Abstract
ΔFosB is a member of the Fos family of transcription factors. While other family members are induced rapidly but transiently in response to a host of acute stimuli, ΔFosB is unique in that it accumulates in response to repeated stimulation due to its unusual protein stability. Such prolonged induction of ΔFosB, within nucleus accumbens (NAc), a key brain reward region, has been most studied in animal models of drug addiction, with considerable evidence indicating that ΔFosB promotes reward and motivation and serves as a mechanism of drug sensitization and increased drug self-administration. In more recent years, prolonged induction of ΔFosB has also been observed within NAc in response to chronic administration of certain forms of stress. Increasing evidence indicates that this induction represents a positive, homeostatic adaptation to chronic stress, since overexpression of ΔFosB in this brain region promotes resilience to stress, whereas blockade of its activity promotes stress susceptibility. Chronic administration of several antidepressant medications also induces ΔFosB in the NAc, and this induction is required for the therapeutic-like actions of these drugs in mouse models. Validation of these rodent findings is the demonstration that depressed humans, examined at autopsy, display reduced levels of ΔFosB within the NAc. As a transcription factor, ΔFosB produces this behavioral phenotype by regulating the expression of specific target genes, which are under current investigation. These studies of ΔFosB are providing new insight into the molecular basis of depression and antidepressant action, which is defining a host of new targets for possible therapeutic development.
Keywords: Fos, nucleus accumbens, prefrontal cortex, epigenetics
1. Introduction
The study of transcriptional mechanisms of depression is based on the hypothesis that regulation of gene expression is one important mechanism by which chronic exposure to stress causes depression or related abnormalities in vulnerable individuals (Krishnan and Nestler, 2010). A corollary of this hypothesis is that the ability of a host of antidepressant medications, after prolonged administration, to reduce the symptoms of depression in some individuals is likewise mediated in part by altered gene expression in relevant brain regions.
Work over the past 20 years has provided increasing evidence for a role of gene regulation in depression models, as several transcription factors—proteins that bind to specific response elements in the promoter regions of target genes and regulate those genes’ expression—have been implicated in these models. Examples of transcription factors that have been studied prominently in stress models include CREB (cAMP response element binding protein), glucocorticoid receptor, and NFκB (nuclear factor kB), among others (Nestler et al., 2002; Carlezon et al., 2005; Holsboer and Ising, 2010; Christoffel et al., 2011; Licznerski and Duman, 2013).
The focus of this review is on another transcription factor, ΔFosB, which has been mostly studied in drug addiction models (Nestler, 2008, 2012). More recent work has demonstrated that ΔFosB, a member of the Fos family of proteins, is also regulated in stress and depression models and appears to play a unique role in promoting resilience and antidepressant responses. This discussion also illustrates the types of experimental approaches that have been used to investigate transcriptional mechanisms of depression in mouse models.
2. Induction of ΔFosB in nucleus accumbens by chronic stress
ΔFosB is encoded by the FosB gene (Figure 1) and shares homology with other Fos family transcription factors, which include c-Fos, FosB, Fra1, and Fra2 (Morgan and Curran, 1995). These Fos family proteins heterodimerize with Jun family proteins (c-Jun, JunB, or JunD) to form active AP1 (activator protein-1) transcription factors that bind to AP1 sites (consensus sequence: TGAC/GTCA) present in the promoters of certain genes to regulate their transcription. Fos family proteins are induced rapidly and transiently in specific brain regions after acute administration of several forms of stress (Perrotti et al., 2004). These responses are seen most prominently in nucleus accumbens (NAc), which is best characterized as an important mediator of reward and motivation. All of these Fos family proteins, however, are highly unstable and return to basal levels within hours of the stress exposure.
Figure 1. Biochemical basis of ΔFosB’s unique stability.
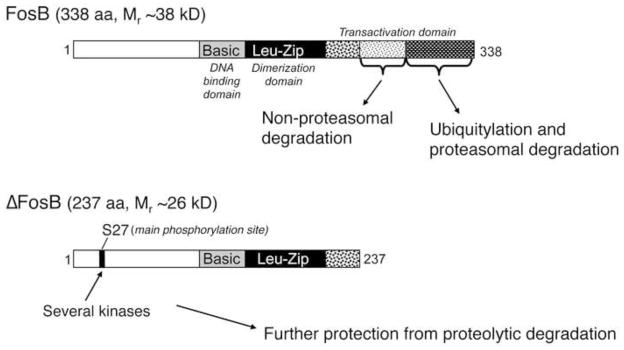
ΔFosB and FosB are encoded by the FosB gene. ΔFosB is generated by alternative splicing and lacks the C-terminal 101 amino acids present in FosB. Two mechanisms are known that account for ΔFosB’s stability. First, ΔFosB lacks two degron domains present in the C-terminus of full length FosB (and found in all other Fos family proteins as well). One of these degron domains targets FosB for ubiquitylation and degradation in the proteasome. The other degron domain targets FosB degradation by a ubiquitin- and proteasome-independent mechanism. Second, ΔFosB is phosphorylated by several protein kinases at its N-terminus which further stabilizes the protein. From Nestler, 2008 with permission.
