Summary
Interactions between cancer cells and their surroundings can trigger essential signaling cues that determine cell fate and influence the evolution of the malignant phenotype. As the primary receptors involved in cell-matrix adhesion, integrins present on the surface of tumor and stromal cells have a profound impact on the ability to survive in specific locations, but in some cases these receptors can also function in the absence of ligand binding to promote stemness and survival in the presence of environmental and therapeutic stresses. Understanding how integrin expression and function is regulated in this context will enable the development of new therapeutic approaches to sensitize tumors to therapy and suppress their metastatic phenotype.
Keywords: Integrins, cancer, drug resistance, stemness
Integrin heterodimers and ligand specificity in cancer
When the extracellular matrix (ECM) is proteolytically degraded or deformed by mechanical forces, cells are prompted to undergo responsive changes that influence remodeling during physiological and pathological events. Integrins are a family of heterodimeric cell surface receptors that sense these changes and trigger a range of cellular responses by forming a physical connection between the inside and outside of a cell to allow the bi-directional “integration” of signals to control cell adhesion, migration, proliferation, survival, and differentiation [1]. While integrins regulate processes important for a range of physiological functions, these receptors also play a crucial role in promoting a more malignant tumor cell phenotype in the setting of cancer [2].
The ability of integrins to dictate cellular responses to a variety of inputs stems from their capacity to differentially recognize distinct environments. To allow for this flexibility, integrins are comprised of 18 α subunits and 8 β subunits that pair to form at least 24 different functional heterodimeric receptors that each bind to one or more ECM ligands. This specificity allows integrin-ligand binding events to enforce distinct niches or boundaries, so that cells expressing only certain integrin heterodimers can pass within an extracellular matrix containing specific components such as laminin, collagen, vitronectin, or fibronectin. Since a given integrin can bind to multiple ligands, and a single ligand can recognize multiple integrin heterodimers, spatio-temporal patterns of integrin vs. ligand expression ultimately determine how a cell senses and responds to its environment. Integrin control of matrix metalloproteinase (MMPs) on the surface of cells is also a determining factor for invasive behavior [3]. In the context of cancer, this cell adhesion-dependent aspect of integrin function plays a critical role in determining a cell’s ability to break through a defined tumor margin in order to locally invade and ultimately metastasize. Ligand binding also controls whether a certain tumor cell can disseminate to a particular metastatic niche, such as bone, brain, or lung environments characterized by distinct ECM signatures.
Upon encountering a specific ligand, integrins undergo a conformational change that switches them from an inactive low avidity state to a high avidity state [4]. This change is based in part on the ability of ligated integrins to cluster in the plane of the membrane leading to “outside-in” signaling via a physical linkage to the actin cytoskeleton (Box 1). Alternatively, intracellular signaling can also activate “inside-out” signals that affect integrin affinity/avidity for extracellular ligands [5]. By selectively recruiting adapter or scaffolding proteins such as CAS, Shc, and Grb2, integrins play an important role in potentiating the activity of receptor tyrosine kinases, including receptors for growth factors such as VEGF, FGF, or EGF [1, 6-9]. Although these “canonical” integrin signaling pathways have been extensively characterized, new specificities and signaling components are still being discovered [10].
Box 1. The canonical integrin signaling cascade.
Integrins are the major cell surface receptors for extracellular matrix molecules, which play critical roles in a variety of biological processes. Focal adhesion kinase (FAK) is a key component of the signal transduction pathways triggered by integrins. When integrins interact with their specific ligands, they recruit FAK through their β subunit. FAK undergoes autophosphorylation that leads to its association with Src, resulting in activation of both kinases. Then, the active FAK/Src complex recruits p130CAS and paxillin that in turn recruit Crk leading to activation of RAC1, p21-activated kinase (PAK), Jun amino-terminal kinase (JNK), and nuclear factor kB (NFκB). Alternatively, the FAK/Src complex can recruit and activate RAP1, which in turn activates extracellular signal-regulated kinase (ERK) and mitogen-activated protein kinase (MAPK) through BRAF. The FAK/Src complex may also lead to its association with growth factor receptor-bound protein 2 (GRB2), which in turn can activate RAS leading to the activation of the RAF-MEK-ERK pathway, and PI 3−kinase (PI3K) has also been shown to bind FAK leading to activation of PI3K and its downstream effectors.
