Abstract
In this article, we present and review the evidence for two major biopsychosocial theories of the onset and course of bipolar spectrum disorders (BSDs) that integrate behavioral, environmental, and neurobiological mechanisms: the reward hypersensitivity and the social and circadian rhythm disruption models. We describe the clinical features, spectrum, age of onset, and course of BSDs. We then discuss research designs relevant to demonstrating whether a hypothesized mechanism represents a correlate, vulnerability, or predictor of the course of BSDs, as well as important methodological issues. We next present the reward hypersensitivity model of BSD, followed by the social/circadian rhythm disruption model of BSD. For each model, we review evidence regarding whether the proposed underlying mechanism is associated with BSDs, provides vulnerability to the onset of BSDs, and predicts the course of BSDs. We then present a new integrated reward/circadian rhythm (RCR) dysregulation model of BSD and discuss how the RCR model explains the symptoms, onset, and course of BSDs. We end with recommendations for future research directions.
Keywords: bipolar spectrum disorder, reward sensitivity, social rhythms, circadian rhythms
Introduction
Emotions vary along a continuum of severity and affective lability is common. However, individuals who experience extreme mood lability may qualify for a diagnosis of bipolar disorder. Bipolar disorder is characterized by extreme swings of mood (euphoria or irritability versus sadness), behavior (excessive goal striving, supercharged energy, and increased talkativeness versus anhedonia, fatigue, and lethargy), and cognition (grandiosity and racing thoughts versus worthlessness) occurring within the same individual. As discussed below, bipolar disorder occurs on a continuum of severity, and bipolar spectrum disorders (BSDs) are relatively common, occurring in 4.4% of the US population (Merikangas et al., 2007). BSDs are among the leading causes of functional disability worldwide for physical and psychological disorders (e.g., Miklowitz & Johnson, 2006) and are linked to high rates of interpersonal dysfunction and divorce, erratic work history, substance abuse, and suicide (for a review, see Miklowitz & Johnson, 2006). However, BSDs also are associated with high creativity and achievement (Miklowitz & Johnson, 2006).
In this article, we describe two of the major biopsychosocial theories of BSD - the behavioral approach system (BAS) or reward hypersensitivity model and the social/circadian rhythm disruption model – and review the evidence for these theories. These models are relatively unique among theories of BSD in that each integrates neurobiological, behavioral, and environmental factors in explaining the onset and course of BSDs and proposes a single underlying mechanism that accounts for both poles of these disorders. In the sections that follow, we first describe the clinical features, spectrum, age of onset, and course of BSDs. We then discuss research designs that are relevant to demonstrating whether a hypothesized mechanism represents a vulnerability to BSD or a course predictor, rather than simply a correlate of BSD, as well as methodological difficulties in investigating mechanisms involved in the onset and course of BSD. We next present the reward hypersensitivity model, followed by the social/circadian rhythm disruption model of BSD. For each model, we review evidence regarding whether the proposed underlying mechanism is associated with BSDs, provides vulnerability to the onset of BSDs, and predicts the course of BSDs. We then present a new integrated reward/circadian rhythm (RCR) dysregulation model of BSD and discuss how the integrated model explains the features, onset, and course of BSDs. We end with recommendations for future research directions.
Bipolar Spectrum Disorders (BSDs): Nature, Onset, and Course
In the sections that follow, we describe the clinical features, spectrum, age of onset, and course of BSDs. These characteristics of BSDs represent clinical features that should be explained by the reward hypersensitivity and social/circadian rhythm models.
Symptoms/Clinical Features
The fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5; American Psychiatric Association, 2013) defines BSDs as encompassing three diagnoses: cyclothymia, bipolar II disorder, and bipolar I disorder. All three diagnoses involve extreme highs (hypomania or mania; in this article, we use “hypo/mania” to refer to either hypomania or mania) and lows (depression) of mood, motivation, cognition, and behavior, but they differ in severity, with bipolar I disorder being the most severe and cyclothymia the least severe. Cyclothymia is diagnosed as the presence of erratic depressive and hypomanic periods in the absence of a history of a full major depressive episode. Bipolar II disorder is diagnosed when there is a history of at least one major depressive episode and one hypomanic episode but no history of a manic episode. Bipolar I disorder is diagnosed when there is a history of at least one manic or mixed episode, as currently defined. A diagnosis of bipolar disorder not otherwise specified (NOS) is reserved for individuals who display bipolar symptomatology that does not meet criteria for any of these three bipolar diagnoses. There is a direct association between the severity of the bipolar diagnosis and indicators of clinical impairment, including number of episodes, chronicity, and symptom severity (Nusslock & Frank, 2011). Epidemiological studies relying on DSM criteria report lifetime prevalence estimates of 1.0% for bipolar I disorder, 1.1% for bipolar II disorder, 2.4% for subthreshold bipolar disorder, and 4.4% overall (Merikangas et al., 2007).
Bipolar Spectrum Concept and Evidence
Having a milder form of bipolar disorder puts an individual at elevated risk for developing a more severe variant of the illness supporting the argument that bipolar disorders are on a spectrum of severity. For example, retrospective studies suggest that individuals with bipolar I disorder first experience less severe symptoms on average 12 years prior to being diagnosed (Berk et al., 2007a). Prospective studies indicate that approximately 5-17% of adults and 20-25% of children/adolescents with bipolar II disorder develop manic or mixed episodes and convert to bipolar I disorder during follow-up (e.g., Alloy et al., 2012b; Birmaher et al., 2009). Rates of conversion from cyclothymia or bipolar disorder NOS to a bipolar II diagnosis may be even higher (Alloy et al., 2012b). Further evidence that cyclothymia is on the bipolar spectrum comes from studies that show elevated familial risk for bipolar I disorder is associated with cyclothymia (e.g., Akiskal et al., 1977; Chiaroni et al., 2005).
Age of Onset
Although the median age of onset for bipolar I and II disorders ranges from 17 to 31, the first peak in rates of BSD is between ages 15 and 19 (see Nusslock & Frank, 2011 for review). This 4- year period is what Weissman et al. (1996) refer to as a “hazard period” for BSD onset. Indeed, admixture analyses indicate that there are three high-risk periods in onset of bipolar disorder, with the earliest around mid-adolescence (e.g., Bellivier et al., 2001). Thus, the transition from adolescence to young adulthood is a critical developmental period constituting an age of risk during which bipolar conditions commence, consolidate, and often progress to a more severe course.
Course
Moreover, BSDs are typically chronic and are characterized by frequent and recurrent symptoms. Prospective naturalistic studies of individuals with BSDs have shown that although rates of recovery from index episodes are high, in the range of 70%-100%, of those who recover, a majority will experience one or more syndromal recurrences over a period of 2 to 5 years (e.g., DelBello et al., 2007). A topic of critical importance is identifying predictors of worsening prognosis. Demographic and clinical factors associated with worse longitudinal outcome include early age at illness onset (childhood/adolescence), cyclothymic temperament, rapid cycling, psychosis, low socioeconomic status, comorbid disorders, and poor adherence to pharmacological treatment (e.g., Birmaher et al., 2009; DelBello et al., 2007). In the present article, we argue that biopsychosocial mechanisms underlying reward sensitivity and circadian/social rhythms also play a central role in the nature, onset, and course of BSDs.
Research Design and Methodological Issues
The two major theoretical models of BSD reviewed below – the reward hypersensitivity and social/circadian rhythm disruption models – hypothesize particular mechanisms as providing vulnerability to the onset and contributing to variations in the course of BSD. In this article, we use the term “vulnerability” to refer to factors that may contribute to the initial onset of BSD and thus may be a causal mechanism, whereas we use the term “predictor of course” to refer to risk factors that predict subsequent symptoms or functional status, relapse and recurrence of mood episodes, or progression to more severe bipolar disorders along the spectrum. Such course predictors may serve as mechanisms that maintain or worsen bipolar symptoms.
Given these distinctions, different research study designs are relevant to establishing that a particular factor is a vulnerability versus a course predictor versus a correlate of BSD. Cross-sectional or retrospective studies that compare individuals with BSD to healthy controls or individuals with another disorder (e.g., unipolar depression) can establish factors that are correlates of BSD, but they are inadequate to demonstrate that these factors serve as vulnerabilities or maintenance factors because they cannot establish temporal precedence for the factor relative to symptom onset or exacerbation/recurrence. Even though cross-sectional or retrospective studies that compare remitted or euthymic BSD individuals with controls, or that compare BSD individuals in depressed, hypo/manic, and euthymic states can demonstrate independence of the potential risk factor from BSD symptoms, they cannot distinguish whether the factor is a vulnerability, a course predictor, or a consequence of BSD. Thus, in our review of the evidence supporting the theoretical models of BSD, we consider cross-sectional and remitted design studies in the sections on associations with BSD.
We also review evidence from truly prospective longitudinal designs, in which the proposed mechanism (e.g., reward hypersensitivity or social/circadian rhythm dysregulation) is assessed prior to first onset of BSD, in the vulnerability sections below. In addition, studies that examine the proposed causal mechanisms in individuals who do not yet exhibit BSD but are known to be at risk for developing BSD, such as the offspring of parents with bipolar disorder or individuals with hypomanic personality, are also relevant to establish vulnerability. Finally, prospective longitudinal studies that assess the proposed mechanism prior to measures of course (relapse or recurrence, symptom exacerbation, progression to more severe bipolar disorder) in samples already exhibiting BSD are reviewed in the sections below on predictors of course.
Moreover, BSDs provide particular methodological challenges for demonstrating vulnerability factors or course predictors that should be considered in interpreting the evidence for the theoretical models. First, these disorders are characterized by significant interepisode symptoms and functional impairment, making it difficult to assess potential vulnerabilities and course predictors at a time when the individual is asymptomatic so as to establish independence of the hypothesized mechanisms from bipolar symptoms. Thus, there is a need to control for symptoms in studies investigating the hypothesized mechanisms. Second, many individuals with BSDs take psychotropic medications and these medications may either directly counteract the hypothesized mechanisms underlying BSD or produce effects that make it difficult to assess the hypothesized mechanisms. In either case, medication use for bipolar disorder may confound study results, particularly studies of correlates of BSD or course predictors, and needs to be statistically controlled.
Reward Hypersensitivity Model of Bipolar Spectrum Disorders: Theory and Evidence
In the sections below, we present the Reward Hypersensitivity Model of BSDs and review evidence on whether reward hypersensitivity is a correlate, vulnerability, or predictor of the course of BSDs. We include evidence on prodromes of bipolar mood episodes, self-report and behavioral task measures, personality and cognitive styles, neurophysiological and neural measures, and life events.
Reward Hypersensitivity Model
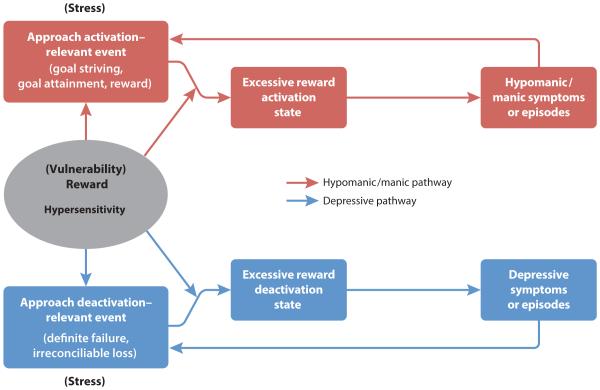
Individuals with BSDs may be characterized by a hypersensitive reward system. The reward system, also known as the BAS, is a biobehavioral system that regulates approach motivation and goal-directed behavior to attain rewards (Gray, 1994). It has been linked to a dopaminergic fronto-striatal neural circuit sensitive to reward-relevant stimuli (e.g., Depue & Collins, 1999; Haber & Knutson, 2010) and is activated by goal- or reward-relevant cues that may be external (e.g., an opportunity for promotion) or internal (e.g., expectancy of winning an award). BAS activation is associated with increased motor behavior, incentive motivation, and positive goal-striving emotions such as hope and happiness (Depue & Collins, 1999; Gray, 1994), as well as with anger when goal striving is frustrated or blocked (Carver, 2004; Harmon-Jones & Sigelman, 2001). Originally proposed by Depue & Iacono (1989) and expanded by Alloy and colleagues (e.g., Alloy & Abramson, 2010; Alloy et al., 2009a; Urošević et al., 2008) and Johnson and colleagues (e.g., Johnson, 2005; Johnson et al., 2012b), the BAS/reward model of BSD suggests a single dimension—approach motivation and reward sensitivity—to organize diverse symptoms and account for both poles of BSD (see Figure 1). Vulnerability to BSDs is hypothesized to be the result of an overly sensitive reward system that is hyperreactive to goal- and reward-relevant cues. This hypersensitivity leads to excessive reward motivation and approach-related affect in response to life events involving rewards or goal striving and attainment, which, in turn, lead to hypomanic or manic symptoms (red pathway in Figure 1). In particular, excessive BAS/reward activation is hypothesized to lead to a cluster of hypo/manic psychomotor activation symptoms (elevated energy, increased goal-directed activity, decreased need for sleep, increased confidence, and irritability when goal-pursuit is thwarted). This model also proposes that BSD individuals experience an excessive downregulation or decrease in behavioral approach in response to nonattainment of goals or rewards (e.g., definite failures), which, in turn, leads to depressive symptoms (blue pathway in Figure 1), particularly anhedonia, psychomotor retardation, low motivation, fatigue, and hopelessness. Thus, the hypothesized vulnerability to BSDs in the reward model is a propensity toward excessive reward system activation and deactivation. It is important to distinguish this vulnerability (trait) from the states of actual activation or deactivation (dysregulation) of the reward system, which are considered the more proximal precursors of mood symptoms/episodes.
Figure 1.
The reward theory of BSD also includes a transactional component in which individuals with BAS or reward hypersensitivity not only react more strongly to goal- or reward-relevant life events, but also are exposed to such events more frequently via “stress generation” (Hammen, 1991) processes (Alloy et al., 2009a; Urošević et al., 2008). That is, vulnerable individuals’ reward hypersensitivity leads them to engage in behaviors that increase their exposure to the very goal- and reward-related events that trigger excessive responses from their reward systems. This exposure to goal- and reward-relevant events, in turn, triggers mood episode onset via a two-hit model (see red and blue pathways in Figure 1).
Considerable evidence has accumulated in support of the reward hypersensitivity of BSDs. We review this evidence below.
Is Reward Hypersensitivity Associated with BSDs?
The association between reward sensitivity and BSDs has been evaluated in multiple ways, including an examination of prodromes for BSDs, self-reports, responses to reward cues on behavioral tasks, measures of reward-relevant personality or cognitive styles, and through electroencephalography (EEG) and both functional and structural neuroimaging. Studies that establish an association between reward hypersensitivity and BSD usually involve correlational or cross-sectional designs that compare individuals with BSDs to healthy controls. In addition, some studies examine reward-relevant constructs in individuals with BSD in a remitted or euthymic state compared with controls or across hypo/manic, euthymic, and depressive mood states. Such studies at least can establish that reward hypersensitivity may be a more stable characteristic of BSD individuals and not a simple consequence of a current mood state.
Evidence from prodromes
Prodromes refer to the earliest signs and symptoms that signal an impending full-blown episode of disorder (Molnar et al., 1988). In line with the potential importance of reward hypersensitivity in bipolar disorders, some of the most common prodromal features of hypo/mania are increased goal-directed activity, extreme goal setting, and increased success expectancies (Lam & Wong, 1997; Lam et al., 2001), whereas anhedonia, decreased motivation and goal-setting, and low self-confidence characterize depressive prodromes in BSDs (e.g., Bauer et al., 2006; Molnar et al., 1988). Moreover, cognitive-behavioral deactivation strategies such as restraining oneself, modifying high success expectancies, and engaging in calming activities during manic prodromes have been found to reduce the likelihood of manic relapse (Lam et al., 2001), whereas behavioral activation techniques have had success in reducing full-blown depression.
Self-report and behavioral task evidence
Consistent with the reward hypersensitivity model, cross-sectional studies find that controlling for bipolar mood symptoms, individuals all along the bipolar spectrum exhibit higher self-reported BAS sensitivity than controls exhibit (e.g., Alloy et al., 2008; Meyer et al., 2001; Salavert et al., 2007; but see Hayden et al., 2008 for contrary findings). Higher BAS sensitivity also distinguished patients with BSD from patients with unipolar depression (Quilty et al., 2014). In addition, parent reports of BAS sensitivity were associated with both manic and depressive symptoms in an outpatient sample of adolescents (Gruber et al., 2013). Even in a euthymic state, individuals with BSDs exhibit higher self-reported BAS sensitivity (Alloy et al., 2008; Salavert et al., 2007), suggesting that the high reward sensitivity may be independent of mood-related report biases. Moreover, self-reported BAS sensitivity is stable across mood states in individuals with BSDs (Alloy et al., 2009a; Urošević et al., 2008), although states of BAS/reward activation fluctuate in response to goal- or reward-relevant events.
Individuals with BSDs also exhibit greater emotional, behavioral, and cognitive responsiveness to rewards on behavioral tasks. For example, Hayden et al. (2008) reported that euthymic bipolar I patients showed greater behavioral responsiveness to small monetary rewards on a card-sorting task. Similarly, Swann and colleagues (2009) found that bipolar I patients exhibited an inability to delay responding for rewards on a task compared to healthy controls. A meta-analysis of performance on the Iowa Gambling Task, a measure of reward sensitivity, also indicated that euthymic individuals with bipolar I disorder make more risky choices than do controls (Edge et al., 2012). Johnson and associates (2005) observed that current hypomanic symptoms were associated with greater positive affect and success expectancies following monetary reward and success feedback on a button-pressing task. And, Ruggero & Johnson (2006) found that bipolar I patients exhibited poorer cognitive performance than did controls following failure feedback on a concept-formation task.
