Abstract
It is well known that the onset of essential hypertension occurs earlier in men than women. Numerous studies have shown sex differences in the vasculature, kidney and sympathetic nervous system contribute to this sex difference in the development of hypertension. The immune system also contributes to the development of hypertension; however, sex differences in immune system modulation of blood pressure (BP) and the development of hypertension has only recently begun to be explored. Here we review findings on the effect of one's sex on the immune system and specifically how these effects impact BP and the development of primary hypertension. We also propose a hypothesis for why mechanisms underlying inflammation-induced hypertension are sex-specific. These studies underscore the value of and need for studying both sexes in the basic science exploration of the pathophysiology of hypertension as well as other diseases.
Keywords: Sex differences, inflammation, T-cells, hypertension, kidney, subfornical organ
1.0 Introduction
The onset of essential hypertension occurs earlier in men than women [1]. This BP sex difference in humans is also observed in experimental animal studies. Females have lower BP than males in numerous animal models of hypertension [2]. There has been an exponential growth over the last ten years in our understanding of how multiple end organs including the kidney, peripheral vasculature and key brain regions involved in central regulation of sympathetic outflow contribute to sex differences in the development of hypertension and within the past couple of years, several excellent reviews have been written on this topic [3–11].
We and others have shown that the gonadal hormones play a key role in sex differences in the development of hypertension. Ovarian hormone deficiency due to premature ovarian failure [12] or menopause [13] is associated with an increased frequency of hypertension in women and numerous animal models of hypertension have shown that 17β-estradiol replacement prevents the rise in BP due to ovarian hormone depletion [2]. For example, we have shown that the increase in BP induced by ovariectomy in the angiotensin II (Ang II) [14]- and aldosterone [15]- infusion models of hypertension can be prevented by 17β-estradiol replacement. As in other models of hypertension, the young female spontaneously hypertensive rat (SHR) has lower BP than the male SHR. Once the female SHR reaches the age at which the estrous cycle ceases, the sex difference in BP disappears [5]. In contrast to many experimental models of hypertension, ovariectomy in the young SHR has no effect on BP, rather studies suggest testosterone plays a key role in the sex differences in BP in SHR. Reducing the levels of testosterone in the male SHR lowered BP [16]. Furthermore, congenic studies in which the SHR Y chromosome was replaced with a Y chromosome from the normotensive Wystar Kyoto (WKY) rat lowered BP as well as testosterone levels [17, 18]. The sex chromosomes can also contribute to sex differences in hypertension independently of the gonadal hormones. Using the four core genotype mouse model, which enables separation of sex chromosome effects from gonadal sex effects, we showed that BP was higher in gonadectomized XX mice compared to gonadectomized XY mice regardless of whether they were male (born with testes) or female (born with ovaries) [19]. Thus, the ovarian and testicular hormone status along with the sex chromosome complement and age of the animal all contribute to sex differences in the development of hypertension.
Recently, studies have demonstrated the immune system is activated in hypertension [20]. Inflammation and adaptive immunity in particular have emerged from both clinical and experimental data as important contributors to the development of hypertension [21, 22]. Inflammatory mechanisms in the kidney, peripheral vasculature, and central nervous system (CNS) all have been shown to be involved [23–27]; however, these studies were conducted primarily in males. Recent studies in females demonstrate sex-specific modulation of the immune system in hypertension. The focus of this review is to examine our current knowledge of the impact of one's sex on immune modulation of BP and the development of the primary cause of hypertension, namely, essential hypertension. Immune modulation of other causes of hypertension such as pulmonary hypertension and preeclampsia are beyond the scope of this review. We also propose a new hypothesis regarding the mechanisms by which the sex chromosomes and gonadal hormones regulate inflammation-induced hypertension. Identification of the cellular mechanisms underlying these robust sex differences in BP may lead to sex-specific preventive strategies and therapeutics that ultimately result in reductions in the incidence of hypertension, delays in the onset of this disease and improved treatments for high blood pressure in both men and women.
2.0 Materials and Methods
2.1 Animals
Rag-1−/− and wild type (WT) mice on the C57BL/6 background were purchased from Jackson Labs. All methods were approved by the Georgetown University and the University of Arizona Animal Care and Use Committees.
2.2 T-cell isolations
Mature CD3+ T-cells were isolated from the spleens of male or female WT mice using Pan T-cell isolation kits (Miltenyi) and negative magnetic sorting for CD3+ isolation. The CD4+ and CD8+ T-cell subpopulations were then isolated by flow cytometry, as described previously [28].
2.3 Adoptive transfer
Both male and female Rag-1−/− mice (10–11 weeks old) were injected via the jugular vein with CD4+ or CD8+ T-cells (2 × 107/mouse), as described previously [28].
2.4 Telemetry
Four weeks after adoptive transfer, radiotransmitters (Data Sciences Int.) were implanted into Rag-1−/− mice. One week later and after a stable baseline was established, osmotic minipumps containing Ang II (490 ng/kg/min) were inserted subcutaneously, then mean arterial pressure (MAP), heart rate (HR), and body weight were recorded for two weeks, as described previously [28].
2.5 Immunohistochemistry assessment of infiltrating T-cells in the subfornical organ
Immunoperoxidase staining
Primary antibodies against CD3+ T-cells were obtained from AbCam. Sections were incubated overnight at 4°C with primary antibody, 1:200, diluted in Tris buffer, pH 7.4, containing 2% normal goat serum and 0.2% Triton X-100. The presence of IgGs in the sections was probed by skipping the primary antibody incubation and directly incubating the tissue samples with biotinylated anti-mouse IgGs. Sections were washed and incubated for 120 min in biotinylated secondary antibodies (1:300; Jackson ImmunoResearch), followed by Avidin/Biotinylated enzyme Complex (ABC) complex for 30 min (Vectastain Elite kit; Vector Laboratories) and reaction with diaminobenzidine tetrahydrochloride (Sigma-Aldrich) for 5–15 min in Tris, pH 7.7. Finally, sections were washed thoroughly, mounted onto gelatin-coated slides, air-dried overnight, dehydrated, and coverslipped. Using an Olympus XI microscope fitted with a digital camera, photomicrographs of the SFO from the different groups following immunohistochemical processing were obtained.