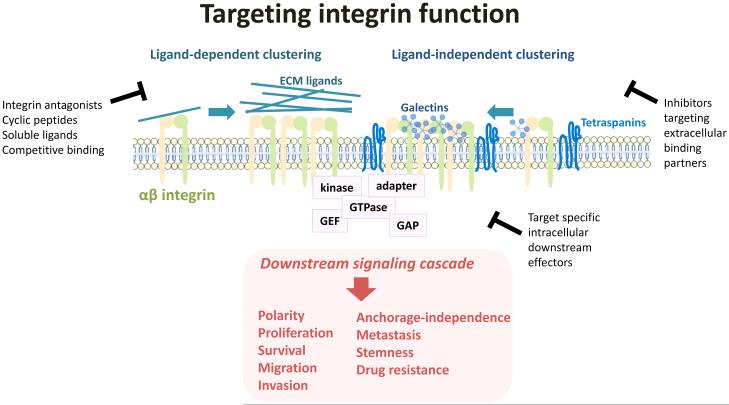
Similar to growth factor receptors, integrin clustering within the plasma membrane is regulated by numerous direct or indirect integrin binding partners, and serves to amplify signal-generating capacity (Figure 1). Accordingly, integrin clustering could represent an Achilles’ heel for integrin function that could be exploited therapeutically. Galectins, a family of β-galactoside-binding lectins recently associated with metastasis [11], influence tumor cell behavior by binding to carbohydrates on the extracellular domain of integrins and regulate clustering. Several galectins have recently been identified to play a role in tumor progression including Galectin-1, which promotes lung cancer metastasis by potentiating integrin α6β4 and Notch1/Jagged2 signaling [12], and Galectin-3, which induces integrin β3-mediated anchorage-independence and drug resistance [13]. Tetraspanins also play key roles in clustering integrins by regulating their trafficking and function [14, 15]. The tetraspanin CD151 in particular shows promise as a diagnostic marker as well as a therapeutic target [16]. Thus, through their effects on integrin clustering, galectins and tetraspanins could provide a means to control integrin signaling independent of ligand binding and promote tumor cell dissemination and metastasis.
Figure 1. Integrin clustering is critical for generation of downstream signals.
Integrin function can be blocked upstream by preventing ligand binding or ligand-independent clustering, or by targeting specific downstream integrin effectors.
Not only can integrin function be suppressed by competitively blocking integrin-ligand binding events, but it is also possible to suppress ligand-independent integrin clustering by manipulating the function of proteins such as galectins or tetraspanins that can cluster and promote integrin activity in the absence of cell adhesion in a permissive microenvironment. In fact, combining these strategies could provide therapeutic opportunities to short circuit the ability of integrins to generate signals across distinct environments. Developing such an approach will require a better understanding of the cues and responses that are spatially and temporally distinct during the course of cancer progression. This review is therefore focused on highlighting newly appreciated roles of integrins in driving a stem phenotype, drug resistance, and metastasis.
Dissecting integrin-dependent regulation of stem cells
Although epithelial stem cells play a critical role in the physiological development, maintenance, and remodeling of organs and tissues [17], their properties are also associated with the initiation and progression of carcinomas [18]. Since the stem cell niche is tightly regulated by signals from the local microenvironment including the ECM, certain integrins may be critical for the ability of stem cells to sense and respond to these cues in both normal tissues and cancer. Indeed, a number of integrins have recently been highlighted as important markers and functional regulators of stem cells, suggesting that additional insight into how integrins contribute to the stem cell phenotype will allow the development of therapeutic approaches to modulate stemness in aggressive cancers.
Integrin regulation of stem cells during development and physiological remodeling
Recent studies have identified specific integrins that are enriched in epithelial stem cells and critical for their behavior. Integrin β1 (CD29) is highly expressed in normal stem cells and regulates their biology in various organs. Stem cells are typically associated with a particular local microenvironment or niche that provides critical signals to direct their self-renewal and pluripotency. For example, ECM proteins such as periostin and tenascin-C are found in stem cell niches [19, 20]. Increasing evidence demonstrates that integrin β1 maintains the stem cell niche, preserves a stable stem cell population, and controls the balance between stem cell renewal and differentiation [21]. In the epidermis, the hair follicle bulge creates a smooth muscle cell niche by expressing integrin α8β1 [22]. Integrin signaling is also involved in proliferation and differentiation of cutaneous epithelial stem cells where integrin β1 promotes keratinocyte adhesion and integrin αvβ6 activates transforming growth factor-β (TGF-β) [23]. In addition, MT1-MMP activates a β1-integrin/RhoGTPase signaling cascade that regulates stem cell shape by controlling skeletal stem cell lineage commitment [24]. In intestinal epithelial stem cells, integrin β1 deletion increases epithelial proliferation suggesting that integrin expression can limit adult stem cell proliferation [25]. Indeed, integrin dependent adhesion to the basement membrane induces cell intrinsic polarity, resulting in asymmetric division, which ultimately allows the continuing maintenance of stem cells and the generation of differentiated cells [26].
In the mammary gland, the microenvironment provides cues to control the behavior of epithelial stem and progenitor cells [27], and integrins are important markers for identifying these cell types, as separate epithelial lineages arising from the same precursors in the breast can be distinguished on the basis of their integrin profiles. Luminal cells express low levels of the integrin α6 (CD49f) and β1 subunits, while cells in the basal layer including mammary stem cells express higher levels [28-30]. Additionally, functional studies have demonstrated that the integrin β1 subunit is essential for the regenerative potential of the adult mammary gland [21].