Very different responses are seen after chronic exposure to stress. Biochemically modified isoforms of ΔFosB (Mr 35–37 kD) accumulate within the same brain regions after repeated stress exposure, whereas other Fos family members show desensitization (i.e., reduced induction compared with initial drug exposures) (Perrotti et al., 2004; Vialou et al., 2010a; Lehmann and Herkenham, 2011). Such accumulation of ΔFosB has been observed for several forms of active stress, such as chronic restraint stress, chronic unpredictable stress, and chronic social defeat stress (Perrotti et al., 2004; Vialou et al., 2010a; Lehmann and Herkenham, 2011), whereas chronic social isolation of adult animals strikingly causes the opposite effect: a decrease in ΔFosB levels in NAc (Vialou et al., 2010a).
For chronic restraint stress and chronic social defeat stress, induction of ΔFosB in NAc has been shown to occur in both major subtypes of medium spiny neurons (MSNs)—those that express predominantly the D1 dopamine receptor (D1-type MSNs) or the D2 dopamine receptor (D2-type MSNs), which together represent ~95% of all neurons in this brain region (Perrotti et al., 2004; Lobo et al., 2013). In contrast, no induction is seen in any of several types of interneurons or in non-neural cells. One of the advantages of the social defeat paradigm is that a subset of mice do not develop depression-like behavioral abnormalities, that is, they remain resilient unlike the majority which are susceptible (Krishnan et al., 2007). Interestingly, the modest induction of ΔFosB seen in the NAc of susceptible mice occurs primarily in D2-type MSNs, where the more robust induction that occurs in resilient mice is specific to D1-type MSNs (Lobo et al., 2013). This cellular specificity of ΔFosB induction has important functional implications as discussed in Section 4.
The 35–37 kD isoforms of ΔFosB dimerize predominantly with JunD to form an active and long-lasting AP1 complex within these brain regions (Chen et al., 1997; Hiroi et al., 1998). However, recent in vitro evidence has indicated that ΔFosB can also form homodimers with distinct physico-chemical properties compared to ΔFosB:JunD heterodimers (Jorrisen et al., 2007). An important focus of current research is to determine whether such ΔFosB homodimers form in vivo and what physiological function they subserve.
The 35–37 kD ΔFosB isoforms accumulate after chronic stress or other repeated stimuli due to their extraordinarily long half-lives, which has been demonstrated both in cultured cells in vitro and within the NAc in vivo (Nestler, 2008). As a result of its stability, therefore, the ΔFosB protein persists in neurons for at least several weeks after cessation of stress exposure. We now know that this stability is due to two factors (Figure 1): (1) the absence from ΔFosB of two degron domains, which are present at the C-terminus of full length FosB and all other Fos family proteins and target those proteins to rapid degradation, and (2) the phosphorylation of ΔFosB at its N-terminus (Ser27) by Ca2+/calmodulin-dependent protein kinase II (CaMKII) and casein kinase 2 and perhaps by other protein kinases (Ulery et al., 2006; Carle et al., 2007; Ulery-Reynolds, 2009; Robison et al., 2013). The mechanism by which phosphorylation of ΔFosB at Ser27 increases its stability remains unknown. The stability of the ΔFosB isoforms provides a novel molecular mechanism by which stress-induced changes in gene expression can persist long after the stress exposure. ΔFosB is also phosphorylated at two threonine residues near its DNA-binding and transactivation domains, and phosphorylation of one of these sites (Thr149) dramatically increases the transcriptional activity of the protein (Cates et al., 2014). Further work is needed to define the role of these phosphorylation sites in stress responses.
3. Induction of ΔFosB in nucleus accumbens by chronic antidepressant treatment
Recent studies have shown that chronic administration of fluoxetine, a serotonin-selective reuptake inhibitor antidepressant, induces ΔFosB in the NAc (Vialou et al., 2010a). ΔFosB is also induced in response to chronic administration of other antidepressants including the tricyclic antidepressant imipramine (unpublished observations) and the rapidly-acting, novel antidepressant ketamine (Donahue et al., 2013). Interestingly, similar to the pattern of induction seen in resilient mice after chronic social defeat stress, ΔFosB induction in NAc in response to chronic fluoxetine treatment is selective for D1-type MSNs, with no induction seen in NAc interneurons or glia (Lobo et al., 2013). It would be interesting moving forward to characterize the induction of ΔFosB in NAc by several other classes of antidepressant medications. Recent work, for example, has shown that environmental enrichment during rearing increases basal levels of ΔFosB in the NAc (Zhang et al., 2014). Since such environmental enrichment is thought to enhance resilience (Lehmann et al., 2012), these findings raise the interesting possibility that non-medication antidepressant approaches might induce ΔFosB in this brain region as well.