3.0 The Role of Immune System in Sex Differences in the Development of Hypertension
One of the earliest studies implicating a role for the immune system in hypertension was performed by Grollman and colleagues in 1967. They demonstrated that hypertension could be induced in a normal male rat by transplanting this animal with lymph cells from the renal infarction rat model of hypertension [29]. Blood pressure was also shown to be increased in a normal rat by adoptive transfer of splenocytes isolated from a male rat made hypertensive by deoxycorticosterone acetate and sodium chloride treatment [30]. The converse to these gain of function studies was shown in the male SHR. Blood pressure was attenuated in the SHR by transplanting the thymus from a normotensive WKY male rat into the male SHR [31]. While these early studies demonstrated the immune system plays an important role in the development of hypertension, the cellular and molecular mechanisms of specific immune cell types responsible for driving the inflammation-induced hypertension was not defined.
A seminal study published by Guzik et al. [23] in 2007 showed that mice which lack both B- and T-cells due to a deficiency in the recombinant activating gene-1 (Rag-1−/−) had lower BP following two weeks of Ang II infusion compared to WT mice. When mature T-cells (CD3+) isolated from WT mouse spleen were replaced in these Rag-1−/− mice through adoptive transfer (CD3→Rag-1−/−), the magnitude of the Ang II-induced hypertension was restored to WT levels. T-cells were also shown to contribute to hypertension in the male Dahl salt-sensitive (DSS) rat. The T-cell surface glycoprotein CD3 zeta chain (CD247) and the Rag-1 gene were recently deleted in DSS rats resulting in the elimination of mature CD3+ T-cells [32, 33]. This new DSS strain exhibited lower BP than their DSS littermates.
In the Guzik et al. paper, the sex of the animals used was not reported. When this experiment was repeated in both male and female mice, we discovered that both the sex of the T-cell and the sex of the Rag-1−/− host were critical biological determinants of susceptibility and resistance to T-cell-induced hypertension. The female Rag-1−/− mouse (Rag-1−/−-F) was resistant to T-cell induced hypertension compared to the male Rag-1−/− mouse (Rag-1−/−-M) independently of the sex of the transferred T-cells, i.e., CD3F→Rag-1−/−-F and CD3M-Rag-1−/−-F both had lower BP than CD3M-Rag-1−/−-M mice after Ang II infusion [34]. We also found that the sex of the T-cells was critical since CD3F→Rag-1−/−-M had lower BP than CD3M→Rag-1−/−-M after Ang II infusion, i.e., the only difference between these hypertensive and normotensive male mice was the sex of the T-cell [28].
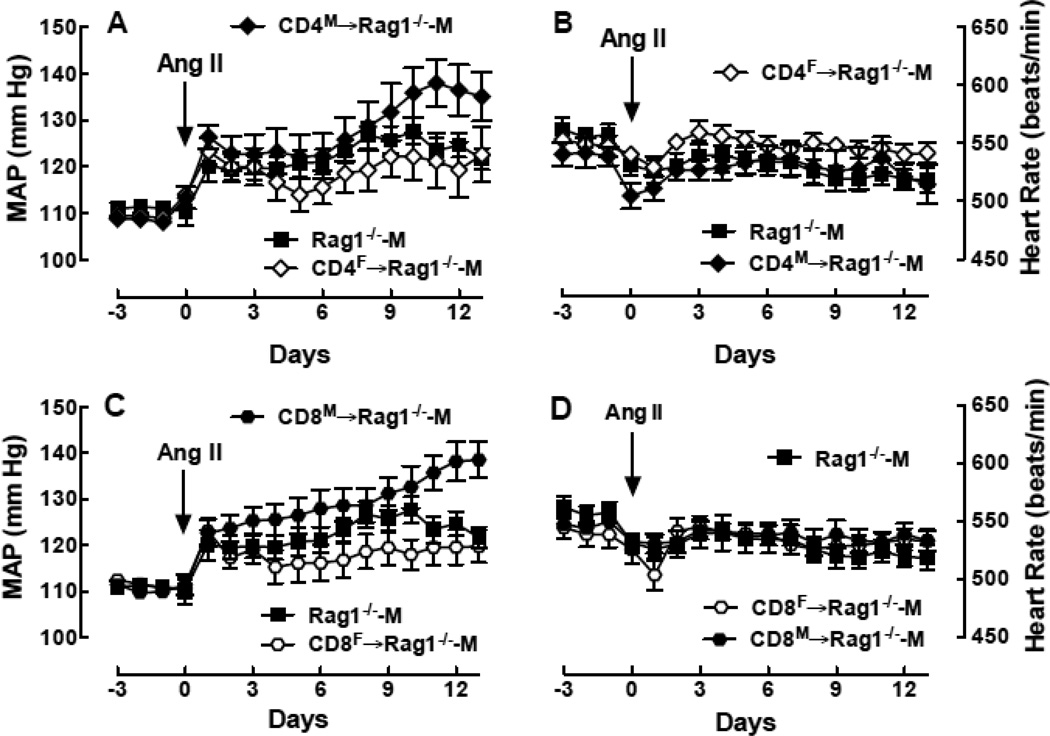
To further investigate which T-cell populations were contributing to the sex-specific T-cell effects on BP, we isolated CD4+ and CD8+ T-cell populations from the spleen of WT male and female mice and transferred these cells into the male Rag-1−/− host. Four weeks after adoptive transfer, there was little difference in basal MAP or basal HR between CD4M→Rag-1−/−-M and CD4F→Rag-1−/−-M or between CD8M→Rag-1−/−-M and CD8F→Rag-1−/−-M. Figure 1 shows that adoptive transfer of both CD4M and CD8M →Rag-1−/−-M resulted in a greater MAP response to Ang II compared to either CD4F or CD8F Tcells. In fact, no differences in MAP were observed in the Ang II time course between the CD4F→Rag-1−/−-M and the Rag-1−/−-M mice and transferring CD8F T-cells actually attenuated the magnitude of Ang II-induced hypertension compared to the Rag-1−/−-M (p<0.01 by 2-way ANOVA). These differences in MAP were not due to differences in body weight since no differences were observed among these four groups (data now shown). Figure 1 also shows that there were no significant differences in the largest drop in HR or in HR at the end of the infusion period between CD4M→Rag-1−/−-M and CD4F→Rag-1−/−-M or between CD8M→Rag-1−/−-M and CD8F→Rag-1−/−-M. Taken together these results indicate that the CD4+ and CD8+ T-cell populations are distinctly different in males and females and sex-specific effects of these T-cells determine the magnitude of Ang II-induced hypertension.
