Abstract
Background
Extended-release naltrexone (XR-NTX, Vivitrol® Alkermes Inc.) is an injectable monthly sustained-release mu opioid receptor antagonist. XR-NTX is a potentially effective intervention for opioid use disorders and as relapse prevention among criminal justice system (CJS) populations.
Methods
This 5-site open-label randomized controlled effectiveness trial examines whether XR-NTX reduces opioid relapse compared with treatment as usual (TAU) among community dwelling, non-incarcerated volunteers with current or recent CJS involvement. The XR-NTX arm receives 6 monthly XR-NTX injections at Medical Management visits; the TAU group receives referrals to available community treatment options. Assessments occur every 2 weeks during a 24-week treatment phase and at 12- and 18-month follow-ups. The primary outcome is a relapse event, defined as either self-report or urine toxicology evidence of ≥10 days of opioid use in a 28-day (4 week) period, with a positive or missing urine test counted as 5 days of opioid use.
Results
We describe the rationale, specific aims, and design of the study. Alternative design considerations and extensive secondary aims and outcomes are discussed.
Conclusions
XR-NTX is a potentially important treatment and relapse prevention option among persons with opioid dependence and CJS involvement.
Keywords: naltrexone, extended-release naltrexone, criminal justice, opioid relapse prevention
1. Introduction and background
Opioid dependence and opioid use disorders are common in the criminal justice system (CJS). Arrested individuals tested positive for opiates at rates of 5–20% in the 2013 US Arrestee Drug Abuse Monitoring Program II.1 Evidence-based treatments including methadone and buprenorphine are usually unavailable during incarceration,2,3 and rates of opioid relapse and overdose death are elevated at release.4 While these medication assisted treatment modalities are associated with improved outcomes,5,6,7,8,9,10,11 community supervision (i.e., parole, probation) authorities typically discourage their use.12 In addition, well-known stigmas and prior negative treatment experiences may bias affected individuals from pursuing these medications.2,13,14
Extended-release (XR-NTX), or sustained-release injectable naltrexone, is a long-acting medication that was approved for the treatment of opioid dependence by the US Food and Drug Administration (FDA) in 2010. XR-NTX may be particularly beneficial to criminal justice system (CJS) and opioid-involved populations, who typically emerge from incarceration ‘drug free’ and no longer physically dependent, lack ready access to agonist treatments, are at high risk for relapse, and for whom psychosocial treatment adherence, opioid-free urine samples, and frequent monitoring are often mandated conditions. Naltrexone is not a controlled substance and requires an active user detoxify prior to induction. For these myriad reasons, XR-NTX may be more readily acceptable and adaptable in CJS and other traditionally ‘drug free’ opioid treatment settings.15
XR-NTX’s sustained-release technology provides gradual release of sufficient naltrexone to block the mu opioid receptor agonist effects of up to 25 mg of intravenous heroin or an equivalent amount of other opioids for at least one month after injection.16,17 In an initial 2-site US randomized placebo-controlled trial, an alternative extended-release naltrexone formulation (Depotrex) was effective at preventing relapse after detoxification among community-recruited heroin users.18 Treatment retention was 68% after 2 months and opioid use outcomes were superior vs. placebo. A large double-blind placebo-controlled randomized trial conducted in Russia established XR-NTX’s superiority over placebo in preventing opioid use and relapse following an inpatient detoxification induction among a general adult opioid (heroin) dependent population, and was the pivotal trial leading to FDA approval.19 However, further US community and criminal justice system effectiveness trials of XR-NTX opioid treatment, including this protocol, are only now underway (NCT01180647, NCT01246401, NCT02032433, NCT01999946, and NCT02110264).
A preceding single-arm observational cohort study conducted by this trial’s 5-site consortium demonstrated the feasibility of inducting community-dwelling parolees and probationers onto XR-NTX (Depotrex).20 Participants remaining on XR-NTX for up to 6 months had lower rates of opioid use vs. earlier treatment drop outs. This earlier pilot experience greatly informed the conception and implementation of this current randomized effectiveness trial.
2. Research Design and Study Population
2.1 Study Design
This is a 5-site open-label, unblinded randomized effectiveness trial that compares 24 weeks of XR-NTX treatment vs. Treatment-as-Usual (TAU) among community-dwelling CJS-involved participants with a history of opioid dependence. The effectiveness trial design intends to estimate the benefit of XR-NTX under real world conditions.
2.2 Research questions and hypotheses
The principal research question is whether assignment of a CJS- and opioid-involved population to the XR-NTX treatment arm reduces the likelihood of an opioid relapse event, and, more generally, of overall rates of opioid use (i.e., % days of use, proportions of urines positive). We hypothesize that XR-NTX treatment assignment will be associated with a significantly lower likelihood of an opioid relapse event, longer relapse-free survival, and lower overall rates of opioid use (i.e., more opioid-free weeks).