Chronic administration of fluoxetine has recently been shown to increase levels of full-length FosB within the NAc 24 hr after the last drug exposure (Vialou et al., 2014b). This is a surprising finding, since full-length FosB is highly unstable and, in chronic stress or chronic drug abuse models, its levels are not elevated beyond 2–4 hr after each exposure. These findings suggest that repeated administration of fluoxetine might cause sustained increases in neural activity in this brain region, which might be expected to drive sustained increases in FosB levels. Further work is needed to study the validity of this possibility as well as to determine if this phenomenon occurs with other antidepressants.
4. Role of ΔFosB in nucleus accumbens in regulating behavioral responses to stress and antidepressant treatments
Insight into the role of ΔFosB in stress and antidepressant responses has come from a combination of experimental approaches. First, we have developed bitransgenic mice in which ΔFosB can be induced selectively within the NAc and dorsal striatum of adult animals (Chen et al., 1995; Kelz et al., 1999). Importantly, these mice overexpress ΔFosB selectively in D1-type MSNs. Second, we have developed a series of viral vectors that selectively overexpress ΔFosB in the NAc, without expression in dorsal striatum, although this expression occurs in all neurons, not just D1-type MSNs (Zachariou et al., 2006). Conversely, we have developed bitransgenic mice that overexpress a truncated Jun protein, called ΔcJun, within NAc, dorsal striatum, and several other brain regions (Peakman et al., 2003), as well as viral vectors that selectively overexpress a different truncated Jun protein, called ΔJunD, in the NAc (Winstanley et al., 2007). These truncated Jun proteins serve as dominant negative antagonists of ΔFosB- or other AP1-mediated transcription.
By use of these complementary approaches, we have established that increased expression of ΔFosB within D1-type MSNs of the NAc reduces an animal’s sensitivity to the deleterious effects of chronic stress, promotes stress resilience, and mediates antidepressant-like responses. Thus, bitransgenic mice inducibly overexpressing ΔFosB in D1-type MSNs, or mice injected intra-NAc with a viral vector encoding ΔFosB, show increased resilience to subsequent chronic social defeat stress (Vialou et al., 2010a; Ohnishi et al., 2014). They also display reversal of depression-like behavioral abnormalities after chronic social defeat stress. Conversely, bitransgenic mice inducibly overexpressing ΔcJun in NAc and several other brain regions, or mice injected intra-NAc with a viral vector encoding ΔJunD, show increased susceptibility to sub-threshold levels of social defeat stress. They also fail to respond to chronic fluoxetine administration, which reverses depression-like behavioral abnormalities, after chronic social defeat stress (Vialou et al., 2010a).
Further support for the view that ΔFosB induction in NAc promotes resilience is the more recent finding that mice that constitutively lack expression of full length FosB, but show increased expression of ΔFosB, display reduced sensitivity to stress (Ohnishi et al., 2011). Likewise, further support for the view that ΔFosB induction in NAc elevates mood and promotes antidepressant-like responses are the findings that ΔFosB overexpression in D1-type MSNs opposes the elevation of brain stimulation reward thresholds induced either by chronic social defeat stress or by a κ opioid agonist which is pro-depressant (Muschamp et al., 2012; Donahue et al., 2013). Moreover, ΔFosB overexpression in NAc promotes several rewarding behaviors, including wheel-running, sucrose drinking, consumption of high-fat food, and sexual activity, with most of these effects seen upon selective expression in D1-type MSNs, while overexpression of ΔcJun or ΔJunD exerts the opposite effect (Werme et al., 2002; Olausson et al., 2006; Teegarden et al., 2008; Wallace et al., 2008; Hedges et al., 2009; Pitchers et al., 2010, 2013; Been et al., 2013).
In contrast to the behavioral phenotype mediated by ΔFosB induction in D1-type MSNs, it has been harder to decipher the effect of ΔFosB induction in D2-type MSNs. As noted earlier, such induction is seen selectively in mice that are susceptible to chronic social defeat stress. However, bitransgenic mice that inducibly overexpress ΔFosB in D2-type MSNs (Chen et al., 1995; Werme et al., 2002) do not show a prominent pro-depression-like phenotype in chronic stress models (unpublished observations), although they do show reduced wheel-running activity (Werme et al., 2003). Further complicating the situation is the observation that several rewarding behaviors, including sucrose drinking and being reared in an enriched environment, induce ΔFosB roughly equally in NAc D1- and D2-type MSNs (Lobo et al., 2013). Additional studies are thus needed to better understand the behavioral consequences of ΔFosB induction in D2-type MSNs. Of note, the recent development of viral vectors, which make it possible for the first time to overexpress ΔFosB selectively in either D1-type MSNs or in D2-type MSNs within the NAc (Grueter et al., 2013), should help in these efforts.
