Abstract
Objective
To investigate the effects of anodal transcranial direct current stimulation (a-tDCS) intensity on corticospinal excitability and affected muscle activation in individuals with chronic spinal cord injury (SCI).
Design
Single blind, randomized, sham-controlled, crossover study.
Setting
Medical Research Institute and Rehabilitation Hospital.
Participants
Nine volunteers with chronic SCI and motor dysfunction in wrist extensor muscles.
Intervention
Three single session exposures to 20 minutes of a-tDCS (anode over the extensor carpi radialis (ECR) muscle representation on the left primary motor cortex, cathode over the right supraorbital area), using 1 mA, 2 mA or sham stimulation, delivered at rest, with at least one week between sessions.
Outcome Measures
Corticospinal excitability was assessed with motor evoked potentials (MEPs) from the ECR muscle using surface electromyography (EMG) following transcranial magnetic stimulation. Changes in spinal excitability, sensory threshold and muscle strength were also investigated.
Results
Mean MEP amplitude significantly increased by ~40% immediately following 2 mA a-tDCS (Pre 0.36±0.1 mV; Post 0.47±0.11 mV; p=0.001), but not with 1 mA or sham. Maximal voluntary EMG measures remained unaltered across all conditions. Sensory threshold significantly decreased over time following 1 mA (p=0.002) and 2 mA (p=0.039) a-tDCS, and did not change with sham. F-wave persistence showed a non-significant trend for increase (Pre: 32±12%; Post: 41±10%; Follow-up: 46±12%) following 2 mA stimulation. No adverse effects were reported with any of the experimental conditions.
Conclusion
Anodal-tDCS can transiently raise corticospinal excitability to affected muscles in chronic SCI patients following 2 mA stimulation. Sensory perception can improve with both 1 and 2 mA stimulation. This study gives support to the safe and effective use of a-tDCS using small electrodes in SCI patients, and highlights the importance of stimulation intensity.
Keywords: transcranial direct current stimulation, spinal cord injury, upper extremity, corticospinal excitability, neuromodulation
In 2013, an estimated 273,000 individuals (range: 238,000 to 332,000) in the United States were reported to be suffering from impairment as a result of spinal cord injury (SCI).1 The estimated incidence rate of new cases was 12,000 per annum, with approximately half of the reported cases resulting in tetraplegia (injury to the cervical spine),1 leading to a loss of arm and/or hand function. This loss of upper-limb function is perceived by many to be the greatest debilitating loss following SCI.2,3 Varying degrees of impairment can severely limit the level of independence4-6 and increase the risk of developing secondary health problems such as cardiovascular disease due to physical inactivity.7 Consequently, recovery of motor activity and residual muscle strength is a major area of interest in rehabilitation aiming to improve the quality of life of individuals with SCI.8
Rehabilitation strategies for individuals with tetraplegia are extensive, involving surgical, pharmacological and/or physical exercise interventions.9 Existing SCI therapies involving exercise training,7 neuromuscular stimulation,10 massed practice11 and robotic-assisted training12 have all shown some degree of improved motor strength and/or function. Despite these exciting results, more effective interventions for improving upper-limb function, and understanding the mechanisms of motor recovery, are still needed.
A previous study by the authors showed that clinically weak muscles due to chronic SCI may still have intact motor evoked responses when tested by transcranial magnetic stimulation (TMS),13 uncovering an anatomical substrate for recovery. Therefore, paralyzed muscles that respond to TMS may have the ability to regain some functionality by exploiting therapeutic approaches targeting the brain like transcranial direct current stimulation (tDCS). The most encouraging evidence for the use of anodal-tDCS (a-tDCS) in patient populations is derived largely from studies conducted in the area of Stroke. Studies show that an increase in cortical excitability targeting areas of the brain controlling muscles with reduced output is correlated with better motor performance.14 Although recovery of motor function following SCI largely depends on the amount of intact anatomic connections, recovery may also depend upon plasticity of the motor cortex and the corticospinal tract (CST)6 as seen in the stroke population.
Neural plasticity occurs spontaneously after SCI, supported by evidence that the sensory-motor cortex can undergo reorganization after SCI.15,16 Other recovery mechanisms may include nerve root recovery, axonal sprouting and changes in gray matter at or neighboring the level of the spinal cord lesion.17-19 Rearrangement or creation of new circuitry within the CST may also be crucial for functional recovery, as shown in rodent studies.20 Despite these findings, more work is needed to understand how plasticity in the human primary motor cortex (M1) and CST is associated with recovery of motor function.
The main aim of this feasibility and proof-of-principle study was to investigate the effectiveness of single-session a-tDCS interventions at different intensities (1 mA, 2 mA, sham) when targeting upper-limb muscles, caudal to the spinal lesion, with diminished motor output in individuals with chronic SCI. Smaller electrodes have been shown to increase focality and local intensity in the produced electric fields, compared to standard larger electrodes (35 cm2),21,22 therefore smaller Pi electrodes (3.14 cm2) have been used to deliver the direct current stimulation in the present study. A secondary aim was to test the safety of 1 and 2 mA a-tDCS using 3.14 cm2 (Pi) electrodes on individuals with chronic SCI. We hypothesized that a-tDCS would be a safe and effective method for enhancing corticospinal excitability and the magnitude of change would be dependent upon stimulation strength.
Methods
Participants and study design
Nine volunteers with SCI (five males, four females) aged 20-56 years participated in the study. Individuals were recruited if they fulfilled the following criteria: traumatic SCI at the cervical level (C4-C7); some degree of motor function in wrist extension scoring 1-4 over 5 on the Medical Research Council scale for motor strength in the right extensor carpi radialis (ECR) muscle; a chronic injury (>8 months after injury); and tolerance to sitting upright for at least one hour. Individuals were excluded if they were medically unstable or had: a change in medication during the study, a progressive neurodegenerative disorder; concomitant traumatic brain injury or stroke; clinically significant cognitive impairment; or presented contraindications to brain stimulation (history of seizures/epilepsy, presence of metallic implants in the brain, pacemaker, pregnancy).