Figure 1.
Effect of the sex of T cell subpopulations on MAP and HR responses to Ang II in the male Rag1−/− host during Ang II infusion. Shown is MAP (A,C) and HR (B,D) as a function of time in Rag1−/−-M (closed square; n=13/group) and in Rag1−/−-M after adoptive transfer of CD4+ (A,B) (diamond; CD4F→Rag1−/−-M; n=11/group) or CD8+ (C,D) (hexagon; CD8F→Rag1−/−-M; n=11/group) T-cells isolated from female (open symbol) or male (closed symbol) WT mouse spleens.
Not all male T-cell populations increase BP in a model of Ang II-dependent hypertension. Increasing the number of regulatory T-cells (Tregs) by adoptive transfer protected mice from both Ang II- and aldosterone-induced hypertension [35, 36]. Furthermore, female SHR have a higher frequency of Tregs in their kidneys than male SHR [37]; however, female T-cell protection from hypertension is not necessarily due to higher levels of Tregs since we have shown the frequency of the Treg population in the circulation and kidneys is actually higher in the male Rag-1−/− mouse after adoptive transfer of CD3M compared to CD3F T-cells. These observations suggest the possibility that the higher frequencies of Tregs in CD3M→Rag-1−/−-M compared to CD4F→Rag-1−/−-M are a compensatory response to the greater magnitude of the hypertension in CD3M→Rag-1−/−-M [28].
Studies showing how the sex of the host and the sex of the T-cell markedly impact the development of hypertension is an example of the rationale underlying why the National Institutes of Health (NIH) announced this past May in the journal Nature that the agency will address the overreliance of male cells and animals in preclinical research [38]. Sex-specific T cell modulation of blood pressure demonstrates how extrapolating to the female from data obtained in males would lead to erroneous conclusions. The NIH announcement is based on the concern that the lack of representation of females in preclinical research could compromise advancements in women's health. Furthermore, the NIH is concerned that not taking into account the influence of biological sex in preclinical research has contributed to the problem of irreproducibility of basic science findings. At the very least, the sex of the experimental animals needs to be reported so that investigators intending to repeat published work as a springboard for further studies can effectively and efficiently do so.
4.0 The Role of the Kidney and Vasculature in Sex-specific T cell regulation of Blood Pressure
There is a growing body of evidence suggesting that T-cell infiltration into end organs is an important contributor to the pathology of hypertension and progression of the disease in males [20]. The initial studies by Guzik et al [23] showed that Ang II infusion in [male] WT mice increased the expression of the chemokine receptor CCR5 and the hyaluronon receptor CD44 in circulating CD4+ lymphocytes and CCR5-ligand and RANTES in vascular tissue. Infiltration of both CD4+ and CD8+ T-cell populations into the vascular endothelium, perivascular fat, renal tissue and recently into brain circumventricular organs correlates with increased BP in male experimental models of hypertension [27, 34, 39]. Greater numbers of CD4+ and CD8+ T-cells were found in the kidney of the male SHR compared to the kidneys of WKY normotensive rats. Similarly, the frequency of CD3+ T-cells was shown to increase in the kidney after sodium-induced hypertension in the male DSS rat [40, 41]. These studies suggest T-cell infiltration into the kidney contributes to hypertension in the male.
Sullivan and colleagues were the first to show that immune cells contribute to hypertension in a female experimental model by demonstrating that the immunosuppressant drug mycophenolate mofetil reduced BP in female SHRs [37]. Furthermore, the authors showed that the T-cell profiles in SHR were sex-specific. Female SHR had more circulating T-cells including the CD4+ and pro-inflammatory CD3+CD4+RORγ+ populations than the male SHR whereas the male SHR had a higher frequency of circulating CD3+CD4+Foxp3+ Tregs. Our studies in male and female WT mice support these observations since we found females had more circulating CD3+ and CD4+ cells than the males after Ang II infusion [28].
In contrast to the circulation, the female SHR kidney had higher frequencies of CD8+ T-cells and Tregs whereas the male SHR kidney had higher frequencies of CD4+ and interleukin (IL)-17+ cells. Mycophenolate mofetil caused greater reductions in BP in the female SHR and this larger drop in BP was associated with greater decreases in CD4+ and IL-17+ cells in the circulation and CD8+ cells in the kidney compared to the male SHR. These findings suggest that sex differences in hypertension may not only be due to male female differences in T cell subtype populations but also to sex-specific regulation of certain T cell subtypes, e.g., Th17 and Treg cells. Furthermore, these comparisons of immune mechanisms in males and females suggests it is not simply the extent of T-cell infiltration into end organ target tissues that dictates the magnitude of the hypertension since greater T-cell infiltration does not necessarily correlate with higher BP when the sex of the T-cell is female. These studies also illustrate the need to investigate immune mechanisms of BP regulation in the female since findings in males are not reproduced in females.