Alongside this hypothesized primary treatment effect on opioid addictions are secondary outcomes, including potential beneficial effects of XR-NTX treatment assignment on rates of HIV risk behaviors, including intravenous (IV) drug use and unsafe sex, heavy alcohol use and non-opioid other drug misuse (i.e. cocaine), continued criminal activities, re-arrest, and re-incarceration, health and social costs, and safety events, including overdose and mortality. We hypothesize that XR-NTX treatment assignment will be associated with significantly lower rates of alcohol and non-opioid drug misuse, HIV risk behaviors, re-arrests and re-incarcerations, lower costs to the extent that XR-NTX treatment will be cost-effective, and lower rates of opioid overdose.
We are also interested in the extent to which CJS-involved participants report feeling coerced or mandated into study participation, given an overall historic bias on the part of CJS authorities against agonist medications and preferences favoring ‘drug free’ recovery. Though participation in this study is voluntary and referrals from CJS authorities are not accepted, we will assess the degree to which participants perceive their decision to participate in the study was coerced or voluntary.
2.3 Study organization and sites
Five independently funded centers are implementing a common protocol under an NIH collaborative clinical trial R01 mechanism (PAR-07-232). The lead site, the University of Pennsylvania (Philadelphia, PA), hosts the regulatory, data management, and statistical cores. The four remaining sites are New York School of Medicine and Bellevue Hospital Center (New York, NY), Brown University and Rhode Island Hospital (Providence, RI), Columbia University (NY, NY), and Friends Research Institute (Baltimore, MD).
2.4 Study population and inclusion/exclusion criteria
Eligible subjects are community-dwelling adults with criminal justice system involvement and a history of opioid dependence. Inclusion/exclusion criteria were designed to assemble a representative sample of CJS opioid dependent adults, including those with significant medical and psychiatric co-morbidities, provided study participation appears safe. Persons excluded are currently on or seeking methadone or buprenorphine treatment by self-report, which are relative contraindications to naltrexone, females planning pregnancy, and persons possibly at high risk for opioid overdose. Notably, participants in either arm are free to reconsider and pursue methadone, buprenorphine or other treatment after randomization.
Eligibility criteria are: 1) current or lifetime opioid dependence (Diagnostic and Statistical Manual, Fourth Edition);21 2) a stated goal of opiate-free treatment rather than opioid agonist/partial agonist maintenance; 3) currently opioid-free with negative urine toxicology for all opioids prior to randomization; 4) currently serving an adjudicated sentence that includes community supervision (e.g., parole, probation, outpatient drug court programs, or other court-mandated treatment) or in the past 12 months arrested or incarcerated; 5) in general good health as determined by history and physical examination; and 6) aged 18 to 60 years and able to provide informed consent. Study exclusion criteria are: 1) current other drug or alcohol dependence requiring medical detoxification or a higher level of care that would interfere with study participation; 2) women who are pregnant, planning conception, lactating, or unable to use adequate contraceptive methods; 3) medical condition that might make participation hazardous, including liver function tests >3x normal; obesity (e.g. BMI > 40) that might make it difficult to deposit the medication safely in the gluteal muscles ; 4) untreated psychiatric disorder that might make participation hazardous; 5) history of allergic reaction to naltrexone PLG, carboxymethylcellulose, or any other components of the diluent; 6) current chronic pain diagnosis for which opioids are necessary; 7) history of a drug overdose in the past 3 years requiring inpatient hospitalization.
2.5 Recruitment Procedures by Study Sites
To minimize the possibility that potential and recruited participants would feel that they had been coerced into participating in the research, study procedures prohibit direct referrals from, or communication of results to, CJS authorities, including departments of corrections, probation, parole, drug courts or other diversion programs. Recruitment procedures vary by site, but generally employ standard outreach to community-dwelling general populations and medical center and addiction treatment programs through general publicity efforts (print, radio, and on-line advertisements) and provider- and patient-level detailing (letters to clinic directors, posting study flyers):
University of Pennsylvania: recruitment at this site focuses on advertising in the waiting rooms of Philadelphia treatment programs, local newspapers, word of mouth and snowballing recruitment (incentivized) where study participants were able to refer up to 5 potential participants to the study.
New York University/Bellevue Hospital Center: located within Adult Primary Care at Bellevue Hospital, recruitment at this site focuses on community outreach (community addiction treatment programs, harm reduction centers, reentry non-government service agencies), recruitment from Bellevue’s high-volume detox unit, and word-of-mouth among current participants, who are encouraged and incentivized to refer potentially eligible acquaintances.
Brown University/Rhode Island Hospital: this site is located in the Research Section of the Division of General Internal Medicine. Participants are recruited from the Providence area through radio and print advertising, referrals from substance abuse treatment centers, and by word of mouth among current participants who are incentivized to refer others. Participants currently dependent on opioids are offered outpatient opioid detoxification treatment.
Columbia University: this site is located at the Substance Treatment and Research Service of Columbia University in Manhattan. Participants are recruited from the community through radio, print and subway advertising, and through two local community-based treatment programs to facilitate recruitment.
Friends Research Institute
The study is being conducted at Maryland Treatment Centers’ Mountain Manor Program, an accredited substance abuse treatment community provider. Participants are recruited primarily from community detoxification and residential treatment programs in Baltimore City.