5. Mechanism of ΔFosB induction in nucleus accumbens
The upstream signaling pathways through which chronic stress or antidepressant treatment induces ΔFosB in NAc remain largely unknown. Recent work, however, has defined the transcription factors that are required for ΔFosB induction by chronic stress. The ability of chronic social defeat stress to induce ΔFosB in NAc requires SRF (serum response factor): local knockout of this transcription factor completely blocks stress induction of ΔFosB, whereas knockout of another transcription factor, CREB, is without effect (Vialou et al., 2010b). Surprisingly, cocaine induction of ΔFosB in this same brain region requires both SRF and CREB: local knockout of both factors completely blocks the ability of cocaine to induce ΔFosB, whereas knockout of either factor alone is without effect (Vialou et al., 2012). These observations demonstrate that different stimuli can invoke different molecular mechanisms to induce ΔFosB even within the same brain region and presumably cell type (D1-type MSNs). Further work is needed to understand the molecular basis of such stimulus-specific actions.
6. Target genes for ΔFosB in nucleus accumbens
Since ΔFosB is a transcription factor, it presumably produces its interesting behavioral phenotypes in NAc by enhancing or repressing expression of other genes. As shown in Figure 1, ΔFosB is a truncated product of the FosB gene that lacks most of the C-terminal transactivation domain present in full-length FosB but retains the dimerization and DNA binding domains (Nestler, 2008, 2012). Some in vitro studies suggest that, because ΔFosB lacks much of its transactivation domain, it functions as a negative regulator of AP-1 activity, while several others show that ΔFosB can activate transcription at AP1 sites (see Nestler, 2008, 2012). An earlier study using our inducible, bitransgenic mice that overexpress ΔFosB or its dominant negative ΔcJun, and analyzing gene expression on Affymetrix chips, demonstrated that—in the NAc in vivo—ΔFosB functions primarily as a transcriptional activator, while it does serve as a repressor for a smaller subset of genes (McClung and Nestler, 2003). Current research, focused on chromatin mechanisms recruited along with ΔFosB to its target genes, is exploring the underlying molecular basis for these opposite, gene-specific actions of ΔFosB (Nestler, 2014).
Several target genes of ΔFosB have been established using a candidate gene approach. One target is GluA2 (GluR2), an AMPA glutamate receptor subunit. ΔFosB overexpression in inducible bitransgenic mice selectively increases GluA2 expression in NAc, with no effect seen for several other AMPA glutamate receptor subunits analyzed (Kelz et al., 1999). AP1 complexes comprised of ΔFosB bind a consensus AP1 site present in the GluA2 promoter. Furthermore, GluA2 overexpression in NAc via viral-mediated gene transfer promotes resilience to chronic social defeat stress (Vialou et al., 2010a), much like ΔFosB overexpression. Since GluA2-containing AMPA channels are impermeable to Ca2+ and have a lower overall conductance compared to AMPA channels that do not contain this subunit, the chronic stress- and ΔFosB-mediated upregulation of GluA2 in NAc could account, at least in part, for the reduced glutamatergic responses seen in these neurons of resilient mice (Vialou et al., 2010a).
Another candidate target gene of ΔFosB in NAc is the opioid peptide, dynorphin, which is suppressed by ΔFosB (Zachariou et al., 2006). Recall that ΔFosB is induced in the NAc of resilient mice after chronic social defeat stress specifically in D1-type MSNs, the subtype of MSNs which also express dynorphin. The suppression of dynorphin by ΔFosB has not yet been demonstrated directly in the context of chronic stress, nevertheless, such dynorphine downregulation would be expected to produce pro-resilient- and antidepressant-like responses given the aversive actions of dynorphin acting at κ opioid receptors in this NAc reward circuit. Work is now needed to directly test this possibility in stress models.