Personality/cognitive style evidence
According to the reward hypersensitivity model, individuals with BSDs should exhibit personality features and cognitive styles related to high drive and incentive motivation, consistent with their highly sensitive reward/approach motivational systems. In line with this hypothesis, individuals along the bipolar spectrum exhibit more ambitious goal striving and higher achievement motivation than do controls, controlling for current mood symptoms (Johnson et al., 2009; 2012a; Lozano & Johnson, 2001). The extreme goal setting is observed most strongly for goals involving financial success and popular fame. In addition, individuals with BSDs are less likely than controls to “coast” (i.e., decrease their goal-striving efforts) following unexpectedly high progress toward a goal (Fulford et al., 2010), suggesting that it is difficult for them to curb their ambitious goal-striving.
Some cross-sectional studies have found that individuals with BSDs exhibit cognitive styles as negative as those of individuals with unipolar depression and more negative than those of controls, even when they are euthymic, but other studies have not obtained this effect (see Alloy et al., 2005; 2006b for reviews). An important contributor to the mixed cognitive style findings in BSDs may be the failure to consider the particular content of the cognitive styles. Indeed, more recent research indicates that BSDs are uniquely associated with cognitive styles that have reward system-relevant themes. Specifically, euthymic bipolar individuals exhibit a distinctive profile of maladaptive cognitive styles characterized by perfectionism, self-criticism, and autonomy rather than the dependency, attachment, and approval-seeking styles observed among unipolar depressed individuals (e.g., Alloy et al., 2009b; Lam et al., 2004; Scott et al., 2000; see Alloy et al., 2009a for review).
Neurophysiological and neuroimaging evidence
Both neurophysiological and neuroimaging research provide additional support for the BAS/reward model of BSD. With respect to neurophysiology, much of this work has focused on relative left versus right frontal EEG activity, a neurophysiological index of approach system sensitivity and reward-related affect (see Coan & Allen, 2004 for review). Increased relative left-frontal EEG activity indicates a propensity to approach or engage a stimulus, whereas decreased relative left-frontal activity indicates a propensity toward reduced approach-related affect or increased withdrawal motivation (Coan & Allen, 2004). In line with the BAS/reward model, Harmon-Jones and colleagues (2008) reported that individuals with BSDs displayed elevated relative left-frontal EEG activity (i.e., more approach motivation) during a reward-related laboratory task compared to healthy controls. Furthermore, BSD individuals in a hypo/manic episode display elevated relative left frontal EEG activity compared to remitted and depressed BSD individuals (Kano et al., 1992; Nusslock et al., 2012b). Thus, frontal EEG asymmetry not only is elevated in bipolar disorder but also appears to be sensitive to one’s hypo/manic status. By contrast, individuals with unipolar depression display decreased relative left frontal EEG activity during both depressive and euthymic states (see Thibodeau et al., 2006 for meta-analytic review), reflecting reduced approach system sensitivity and blunted reward-related affect (Kasch et al., 2002).1
With respect to neuroimaging, considerable data link the BAS, and reward processing more generally, to a fronto-striatal neural circuit involving the ventral striatum (VS) and the orbitofrontal cortex (OFC), among other regions (Haber & Knutson, 2010; Kringelbach & Rolls, 2004). Research in both animals and humans indicates the fronto-striatal circuit plays a central role in dopamine transmission, which, in the context of this circuit, helps facilitate reward-responsivity, reward-driven learning, prediction error, and goal-directed behavior (Haber & Knutson, 2010; Schultz, 2002). The VS is involved in processing both primary and secondary (e.g., monetary) rewards and plays a central role in reward anticipation (Haber & Knutson, 2010; Knutson et al., 2001). The OFC appears to be particularly important for encoding reward value and assessing probability of reward receipt (Haber & Knutson, 2010). Drawing from research on reward-related neural activation in bipolar disorder (Bermpohl et al., 2010; Nusslock et al., 2012a), we define the OFC as Brodmann area (BA) 10, 11, and 47 for the present article. Elevated VS activity during reward anticipation is associated with elevated self-reported BAS/reward sensitivity (Caseras et al., 2013; Hahn et al., 2009), and connectivity between the VS and OFC predicts individual differences in reward dependence (Cohen & Ranganath, 2007).
Structural and functional neuroimaging studies implicate the fronto-striatal neural circuit in the pathophysiology of BSD. Structural imaging studies report abnormalities in prefrontal volume (Lopez-Larson et al., 2002) and increased striatal size (Strakowski et al., 2002) in individuals with bipolar disorder. Positron emission tomography studies report increased metabolism in the striatum (among other regions) in both currently depressed (e.g., Ketter et al., 2001; Mah et al., 2007) and manic (Blumberg et al., 2000) bipolar individuals, suggesting that elevated metabolic activity in the striatum may be a state-independent marker of bipolar disorder (although see Brooks et al., 2009 for evidence of decreased metabolic activity in the striatum in bipolar depression).
In line with the BAS/reward model of BSD, functional MRI (fMRI) research indicates that BSD is associated with an excessive increase in fronto-striatal reward-related neural activation to positive or approach-related stimuli. For example, bipolar individuals display elevated striatal (Hassel et al., 2008; Lawrence et al., 2004) and OFC (Elliott et al., 2004) activation to pictures of happy faces or pleasant stimuli compared to healthy controls. The handful of studies on bipolar disorder that have employed established fMRI reward paradigms provide additional evidence that BSD is characterized by a fronto-striatal hypersensitivity to reward-relevant cues. Individuals with euthymic bipolar I (Nusslock et al., 2012a) and bipolar II (Caseras et al., 2013) disorder display greater VS, medial OFC (BA 10), and left lateral OFC (BA 47) activation during the anticipation of monetary reward compared to healthy controls. The fact that reward-related neural activation was abnormally elevated in bipolar individuals during remission suggests that this fronto-striatal hyperactivity reflects a trait-like profile of bipolar disorder. With respect to hypo/mania, Bermpohl and colleagues (2010) reported that 15 bipolar I individuals in a manic episode displayed elevated left lateral OFC (BA 47) activation during reward anticipation, whereas healthy participants showed the inverse effect. In a second study, Abler and colleagues (2008) reported increased activation in the VS coupled with reward omission in manic versus healthy participants, suggesting that bipolar individuals in a manic episode have a reduced capacity to discriminate between rewards on the basis of their actual value and relevance. Finally, currently depressed bipolar I individuals displayed elevated left lateral OFC (BA 47) activation during anticipation, collapsing across reward and loss trials, compared to both currently depressed individuals with major depression and healthy controls (Chase et al., 2013). Thus, even during depression, individuals with bipolar I disorder maintain heightened activation in regions of the fronto-striatal neural circuit. We propose that elevated reward-related neural activation is likely the biological mechanism underlying elevated self-report, behavioral, and neurophysiological indices of reward sensitivity that have been documented in research on BSDs (see above sections).
Is Reward Hypersensitivity a Vulnerability for BSDs?
The strongest evidence for the role of reward hypersensitivity as a vulnerability for BSDs comes from prospective studies that examine predictors of first onset of BSD, because such studies can establish the temporal precedence of reward hypersensitivity relative to disorder onset. Additional relevant evidence derives from research that examines reward sensitivity and related constructs in samples at genetic, psychological, or behavioral risk for BSD, even if these studies do not demonstrate prediction of BSD onset. We review research relevant to establishing reward hypersensitivity as a vulnerability factor for BSD in this section.
Self-report and behavioral task evidence
In a retrospective behavioral high-risk design, Alloy and colleagues (2006a) selected late adolescents (ages 18-24) with high versus moderate levels of self-reported reward sensitivity (based on two different self-report BAS questionnaires) and compared their lifetime histories of mood disorders, blind to their BAS scores. High-BAS- sensitivity adolescents were six times more likely to meet DSM-IV-TR diagnostic criteria for a BSD (50%) than were moderate-BAS-sensitivity adolescents (8.3%). Although these findings are consistent with high reward sensitivity as a vulnerability for BSDs, the causal direction of the association between high reward sensitivity and increased rates of BSD is unclear. Consequently, in a fully prospective design, Alloy et al. (2012a) selected younger adolescents (ages 14-19) who scored in the top 15% on two different self-report measures of BAS/reward sensitivity or who scored in the middle 40-60% on both measures, but who had no prior lifetime history of a BSD or even a single episode of hypomania, and followed them for over a year. Participants also completed measures of goal setting and reward responsiveness on a behavioral task at baseline. Alloy et al. (2012a) found that controlling for family history of BSD and baseline hypo/manic and depressive symptoms, the high-BAS group was significantly more likely to develop a BSD (12.3% vs. 4.2%) and had a shorter time to first onset of a BSD than the moderate-BAS group. Greater reward responsiveness on the behavioral task also predicted a greater likelihood and shorter time to first onset of BSD, even after controlling for BAS-risk group status. This is the first study to identify psychological vulnerabilities that predict first lifetime onset of BSD.
Although the Alloy et al. (2012a) findings provide the strongest evidence for reward hypersensitivity as a vulnerability to BSD, studies of self-reported and behavioral reward responsiveness in samples at risk for BSD are also relevant. In genetic high-risk samples, Jones and colleagues (2006) did not report differential self-reported BAS sensitivity in adolescent offspring of bipolar vs. control parents, whereas Chang and associates (2003) observed that children of bipolar parents reported a greater tendency to approach novel, rewarding situations on a temperament scale than controls reported. Nurnberger et al. (1988b) also found greater self-reported sensation seeking in the offspring of bipolar parents than of control parents. In addition, in comparison with controls, individuals who have a high score on the Hypomanic Personality Scale (HPS; Eckblad & Chapman, 1986), a demonstrated risk factor for BSD (Kwapil et al., 2000), also exhibit higher self-reported BAS sensitivity than controls (e.g., Johnson & Carver, 2006; Meyer et al., 1999) and greater approach motivation in response to threatening vignettes (Meyer et al., 2007). Participants with hypomanic personality also show greater positive generalization and cognitive reactivity to success experiences on a behavioral task (Carver & Johnson, 2009; Eisner et al., 2008; Johnson et al., 2005).
Personality/cognitive style evidence
In the Alloy et al. (2012a) prospective behavioral high-risk study, ambitious goal striving at baseline also predicted a greater likelihood and shorter time to first onset of BSD, controlling for family history of BSD and initial mood symptoms. Moreover, ambitious goal striving mediated the predictive association of BAS risk group status with first onset of BSD, indicating that highly ambitious goal setting also is a vulnerability to BSD and may account for some of the risk effect related to self-reported reward sensitivity. In addition, ambitious goal setting also has been observed in individuals at risk for BSD based on high HPS scores (e.g., Carver & Johnson, 2009; Johnson & Carver, 2006; Johnson et al., 2005; Meyer & Krumm-Merabet, 2003) and in adolescents at high risk for BSD based on high self-reported reward sensitivity (Stange et al., 2013). Stange et al. (2013) also found that participants who were high in self-reported BAS sensitivity and at risk for BSD reported more perfectionistic cognitive styles and tendencies to engage in positive overgeneralization than did participants who were moderate in BAS sensitivity. Moreover, a cognitive style to overgeneralize from positive experiences interacted with self-reported high reward sensitivity to predict increases in hypomanic symptoms among adolescents with no prior history of BSD, controlling for baseline hypomanic symptoms (Stange et al., 2012). Finally, other studies of individuals at genetic or behavioral high risk for BSD have reported that high-risk participants exhibit more dysfunctional attitudes than do low-risk participants (e.g., Jones et al., 2006; Knowles et al., 2005), but these studies didn’t examine reward-relevant cognitive styles specifically.
Neurophysiological and neuroimaging evidence
To our knowledge, no studies have examined neurobiological indices of reward sensitivity as predictors of first onset of BSD. There is, however, preliminary evidence of elevated neurophysiological and neural indices of approach motivation and reward-related affect in individuals at psychological and genetic risk for BSD. At the neurophysiological level, Harmon-Jones et al (2002) examined the relationship between self-reported proneness to hypo/mania and relative left frontal EEG activity (a neurophysiological index of BAS sensitivity) to an anger evoking laboratory task. Anger is an approach-oriented emotion, despite its negative valence (Carver, 2004). As predicted, individuals prone to hypo/manic symptoms, as assessed by the General Behavior Inventory (GBI; Depue et al., 1989), had a greater increase in relative left frontal EEG activity to the anger-provoking event.
Complementing neurophysiological research on relative left frontal EEG activity is work employing event-related potentials (ERP). Embedded within EEG data, ERPs are electrical potentials that occur in preparation for, or in response to, discrete events, whether they are internal or external to the participant. Increasing evidence suggests that reward sensitivity can be measured using the feedback negativity (FN), an ERP component elicited by stimuli that indicate monetary gain versus loss (Hajcak et al., 2007). Mason and colleagues (2012) recently examined the relationship between the FN to monetary gain/loss stimuli and hypomanic traits, as assessed by the HPS (Eckblad & Chapman, 1986). In line with the BAS/reward model, individuals with elevated HPS scores displayed greater reward responsiveness, as indexed by the FN, compared to those with both moderate and low HPS scores.
At the neural level, gray matter deficits in the VS and the anterior cingulate cortex (ACC), a region implicated in the fronto-striatal circuit (Haber & Knutson, 2010), are present in individuals at genetic risk for bipolar disorder but who have not yet developed the illness (McDonald et al., 2004). Furthermore, research employing established fMRI reward paradigms indicates that both individuals with subsyndromal hypomanic symptoms (O’Sullivan et al., 2011) and individuals with a hypomanic temperament who have not yet developed BSD (Harada et al., 2013) display elevated VS activation and left-lateral OFC activation during reward processing. These findings suggest that abnormalities in fronto-striatal neural circuitry reflect preexisting risk factors for BSD as opposed to a consequence of the illness.
One of the most reliable findings to emerge from research on reward-related neural activation in bipolar disorder is that the illness is associated with an abnormal increase in left lateral OFC activation during reward anticipation. This has been observed across the entire bipolar spectrum, including in individuals with bipolar I (Bermpohl et al., 2010; Nusslock et al., 2012a) and bipolar II disorder (Caseras et al., 2013), and in individuals at risk for BSD who have not developed the illness (Harada et al., 2013). Elevated left lateral OFC activation during reward anticipation also has been observed across all phases of bipolar disorder, including mania (Bermpohl et al., 2010), euthymia (Nusslock et al., 2012a), and depression (Chase et al., 2013). Collectively, these findings suggest that there may be a trait-like, and perhaps endophenotypic, abnormality during reward processing in this region in individuals at risk for BSD. Given the role of the lateral OFC in arousal and salience processing (Kringelbach & Rolls, 2004), these data suggest that individuals at risk for BSD may be characterized by abnormalities in regulating arousal and behavioral activation during reward processing and goal pursuit. Furthermore, the left lateralization of this effect is consistent with neurophysiological data summarized above indicating that BSD is characterized by elevated relative left frontal EEG activity (Harmon-Jones et al., 2002, 2008; Nusslock et al., 2012b).
Life events evidence
Characteristics of an individual’s environment influence the onset, expression, and course of BSDs. In particular, the occurrence of life events is associated with first onset and recurrences of bipolar mood episodes (see Alloy et al., 2005; 2006b; 2009a; Johnson, 2005 for reviews). There is evidence that both positive and negative life events precede onsets of hypo/manic episodes, whereas only negative events precede depressive episode onset in BSD individuals (see Alloy et al., 2005; 2006b; 2009a for reviews). An advantage of the reward hypersensitivity model is that it makes predictions about the specific types of life events that should trigger bipolar mood episodes. Hypo/manic episodes should be precipitated by BAS/reward-activation events involving goal striving, goal attainment, or goal obstacles evoking anger/irritability, whereas depressive episodes should be triggered by BAS/reward-deactivation events involving definite failures or losses that cannot be remedied.
Although several studies find that reward-relevant events in particular predict the course of BSDs (reviewed below), no study to date has examined goal- or reward-relevant events specifically as predictors of first onset of BSD so as to demonstrate their role as vulnerabilities to BSD. Thus, life event studies on first onset of BSDs are needed. However, evidence of stress generation, particularly in individuals at risk for BSDs, may have relevance to the vulnerability status of life events. Consistent with the transactional component of the reward hypersensitivity model, individuals with BSDs experienced more BAS/reward-activation and more BAS/reward-deactivation life events over a six-month follow-up than do demographically similar controls (Urošević et al., 2010). Moreover, high-BAS-sensitivity adolescents at risk for BSD also experienced more BAS/reward-activation and -deactivation events than did moderate-BAS-sensitivity adolescents (Boland et al., 2014).
Does Reward Hypersensitivity Predict the Course of BSDs?
Self-report and behavioral task evidence
Several studies have examined whether self-reported reward sensitivity predicts the course of BSD. Among bipolar I patients, Meyer et al. (2001) found that BAS sensitivity following recovery from a mood episode predicted greater manic symptoms over follow-up. Also, over an 18-month follow-up of bipolar I patients, Salavert et al. (2007) reported that higher baseline BAS sensitivity predicted relapse with hypomanic or manic episodes, whereas lower baseline BAS sensitivity predicted depressive episode relapse compared with patients who remained asymptomatic. The depressive relapse finding is inconsistent with the reward hypersensitivity model, which would predict that high, rather than low, initial BAS sensitivity would predict depressive relapse. Consistent with the reward hypersensitivity model, in a sample of late adolescents with BSDs, Alloy et al. (2008) found that controlling for initial hypo/manic and depressive symptoms, higher Time 1 BAS sensitivity predicted a shorter time to relapse of hypomanic and manic episodes over a three year follow-up and higher Time 1 BAS reward responsiveness (a subscale of the BAS questionnaire) marginally predicted a shorter time to relapse of depressive episodes. High BAS sensitivity also predicted progression to more severe disorders along the bipolar spectrum. Controlling for initial hypo/manic and depressive symptoms, treatmentseeking, and family history of BSD, Alloy et al. (2012b) reported that high baseline BAS sensitivity (and especially the fun-seeking subscale) predicted a greater likelihood of progression to bipolar II disorder (onset of a major depressive episode) among individuals with cyclothymia or bipolar NOS and a greater likelihood of progression to bipolar I disorder (onset of a manic episode) among participants with bipolar II, cyclothymia, and bipolar NOS (when family history was not controlled). In addition, the combination of high BAS sensitivity and high behavioral inhibition system or threat/punishment sensitivity also predicted a greater likelihood of progression to bipolar I disorder. Furthermore, high baseline BAS sensitivity (especially the fun-seeking subscale) also predicted increased substance use problems prospectively (Alloy et al., 2009c).
