Abstract
OBJECTIVE
Evaluate effects of a Lynch syndrome universal screening protocol in newly diagnosed endometrial cancers on subsequent genetic counseling (GC) and germline testing (GT) referral and acceptance rates.
METHODS
We performed a retrospective cohort study of women who underwent a hysterectomy for endometrial cancer at Barnes Jewish Hospital in St. Louis, MO between 1/1/2011 and 12/31/2013 (n=637). An immunohistochemistry-based (IHC) universal screening protocol for Lynch syndrome was initiated on 12/17/2012. The cohorts consisted of women presenting prior to (Pre Em-USP; n=395) and those presenting following (Em-USP; n=242) initiation of the universal screening protocol. GC and GT referrals were based on risk factors and/or IHC results. Comparisons were made using the Fisher’s exact test and the Kruskal-Wallis test.
RESULTS
A greater proportion of individuals in the Em-USP cohort underwent GT than in Pre Em-USP (9.1% vs 4.8%, p<0.05). Of individuals with an IHC screening result suggestive of LS, those within the Em-USP cohort were significantly more likely to accept GC compared to those in the Pre-Em-USP cohort (95% vs 64%, p=0.02). Specifically within the Em-USP cohort, patients referred to GC due to a concerning IHC screening result, versus those who were referred based on other risk factors, had a higher counseling acceptance rate (95% vs 61%, p=0.03) and underwent genetic testing more readily (76% vs 30%, p<0.001).
CONCLUSIONS
Implementation of an IHC-based universal screening protocol for LS in endometrial cancer leads to higher acceptance of genetic counseling and higher rates of genetic testing compared to referral based on risk factors alone.
Keywords: Endometrial cancer, Lynch syndrome, genetic counseling, genetic testing
Introduction
Lynch syndrome (LS), also known as hereditary non-polyposis colorectal cancer (HNPCC), is an autosomal dominant cancer syndrome, caused by inactivating germline mutations in one or more mismatch repair (MMR) genes (1). These genes behave as tumor suppressors and the most clinically relevant include MLH1, MSH2, MSH6, and PMS2. Women with LS are at an increased risk of developing colorectal, endometrial, ovarian, gastric, urinary tract and other cancers (2, 3). A mismatch repair defect in one of the four most commonly mutated genes confers a significantly increased lifetime risk of developing endometrial cancer and 2–5% of all patients with endometrial cancer are mutation carriers (4, 5). Since almost half of women with LS will present with endometrial cancer as their first malignancy (6), it is essential to identify these individuals in order to refer these women and their family members for proper cancer screening and prevention.
Screening for LS was traditionally based on family history using Amsterdam Criteria, initially developed primarily for individuals presenting with colorectal cancer (7–9). These methods have been found to have low sensitivity, particularly in endometrial cancer patients, and may miss a significant number of patients with a mismatch repair defect (4, 10). Patient derived histories are also fraught with errors arising from patients’ lack of knowledge or recall of family history and providers’ difficulty with eliciting a good family history (11, 12). Molecular screening of the tumors for presence of MMR proteins in the nuclei using immunohistochemistry (IHC) is an alternative method of screening with sensitivity ranging between 86–100% (13). IHC has been implemented as part of the universal screening protocols in colorectal cancers after a recommendation from the Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group suggested that it should be performed on all newly diagnosed colon cancer patients (14). While there is no consensus regarding what the optimal methods of LS screening should be for endometrial cancer patients, the Society of Gynecologic Oncology recommended selectively screening all new endometrial cancer patients younger than 60 years old for LS using IHC (15). However, there is a growing interest in implementation of IHC several as part of the screening protocols for LS in endometrial cancer patients (16–19).
Screening success should reflect not only detection rates of LS, but also whether the information is appropriately utilized in order to yield clinical relevance. One of the goals of obtaining a sensitive screening strategy is to be able to provide the appropriate counseling regarding genetic testing and subsequent cancer screening and prevention to patients and their families. This is best accomplished when access to a genetic counselor is easily available and when these counselors are involved in the referral process (20).