Expression levels of integrin β3 (CD61) can distinguish mammary luminal progenitors (β3−) from mature, differentiated luminal (β3−) cells [31]. There is also evidence that an αvβ3-mediated stemness pathway is important for physiological remodeling events mediated by adult stem cells, as TGFβ2 induces αvβ3 expression on adult mammary stem cells during mid-pregnancy to promote mammary stem cell expansion, clonogenicity, and expression of the master stem cell regulator Slug [32]. Whereas virgin mice lacking integrin αvβ3 develop normal mammary glands, mammary remodeling during pregnancy is defective [32]. This pathway can be usurped during breast cancer, as αvβ3 contributes to cancer stem cell properties including tumorsphere formation, tumor initiation [32] and metastasis [33]. These examples illustrate the capacity of specific integrins to influence stem cell behavior during maintenance or remodeling of normal tissues, roles that may be conserved in “stem-like” cancer cells.
Integrin regulation of cancer stem cells during tumor initiation
Accumulating evidence suggests that a relatively small number of tumor initiating cells (TICs) within a given tumor represents the subpopulation capable of self-renewal, tumorigenesis, and generation of the heterogeneous tumor cell populations observed in many cancer types. Also known as cancer stem cells (CSCs), TIC have been established as drivers of tumor progression, drug resistance, and disease relapse [18]. Although integrins are well-known for their contributions to tumor progression, it is unclear whether these effects are related to their role in CSCs. Many of the same integrins that are enriched on normal adult stem and progenitor cells are also markers of CSCs, including integrin subunits β1, α6, and β3 [34]. Among these, α6 is the most widely observed, enriching for CSCs in breast [35], prostate [36], squamous cell carcinoma [37] and colorectal [38] cancers. Recent studies have also characterized integrin β4 as a new CSC marker in lung cancer, where it is involved in self-renewal, tumor propagation, and chemotherapy resistance [39]. Therefore, differential surface expression of specific integrins may identify a small sub-population of the most aggressive and dangerous tumor cells.
In addition to serving as cancer stem markers, there is recent evidence that integrins also potentiate cancer stem cell function. In glioblastoma, disrupting integrin α6 function suppresses the cancer stem cell phenotype, highlighting the role of this integrin for the maintenance of glioblastoma stem cells [40]. Integrin α6 also contributes to breast cancer initiation by regulating a FAK-mediated induction of the Polycomb complex protein BMI-1, necessary for cancer cell self-renewal [41]. In fact, two alternative splice variants of integrin α6 drive opposite phenotypes. Whereas the α6A variant promotes an epithelial phenotype, the α6B variant is essential for establishing a stem-like mesenchymal phenotype [42]. Interestingly, these variants differ only in the cytoplasmic tail of integrin α6. Simply disabling the signaling domain of integrin β4 abrogates the CSC capabilities of prostate cancer cells, without impacting the development of the prostate [43]. Moreover, integrin β3 is necessary and sufficient for the CSC phenotype in breast [32, 44], pancreas [13], and lung [13] cancers. Thus, targeting the ability of specific integrins to modulate cell adhesion events important for cancer stem cells by manipulating integrin/ECM interactions may represent a therapeutic strategy to suppress the function of cancer stem cells.
Ligand independent functions
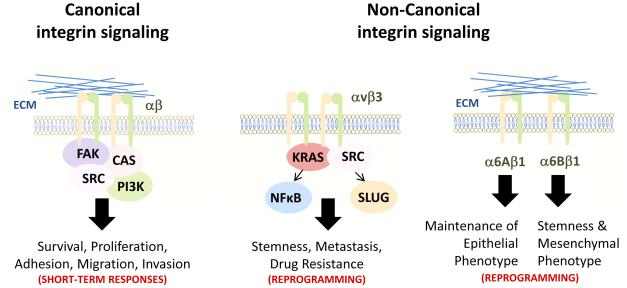
Several studies now show that integrins may influence cancer stem cells independent from their capacity to interact with the ECM. In lung and pancreatic cancers, integrin αvβ3 forms cluster on the surface of cells growing in suspension, without any physical link to ECM ligands. This clustering is mediated by a Galectin-3 interaction with αvβ3 independent of its ligand binding domain, which in turn promotes recruitment of activated KRAS and ultimately leads to a “stemness” phenotype [13]. Treating cells with integrin antagonists that compete for ligand binding or expressing mutant integrin constructs with impaired ligand binding ability do not compromise the ability of αvβ3 to drive this stem phenotype, suggesting that it is ligation independent [13]. These recent examples suggest that tumor cells may utilize certain integrins independent from their role as adhesion receptors to promote survival within non-permissive environments, such as those encountered during metastatic dissemination or tumor initiation (Figure 2).
Figure 2. Canonical and non-canonical role of integrin αvβ3.