Sex differences are also observed in renal infiltration of CD4+, CD8+, and CD4+ Foxp3+-Tregs after adoptive transfer of male CD3+ T-cells into male and female Rag-1−/− mice and two weeks of Ang II infusion [34]. We found a higher frequency of these T-cell populations in the kidneys of male Rag-1−/− mice compared to female Rag-1−/− mice. In addition, Ang II infusion induced increases in renal expression of interleukin-2, tumor necrosis factor-α and monocyte chemoattractant protein-1 in the male Rag-1−/− mice but not in the females. These results suggest that the pro-hypertensive effects of male T-cells during Ang II infusion are inhibited in females and that reduced T-cell infiltration of the female kidneys may protect females against the hypertensive actions of Ang II.
Importantly, when the subpopulations of T-cells were compared between CD3M→Rag-1−/−-M and CD3F→Rag-1−/−-M, the Rag-1−/− mice receiving male T-cells had significantly higher frequencies of T-cells producing the pro-inflammatory cytokines including tumor necrosis factor and IL-17 and the mice receiving female T-cells had significantly higher levels of circulating IL-10 compared to mice receiving male T-cells. These differential T-cell subtype responses are likely contributors to male susceptibility and female resistance to T-cell mediated hypertension.
T-cell renal infiltration during the development of hypertension was shown to require nitric oxide synthase (NOS) activation in the female SHR [42]. When nitric oxide production was inhibited by treatment with the NOS inhibitor NG-nitro-L-arginine methyl ester (L-NAME), BP increased in both males and females; however, the increase in BP was greater in the females. This L-NAME-induced increase in BP in the SHR was accompanied by increases in renal T-cell infiltration in both males and females; however, there was a higher frequency of Th17 cells and a lower frequency of Tregs in the kidneys of female rats compared to males. Interestingly, when the L-NAME induced increase in BP was prevented pharmacologically, the numbers of T-cells infiltrating the kidney were reduced suggesting that T-cell infiltration of the kidney requires both increases in BP and NOS inhibition. These findings suggest the higher levels of nitric oxide in the kidney of females compared to males may contribute to the sex differences in renal T-cell infiltration and BP regulation.
The role of BP and gonadal hormones in T-cell kidney infiltration has also been examined [43]. In studies using male and female SHR, decreasing BP with hydrochlorothiazide and reserpine did not alter the frequency of the two major CD3+ T-cell populations, i.e., CD4+ or CD8+, in the kidney. The drop in BP though was associated with a greater decrease in the CD4+Foxp3+ Treg population in the female kidney as compared to the males. Gonadectomy increased the proinflammatory markers in both male and female SHR; however, little is known regarding the role gonadal hormones and sex chromosomes on the regulation of the immune system in hypertension.
5.0 The Role of the Brain in Sex-specific T-cell modulation of Blood Pressure
A number of studies suggest sex hormones defend the brain against pro-inflammatory processes and stimulate cell survival by activating anti-apoptotic pathways, decreasing reactive oxygen species (ROS) production and reducing glutamate excitotoxicity [44, 45]. Both astrocytes and the brain resident macrophage, microglia, express the two estrogen receptor (ER) subtypes (ERα and ERβ). Furthermore, 17β-estradiol actions on these cells contributes to the sex differences in brain inflammatory responses [46]. For example, in the experimental autoimmune encephalomyelitis mouse model for neuroinflammatory multiple sclerosis, specific expression of ERα on astroglia but not on neurons is required for 17β-estradiol protection from inflammation, T-cell infiltration and axonal loss in the CNS [47].
Sex differences in the ability of Ang II to induce hypertension has been shown to involve a sex difference in the ability of Ang II to increase sympathetic outflow and activate central neurons involved in BP regulation [48, 49]. Recent studies by Harrison and colleagues suggest that T-cell facilitation of Ang II-induced hypertension in males requires an increase in sympathetic outflow [50]. Indeed, lesioning of the anteroventral third cerebral ventricle (AV3V) in male mice, prevented not only the hypertension but also the Ang II-induced activation of circulating T-cells and vascular inflammation [51]. These findings in males suggest that Ang II-induced increases in sympathetic outflow is required in order to observe the full extent of the proinflammatory effects and T-cell activation triggered by Ang II. We know much less about immune modulation of hypertension in the female and in particular, the role of the CNS in female protection from T-cell facilitated hypertension remains poorly understood.
Central brain regions involved in the regulation of sympathetic neuronal activity and therefore BP include numerous brain stem and forebrain nuclei including the nucleus of the solitary tract (NTS), caudal ventral lateral medulla (CVLM), rostral ventral lateral medulla (RVLM), paraventricular nucleus of the hypothalamus (PVN), and circumventricular organs which lack a blood-brain barrier such as the area postrema (AP) and the SFO. Importantly, all of these key structures involved in BP regulation express ERs [52, 53].
The role of the CNS in sex differences in hypertension and the role of sex hormones was shown in a number of studies in which 17β-estradiol replacement was limited to the brain [54]. For example in both male and ovariectomized female mice, central intracerebroventricular infusion of estradiol attenuated the pressor effect of Ang II and this effect was prevented if brain ERs were blocked [55]. Similarly, in aldosterone/salt-induced hypertension, sex differences observed in this model were normalized by blocking brain ERs in females or by activating brain ERs in males [15]. Thus, brain ERs in both males and females modulate the development of multiple forms of hypertension and therefore, these receptors may also modulate the brain inflammatory mechanisms contributing to hypertension.
The role of central ERs in the sex differences in T-cell mediated hypertension has not been explored. However, in studies of intact male and female Rag-1−/− mice [34], we hypothesize that the inability of male T-cells to increase the magnitude of Ang II-induced hypertension in female Rag-1−/− mice is due to reduced levels of sympathoexcitation following Ang II infusion in females compared to the levels in males. It has been suggested that ER action at the level of both neurons and microglia in brain regions involved in BP regulation contribute to the mechanisms underlying female protection from the development of hypertension [54, 56]. Indeed preliminary studies have shown that microglia activation and proliferation in the NTS, AP and SFO by Ang II infusion in male rats is significantly higher than the levels observed in female rats [57].