Personality/cognitive style evidence
Only a few studies have investigated whether goal- or reward-relevant cognitive styles predict the course of BSDs. In a bipolar I sample, Lozano & Johnson (2001) found that the goal-relevant style of high achievement motivation predicted increases in manic symptoms over a six-month follow-up. In addition, Johnson et al. (2012a) observed that ambitious goal setting for popular fame and financial success predicted increases in manic symptoms over a three-month follow-up in individuals with bipolar I disorder. Among late adolescents with BSDs controlling for initial hypo/manic and depressive symptoms and past history of mood episodes, Alloy et al. (2009b) found that BAS-relevant cognitive styles involving high self-criticism and autonomy predicted a greater likelihood of hypomanic and manic episodes over a three-year follow-up. Moreover, an autonomous cognitive style mediated the predictive association between high self-reported BAS sensitivity and prospective hypomanic and manic episodes.
Neurophysiological and neuroimaging evidence
To date, only one study has investigated whether neural or neurophysiological indices of approach/reward-related affect predict the course of BSDs (Nusslock et al., 2012b). In this study, 58 individuals with cyclothymia or bipolar II disorder completed resting EEG recordings. In line with the BAS/reward model, elevated relative left frontal EEG activity (a neurophysiological index of BAS sensitivity) prospectively predicted a greater likelihood of converting from cyclothymia or bipolar II disorder to bipolar I disorder over the 4.7-year follow-up. This is the first study to identify a neurophysiological marker that prospectively predicts conversion to bipolar I disorder.
Pharmacological studies also are relevant to understanding whether reward sensitivity predicts the course of BSD. As indicated, dopamine transmission is at the heart of the fronto-striatal reward circuit and reward processing more generally (Haber & Knutson, 2010; Schultz, 2002). In line with the BAS/reward model of BSD, converging data highlight the central role of dopamine in bipolar disorder, and pharmacological models suggest a role for increased dopaminergic drive in the onset of hypo/mania and the converse in the onset of bipolar depression (see Berk et al., 2007b for review). For example, administration of the dopamine precursor levdopa (L-DOPA) to patients with bipolar disorder produces hypo/manic episodes (van Praag & Korf, 1975). Similarly, amphetamine, which increases levels of synaptic dopamine, can produce a hypomanic-like state in otherwise healthy individuals (Jacobs & Silverstone, 1986) and hyperactivity in an animal model of hypo/mania (Frey et al., 2006). By contrast, dopamine deficits play an important role in both bipolar and unipolar depression, and symptoms of depression can arise in the context of lowered dopamine transmission (Berk et al., 2007b). Furthermore, psychiatric medications that target dopamine neurotransmission are central to managing both the manic and depressive symptoms of BSDs (e.g., Tohen et al., 2003; Vieta et al., 2005), and there is preliminary evidence that lithium, perhaps the most effective mood stabilizer, decreases the amount of dopamine and its metabolites in women with unipolar and bipolar disorders (Tohen et al., 2003).
Life events evidence
Consistent with the reward hypersensitivity model, in two studies of patients with bipolar I disorder Johnson and colleagues (2000; 2008) found that goal-attainment life events predicted increases in manic but not depressive symptoms over follow-up; general positive events did not predict manic symptom increases. Similarly, Nusslock and associates (2007) reported that late-adolescent students with BSDs were more likely to develop a new episode of hypomania but not depression following a goal-striving event (studying for and taking final exams) than were other nonstudent bipolar adolescents who did not experience this event (42% vs. 4%). Anger-provocation events that also activate the BAS/reward system (e.g., goal obstacles, insults) also have been found to predict increased hypomanic symptoms (e.g., Carver, 2004; Harmon-Jones et al., 2002). In contrast, failure or loss events frequently have been found to trigger depressive episodes (e.g., see Alloy et al., 2005; 2006b; 2009a for review).
Cognitive styles also have been found to interact with life events to predict bipolar mood symptoms and episodes (see Alloy et al., 2005; 2006b; 2009a for review), but only a few studies have specifically examined BAS/reward-relevant cognitive styles in combination with life events as predictors of BSD course. Hammen and colleagues (1989; 1992) reported that autonomous cognitive styles did not interact with achievement events to predict symptom severity in individuals with BSDs; however, they did not examine prediction to hypo/manic and manic symptoms separately. In contrast, in a late adolescent BSD sample, Francis-Raniere et al. (2006) found that controlling for initial hypo/manic and depressive symptoms and the total number of events experienced, BAS/reward-relevant self-critical and perfectionistic cognitive styles interacted with BAS/reward-activating positive events to predict increases in hypo/manic symptoms and with BAS/reward-deactivating negative events to predict increases in depressive symptoms over follow-up. Further studies of reward-relevant life events in combination with reward-related personality or cognitive styles as predictors of BSD course are needed. Moreover, studies that examine other self-report, behavioral, and neural indicators of reward sensitivity in combination with goal- and reward-relevant events as predictors of BSD course also are yet to be conducted.
Social/Circadian Rhythm Model of Bipolar Spectrum Disorders: Theory and Evidence
In the following sections, we describe the Social/Circadian Rhythm Disruption Model of BSDs and review evidence on whether social and circadian rhythm disruption is associated with BSDs or is a vulnerability for or predictor of the course of BSDs. We include evidence from prodromes of bipolar mood episodes, self-report measures, circadian rhythm-relevant personality, sleep and actigraphy measures, life events, and neurobiological and genetic studies
Social/Circadian Rhythm Disruption Model
Biological processes that repeat about every 24 hours and persist with the same period in the absence of external cues are defined as circadian rhythms (e.g., Czeisler & Gooley, 2007; Wever, 1979). The suprachiasmatic nucleus (SCN), or biological clock, located in the anterior hypothalamus, regulates circadian rhythms (e.g., Reppert & Wever, 2001). The SCN functions autonomously, but under normal conditions, is entrained by external zeitgebers (German for “time givers”), such as light (e.g., Roenneberg & Merrow, 2007; Wever, 1989), yielding a period of slightly more than 24 hours in humans (e.g., Panda et al., 2002; Wever, 1979). Circadian rhythms also can be entrained or phase-shifted by non-photic cues, including social stimuli, auditory stimuli, exercise, and daily schedules or social rhythms (see Grandin et al., 2006 for review). The sleep/wake cycle, body temperature, cortisol, norepinephrine, and melatonin all show circadian rhythms (Reppert & Wever, 2001), with melatonin considered an accurate physiological marker of endogenous circadian function (Lewy & Sack, 1989).
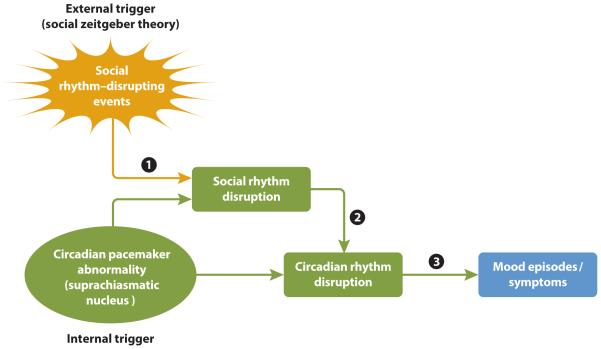
Circadian rhythm disruption (CRD) has been proposed to be a key mechanism in the neurobiological vulnerability to BSDs (e.g., McClung, 2007; Murray & Harvey, 2010) and may reflect either abnormalities in the SCN itself or in the external entrainment of the SCN (Grandin et al., 2006; see Figure 2). According to the social zeitgeber theory of mood disorders (Ehlers et al., 1988; Grandin et al., 2006), depicted in Figure 2, life events that disrupt social zeitgebers, defined as daily social rhythms or schedules (e.g., bedtimes, mealtimes, start and end of school or a job; path 1 in Figure 2), precipitate BSD symptoms, particularly somatic symptoms such as sleep changes, because they disturb circadian rhythms (paths 2 and 3 in Figure 2). Events that disrupt social rhythms (e.g., causing a change in bedtime) may be associated with increased light exposure at certain times of day, which can phase shift melatonin and other circadian rhythms (e.g., Roenneberg & Merrow, 2007; Stetler et al., 2004; Wever, 1989). Similarly, social rhythm disruption (SRD) events that change daily schedules (e.g., skipping a meal) may disrupt circadian rhythms through their effects on non-photic zeitgebers (e.g., Goel, 2005). Indeed, changes in daily activity patterns or social contacts, whether naturally occurring or manipulated, are associated with changes in circadian rhythms (see Stetler et al., 2004, for review) in healthy individuals.
Figure 2.
Much evidence is consistent with the social/circadian rhythm disruption model of BSD. We review this evidence below.
Is Social/Circadian Rhythm Dysregulation Associated with BSDs?
Studies of prodromal features, self-reports of social rhythm regularity, circadian rhythm-relevant personality, sleep and actigraphy, circadian system-relevant hormones (e.g., melatonin and cortisol), circadian relevant neurotransmitters (e.g., dopamine and serotonin), and genetic evidence have established an association between social or circadian rhythm disturbance and BSDs. In this section, we review evidence from cross-sectional studies that compare individuals with BSD either in episode or in a euthymic state to controls on social rhythm-relevant or circadian rhythm-relevant constructs.
Evidence from prodromes
In addition to increased goal-directed activity, the other most common prodrome of manic episodes is decreased need for sleep (Lam & Wong, 1997; Jackson et al., 2003; Molnar et al., 1988). Indeed, decreased need for sleep occurs as a prodromal feature of hypo/mania in 80% of individuals with BSD (Jackson et al., 2003). Sleep disturbance also is a common prodromal symptom prior to depressive episodes (e.g., Jackson et al., 2003). This is consistent with the hypothesis that disturbances of circadian rhythms, such as the sleep-wake cycle, are central mechanisms in BSD.
Self-report evidence
Several studies have compared individuals with BSDs and controls on self-reported regularity of their social rhythms using the Social Rhythm Metric (SRM; Monk et al., 1990). The SRM assesses the frequency (number of days per week) with which activities (e.g., getting out of bed, eating dinner, first social contact) are performed and the degree of regularity (occurrence of the activity within 45 minutes of the average time) of these activities. Social rhythm regularity is defined as the number of activities that occur at least three days per week within 45 minutes of the average time. The SRM is moderately consistent over time and correlates with other indices of social rhythm regularity (see Grandin et al., 2006 for review). SRM studies indicate that consistent with the social/circadian rhythm disruption model, individuals with BSDs exhibit lower social rhythm regularity than do healthy controls (Ashman et al., 1999; Jones et al., 2005; Szuba et al., 1992). Even during the euthymic state and controlling for subsyndromal depressive and hypo/manic symptoms, Shen and colleagues (2008a) reported that individuals with bipolar II or cyclothymic disorders had lower social rhythm regularity on the SRM than did sex- and age-matched controls. Moreover, these individuals with BSDs continued to exhibit stable lower SRM regularity over one year of follow-up (Sylvia et al., 2009).
Personality (chronotype) evidence
Chronotype, also known as morningness/eveningness, is a personality/temperament relevant to circadian rhythms. Chronotype refers to an individual’s relative preference for engaging in activity in the morning versus the evening (Cavallera & Giudici, 2008). Individuals with a preference for evening activity tend to go to bed and wake up later, and their alertness and performance peaks later in the day. Individual differences in chronotype, as assessed with self-report questionnaires, correlate with physiological markers (e.g., melatonin, cortisol awakening response) of circadian phase (e.g., Bernert et al., 2006; Randler & Schaal, 2010), and the eveningness chronotype is thought to reflect phase-delayed circadian rhythms (Bullock et al., 2014).
Several studies indicate that individuals with BSDs exhibit greater evening chronotype than controls. For example, Mansour et al. (2005b) compared chronotypes among individuals with bipolar I disorder, schizophrenia, and controls and found that the bipolar I participants exhibited greater eveningness than the schizophrenia participants and controls exhibited, controlling for age. Rapid-cycling bipolar participants were most likely to exhibit an evening preference. Greater eveningness for bipolar participants compared to controls has been replicated by others (e.g., Ahn et al., 2008; Chung et al., 2012; Wood et al., 2009), but Ahn et al. (2008) did not obtain a chronotype difference between bipolar I and schizophrenia patients. Moreover, even when in remission, bipolar participants exhibited a greater preference for evening activities in comparison with controls (Boudebesse et al., 2013; Giglio et al., 2010). However, a limitation of these chronotype studies is that they didn’t control for the effects of medication.
Sleep and actigraphy evidence
There is a substantial literature pointing toward persistent sleep disruption, a key circadian component, among individuals with BSDs. Studies suggest that sleep disturbance is present throughout all phases of BSDs, including the euthymic period (Harvey, 2008). Sleep disturbance among individuals with BSDs has been observed via self-report as well as objective measures (e.g., polysomnography, actigraphy), although some discrepancies have been noted between subjective and objective assessments of sleep disruption. In the insomnia literature, individuals with insomnia have been observed to underestimate their total sleep time and overestimate the time it takes to fall asleep (e.g., Mercier et al., 2002); however, recent research into the validity of actigraphy in bipolar samples has found actigraphy to be an adequate tool for measuring sleep disturbance relative to self-report and polysomnography (Kaplan et al., 2012).
Studies utilizing actigraphy have yielded mixed findings regarding the nature of sleep among individuals with BSDs. Millar and associates (2004) found that individuals in the euthymic phase of BSD exhibited trends toward longer total sleep time, longer sleep onset latency, and less efficient sleep as well as significantly more variable sleep duration and night waking time relative to healthy control participants. Greater variability of time in bed also was observed via actigraphy in a prospective longitudinal study of sleep and mood in the euthymic phase (Gershon et al., 2012). A naturalistic examination of sleep among individuals in remission from affective episodes reported significantly greater sleep duration and fragmentation of sleep, as well as reduced sleep efficiency and daily activity relative to controls (Geoffroy et al., 2014). Harvey and colleagues (2005) conducted a similar analysis of sleep parameters but incorporated a comparison group of individuals with a diagnosis of primary insomnia. Individuals with BSDs had longer total sleep times as well as lower average daytime activity levels relative to both healthy controls and individuals with insomnia on actigraphy. Individuals with a bipolar phenotype who were medication free experienced more physical activity during sleep compared to a control group, but did not differ in other sleep indices as measured by actigraphy (Rock et al., 2014).
Not all studies utilizing actigraphy have yielded significant findings, however. Utilizing the same three-group design as that of the Harvey et al. (2005) report (e.g., insomnia, bipolar, and healthy sleeper groups), St-Amand et al. (2013) found no significant differences between bipolar participants and controls on any actigraphy measure. Compared to adolescents with attention-deficit/hyperactivity disorder and normal controls, adolescents with BSD likewise didn’t differ in sleep parameters via actigraphy despite subjective reports of sleep disturbance among bipolar participants (Mullin et al., 2011). Jones and colleagues (2005) similarly reported no differences in sleep parameters in euthymic bipolar participants and healthy controls; however, they did report less stable and more variable daily activity patterns among individuals with BSD relative to controls, and these activity patterns were significant predictors of group status. Similarly, Salvatore et al. (2008) reported abnormal activity rhythms, including phase advances, in recovered bipolar I patients compared with healthy controls. Given putative associations between daily social rhythms and circadian rhythms, these results are in line with reports of circadian instability among individuals with BSDs.
Self-reports of sleep disturbance have been observed more consistently among individuals with BSD. Harvey (2008) conducted a thorough review of the literature on sleep disturbance across phases of BSD that revealed a high percentage of self-reported sleep disturbances in depression and hypo/mania. Reduced need for sleep was the most frequently reported symptom in hypo/mania, ranging from 69% to 94% of participants across six studies. Sleep disturbance was much more varied in the depressive phase, with participants reporting initial, middle, and terminal insomnia, as well as global sleep disturbance and hypersomnia. Self-reports of sleep disturbance during the euthymic phase are likewise prevalent and include reports of increased sleep onset latency, poor sleep quality, and increased daytime disturbance as a consequence of poor sleep (Harvey et al., 2005; Millar et al., 2004).
Life events evidence
Research also suggests that individuals with BSDs may have an underlying sensitivity to life event-specific SRD. Boland and colleagues (2012) examined the impact of life events on SRD and sleep loss in a sample of individuals with BSDs versus sex-, race-, and age-matched healthy controls. They found that following identical intensity (high or low) and valence (negative or positive) life events, individuals with BSDs experienced significantly more SRD and sleep loss than healthy individuals did even when controlling for group differences in overall trait social rhythm regularity. This is consistent with the results of Saunders and colleagues (2013), who found stressful life events to be associated with self-reports of poor sleep quality among individuals with BSDs in the euthymic phase, but not in a comparison group of healthy controls. Thus, individuals with BSDs also may possess an inherent vulnerability to the social rhythm-disrupting effects of life events above and beyond an already lower baseline regularity of social rhythms.