2.6 Informed Consent
Telephone or in-person pre-screening briefly evaluates potentially eligible individuals in anonymous fashion and an in-person screening visit is scheduled. Per standard Good Clinical Practice guidelines, study staff administers informed consent at the initial in-person visit. The study is described in summary, and the Informed Consent Form is reviewed, including a discussion of potential risks and benefits, and emphasizing conditions of voluntary, anonymous participation. Interested participants must sign the Informed Consent Form as well as pass a Consent Quiz, which documents a basic understanding of study conditions and procedures by the participant.
2.7 Screening, Randomization, and Follow-up Procedures
Following completion of informed consent, participants are given baseline evaluations, including medical history and physical assessment, blood and urine testing, and an EKG. Study eligibility is determined after agreement between the research coordinator and study clinician, and documented using an eligibility checklist, including confirmation of recent opioid abstinence and a negative opioid urine toxicology. At a subsequent randomization visit, participants are randomized to XR-NTX vs. TAU in a 1:1 ratio stratified by gender, site, and pre-randomization detox status (needed to detox prior to study entry [yes/no]; a marker of recent active opioid use and physical dependence) via the urn method22 and using the Perry Point Veterans Administration Cooperative Studies Program Coordinating Center’s telephone randomization system.23 All screening, randomization, and follow-up visits are incentivized. Cash vs. vouchers and maximum payments across 17 scheduled visits (range, $385–820) vary by site and local standards.
3. Data management
Research coordinators and study clinicians complete assessment instruments and data entry using a direct-entry web-based data management system at the Univ. Pennsylvania Center for Studies on Addictions data coordinating center.
4. Regulatory Affairs and Data and Safety Monitoring
4.1 Approvals and Certifications
Institutional Review Board (IRB) approval is obtained at each of the 5 sites based on a common study protocol. In addition, the Univ. Pennsylvania lead site and IRB coordinate and monitor data and safety logs across the trial and host the Data and Safety Monitoring Board (DSMB). Each site submits changes to the common protocol to their respective IRBs in timely fashion. Site-specific issues, i.e. flyers or local recruitment methods, are considered by the site’s IRB only. Regulatory affairs and study status are discussed on recurring calls and periodic in-person investigator meetings.
The study initiated prior to XR-NTX’s 2010 US approval for the treatment of opioid dependence and under an Investigator New Drug application held by Dr. O’Brien (IND 102711). Clinical trial registration was completed at ClinicalTrials.gov (NCT00781898). Department of Human Services Office of Human Research Protections reviewed the approved protocol and concurred that the study met conditions (45 CRF 46.306(a)(2)(iv)) for the ethical conduct of research among prisoners (including persons mandated to community residential drug treatment or incarcerated after study enrollment). A federal Certificate of Confidentiality prevents disclosure of individual study data.
4.2 Data and Safety Monitoring
A Data and Safety Monitoring Board (DSMB) is at the Univ. Pennsylvania. Each site’s team, principal investigator, and site IRB monitor local recruitment, retention, and safety outcomes. Site and independent outside monitors annually review procedures and data quality. The Univ. Pennsylvania IRB and DSMB monitors study progress and adverse events (AE) across all sites. Adverse events (AE) and serious adverse events (SAEs) were logged in sequential, open-ended fashion during monthly assessments using AE and SAE logs. AE/SAE were determined to be medication-related by the study clinician and/or site PI. SAE are reported by each site to their own IRBs, the Univ. Pennsylvania IRB, the DSMB, and the study sponsor (NIDA). Additionally, medication-related AE are reported to the drug manufacturer (Alkermes, Inc.) and the US Food and Drug Administration.
5. Study treatments
5.1 Medical Management and XR-NTX Injection Visits
XR-NTX (Vivitrol®, Alkermes, Inc.) 380mg IM injections occur once every four weeks within a simple Medical Management (MM) visit, the first of which occurs as part of the randomization visit. Prior to the initial injection, XR-NTX participants receive a standard naloxone challenge, consisting of >0.8mg naloxone IV (SC or IM if no IV access) with a pre/post withdrawal symptom checklist. One site (FRI) then administers a 12.5mg oral naltrexone low-dose challenge followed by a 2-hour observation period, reflecting local site and IRB preferences. Following a negative challenge (defined as no significant new-onset opioid withdrawal symptoms), the initial XR-NTX IM injection is given to the outer, upper gluteal region. Medical Management counseling encompasses expected side effects (injection site pain, potential nausea and malaise following the initial injection), support for recovery and community treatment participation, and relapse and overdose risk reduction within a typical ambulatory care office visit.24 Subsequent injections occur every 4 weeks during the 6-month treatment phase (6 monthly injections). Study physicians or nurses provide MM counseling and XR-NTX injections.
TAU participants are encouraged by study staff to access appropriate community treatment and relapse prevention resources, including buprenorphine or methadone treatment providers if participants’ treatment preferences change post-randomization. All participants are given referrals to available community treatment, including preferred medication treatments, following the 24-week treatment phase. XR-NTX study treatment was not available during the subsequent 12-month long-term follow-up phase.