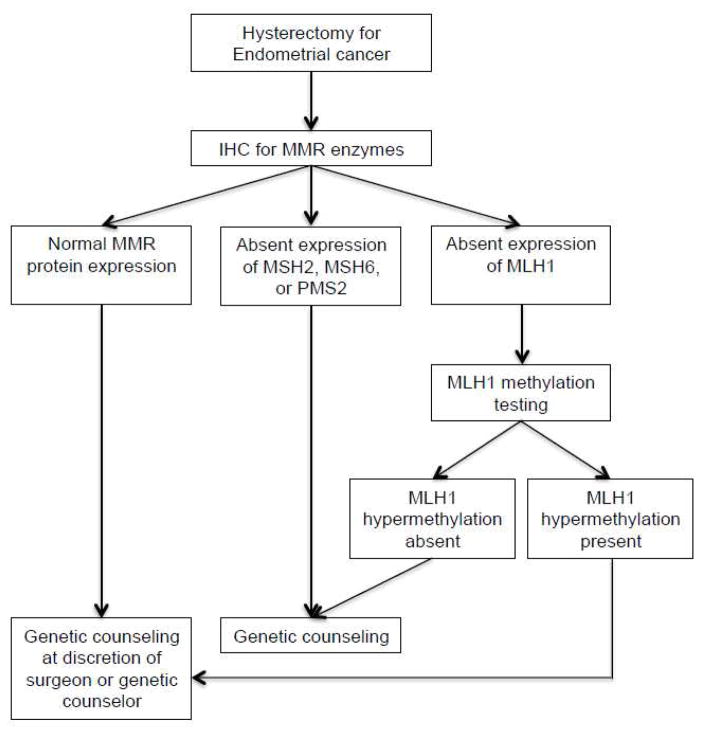
Our institution implemented a universal screening protocol (Em-USP) for all endometrial cancer patients undergoing hysterectomy (Figure 1). Prior to the Em-USP, IHC for MMR enzymes and/or genetic counseling referrals were initiated by the gynecologic oncology surgeon and genetic counselor after review of endometrial pathology and reported family or personal history of cancer. Here we report our experience with LS detection and genetic counseling referral prior to and following the Em-USP in order to compare the two different screening methods. Our primary objective was to determine whether rates of genetic counseling and genetic testing were affected by initiation of Em-USP.
Figure 1.
Endometrial cancer universal screening protocol.
IHC, Immunohistochemistry; MMR, Mismatch repair
Methods
We conducted a retrospective cohort study of all women who underwent a hysterectomy for endometrial cancer at Barnes Jewish Hospital in St. Louis Missouri between January 1, 2011 and December 31, 2013. Prior to initiation of the study, Institutional Review Board approval was obtained from the Human Research Protection Office at Washington University in St. Louis. Women included in the study had a new diagnosis of endometrial carcinoma. All histologies of endometrial carcinomas, endometrioid and non-endometrioid, were included. Patients with uterine sarcomas were excluded.
Prior to the implementation of Em-USP, cases were referred to IHC for MMR enzymes based on based on age of diagnosis, tumor histology, personal history of other LS-associated cancers or reported family history. All tumor pathology results were reviewed by the surgeon and genetic counselor to determine eligibility for IHC screening. IHC for MMR enzymes (MLH1, MSH2, MSH6, and PMS2) was performed on selected cases. If MLH1 protein expression was absent, testing for MLH1 promoter methylation was based on genetic counselor recommendation. Following Em-USP initiation, IHC for MMR enzymes was performed on all pathology samples from hysterectomy specimens with an endometrial carcinoma diagnosis. If tumor tissue sample was inadequate, the prior endometrial biopsy was to be used for the IHC screen. If MLH1 protein expression was absent, the sample was automatically triaged to test for MLH1 promoter methylation. An IHC screen was concerning for LS if any of the enzymes showed absence of staining and in the cases of MLH1 absence, if the MLH1 promoter was negative for methylation. If MLH1 promoter methylation studies were not conducted, absence of MLH1 staining was considered concerning for LS. Genetic testing was performed at Myriad Genetics, Ambry Genetics or GeneDx.
Demographic information was extracted from the patient records. Age and BMI were based on patient data at the office visit directly prior to scheduled surgery. Family history was ascertained either based on the initial family history form filled out by the patient or on family history collected by the genetic counselor during a scheduled GC appointment. Tumor histology, stage, invasion characteristics and IHC for MMR enzymes were extracted from the surgical pathology reports.
Demographic and clinical characteristics of the sample were summarized using descriptive statistics and the differences between Em-USP and Pre Em-USP cohorts were compared using two-sample t-test and Mann-Whitney rank-sum test (for continuous variables) or Chi-square test and Fisher’s exact test (for categorical data) as appropriate. All analyses were two-sided and significance was set at a P-value of 0.05. Statistical analyses were performed using statistical packages SAS 9.2 (SAS Institutes, Cary, NC).
Results
The two cohorts consisted of 637 total patients divided as follows: 395 cases of primary endometrial cancers diagnosed prior to initiation of Em-USP protocol (Pre Em-USP cohort) and 242 cases diagnosed following initiation of the protocol (Em-USP cohort). There were no significant differences in the patient and tumor characteristics, including age, BMI, tumor histology, or tumor stage between these two cohorts (Table 1). There was also no significant difference in the proportion of cases noted to have lymphovascular space invasion (LVSI), but a slightly larger proportion of cases in the Pre Em-ESP cohort were noted to involve the lower uterine segment (LUS) (p=0.02).