Neurobiological and genetic evidence
Several hormones, neurotransmitters, and genes implicated in the generation and regulation of circadian rhythms and the sleep system are associated with BSD. The most direct measure of the circadian system is the hormone melatonin, which is released by the pineal gland. Melatonin has a time-keeping function in many mammals and appears to adjust the timing of circadian rhythm information transmitted from the SCN of the hypothalamus to entrain physiological rhythms (Lewy & Sack, 1989). Euthymic bipolar individuals exhibit lower melatonin levels and a later peak time for melatonin during the night relative to healthy controls (Nurnberger et al., 2000) and to unipolar depressed individuals (Robillard et al., 2013). Furthermore, light suppression of melatonin (percentage reduction in melatonin following light exposure) is greater in bipolar patients than in controls (Lewy et al., 1981). Low melatonin levels are associated with elevated cortisol secretion, another hormone implicated in sleep regulation (Wetterberg et al., 1979). Cortisol is a steroid hormone that plays an important role in the stress response. Photoreceptors in the eye communicate information about ambient light to the circadian clock in the SCN, which connects directly and indirectly with the paraventricular nucleus (PVN) of the hypothalamus, driving the eventual release of cortisol. Thus, there is an important coupling between circadian processes and stress hormones. Circadian rhythms of cortisol are altered in BSD (e.g., Jones, 2001), and abnormalities in cortisol secretion and regulation are considered to be a trait marker of bipolar disorder (Daban et al., 2005). In another study, bipolar patients in a manic episode exhibited higher cortisol levels during the night and an earlier nadir for plasma cortisol relative to healthy controls (Nurnberger et al., 2000).
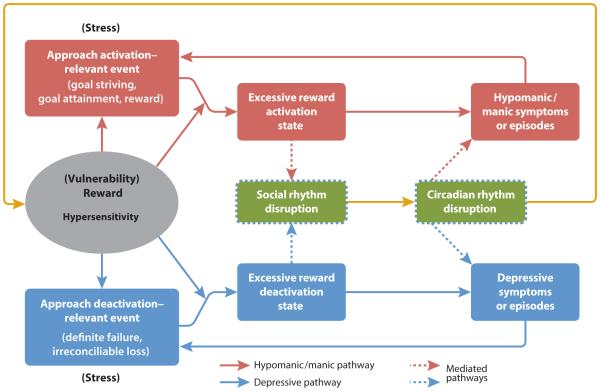
Two neurotransmitters that are critical in the link between biological rhythms and emotion are dopamine and serotonin. Dopamine, which is central to reward processing and the fronto-striatal reward circuit, plays a key role in the regulation of the sleep-wake cycle (Lima et al., 2008). Highlighting the important coupling between reward processes and sleep, dopaminergic neurons in fronto-striatal regions including the ventral tegmental area and the substantia nigra are implicated in REM (rapid eye movement) sleep (Dahan et al., 2007), and dopaminergic pathways have multiple interactions with the SCN (e.g., Murray et al., 2009). Sleep deprivation, a key symptom of hypo/mania, seems to activate limbic dopamine pathways as indexed by increased limbic blood flow, increased dopamine D2 receptor occupancy, and increased eye blink rates after total sleep deprivation (Ebert et al., 1996). Furthermore, as outlined in this article, there is considerable evidence that BSD is characterized by abnormalities in fronto-striatal neural circuitry and dopamine transmission more specifically [we discuss the relationship between reward processing, dopamine transmission, and circadian processes in BSD below in more detail in our integrated reward/circadian rhythm (RCR) dysregulation model]. Evidence for the involvement of serotonin in sleep includes the finding that serotonin turnover increases immediately after light exposure, and the highest concentrations of central nervous system serotonin are in the SCN and raphe nucleus (for a review, see Harvey, 2008). Considerable research indicates that serotonergic circuits are critical pathways in bipolar disorder (e.g., Oquendo et al., 2007).
Several genes known to be important in the generation and regulation of circadian rhythms and the sleep system are associated with bipolar disorder, including the circadian locomotor output cycles kaput (CLOCK) gene (Kripke et al., 2009; Shi et al., 2008). Other circadian genes for which some support exists include GSK3-β (Benedetti et al., 2003), PER3 (Nievergelt, et al., 2006), ARNTL (Nievergelt et al., 2006), and TIMELESS (Mansour et al., 2005a). In preclinical studies, mice with a mutation in the CLOCK gene display mania like behaviors (Roybal et al., 2007). These associations are modest, however, which is consistent with the notion that BSD is inherited polygenetically, with numerous genes adding small effects to overall risk (Murray & Harvey, 2010).
Is Social/Circadian Rhythm Disruption a Vulnerability for BSDs?
There are no truly prospective studies to date that examine whether social or circadian rhythm disruption predicts first onset of BSD and, thus, that strongly demonstrate that such disruption is a vulnerability for BSD. However, evidence relevant to whether social or circadian rhythm disruption is a vulnerability comes from studies in samples at genetic or behavioral risk for BSD. We review these studies in this section.
Self-report evidence
Three studies have examined social rhythm regularity in individuals at behavioral risk for BSDs. In a short-term diary study, Meyer & Maier (2006) compared participants at risk for BSD based on exhibiting hypomanic personality to those at risk for unipolar depression based on scoring high on rigidity and to controls scoring low on both risk measures on daily ratings of activities over 28 days. The group at risk for BSD reported lower regularity of their daily activities in comparison with the controls, whereas the group at risk for unipolar depression did not differ from the controls on activity regularity. Bullock et al. (2011) compared SRM scores among three groups of participants: bipolar disorder patients and two groups at high or low risk for BSD based on high versus low scores on the GBI (Depue et al., 1989), a demonstrated measure of risk for BSD (Alloy et al., 2008; Depue et al., 1989). The high-risk group exhibited lower social rhythm regularity than the low-risk group, but the bipolar group did not show lower regularity. Similarly, Shen et al. (2008b) reported that in a sample of undergraduates at risk for BSD based on exhibiting high GBI scores, lower SRM regularity scores were associated with higher depressive symptoms and greater across-day mood symptom lability over a two-week period. Moreover, increases in social rhythm regularity in response to an intervention to regularize daily activity schedules were associated with decreases in depressive symptoms and within-day symptom lability (Shen et al., 2008b). In contrast, a genetic risk study of the children of parents with bipolar disorder did not find differences in their social rhythm regularity compared to the age- and sex-matched children of healthy control parents (Jones et al., 2006).
Personality (chronotype) evidence
To our knowledge, only one study has been conducted that is relevant to whether evening chronotype is a vulnerability to BSD. Bullock and colleagues (2014) examined the association between chronotype as assessed by self-report questionnaire and vulnerability to BSD on the GBI. They found that high GBI depression scores, but not high GBI hypo/mania scores, were significantly associated with greater eveningness. In addition, given that chronotype has been posited to be heritable in the general population (e.g., Barclay et al., 2010), the association of evening chronotype with BSDs may suggest that it is a trait characteristic that could provide vulnerability to onset of BSDs.
Sleep and actigraphy evidence
There is evidence from high-risk samples to suggest that sleep and circadian disruption may be a vulnerability factor for BSDs (see Ng et al., in press for a review and meta-analysis). Ritter and colleagues (2012) examined subjective and objective sleep parameters in individuals with bipolar I and II disorder, individuals at high risk of developing bipolar disorder, and healthy controls. They found that both the bipolar and the high-risk groups reported more frequent and intense sleep disturbances, lifetime histories of insomnia and hypersomnia, as well as high levels of nonrestorative sleep compared to healthy controls. However, when observed via actigraphy, the high-risk group more closely resembled the healthy control group on measures of sleep disturbance. As noted previously, this difference in subjective versus objective assessments of sleep disturbance is not uncommon. However, Ankers & Jones (2009) did find actigraphic evidence of circadian rhythm disturbances in individuals at high risk for BSD compared to sex- and age-matched controls. The high-risk participants exhibited greater variability in duration, efficiency, and fragmentation of sleep, shorter sleep duration, and later and more variable bedtimes than controls, as well as lower relative amplitude of activity patterns. Similarly, Bullock & Murray (2014) reported that individuals at high risk for BSD based on high scores on the GBI exhibited a lower 24-hour activity rhythm amplitude (indicating a less stable activity pattern) during a week of actigraphic recording in comparison with a low-risk group. Moreover, the low activity rhythm amplitude was more strongly associated with GBI hypo/mania-proneness than depression proneness.
Children considered at high genetic risk for developing bipolar disorder also have been found to experience disturbed sleep relative to controls. Utilizing the Pittsburgh Sleep Quality Index, Jones et al. (2006) found trends toward greater sleep disturbance and poorer overall sleep among children of bipolar parents. Those high-risk children who were symptomatic demonstrated greater sleep disturbance relative to non-symptomatic high-risk children. Actigraphic measures of sleep demonstrated the reverse, however, suggesting that the sleep of the high-risk children was better than that of the control children. In light of these data, in addition to the Ritter et al. (2012) report, the trend toward a subjective experience of inadequate sleep despite objective assessments to the contrary seems to persist even in individuals who have yet to develop BSDs.
Life events evidence
No studies to date have examined whether SRD events predict first onset of BSD. However, one study found that controlling for baseline mood symptoms, individuals at demonstrated high risk for the development of first onset of BSD based on exhibiting high self-reported BAS sensitivity experienced more SRD events over prospective follow-up than did moderate-BAS-sensitivity participants at low risk for BSD (Boland et al., 2014). This latter finding is consistent with the idea that individuals vulnerable to BSD experience more SRD events, which, in turn, could contribute to bipolar mood episode onset.
Neurobiological and genetic evidence
Only a handful of studies have examined hormonal indices of CRD as a vulnerability for BSDs, and, to the best of our knowledge, no studies have directly examined neurotransmitter or genetic indices of such a vulnerability. Nurnberger and colleagues (1988a) reported that 15-25 year old offspring of individuals with bipolar disorder had increased suppression of melatonin compared to healthy controls, suggesting that sensitivity to melatonin suppression by light may reflect a preexisting vulnerability for BSD. Likewise, individuals with a family history of bipolar disorder, but who did not yet have the illness themselves, had higher salivary cortisol levels following awakening and during the day than offspring of parents with no psychiatric history (Ellenbogen et al., 2006). Future research on neurobiological/genetic indices of CRD as a vulnerability for BSDs is warranted.
Does Social/Circadian Rhythm Disruption Predict the Course of BSDs?
Self-report evidence
Two studies have directly investigated the role of social rhythm regularity as a predictor of the course of BSD. In a sample of euthymic individuals with bipolar II or cyclothymic disorders, controlling for family history of BSD and baseline subsyndromal depressive and hypo/manic symptoms, Shen et al. (2008a) found that lower SRM regularity scores at baseline predicted a greater likelihood and shorter time to recurrence of both major depressive and hypo/manic episodes over prospective follow-up. In addition, Sylvia et al. (2009) reported that lower SRM regularity scores predicted increases in depressive symptoms four months later in a sample of individuals with bipolar II or cyclothymia.
Intervention studies that attempt to stabilize social rhythms to affect bipolar mood symptoms or relapse rates are also relevant to understanding the course of BSD. Shen et al. (2008b) found that individuals with high GBI scores and subsyndromal symptoms at risk for BSD randomly assigned to an intervention to increase social rhythm regularity were successful at increasing the regularity of their daily activities compared to those in the control condition, but the increase in regularity did not predict changes in depressive or hypomanic symptoms; this finding is inconsistent with the social/circadian rhythm disruption model. However, consistent with the model, several randomized controlled trials of interpersonal and social rhythm therapy for BSDs, designed to specifically maintain social rhythm regularity and reduce potential life event triggers of social/circadian rhythm disruption, indicate that the therapy is efficacious in reducing relapse and improving symptoms and functioning of bipolar individuals (see Nusslock & Frank, 2012 for review).
Personality (chronotype) evidence
We know of only one study to date that examined whether chronotype relates to the course of BSD. Mansour et al. (2005b) found that greater evening chronotype was associated with an earlier age of onset and with longer-duration depressive episodes in a sample of bipolar I patients.
Sleep and actigraphy evidence
Sleep disturbance does affect the course of BSD (Boland & Alloy, 2013). Studies suggest that sleep remains disturbed throughout the inter-episode period of BSD and intensifies during the prodromal period just prior to episode onset. Bauer and colleagues (2006) examined the temporal relationship between sleep and mood changes among individuals with BSD and found that a decrease in sleep or bed rest preceded a mood shift toward hypo/mania the following day, whereas an increase in sleep or bed rest preceded mood shifts toward depression. Gershon et al. (2012) also found that longer sleep onset latency as assessed by actigraphy was coupled with higher negative affect among remitted bipolar I participants in comparison with healthy controls, and associations between decreased sleep and increase in hypo/manic symptoms have been observed elsewhere (e.g., Barbini et al., 1996). Moreover, therapeutically induced sleep deprivation has been shown to produce a shift from depression into hypo/mania (e.g., Bunney & Bunney, 2013; Colombo et al., 1999). Among individuals with co-occurring bipolar disorder and substance use diagnoses, poor sleep at baseline, as measured by the Pittsburgh Sleep Quality Index was associated with increased length of illness (Putnins et al., 2012). Pittsburgh Sleep Quality Index variables of sleep quality, sleep latency, and sleep duration significantly predicted worse mood symptoms during treatment and at follow-up, and a three-point increase in sleep symptoms at baseline was associated with a 60% greater likelihood of an additional week of illness.
Sleep disturbance also has been associated with worse illness history as well as greater risk for relapse. Eidelman and colleagues (2010) found that among 21 individuals with bipolar disorder in the euthymic phase, more variable sleep efficiency and more variable total wake times were associated with a greater number of past depressive episodes. Among 483 participants with BSDs in the euthymic phase, 15% (n= 72) of individuals who had histories of suicide attempts as well as hypomanic symptoms had more prevalent accounts of sleep disturbance (Sylvia et al., 2012). Moreover, survival analyses revealed a significant association between sleep disturbance and increased risk for mood episode recurrence.
Life events evidence
As posited by the social zeitgeber theory, life events are hypothesized to play a key role in the initiation or exacerbation of affective symptoms and episodes in BSDs by triggering a disruption in social rhythms and, by extension, circadian rhythms. Indeed, research has found significant relationships between life event-induced SRD and episode onset in BSDs, although the specificity of the effect of SRD on bipolar episodes is unclear. Malkoff-Schwartz and colleagues (1998) assessed the presence of severe events and SRD events in the 8-week period prior to episode onset as well as during a control period not related to episode onset. More SRD events were indicated in the pre-onset compared to the control period for all bipolar participants, and when participants were divided by episode polarity, a significant relationship between SRD events and episode onset was observed among manic participants. In a separate study, the authors included a unipolar depression comparison group to examine whether this effect would also be observed relative to other types of bipolar episodes (e.g., depressive, cycling) or in unipolar depression. The authors found that manic participants had more SRD events in the 8- and 20-week periods prior to onset than unipolar participants and cycling bipolar participants had: the authors also replicated the 1998 finding about greater numbers of SRD events in the pre-onset vs. control period (Malkoff-Schwartz et al., 2000). In contrast, Sylvia and colleagues (2009) found that among a sample of individuals with BSDs, the occurrence of SRD events significantly predicted prospective onset of depressive episode recurrences, but the events were inconsistent predictors of hypomanic episodes.
Neurobiological and genetic evidence
Much of the research on biological indices of social/circadian rhythm disruptions as predictors of BSD course comes from genetic studies. Variants in circadian genes have been associated with an earlier age at onset of BSD (PER3, GSK-3β) (e.g. Benedetti, et al., 2008a; Benedetti et al., 2004a), a higher recurrence of bipolar episodes (CLOCK) (Benedetti et al., 2003), delayed sleep onset (CLOCK; Benedetti et al., 2007), and changes in neural responses (CLOCK; Benedetti et al., 2008b). Circadian genes also have been implicated in the mechanisms through which biological treatments for bipolar disorder act. For example, biological treatments for BSD (e.g., lithium, valproic acid, antidepressants, electroconvulsive therapy) affect circadian function (e.g., Dallaspezia & Benedetti, 2009). Given evidence across species that lithium slows down circadian periodicity and can modify circadian cycle length (Abe et al., 2000), it may target dysregulated circadian rhythms. Indeed, in a case series of seven rapid-cycling bipolar patients, five exhibited a circadian rhythm that ran fast, and in these participants lithium slowed the rhythm (Kripke et al., 1978). GSK-3β, which plays an important role in the regulation of the molecular clock, codes for an enzyme that is a target for the action of lithium (Benedetti et al., 2004b), and variants of GSK-3β modulate the therapeutic response to a sleep deprivation treatment for bipolar depression (Benedetti et al., 2004b). Finally, preliminary evidence indicates that melatonin agonists help manage insomnia during mania (Bersani & Garavini, 2000).
An Integrated Reward/Circadian Rhythm Dysregulation Model
The reward hypersensitivity and social/circadian rhythm disruption models of BSD can be integrated in a novel way involving bidirectional associations between reward sensitivity and circadian dysregulation (see Figure 3). Here, we describe a new, integrated reward/circadian rhythm (RCR) dysregulation model of BSD and evidence relevant to this model.
Figure 3.