Circulating peptides such as Ang II can act on the SFO because it is a forebrain circumventricular organ that lacks a blood-brain barrier. The SFO has primary projections to the PVN and Ang II acting at the level of SFO neurons is required for Ang II-mediated increases in sympathetic outflow and hypertension [58]. Increases in circulating Ang II were shown to increase the firing rate of SFO neurons [59, 60]. Importantly, the SFO also expresses ERs which co-localize on the same neurons that express angiotensin type 1 receptors (AT1R) [61]. In male animals, maintaining Ang II-induced hypertension requires activation of inflammatory processes and ROS production within the SFO [58, 62, 63]. Furthermore, it has been shown that 17β-estradiol acting in the SFO via ERs inhibits Ang-II-induced inflammatory processes such as Ang II-induced production of ROS [49, 55]. Thus, 17β-estradiol inhibition of Ang II induced increases in brain ROS and neuronal activation may be a contributory mechanism underlying sex differences in Ang II hypertension.
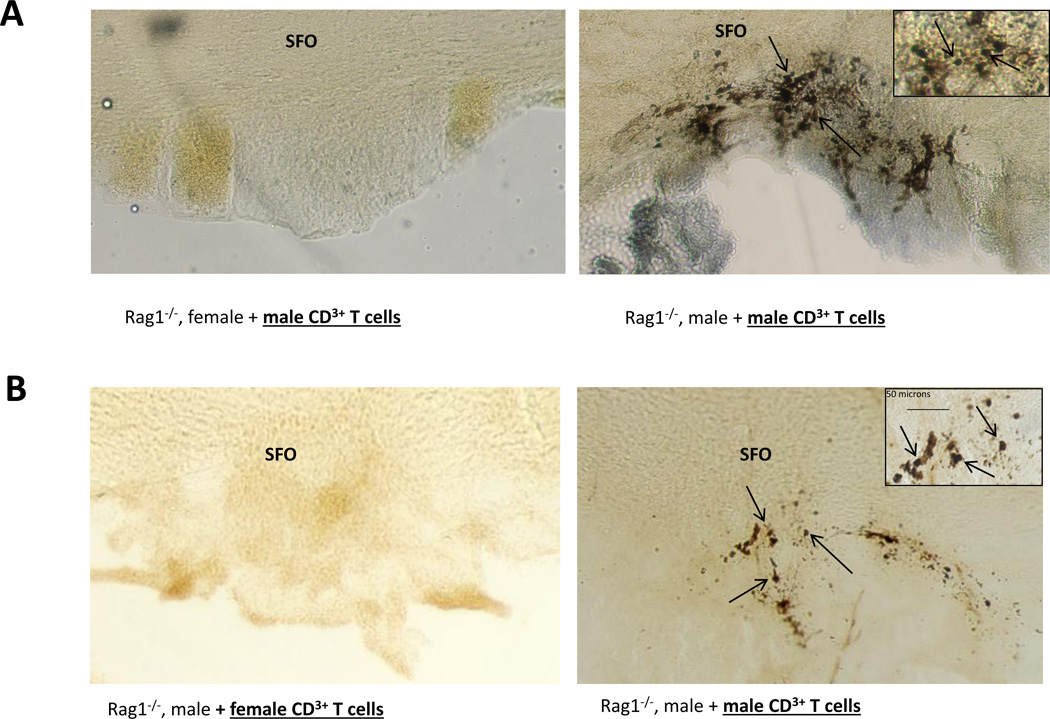
The role of T-cell infiltration into brain regions known to be involved in Ang II-induced hypertension has been studied in both male and female Rag-1−/− mice. In studies employing Rag-1−/− mice, when male T-cells were transferred into males and females during Ang II-induced hypertension, we found significant sex differences in T-cell trafficking into the SFO [34]. Figure 2A is a photomicrograph example of CD3+ T-cell infiltration into the SFO from male and female Rag-1−/− mice following Ang II infusion. The males show significantly more T-cells within the perinchyma of the SFO compared to the females. In a second set of studies investigating the effects of the sex of the T-cell on Ang II dependent hypertension, T-cells from males and females differed markedly in their ability to induce hypertension in the Rag-1−/− mouse [28]. When T-cell infiltration was assessed in the brains from these animals, only the mice receiving male T-cells exhibited T-cell infiltration into the SFO. Figure 2B is a photomicrograph example of CD3+ T-cell infiltration into the SFO compared to a male Rag-1−/− receiving female T-cells. Together, these results suggest that both the sex of the animal and the sex of the T-cell are critically important in regulating T-cell infiltration into the brain during Ang II-induced hypertension.
Figure 2.
CD3+ T-cell infiltration into the SFO of male Rag-1−/− mice. Shown are photomicrographs of representative CD3+ immunohistochemical stained 30 micron sections from the SFO of a Rag-1−/−-F (A, left), and a Rag-1−/−-M (A, right) mouse following Ang II infusion. (B) Photomicrographs of representative CD3+ immunohistochemical stained 30 micron sections from the SFO of Rag-1−/−-M after adoptive transfer of female CD3+ cells (C, left), or male CD3+ cells (C, right) following Ang II infusion.
From these studies reviewed above and from others in the literature, we have hypothesized that in males, Ang II activation of NADPH oxidase and ROS production in the SFO and cytokine production in the kidney acts as a signal to attract specific populations of T-cells to traffic into these end organs. This infiltration of T-cells results in an escalation of the inflammatory cascade during Ang II infusion resulting in a sustained increase in sympathetic outflow and hypertension. Figure 3 illustrates this T-cell-mediated “priming” of the inflammatory cascade within the SFO and potential sites where activation of ERs via 17β-estradiol inhibits the pro-inflammatory pathway. The T-cells, attracted by AT1R-activated brain endothelial cells, neurons and increases in chemokines such as CCL2, migrate across the open blood-brain barrier of the SFO. T-cell infiltration into the SFO results in microglia and astrocyte activation thus further increasing cytokine production and activation of neurons. These events ultimately result in sustained increases in sympathetic outflow and BP. We hypothesize that in ovarian hormone replete females, 17β-estradiol activates ERs leading to inhibition of Ang II-induced increases in intracellular calcium and ROS at the level of the SFO along with inhibition of proinflammatory chemokines and cytokine expression, decreases in T-cell trafficking into the SFO. Accordingly, decreased neuronal activation prevents increases in sympathetic outflow thereby protecting the female from hypertension.