Table 1.
Patient and tumor characteristics
| Pre Em-USP (n=395) | Em-USP (n=242) | |
|---|---|---|
| Median age | 61 | 62 |
| BMI (kg/m2) mean (SD) | 36.2 (10) | 35.3 (9) |
| Tumor Histology (%) | ||
| Endometrioid | 297 (75) | 193 (80) |
| UPSC | 22 (6) | 18 (7) |
| Clear cell | 3 (1) | 0 |
| Carcinosarcoma | 27 (7) | 16 (7) |
| Other | 8 (2) | 2 (1) |
| Mixed | 38 (10) | 13 (5) |
| Stage (%) | ||
| I | 289 (73) | 188 (78) |
| II | 23 (6) | 11 (5) |
| III | 52 (13) | 23 (10) |
| IV | 31 (8) | 20 (8) |
| LVSI (%) | 143 (36) | 91 (38) |
| LUS involved (%) * | 148 (37) | 67 (28) |
, p-value = 0.01
BMI, Body mas index; UPSC, Uterine papillary serous carcinoma; LVSI, Lymphovascular space invasion; LUS, Lower uterine segment.
Screening results
In the Pre Em-USP cohort, 141/395 (36%) newly diagnosed endometrial cancer patients had IHC performed, usually at the request of the gynecologic oncologist performing the surgery or the genetic counselor reviewing the chart and pathology (Table 2). Of these patients, 20 (14%) had a result concerning for LS. Of note, MLH1 promoter methylation testing was not routinely utilized until initiation of Em-USP and therefore, 12 of the cases with MLH1 negative staining did not have MLH1 promoter methylation studies available in this cohort. Following initiation of Em-USP, 234/242 (97%) patients underwent IHC screening. Of these 234 patients, 22 (9%) had an IHC result concerning for LS, a proportion that did not significantly differ from the Pre Em-USP cohort. Since the Em-USP algorithm triaged all cases with negative MLH1 staining to MLH1 promoter methylation testing, an additional 29/32 cases were found to have MLH1 promoter hypermethylation and could be assumed to have a sporadic endometrial cancer, further reducing the number of patients that necessitated genetic testing (Table 2). Of the eight cases where screening was not performed, five did not have any residual tumor at time of hysterectomy, either due to neoadjuvant chemotherapy or complete removal during biopsy. The remaining three cases (1.2%) were missed for unknown reasons.
Table 2.
Screening results
| Pre Em-USP (n=395) | Em-USP (n=242) | |
|---|---|---|
| IHC screen performed (%)* | 141 (36) | 234 (97) |
| IHC concerning for LS | 20 | 22 |
| MLH1 | 2 | 2 |
| MLH1/PMS2 | 20 | 29 |
| MSH6/MLH1 | 1 | 0 |
| MSH6/PMS2/MLH1 | 1 | 1 |
| MLH1 methylation referral (%) * | 12 (50) | 32 (100) |
| MLH1 methylation present* | 10 | 29 |
| PMS2 | 1 | 8 |
| MSH2 | 0 | 1 |
| MSH6 | 3 | 3 |
| MSH2/MSH6 | 4 | 5 |
| MSH6/PMS2 | 0 | 1 |
, p-value < 0.001
IHC, Immunohistochemistry
Overall, there was no difference between the cohorts in the number of specific MMR proteins that were found to have loss of expression by IHC (Table 2). There was a significant difference between the cohorts in the number of cases with absent MLH1 expression that were referred for MLH1 promoter methylation testing, likely because this testing became part of the standard protocol following implementation of Em-USP.
Genetic counseling
In the Pre EM-USP cohort, all 22 of the individuals who had an IHC screening concerning for LS were offered genetic counseling (GC) and 14 (64%) accepted the counseling (Figure 2). Germline testing (GT) was performed on 10/14 (71%) patients and five (1.3% of total population) were found to carry a known deleterious mutation or a variant of unknown significance (VUS) (Figure 2). Within the Em-USP cohort, all 22 individuals who had an IHC screen concerning for LS were also referred to GC and 21 (95%) of these individuals accepted the referral, a significantly higher proportion than the Pre Em-USP cohort (p=0.02) (Figure 2). Following GC, 16 (76%) of the patients underwent GT and 5 were found to have a mutation or a VUS in one of the MMR genes and one result was still pending (Table 3).
Figure 2.