Reward Hypersensitivity and Reward-Relevant Life Events May Affect Social/Circadian Rhythm Disruption
In individuals with a hypersensitive reward system, BAS-activating (BAS-A) and BAS-deactivating (BAS-D) events lead to states of excessive reward activation and deactivation, respectively, which in turn are likely to disrupt social rhythms and thus circadian rhythms (green boxes in Figure 3). For example, when high-BAS or reward-hypersensitive individuals experience excessive reward activation in response to BAS-A events, they should exhibit excessively high appetitive motivation, goal striving, and response initiation (Alloy & Abramson, 2010; Berridge & Robinson, 2003; Johnson et al., 2012b), tendencies incongruent with maintaining regular social rhythms. Thus, they may work excessively long hours and neglect normal social routines (e.g., bedtime), which in turn may disrupt circadian rhythms and trigger mood symptoms (Nusslock et al., 2009). Similarly, in vulnerable reward-hypersensitive individuals, BAS-D events (e.g., failure) can lead to excessive reward deactivation involving decreased approach/response initiation and disregard of social routines. Indeed, increased and decreased goal striving is one of the most common prodromes of hypo/manic and depressive episodes, respectively (Lam & Wong, 1997; Lam et al., 2001).
Consistent with the integrative RCR model, adolescents at demonstrated risk for developing BSD based on exhibiting high self-reported BAS sensitivity (Alloy et al., 2012a) experienced significantly more SRD following the occurrence of both BAS-A and BAS-D events than did moderate BAS sensitivity adolescents at low risk for BSD (Boland et al., 2014). Thus, reward-hypersensitive adolescents were more vulnerable to SRD produced by BAS-A and BAS-D events. In addition, Boland et al. (2014) found that high-BAS-sensitivity adolescents generated greater numbers of BAS-A and BAS-D events than did moderate-BAS-sensitivity adolescents, thus increasing their risk for experiencing social rhythm disruption. Finally, Boland et al. (2014) also found that BAS-A and BAS-D events predicted prospective increases in hypomanic and depressive symptoms, respectively, in high-BAS adolescents more strongly than in moderate-BAS adolescents, and these prospective associations were mediated by SRD. Providing further support for the integrated RCR model, Alloy et al. (2014) found that among high-BAS-sensitivity adolescents, already shown to be at increased risk for first lifetime onset of BSD (Alloy et al., 2012a), those who also exhibited low social rhythm regularity on the SRM were significantly more likely to develop first onset of BSD over one year of follow-up, controlling for age, time in follow-up, initial depressive and hypo/manic symptoms, and family history of bipolar disorder. That is, the combination of reward hypersensitivity and low social rhythm regularity predicted first onset of BSD even better than did just reward hypersensitivity alone. However, no study has yet examined the SRD – CRD link and whether CRD further mediates the effects of reward hypersensitivity and reward relevant events on bipolar mood symptoms or episodes as predicted by the RCR model.
Circadian Rhythms May Influence Positive Affect and Reward Motivation
According to the RCR model, CRD in turn may affect levels of reward sensitivity and activation (dark yellow feedback path in Figure 3). Reward system activation may be influenced in part by timing information generated by the SCN, such that reward motivation is greatest when engagement with the environment is most likely to lead to rewards (Clark et al., 1989; Murray et al., 2009; Thayer et al., 1988), namely during daytime for diurnal species such as humans. Thus, reward sensitivity levels also may exhibit a circadian rhythm.
Consistent with this idea, animal studies show circadian influences on reward motivation (Murray et al., 2009), and there are associations between reward-related brain activity and both sleep variables (Holm et al., 2009) and circadian clock genes (Forbes et al., 2012). Indeed, clock genes are expressed in many brain areas involved in the reward system, such as the ventral tegmental area, nucleus accumbens, and amygdala (Albrecht, 2011), and dopaminergic reward circuits have multiple interactions with the SCN (Murray & Harvey, 2010; Murray et al., 2009; Sleipness et al., 2007; Yujnovsky et al., 2006). In addition, growing evidence in humans indicates that positive affect and subjective alertness (indicators of BAS/reward activation) exhibit a circadian rhythm and reach their zenith in the late afternoon, whereas negative affect does not show systematic daily variation (Clark et al., 1989; Hasler et al., 2008; 2010; Murray et al., 2002; 2009). Reaction times on a reward task also vary systematically with circadian phase (Murray et al., 2009).
The Integrated Reward/Circadian Rhythm Model’s Explanation of the Symptoms, Onset, and Course of Bipolar Spectrum Disorders
The research reviewed above that demonstrates influences of the reward and circadian systems on each other supports the RCR model. However, inasmuch as the RCR model is an integration of the reward hypersensitivity and social/circadian disruption theories, evidence that supports either the reward or social/circadian rhythm theory reviewed above also supports the RCR model. In addition, the RCR model makes use of mechanisms featured in both the reward and social/circadian rhythm models to explain the symptoms, age of onset, and course of BSDs.
With regard to the symptoms of BSD, we propose that there are specific clusters of symptoms that may be explained by either the reward or the social/circadian rhythm components of the RCR model. Based on theory and evidence from the reward model, hypersensitivity to reward can lead to excessive approach-related affect and motivation when individuals experience BAS-activating internal stimuli or external events. We predict that this excessive activation of the reward system should be associated with the occurrence of hypo/manic symptoms characterized specifically by psychomotor hyperactivation involving elevated energy, increased goal-directed activity, decreased need for sleep, increased self-confidence, and irritability when goal pursuit is thwarted. This prediction is based on evidence that both the BAS (Depue & Collins, 1999: Urošević et al., 2008) and the fronto-striatal neural circuit underlying reward processing (e.g., Haber & Knutson, 2010) are directly involved in generating incentive motivation and goal-pursuit emotions (i.e., wanting), rather than positive or hedonic mood per se (i.e., liking) (Berridge & Robinson, 2003). Reward processing also has not been directly implicated in cognitive activity (Johnson et al., 2012b), and thus hypo/manic symptoms of elation and expansiveness, as well as cognitive symptoms involving distractibility and flight of ideas, should be less related to reward sensitivity than are hypo/manic hyperactivation symptoms. Decreased need for sleep is included in the hyperactivation cluster given the coupling of reward processing with sleep variables, circadian influences, and circadian genes (see Murray et al., 2009 for review) as well as because hyperactivation interferes with sleep. Increased confidence is included in this cluster given that both elevated reward sensitivity and BSDs are linked with elevated confidence following goal attainment (Eisner et al., 2008). Irritability is included given that there is a neurophysiological overlap between anger and approach-related affect (Carver, 2004) and an increase in approach-related neural activity if goal pursuit is thwarted (Harmon-Jones & Sigelman, 2001). By contrast, in response to BAS-deactivating internal cues or external events, individuals with hypersensitive reward systems should experience excessive deactivation of approach motivation and affect, leading specifically to depressive symptoms of anhedonia, psychomotor retardation, hypersomnia, and low energy.
Similarly, based on theory and evidence from the social/circadian rhythm disruption model, either abnormalities in the circadian pacemaker (SCN) leading to circadian rhythm disturbances or disruptions of circadian rhythms brought about by SRD events should lead to specific bipolar symptoms of sleep disturbances, positive affect swings, changes in goal-directed behavior, and increases or decreases in concentration and cognitive functioning (Boland & Alloy, 2013; Grandin et al., 2006; Murray & Harvey, 2010). Given that the sleep-wake cycle is one of the body’s circadian rhythms, it follows that disruptions in circadian functioning would lead to the symptom of sleep disturbance (Murray & Harvey, 2010). In addition, the evidence reviewed above indicating that positive affect, reward-motivated behavior, and subjective alertness and concentration display circadian rhythms (e.g., Murray et al., 2009) suggests that circadian rhythm disturbances would be manifest in symptoms of high and low positive affect and increases and decreases in goal-directed behavior, concentration levels, and cognitive functioning (Boland & Alloy 2013).
The evidence reviewed above indicating that the reward and circadian systems interact with and bidirectionally influence one another may explain why the specific symptom profiles hypothesized to be produced by changes in reward system activation and by CRDs, respectively, can occur together in a given mood episode. For example, as discussed in our description of the RCR model, excessive reward activation or deactivation brought about by BAS-A and BAS-D events, respectively, can lead an individual to neglect normal daily activity routines, which, in turn, may lead to CRD. The severity and duration of symptoms experienced by an individual with a BSD should be a function of the extent and duration of reward system activation or deactivation and of circadian rhythm disturbances as well as the extent to which perturbations of one system in turn cause perturbations in the other system.
The reward hypersensitivity component of the RCR model is particularly relevant to an understanding of why the initial age of onset of BSDs is typically in adolescence. Adolescence is a developmental period involving heightened reward sensitivity. In early adolescence, neural circuitry implicated in reward processing (Haber & Knutson, 2010) undergoes rapid development, resulting in normative increases in sensitivity to and motivation for rewards (e.g., Steinberg, 2008; Urošević et al., 2012). Thus, those individuals who exhibit particularly sensitive reward systems may be the ones who are especially vulnerable to developing a first onset of BSD during adolescence, when normative increases in reward motivation occur. However, the social/circadian rhythm component of the RCR model also may be relevant to understanding the typical onset of BSD during adolescence. Adolescence is also a developmental period in which youth begin to have greater independence and more autonomous control over their lives. Prior to adolescence, children’s sleep and wake schedules, meal times, social contact times, etc. are more regimented and regularized by their parents and the school day. Thus, beginning in adolescence, as youth become more autonomous, they may be more exposed to SRD events and may generate through their own behavior more SRD events; therefore, adolescents should be more likely to experience circadian rhythm disturbances and the onset of BSD. Given that some cases of BSD have their onset in prepubertal childhood (see Youngstrom et al., 2008), it is possible that an abnormal circadian pacemaker (SCN) related to risk alleles on clock genes may lead to earlier occurrence of CRD in some vulnerable individuals and, in turn, bipolar symptoms.
BSD usually exhibits a recurrent course, with individuals experiencing multiple episodes of hypo/mania and depression in their lifetimes. According to the RCR model, a mood episode recurrence is likely to occur when individuals with hypersensitive reward systems experience either BAS-A or BAS-D events that lead to excessive reward activation or deactivation that, in turn, may trigger social/circadian rhythm disruption. In addition, SRD events, even if they are not BAS-A or BAS-D events, also may lead to social/circadian rhythm disruption directly, which, in turn, may perturb a hypersensitive reward system.
Some individuals with BSD have a worsening course and progress along the bipolar spectrum to a more severe diagnosis (e.g., from cyclothymia to bipolar II or I). In a previous section, we reviewed evidence that two indicators of a hypersensitive reward system (i.e., high self-reported BAS sensitivity and relative left frontal cortical asymmetry on EEG in the resting state) predicted progression to more severe diagnoses on the spectrum (Alloy et al., 2012b; Nusslock et al., 2012b). It is possible that through a kindling process of stress sensitization (Post, 1992; see Bender & Alloy, 2011 for review of kindling in BSD), individuals with a hypersensitive reward system who experience repeated milder mood episodes following exposure to major BAS-A and BAS-D events become sensitized to reward-relevant events, leading to more severe mood episodes in response to more minor BAS-A and BAS-D events. Weiss et al. (in press) obtained some support for such stress sensitization processes in a sample of individuals with bipolar II disorder. Stress sensitization to more minor BAS-A and BAS-D events also could lead to a rapid cycling course in some BSD individuals with hypersensitive reward systems. In the section titled Course above, we indicated that an earlier age of first onset also predicts a worse course and progression to a worse diagnosis on the spectrum (e.g., Alloy et al., 2012b; Birmaher et al., 2009), and homozygous carriers of the clock gene Per35 variant are more likely to have an early age of onset (Benedetti et al., 2008a). Thus, individuals with a genetic risk for abnormalities of the circadian pacemaker also may have an earlier onset of BSD and may be vulnerable to experiencing a worsening course, particularly in response to social rhythm disruptions.
Directions for Future Research
Although considerable support has accumulated for both the reward hypersensitivity and social/circadian rhythm disruption theories of BSD, there are many gaps in the evidence available for these models. Most of the existing literature only has demonstrated that the hypothesized risk factors or mechanisms in each model are associated with or correlates of BSD. Much less research, and in some cases, no research exists that directly tests whether the hypothesized risk factors and mechanisms are vulnerabilities for the initial development of BSD or are causal factors that affect the course of BSD. And, of course, the RCR model represents a new integration of the reward hypersensitivity and social/circadian rhythm disruption models. Although a growing body of evidence supports the bidirectional influence of the reward and circadian systems on each other, there is as yet only little direct evidence for the new RCR model. Consequently, here we highlight some important major directions for future research needed to more fully investigate the three models of BSD discussed herein.
For the reward hypersensitivity model of BSD, only one study exists that demonstrated that reward-relevant mechanisms (self-reported BAS sensitivity, ambitious goal striving, and high reward responsiveness on a behavioral task) are vulnerabilities to bipolar disorder and predict first lifetime onset of BSD (Alloy et al., 2012a). Thus, future research needs to further examine indicators of reward hypersensitivity, particularly neurophysiological, neuroimaging, or dopaminergic indicators of reward hypersensitivity, as well as BAS-A and BAS-D events as predictors of first onset of BSD. In addition, given that there is only one study demonstrating that a neurophysiological indicator of reward hypersensitivity predicts conversion to bipolar I disorder (Nusslock et al., 2012b), further work is also needed to test reward system measures as predictors of the course of BSD. Evidence also is needed to explicitly test the hypothesized link between exposure to BAS-A or BAS-D events and activation and deactivation of the reward system, respectively, as well as the further link of reward system activation and deactivation to specific symptom profiles and whether these links are stronger in individuals with indicators of hypersensitive reward systems.
A particular puzzle for the reward hypersensitivity model of BSD that needs to be resolved by future research is whether reward hypersensitivity or hyposensitivity is a vulnerability factor for bipolar depressive episodes. According to the BAS/reward model of BSD, a hypersensitive reward system provides vulnerability to bipolar depression when the system is deactivated too strongly by BAS-D events. The basis for this prediction is that reward hypersensitivity should make individuals hyperreactive to cues signaling the loss of reward as well as those signaling possible reward attainment. However, there is evidence that reward hyposensitivity is associated with unipolar depression (e.g., Treadway & Zald, 2011). Thus, further research is needed to determine whether hyper- or hypo-sensitivity of the reward system provides vulnerability to bipolar depression. In this regard, it may be crucial to distinguish between the trait-like sensitivity of the reward system (i.e., its vulnerability to being excessively activated and deactivated) and state activation levels of the system. Bipolar depression may be related to excessive hypo-activation of a hyper-sensitive reward system.
Similarly, most research testing components of the social/circadian rhythm disruption theory of BSD demonstrates an association between social/circadian rhythms and BSD. There are no prospective studies of social/circadian rhythm disruptions as predictors of first onset of BSD, and very few studies have examined circadian-relevant constructs such as social rhythm regularity, chronotype, and melatonin in individuals at demonstrated high risk for BSD (but see Alloy et al., 2014; Boland et al., 2014). Likewise, although SRD events have been found to predict the course of BSD, no studies have tested whether SRD events predict the initial onset of BSD. Moreover, relatively little research has examined circadian system indicators as predictors of the course of BSD. Consequently, much future research is needed to test whether social/circadian rhythm disruption and circadian system constructs do, in fact, provide vulnerability to BSD and causal mechanisms for the course of BSD. Furthermore, although a central hypothesis of the social/circadian rhythm model of BSD is that disruption of daily social routines triggers CRD in turn, as yet there is no direct evidence that SRD leads to CRD. Studies providing such evidence are clearly needed.
Finally, although we reviewed evidence for the effects of the reward system on the circadian system and vice versa, the integrated RCR theory of BSD presented here is new. More research is needed to examine the bidirectional influences of the reward and circadian systems on each other, particularly studies that explore potential behavioral, neural, and neurobiological mechanisms for their reciprocal interactions. In addition, only one study (Boland et al., 2014) has directly tested and supported the hypothesized links between reward hypersensitivity, reward-relevant life events, social rhythm disruption, and hypo/manic and depressive symptoms. Consequently, much additional research is needed to test and verify these hypothesized mechanisms of mood episodes in BSD. For example, future studies should examine whether SRD and CRD mediate the effects of reward sensitivity and BAS-A and BAS-D events on BSD symptoms in daily life. Similarly, studies might examine whether circadian phase affects reward sensitivity and activation and whether changes in social/circadian rhythms produced by reward-relevant events influence reward activation and BSD symptoms.
In conclusion, the reward hypersensitivity and social/circadian rhythm disruption theories of BSDs both have considerable empirical support and have contributed importantly to an understanding of bipolar disorders. Moreover, an integration of the reward and social/circadian rhythm models such as the RCR model proposed herein has great promise for more fully elucidating the mechanisms involved in the initial development and course of BSDs and, in turn, for suggesting more targeted and effective interventions for bipolar disorders. Further research designed to test these models of BSDs in the manner suggested here is likely to be fruitful and lead to important new discoveries regarding the nature of these puzzling conditions.
Acknowledgments
Preparation of this article was supported by National Institute of Mental Health Grants MH077908 and MH102310 to Lauren B. Alloy.
Footnotes
Disclosure Statement
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Most studies on relative left frontal EEG activity in unipolar depression and bipolar disorder utilize a difference score (ln(right) – ln(left) alpha power) to conveniently summarize the relative activity at homologous right and left electrodes. The difference score, commonly referred to as an asymmetry index, provides a simple unidimensional scale representing the relative activity of the right and left hemisphere. Given that alpha power is inversely related to cortical activity, higher scores on this difference score reflect relatively greater left hemispheric activity. A limitation of this difference score, however, is that it makes it difficult to identify the neuronal source underlying frontal EEG asymmetry. That is, elevated relative left frontal EEG activity could be driven by elevated neuronal activation in the left prefrontal cortex, or decreased activation in the right prefrontal cortex, or both. This issue, combined with the poor spatial resolution associated with EEG data more generally, makes it difficult to draw inferences about the pathophysiological processes underlying abnormally increased or decreased relative left frontal EEG activity. Future research using source localization or equivalent techniques is warranted to more precisely identify the neuronal source underlying frontal EEG asymmetry in mood disorders.