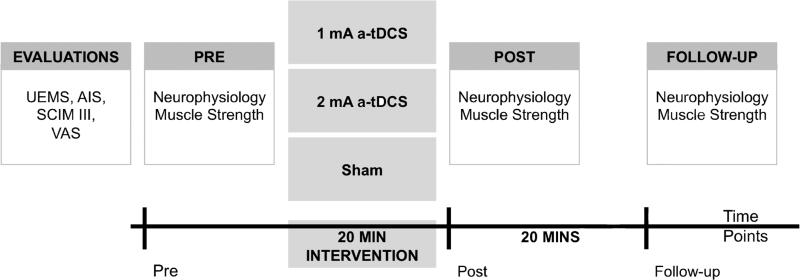
Participants randomly receive either 1 or 2 mA a-tDCS, or sham stimulation. Clinical and functional evaluations were performed prior to the brain stimulation intervention, and included the Upper Extremity Motor Score (UEMS), American Spinal Injury Association Impairment Scale (AIS), Spinal Cord Independence Measure (SCIM III) and Visual Analog Scale (VAS) pain questionnaires. Outcome measures included changes in: a) corticospinal excitability, b) spinal excitability, c) sensory threshold, and d) muscle maximum voluntary contraction (MVC). These measures were recorded before (pre), immediately after (post) and 20 minutes following (follow-up) the end of each intervention (Figure 1).
Figure 1.
Study design schematic. During the first visit, initial evaluations (Upper Extremity Motor Score: UEMS; American Spinal Injury Association Impairment Scale: AIS) and questionnaires (Spinal Cord Independence Measure: SCIM III; and Visual Analog Scale: VAS) were completed. During the 20-minute intervention period, participants received either 1 or 2 mA of anodal transcranial direct current stimulation (a-tDCS) or sham. Neurophysiology and muscle strength measures were recorded at three time points (pre, post and follow-up).
The study was approved by the Burke Medical Rehabilitation Institutional Review Board and conformed to the standards set out by the 1964 Declaration of Helsinki.
Transcranial direct current stimulation (tDCS) intervention
Participants remained seated in their own wheelchair or were provided with a comfortable chair. The StarstimNE non-invasive wireless tDCS neurostimulator (NE Neuroelectrics®, Barcelona, Spain)a was used to deliver the direct current. The StarstimNE neurostimulator included a wireless neoprene cap based on the International 10-20 System, which was placed on each participant's head by aligning the central CZ electrode position with the vertex.
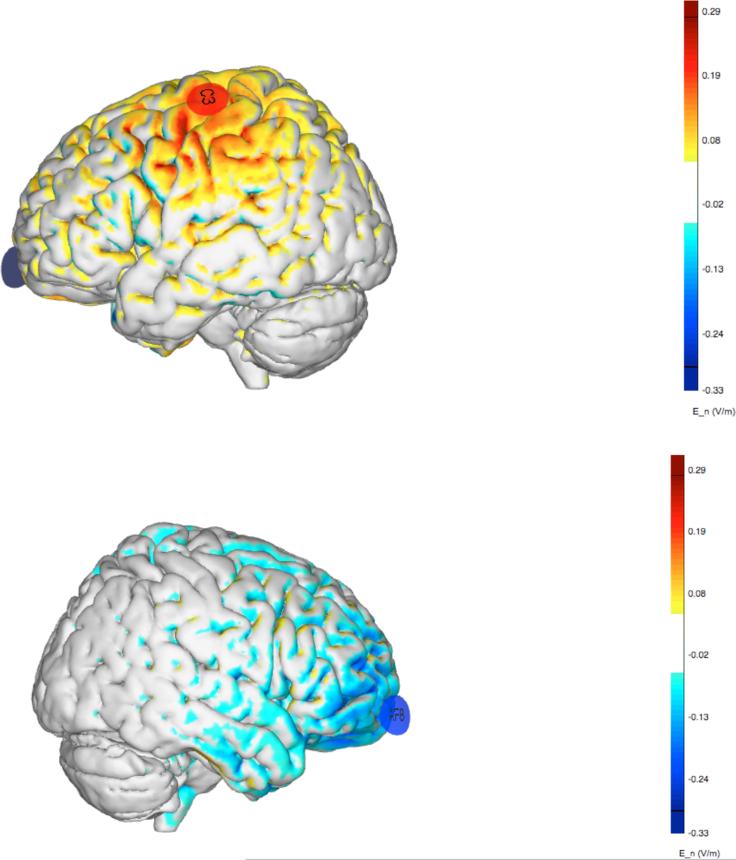
Small Ag/AgCl gelled electrodes, with a surface contact area of 3.14 cm2, specific to the StarstimNE device (Pi electrodes, Neuroelectrics®), were placed over the left M1 at the optimal site for the right ECR muscle (C3; anode) and the contralateral supraorbital area (AF8; cathode, Figure 2). The electrodes were connected to a control device, which was wirelessly connected to a computer with NIC software (version 1.2, Neuroelectrics®)a.
Figure 2.
Electric field (normal component) generated by the montage (+C3, −AF8) using Pi electrodes (3.14 cm2 Ag/AgCl electrodes). Positive/negative values indicate anodal/cathodal stimulation (normal component of the electric field pointing inward/outward at the cortical surface).
During anodal stimulation, direct current was delivered from a current-control circuit in a battery-driven stimulator inside the control device. The current was set at either 1 or 2 mA intensity and applied for 20 minutes. For the sham stimulation, electrodes were placed in the same position and participants received a short ramp up/down event at the beginning and end of the stimulation period without any current between the two events.23
Electromyography (EMG) and transcranial magnetic stimulation (TMS)
A bipolar surface EMG electrode (1 cm diameter, 2 cm inter-pole distance; Biometrics Ltd, UK)b was placed over the right ECR muscle, with the forearm relaxed in a pronated position and supported by a cushion. The EMG activity was amplified and filtered on site (x1000 gain, band-pass filter 20-400 Hz), digitized at 2 kHz (CED 1401, Cambridge Electronic Design, Cambridge, UK)c and stored for offline analysis using Spike 2.6 software. Measurements were performed at rest and during a maximal muscle contraction. During the experiment, free running EMG was continuously monitored with visual feedback of EMG silence to ensure complete muscle relaxation during resting trials.