We recently showed that Camk2a is a target gene for ΔFosB within D1-type MSNs in drug abuse models. Chronic cocaine administration induces CaMKII in these neurons of the NAc and, interestingly, functions as part of a positive feed-forward loop, whereby CaMKII phosphorylates and stabilizes ΔFosB (see above), promoting further CaMKII induction (Robison et al., 2013). In the context of chronic stress and chronic fluoxetine administration, however, the two proteins function very differently (Robison et al., 2014). Chronic social defeat stress does not alter ΔFosB binding to the Camk2a gene promoter, while chronic administration of fluoxetine decreases ΔFosB binding to the promoter, despite the fact that ΔFosB levels are increased under both of these conditions. The depletion of ΔFosB from the Camk2a promoter in response to chronic fluoxetine occurs in tandem with reduced histone H3 acetylation and increased H3 methylation (at Lys9) at the Camk2a promoter and with suppression of Camk2a expression. Indeed, we know that these chromatin modifications in the NAc have broad effects on depression-related behavioral abnormalities (Covington et al., 2009, 2011). These findings suggest that specific chromatin modifications at subsets of genes are responsible for gene-specific effects of transcriptional regulation, in this case, with ΔFosB binding to and activating Camk2a in D1-type MSNs after chronic cocaine, but being depleted from the Camk2a gene in concert with its repression within the same neuronal cell type after chronic fluoxetine. These actions of fluoxetine appear to be functional important. First, the same pattern of chromatin modifications and CaMKII repression is seen in the NAc of depressed patients treated chronically with antidepressant medications but not n medication-free individuals (Robison et al., 2014). Second, viral-mediated overexpression of CaMKII in the NAc prevents the antidepressant-like effects of fluoxetine in the chronic social defeat procedure (Robison et al., 2014). It will be interesting in future studies to identify the proteins whose reduced phosphorylation as a consequence of suppressed CaMKII levels contributes to antidepressant action.
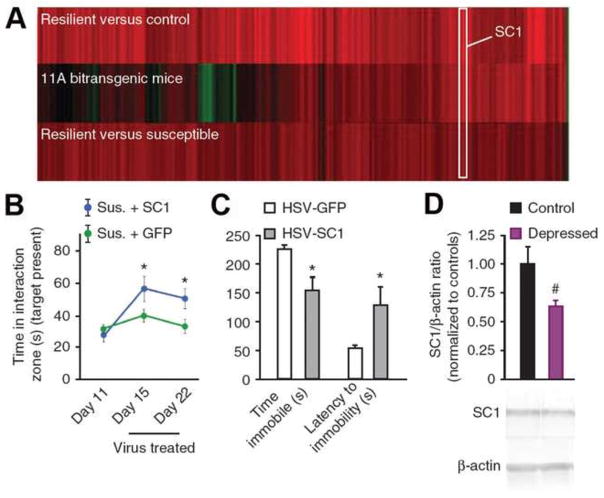
The second approach used to identify target genes of ΔFosB in NAc is unbiased and utilizes genome-wide methods. First, as noted earlier, we have identified genes whose expression levels are up- or downregulated upon the inducible overexpression of ΔFosB (or ΔcJun) in D1-type NAc MSNs using DNA expression arrays (McClung and Nestler, 2003). More recently, we overlaid this list of ΔFosB-regulated genes in NAc with the list of genes that are altered in the NAc of resilient mice, based on the hypothesis, stated earlier, that ΔFosB induction in D1-type MSNs mediates resilience. The results identify numerous putative resilience genes whose regulation might be mediated by ΔFosB (Figure 4). One of the genes that is most robustly induced in the NAc under resilience and upon ΔFosB overexpression is Sparcl1, which encodes Sparc-like 1 (also known as hevin). Sparcl1 is an anti-adhesive matrix molecule that is highly expressed in adult brain, where it localizes in the postsynaptic density and is implicated in synaptic plasticity (Lively et al., 2008). Indeed, based on our unbiased discovery, we went on to show that Sparc1 levels are downregulated in the NAc of depressed humans and that overexpression of Sparcl1 in mouse NAc exerts potent antidepressant-like effects (Figure 4).
Figure 4. Sparcl1 has pro-resilience, antidepressant-like effects in NAc.
A. Changes in gene expression observed in NAc during resilience overlap with those observed upon overexpression of ΔFosB (comparison of data sets in McClung and Nestler, 2003 and Krishnan et al., 2007). Shown are 106 genes that are significantly regulated (>1.5 fold; *p < 0.05) in NAc by social defeat in resilient mice as compared with controls (upper heat map) and how these genes are regulated both in resilient mice versus susceptible mice (lower heat map) and by overexpression of ΔFosB in D1-type MSNs of NAc (middle heat map). The position of Scarpl1 (SC1) on the heat maps is indicated. (b) Viral overexpression of Scarpl1 in NAc reverses the social avoidance induced by chronic (10 days) social defeat. C. Overexpression of Scarpl1 has an antidepressant-like effect as measured by a decrease in time spent immobile and an increase in latency to immobility in the forced swim test. Rats were injected with HSV-GFP or HSV-Scarpl1 into NAc before the test. D. Human NAc samples from depressed individuals show a strong trend for lower Scarpl1 concentrations as compared with matched controls. From Vialou et al., 2010a with permission.
These findings illustrate the potential power of open-ended approaches in driving innovative drug discovery efforts. In parallel, we are now combining such studies of gene expression with chromatin immunoprecipitation (ChIP) coupled with deep sequencing (ChIP-seq) to identify genes where the binding of endogenous ΔFosB in NAc is regulated by chronic stress or antidepressant exposure. Together, we expect these unbiased approaches to enable the identification of a large number of previously unappreciated target genes for ΔFosB in NAc in the context of depression-related behavioral abnormalities, as we have accomplished in drug abuse models (see Renthal et al., 2009).