6. Assessments
Assessments occur at screening and randomization visits, then bi-weekly, monthly (every 4 weeks), and every 6 months (Table 1). Monthly visits included XR-NTX injections and MM visits among the XR-NTX treatment arm; all participants are otherwise assessed at the same intervals. Longer research follow-up and medical assessment visits occur at week 27 (end-of-treatment, 6 months from randomization), week 52 (12 months), and week 78 (end-of-study, 18 months) (Table 1).
Table 1.
Schedule of Assessments and Procedures
| Assessments (study weeks) | Baseline (0/1) | Monthly (3–27) | Mo. 5 (21) | Mo. 6 (27) | Mo.12 (52) | Mo. 18 (78) |
|---|---|---|---|---|---|---|
| Screening and Safety | ||||||
| Informed Consent | X | |||||
| Consent Quiz | X | X | ||||
| Vital Signs/Laboratory Test | ||||||
| Liver function | X | X | ||||
| Liver Profile (weeks) | X | X (8,16,26) | X | |||
| Hepatitis B & C, HIV | X | |||||
| Vital Signs | X | X | X | |||
| Pregnancy | X | X | X | |||
| Blood Alcohol Level (BAC) | X | 2X | X | X | X | |
| Hair Toxicology | X | X | X | X | X | |
| Urine Toxicology | X | 2X | X | X | X | |
| Research Assessments | ||||||
| Timeline Follow-Back | X | 2X | X | X | X | |
| Mini-International Neuropschiatric Interview | X | |||||
| Risk Assessment Battery | X | X | X | X | ||
| Addiction Severity Index | X | X | X | X | ||
| California Psychological Inventory-Socialization Scale | X | |||||
| Beck Depression Inventory | X | X | X | X | X | |
| Crime and Legal Activities | X | X | X | X | X | |
| Criminal Records Review** | X | |||||
| Non-Study Medical and Other Services | X | X | X | X | X | |
| Quality of Life | X | X | X | X | X | |
| Motivation for Research | X | X | ||||
| Decision Making Scale | X | X | ||||
| Adverse Events | X | X | X | X | X | |
| Procedures | ||||||
| Naloxone challenge* | X | |||||
| XR-NTX Injection* | X | X |
XR-NTX arm only.
6.1 Primary Outcome
The primary outcome is the likelihood of opioid relapse during weeks 0–25. The relapse event is defined as either self-report or urine toxicology evidence of >10 days of opioid use in a 28-day (4 week) period, with a positive or missing urine test counted as 5 days of opioid use. This primary relapse outcome is similarly defined in other related and on-going clinical trials (NCT01180647, NCT0203243). Other opioid use outcomes are rates of confirmed opioid abstinence, defined as the proportion of 2-week assessment intervals with self-report of no opioid use and negative urine results, the proportions of days of self-reported opioid use vs. abstinent days, rates of urine opioid positive or missing vs. negative results, and hair analysis.
Research assessment visits occur every two weeks to obtain urine toxicology samples and query opioid and other drug use self-report using the Timeline Follow-back (TLFB).25 Urine samples are tested using a point-of-care dip card for opiates (300ng/ml), oxycodone, methadone, and buprenorphine metabolites. Hair samples (0.5–1.5 inches from scalp) are scheduled monthly, if available, and assessed for extended opioids (codeine, morphine, heroin, hydrocodone, hydromorphone, oxycodone), cocaine, methamphetamines, PCP and THC metabolites at a central laboratory (Omega Laboratories, Inc.). Persons with minimal scalp hair are not expected to provide hair samples.
6.2 Secondary Outcomes
Secondary outcomes of interest determine the effectiveness of XR-NTX in reducing: a) HIV risk behaviors; b) alcohol and other non-opioid drug misuse; c) rates of arrests and re-incarceration; and d) costs (cost-effectiveness). The study also assesses self-reported factors influencing the decision to participate in the study, participants’ perceived voluntariness of this decision, and related ethical concerns. The same bi-weekly TLFB and urine sample assessments catalog days and quantities of alcohol use, days of cocaine use, and the presence of urine cannabis, cocaine, benzodiazepines, amphetamines, and barbiturate metabolites. Bi-weekly alcohol breathalyzer testing estimates blood alcohol levels. Rates of IV drug use and unsafe sex are captured using the Risk Assessment Battery (RAB).26 Criminal activity, re-arrest, and re-incarceration are assessed using the Addiction Severity Index Lite (ASI),27 the Crime and Legal Activities Report,28 as well as audits of state criminal records databases. Health services, employment, financial support, and quality of life are assessed using the ASI, the Euroqol EQ-5D scale, 29 and the Non-study Other Medical Service Form.30 The Motivation for Research and Decision Making self-reported scales assess factors influencing the decision to participate in the study including perceived voluntariness and coercion. They are adapted from the MacArthur Perceived Coercion Scale and previous theoretical conceptualization of factors influencing voluntariness of consent to research.31,32,33
7. Statistical analysis
Primary analyses will compare overall rates of the opioid relapse by intervention using mixed effects logistic regression models and an intent-to-treat approach. We will test whether XR-NTX prolongs time-to-relapse using Cox proportional hazards regression models, and total self-reported days of opioid use and positive urine tests using mixed linear effects model for count data.