Integrin signaling generated by binding to extracellular matrix ligands occurs via focal adhesion complexes leading to physical changes in cellular movement and activity. In the absence of ligand binding, αvβ3 integrin instead recruits KRAS and Src to drive cellular reprogramming events that lead to phenotypic changes that promote stemness, metastasis, and drug resistance. The 6A and 6B splice variants of α6β1 integrin serve as a phenotypic switch between epithelial vs. mesenchymal states.
For the case of αvβ3, its adhesion-independent functions trigger pathways distinct from the typical RAS signaling cascade and cytoskeletal links. Expression of αvβ3 is necessary and sufficient to drive tumor cell metastasis by virtue of β3-mediated the recruitment and activation of Src kinase in a manner that is not dependent on FAK or MEK/ERK signaling [33]. Furthermore, only unligated αvβ3 can form a complex with KRAS to recruit RALB and TBK1 and promote NFκB activity [13]. Therefore, while the activation of RAS family members is triggered downstream of multiple integrins, it is becoming clear that integrin-mediated signaling pathways are diverse and context-dependent, with certain integrins capable of directing specific stemness-related reprogramming. While this mode of integrin function provides an important role during development, its usurpation by cancer stem cells likely allows their survival and transit into inappropriate locations that exacerbates metastasis and tumor progression, and renders tumors highly resistant to therapies that fail to target this tumor subpopulation.
Connecting integrins and drug resistance
Despite advances in cancer treatment, many cancer therapies are limited by the development of resistance that results from a variety of factors, including alterations in the drug target, activation of pro-survival pathways, and ineffective induction of cell death. Resistance to anticancer therapeutics can be divided into two categories: intrinsic resistance derived from genetic or environmental factors pre-existing in the tumor, or acquired resistance resulting from adaptive responses, activation of alternative pathways, and selection of resistant sub-populations.
Cell adhesion-mediated drug resistance, a pro-survival and anti-apoptotic program, is dictated by integrins/ECM interactions [45]. This evasion strategy can select for cells already expressing certain integrins and/or cells capable of inducing integrin gene expression. For example, integrin β1 has been implicated as a driver of resistance to radiotherapy in head and neck cancer [46], lapatinib and trastuzumab resistance in breast cancer [47], and erlotinib resistance in lung cancer [48]. In lung cancer, erlotinib increased expression of β1, α2, and α5 and enhanced cell adhesion, while silencing integrin β1 restored erlotinib sensitivity by reducing Src and Akt activity, implicating the β1/Src signaling pathway as a key mediator of acquired resistance to EGFR targeted therapies [48]. It is also likely that multiple integrins cooperatively contribute to drug resistance. Indeed, matrix-attached ovarian carcinoma cells tolerate dual PI3K/mTOR inhibition by inducing an adaptive pro-survival response, which can be blocked by simultaneous inhibition integrin β1, integrin β4, ILK, and FAK [49]. There is also evidence that the ECM can directly modulate cell sensitivity to treatment therapies. In ovarian cancer, the matrix-associated growth factor TGF-β, sensitizes cells to paclitaxel-induced cell death by mediating FAK/Rho signaling pathway through preferential ligation to integrin αvβ3 [50].
The involvement of integrins in the promotion of a CSC phenotype is likely an additional contributor to chemoresistance and tumor relapse. In breast cancer, an integrin α6− CSC population is enriched after taxol treatment [42]. Although several studies suggest that CSCs are enriched after cancer therapy, this phenotype has not been confirmed using spontaneous tumorigenesis mouse models or in human cancers. A recent study demonstrates that CSC expressing integrin β4 are enriched after cisplatin treatment in the KrasG12D;Trp53fl/fl spontaneous lung cancer model [39]. β3 is also involved in intrinsic and acquired resistance to erlotinib and lapatinib, as this integrin is highly expressed after acquired resistance to EGFR inhibitors where it drives an NFκB signaling pathway leading to erlotinib resistance [13]. Together, these findings suggest that several integrins play a role in cancer drug resistance, possibly through controlling CSC behavior.
Integrin involvement in drug resistance may also depend on modulation of the immune response. A variety of drugs induce a DNA damage response that enhances the expression of integrin αvβ3 on tumor cells as they acquire drug resistance, and these therapy-resistant tumor cells are more readily phagocytized by dendritic cells to suppress the immune response [51]. In contrast, monoclonal antibodies that block integrin adhesion may also act to enhance immune response. For example, when tumors highly express β3 integrin, systemic therapy with the anti-αvβ3 antibody LM609 invokes host defense mechanisms and triggers antibody-dependent cellular cytotoxicity [52]. Understanding how tumor cells are recognized by the immune system can provide novel therapeutic strategies to combat drug resistance.