The ECR muscle was selected for recording because restoration of motor function in this muscle can help increase independence with activities of daily living, such as self-feeding, bathing, dressing, and toileting; and with mobility needs, such as surface transfers, transitional movements, crutch walking, and wheeled mobility.3
A figure-of-eight coil (Model DB-80, Tonika Elektronik A/S, Farum, Denmark)d, connected to a MagPro X100 Series magnetic stimulator (MagVenture A/S, Farum, Denmark)d was placed congruent to the head with the handle rotated 45° lateral from mid-sagittal to induce currents in the brain perpendicular to the central sulcus. The optimal site for eliciting the greatest motor evoked potential (MEP) amplitude from the right ECR muscle was identified by moving the coil in 1 cm steps around the initial stimulation site while delivering single TMS pulses at constant suprathreshold intensity. Resting motor threshold (rMT) was defined as the minimum TMS intensity required to elicit a reliable MEP amplitude of >50 μV in at least 50% of consecutive trials.
Care was taken to control the stimulus parameters, time of day, equipment, and procedure between sessions as well as the participant's arousal level.
Peripheral nerve stimulation
Electrical stimulation (ES) to the right radial nerve was delivered using a Digitimer DS7AH constant current stimulator (Digitimer Ltd., UK; 200 μs duration; square pulses)e with surface bipolar electrodes secured in place 8-10 cm above the elbow on the lateral upper arm. The same intensity was used throughout the session and supramaximal M-wave amplitude was monitored to ensure it remained constant across each time point.
Outcome measures
Neurophysiological outcomes
The neurophysiology evaluation consisted of: a) corticospinal excitability: resting MEP amplitude, b) sensory threshold and c) spinal excitability: F-wave persistence.
Resting MEP amplitude was measured during 12 single-pulse TMS stimuli set at 130% of the rMT and applied to the left M1 optimal site for the right ECR muscle.
Sensory perceptual threshold was measured using ES to the right radial nerve. Sensory threshold was determined by decreasing the stimulation intensity in large decrements every 5 seconds, with smaller steps of 0.5 mA when approaching the threshold. At every step, the participant was asked if they could still feel the ES. The lowest stimulation perceived by the participant was recorded.
In order to investigate the effects of a-tDCS on spinal excitability, F-wave persistence was calculated by applying supramaximal ES over the right radial nerve during 20 consecutive stimuli, separated by a 5 second rest period.
Maximal voluntary contraction (MVC)
To determine the effects of a-tDCS on voluntary motor activity, root mean square (RMS) measured surface EMG activity during three attempted MVCs of the right ECR muscle.
Safety
Safety of using 1 and 2 mA a-tDCS was assessed through a standard adverse event report questionnaire recording responses to: Did you experience any headaches, neck and scalp pain, scalp redness or burns, tingling sensations, sleepiness, trouble concentrating, or acute mood changes as a direct result of the tDCS stimulation?24
Data analysis
Average peak-to-peak amplitude was determined for MEPs during rest and maximal contraction. The first two resting responses were excluded to allow responses to settle, resulting in 10 MEPs being used for analysis. During each attempted MVC, voluntary motor activity measured by RMS was assessed (rectified, average EMG over 0.5 second window).
Sensory threshold was recorded as a single value at each time point. Supramaximal M-wave amplitude was measured and averaged from 20 stimuli. F-wave persistence was calculated by dividing the number of present F-waves by the number of peripheral stimuli (20 stimuli), and representing the value as a percentage.
Raw and normalized values were used for analysis. Results are presented as mean ± SD unless otherwise stated.
Statistical analysis
A two-way repeated measures ANOVA (rmANOVA) was used to compare changes in outcome measures induced by the three interventions (1 mA/2 mA/sham; n=9) at the three different time points (pre, post and follow-up). Multiple two-way rmANOVAs were also performed to compare changes between pairs of interventions (1 mA/sham; 2 mA/sham; 1 mA/2 mA) at three different time points. One-way rmANOVAs of individual interventions were performed when a significant effect was found in the pairs. Two-tailed paired t-Tests of individual time points, between different interventions or within the same intervention, were also performed. The stimulus intensities of both the cortical and peripheral stimuli were analyzed by a two-tailed paired t-Test between sessions.
When a significant interaction effect was found, post-hoc comparisons were performed using a Bonferroni correction for multiple comparisons. If the Mauchly's test for sphericity was violated, the Huynh-Feldt correction was used. Statistical analysis was carried out with Predictive Analytics Software IBM (SPSS) Statistics Version 21.0f. Significance was set at p<0.05.
Results
Participant clinical characteristics, baseline data
Nine SCI participants (5 males, 4 females; 40.8±14.2 years, range 20-56 years) with motor complete or incomplete (5 AIS-B, 4 AIS-C) chronic traumatic lesions at the cervical level (C4-C6) completed the study (see Table 1). All but one was right-handed prior to injury, and the average time since injury was 5.9±2.9 years (range 0.75-10.5 years).
Table 1.
Patient characteristics
| ID | Age (yrs) | Gender | Handedness | Time since injury | Level of injury | AIS | Right ECR Motor Power | UEMS score | SCIM-III score | VAS score |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 34 | M | R | 10Y 6M | C4 | B | 2 | 21 | 31 | PF |
| 2 | 32 | F | R | 5Y 8M | C6 | B | 3 | 22 | 29 | HI |
| 3 | 55 | F | R | 5Y 0M | C6 | B | 3 | 24 | 31 | HI |
| 4 | 55 | M | R | 4Y 11M | C5 | B | 4 | 28 | 20 | PF |
| 5 | 45 | M | R | 8Y 10M | C5 | C | 4 | 27 | 52 | HI |
| 6 | 22 | M | L | 6Y 10M | C5 | C | 4 | 25 | 43 | PF |
| 7 | 20 | M | R | 0Y 9M | C5 | B | 4 | 27 | 68 | LI |
| 8 | 48 | F | R | 5Y 6M | C4 | C | 4 | 25 | 88 | LI |
| 9 | 56 | F | R | 5Y 6M | C5 | C | 4 | 45 | 83 | HI |
Note. ECR: Extensor Carpi Radialis [right; 0-5]. AIS: American Spinal Injury Association Impairment Scale (B, motor complete; C, sensory and motor incomplete). UEMS: Upper Extremity Motor Scores form the American Spinal Injury Association Scale [right and left; 0-50]. SCIM-III: Spinal Cord Independence Measure questionnaire [0-100]. VAS: Visual Analogue Scale. PF: Pain free. LI: Low intensity pain. HI: High intensity pain.