Missed visits and missing urine data will be counted as positive for opioid use, thus drop-out will contribute to the relapse primary outcome. This is akin to a ‘present and sober (yes/no)’ approach to drug treatment outcomes and missing data. We will also model missing data as missing at random (MAR) and missing as missing (in which only obtained self-report and urine samples contribute to a primary relapse outcome) in order to estimate the impact of drop out on main effects. Likewise, we will examine rates of self-reported non-study agonist treatment to differentiate legitimate prescribing from illicit ‘street’ buprenorphine and methadone use, the former of which contributes to rates of misuse and relapse.
Rates of other drug and alcohol misuse will be examined in a similar approach to that of opioid use self-report days and urine results. We will compare the two groups on the number of arrests and re-incarceration during the 6-month treatment and 6–18-month follow-up phases of the study using Poisson or negative binomial regression models for count outcomes and Cox regression models for time to relapse, arrest and re-incarceration. HIV risk behavior analysis will compare count data of both the number of days injecting drugs and RAB risk scores. Economic and cost-effectiveness analysis will estimate the downstream benefits (i.e. costs avoided) of XR-NTX vs. TAU from societal and criminal justice system perspectives. Perceived coercion and study ’voluntariness’ will be described at baseline and follow-up.
8. Sample size, power, and effect size
The recruitment plan targets 360 subjects randomized equally to two arms across the 5 sites. The 5-site XR-NTX pilot study observed 6-month treatment retention rates of 66% (N=61), with overall relapse-free survival as defined in this protocol estimated at 30–45%.20 We expect rates of relapse-free survival to be lower among TAU, similar to rates seen in the pivotal trial’s placebo arm (20–30%).19 A sample size of 164 per group, with an assumed loss to attrition of about 5% per month, would provide 80% power to detect a hazard ratio of 1.53 or higher between the hazards in the two groups, equivalent to a 30% vs. 45% difference in relapse rates.
Results
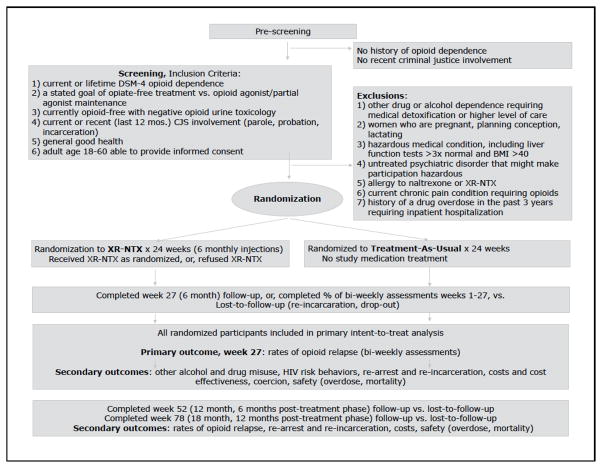
Recruitment began in February 2009 and concluded in November 2013. The 5 sites obtained informed consent from, screened, and randomized 308 eligible participants; 153 XR-NTX, 155 TAU (Fig 1). Final 18-month follow-up continues until May 2015. Retention through the 6-month treatment phase has been robust; treatment exposure among XR-NTX participants was 77% (of 6 scheduled monthly injections per participant).
Figure 1.
Study Flow, Inclusion/Exclusion Criteria, and Outcomes
Discussion
This US multisite randomized effectiveness trial of extended-release naltrexone to prevent opioid relapse among CJS-involved, community-dwelling adults is the largest such evaluation of XR-NTX’s effectiveness to date. By examining rates of opioid and other drug use, as well as HIV risk behaviors rearrest, re-incarceration, costs and cost-effectiveness, this study aims to characterize the overall impact of XR-NTX vs. usual care in a high-cost and high-risk group.
Opioid use disorders are very common among CJS populations, but access to evidence-based treatment with pharmacotherapies is poor. 34,35 CJS staff commonly view methadone and buprenorphine with suspicion, even hostility. Despite data consistently supporting agonist medications’ effectiveness, offenders are rarely required to take them as a condition of probation or parole. Recent developments in Missouri and other states suggest that antagonist therapy with naltrexone might be more acceptable to both correctional systems and CJS patient populations.15 Our previous 5-site pilot demonstrated the feasibility of outpatient sustained-release naltrexone induction and monthly treatment among parolees and probationers, and found significantly less opioid use among participants retained in naltrexone treatment for 6 months.20
The limited XR-NTX efficacy data available at this study’s proposal and launch, followed by the pivotal efficacy trial results, increased our enthusiasm for an effectiveness design. Avoiding the stricter placebo and blinding controls, adherence expectations and exclusion criteria of an efficacy trial, we have pursued greater generalizability. Eligibility criteria are broad and likely characterize a large proportion of opioid-CJS-involved individuals currently not interested in or able to access agonist treatment. Treatment assignment is un-blinded as in real world settings. Participants are encouraged to pursue a wide range of non-study community treatment. For example, subjects in either arm may choose to reconsider study participation and instead access buprenorphine and methadone treatment post-randomization, options that were not available in the Russian trial.