Critical roles for integrins during the metastatic cascade
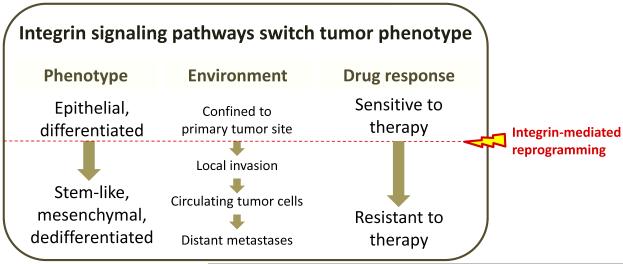
Metastasis is a multistep process that requires a cancer cell to escape from the primary tumor, survive in the circulation, colonize distant sites, and proliferate. Since integrin function can enable and enhance many facets of these steps, it is not surprising that increased expression of certain integrins within the primary tumor are associated with poor prognosis and enhanced metastasis in a variety of cancers [1] (Figure 3). This increased expression may reflect higher numbers of CSCs, with their enriched expression of certain integrins [34] and may explain why some tumors progress, while others do not. Additionally, integrin expression in both CSCs and other tumor cells can be induced by cues from the microenvironment [53, 54] resulting in increased integrin signaling that promotes the various steps responsible for metastatic progression.
Figure 3. Reprogramming of cancer cells by integrin signaling pathways.
Integrin signaling is capable of reprogramming tumor cells to promote invasion, hematogeneous dissemination, and seeding of distant metastatic sites. Similarly, stemness and drug resistance can be triggered by changes in integrin expression and function. Understanding these events offers new therapeutic opportunities for cancer.
Local invasion
The initial phase of tumor dissemination from a primary site involves a variety of signals that can be modulated by the function of multiple integrins [1, 55]. During local invasion, a cancer cell uses migration mechanisms similar to those in non-neoplastic cells during physiological processes such as embryonic morphogenesis, e.g., a cell must acquire a capacity to spread within the tissue and invade the matrix extracellular. To migrate, the cancer cell modifies its shape to interact with the surrounding tissue. This initial step requires a phenotypic conversion known as the epithelial-to-mesenchymal transition (EMT). This process is critical during development, but is also frequently triggered during metastasis. EMT is characterized by the transition to a mesenchymal phenotype, involving the disassembly of cell-cell contacts, cytoskeletal reorganization, and acquisition of mesenchymal markers and migratory properties [56]. It is not surprising that the mechanisms critical for EMT, stemness, and drug resistance demonstrate significant overlap [57], and it is likely that integrins play a critical role in allowing tumor cells to become more aggressive and therapy-resistant.
Anchorage-independent survival in the bloodstream
Non-transformed cells depend on integrins to relay cues from the ECM to maintain organ integrity and prevent cells from inappropriately wandering to other tissues. While tumor cells display some degree of anchorage-independence, their detachment from the ECM can promote cell death. Once tumor cells escape from a primary site and intravasate into blood or lymphatic vessels, they must therefore adapt to survive in the absence of adhesion to ECM. Growing evidence supports a central role for integrins in controlling growth and survival under anchorage-independent conditions, a property critical for hematogeneous metastasis. For example, integrin β1 promotes anchorage-independent growth in prostate [58] and breast cancer cells by activating a FAK/PAK/MAPK signaling cascade [59], while integrin β3 interacts with c-Src independently of FAK signaling to drive increased anchorage-independence and lymph node metastasis in pancreatic and breast cancer tumor models [32]. The paradigm of integrin-mediated anchorage-independent growth may be explained in part by the ability of specific integrins to form cell surface clusters in response to growth factors or oncogenes and drive downstream signaling [13, 60].
Colonization in the metastatic niche
Metastatic colonization of distant organs requires the cell survival and expansion of cancer cells at each secondary site. This process is successfully accomplished by only a minority of cancer cells that reach the distant organ, as seeding a “metastatic niche” requires specific recognition between cancer cells and their surrounding ECM. Since the integrin repertoire expressed by a given tumor cell may dictate that cell’s ability to respond to a particular niche and initiate metastatic colonization, integrins may be critical for the “homing” of tumor cells to organ environments that promote metastasis.
The propensity for metastasis has recently been linked to the accumulation of certain ECM proteins within a particular metastatic niche. Tenascin C is a ligand for β1 and β3 integrins that is produced within the lung metastatic niche and correlates with poor outcome for breast cancer [61]. Periostin is a ligand for αvβ3 and αvβ5 that is enriched in breast cancer lymph node metastases [62]. Expressed on breast cancer cells, the αvβ3 ligand L1-CAM is required for breast cancer metastasis to the lungs, allowing tumor cells to bind and extravasate through the lung endothelium [63]. VCAM-1 drives the metastatic spread of tumor cells to lymph nodes where α4β1 is expressed on lymph node endothelial cells. VEGF-C/PI3Kα-driven remodeling of lymph nodes activates integrin α4β1 on lymph node lymphatic endothelium, which in turn serves as an adhesive ligand for VCAM− tumor cells [64]. Since binding of α4β1 to VCAM-1 also contributes to the vascular arrest and extravasation of melanoma or lymphoma cells to the lung or spleen, this integrin-mediated binding event is gaining interest as a possible target for cancer therapy [65]. Similarly, integrins αvβ3, α2β1, and α4β1 play key roles in bone metastasis, as their collective ligands represent ECM proteins normally expressed by bone-associated cells: αvβ3 binds osteopontin, while α2β1 and α4β1 bind type I collagen on the bone matrix and VCAM-1 on bone endothelial cells [66].