7. Induction of ΔFosB in other brain regions
The discussion up to now has focused solely on NAc. While this is a key brain reward region and important for depression and antidepressant action, many other brain regions are also crucial. A central question, then, is whether ΔFosB acting in other brain regions beyond the NAc may also influence depression-related behavioral abnormalities. Increasing evidence suggests that this is the case.
We have mapped the induction of ΔFosB throughout the brain in response to chronic restraint stress or chronic social defeat stress and have demonstrated robust ΔFosB induction in numerous brain regions in both stress models (Perrotti et al., 2004; Vialou et al., 2014a). We have also demonstrated ΔFosB induction in numerous brains regions in addition to the NAc after chronic fluoxetine administration (Vialou et al., 2014b). A major goal for future research is to carry out studies, analogous to those described above for NAc, to delineate the neural and behavioral phenotype mediated by ΔFosB for each of the brain regions implicated. This represents an enormous undertaking, yet it is crucial for understanding the global influence of ΔFosB in depression.
An early example of this effort is a recent study where we characterized the influence of ΔFosB, acting in the prelimbic region of medial prefrontal cortex (mPFC) in chronic social defeat stress. We had shown previously that decreased neuronal activity, as inferred from immediate early gene expression levels, in medial prefrontal cortex (mPFC) is associated with social defeat-induced depression- and anxiety-like behaviors in mice (Covington et al., 2010). More recently, we demonstrated that ΔFosB is induced in the pre-limbic mPFC selectively in susceptible mice, and that overexpression of ΔFosB in this region, but not in the nearby infra-limbic area, enhances stress susceptibility (Vialou et al., 2014a). ΔFosB produces these effects partly through induction of the cholecystokinin (CCK)-B receptor: CCKB blockade in mPFC induces a resilient phenotype, whereas CCK administration into mPFC mimics the anxiogenic- and depressant-like effects of social stress. We previously found that optogenetic stimulation of mPFC neurons in susceptible mice reverses several behavioral abnormalities seen after chronic social defeat stress (Covington et al., 2010). We therefore hypothesized that optogenetic stimulation of prelimbic mPFC projections would rescue the pathological effects of CCK within this brain region. Indeed, following CCK infusion in mPFC, we optogenetically stimulated mPFC projections to NAc or basolateral amygdala. Stimulation of cortico-NAc projections reversed CCK-induced depression-like but not anxiogenic-like effects, whereas stimulation of cortico-amygdala projections blocked the anxiogenic- but not depression-like effects of CCK (Vialou et al., 2014a). Thus, the pro-susceptible role of ΔFosB acting in the prelimbic mPFC is opposite to ΔFosB’s pro-resilience actions in the NAc, emphasizing the highly region-specific effects that should be expected from many regulatory proteins.
8. Future Directions
Beyond characterizing the role played by ΔFosB, acting in several regions of the brain, in mediating depression- and antidepressant responses, an important question is how this information can be mined to improve clinical management of depression and related disorders. We believe that work on ΔFosB can contribute to such clinical efforts. First, it would be interesting to develop imaging ligands for ΔFosB, which might be used in conjunction with PET or MRI, for example, to determine an individual’s susceptibility to depression and to track antidepressant responses based on levels of ΔFosB induction throughout the brain. As well, we are interested in developing small molecules that directly inhibit or potentiate the actions of ΔFosB. We have made early progress in this regard (Wang et al., 2012). As test compounds of higher affinity and greater brain penetration are generated, it would be useful to administer such molecules systemically to determine the net effect of increasing or decreasing ΔFosB activity throughout the brain. One striking feature of ΔFosB is that it is expressed at many fold higher levels in NAc and dorsal striatum than any other brain region or peripheral tissue studied; we would thus expect effects in NAc to predominate. Moreover, studies of ΔFosB are revealing numerous target genes whose encoded proteins show greater region-specific regulation by stress and antidepressants than that exhibited by ΔFosB itself. Such targets should be used to help drive antidepressant drug discovery efforts in future years. Finally, studies of ΔFosB illustrate the ways in which it is possible to elaborate detailed transcriptional mechanisms of depression and antidepressant action.