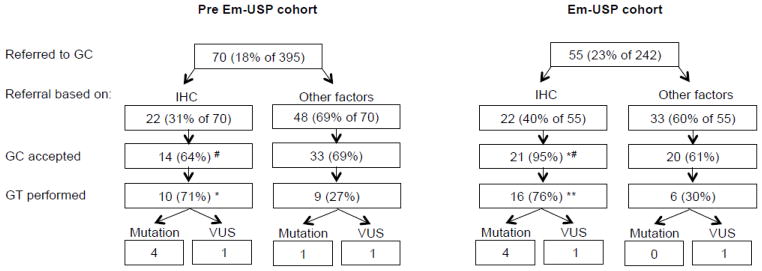
IHC GC results
*, p-value <0.05; **, p-value <0.005 (comparison within cohort)
#, p-value 0.02 (between cohorts)
GC, Genetic counseling; IHC, Immunohistochemistry; GT, Germline mutation testing; VUS, Variant of unknown significance
Table 3.
Identified mutations and variants of uncertain significance and patient characteristics.
| Patient # | Cohort | Age | Histology | Stage | Grade | Met Amsterdam II Criteria – Pre-GC | Met Amsterdam II Criteria – Post-GC | Personal History of Cancer | Family History of LS-associated Cancer | IHC Results | Genetic Testing Results - Mutations | Genetic Testing Results - VUS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Pre EM-USP | 45 | Endometrioid | IA | 1 | No | No | None | No | Loss of MSH2/MSH6 | Mutation in MSH2 – del exons 1–6 | |
| 2 | Pre EM-USP | 52 | Endometrioid | IB | 3 | No | Yes | Breast age 46 | Yes | Loss of MLH1/PMS2 | Mutation in MLH1 – c.588+1_+2delGTinsTA | |
| 3 | Pre EM-USP | 73 | UPSC | IVB | 3 | No | No | None | Yes | N/A | Mutation in PMS2 – p.S461 | |
| 4 | Pre EM-USP | 56 | Endometrioid | IA | 1 | No | Yes | None | Yes | Loss of MLH1/PMS2 | Mutation in MLH1 – 1989del9 | |
| 5 | Pre EM-USP | 60 | Endometrioid | IA | 1 | Yes | Yes | Colon age 40 | Yes | Loss of MSH2/MSH6 | Mutation in MSH2 – 5′UTR_EX6del | |
| 6 | Pre EM-USP | 50 | Endometrioid | IA | 1 | No | No | Colon age 39 | Yes | Normal | VUS in MSH6 – A20V (59C>T) | |
| 7 | Pre EM-USP | 61 | Endometrioid | IB | 1 | No | No | None | Yes | Loss of MSH6 | VUS in MSH6 – p.L585P | |
|
| ||||||||||||
| 8 | EM-USP | 46 | Endometrioid | II | 1 | No | Yes | None | Yes | Loss of MSH2/MSH6 | Mutation in MSH2 – del exons 1–6 | |
| 9 | EM-USP | 61 | Carcinosarcoma | IVB | 3 | No | No | Ovarian age 61, Breast age 60 | Yes | Loss of MSH2 | Mutation in MSH6 – c.1135_1139delAGAGA | |
| 10 | EM-USP | 35 | Endometrioid | IA | 2 | No | No | None | Yes | Loss of MLH1/PMS2, MLH1 methylation absent | Mutation in MLH1 – G67E (200G>A) | |
| 11 | EM-USP | 54 | Mixed clear cell/ endometrioid | IIIC | 1 | Yes | Yes | Colon age 32 | Yes | Loss of MSH2/MSH6 | Mutation in MSH2 – c.1982_1985delAACA (p.K661RfsX23) (Lys661ArgfsX23) | |
| 12 | EM-USP | 76 | Endometrioid with focal clear cell | IA | 1 | No | No | None | Yes | Normal | VUS in MLH1 – c.1709A>G (p.Asn570Ser) | |
| 13 | EM-USP | 75 | UPSC | IA | 3 | No | No | None | Yes | Loss of MSH6 | VUS in MSH6 – p.P199L | |
UPSC, Uterine Papillary Serous Carcinoma; GC, Genetic Counseling; IHC, Immunohistochemistry; VUS, Variant of Uncertain Significance.
Both cohorts had a number of individuals who were recommended for genetic counseling due to risk factors other than IHC screening, including family history (concerning for LS or BRCA mutations), personal history (early onset of endometrial cancer or prior history of Lynch syndrome associated cancers) or self-referral. Within the Em-USP cohort, 33 (12% of 242) additional individuals who had normal IHC staining patterns were referred to GC (Figure 2). Following referral, 20 (61%) accepted counseling, 9 were offered GT and 6 underwent GT. None of these individuals had a deleterious mutation consistent with LS; however, one had a VUS in MLH1. Similarly, in the Pre Em-USP cohort, 48 (12% of 395) individuals were referred to GC based on indications other than IHC screening for LS. There was no significant difference in the proportion of individuals who were offered of who underwent GT compared to the Em-USP cohort. However, one additional individual, who was not tested for IHC, was found to have a deleterious mutation in one of the MMR genes consistent with LS (Figure 2 and Table 3). An additional individual was found to have a VUS, later determined to be a likely polymorphism. The additional patient found to have LS did not identify any family history of endometrial cancer or colorectal cancer on the initial patient survey and did not meet Amsterdam II criteria. The patient did report a cousin with bilateral breast cancer in her 40s and was referred to GC for this family history. Upon meeting with the genetic counselor, 10 additional family members with Lynch-associated cancers were identified; however, Amsterdam II criteria were still not met.