Figure 3.
Hypothesis of the 17β-estradiol inhibitory mechanism of T cell “priming” of SFO inflammatory pathways. 17β-Estradiol activation of ERs in the SFO (green pentagons) acts at multiple sites within the SFO to modulate Ang II-induced brain inflammation, T cell infiltration, microglia activation, increased sympathetic activity and BP. 17β-Estradiol: 1) inhibits AT1R-mediated increases in intracellular calcium; 2) inhibits Ang II-induced ROS formation in the SFO and decreases microglia activation; 3) inhibits expression of CCL2 and blocks translocation of T-cells; and, 4) inhibits Ang IIinduced activation of SFO neurons, thereby ultimately preventing increases in sympathetic outflow and subsequent hypertension.
6.0 Concluding Remarks
In summary, there are significant sex differences in the role of the adaptive immune system in the modulation of BP and the development of essential hypertension. These sex differences may explain why males are more susceptible to hypertension than ovarian hormone replete females. The immune system mechanisms that result in hypertension in males are not extrapolatable to females. The studies reviewed above suggest that multiple factors contribute to these sex differences in immune modulation of BP including, but not limited to: 1) the ratio of Tregs to T helper cells and the infiltration of these cells into end organs; 2) sex differences in tissue expression of NOS and ROS modulation; 3) the sex of the T-cell itself; 4) the ability of female T-cells to mobilize anti-inflammatory T-cells including IL-10-producing cells; and, 5) the endogenous hormonal milieu of the host. The underlying role of sex hormones and sex chromosomes in these observed sex differences in inflammation-related hypertension have yet to be explored. These studies clearly underscore the importance and need for the study of both sexes as the cellular and molecular mechanisms underlying hypertension are further explored. This will be essential for the translation of these findings into improved antihypertensive treatments for both men and women.
Highlights.
Critical sex differences exist in the immune system in hypertension.
New model for how sex impacts T-cell end organ infiltration and hypertension.
Sex and the immune system contribute to hypertension differences in men and women.
Acknowledgments
This work was supported by NIH grants to KS (AG/HL-19291, AG-039779 & AG-16902).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension. 2008;52:818–827. doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- 2.Sandberg K, Ji H. Sex differences in primary hypertension. Biology of sex differences. 2012;3:7. doi: 10.1186/2042-6410-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coutinho T. Arterial stiffness and its clinical implications in women. The Canadian journal of cardiology. 2014;30:756–764. doi: 10.1016/j.cjca.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 4.Zimmerman MA, Sullivan JC. Hypertension: what's sex got to do with it? Physiology. 2013;28:234–244. doi: 10.1152/physiol.00013.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maranon R, Reckelhoff JF. Sex and gender differences in control of blood pressure. Clinical science. 2013;125:311–318. doi: 10.1042/CS20130140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xue B, Johnson AK, Hay M. Sex differences in angiotensin II- and aldosterone-induced hypertension: the central protective effects of estrogen. American journal of physiology. Regulatory, integrative and comparative physiology. 2013;305:R459–R463. doi: 10.1152/ajpregu.00222.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kittikulsuth W, Sullivan JC, Pollock DM. ET-1 actions in the kidney: evidence for sex differences. British journal of pharmacology. 2013;168:318–326. doi: 10.1111/j.1476-5381.2012.01922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wenner MM, Stachenfeld NS. Blood pressure and water regulation: understanding sex hormone effects within and between men and women. The Journal of physiology. 2012;590:5949–5961. doi: 10.1113/jphysiol.2012.236752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reckelhoff JF, Maric C. Sex and gender differences in cardiovascular-renal physiology and pathophysiology. Steroids. 2010;75:745–746. doi: 10.1016/j.steroids.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Miller VM, Black DM, Brinton EA, Budoff MJ, Cedars MI, Hodis HN, Lobo RA, Manson JE, Merriam GR, Naftolin F, Santoro N, Taylor HS, Harman SM. Using basic science to design a clinical trial: baseline characteristics of women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS) Journal of cardiovascular translational research. 2009;2:228–239. doi: 10.1007/s12265-009-9104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lima R, Wofford M, Reckelhoff JF. Hypertension in postmenopausal women. Current hypertension reports. 2012;14:254–260. doi: 10.1007/s11906-012-0260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pal L, Santoro N. Premature ovarian failure (POF): discordance between somatic and reproductive aging. Ageing Res Rev. 2002;1:413–423. doi: 10.1016/s1568-1637(02)00009-0. [DOI] [PubMed] [Google Scholar]
- 13.Staessen JA, Ginocchio G, Thijs L, Fagard R. Conventional and ambulatory blood pressure and menopause in a prospective population study. Journal of human hypertension. 1997;11:507–514. doi: 10.1038/sj.jhh.1000476. [DOI] [PubMed] [Google Scholar]
- 14.Xue B, Pamidimukkala J, Lubahn DB, Hay M. Estrogen receptor-alpha mediates estrogen protection from angiotensin II-induced hypertension in conscious female mice. American journal of physiology. Heart and circulatory physiology. 2007;292:H1770–H1776. doi: 10.1152/ajpheart.01011.2005. [DOI] [PubMed] [Google Scholar]