These examples portray the complexity of integrin-ligand binding events that may govern how and where circulating tumor cells travel to form new micrometastatic colonies. Once entrenched within an appropriate metastatic niche, tumor cells must adapt again to allow their survival and proliferation. Integrin β1-mediated filopodium-like protrusions that support the initial interactions between the extravasated cancer cells and ECM components of the host help these cells to trigger adhesion-dependent signaling events including FAK activation, which then phosphorylates and activates ERKs leading to rapid proliferation of these cancer cells within the host tissue [67, 68]. It is also likely that integrins that provide anchorage-independent growth advantages for circulating tumor cells will have less influence over the later steps of metastasis when adhesion-dependent growth may again prevail. However, it is important to point out that a single integrin can regulate very different aspects of tumor progression. For example, unligated integrin αvβ3 promotes tumor cell reprogramming to a stem-like cell fate, while ligated αvβ3 can produce distinct cues derived from the ECM driving cell invasion and proliferation (Figure 2).
Concluding remarks
Recent findings have demonstrated that integrins participate in the regulation of stem-cell and cancer stem-cell biology and are required for cancer progression and drug resistance (Figure 3). Further understanding of which specific integrins are required for these events, whether these integrins are interchangeable or specifically required, whether these integrins define a subset of cells that expand in response to changes in the microenvironment or whether a dynamic program allows the cells to turn on and off their expressions as well as the complete integrin “signalosome” will open up new avenues for cancer treatment (Box 2). The development of new agents to target integrins and their signals should consider the context dependency of integrin function, including 2D, 3D, and in vivo assays that better reflect the tumor microenvironment or different locales encountered during the metastatic cascade.
Box 2. Outstanding questions.
- Which integrins are required to promote stemness, drug resistance, and metastasis?
- Are integrins interchangeable or specifically required?
- Do integrins define a subset of cells with stemness properties?
- Do cancer cells can turn on and off integrin expression when required?
- What is the complete integrin signalosome?
Highlights.
Integrins contribute to cancer progression via adhesion-dependent and -independent pathways.
Specific integrins not only represent stem cell markers, but also dictate stem cell behavior.
Integrins drive therapeutic resistance through canonical and non-canonical mechanisms.
Integrin expression contributes to multiple steps of the metastatic cascade.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nature reviews. Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mouw JK, et al. Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nat Med. 2014;20:360–367. doi: 10.1038/nm.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks PC, et al. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell. 1996;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- 4.Shattil SJ, et al. The final steps of integrin activation: the end game. Nature reviews. Molecular cell biology. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barczyk M, et al. Integrins. Cell and tissue research. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang M, et al. EGFR-dependent pancreatic carcinoma cell metastasis through Rap1 activation. Oncogene. 2012;31:2783–2793. doi: 10.1038/onc.2011.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goel HL, Mercurio AM. Enhancing integrin function by VEGF/neuropilin signaling: implications for tumor biology. Cell adhesion & migration. 2012;6:554–560. doi: 10.4161/cam.22419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hood JD, et al. Differential alphav integrin-mediated Ras-ERK signaling during two pathways of angiogenesis. The Journal of cell biology. 2003;162:933–943. doi: 10.1083/jcb.200304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nature reviews. Molecular cell biology. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 10.Morse EM, et al. Integrin cytoplasmic tail interactions. Biochemistry. 2014;53:810–820. doi: 10.1021/bi401596q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reticker-Flynn NE, et al. A combinatorial extracellular matrix platform identifies cell-extracellular matrix interactions that correlate with metastasis. Nature communications. 2012;3:1122. doi: 10.1038/ncomms2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu YL, et al. Galectin-1 promotes lung cancer tumor metastasis by potentiating integrin alpha6beta4 and Notch1/Jagged2 signaling pathway. Carcinogenesis. 2013;34:1370–1381. doi: 10.1093/carcin/bgt040. [DOI] [PubMed] [Google Scholar]
- 13.Seguin L, et al. An integrin beta(3)-KRAS-RalB complex drives tumour stemness and resistance to EGFR inhibition. Nature cell biology. 2014;16:457–468. doi: 10.1038/ncb2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charrin S, et al. Tetraspanins at a glance. Journal of cell science. 2014;127:3641–3648. doi: 10.1242/jcs.154906. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, et al. Tspan8, CD44v6 and alpha6beta4 are biomarkers of migrating pancreatic cancer-initiating cells. International journal of cancer. Journal international du cancer. 2013;133:416–426. doi: 10.1002/ijc.28044. [DOI] [PubMed] [Google Scholar]