In comparing the two cohorts as a whole, while the overall proportion of patients referred to and presenting for GC did not differ, significantly more women underwent GT in the Em-USP cohort (9.1% vs 4.8%, p<0.05).
Discussion
Universal screening for LS is recommended for all patients with colorectal cancer and has been shown to be feasible and cost-effective (14, 21–23). Screening strategies for endometrial cancer patients have not been as well established, however, IHC of MMR enzyme expression is evolving as an important strategy and universal screening using this method has been proposed and tested by several groups (16–18). Implementation of a universal screening protocol using IHC for MMR enzymes at our institution resulted in 97% of patients being successfully screened and all of the patients with IHC results concerning for LS being referred for GC. The frequency of germline mutations associated with LS in our population of newly diagnosed endometrial cancer patients was 1.3–1.7% (Figure 2), which is similar to previous reports (4). These data suggest that a screening strategy such as this, with the goal of providing genetic counseling to all patients with screening results concerning for LS, is feasible even at a large tertiary referral medical center with a high volume of new endometrial cancer diagnoses.
Despite our success in screening almost all endometrial cancer samples for MMR protein expression, we found that there was no difference in the number of patients referred to GC between the two cohorts (18% Pre EM-USP vs 22% Em-USP) (Figure 2). This likely represents the success of our genetic counseling program prior to initiation of Em-USP as we have an on-site genetic counselor available and each patient referred for GC is contacted by a counselor via telephone and follow-up letter. The genetic counselor is also heavily involved in the referral process and reviews all pathology and reported family histories for new endometrial cancer patients in order to determine whether additional patients need to be contacted. A prior study evaluating screening strategies in colorectal cancer patients showed that having a genetic counselor involved in facilitating the GC referral process increased both the referral rates for qualifying individuals (55% to 100%) and acceptance rates of GC (32% to 71%) (20).
In analyzing the outcomes of our universal screening protocol in detecting endometrial cancer patients at risk for LS, we found no additional patients who were positive for LS-associated mutations outside of those who had concerning IHC results in the Em-USP cohort. In contrast, one additional patient with an LS-associated mutation was identified in the Pre Em-UPS cohort after being triaged to GC based on family and personal history. This brings up an important consideration of resource availability and success of various screening strategies. Our institution benefits from an on-site genetic counseling program, which allows for close follow up after a referral is placed and convenient appointment times as the counselor is able to see patients on the same day as their appointment with the gynecologic oncologist and in multiple locations, including the chemotherapy infusion center. The results presented here suggest that selective IHC screening, such as our strategy in the Pre Em-USP cohort, may miss LS carriers unless family history is carefully elicited by the providers, thereby resulting in adequate referral to GC. Therefore, institutions with fewer genetic counseling resources may have lower referral and uptake rates for GC based on family and personal history of cancer and could gain a tremendous benefit from universal IHC screening for MMR enzyme expression in endometrial cancer cases as a triage method for GC. Alternatively, we can suggest that IHC for MMR enzyme expression could be used as a sole referral method at any institution. This would not preclude the need for extensive genetic counseling once an IHC result concerning for LS is identified. A recent meta-analysis of genetic counseling utilization by first degree relatives of LS probands within colorectal cancer patients also showed uptake rates of 50% or less among these family members (24). We should, therefore, consider that prioritizing genetic counselors towards meeting individuals at risk for LS and their families as opposed to triaging patients for initial evaluation could provide for an opportunity to better utilize counseling resources and improve patient care. Numerous studies in colon cancer patients with LS have shown that uptake of genetic counseling and testing among relatives of probands is underutilized (24).
Following initiation of Em-USP, the referral process at our institution did not significantly differ and there was a large proportion of individuals referred to GC based on family or personal history (other factors) despite normal IHC results (Figure 2). In the Em-USP cohort, the acceptance rate among individuals referred to GC based on a concerning IHC screen was significantly higher than among patients who were referred based on these other factors (95% vs 61%, p=0.004). This suggests that the concrete evidence of a concerning IHC screen result in the setting of universal screening is a stronger incentive for patients to follow-up with GC than a provider’s recommendation based on family or personal history. Since previous studies indicate acceptance of GC is an important hurdle to overcome among endometrial cancer patients in order to increase detection rates of LS (25), screening methods involving IHC may be a critically helpful tool.