Contributor Information
Lauren B. Alloy, Temple University
Robin Nusslock, Northwestern University
Elaine M. Boland, Temple University
Literature Cited
- Abe M, Herzog ED, Block GD. Lithium lengthens the circadian period of individual suprachiasmatic nucleus neurons. Neuroreport. 2000;11:3261–3264. doi: 10.1097/00001756-200009280-00042. [DOI] [PubMed] [Google Scholar]
- Abler B, Greenhouse I, Ongur D, Walter H, Heckers S. Abnormal reward system activation in mania. Neuropsychopharm. 2008;33:2217–2227. doi: 10.1038/sj.npp.1301620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn YM, Chang J, Joo YH, Kim SC, Lee KY, Kim YS. Chronotype distribution in bipolar I disorder and schizophrenia in a Korean sample. Bipolar Disord. 2008;10:271–275. doi: 10.1111/j.1399-5618.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- Akiskal HS, Djenderedjian AH, Rosenthal RH, Khani MK. Cyclothymic disorder: Validating criteria for inclusion in the bipolar affective group. Am J Psychiatry. 1977;134:1227–1233. doi: 10.1176/ajp.134.11.1227. [DOI] [PubMed] [Google Scholar]
- Albrecht U. The circadian clock, reward, and memory. Front. Molec. Neurosci. 2011;4:41. doi: 10.3389/fnmol.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY. The role of the behavioral approach system (BAS) in bipolar spectrum disorders. Curr. Direc. Psych. Sci. 2010;19:189–194. doi: 10.1177/0963721410370292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Urošević S, Bender RE, Wagner CA. Longitudinal predictors of bipolar spectrum disorders: A behavioral approach system (BAS) perspective. Clin. Psychol. Sci. Prac. 2009a;16:206–226. doi: 10.1111/j.1468-2850.2009.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Urošević S, Walshaw PD, Nusslock R, et al. The psychosocial context of bipolar disorder: Environmental, cognitive, and developmental risk factors. Clin. Psychol. Rev. 2005;25:1043–1075. doi: 10.1016/j.cpr.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Grandin LD, et al. Behavioral approach system and behavioral inhibition system sensitivities: Prospective prediction of bipolar mood episodes. Bipolar Disord. 2008;10:310–322. doi: 10.1111/j.1399-5618.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Smith J, et al. Behavioral approach system (BAS) sensitivity and bipolar spectrum disorders: A retrospective and concurrent behavioral high-risk design. Motiv. Emot. 2006a;30:143–155. [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Gerstein RK, Keyser JD, et al. Behavioral approach system (BAS) – relevant cognitive styles and bipolar spectrum disorders: Concurrent and prospective associations. J. Abnorm. Psychol. 2009b;118:459–471. doi: 10.1037/a0016604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Keyser J, Gerstein RK. A cognitive vulnerability-stress perspective on bipolar spectrum disorders in a normative adolescent brain, cognitive, and emotional development context. Dev. Psychopathol. 2006b;18:1055–1103. doi: 10.1017/S0954579406060524. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Bender RE, Wagner CA, Whitehouse WG, Abramson LY, et al. Bipolar spectrum – substance use co-occurrence: Behavioral approach system (BAS) sensitivity and impulsiveness as shared personality vulnerabilities. J. Personal. Soc. Psychol. 2009c;97:549–565. doi: 10.1037/a0016061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Bender RE, Whitehouse WG, Wagner CA, Liu RT, et al. High behavioral approach system (BAS) sensitivity, reward responsiveness, and goal-striving predict first onset of bipolar spectrum disorders: A prospective behavioral high-risk design. J. Abnorm. Psychol. 2012a;121:399–351. doi: 10.1037/a0025877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Boland EM, Ng T, Whitehouse WG, Abramson LY. Low social rhythm regularity predicts first onset of bipolar spectrum disorder among individuals with high reward sensitivity. 2014 doi: 10.1037/abn0000107. Manuscript in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Urošević S, Abramson LY, Jager-Hyman S, Nusslock R, et al. Progression along the bipolar spectrum: A longitudinal study of predictors of conversion from bipolar spectrum conditions to bipolar I and II disorders. J. Abnorm. Psychol. 2012b;121:16–27. doi: 10.1037/a0023973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th American Psychiatric Association Press; Arlington, VA: 2013. [Google Scholar]
- Ankers D, Jones SH. Objective assessment of circadian activity and sleep patterns in individuals at behavioral risk of hypomania. J. Clin. Psychol. 2009;65:1071–1086. doi: 10.1002/jclp.20608. [DOI] [PubMed] [Google Scholar]
- Ashman SB, Monk TH, Kupfer DJ, Clark CN, Myers FS, et al. Relationship between social rhythms and mood in patients with rapid cycling bipolar disorder. Psychiatr. Res. 1999;86:1–8. doi: 10.1016/s0165-1781(99)00019-0. [DOI] [PubMed] [Google Scholar]
- Barbini B, Bertelli S, Colombo C, Smeraldi E. Sleep loss, a possible factor in augmenting manic episode. Psychiatr. Res. 1996;65:121–125. doi: 10.1016/s0165-1781(96)02909-5. [DOI] [PubMed] [Google Scholar]
- Barclay NL, Eley TC, Buysse DJ, Archer SN, Gregory AM. Diurnal preference and sleep quality: Same genes? A study of young adult twins. Chronobiol. Int. 2010;27:278–296. doi: 10.3109/07420521003663801. [DOI] [PubMed] [Google Scholar]
- Bauer M, Grof P, Rasgon N, Bschor T, Glenn T, Whybrow P. Temporal relation between sleep and mood in patients with bipolar disorder. Bipolar Disord. 2006;8:160–167. doi: 10.1111/j.1399-5618.2006.00294.x. [DOI] [PubMed] [Google Scholar]
- Bellivier F, Golmard J, Henry C, Leboyer M, Schurhoff F. Admixture analysis of age at onset in bipolar I affective disorder. Arch. Gen. Psychiatry. 2001;58:510–512. doi: 10.1001/archpsyc.58.5.510. [DOI] [PubMed] [Google Scholar]
- Bender RE, Alloy LB. Life stress and kindling in bipolar disorder: Review of the evidence and integration with emerging biopsychosocial theories. Clin. Psychol. Rev. 2011;31:383–398. doi: 10.1016/j.cpr.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Bernasconi A, Lorenzi C, Pontigga A, Serretti A, et al. A single nucleotide polymorphism in glycogen synthase kinase 3-β promoter gene influences onset of illness in patients affected by bipolar disorder. Neurosci. Letters. 2004a;355:37–40. doi: 10.1016/j.neulet.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Dallaspezia S, Colombo C, Pirovano A, Marino E, Smeraldi E. A length polymorphism in the circadian clock gene Per3 influences age at onset of bipolar disorder. Neurosci. Letters. 2008a;445:184–187. doi: 10.1016/j.neulet.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Serretti A, Colombo C, Lorenzi C, Tubazio V, Smeraldi E. A glycogen synthase kinase 3-beta promoter gene single nucleotide polymorphism is associated with age at onset and response to total sleep deprivation in bipolar depression. Neurosci. Letters. 2004b;368:123–126. doi: 10.1016/j.neulet.2004.06.050. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Serretti A, Colombo C, Barbini B, Lorenzi C, et al. Influence of CLOCK gene polymorphism on circadian mood fluctuation and illness recurrence in bipolar depression. Am. J. Med. Genetics. 2003;123:23–26. doi: 10.1002/ajmg.b.20038. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Dallaspezia S, Fulgosi MC, Lorenzi, Serretti A, et al. Actimetric evidence that CLOCK 3111 T ⁄C SNP influences sleep and activity patterns in patients affected by bipolar depression. Am. J. Med. Genetics. 2007;144B:631–635. doi: 10.1002/ajmg.b.30475. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Radaelli D, Bernasconi A, Dallaspezia S, Falini A, et al. Clock genes beyond the clock: CLOCK genotype biases neural correlates of moral valence decision in depressed patients. Genes Brain Behav. 2008b;7:20–25. doi: 10.1111/j.1601-183X.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- Berk M, Dodd S, Callaly P, Berk L, Fitzgerald P, et al. History of illness prior to a diagnosis of bipolar disorder or schizoaffective disorder. J. Affect. Disord. 2007a;103:181–186. doi: 10.1016/j.jad.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Berk M, Dodd S, Kauer-Saint’Anna M, Malhi GS, Bourin M, et al. Dopamine dysregulation syndrome: Implications for a dopamine hypothesis of bipolar disorder. Acta Psychiatr. Scand. 2007b;116:41–49. doi: 10.1111/j.1600-0447.2007.01058.x. [DOI] [PubMed] [Google Scholar]
- Bermpohl F, Kahnt T, Dalanay U, Hagele C, Sajonz B, et al. Altered representation of expected value in the orbitofrontal cortex in mania. Human Brain Map. 2010;31:958–969. doi: 10.1002/hbm.20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernert RA, Hasler BP, Cromer KR, Joiner TE. Diurnal preferences and circadian phase: A meta-analysis. Sleep. 2006;29:A54–A55. [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Bersani G, Garavini A. Melatonin add-on in manic patients with treatment resistant insomnia. Prog. Neuro-Psychopharm. Biol. Psychiatry. 2000;24:186–191. doi: 10.1016/s0278-5846(99)00097-4. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Goldstein B, Strober M, Gill MK, et al. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: The course and outcome of bipolar youth (COBY) study. Am. J. Psychiatry. 2009;166:795–804. doi: 10.1176/appi.ajp.2009.08101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg HP, Stern E, Martinez D, Ricketts S, Asis J, et al. Increased anterior cingulate and caudate activity in bipolar mania. Biol. Psychiatry. 2000;48:1045–1052. doi: 10.1016/s0006-3223(00)00962-8. [DOI] [PubMed] [Google Scholar]
- Boland EM, Alloy LB. Sleep disturbance and cognitive deficits in bipolar disorder: Toward an integrated examination of disorder maintenance and functional impairment. Clin. Psychol. Rev. 2013;33:33–44. doi: 10.1016/j.cpr.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland EM, Bender RE, Alloy LB, Conner BT, LaBelle DR, Abramson LY. Life events and social rhythms in bipolar spectrum disorders: An examination of social rhythm sensitivity. J. Affec. Disord. 2012;139:264–272. doi: 10.1016/j.jad.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland EM, Stange JS, LaBelle DR, Shapero BG, Weiss RB, et al. Affective disruption from social rhythm and behavioral approach system (BAS) sensitivities: A test of an integration of the social zeitgeber and reward theories of bipolar disorder. 2014 doi: 10.1177/2167702615603368. Manuscript under editorial review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudebesse C, Lajnet M, Geoffroy PA, Bellivier F, Nieto I, et al. Chronotypes of bipolar patients in remission: Validation of the French version of the Circadian Type Inventory in the FACE-BD sample. Chronobiol. Int. 2013;30:1042–1049. doi: 10.3109/07420528.2013.798330. [DOI] [PubMed] [Google Scholar]
- Brooks JO, Wang PW, Bonner JC, Rosen AC, Hoblyn JC, et al. Decreased prefrontal, anterior cingulate, insula, and ventral striatal metabolism in medication-free depressed outpatients with bipolar disorder. J. Psychiatr. Res. 2009;43:181–188. doi: 10.1016/j.jpsychires.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock B, Corlass-Brown J, Murray G. Eveningness and seasonality are associated with the bipolar vulnerability trait. J. Psychopathol. Behav. Assess. 2014;36(3):443–51. [Google Scholar]
- Bullock B, Judd F, Murray G. Social rhythms and vulnerability to bipolar disorder. J. Affec. Disord. 2011;135:384–388. doi: 10.1016/j.jad.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Bullock B, Murray G. Reduced amplitude of the 24 hour activity rhythm: A biomarker of vulnerability to bipolar disorder? Clin. Psychol. Sci. 2014;2:86–96. [Google Scholar]
- Bunney BG, Bunney WE. Mechanisms of rapid antidepressant effects of sleep deprivation therapy: Clock genes and circadian rhythms. Biol. Psychiatry. 2013;73:1164–1171. doi: 10.1016/j.biopsych.2012.07.020. [DOI] [PubMed] [Google Scholar]
- Carver CS. Negative affect deriving from the behavioral approach system. Emotion. 2004;4:3–22. doi: 10.1037/1528-3542.4.1.3. [DOI] [PubMed] [Google Scholar]
- Carver CS, Johnson SL. Tendencies toward mania and tendencies toward depression have distinct motivational, affective, and cognitive correlates. Cogn. Ther. Res. 2009;33:552–569. doi: 10.1007/s10608-008-9213-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caseras X, Lawrence NS, Murphy K, Wise RG, Phillips ML. Ventral striatum activity in response to reward: Differences between bipolar I and bipolar II disorders. Am. J. Psychiatry. 2013;17:533–541. doi: 10.1176/appi.ajp.2012.12020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallera GM, Giudici S. Morningness and eveningness personality: A survey in literature from 1995 up till 2006. Personal. Individ. Differ. 2008;44:3–21. [Google Scholar]
- Chang KD, Blasey CM, Ketter TA, Steiner H. Temperament characteristics of child and adolescent bipolar offspring. J. Affec. Disord. 2003;77:11–19. doi: 10.1016/s0165-0327(02)00105-2. [DOI] [PubMed] [Google Scholar]
- Chase H, Nusslock R, Almeida JRC, Forbes EE, LaBarbara EJ, Phillips ML. Dissociable patterns of abnormal frontal cortical activation during anticipation of an uncertain reward or loss in bipolar versus major depression. Bipolar Disord. 2013;15:839–854. doi: 10.1111/bdi.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaroni P, Hantouche EG, Gouvernet J, Azorin JM, Akiskal HS. The cyclothymic temperament in healthy controls and familially at risk individuals for mood disorder: Endophenotype for genetic studies? J. Affec. Disord. 2005;85:135–145. doi: 10.1016/j.jad.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Chung JK, Lee KY, Kim SH, Kim E, Jeong SH, et al. Circadian rhythm characteristics in mood disorders: Comparison among bipolar I disorder, bipolar II disorder, and recurrent major depressive disorder. Clin. Psychopharm. Neurosci. 2012;10:110–116. doi: 10.9758/cpn.2012.10.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LA, Watson D, Leeka J. Diurnal variation in the positive affects. Motiv. Emot. 1989;13:205–234. [Google Scholar]
- Coan JA, Allen JJB. Frontal EEG asymmetry as a moderator and mediator of emotion. Biol. Psychol. 2004;67:7–49. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Ranganath C. Reinforcement learning signals predict future decisions. J. Neurosci. 2007;27:371–378. doi: 10.1523/JNEUROSCI.4421-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo C, Benedetti F, Barbini B, Campori E, Smeraldi E. Rate of switch from depression into mania after therapeutic sleep deprivation in bipolar depression. Psychiatr. Res. 1999;86:267–270. doi: 10.1016/s0165-1781(99)00036-0. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Gooley JJ. Sleep and circadian rhythms in humans. Cold Spring Harbor Symp. Quant. Biol. 2007;72:579–597. doi: 10.1101/sqb.2007.72.064. [DOI] [PubMed] [Google Scholar]
- Daban C, Vieta E, Mackin P, Young AH. Hypothalamic-pituitary-adrenal axis and bipolar disorder. Psychiatr. Clin. North Am. 2005;28:469–480. doi: 10.1016/j.psc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Dahan L, Astier B, Vautrelle N, Urbain N, Kocsis B, Chouvet G. Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharm. 2007;32:1232–1241. doi: 10.1038/sj.npp.1301251. [DOI] [PubMed] [Google Scholar]
- Dallaspezia S, Benedetti F. Melatonin, circadian rhythms, and the clock genes in bipolar disorder. Curr. Psychiatr. Rep. 2009;11:488–493. doi: 10.1007/s11920-009-0074-1. [DOI] [PubMed] [Google Scholar]
- DelBello MP, Hanseman D, Adler CM, Fleck DE, Strakowski SM. Twelve-month outcome of adolescents with bipolar disorder following first hospitalization for a manic or mixed episode. Am. .J Psychiatry. 2007;164:582–590. doi: 10.1176/ajp.2007.164.4.582. [DOI] [PubMed] [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behav. Brain Sci. 1999;22:491–517. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- Depue RA, Iacono WG. Neurobehavioral aspects of affective disorders. Ann. Rev. Psychol. 1989;40:457–492. doi: 10.1146/annurev.ps.40.020189.002325. [DOI] [PubMed] [Google Scholar]
- Depue RA, Krauss S, Spoont MR, Arbisi P. General Behavior Inventory identification of unipolar and bipolar affective conditions in a nonclinical university population. J. Abnorm. Psychol. 1989;98:117–126. doi: 10.1037//0021-843x.98.2.117. [DOI] [PubMed] [Google Scholar]
- Ebert D, Albert R, Hammon G, Strasser B, May A, Merz A. Eyeblink rates and depression: Is the antidepressant effect of sleep deprivation mediated by the dopamine system? Neuropsychopharm. 1996;15:332–339. doi: 10.1016/0893-133X(95)00237-8. [DOI] [PubMed] [Google Scholar]
- Eckblad M, Chapman LJ. Development and validation of a scale for hypomanic personality. J. Abnorm. Psychol. 1986;95:214–222. doi: 10.1037//0021-843x.95.3.214. [DOI] [PubMed] [Google Scholar]
- Edge MD, Johnson SL, Ng T, Carver CS. Iowa gambling task perfomance in euthymic bipolar I disorder: A meta-analysis and empirical study. J. Affec. Disord. 2012;150:115–122. doi: 10.1016/j.jad.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Frank E, Kupfer DJ. Social zeitgebers and biological rhythms: A unified approach to understanding the etiology of depression. Arch. Gen. Psychiatry. 1988;45:948–952. doi: 10.1001/archpsyc.1988.01800340076012. [DOI] [PubMed] [Google Scholar]