All participants had severe upper limb impairment, with lack of motor control in forearm muscles. The UEMS graded five muscles from 0 (total paralysis) to 5 (full range active movement against gravity and normal resistance).25 The total UEMS for the right arm was 13.7±3.9 (median 12; range 10-21) and for the left arm 13.4±4.8 (13; 6-24). More specifically, motor power for the five muscles on the right were: elbow flexors 4.9±0.3 (5; 4-5); wrist extensors 3.6±0.7 (4; 2-4); elbow extensors 3.2±1.1 (3; 2-5); finger flexors 0.9±1.4 (0; 0-4); and finger abductors 1.1±1.5 (1; 0-4).
The SCIM-III questionnaire was completed to assess three areas of function (self care, respiration and sphincter management, and mobility) with an overall score ranging from 0 (total dependence) to 100 (complete independence). The total and three sub-domain scores were 49.4±24.9 (SCIM-III total), 9.9±6.9 (self-care), 27.1±8.7 (respiratory and sphincter management), and 12.4±10.3 (mobility – ‘in’ and ‘out’). Based on the VAS, three participants were classified as ‘pain free’, two as ‘low intensity pain’, and four with ‘high intensity pain’.
Corticospinal excitability results
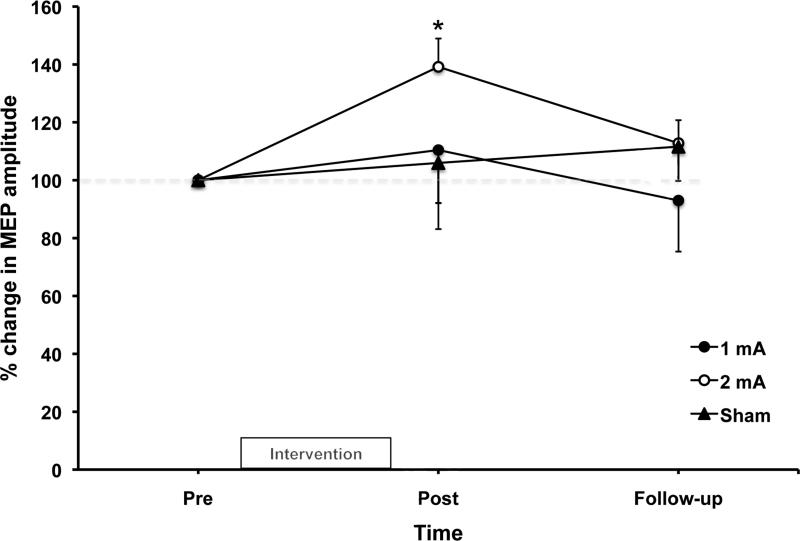
Baseline values for resting MEP amplitude were similar between interventions (average: 0.37±0.05 mV; mean±SE). A significant interaction effect (F(4,32)=4.955; p=0.003) was found for the changes in MEP amplitude among the interventions. Further analysis showed a significant mean increase of 21% post 2 mA a-tDCS when compared to baseline (F(2,16)=7.377; p=0.005; see Figure 3), with a significant increase of ~40% seen from pre to post (0.36±0.1 to 0.47±0.11 mV; mean±SE; p=0.001). No changes were observed for 1 mA a-tDCS or sham.
Figure 3.
Normalized motor evoked potential (MEP) amplitude changes over time (n=9). Values are presented as mean ± SE.
The stimulation intensities used to obtain the rMT were not significantly different between 1 mA (64±17% maximal stimulator output; MSO), 2 mA (59±9% MSO) or sham (66±16% MSO). All participants presented MEP responses.
Sensory threshold
Baseline sensory threshold was similar between interventions. A rmANOVA showed a significant difference between interventions (F(2,16)=13.63; p=0.000). Further analysis showed significant changes for both 1 mA (F(2,16)=9.673; p=0.002) and 2 mA (F(2,16)=4.0; p=0.039) a-tDCS. Additional t-Test analysis revealed a significant difference between pre and post (4.7±1.2 to 4.2±1.2; mean±SE; p=0.009) and pre and follow-up (4.7±1.2 to 4.0±1.2; mean±SE; p=0.012) for 1 mA a-tDCS; and pre and follow-up (5.2±1.9 to 4.4±1.2; mean±SE; p=0.05) for 2 mA a-tDCS. No changes were observed following sham.
Spinal excitability results
F-waves were present in ~33% of stimuli and remained constant throughout the study. Despite the lack of significant changes, 2 mA a-tDCS displayed a tendency for increased spinal excitability in F-wave persistence (pre: 32±12%; post: 41±10%; follow-up: 46±12%; mean±SE). Supramaximal M-wave amplitude was not significantly different across interventions and remained consistent throughout the study.
Muscle strength results: Maximum voluntary contraction (MVC)
No changes in RMS were found among the three interventions.
Safety assessment
Overall, participants tolerated the intervention well. One participant reported a dull headache around the right supraorbital region following 2 mA a-tDCS. Another reported sensitivity to light following 1 mA a-tDCS. The same individual reported a mild transient headache following sham. Four participants reported itching under the electrodes during 1 mA a-tDCS and five reported itching under the electrodes during 2 mA a-tDCS. All symptoms dissipated soon after the cessation of the intervention and ranged from mild to moderate in intensity. Importantly, active a-tDCS did not worsen pain in any of the participants, with two participants (1 high intensity pain, 1 low intensity pain) reporting a decrease in pain symptoms the next day following 1 mA a-tDCS. Only one of these participants (1 high intensity pain) reported a further decrease in pain symptoms following 2 mA a-tDCS.
Discussion
The observed transient improvements in the human motor and sensory systems following a-tDCS for 20 minutes, supports the application of a-tDCS in individuals following chronic SCI. The magnitude of change in corticospinal excitability appeared to be intensity dependent and improvements in sensory perception were more sensitive. These findings lend support to the theory that muscles with reduced motor output can demonstrate an a-tDCS-related improvement in corticospinal activation, regardless of the pre-existing deficit in motor performance/strength.