Another important finding to address is that in the Pre Em-USP cohort, the acceptance rate for GC among patients with concerning IHC screens was only 64%, significantly lower than within the Em-USP cohort, 95% (p<0.05, Figure 2). It is difficult to predict why this is the case and while a few patients cited financial concerns or specifically not wanting to know genetic information, the majority simply did not respond to multiple contact attempts made by the genetic counseling counselor. We cannot exclude the confounding variable of time between the two cohorts and can speculate that perhaps genetic counseling and testing may be becoming more accepted among patients in recent years. Additionally, a notable difference between the two protocols is that referral for IHC in the Pre Em-USP cohort was often made following pathology results and further into the patient’s treatment course. This may indicate a benefit specific to universal screening as patients in the Em-USP cohort were presented with IHC results as part of their standard pathology in a timely manner, eliminating any delays in the referral process.
Overall, the proportion of individuals undergoing GT in the Em-USP cohort was higher than the Pre Em-USP cohort (22/242, 9.1% vs 19/395, 4.8%; p=0.04). The data discussed above would suggest that this is largely due to a higher GC acceptance rate since the uptake of GT following GC was largely unchanged between the two cohorts. Additionally, there was a significant difference in the proportion of individuals accepting GT among individuals who had IHC results suggestive of LS versus those who were offered GT based on other risk factors in both cohorts (71% vs 27%, p<0.05 in Pre Em-USP and 76% vs 30%, p<0.005 in Em-USP) (Figure 2). Several individuals were referred based on family history of breast cancer and other BRCA associated cancers. A number of the individuals referred to GC for family or personal history on intake forms were found to be low risk for LS by the genetic counselor and were subsequently not recommended for LS germline mutation testing. In fact, if we take this into account, the uptake of GT was similar between those who were offered germline testing based on IHC results versus those who were offered based on other factors. However, many more low-risk individuals underwent genetic counseling if they did not have a concerning IHC screen. Among patients who declined genetic testing despite recommendations, financial reasons were the most commonly cited. The data again suggest a benefit of the IHC screening results in triaging patients towards definitive testing for LS as they are more likely to not only comply with the referral for GC, but to also qualify for and undergo GT. The results highlight an opportunity to use genetic counseling offices most efficiently to counsel individuals with positive IHC screens and their relatives towards germline mutation testing and subsequent increased cancer screening and prevention.
Our study showed that approximately 69.2% of individuals with IHC screens suggestive of LS did not have germline mutations in MMR genes. Similar findings were reported by Rodriguez-Soler et al, who reported a 71% incidence of these patients (26). This group of individuals has been termed to have a “Lynch-like syndrome” and have an increased risk of LS-associated cancers that is still much lower than those with mutation-proven LS. While about half of these individuals have recently been shown to have somatic mutations (27), the other half may have underlying germline mutations that we are not yet capable of detecting, such as mutations in the promoter regions or other activating genes. This is an ongoing area of research and our inability to detect these possible variations is an inherent weakness in our study. However, our ability to detect the associated IHC abnormalities and successfully triage patients to a genetic counselor who can then carefully review family history and make screening recommendations is another benefit of a universal screening approach.
Some strengths of our study are the availability of a comparison cohort prior to initiation of Em-USP and large sample sizes in both cohorts. This allowed for identification of at least one individual with LS who would have been missed by a selective IHC-screening protocol. One weakness of this study is its retrospective design. Another weakness is that germline testing results are not known for all patients and therefore, we cannot exclude the possibility that mutations were missed in the Em-USP cohort. In 2009, the EGAPP Working Group reported that IHC for MMR enzyme expression in colorectal cancer had a sensitivity of 83% based on a review of nine studies and 149 total patients (14). A recent review of screening strategies in endometrial cancers reported sensitivities of IHC for MMR proteins ranging from 86–100% (13). Mercado et al. reported a negative predictive value (NPV) of 100% for IHC among the general population and 76% among a high-risk population in the Colon Cancer Family Registry (28). Since this study was in an unselected cohort of endometrial cancer patients, but at a tertiary referral center with likely an increased proportion of high-risk patients, we suspect that the NPV of our population is somewhere in between 76% and 100%, but closer to latter.
Lastly, while we suggest that universal screening may lead to improved use of genetic counselors and better resource allocation, this study did not compare cost-effectiveness of the different screening strategies. One group looking at colorectal cancer patients showed that universal screening is cost effective for the US health care system since this strategy leads to more cases of LS being detected allowing for better preventative strategies (22). Another group conducted an estimate cost-effectiveness analysis in endometrial cancer patients using Medicare reimbursements as the cost estimates. This group showed that universal screening using IHC followed by single gene sequencing was most cost-effective when compared to screening by Amsterdam criteria, age or universal sequencing (29). Another group showed incremental increases in effectiveness and cost-effectiveness of IHC based screening for LS in colorectal patients when more relatives presented for subsequent genetic testing (23). This is encouraging since our data shows increased genetic counseling uptake in the setting of universal screening for LS.