- Eidelman P, Talbot LS, Gruber J, Hairston I, Harvey AG. Sleep architecture as correlate and predictor of symptoms and impairment in inter-episode bipolar disorder: Taking on the challenge of medication effects. J. Sleep Res. 2010;19:516–524. doi: 10.1111/j.1365-2869.2010.00826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner L, Johnson SL, Carver CS. Cognitive responses to failure and success relate uniquely to bipolar depression versus mania. J. Abnorm. Psychol. 2008;117:154–163. doi: 10.1037/0021-843X.117.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbogen MA, Hodgins S, Walker C-D, Adam S, Couture S. Daytime cortisol and stress reactivity in the offspring of parents with bipolar disorder. Psychoneuroendocrin. 2006;31:1164–1180. doi: 10.1016/j.psyneuen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Elliott R, Ogilvie A, Rubinsztein JS, Calderon G, Dolan RJ, Sahakian BJ. Abnormal ventral frontal response during performance of an affective go/no go task in patients with mania. Biol. Psychiatry. 2004;55:1163–1170. doi: 10.1016/j.biopsych.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE, Almeida JRC, Ferrell RE, Nimgaonkar VL, et al. PER2 rs2304672 polymorphism moderates circadian-relevant reward circuitry activity in adolescents. Biol. Psychiatry. 2012;71:451–457. doi: 10.1016/j.biopsych.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis-Raniere EL, Alloy LB, Abramson LY. Depressive personality styles and bipolar spectrum disorders: Prospective tests of the event congruency hypothesis. Bipolar Disord. 2006;8:382–399. doi: 10.1111/j.1399-5618.2006.00337.x. [DOI] [PubMed] [Google Scholar]
- Frey BN, Andreazza AC, Cereser KM, Martins MR, Valvasson SS, et al. Effects of mood stabilizers on hippocampus BDNF levels in an animal model of mania. Life Sci. 2006;79:281–286. doi: 10.1016/j.lfs.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Fulford D, Johnson SL, Llabre MM, Carver CS. Pushing and coasting in dynamic goal pursuit: Coasting is attenuated in bipolar disorder. Psychol. Sci. 2010;21:1021–1027. doi: 10.1177/0956797610373372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy PA, Boudebesse C, Bellivier F, Lainef M, Henry C, et al. Sleep in remitted bipolar disorder: A naturalistic case-control study using actigraphy. J. Afec.f Disord. 2014;158:1–7. doi: 10.1016/j.jad.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Gershon A, Thompson W, Eidelman P, McGlinchey EL, Kaplan KA, et al. Restless pillow, ruffled mind: Sleep and affect coupling in interepisode bipolar disorder. J. Abnorm. Psychol. 2012;121:863–873. doi: 10.1037/a0028233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giglio LMF, Magalhaes PVS, Andersen ML, Walz JC, Jakobson Kapczinski F. Circadian preference in bipolar disorder. Sleep Breath. 2010;14:153–155. doi: 10.1007/s11325-009-0301-3. [DOI] [PubMed] [Google Scholar]
- Goel N. Late-night presentation of an auditory stimulus phase delays human circadian rhythms. Am. J. Physiol. – Reg. Integrat. Compa.r Physiol. 2005;289:R209–R216. doi: 10.1152/ajpregu.00754.2004. [DOI] [PubMed] [Google Scholar]
- Grandin LD, Alloy LB, Abramson LY. The social zeitgeber theory, circadian rhythms, and mood disorders: Review and evaluation. Clin. Psychol. Rev. 2006;26:679–694. doi: 10.1016/j.cpr.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Gray JA. Three fundamental emotion systems. In: Eckman P, Davidson RJ, editors. The nature of emotion: Fundamental questions. Oxford University Press; New York: 1994. pp. 243–247. [Google Scholar]
- Gruber J, Gilbert KE, Youngstrom E, Youngstrom JK, Feeny N, Findling RJ. Reward dysregulation and mood symptoms in an adolescent outpatient sample. J. Abnorm. Child Psychol. 2013;41:1053–1065. doi: 10.1007/s10802-013-9746-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharm. Rev. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn T, Dresler T, Ehlis AC, Plichta MM, Heinzel S, et al. Neural response to reward anticipation is modulated by Gray’s impulsivity. Neuroimage. 2009;46:1148–1153. doi: 10.1016/j.neuroimage.2009.03.038. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF. It’s worse than you thought: The feedback negativity and violations of reward prediction in gambling tasks. Psychophysiol. 2007;44:905–912. doi: 10.1111/j.1469-8986.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- Hammen C. Generation of stress in the course of unipolar depression. J. Abnorm. Psychol. 1991;100:555–561. doi: 10.1037//0021-843x.100.4.555. [DOI] [PubMed] [Google Scholar]
- Hammen C, Ellicott A, Gitlin M. Stressors and sociotropy/autonomy: A longitudinal study of their relationship to the course of bipolar disorder. Cogn. Ther. Res. 1992;16:409–418. [Google Scholar]
- Hammen C, Ellicott A, Gitlin M, Jamison K. Sociotropy/autonomy and vulnerability to specific life events in patients with unipolar depression and bipolar disorders. J. Abnorm. Psychol. 1989;98:154–160. doi: 10.1037//0021-843x.98.2.154. [DOI] [PubMed] [Google Scholar]
- Harada M, Hoaki N, Tero T, Takeshi T, Hatano K, et al. Hyperthymic temperament and brightness judgment in healthy subjects: Involvement of left inferior orbitofrontal cortex. J. Affec. Disord. 2013;151:143–148. doi: 10.1016/j.jad.2013.05.066. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Abramson LY, Nusslock R, Sigelman JD, Urošević S, et al. Effect of bipolar disorder on left frontal cortical responses to goals differing in valence and task difficulty. Biol. Psychiatry. 2008;63:693–698. doi: 10.1016/j.biopsych.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Abramson L, Sigelman JD, Bohlig A, Hogan ME, Harmon-Jones C. Proneness to hypomania/mania symptoms or depression symptoms and asymmetrical frontal cortical responses to an anger-evoking event. J. Personal. Soc. Psychol. 2002;82:610–618. [PubMed] [Google Scholar]
- Harmon-Jones E, Sigelman JD. State anger and prefrontal brain activity: Evidence that insult-related relative left prefrontal activity is associated with experienced anger and aggression. J. Personal. Soc. Psychol. 2001;80:797–803. [PubMed] [Google Scholar]
- Harvey AG. Sleep and circadian rhythms in bipolar disorder: Seeking synchrony, harmony, and regulation. Am. J. Psychiatry. 2008;165:820–829. doi: 10.1176/appi.ajp.2008.08010098. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Schmidt DA, Scarna A, Semler CN, Goodwin GM. Sleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problems. Am. J. Psychiatry. 2005;162:50–57. doi: 10.1176/appi.ajp.162.1.50. [DOI] [PubMed] [Google Scholar]
- Hasler BP, Allen JJB, Sbarra DA, Bootzi RR, Bernert RA. Morningness-eveningness and depression: Preliminary evidence for the role of the behavioral activation system and positive affect. Psychiatr. Res. 2010;176:166–173. doi: 10.1016/j.psychres.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Mehl MR, Bootzin RR, Vazire S. Preliminary evidence of diurnal rhythms in everyday behaviors associated with positive affect. J. Res. Personal. 2008;42:1537–1546. [Google Scholar]
- Hassel S, Almeida JR, Kerr N, Nau S, Ladouceur CD, et al. Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: No associations with psychotropic medication load. Bipolar Disord. 2008;10:916–927. doi: 10.1111/j.1399-5618.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden EP, Bodkins M, Brenner C, Shekhar A, Nurnberger JI, et al. A multimethod investigation of the behavioral activation system in bipolar disorder. J. Abnorm. Psychol. 2008;117:164–170. doi: 10.1037/0021-843X.117.1.164. [DOI] [PubMed] [Google Scholar]
- Holm SM, Forbes EE, Ryan ND, Phillips ML, Tarr JA, Dahl RE. Reward-related brain function and sleep in pre/early pubertal and mid/late pubertal adolescents. J. Adolesc. Health. 2009;45:326–334. doi: 10.1016/j.jadohealth.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs D, Silverstone T. Dextroamphetamine-induced arousal in human subjects as a model for mania. Psychol. Med. 1986;16:323–329. doi: 10.1017/s0033291700009132. [DOI] [PubMed] [Google Scholar]
- Jackson A, Cavanaugh J, Scott J. A systematic review of manic and depressive prodromes. J. Affec. Disord. 2003;74:209–217. doi: 10.1016/s0165-0327(02)00266-5. [DOI] [PubMed] [Google Scholar]
- Johnson SL. Mania and dysregulation in goal pursuit: A review. Clin. Psychol. Rev. 2005;25:241–262. doi: 10.1016/j.cpr.2004.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Carver CS. Extreme goal setting and vulnerability to mania among undiagnosed young adults. Cogn. Ther. Res. 2006;30:377–395. doi: 10.1007/s10608-006-9044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Carver CS, Gotlib IH. Elevated ambitions for fame among individuals diagnosed with bipolar I disorder. J. Abnorm. Psychol. 2012a;121:602–609. doi: 10.1037/a0026370. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Cueller AK, Ruggero C, Winett-Perlman C, Goodnick P, et al. Life events as predictors of mania and depression in bipolar I disorder. J. Abnorm. Psychol. 2008;117:268–277. doi: 10.1037/0021-843X.117.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Edge MD, Holmes MK, Carver CS. The behavioral activation system and mania. Ann. Rev. Clin. Psychol. 2012b;8:243–267. doi: 10.1146/annurev-clinpsy-032511-143148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Eisner LR, Carver CS. Elevated expectancies among persons diagnosed with bipolar disorder. Br. J. Clin. Psychol. 2009;48:217–222. doi: 10.1348/014466509X414655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Ruggero C, Carver CS. Cognitive, behavioral and affective responses to reward: Links with hypomanic vulnerability. J. Soc. Clin. Psych.ol. 2005;24:894–906. [Google Scholar]
- Johnson SL, Sandrow D, Meyer B, Winters R, Miller I, et al. Increases in manic symptoms after life events involving goal attainment. J. Abnorm. Psychol. 2000;109:721–727. doi: 10.1037//0021-843x.109.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SH. Circadian rhythms, multilevel models of emotion and bipolar disorder: An initial step towards integration? Clin. Psychol. Rev. 2001;21:1193–1209. doi: 10.1016/s0272-7358(01)00111-8. [DOI] [PubMed] [Google Scholar]
- Jones SH, Hare DJ, Evershed K. Actigraphic assessment of circadian activity and sleep patterns in bipolar disorder. Bipolar Disord. 2005;7:1–11. doi: 10.1111/j.1399-5618.2005.00187.x. [DOI] [PubMed] [Google Scholar]
- Jones SH, Tai S, Evershed K, Knowles R, Bentall R. Early detection of bipolar disorder: A pilot familial high-risk study of parents with bipolar disorder and their adolescent children. Bipolar Disord. 2006;8:362–372. doi: 10.1111/j.1399-5618.2006.00329.x. [DOI] [PubMed] [Google Scholar]
- Kano K, Nakamura M, Matsuoka T, Iida H, Nakajima T. The topographical features of EEGs in patients with affective disorders. Electroenceph. Clin. Neurophys. 1992;83:124–129. doi: 10.1016/0013-4694(92)90025-d. [DOI] [PubMed] [Google Scholar]
- Kaplan KA, Talbot LS, Gruber J, Harvey AG. Evaluating sleep in bipolar disorder: comparison between actigraphy, polysomnography, and sleep diary. Bipolar Disord. 2012;14:870–879. doi: 10.1111/bdi.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasch KL, Rottenberg J, Arnow BA, Gotlib IH. Behavioral activation and inhibition systems and the severity and course of depression. J. Abnorm. Psychol. 2002;111:589–597. doi: 10.1037//0021-843x.111.4.589. [DOI] [PubMed] [Google Scholar]
- Ketter TA, Kimbrell TA, George MS, Dunn RT, Speer AM, et al. Effects of mood and subtype on cerebral glucose metablism in treatment-resistant bipolar disorder. Bio.l Psychiatry. 2001;49:97–109. doi: 10.1016/s0006-3223(00)00975-6. [DOI] [PubMed] [Google Scholar]
- Knowles R, Tai S, Christensen I, Bentall R. Coping with depression and vulnerability to mania: A factor analytic study of the Response Styles Questionnaire. Br. J. Clin. Psychol. 2005;44:99–112. doi: 10.1348/014466504X20062. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner J, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001a;1:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog. Neurobiol. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Mullaney DJ, Atkinson ML, Wolf S. Circadian rhythm disorders in manic depressives. Biol. Psychiatry. 1978;13:335–351. [PubMed] [Google Scholar]
- Kripke DF, Nievergelt CM, Joo E, Shekhtman T, Kelsoe JR. Circadian polymorphisms associated with affective disorders. J. Circad. Rhythms. 2009;7:2. doi: 10.1186/1740-3391-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapil TR, Miller MB, Zinser MC, Chapman LJ, Chapman J, Eckblad M. A longitudinal study of high scorers on the Hypomanic Personality Scale. J. Abnorm. Psychol. 2000;109:222–226. [PubMed] [Google Scholar]
- Lam D, Wong G. Prodromes, coping strategies, insight and social functioning in bipolar affective disorders. Psychol. Med. 1997;27:1091–1100. doi: 10.1017/s0033291797005540. [DOI] [PubMed] [Google Scholar]
- Lam D, Wong G, Sham P. Prodromes, coping strategies and course of illness in bipolar affective disorder—a naturalistic study. Psychol. Med. 2001;31:1397–1402. doi: 10.1017/s003329170100472x. [DOI] [PubMed] [Google Scholar]
- Lam D, Wright K, Smith N. Dysfunctional assumptions in bipolar disorder. J. Affec. Disord. 2004;79:193–199. doi: 10.1016/S0165-0327(02)00462-7. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol. Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Sack RL. The dim light melatonin onset as a marker for circadian phase position. Chronobiol. Int. 1989;6:93–102. doi: 10.3109/07420528909059144. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Wehr TA, Goodwin FK, Newson DA. Manic depressive patients may be supersensitive to light. Lancet. 1981;1:383–384. doi: 10.1016/s0140-6736(81)91697-4. [DOI] [PubMed] [Google Scholar]
- Lima MM, Andersen M, Reksidler AB, Silva A, Zager A, et al. Blockage of dopaminergic D(2) receptors produces decrease of REM but not of slow wave sleep in rats after REM sleep deprivation. Behav. Brain Res. 2008;188:406–411. doi: 10.1016/j.bbr.2007.11.025. [DOI] [PubMed] [Google Scholar]
- Lopez-Larson MP, DelBello MP, Zimmerman ME, Schwiers ML, Strakowski SM. Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biol. Psychiatry. 2002;52:93–100. doi: 10.1016/s0006-3223(02)01350-1. [DOI] [PubMed] [Google Scholar]
- Lozano BE, Johnson SL. Can personality traits predict increases in manic and depressive symptoms? J. Affec. Disord. 2001;63:103–111. doi: 10.1016/s0165-0327(00)00191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah L, Zarate CA, Singh J, Duan Y, Luckenbaugh DA, et al. Regional cerebral glucose metabolic abnormalities in bipolar II depression. Biol. Psychiatry. 2007;61:765–775. doi: 10.1016/j.biopsych.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Malkoff-Schwartz S, Frank E, Anderson BP, Hlastala SA, Luther JF, et al. Social rhythm disruption and stressful life events in the onset of bipolar and unipolar episodes. Psychol. Med. 2000;30:1005–1010. doi: 10.1017/s0033291799002706. [DOI] [PubMed] [Google Scholar]
- Malkoff-Schwartz S, Frank E, Anderson BP, Sherrill JT, Siegel L, et al. Stressful life events and social rhythm disruption in the onset of manic and depressive bipolar episodes: A preliminary investigation. Arch. Gen. Psychiatry. 1998;55:702–707. doi: 10.1001/archpsyc.55.8.702. [DOI] [PubMed] [Google Scholar]
- Mansour HA, Monk TH, Nimgaonkar VL. Circadian genes and bipolar disorder. Annals Med. 2005a;37:196–205. doi: 10.1080/07853890510007377. [DOI] [PubMed] [Google Scholar]
- Mansour HA, Wood J, Chowdari KV, Dayal M, Thase ME, et al. Circadian phase variation in bipolar I disorder. Chronobiol. Int. 2005b;22:571–584. doi: 10.1081/CBI-200062413. [DOI] [PubMed] [Google Scholar]
- Mason L, O’Sullivan N, Bentall RP, El-Deredy W. Better than I thought: Positive evaluation bias in hypomania. PLOS One. 2012;7:1–8. doi: 10.1371/journal.pone.0047754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacol. & Ther. 2007;114:222–232. doi: 10.1016/j.pharmthera.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald C, Bullmore ET, Sham PC, Chitnis X, Wickham H, et al. Association of genetic risks for schizophrenia and bipolar disorder with specific and generic brain structural endophenotypes. Arch. Gen. Psychiatry. 2004;61:974–984. doi: 10.1001/archpsyc.61.10.974. [DOI] [PubMed] [Google Scholar]
- Mercier JD, Bootzin RR, Lack LC. Insomniacs’ perception of wake instead of sleep. Sleep. 2002;25:559–566. [PubMed] [Google Scholar]