- 15.Xue B, Badaue-Passos D, Jr, Guo F, Gomez-Sanchez CE, Hay M, Johnson AK. Sex differences and central protective effect of 17beta-estradiol in the development of aldosterone/NaCl-induced hypertension. American journal of physiology. Heart and circulatory physiology. 2009;296:H1577–H1585. doi: 10.1152/ajpheart.01255.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reckelhoff JF, Zhang H, Granger JP. Testosterone exacerbates hypertension and reduces pressure-natriuresis in male spontaneously hypertensive rats. Hypertension. 1998;31:435–439. doi: 10.1161/01.hyp.31.1.435. [DOI] [PubMed] [Google Scholar]
- 17.Ely DL, Turner ME. Hypertension in the spontaneously hypertensive rat is linked to the Y chromosome. Hypertension. 1990;16:277–281. doi: 10.1161/01.hyp.16.3.277. [DOI] [PubMed] [Google Scholar]
- 18.Davidson AO, Schork N, Jaques BC, Kelman AW, Sutcliffe RG, Reid JL, Dominiczak AF. Blood pressure in genetically hypertensive rats. Influence of the Y chromosome. Hypertension. 1995;26:452–459. doi: 10.1161/01.hyp.26.3.452. [DOI] [PubMed] [Google Scholar]
- 19.Ji H, Zheng W, Wu X, Liu J, Ecelbarger CM, Watkins R, Arnold AP, Sandberg K. Sex chromosome effects unmasked in angiotensin II-induced hypertension. Hypertension. 2010;55:1275–1282. doi: 10.1161/HYPERTENSIONAHA.109.144949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison DG. The immune system in hypertension. Transactions of the American Clinical and Climatological Association. 2014;125:130–138. discussion 138–140. [PMC free article] [PubMed] [Google Scholar]
- 21.Harrison DG, Gongora MC, Guzik TJ, Widder J. Oxidative stress and hypertension. J Am Soc Hypertens. 2007;1:30–44. doi: 10.1016/j.jash.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Lob HE, Marvar PJ, Guzik TJ, Sharma S, McCann LA, Weyand C, Gordon FJ, Harrison DG. Induction of hypertension and peripheral inflammation by reduction of extracellular superoxide dismutase in the central nervous system. Hypertension. 2010;55:277–283. doi: 10.1161/HYPERTENSIONAHA.109.142646. 276p following 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. The Journal of experimental medicine. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paton JF, Waki H. Is neurogenic hypertension related to vascular inflammation of the brainstem? Neurosci Biobehav Rev. 2009;33:89–94. doi: 10.1016/j.neubiorev.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 25.Zubcevic J, Waki H, Raizada MK, Paton JF. Autonomic-immune-vascular interaction: an emerging concept for neurogenic hypertension. Hypertension. 2011;57:1026–1033. doi: 10.1161/HYPERTENSIONAHA.111.169748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2:247–257. doi: 10.1002/emmm.201000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji H, Zheng W, Li X, Liu J, Wu X, Zhang MA, Umans JG, Hay M, Speth RC, Dunn SE, Sandberg K. Sex-Specific T-Cell Regulation of Angiotensin II-Dependent Hypertension. Hypertension. 2014 doi: 10.1161/HYPERTENSIONAHA.114.03663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okuda T, Grollman A. Passive transfer of autoimmune induced hypertension in the rat by lymph node cells. Tex Rep Biol Med. 1967;25:257–264. [PubMed] [Google Scholar]
- 30.Olsen F. Transfer of arterial hypertension by splenic cells from DOCA-salt hypertensive and renal hypertensive rats to normotensive recipients. Acta Pathol Microbiol Scand C. 1980;88:1–5. doi: 10.1111/j.1699-0463.1980.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 31.Ba D, Takeichi N, Kodama T, Kobayashi H. Restoration of T cell depression and suppression of blood pressure in spontaneously hypertensive rats (SHR) by thymus grafts or thymus extracts. J Immunol. 1982;128:1211–1216. [PubMed] [Google Scholar]
- 32.Rudemiller N, Lund H, Jacob HJ, Geurts AM, Mattson DL, Program PK. CD247 modulates blood pressure by altering T-lymphocyte infiltration in the kidney. Hypertension. 2014;63:559–564. doi: 10.1161/HYPERTENSIONAHA.113.02191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM, Jacob H. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am J Physiol Regul Integr Comp Physiol. 2013;304:R407–R414. doi: 10.1152/ajpregu.00304.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pollow DP, Uhrlaub J, Romero-Aleshire MJ, Sandberg K, Nikolich-Zugich J, Brooks HL, Hay M. Sex Differences in T-Lymphocyte Tissue Infiltration and Development of Angiotensin II Hypertension. Hypertension. 2014;64:384–390. doi: 10.1161/HYPERTENSIONAHA.114.03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kasal DA, Barhoumi T, Li MW, Yamamoto N, Zdanovich E, Rehman A, Neves MF, Laurant P, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension. 2012;59:324–330. doi: 10.1161/HYPERTENSIONAHA.111.181123. [DOI] [PubMed] [Google Scholar]
- 36.Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension. 2011;57:469–476. doi: 10.1161/HYPERTENSIONAHA.110.162941. [DOI] [PubMed] [Google Scholar]
- 37.Tipton AJ, Baban B, Sullivan JC. Female Spontaneously Hypertensive Rats Have Greater Renal Anti-Inflammatory T Lymphocyte Infiltration Than Males. Am J Physiol Regul Integr Comp Physiol. 2012 doi: 10.1152/ajpregu.00246.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tipton AJ, Sullivan JC. Sex Differences in Blood Pressure Control: Are T Lymphocytes the Missing Link? Hypertension. 2014 doi: 10.1161/HYPERTENSIONAHA.114.03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schiffrin EL. Immune mechanisms in hypertension: how do T-regulatory lymphocytes fit in? J Hypertens. 2013;31:1944–1945. doi: 10.1097/HJH.0b013e3283638b52. [DOI] [PubMed] [Google Scholar]
- 41.Schiffrin EL. Immune mechanisms in hypertension and vascular injury. Clin Sci (Lond) 2014;126:267–274. doi: 10.1042/CS20130407. [DOI] [PubMed] [Google Scholar]