The psychosocial intervention among XR-NTX participants is a simplified Medical Management protocol. This platform was adapted for the trial from prior research and clinical experience with opioid and alcohol pharmacotherapies and intended to reflect the usual practice of office-based providers (i.e., general practitioners) while remaining relevant to specialty addiction treatment.24,36 We considered and rejected a standardized, more intensive and manualized psychosocial treatment, as well as more assertive ‘warm’ referrals to non-study community treatment. In both cases, investigators believed that these control conditions would far exceed community standards, which are characterized by very low rates of post-incarceration linkages to aftercare and high rates of drop out from psychosocial treatment. The intention was that actual TAU, rather than an enhanced, study-provided psychosocial intervention control, would provide a better comparison arm for a health services study and an actual test of XR-NTX’s effectiveness as it might be layered into existing community treatment.
In response to ethical concerns about potential coercion into and adverse consequences from study participation raised by the scientific reviewers of our original proposal, the study accepts no referrals from parole staff, and research staff does not communicate anything about participants in the study to the officials monitoring their parole. To examine whether these concerns are valid, a secondary study objective investigates factors influencing participants’ decision to participate in the study, the level of perceived coercion and voluntariness of this decision, participants’ understanding of risks and benefits of study participation, and social and legal circumstances that may affect study participation. Few empirical studies have examined whether situational factors, such as criminal justice supervision, impair participants’ abilities to make voluntary choices about participating in research and whether these factors distort participants’ weighing of the risks and benefits of study participation, though theoretical concerns abound.37,38,39,40 We examine participants’ perceptions of various pressures and influences on their decision to participate in the study. This includes perceptions of the voluntariness of their decision to enroll in the research and its relationship to an understanding of risks and benefits, participants’ social and legal characteristics, and reasons and motivations for enrolling in the research study, including the study’s financial incentives.41,42,43
Study limitations arise from the open-label effectiveness design, which is more vulnerable to differential attention, recall and assessment biases than an efficacy design. However, study treatment including an expensive medication is free and financially incentivized, traditional clinical trial features which are not characteristic of community treatment or an optimal pragmatic trial design. The large cohort will allow tests for multiple confounders and interactions, such as parole vs. probation status and degrees of coercion; randomization strata are otherwise few (site, gender, pre-randomization detox). Most participants are not expected to have required pre-randomization detox, meaning generalizability to the general population of active opioid users will be more limited, vs. recent or former users presently detoxed and likely to benefit from a relapse prevention intervention.
In summary, XR-NTX is increasingly considered as a therapeutic option for opioid use disorders in criminal justice settings. This multi-site, open-label randomized effectiveness trial will provide valuable evidence as to whether XR-NTX reduces relapse compared with treatment as usual among criminal-justice involved persons with opioid use disorders. Results should have important implications for state and federal agencies that seek to reduce incarceration and enhance effective community-based treatment for opioid-dependent populations in the criminal justice system.
Acknowledgments
The funding for this manuscript is provided by NIDA through a collaborative clinical trial mechanism, PAR-07-232 (R01DA024549 [PDF], R01DA024550 [TWK], R01DA024553 [CPO], R01DA024554 [EVN], R01024555 [JDL]), and additional support (K24DA022412 [EVN]). Study medication is provided in-kind from an investigator-initiated grant from Alkermes, Inc. Funding from the Dana Foundation to COB supported the conduct of the 5-site pilot study. The authors would like to thank the study participants and combined research staff across the 5 sites.