In conclusion, our retrospective review of endometrial cancer screening strategies for LS before and after initiation of universal IHC for MMR proteins showed that universal IHC screening is feasible in the setting of a large academic institution with a high patient volume. It also showed that referral for genetic counseling based on IHC screening results in the setting of universal screening leads to higher patient compliance with counseling and a higher rate of subsequent genetic testing. Lastly, our data suggest that using a selective IHC screening protocol may miss identification of patients with LS unless a rigorous referral to genetic counseling based on family history is also available.
Highlights.
Universal screening of endometrial cancer for Lynch syndrome using an immunohistochemistry-based protocol is feasible in a tertiary referral medical center.
Triaging patients to genetic counseling based on immunohistochemistry screening results for Lynch syndrome is associated with higher patient follow-up.
Universal screening of newly diagnosed endometrial cancer cases for Lynch syndrome leads to higher rates of germline genetic testing.
Acknowledgments
This research is in part supported by the National Institutes of Health SPORE in Endometrial Cancer, grant no. NIH P50CA134254-01A1
Footnotes
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lynch HT, de la Chapelle A. Genetic susceptibility to non-polyposis colorectal cancer. J Med Genet. 1999 Nov;36(11):801–18. [PMC free article] [PubMed] [Google Scholar]
- 2.Aarnio M, Sankila R, Pukkala E, Salovaara R, Aaltonen LA, de la Chapelle A, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999 Apr 12;81(2):214–8. doi: 10.1002/(sici)1097-0215(19990412)81:2<214::aid-ijc8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 3.Dunlop MG, Farrington SM, Carothers AD, Wyllie AH, Sharp L, Burn J, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997 Jan;6(1):105–10. doi: 10.1093/hmg/6.1.105. [DOI] [PubMed] [Google Scholar]
- 4.Hampel H, Frankel W, Panescu J, Lockman J, Sotamaa K, Fix D, et al. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res. 2006 Aug 1;66(15):7810–7. doi: 10.1158/0008-5472.CAN-06-1114. [DOI] [PubMed] [Google Scholar]
- 5.Ollikainen M, Abdel-Rahman WM, Moisio AL, Lindroos A, Kariola R, Jarvela I, et al. Molecular analysis of familial endometrial carcinoma: a manifestation of hereditary nonpolyposis colorectal cancer or a separate syndrome? J Clin Oncol. 2005 Jul 20;23(21):4609–16. doi: 10.1200/JCO.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 6.Lu KH, Dinh M, Kohlmann W, Watson P, Green J, Syngal S, et al. Gynecologic cancer as a “sentinel cancer” for women with hereditary nonpolyposis colorectal cancer syndrome. Obstet Gynecol. 2005 Mar;105(3):569–74. doi: 10.1097/01.AOG.0000154885.44002.ae. [DOI] [PubMed] [Google Scholar]
- 7.Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999 Jun;116(6):1453–6. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Bigas MA, Boland CR, Hamilton SR, Henson DE, Jass JR, Khan PM, et al. A National Cancer Institute Workshop on Hereditary Nonpolyposis Colorectal Cancer Syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst. 1997 Dec 3;89(23):1758–62. doi: 10.1093/jnci/89.23.1758. [DOI] [PubMed] [Google Scholar]
- 9.Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Ruschoff J, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004 Feb 18;96(4):261–8. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Syngal S, Fox EA, Eng C, Kolodner RD, Garber JE. Sensitivity and specificity of clinical criteria for hereditary non-polyposis colorectal cancer associated mutations in MSH2 and MLH1. J Med Genet. 2000 Sep;37(9):641–5. doi: 10.1136/jmg.37.9.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziogas A, Anton-Culver H. Validation of family history data in cancer family registries. Am J Prev Med. 2003 Feb;24(2):190–8. doi: 10.1016/s0749-3797(02)00593-7. [DOI] [PubMed] [Google Scholar]
- 12.Sijmons RH, Boonstra AE, Reefhuis J, Hordijk-Hos JM, de Walle HE, Oosterwijk JC, et al. Accuracy of family history of cancer: clinical genetic implications. Eur J Hum Genet. 2000 Mar;8(3):181–6. doi: 10.1038/sj.ejhg.5200441. [DOI] [PubMed] [Google Scholar]