- 16.Sadej R, et al. CD151 in cancer progression and metastasis: a complex scenario. Laboratory investigation; a journal of technical methods and pathology. 2014;94:41–51. doi: 10.1038/labinvest.2013.136. [DOI] [PubMed] [Google Scholar]
- 17.Blanpain C, Fuchs E. Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science. 2014;344:1242281. doi: 10.1126/science.1242281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell stem cell. 2012;10:717–728. doi: 10.1016/j.stem.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Malanchi I, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2012;481:85–89. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- 20.Oskarsson T, et al. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nature medicine. 2011;17:867–874. doi: 10.1038/nm.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taddei I, et al. Beta1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nature cell biology. 2008;10:716–722. doi: 10.1038/ncb1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujiwara H, et al. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell. 2011;144:577–589. doi: 10.1016/j.cell.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rognoni E, et al. Kindlin-1 controls Wnt and TGF-beta availability to regulate cutaneous stem cell proliferation. Nature medicine. 2014;20:350–359. doi: 10.1038/nm.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang Y, et al. MT1-MMP-dependent control of skeletal stem cell commitment via a beta1-integrin/YAP/TAZ signaling axis. Developmental cell. 2013;25:402–416. doi: 10.1016/j.devcel.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones RG, et al. Conditional deletion of beta1 integrins in the intestinal epithelium causes a loss of Hedgehog expression, intestinal hyperplasia, and early postnatal lethality. The Journal of cell biology. 2006;175:505–514. doi: 10.1083/jcb.200602160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goulas S, et al. The Par complex and integrins direct asymmetric cell division in adult intestinal stem cells. Cell stem cell. 2012;11:529–540. doi: 10.1016/j.stem.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kessenbrock K, et al. A role for matrix metalloproteinases in regulating mammary stem cell function via the Wnt signaling pathway. Cell stem cell. 2013;13:300–313. doi: 10.1016/j.stem.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shackleton M, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 29.Stingl J, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 30.Visvader JE. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes & development. 2009;23:2563–2577. doi: 10.1101/gad.1849509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asselin-Labat ML, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nature cell biology. 2007;9:201–209. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- 32.Desgrosellier JS, et al. Integrin alphavbeta3 drives slug activation and stemness in the pregnant and neoplastic mammary gland. Developmental cell. 2014;30:295–308. doi: 10.1016/j.devcel.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desgrosellier JS, et al. An integrin alpha(v)beta(3)-c-Src oncogenic unit promotes anchorage-independence and tumor progression. Nature medicine. 2009;15:1163–1169. doi: 10.1038/nm.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medema JP. Cancer stem cells: the challenges ahead. Nature cell biology. 2013;15:338–344. doi: 10.1038/ncb2717. [DOI] [PubMed] [Google Scholar]
- 35.Martin TA, Jiang WG. Evaluation of the expression of stem cell markers in human breast cancer reveals a correlation with clinical progression and metastatic disease in ductal carcinoma. Oncology reports. 2014;31:262–272. doi: 10.3892/or.2013.2813. [DOI] [PubMed] [Google Scholar]
- 36.Hoogland AM, et al. Validation of stem cell markers in clinical prostate cancer: alpha6-integrin is predictive for non-aggressive disease. The Prostate. 2014;74:488–496. doi: 10.1002/pros.22768. [DOI] [PubMed] [Google Scholar]
- 37.Schober M, Fuchs E. Tumor-initiating stem cells of squamous cell carcinomas and their control by TGF-beta and integrin/focal adhesion kinase (FAK) signaling. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:10544–10549. doi: 10.1073/pnas.1107807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haraguchi N, et al. CD49f-positive cell population efficiently enriches colon cancer-initiating cells. International journal of oncology. 2013;43:425–430. doi: 10.3892/ijo.2013.1955. [DOI] [PubMed] [Google Scholar]
- 39.Zheng Y, et al. A rare population of CD24(−)ITGB4(−)Notch(hi) cells drives tumor propagation in NSCLC and requires Notch3 for self-renewal. Cancer cell. 2013;24:59–74. doi: 10.1016/j.ccr.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lathia JD, et al. Integrin alpha 6 regulates glioblastoma stem cells. Cell stem cell. 2010;6:421–432. doi: 10.1016/j.stem.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goel HL, et al. GLI1 regulates a novel neuropilin-2/alpha6beta1 integrin based autocrine pathway that contributes to breast cancer initiation. EMBO molecular medicine. 2013;5:488–508. doi: 10.1002/emmm.201202078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goel HL, et al. Regulated splicing of the alpha6 integrin cytoplasmic domain determines the fate of breast cancer stem cells. Cell reports. 2014;7:747–761. doi: 10.1016/j.celrep.2014.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshioka T, et al. beta4 Integrin signaling induces expansion of prostate tumor progenitors. The Journal of clinical investigation. 2013;123:682–699. doi: 10.1172/JCI60720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lo PK, et al. CD49f and CD61 identify Her2/neu-induced mammary tumor-initiating cells that are potentially derived from luminal progenitors and maintained by the integrin-TGFbeta signaling. Oncogene. 2012;31:2614–2626. doi: 10.1038/onc.2011.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Damiano JS. Integrins as novel drug targets for overcoming innate drug resistance. Current cancer drug targets. 2002;2:37–43. doi: 10.2174/1568009023334033. [DOI] [PubMed] [Google Scholar]