Footnotes
ClinicalTrials.gov: NCT00781898
Conflicts of Interest: Dr. Lee has received investigator initiated study funding and study drug in-kind from Alkermes and Reckitt Benckiser for additional studies. Drs. Gordon and Kinlock received investigator initiated study funding and study drug in-kind from Alkermes for an additional study. Dr. Nunes has received: medication in-kind for research studies from Alkermes/Cephalon, Inc., Reckitt-Benckiser, and Duramed Pharmaceuticals; web-based behavioral intervention for research study from HealthSim, LLC; devices under investigation and reimbursement for travel for investigators’ meeting from Brainsway. He was paid an honorarium and received reimbursement for travel for attendance at a Lilly Advisory Board Meeting in January 2012 and received educational materials from Otsuka America Pharmaceutical, Inc. in 2013. He plans to serve on Advisory Board for Alkermes in October 2014. Dr. O’Brien has served as a consultant to Alkermes.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Office of National Drug Control Policy. 2013 Annual Report, Arrestee Drug Abuse Monitoring Program II. Washington, DC: Executive Office of the President; 2014. [Google Scholar]
- 2.Lee JD, Rich JD. Opioid pharmacotherapy in criminal justice settings: now is the time. Subst Abus. 2012;33(1):1–4. doi: 10.1080/08897077.2011.616797. [DOI] [PubMed] [Google Scholar]
- 3.Nunn A, Zaller N, Dickman S, Trimbur C, Nijhawan A, Rich JD. Methadone and buprenorphine prescribing and referral practices in US prison systems: results from a nationwide survey. Drug Alcohol Depend. 2009;105(1–2):83–8. doi: 10.1016/j.drugalcdep.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binswanger IA, Blatchford PJ, Mueller SR, Stern MF. Mortality after prison release: opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Ann Intern Med. 2013;159(9):592–600. doi: 10.7326/0003-4819-159-9-201311050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Degenhardt L, Larney S, Kimber J, et al. The impact of opioid substitution therapy on mortality post-release from prison: retrospective data linkage study. Addiction. 2014 Aug;109(8):1306–17. doi: 10.1111/add.12536. [DOI] [PubMed] [Google Scholar]
- 6.Perry AE, Neilson M, Martyn-St James M, et al. Pharmacological interventions for drug-using offenders. Cochrane Database Syst Rev. 2013;12:CD010862. doi: 10.1002/14651858.CD010862. [DOI] [PubMed] [Google Scholar]
- 7.Chandler RK, Fletcher BW, Volkow ND. Treating drug abuse and addiction in the criminal justice system: improving public health and safety. JAMA. 2009;301(2):183–90. doi: 10.1001/jama.2008.976. Erratum in: JAMA 2009, 301(10),1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magura S, Lee JD, Hershberger J, et al. Buprenorphine and methadone maintenance in jail and post-release: a randomized clinical trial. Drug Alcohol Depend. 2009;99(1–3):222–30. doi: 10.1016/j.drugalcdep.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon MS, Kinlock TW, Schwartz RP, Couvillion KA, Sudec LJ, O’Grady KE, Vocci FJ, Shabazz H. Buprenorphine Treatment for Probationers and Parolees. Subst Abus. 2014;4:0. doi: 10.1080/08897077.2014.902787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon MS, Kinlock TW, Schwartz RP, Fitzgerald TT, O’Grady KE, Vocci FJ. A randomized controlled trial of prison-initiated buprenorphine: Prison outcomes and community treatment entry. Drug Alcohol Depend. 2014;142:33–40. doi: 10.1016/j.drugalcdep.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon MS, Kinlock TW, Schwartz RP, O’Grady KE. A randomized clinical trial of methadone maintenance for prisoners: findings at 6 months post-release. Addiction. 2008;103(8):1333–42. doi: 10.1111/j.1360-0443.2008.002238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedmann PD, Hoskinson R, Gordon M, et al. Mat Working Group Of CJ-DATS. Medication-assisted treatment in criminal justice agencies affiliated with the criminal justice-drug abuse treatment studies (CJ-DATS): availability, barriers, and intentions. Subst Abus. 2012;33(1):9–18. doi: 10.1080/08897077.2011.611460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Awgu E, Magura S, Rosenblum A. Heroin-dependent inmates’ experiences with buprenorphine or methadone maintenance. J Psychoactive Drugs. 2010;42(3):339–46. doi: 10.1080/02791072.2010.10400696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell SG, Kelly SM, Brown BS, et al. Incarceration and opioid withdrawal: the experiences of methadone patients and out-of-treatment heroin users. J Psychoactive Drugs. 2009;41(2):145–52. doi: 10.1080/02791072.2009.10399907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finigan MW, Perkins T, Zold-Kilbourn P, Parks J, Stringer M. Preliminary evaluation of extended-release naltrexone in Michigan and Missouri drug courts. J Subst Abuse Treat. 2011;41(3):288–93. doi: 10.1016/j.jsat.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Comer SD, Collins ED, Kleber HD, Nuwayser ES, Kerrigan JH, Fischman MW. Depot naltrexone: long-lasting antagonism of the effects of heroin in humans. Psychopharmacology. 2002;159(4):351–60. doi: 10.1007/s002130100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bigelow GE, Preston KL, Schmittner J, Dong Q, Gastfriend DR. Opioid challenge evaluation of blockade by extended-release naltrexone in opioid-abusing adults:dose-effects and time-course. Drug Alcohol Depend. 2012;123(1–3):57–65. doi: 10.1016/j.drugalcdep.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, Kampman K, Dackis C, O’Brien CP. Injectable, sustained-release naltrexone for the treatment of opioid dependence: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2006;63(2):210–8. doi: 10.1001/archpsyc.63.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377(9776):1506–13. doi: 10.1016/S0140-6736(11)60358-9. [DOI] [PubMed] [Google Scholar]
- 20.Coviello DM, Cornish JW, Lynch KG, Boney TY, Clark CA, Lee JD, Friedmann PD, Nunes EV, Kinlock TW, Gordon MS, Schwartz RP, Nuwayser ES, O’Brien CP. A multisite pilot study of extended-release injectable naltrexone treatment for previously opioid-dependent parolees and probationers. Subst Abus. 2012;33(1):48–59. doi: 10.1080/08897077.2011.609438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 22.Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol Suppl. 2004;12:70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- 23.Veterans Administration, Office of Research and Development, CSP Coordinating Center, Perry Point, MD. [accessed Jan. 1, 2015]; http://www.research.va.gov/programs/csp/perrypoint.cfm.