Corticospinal excitability following anodal transcranial direct current stimulation (a-tDCS)
Several studies have investigated corticospinal excitability following 1 mA26-28 and 2 mA29-33 a-tDCS, usually with large sponge electrodes (25 or 35 cm2).22 However there are some studies investigating stimulation strength as low as 0.2 mA,14,28 and as high as 5 mA.26 Stimulation duration is commonly reported between 10 and 20 minutes, although shorter durations of 5 minutes or less have also been used.28,34 The results of these studies suggest that longer-lasting robust effects are usually found with higher intensities (2 mA)28 and/or longer (≥10 minutes) durations,28,34 though higher intensities and longer durations have not been extensively tested. Nitsche and Paulus28 attribute the enhanced effects to more robust neurophysiological changes. However, the relationship of physiological changes to stimulation is less understood in neurological populations, and no study to date has systematically investigated corticospinal excitability following 20 minutes of 1 and 2 mA a-tDCS in a single-session randomized, sham-controlled study in chronic SCI.
In the present study, increased MEP amplitude was observed following 20 minutes of 2 mA a-tDCS, in line with the assumption that motor excitability is dependent on stimulation intensity; as 1 mA failed to significantly increase responses. Based on healthy studies, increased corticospinal excitability following brain stimulation can be associated with increased spontaneous firing rates, prolonged membrane potential shifts,28,35 long-term potentiation (LTP)-like mechanisms,35,36 and/or decreased inhibitory interneuronal activity.37,38 After SCI, some axons of the CST at the site of the injury will be damaged. It is possible that spontaneous creation of alternate circuits may restore some function by rerouting the signals from above to below the injury.17 However as the study involved a single session of a-tDCS, sprouting of corticospinal axons is unlikely to have occurred due to the effects of stimulation. More research is needed to further elucidate the part that each mechanism plays.
Despite the post-intervention MEP amplitude increase following 2 mA, changes in corticospinal excitability were relatively short-lived. A possible explanation for the lack of prolonged effects is that the responses following a-tDCS ceased prematurely. In the present study, excitability was measured immediately and 20 minutes after the application of a-tDCS. These time points may not have been long enough to uncover tDCS-related effects. Based on the findings of Batsikadze and colleagues33 with healthy subjects, 2 mA a-tDCS over the first dorsal interosseous motor area of the left M1 for 20 minutes led to significant increases in MEP amplitudes at 60 and 90 minutes, and not before. This may also be true for lower intensities. Alternatively, extending the intervention for another 10 minutes may have produced a more robust effect. Although, Nitsche and Paulus28 previously showed that 3 minutes of 1 mA, or 5 minutes of 0.6 mA, was enough to induce after effects in healthy subjects. It is possible that 1 mA was ineffective at reducing intracortical inhibition, compared to 2 mA, which may have been more prominent at increasing activity in the excitatory circuits. An additional explanation may be that insufficient current was delivered to the targeted motor area (due to shunting), although the use of small electrodes should decrease this effect with respect to traditional large sponges (depending on factors such as inter-electrode distance). However, more studies are needed to test these theories in patient populations.
The stimulation parameters used in the present study failed to produce changes in voluntary muscle activation measured by RMS. Despite the increase of MEP amplitude in the wrist extensor muscle after a-tDCS stimulation, there was no parallel increase in the generation of muscle voluntary activation.
Peripheral nerve stimulation following anodal transcranial direct current stimulation (a-tDCS)
To see if changes in the MEP responses are attributed to changes at the spinal level, peripheral stimulation of the radial nerve was used to measure sensory threshold and spinal excitability.
In the present study, sensory threshold significantly decreased irrespective of stimulation intensity when compared to sham, supporting heightened somatosensory ability following a-tDCS. Increases in spinal excitability lacked significance but showed a tendency for increased F-wave persistence (+33%) following 2 mA a-tDCS. Continued post-stimulation may have significantly changed spinal excitability due to a possible delay in responses, as previously seen with TMS-elicited MEP amplitudes.33 The results of the present study suggest that a-tDCS at higher intensities (2 mA) may stimulate spinal pathways whereas stimulation at lower intensities (1 mA) are insufficient at producing spinal effects.
The theory that non-invasive brain stimulation techniques, such as repetitive TMS, can modify both cortical and spinal network excitability is further strengthened by results of several studies where transcranial stimulation, either above or below rMT, changed excitability of non-monosynaptic and monosynaptic spinal reflex pathways.39,40 Although the study design did not allow us to draw these conclusions, if the two networks overlap following a-tDCS, changes in both cortical and spinal motor circuits should be considered when interpreting results and when designing future studies. Overall, the results of the present study reveal that spinal as well as cortical networks may benefit from a-tDCS interventions at higher intensities.
Safety aspects of anodal transcranial direct current stimulation (a-tDCS)
To date, all tDCS studies have been performed free of serious adverse events, such as psychotic episodes or seizures.41 Commonly reported side effects include transient skin reactions below the stimulating electrodes, such as local erythema,42 as well as focal tingling (70.6%), fatigue (35.3%), itching (30.4%), slight burning (21.6%), or mild pain sensations (15.7%) under the electrodes, and headaches (4.9%) following tDCS.41,43 However, it is important to note that these effects are also reported following sham, consisting of the ramp up/down events without sustained current.
Skin lesion following tDCS is rare, but has been reported.44,45 Previous studies have shown no evidence of neuronal damage46 or magnetic resonance imaging measured cerebral edema47 following the application of 1 mA a-tDCS. Increasing anodal stimulation to 2 mA for 20 minutes has also shown no evidence of heating under the electrodes,37 or pathological waveforms during electroencephalography recordings.48 Other side effects such as nausea, sleepiness and difficulties with concentration are rare.43 In addition, single and repeated sessions (5 days) of 1 and 2 mA are reported safe.33,49,50
Despite the known safety aspects of a-tDCS, stimulation paradigms tend to differ between both healthy and patient population studies. Therefore, it is important to include the safety aspects of the present study. All participants tolerated 20 minutes of active a-tDCS with ease, confirming the safe use of a-tDCS in chronic SCI populations whilst using small gelled electrodes.