- 13.Stewart AP. Genetic Testing Strategies in Newly Diagnosed Endometrial Cancer Patients Aimed at Reducing Morbidity or Mortality from Lynch Syndrome in the Index Case or Her Relatives. PLoS Curr. 2013:5. doi: 10.1371/currents.eogt.b59a6e84f27c536e50db4e46aa26309c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med. 2009 Jan;11(1):35–41. doi: 10.1097/GIM.0b013e31818fa2ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lancaster JM, Powell CB, Kauff ND, Cass I, Chen LM, Lu KH, et al. Society of Gynecologic Oncologists Education Committee statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol. 2007 Nov;107(2):159–62. doi: 10.1016/j.ygyno.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 16.Backes FJ, Leon ME, Ivanov I, Suarez A, Frankel WL, Hampel H, et al. Prospective evaluation of DNA mismatch repair protein expression in primary endometrial cancer. Gynecol Oncol. 2009 Sep;114(3):486–90. doi: 10.1016/j.ygyno.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 17.Batte BA, Bruegl AS, Daniels MS, Ring KL, Dempsey KM, Djordjevic B, et al. Consequences of universal MSI/IHC in screening ENDOMETRIAL cancer patients for lynch syndrome. Gynecol Oncol. 2014 Jun 14; doi: 10.1016/j.ygyno.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moline J, Mahdi H, Yang B, Biscotti C, Roma AA, Heald B, et al. Implementation of tumor testing for lynch syndrome in endometrial cancers at a large academic medical center. Gynecol Oncol. 2013 Jul;130(1):121–6. doi: 10.1016/j.ygyno.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 19.Rabban JT, Calkins SM, Karnezis AN, Grenert JP, Blanco A, Crawford B, et al. Association of tumor morphology with mismatch-repair protein status in older endometrial cancer patients: implications for universal versus selective screening strategies for Lynch syndrome. Am J Surg Pathol. 2014 Jun;38(6):793–800. doi: 10.1097/PAS.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 20.Heald B, Plesec T, Liu X, Pai R, Patil D, Moline J, et al. Implementation of universal microsatellite instability and immunohistochemistry screening for diagnosing lynch syndrome in a large academic medical center. J Clin Oncol. 2013 Apr 1;31(10):1336–40. doi: 10.1200/JCO.2012.45.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005 May 5;352(18):1851–60. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 22.Mvundura M, Grosse SD, Hampel H, Palomaki GE. The cost-effectiveness of genetic testing strategies for Lynch syndrome among newly diagnosed patients with colorectal cancer. Genet Med. 2010 Feb;12(2):93–104. doi: 10.1097/GIM.0b013e3181cd666c. [DOI] [PubMed] [Google Scholar]
- 23.Ladabaum U, Wang G, Terdiman J, Blanco A, Kuppermann M, Boland CR, et al. Strategies to identify the Lynch syndrome among patients with colorectal cancer: a cost-effectiveness analysis. Ann Intern Med. 2011 Jul 19;155(2):69–79. doi: 10.7326/0003-4819-155-2-201107190-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharaf RN, Myer P, Stave CD, Diamond LC, Ladabaum U. Uptake of genetic testing by relatives of lynch syndrome probands: a systematic review. Clin Gastroenterol Hepatol. 2013 Sep;11(9):1093–100. doi: 10.1016/j.cgh.2013.04.044. [DOI] [PubMed] [Google Scholar]
- 25.Backes FJ, Mitchell E, Hampel H, Cohn DE. Endometrial cancer patients and compliance with genetic counseling: room for improvement. Gynecol Oncol. 2011 Dec;123(3):532–6. doi: 10.1016/j.ygyno.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Soler M, Perez-Carbonell L, Guarinos C, Zapater P, Castillejo A, Barbera VM, et al. Risk of cancer in cases of suspected lynch syndrome without germline mutation. Gastroenterology. 2013 May;144(5):926–32. e1. doi: 10.1053/j.gastro.2013.01.044. quiz e13–4. [DOI] [PubMed] [Google Scholar]
- 27.Mensenkamp AR, Vogelaar IP, van Zelst-Stams WA, Goossens M, Ouchene H, Hendriks-Cornelissen SJ, et al. Somatic mutations in MLH1 and MSH2 are a frequent cause of mismatch-repair deficiency in Lynch syndrome-like tumors. Gastroenterology. 2014 Mar;146(3):643–6. e8. doi: 10.1053/j.gastro.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Mercado RC, Hampel H, Kastrinos F, Steyerberg E, Balmana J, Stoffel E, et al. Performance of PREMM(1,2,6), MMRpredict, and MMRpro in detecting Lynch syndrome among endometrial cancer cases. Genet Med. 2012 Jul;14(7):670–80. doi: 10.1038/gim.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Resnick K, Straughn JM, Jr, Backes F, Hampel H, Matthews KS, Cohn DE. Lynch syndrome screening strategies among newly diagnosed endometrial cancer patients. Obstet Gynecol. 2009 Sep;114(3):530–6. doi: 10.1097/AOG.0b013e3181b11ecc. [DOI] [PubMed] [Google Scholar]