- 46.Eke I, et al. beta(1)Integrin/FAK/cortactin signaling is essential for human head and neck cancer resistance to radiotherapy. The Journal of clinical investigation. 2012;122:1529–1540. doi: 10.1172/JCI61350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang C, et al. beta1 integrin mediates an alternative survival pathway in breast cancer cells resistant to lapatinib. Breast cancer research : BCR. 2011;13:R84. doi: 10.1186/bcr2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanda R, et al. Erlotinib resistance in lung cancer cells mediated by integrin beta1/Src/Akt-driven bypass signaling. Cancer research. 2013;73:6243–6253. doi: 10.1158/0008-5472.CAN-12-4502. [DOI] [PubMed] [Google Scholar]
- 49.Mouneimne G, et al. Differential remodeling of actin cytoskeleton architecture by profilin isoforms leads to distinct effects on cell migration and invasion. Cancer cell. 2012;22:615–630. doi: 10.1016/j.ccr.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tumbarello DA, et al. ss3 integrin modulates transforming growth factor beta induced (TGFBI) function and paclitaxel response in ovarian cancer cells. Molecular cancer. 2012;11:36. doi: 10.1186/1476-4598-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jinushi M, et al. ATM-mediated DNA damage signals mediate immune escape through integrin-alphavbeta3−dependent mechanisms. Cancer research. 2012;72:56–65. doi: 10.1158/0008-5472.CAN-11-2028. [DOI] [PubMed] [Google Scholar]
- 52.Mulgrew K, et al. Direct targeting of alphavbeta3 integrin on tumor cells with a monoclonal antibody, Abegrin. Molecular cancer therapeutics. 2006;5:3122–3129. doi: 10.1158/1535-7163.MCT-06-0356. [DOI] [PubMed] [Google Scholar]
- 53.Finger EC, Giaccia AJ. Hypoxia, inflammation, and the tumor microenvironment in metastatic disease. Cancer metastasis reviews. 2010;29:285–293. doi: 10.1007/s10555-010-9224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ziaee S, Chung LW. Induction of integrin alpha2 in a highly bone metastatic human prostate cancer cell line: roles of RANKL and AR under three-dimensional suspension culture. Molecular cancer. 2014;13:208. doi: 10.1186/1476-4598-13-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weis SM, Cheresh DA. A wake-up call for hibernating tumour cells. Nature cell biology. 2013;15:721–723. doi: 10.1038/ncb2794. [DOI] [PubMed] [Google Scholar]
- 56.Lamouille S, et al. Molecular mechanisms of epithelial-mesenchymal transition. Nature reviews. Molecular cell biology. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schooley AM, et al. beta1 integrin is required for anchorage-independent growth and invasion of tumor cells in a context dependent manner. Cancer letters. 2012;316:157–167. doi: 10.1016/j.canlet.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 59.Cagnet S, et al. Signaling events mediated by alpha3beta1 integrin are essential for mammary tumorigenesis. Oncogene. 2014;33:4286–4295. doi: 10.1038/onc.2013.391. [DOI] [PubMed] [Google Scholar]
- 60.Shin DH, et al. Combating resistance to anti-IGFR antibody by targeting the integrin beta3−Src pathway. Journal of the National Cancer Institute. 2013;105:1558–1570. doi: 10.1093/jnci/djt263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oskarsson T, et al. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med. 2011;17:867–874. doi: 10.1038/nm.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malanchi I, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2012;481:85–89. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- 63.Zhang H, et al. HIF-1-dependent expression of angiopoietin-like 4 and L1CAM mediates vascular metastasis of hypoxic breast cancer cells to the lungs. Oncogene. 2012;31:1757–1770. doi: 10.1038/onc.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Garmy-Susini B, et al. PI3Kalpha activates integrin alpha4beta1 to establish a metastatic niche in lymph nodes. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9042–9047. doi: 10.1073/pnas.1219603110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schlesinger M, Bendas G. Vascular cell adhesion molecule-1 (VCAM-1)-An increasing insight into its role in tumorigenicity and metastasis. International journal of cancer. Journal international du cancer. 2014 doi: 10.1002/ijc.28927. [DOI] [PubMed] [Google Scholar]
- 66.Esposito M, Kang Y. Targeting tumor-stromal interactions in bone metastasis. Pharmacology & therapeutics. 2014;141:222–233. doi: 10.1016/j.pharmthera.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shibue T, et al. The outgrowth of micrometastases is enabled by the formation of filopodium-like protrusions. Cancer discovery. 2012;2:706–721. doi: 10.1158/2159-8290.CD-11-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shibue T, Weinberg RA. Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:10290–10295. doi: 10.1073/pnas.0904227106. [DOI] [PMC free article] [PubMed] [Google Scholar]