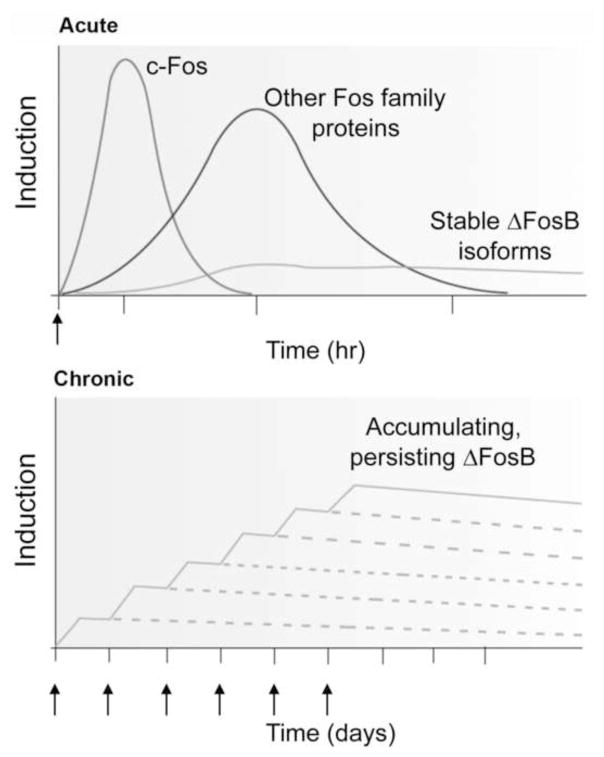
Figure 2. Scheme showing the gradual accumulation of ΔFosB versus the rapid and transient induction of other Fos family proteins in response to stress or another stimulus.
The upper graph shows that several waves of Fos family proteins (comprised of c-Fos, FosB, ΔFosB [33 kD isoform], Fra1, and Fra2) are induced in NAc and dorsal striatal neurons by acute stress. Also induced are biochemically modified isoforms of ΔFosB (35–37 kD); they are induced at low levels by acute stress, but persist in brain for long periods due to their stability. The lower graph shows that with repeated (e.g., twice daily) stress exposure, each acute stimulus induces a low level of the stable ΔFosB isoforms. This is indicated by the lower set of overlapping lines, which indicate ΔFosB induced by each acute stimulus. The result is a gradual increase in the total levels of ΔFosB with repeated stimuli during a course of chronic treatment. This is indicated by the increasing stepped line in the graph. From Nestler, 2008 with permission.
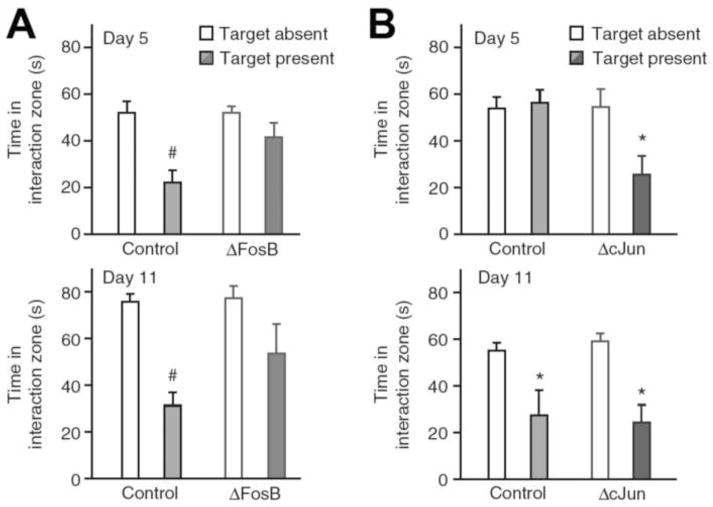
Figure 3. ΔFosB induction in NAc by chronic social defeat stress mediates resilience.
A. Inducible bitransgenic mice overexpressing ΔFosB in D1-type MSNs do not develop the social aversion which is a hallmark of social defeat stress after either 4 or 10 days of defeat with mice tested on days 5 or 11, respectively. B. Conversely, overexpression of ΔcJun increases susceptibility to social defeat with increased social aversion seen after 4 days of defeat with mice examined on day 5. By day 11, both control and ΔcJun-expressing mice exhibit comparable levels of social avoidance. *p<0.05. From Vialou et al., 2010a with permission.