- 42.Brinson KN, Elmarakby AA, Tipton AJ, Crislip GR, Yamamoto T, Baban B, Sullivan JC. Female SHR have greater blood pressure sensitivity and renal T cell infiltration following chronic NOS inhibition than males. Am J Physiol Regul Integr Comp Physiol. 2013;305:R701–R710. doi: 10.1152/ajpregu.00226.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have a compensatory increase in renal regulatory T cells in response to elevations in blood pressure. Hypertension. 2014;64:557–564. doi: 10.1161/HYPERTENSIONAHA.114.03512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johann S, Beyer C. Neuroprotection by gonadal steroid hormones in acute brain damage requires cooperation with astroglia and microglia. J Steroid Biochem Mol Biol. 2013;137:71–81. doi: 10.1016/j.jsbmb.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 45.DonCarlos LL, Azcoitia I, Garcia-Segura LM. Neuroprotective actions of selective estrogen receptor modulators. Psychoneuroendocrinology. 2009;34(Suppl 1):S113–S122. doi: 10.1016/j.psyneuen.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Habib P, Beyer C. Regulation of brain microglia by female gonadal steroids. J Steroid Biochem Mol Biol. 2014 doi: 10.1016/j.jsbmb.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 47.Spence RD, Hamby ME, Umeda E, Itoh N, Du S, Wisdom AJ, Cao Y, Bondar G, Lam J, Ao Y, Sandoval F, Suriany S, Sofroniew MV, Voskuhl RR. Neuroprotection mediated through estrogen receptor-alpha in astrocytes. Proc Natl Acad Sci U S A. 2011;108:8867–8872. doi: 10.1073/pnas.1103833108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xue B, Pamidimukkala J, Hay M. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. American journal of physiology. Heart and circulatory physiology. 2005;288:H2177–H2184. doi: 10.1152/ajpheart.00969.2004. [DOI] [PubMed] [Google Scholar]
- 49.Xue B, Singh M, Guo F, Hay M, Johnson AK. Protective actions of estrogen on angiotensin II-induced hypertension: role of central nitric oxide. Am J Physiol Heart Circ Physiol. 2009;297:H1638–H1646. doi: 10.1152/ajpheart.00502.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marvar PJ, Lob H, Vinh A, Zarreen F, Harrison DG. The central nervous system and inflammation in hypertension. Curr Opin Pharmacol. 2011;11:156–161. doi: 10.1016/j.coph.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, Gordon FJ, Harrison DG. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circulation research. 2010;107:263–270. doi: 10.1161/CIRCRESAHA.110.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S. Expression and neuropeptidergic characterization of estrogen receptors (ERalpha and ERbeta) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurobiol. 1998;36:357–378. doi: 10.1002/(sici)1097-4695(19980905)36:3<357::aid-neu5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 53.Spary EJ, Maqbool A, Batten TF. Oestrogen receptors in the central nervous system and evidence for their role in the control of cardiovascular function. J Chem Neuroanat. 2009;38:185–196. doi: 10.1016/j.jchemneu.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 54.Xue B, Zhang Z, Beltz TG, Guo F, Hay M, Johnson AK. Estrogen regulation of the brain renin-angiotensin system in protection against angiotensin II-induced sensitization of hypertension. Am J Physiol Heart Circ Physiol. 2014;307:H191–H198. doi: 10.1152/ajpheart.01012.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xue B, Zhao Y, Johnson AK, Hay M. Central estrogen inhibition of angiotensin II-induced hypertension in male mice and the role of reactive oxygen species. American journal of physiology. Heart and circulatory physiology. 2008;295:H1025–H1032. doi: 10.1152/ajpheart.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hay M, Xue B, Johnson AK. Yes! Sex matters: sex, the brain and blood pressure. Curr Hypertens Rep. 2014;16:458. doi: 10.1007/s11906-014-0458-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Samareh- Jahani F, Xue B, Johnson AK, Hay M. Sex Differences in Microglia Activation During Subpressor Dose of Angiotensin (Ang) II Sensitization of Subsequent Pressor Dose of Ang II. Council on High Blood Pressure Research. 2014 [Google Scholar]
- 58.Lob HE, Schultz D, Marvar PJ, Davisson RL, Harrison DG. Role of the NADPH oxidases in the subfornical organ in angiotensin II-induced hypertension. Hypertension. 2013;61:382–387. doi: 10.1161/HYPERTENSIONAHA.111.00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferguson AV. Angiotensinergic regulation of autonomic and neuroendocrine outputs: critical roles for the subfornical organ and paraventricular nucleus. Neuroendocrinology. 2009;89:370–376. doi: 10.1159/000211202. [DOI] [PubMed] [Google Scholar]
- 60.Ciriello J. Afferent renal inputs onto subfornical organ neurons responsive to angiotensin II. Am J Physiol. 1997;272:R1684–R1689. doi: 10.1152/ajpregu.1997.272.5.R1684. [DOI] [PubMed] [Google Scholar]
- 61.Rosas-Arellano MP, Solano-Flores LP, Ciriello J. Co-localization of estrogen and angiotensin receptors within subfornical organ neurons. Brain Res. 1999;837:254–262. doi: 10.1016/s0006-8993(99)01672-8. [DOI] [PubMed] [Google Scholar]
- 62.Wang G, Sarkar P, Peterson JR, Anrather J, Pierce JP, Moore JM, Feng J, Zhou P, Milner TA, Pickel VM, Iadecola C, Davisson RL. COX-1-derived PGE2 and PGE2 type 1 receptors are vital for angiotensin-II-induced formation of reactive oxygen species and Ca2+ influx in the subfornical organ. Am J Physiol Heart Circ Physiol. 2013 doi: 10.1152/ajpheart.00238.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cao X, Peterson JR, Wang G, Anrather J, Young CN, Guruju MR, Burmeister MA, Iadecola C, Davisson RL. Angiotensin II-dependent hypertension requires cyclooxygenase 1-derived prostaglandin E2 and EP1 receptor signaling in the subfornical organ of the brain. Hypertension. 2012;59:869–876. doi: 10.1161/HYPERTENSIONAHA.111.182071. [DOI] [PMC free article] [PubMed] [Google Scholar]