Before the application of 2 mA a-tDCS, great care and consideration was given to safety. Since several studies using smaller Ag/AgCl Pi gelled electrodes51-53 have been performed without relevant side effects, we considered it safe to apply a single session of 1 and 2 mA a-tDCS using 3.14 cm2 Ag/AgCl Pi electrodes, for 20 minutes. Furthermore, it is important to note that current density (current intensity to electrode contact area) is not a good parameter to linearly extrapolate the magnitude of the generated electric fields54 in the brain or levels of discomfort.55
Study limitations
There are several limitations of this study that need to be considered when interpreting the results. Small numbers as well as the highly heterogeneous clinical presentation, even for participants’ with the same level of injury, may have contributed to the lack of significant differences seen in some measures. The findings are also limited by the study design, with only two post measures performed, conclusions regarding the long-term effects cannot be made. Stimulation parameters used in the current study (20 min; 1 mA, 2 mA, sham tDCS; left M1; anode C3 and cathode AF8 placement in the 10/20 system; 3.14 cm2 gelled electrodes) have not been performed before, and therefore the results cannot be directly compared to other studies. Given the placement of the anode electrode over the left M1 (C3), the precise targeting of the ECR muscle may have been different for each participant due to a possible cortical reorganization after injury. Moreover, the investigator was not blinded to the intervention, and it was not verified whether the participants were effectively blinded. Despite these limitations, the randomized sham-controlled nature of the study supports the significance of the findings.
Conclusions
The findings of the present study demonstrate for the first time that a 20-minute single session of a-tDCS leads to increases in corticospinal excitability for individuals with chronic SCI. Not only does a-tDCS modulate activity in the motor system, but changes in the sensory systems also occur. The magnitude of these changes may be intensity dependent, although future studies should not rule out the potential of stimulation strength, duration, or frequency of sessions when investigating other experimental conditions. Overall, the study demonstrates the safety and efficacy of using a-tDCS to modulate changes in both motor and sensory systems following chronic SCI. It remains to be tested if the study findings translate into a long-term rehabilitative therapy, where multiple sessions of a-tDCS yield stronger and longer-lasting changes in sensorimotor physiology and function. More studies are warranted to confirm the therapeutic effect of a-tDCS at enhancing motor function in chronic SCI.
Acknowledgments
This work was supported by NIH Grants R01HD069776 and R21HD077616 for DJE, MC, and APL; and the Burke Foundation.
Abbreviations
- AIS
American Spinal Injury Association impairment scale
- a-tDCS
anodal transcranial direct current stimulation
- CST
corticospinal tract
- ECR
extensor carpi radialis
- EMG
electromyography
- ES
electrical stimulation
- LTP
long-term potentiation
- M1
primary motor cortex
- mA
milliampere
- MEP
motor evoked potential
- MSO
maximal stimulator output
- MVC
maximum voluntary contraction
- rmANOVA
repeat measures ANOVA
- rMT
resting motor threshold
- RMS
root mean square
- SCI
spinal cord injury
- SCIM III
Spinal cord independence measure
- tDCS
transcranial direct current stimulation
- TMS
transcranial magnetic stimulation
- UEMS
upper extremity motor score
- VAS
visual analog scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This research was performed at the Burke Medical Research Institute, in the Non-invasive Brain Stimulation and Human Motor Control Laboratory, White Plains, NY.
Preliminary results were presented in a poster at the ASNR (American Society of Neurorehabilitation) 2013 meeting in San Diego and the 30th International Congress of Clinical Neurophysiology Conference 2014.
Giulio Ruffini is a cofounder of Neuroelectrics, a company that manufactures the tDCS technology used in the study. Alvaro Pascual-Leone has a financial involvement with Neuroelectrics. The remaining authors have no conflict of interest in the submission of this manuscript.
- NE Neuroelectrics®, Barcelona, Spain
- Biometrics Ltd, UK
- CED 1401, Cambridge Electronic Design, Cambridge, UK
- Model DB-80, Tonika Elektronik A/S, Farum, Denmark
- Digitimer Ltd., UK
- IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.
Device Status Statement
The device that is the subject of this manuscript is exempt from FDA regulations because it has gone through the IRB process.
References
- 1.NSCISC . Spinal cord injury facts and figures at a glance. University of Alabama at Birmingham. National Spinal Cord Injury Statistical Center [NSCISC]; Birmingham, AL: 2013. [Google Scholar]
- 2.Becker D, Sadowsky CL, McDonald JW. Restoring function after spinal cord injury. The Neurologist. 2003;9:1–15. doi: 10.1097/01.nrl.0000038587.58012.05. [DOI] [PubMed] [Google Scholar]
- 3.Snoek GJ, IJzerman MJ, Hermens HJ, Maxwell D, Biering-Sorensen F. Survey of the needs of patients with spinal cord injury: impact and priority for improvement in hand function in tetraplegics. Spinal Cord. 2004;42(9):526–32. doi: 10.1038/sj.sc.3101638. [DOI] [PubMed] [Google Scholar]
- 4.Curt A, Keck ME, Dietz V. Functional outcome following spinal cord injury: Significance of motor-evoked potentials and ASIA scores. Archives of Physical Medicine and Rehabilitation. 1998;79(1):81–6. doi: 10.1016/s0003-9993(98)90213-1. [DOI] [PubMed] [Google Scholar]
- 5.Kadivar Z, Sullivan JL, Eng DP, Pehlivan AU, O'Malley MK, Yozbatiran N, Francisco GE. Robotic training and kinematic analysis of arm and hand after incomplete spinal cord injury: A case study.. Proceedings of the International Conference on Rehabilitation Robotics; Zurich, Switzerland. 2011; [DOI] [PubMed] [Google Scholar]
- 6.Beekhuizen KS. New perspectives on improving upper extremity function after spinal cord injury. Journal of Neurologic Physical Therapy. 2005;29(3):157–62. doi: 10.1097/01.npt.0000282248.15911.38. [DOI] [PubMed] [Google Scholar]
- 7.Hicks AL, Martin KA, Ditor DS, Latimer AE, Craven C, Bugaresti J, McCartney N. Long-term exercise training in persons with spinal cord injury: Effects on strength, arm ergometry performance and psychological well-being. Spinal Cord. 2003;41(1):34–43. doi: 10.1038/sj.sc.3101389. [DOI] [PubMed] [Google Scholar]