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RMA, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B, Beevers CG, Johnson SL. Unique association of approach motivation and mania vulnerability. Cogn. Emot. 2007;21:1647–1668. doi: 10.1080/02699930701252686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B, Johnson SL, Carver CS. Exploring behavioral activation and inhibition sensitivities among college students at risk for bipolar spectrum symptomatology. J. Psychopathol. Behav. Assess. 1999;21:275–292. doi: 10.1023/A:1022119414440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B, Johnson SL, Winters R. Responsiveness to threat and incentive in bipolar disorder: Relations of the BIS/BAS scales with symptoms. J. Psychopathol. Behav. Assess. 2001;23:133–143. doi: 10.1023/A:1010929402770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TD, Krumm-Merabet C. Academic performance and expectations for the future in relation to a vulnerability marker for bipolar disorders: The hypomanic temperament. Personal. Individ. Differ. 2003;35:785–796. [Google Scholar]
- Meyer TD, Maier S. Is there evidence for social rhythm instability in people at risk for affective disorders? Psychiatr. Res. 2006;141:103–114. doi: 10.1016/j.psychres.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Miklowitz DJ, Johnson SL. The psychopathology and treatment of bipolar disorder. Ann. Rev. Clin. Psychol. 2006;2:199–235. doi: 10.1146/annurev.clinpsy.2.022305.095332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar A, Espie CA, Scott J. The sleep of remitted bipolar outpatients: A controlled naturalistic study using actigraphy. J. Affec. Disord. 2004;80:145–153. doi: 10.1016/S0165-0327(03)00055-7. [DOI] [PubMed] [Google Scholar]
- Molnar GJ, Feeney MG, Fava GA. Duration and symptoms of bipolar prodromes. Am. J. Psychiatry. 1988;145:1576–1578. doi: 10.1176/ajp.145.12.1576. [DOI] [PubMed] [Google Scholar]
- Monk TH, Flaherty JF, Frank E, Hoskinson K, Kupfer DJ. The Social Rhythm Metric: An instrument to quantify the daily rhythms of life. J. Nerv. Ment. Dis. 1990;178:120–126. doi: 10.1097/00005053-199002000-00007. [DOI] [PubMed] [Google Scholar]
- Mullin BC, Harvey AG, Hinshaw SP. A preliminary study of sleep in adolescents with bipolar disorder, ADHD, and non-patient controls. Bipolar Disord. 2011;13:425–432. doi: 10.1111/j.1399-5618.2011.00933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray G, Allen NB, Trinder J. Mood and the circadian system: Investigation of a circadian component in positive affect. Chronobiol. Int. 2002;19:1151–1169. doi: 10.1081/cbi-120015956. [DOI] [PubMed] [Google Scholar]
- Murray G, Harvey A. Circadian rhythms and sleep in bipolar disorder. Bipolar Disord. 2010;12:459–472. doi: 10.1111/j.1399-5618.2010.00843.x. [DOI] [PubMed] [Google Scholar]
- Murray G, Nicholas CL, Kleiman J, Dwyer R, Carrington MJ, et al. Nature’s clock and human mood: The circadian system modulates reward motivation. Emotion. 2009;9:705–716. doi: 10.1037/a0017080. [DOI] [PubMed] [Google Scholar]
- Ng TH, Chung K, Yan-Yee Ho F, Yeung W, Yung K, Lam T. Sleep-wake disturbance in interepisode bipolar disorder and high-risk individuals: A systematic review and meta-analysis. Sleep Med. Rev. doi: 10.1016/j.smrv.2014.06.006. in press. [DOI] [PubMed] [Google Scholar]
- Nievergelt CM, Kripke DF, Barrett TB, Burg E, Remick RA, et al. Suggestive evidence for association of the circadian genes PERIOD3 and ARNTL with bipolar disorder. Am. J. Med. Genetics. 2006;141B:234–241. doi: 10.1002/ajmg.b.30252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberger J, Adkins S, Lahiri DK, Mayeda A, Hu K, et al. Melatonin suppression by light in euthymic bipolar and unipolar patients. Arch. Gen. Psychiatry. 2000;57:572–579. doi: 10.1001/archpsyc.57.6.572. [DOI] [PubMed] [Google Scholar]
- Nurnberger JL, Berrettini W, Tamarkin L, Hamovit J, Norton J, Gershon E. Supersensitivity to melatonin suppression by light in young people at high risk for affective disorder. A preliminary report. Neuropsychopharm. 1988a;1:217–223. doi: 10.1016/0893-133x(88)90020-6. [DOI] [PubMed] [Google Scholar]
- Nurnberger J, Hamovit J, Hibbs ED, Pelligrini D, Guroff JJ, et al. A high-risk study of primary affective disorder: Selection of subjects, initial assessment and 1- to 2-year follow-up. In: Dunner DL, Gershon E, Barrett JE, editors. Relatives at risk for mental disorder. Raven Press; New York: 1988b. pp. 161–177. [Google Scholar]
- Nusslock R, Abramson LY, Harmon-Jones E, Alloy LB, Coan JA. Psychosocial interventions for bipolar disorder: perspective from the behavioral approach system (BAS) dysregulation theory. Clin Psychol. Sci. Prac. 2009;16:449–469. doi: 10.1111/j.1468-2850.2009.01184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Abramson LY, Harmon-Jones E, Alloy LB, Hogan ME. A goal-striving life event and the onset of bipolar episodes: Perspective from the behavioral approach system (BAS) dysregulation theory. J. Abnorm. Psychol. 2007;116:105–115. doi: 10.1037/0021-843X.116.1.105. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Almeida JRC, Forbes EE, Versace A, LaBarbara EJ, et al. Waiting to win: Elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar adults. Bipolar Disord. 2012a;14:249–260. doi: 10.1111/j.1399-5618.2012.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Frank E. Subthreshold bipolarity: Diagnostic issues and challenges. Bipolar Disord. 2011;13:587–603. doi: 10.1111/j.1399-5618.2011.00957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Frank E. Interpersonal social rhythm therapy for bipolar disorder: An empirical review and case conceptualization. In: Markowitz JC, Weisman M, editors. Casebook of interpersonal psychotherapy. Oxford University Press; New York: 2012. pp. 103–121. [Google Scholar]
- Nusslock R, Harmon-Jones E, Alloy LB, Urošević S, Goldstein K, Abramson LY. Elevated left mid-frontal cortical activity prospectively predicts conversion to bipolar I disorder. J. Abnorm. Psychol. 2012b;121:592–601. doi: 10.1037/a0028973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo MA, Hastings RS, Huang YY, Simpson N, Ogden RT, et al. Brain serotonin transporter binding in depressed patients with bipolar disorder using positron emission tomography. Arch. Gen. Psychiatry. 2007;64:201–208. doi: 10.1001/archpsyc.64.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan N, Szczepanowski R, El-Deredy W, Mason L. fMRI evidence of a relationship between hypomania and both increased goal-sensitivity and positive outcome-expectancy bias. Neuropsychologia. 2011;49:2825–2835. doi: 10.1016/j.neuropsychologia.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Panda S, Hogenesch JB, Kay SA. Circadian rhythms from flies to humans. Nature. 2002;417:329–335. doi: 10.1038/417329a. [DOI] [PubMed] [Google Scholar]
- Post R. Transduction of psychosocial stress into the neurobiology of recurrent affective disorders. Am. J. Psychiatry. 1992;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- Putnins SI, Griffin ML, Fitzmaurice GM, Dodd DR, Weiss RD. Poor sleep at baseline predicts worse mood outcomes in patients with co-occurring bipolar disorder and substance dependence. J. Clin. Psychiatry. 2012;71:703–708. doi: 10.4088/JCP.11m07007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilty LC, Mackew L, Bagby RM. Distinct profiles of behavioral inhibition and activation system sensitivity in unipolar vs. bipolar mood disorders. Psychiatr. Res. 2014;219:228–231. doi: 10.1016/j.psychres.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Randler C, Schaal S. Morningness-eveningness, habitual sleep-wake variables, and cortisol level. Biol. Psychol. 2010;85:14–18. doi: 10.1016/j.biopsycho.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Wever DR. Molecular analysis of mammalian circadian rhythms. Ann. Rev. Physiol. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- Ritter PS, Marx C, Lewtschenko N, Pfeiffer S, Leopold K, et al. The characteristics of sleep in patients with manifest bipolar disorder, subjects at high risk of developing the disease and healthy controls. J. Neur Transm. 2012;119:1173–1184. doi: 10.1007/s00702-012-0883-y. [DOI] [PubMed] [Google Scholar]
- Robillard R, Naismith SL, Rogers NL, Scott EM, Ip TKC, et al. Sleep-wake cycle and melatonin rhythms in adolescents and young adults with mood disorders: Comparison of unipolar and bipolar phenotypes. Eur. Psychiatry. 2013;28:412–416. doi: 10.1016/j.eurpsy.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Rock P, Goodwin G, Harmer Wulff K. Daily rest-activity patterns in the bipolar phenotype: A controlled actigraphy study. Chronobiol. Int. 2014;31:290–296. doi: 10.3109/07420528.2013.843542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Merrow M. Entrainment of the human circadian clock. Cold Spring Harbor Symp. Quant. Biol. 2007;72:293–299. doi: 10.1101/sqb.2007.72.043. [DOI] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, et al. Mania-like behavior induced by disruption of CLOCK. Proc. Nat. Acad. Sci. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero C, Johnson SL. Reactivity to a laboratory stressor among individuals with bipolar I disorder in full or partial remission. J. Abnorm. Psychol. 2006;115:539–544. doi: 10.1037/0021-843X.115.3.539. [DOI] [PubMed] [Google Scholar]
- Salavert J, Caseras X, Torrubia R, Furest S, Arranz B, et al. The functioning of the behavioral activation and inhibition systems in bipolar I euthymic patients and its influence in subsequent episodes over an 18-month period. Personal. Individ. Differ. 2007;42:1323–1331. [Google Scholar]
- Salvatore P, Ghidini S, Zita G, De Panfills C, Lambertino S, et al. Circadian activity rhythm abnormalities in ill and recovered bipolar I disorder patients. Bipolar Disord. 2008;10:256–265. doi: 10.1111/j.1399-5618.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- Saunders EFH, Novick DM, Fernandez-Mendoza J, Masoud K, Ryan KA, et al. Sleep quality during euthymia in bipolar disorder: the role of clinical features, personality traits, and stressful life events. Int. J. Bipolar Disord. 2013;1:16. doi: 10.1186/2194-7511-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Scott J, Stanton B, Garland A, Ferrier IN. Cognitive vulnerability in patients with bipolar disorder. Psychol. Med. 2000;30:467–472. doi: 10.1017/s0033291799008879. [DOI] [PubMed] [Google Scholar]
- Shen GC, Alloy LB, Abramson LY, Sylvia LG. Social rhythm regularity and the onset of affective episodes in bipolar spectrum individuals. Bipolar Disord. 2008a;10:520–529. doi: 10.1111/j.1399-5618.2008.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen GC, Sylvia LG, Alloy LB, Barrett F, Kohner M, et al. Lifestyle regularity and cyclothymic symptomatology. J. Clin. Psychol. 2008b;64:482–500. doi: 10.1002/jclp.20440. [DOI] [PubMed] [Google Scholar]
- Shi J, Wittke-Thompson JK, Badner JA, Hattori E, Potash JB, et al. Clock genes may influence bipolar disorder susceptibility and dysfunctional circadian rhythm. Am. J. Med. Genetics. 2008;147B:1047–1055. doi: 10.1002/ajmg.b.30714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleipness EP, Sorg BA, Jansen HT. Diurnal differences in dopamine transporter and tyrosine hydroxylase levels in rat brain: Dependence on the suprachiasmatic nucleus. Brain Res. 2007;1129:34–42. doi: 10.1016/j.brainres.2006.10.063. [DOI] [PubMed] [Google Scholar]
- St-Amand J, Provencher MD, Belanger L, Morin CM. Sleep disturbances in bipolar disorder during remission. J. Affec. Disord. 2013;20:112–119. doi: 10.1016/j.jad.2012.05.057. [DOI] [PubMed] [Google Scholar]
- Stange JP, Molz AR, Black CL, Shapero BG, Bacelli JM, et al. Positive overgeneralization and behavioral approach system (BAS) sensitivity interact to predict prospective increases in hypomanic symptoms: A behavioral high-risk design. Behav. Res. Ther. 2012;50:231–239. doi: 10.1016/j.brat.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange JP, Shapero BG, Jager-Hyman SG, Grant DA, Abramson LY, Alloy LB. Behavioral approach system (BAS) – relevant cognitive styles in individuals with high vs. moderate BAS sensitivity: A behavioral high-risk design. Cogn. Ther. Res. 2013;37:139–149. doi: 10.1007/s10608-012-9443-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Dev. Rev. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler C, Dickerson SS, Miller GE. Uncoupling of social zeitgebers and diurnal cortisol secretion in clinical depression. Psychoneuroendocrinol. 2004;29:1250–1259. doi: 10.1016/j.psyneuen.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Zimmerman ME, Getz GE, Mills NP, et al. Ventricular and periventricularstructural volumes in first- versus multiple-episode bipolar disorder. Am. J. Psychiatry. 2002;15:1841–1847. doi: 10.1176/appi.ajp.159.11.1841. [DOI] [PubMed] [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Steinberg JL, Moeller FG. Severity of bipolar disorder is associated with impairment of response inhibition. J. Affec. Disord. 2009;116:30–36. doi: 10.1016/j.jad.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvia LG, Alloy LB, Hafner JA, Gauger MC, Verdon K, Abramson LY. Life events and social rhythms in bipolar spectrum disroders: A prospective study. Behav. Ther. 2009;40:131–141. doi: 10.1016/j.beth.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvia LG, Dupuy JM, Ostacher MJ, Cowperthwait CM, Hay AC, et al. Sleep disturbance in euthymic bipolar patients. J. Psychopharm. 2012;26:1108–1112. doi: 10.1177/0269881111421973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szuba MP, Yager A, Guze BH, Allen EM, Baxter LR. Disruption of social circadian rhythms in major depression: A preliminary report. Psychiatr. Res. 1992;42:221–230. doi: 10.1016/0165-1781(92)90114-i. [DOI] [PubMed] [Google Scholar]
- Thayer RE, Takahashi PJ, Pauli JA. Multidimensional arousal states, diurnal rhythms, cognitive and social processes, and extraversion. Personal. Individ. Differ. 1988;9:15–24. [Google Scholar]
- Thibodeau R, Jorgensen RS, Kim S. Depression, anxiety, and resting frontal EEG asymmetry: A meta-analytic review. J. Abnorm. Psychol. 2006;115:715–729. doi: 10.1037/0021-843X.115.4.715. [DOI] [PubMed] [Google Scholar]
- Tohen M, Goldberg JF, Gonzalez-Pinto Arrillaga AM, Azorin JM, Vieta E, et al. A 12-week, double-blind comparison of olanzapine vs. haloperidol in the treatment of acute mania. Arch. Gen. Psychiatry. 2003;60:1218–1226. doi: 10.1001/archpsyc.60.12.1218. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neurosci. Biobehav. Rev. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urošević S, Abramson LY, Alloy LB, Nusslock R, Harmon-Jones E, et al. Increased rates of events that activate or deactivate the behavioral approach system, but not events related to goal attainment, in bipolar spectrum disorders. J. Abnorm. Psychol. 2010;119:610–615. doi: 10.1037/a0019533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urošević S, Abramson LY, Harmon-Jones E, Alloy LB. Dysregulation of the behavioral approach system (BAS) in bipolar spectrum disorders: Review of theory and evidence. Clin. Psychol. Rev. 2008;28:1188–1205. doi: 10.1016/j.cpr.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urošević S, Collins P, Muetzel R, Lim K, Luciana M. Longitudinal changes in behavioral approach system sensitivity and brain structures involved in reward processing during adolescence. Dev. Psychol. 2012;48:1488–1500. doi: 10.1037/a0027502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag HM, Korf J. Central monoamine deficiency indepressions: causative of secondary phenomenon? Pharmakopsychiat Neuropsychopharm. 1975;8:322–326. doi: 10.1055/s-0028-1094463. [DOI] [PubMed] [Google Scholar]
- Vieta E, Ros S, Goikolea JM, Benabarre A, Popova E, et al. An open-label study of amisulpride in the treatment of mania. J. Clin. Psychiatry. 2005;66:575–578. doi: 10.4088/jcp.v66n0505. [DOI] [PubMed] [Google Scholar]
- Weiss RB, Stange JP, Boland EM, Black SK, LaBelle DR, et al. Kindling of life stress in bipolar disorder: Comparison of sensitization and autonomy models. J. Abnorm. Psychol. doi: 10.1037/abn0000014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, et al. Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996;276:293–299. [PubMed] [Google Scholar]
- Wetterberg L, Beck-Friis J, Aperiam B, Petterson U. Melatonin/cortisol ratio in depression. Lancet. 1979;2:1361. doi: 10.1016/s0140-6736(79)92837-x. [DOI] [PubMed] [Google Scholar]
- Wever RA. The circadian system of man: Results from experiments under temporal isolation. Springer Verlag; New York, NY: 1979. [Google Scholar]
- Wever RA. Light effects on human circadian rhythms: A review of recent Andechs experiments. J. Biol. Rhythms. 1989;4:161–185. [PubMed] [Google Scholar]
- Wood J, Birmaher B, Axelson D, Ehmann M, Kalas C, et al. Replicable differences in preferred circadian phase between bipolar disorder patients and control individuals. Psychiatr. Res. 2009;166:201–209. doi: 10.1016/j.psychres.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: Validity, phenomenology, and recommendations for diagnosis. Bipolar Disord. 2008;10:194–214. doi: 10.1111/j.1399-5618.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yujnovsky I, Hirayama J, Doi M, Borrelli E, Sassone-Corsi P. Signaling mediated by the dopamine D2 receptor potentiates circadian regulation by CLOCK: BMAL1. Proc. Nat. Acad. Sci. 2006;103:6386–6391. doi: 10.1073/pnas.0510691103. [DOI] [PMC free article] [PubMed] [Google Scholar]