284:31972–31981. doi: 10.1074/jbc.M109.056838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maines MD, Miralem T, Lerner-Marmarosh N, Shen J, Gibbs PE. Human biliverdin reductase, a previously unknown activator of protein kinase C betaII. The Journal of biological chemistry. 2007;282:8110–8122. doi: 10.1074/jbc.M513427200. [DOI] [PubMed] [Google Scholar]
- 58.Liberman Z, Plotkin B, Tennenbaum T, Eldar-Finkelman H. Coordinated phosphorylation of insulin receptor substrate-1 by glycogen synthase kinase-3 and protein kinase C betaII in the diabetic fat tissue. American journal of physiology. Endocrinology and metabolism. 2008;294:E1169–1177. doi: 10.1152/ajpendo.00050.2008. [DOI] [PubMed] [Google Scholar]
- 59.Jaishy B, Zhang Q, Chung HS, Riehle C, Soto J, Jenkins S, Abel P, Cowart LA, Van Eyk JE, Abel ED. Lipid-induced NOX2 activation inhibits autophagic flux by impairing lysosomal enzyme activity. Journal of lipid research. 2014 doi: 10.1194/jlr.M055152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miralem T, Lerner-Marmarosh N, Gibbs PE, Tudor C, Hagen FK, Maines MD. The human biliverdin reductase-based peptide fragments and biliverdin regulate protein kinase Cdelta activity: the peptides are inhibitors or substrate for the protein kinase C. The Journal of biological chemistry. 2012;287:24698–24712. doi: 10.1074/jbc.M111.326504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lerner-Marmarosh N, Miralem T, Gibbs PE, Maines MD. Regulation of TNF-alpha-activated PKC-zeta signaling by the human biliverdin reductase: identification of activating and inhibitory domains of the reductase. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2007;21:3949–3962. doi: 10.1096/fj.07-8544com. [DOI] [PubMed] [Google Scholar]
- 62.Marino JS, Hinds TD, Jr., Potter RA, Ondrus E, Onion JL, Dowling A, McLoughlin TJ, Sanchez ER, Hill JW. Suppression of protein kinase C theta contributes to enhanced myogenesis in vitro via IRS1 and ERK1/2 phosphorylation. BMC cell biology. 2013;14:39. doi: 10.1186/1471-2121-14-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maines MD. Biliverdin reductase: PKC interaction at the cross-talk of MAPK and PI3K signaling pathways. Antioxidants & redox signaling. 2007;9:2187–2195. doi: 10.1089/ars.2007.1805. [DOI] [PubMed] [Google Scholar]
- 64.Kapitulnik J, Maines MD. Pleiotropic functions of biliverdin reductase: cellular signaling and generation of cytoprotective and cytotoxic bilirubin. Trends in pharmacological sciences. 2009;30:129–137. doi: 10.1016/j.tips.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 65.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. The Journal of clinical investigation. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singh AK, Jiang Y. Lipopolysaccharide (LPS) induced activation of the immune system in control rats and rats chronically exposed to a low level of the organothiophosphate insecticide, acephate. Toxicology and industrial health. 2003;19:93–108. doi: 10.1191/0748233703th181oa. [DOI] [PubMed] [Google Scholar]
- 67.Florczyk U, Golda S, Zieba A, Cisowski J, Jozkowicz A, Dulak J. Overexpression of biliverdin reductase enhances resistance to chemotherapeutics. Cancer letters. 2011;300:40–47. doi: 10.1016/j.canlet.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 68.Melle C, Ernst G, Scheibner O, Kaufmann R, Schimmel B, Bleul A, Settmacher U, Hommann M, Claussen U, von Eggeling F. Identification of specific protein markers in microdissected hepatocellular carcinoma. Journal of proteome research. 2007;6:306–315. doi: 10.1021/pr060439b. [DOI] [PubMed] [Google Scholar]
- 69.Pallua JD, Schaefer G, Seifarth C, Becker M, Meding S, Rauser S, Walch A, Handler M, Netzer M, Popovscaia M, Osl M, Baumgartner C, Lindner H, Kremser L, Sarg B, Bartsch G, Huck CW, Bonn GK, Klocker H. MALDI-MS tissue imaging identification of biliverdin reductase B overexpression in prostate cancer. Journal of proteomics. 2013;91:500–514. doi: 10.1016/j.jprot.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 70.Lemberg R, Wyndham RA. Reduction of biliverdin to bilirubin in tissues. The Biochemical journal. 1936;30:1147–1170. doi: 10.1042/bj0301147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singleton JW, Laster L. Biliverdin reductase of guinea pig liver. The Journal of biological chemistry. 1965;240:4780–4789. [PubMed] [Google Scholar]
- 72.Yamaguchi T, Komoda Y, Nakajima H. Biliverdin-IX alpha reductase and biliverdin-IX beta reductase from human liver. Purification and characterization. The Journal of biological chemistry. 1994;269:24343–24348. [PubMed] [Google Scholar]
- 73.Saito F, Yamaguchi T, Komuro A, Tobe T, Ikeuchi T, Tomita M, Nakajima H. Mapping of the newly identified biliverdin-IX beta reductase gene (BLVRB) to human chromosome 19q13.13-->q13.2 by fluorescence in situ hybridization. Cytogenetics and cell genetics. 1995;71:179–181. doi: 10.1159/000134102. [DOI] [PubMed] [Google Scholar]
- 74.Parkar M, Jeremiah SJ, Povey S, Lee AF, Finlay FO, Goodfellow PN, Solomon E. Confirmation of the assignment of human biliverdin reductase to chromosome 7. Annals of human genetics. 1984;48:57–60. doi: 10.1111/j.1469-1809.1984.tb00834.x. [DOI] [PubMed] [Google Scholar]
- 75.Mizukami Y, Kobayashi S, Uberall F, Hellbert K, Kobayashi N, Yoshida K. Nuclear mitogen-activated protein kinase activation by protein kinase czeta during reoxygenation after ischemic hypoxia. The Journal of biological chemistry. 2000;275:19921–19927. doi: 10.1074/jbc.M907901199. [DOI] [PubMed] [Google Scholar]
- 76.Jacobs D, Glossip D, Xing H, Muslin AJ, Kornfeld K. Multiple docking sites on substrate proteins form a modular system that mediates recognition by ERK MAP kinase. Genes & development. 1999;13:163–175. [PMC free article] [PubMed] [Google Scholar]