- 8.Barbeau H, Nadeau S, Garneau C. Physical determinants, emerging concepts, and training approaches in gait of individuals with spinal cord injury. Journal of Neurotrauma. 2006;23(3-4):571–85. doi: 10.1089/neu.2006.23.571. [DOI] [PubMed] [Google Scholar]
- 9.Scholtes F, Brook G, Martin D. Spinal cord injury and its treatment: Current management and experimental perspectives. Advances and Technical Standards in Neurosurgery. 2012;38:29–56. doi: 10.1007/978-3-7091-0676-1_2. [DOI] [PubMed] [Google Scholar]
- 10.Martin R, Johnston K, Sadowsky C. Neuromuscular electrical stimulation-assisted grasp training and restoration of function in the tetraplegic hand: A case series. The American Journal of Occupational Therapy. 2012;66(4):471–7. doi: 10.5014/ajot.2012.003004. [DOI] [PubMed] [Google Scholar]
- 11.Beekhuizen KS, Field-Fote EC. Massed practive versus massed practice with stimulation: Effects on upper extremity function and cortical plasticity in individuals with incomplete cervical spinal cord injury. Neurorehabilitation and Neural Repair. 2005;19:33–45. doi: 10.1177/1545968305274517. [DOI] [PubMed] [Google Scholar]
- 12.Cortes M, Elder J, Rykman A, Murray L, Avedissian M, Stampas A, Thickbroom GW, Pascual-Leone A, Krebs HI, Valls-Sole J, Edwards DJ. Improved motor performance in chronic spinal cord injury following upper-limb robotic training. NeuroRehabilitation. 2013;33:57–65. doi: 10.3233/NRE-130928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards DJ, Cortes M, Thickbroom GW, Rykman A, Pascual-Leone A, Volpe BT. Preserved corticospinal conduction without voluntary movement after spinal cord injury. Spinal Cord. 2013;51(10):765–7. doi: 10.1038/sc.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bastani A, Jaberzadeh S. Does anodal transcranial direct current stimulation enhance excitability of the motor cortex and motor function in healthy individuals and subjects with stroke: A systematic review and meta-analysis. Journal of Clinical Neurophysiology. 2012;123(4):644–57. doi: 10.1016/j.clinph.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 15.Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nature Reviews Neuroscience. 2001;2:263–73. doi: 10.1038/35067570. [DOI] [PubMed] [Google Scholar]
- 16.Kaas JH, Qi HX, Burish MJ, Gharbawie OA, Onifer SM, Massey JM. Cortical and subcortical plasticity in the brains of humans, primates, and rats after damage to sensory afferents in the dorsal columns of the spinal cord. Experimental Neurology. 2008;209(2):407–16. doi: 10.1016/j.expneurol.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nature Neuroscience. 2004;7(3):269–77. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- 18.Ding Y, Kastin AJ, Pan W. Neural plasticity after spinal cord injury. Current Pharmaceutical Design. 2005;11:1441–50. doi: 10.2174/1381612053507855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagg T, Oudega M. Degenerative and spontaneous regenerative processes after spinal cord injury. Journal of Neurotrauma. 2006;23(3-4):264–80. doi: 10.1089/neu.2006.23.263. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh A, Sydekum E, Haiss F, Peduzzi S, Zörner B, Schneider R, Baltes C, Rudin M, Weber B, Schwab ME. Functional and anatomical reorganization of the sensory-motor cortex after incomplete spinal cord injury in adult rats. The Journal of Neuroscience. 2009;29(39):12210–9. doi: 10.1523/JNEUROSCI.1828-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miranda PC, Mekonnen A, Salvador R, Ruffini G. The electric field in the cortex during transcranial current stimulation. Neuroimage. 2013;70:48–58. doi: 10.1016/j.neuroimage.2012.12.034. [DOI] [PubMed] [Google Scholar]
- 22.Ruffini G, Wendling F, Merlet I, Molaee-Ardekani B, Mekonnen A, Salvador R, Soria-Frisch A, Grau C, Dunne S, Miranda PC. Transcranial current brain stimulation (tCS): Models and technologies. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2013;21(3):333–45. doi: 10.1109/TNSRE.2012.2200046. [DOI] [PubMed] [Google Scholar]
- 23.Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clinical Neurophysiology. 2006;117(4):845–50. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. International Journal of Neuropsychopharmacology. 2011;14:1133–45. doi: 10.1017/S1461145710001690. [DOI] [PubMed] [Google Scholar]
- 25.Burns S, Biering-Sørensen F, Donovan W, Graves DE, Jha A, Johansen M, Jones L, Krassioukov A, Kirshblum S, Mulcahey MJ. Schmidt Read M, Waring W. International standards for neurological classification of spinal cord injury. Topics in Spinal Cord Injury Rehabilitation. 2011;18(1):85–99. doi: 10.1310/sci1801-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furubayashi T, Terao Y, Arai N, Okabe S, Mochizuki H, Hanajima R, Hamada M, Yugeta A, Inomata-Terada S, Ugawa Y. Short and long duration transcranial direct current stimulation (tDCS) over the human hand motor area. Experimental Brain Research. 2008;185:279–86. doi: 10.1007/s00221-007-1149-z. [DOI] [PubMed] [Google Scholar]
- 27.Kidgell DJ, Goodwill AM, Frazer AK, Robin MD. Induction of cortical plasticity and improved motor performance following unilateral and bilateral transcranial direct current stimulation of the primary motor cortex. BMC Neuroscience. 2013;14:64. doi: 10.1186/1471-2202-14-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. Journal of Physiology. 2000;527(3):633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bastani A, Jaberzadeh S. Differential modulation of corticospinal excitability by different current densities of anodal transcranial direct current stimulation. PLoS ONE. 2013;8(8):e72254. doi: 10.1371/journal.pone.0072254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuo H, Bikson M, Datta A, Minhas P, Paulus W, Kuo M, Nitsche M. Comparing cortical plasticity induced by conventional and high-definition 4 × 1 ring tDCS: A neurophysiological study. Brain Stimulation. 2013;6(4):644–8. doi: 10.1016/j.brs.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 31.Miyaguchi S, Onishi H, Kojima S, Sugawara K, Tsubaki A, Kirimoto H, Tamaki H, Yamamoto N. Corticomotor excitability induced by anodal transcranial direct current stimulation with and without non-exhaustive movement. Brain Research. 2013;1529:83–91. doi: 10.1016/j.brainres.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 32.Mordillo-Mateos L, Turpin-Fenoll L, Millán-Pascual J, Núñez-Pérez N, Panyavin I, Gómez-Argüelles JM, Botia-Paniagua E, Foffani G, Lang N, Oliviero A. Effects of simultaneous bilateral tDCS of the human motor cortex. Brain Stimulation. 2012;5(3):214–22. doi: 10.1016/j.brs.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Batsikadze G, Moliadze V, Paulus W, Kuo MF, Nitsche MA. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. Journal of Physiology. 2013;591(7):1987–2000. doi: 10.1113/jphysiol.2012.249730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fricke K, Seeber AA, Thirugnanasambandam N, Paulus W, Nitsche MA, Rothwell JC. Time course of the induction of homeostatic plasticity generated by repeated transcranial direct current stimulation of the human motor cortex. Journal of Neurophysiology. 2011;105(3):1141–9. doi: 10.1152/jn.00608.2009. [DOI] [PubMed] [Google Scholar]