- 24.Lee JD, Grossman E, DiRocco D, et al. Extended-release naltrexone for treatment of alcohol dependence in primary care. J Subst Abuse Treat. 2010;39(1):14–21. doi: 10.1016/j.jsat.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Sobell LC, Sobell MB. Alcohol Timeline Followback Users’ Manual. Toronto, Canada: Addiction Research Foundation; 1995. [Google Scholar]
- 26.Watkins KE, Metzger D, Woody G, McLellan AT. High-risk sexual behaviors of intravenous drug users in- and out-of-treatment: implications for the spread of HIV infection. Am J Drug Alcohol Abuse. 1992;18(4):389–98. doi: 10.3109/00952999209051037. [DOI] [PubMed] [Google Scholar]
- 27.McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 28.Nurco DN, Kinlock TW, Balter MB. The severity of preaddiction criminal behavior among urban, male narcotic addicts and two nonaddicted control groups. Journal of Research in Crime and Delinquency. 1993;30(3):293–316. [Google Scholar]
- 29.EuroQol Group. EuroQol-A new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 30.McLellan AT, Alterman AI, Cacciola J, Metzger D, O’Brien CP. A new measure of substance abuse treatment: initial studies of the treatment services review. J Nervous Mental Dis. 1992;180(2):101–110. doi: 10.1097/00005053-199202000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Gardner W, Hoge S, Bennett N, Roth L, Lidz C, Monahan J, Mulvey E. Two scales for measuring patients’ performance perceptions of coercion during hospital admission. Behavioral Sciences and the Law. 1993;20:307–321. doi: 10.1002/bsl.2370110308. [DOI] [PubMed] [Google Scholar]
- 32.Nelson RM, Merz JF. Voluntariness of consent for research: an empirical and conceptual review. Med Care. 2002;40(9 Suppl):V69–80. doi: 10.1097/01.MLR.0000023958.28108.9C. [DOI] [PubMed] [Google Scholar]
- 33.Appelbaum PS, Lidz CW, Klitzman R. Voluntariness of consent to research: a conceptual model. Hastings Cent Rep. 2009;39(1):30–9. doi: 10.1353/hcr.0.0103. [DOI] [PubMed] [Google Scholar]
- 34.Kinlock TW, Battjes RJ, Schwartz RP MTC Project Team. A novel opioid maintenance program for prisoners: report of post-release outcomes. Am J Drug Alcohol Abuse. 2005;31(3):433–54. doi: 10.1081/ada-200056804. [DOI] [PubMed] [Google Scholar]
- 35.Friedmann PD, Hoskinson R, Gordon M, et al. Mat Working Group Of CJ-DATS. Medication-assisted treatment in criminal justice agencies affiliated with the criminal justice-drug abuse treatment studies (CJ-DATS): availability, barriers, and intentions. Subst Abus. 2012;33(1):9–18. doi: 10.1080/08897077.2011.611460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anton RF, O’Malley SS, Ciraulo DA, et al. COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003–17. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 37.Kipnis K. Ethical and Policy Isuses in Research Involving Human Research Participants. Bethesda: National Bioethics Advisory Commission; 2001. Vulnerability in research subjects: a bioethical taxonomy; pp. G1–G13. [Google Scholar]
- 38.Pace C, Miller F, Danis M. Enrolling the uninsured in clinical trials: an ethical perspective. Crit Care Med. 2003;31(3):S121–125. doi: 10.1097/01.CCM.0000054907.33928.48. [DOI] [PubMed] [Google Scholar]
- 39.London A. Undue inducements and reasonable risks: Will the dismal science lead to dismal research ethics? Am J Bioeth. 2005;5(5):29–32. doi: 10.1080/15265160500245105. [DOI] [PubMed] [Google Scholar]
- 40.McGregor J. “Undue inducement” as coecive offers. Am J Bioeth. 2005;5(5):24–25. doi: 10.1080/15265160500245048. [DOI] [PubMed] [Google Scholar]
- 41.Appelbaum PS, Lidz CW, Klitzman R. Voluntariness of consent to research: a preliminary empirical investigation. IRB. 2009;31(6):10–4. [PubMed] [Google Scholar]
- 42.Dugosh KL, Festinger DS, Croft JR, Marlowe DB. Measuring coercion to participate in research within a doubly vulnerable population: initial development of the coercion assessment scale. J Empir Res Hum Res Ethics. 2010;5(1):93–102. doi: 10.1525/jer.2010.5.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller VA, Ittenbach RF, Harris D, Reynolds WW, Beauchamp TL, Luce MF, Nelson RM. The decision making control instrument to assess voluntary consent. Med Decis Making. 2011;31(5):730–41. doi: 10.1177/0272989X11398666. [DOI] [PMC free article] [PubMed] [Google Scholar]