- 35.Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Henning S, Tergau F, Paulus W. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. Journal of Physiology (London) 2003;553:293–301. doi: 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lisman JE. Three Ca2+ levels affect plasticity differently: the LTP zone, the LTD zone and no man's land. Journal of Physiology (London) 2001;532:285. doi: 10.1111/j.1469-7793.2001.0285f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stagg CJ, Best JG, Stephenson MC, O'Shea J, Wylezinska M, Kincses ZT, Morris PG, Matthews PM, Johansen-Berg H. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. Journal of Neuroscience. 2009;29:5202–6. doi: 10.1523/JNEUROSCI.4432-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nitsche MA, Seeber A, Frommann K, Klein CC, Rochford C, Nitsche MS, Fricke K, Liebetanz D, Lang N, Antal A, Paulus W, Tergau F. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. Journal of Physiology. 2005;568(1):291–303. doi: 10.1113/jphysiol.2005.092429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berardelli A, Inghilleri M, Rothwell JC, Romeo S, Currà A, Gilio F, Modugno N, Manfredi M. Facilitation of muscle evoked responses after repetitive cortical stimulation in man. Experimental Brain Research. 1998;122:79–84. doi: 10.1007/s002210050493. [DOI] [PubMed] [Google Scholar]
- 40.Valero-Cabré A, Oliveri M, Gangitano M, Pascual-Leone A. Modulation of spinal cord excitability by subthreshold repetitive transcranial magnetic stimulation of the primary motor cortex in humans. Neuroreport. 2001;12:3845–8. doi: 10.1097/00001756-200112040-00048. [DOI] [PubMed] [Google Scholar]
- 41.Madhavan S, Shah B. Enhancing motor skill learning with transcranial direct current stimulation – A concise review with applications to stroke. Frontiers in Psychiatry. 2012;3(66) doi: 10.3389/fpsyt.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Priori A, Hallett M, Rothwell JC. Repetitive transcranial magnetic stimulation or transcranial direct current stimulation? Brain Stimulation. 2009;2:241–5. doi: 10.1016/j.brs.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Poreisz C, Boros K, Antal A, Paulus W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Research Bulletin. 2007;72:108–14. doi: 10.1016/j.brainresbull.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 44.Palm U, Keeser D, Schiller C, Fintescu Z, Reisinger E, Nitsche M. Skin lesions after treatment with transcranial direct current stimulation (tDCS). Brain Stimulation. 2008;1:386–7. doi: 10.1016/j.brs.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 45.Frank E, Wilfurth S, Landgrebe M, Eichhammer P, Hajak G, Langguth B. Anodal skin lesions after treatment with transcranial direct current stimulation. Brain Stimulation. 2010;3(1):58–9. doi: 10.1016/j.brs.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57(10):1899–901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- 47.Nitsche M, Niehaus L, Hoffmann K, Hengst S, Liebetanz D, Paulus W, Meyer BU. MRI study of human brain exposed to weak direct current stimulation of the frontal cortex. Clinical Neurophysiology. 2004;115:2419–23. doi: 10.1016/j.clinph.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Iyer MB, Mattu U, Grafman J, Lomarev M, Sato S, Wassermann EM. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology. 2005;64(5):872–5. doi: 10.1212/01.WNL.0000152986.07469.E9. [DOI] [PubMed] [Google Scholar]
- 49.Fregni F, Boggio PS, Lima MC, Ferreira MJ, Wagner T, Rigonatti SP, Castro AW, Souza DR, Riberto M, Freedman SD, Nitsche MA, Pascual-Leone A. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain. 2006;122(1-2):197–209. doi: 10.1016/j.pain.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 50.Fregni F, Boggio PS, Nitsche M, Marcolin MA, Rigonatti SP, Pascual-Leone A. Treatment of major depression with transcranial direct current stimulation. Bipolar Disorders. 2006;8(2):203–4. doi: 10.1111/j.1399-5618.2006.00291.x. [DOI] [PubMed] [Google Scholar]
- 51.Minhas P, Bansal V, Patel J, Ho JS, Diaz J, Datta A, Bikson M. Electrodes for high-definition transcutaneous DC stimulation for applications in drug delivery and electrotherapy, including tDCS. Journal of Neuroscience Methods. 2010;190:188–97. doi: 10.1016/j.jneumeth.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borckardt JJ, Bikson M, Frohman H, Reeves ST, Datta A, Bansal V, Madan A, Barth K, George MS. A pilot study of the tolerability and effects of high-definition transcranial direct current stimulation (HD-tDCS) on pain perception. The Journal of Pain. 2012;13(2):112–20. doi: 10.1016/j.jpain.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 53.Faria P, Fregni F, Sebastião F, Dias AI, Leal A. Feasibility of focal transcranial DC polarization with simultaneous EEG recording: Preliminary assessment in healthy subjects and human epilepsy. Epilepsy & Behavior. 2012;25:417–25. doi: 10.1016/j.yebeh.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 54.Miranda PC, Faria P, Hallett M. What does the ratio of injected current to electrode area tell us about current density in the brain during tDCS? Clinical Neurophysiology. 2009;120(6):1183–7. doi: 10.1016/j.clinph.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turi Z, Ambrus GG, Ho KA, Sengupta T, Paulus W, Antal A. When size matters: Large electrodes induce greater stimulation-related cutaneous discomfort than smaller electrodes at equivalent current density. Brain Stimulation. 2014;7(3):460–7. doi: 10.1016/j.brs.2014.01.059. [DOI] [PubMed] [Google Scholar]