Acknowledgments
Preparation of this review was supported by grants from the National Institute of Mental Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Been LE, Hedges VL, Vialou V, Nestler EJ, Meisel RL. Delta JunD expression in the nucleus accumbens prevents sexual reward in female Syrian hamsters. Genes Brain Behav. 2013;12:666–672. doi: 10.1111/gbb.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carle TL, Ohnishi YN, Ohnishi YH, Alibhai IN, Wilkinson MB, Kumar A, Nestler EJ. Absence of conserved C-terminal degron domain contributes to ΔFosB’s unique stability. Eur J Neurosci. 2007;25:3009–3019. doi: 10.1111/j.1460-9568.2007.05575.x. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Cates H, Thibault M, Pfau M, Heller E, Eagle A, Gajewski P, Bagot R, Colangelo C, Abbott T, Rudenko G, Neve R, Nestler EJ, Robison AJ. Threonine 149 phosphorylation enhances ΔFosB transcriptional activity to control psychomotor responses to cocaine. J Neurosci. 2014;34:11461–11469. doi: 10.1523/JNEUROSCI.1611-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JS, Nye HE, Kelz MB, Hiroi N, Nakabeppu Y, Hope BT, Nestler EJ. Regulation of ΔFosB and FosB-like proteins by electroconvulsive seizure (ECS) and cocaine treatments. Mol Pharmacol. 1995;48:880–889. [PubMed] [Google Scholar]
- Chen J, Kelz MB, Hope BT, Nakabeppu Y, Nestler EJ. Chronic FRAs: Stable variants of ΔFosB induced in brain by chronic treatments. J Neurosci. 1997;17:4933–4941. doi: 10.1523/JNEUROSCI.17-13-04933.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, Krishnan V, Reyes CM, Han MH, Ables JL, Eisch AJ, Dietz DM, Ferguson D, Neve RL, Greengard P, Kim Y, Morrison JH, Russo SJ. IκB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci. 2011;31:314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, III, Maze I, LaPlant QC, Vialou VF, Yoshinori ON, Berton O, Fass DM, Renthal W, Rush AJ, III, Wu EY, Ghose S, Krishnan V, Russo SJ, Tamminga C, Haggarty SJ, Nestler EJ. Antidepressant actions of HDAC inhibitors. J Neurosci. 2009;29:11451–11460. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, III, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, LaPlant Q, Mouzon E, Ghose S, Tamminga CA, Neve RL, Deisseroth K, Nestler EJ. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J Neurosci. 2010;30:16082–16090. doi: 10.1523/JNEUROSCI.1731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, III, Maze I, Sun HS, Wu EY, Dietz D, Lobo MK, Ghose S, Neve R, Tamminga CA, Nestler EJ. A role for repressive histone methylation in cocaine-induced vulnerability to stress. Neuron. 2011;71:656–670. doi: 10.1016/j.neuron.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue RJ, Muschamp JW, Russo SR, Nestler EJ, Carlezon WA. Effects of striatal ΔFosB overexpression and ketamine on social defeat stress-induced anhedonia in mice. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2013.12.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, Robison AJ, Neve RL, Nestler EJ, Malenka RC. ΔFosB differentially modulates nucleus accumbens direct and indirect pathway function. Proc Natl Acad Sci USA. 2013;110:1923–1928. doi: 10.1073/pnas.1221742110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges VL, Chakravarty S, Nestler EJ, Meisel RL. ΔFosB overexpression in the nucleus accumbens enhances sexual reward in female Syrian hamsters. Genes Brain Behav. 2009;8:442–449. doi: 10.1111/j.1601-183X.2009.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi N, Brown J, Ye H, Saudou F, Vaidya VA, Duman RS, Greenberg ME, Nestler EJ. Essential role of the fosB gene in molecular, cellular, and behavioral actions of electroconvulsive seizures. J Neurosci. 1998;18:6952–6962. doi: 10.1523/JNEUROSCI.18-17-06952.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsboer F, Ising M. Stress hormone regulation: biological role and translation into therapy. Annu Rev Psychol. 2010;61:81–109. doi: 10.1146/annurev.psych.093008.100321. [DOI] [PubMed] [Google Scholar]
- Jorissen H, Ulery P, Henry L, Gourneni S, Nestler EJ, Rudenko G. Dimerization and DNA-binding properties of the transcription factor deltaFosB. Biochemistry. 2007;46:8360–8372. doi: 10.1021/bi700494v. [DOI] [PubMed] [Google Scholar]
- Kelz MB, Chen JS, Carlezon WA, Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang YJ, Marotti L, Self DW, Tkatch R, Baranauskas G, Surmeier DJ, Neve RL, Duman RS, Picciotto MR, Nestler EJ. Expression of the transcription factor ΔFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, LaPlant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Susceptibility and resistance to social defeat are mediated through molecular adaptations in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. Linking molecules to mood: New insight into the biology of depression. Am J Psychiatry. 2010;167:1305–1320. doi: 10.1176/appi.ajp.2009.10030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licznerski P, Duman RS. Remodeling of axo-spinous synapses in the pathophysiology and treatment of depression. Neuroscience. 2013;251:33–50. doi: 10.1016/j.neuroscience.2012.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lively S, Brown IR. The extracellular matrix protein SC1/hevin localizes to excitatory synapses following status epilepticus in the rat lithium-pilocarpine seizure model. J Neurosci Res. 2008;86:2895–2905. doi: 10.1002/jnr.21735. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Zaman S, Damez-Werno DM, Koo JW, Bagot RC, DiNieri JA, Nugent A, Finkel E, Chaudhury D, Chandra R, Ribeiro E, Rabkin J, Mouzon E, Cachope R, Cheer JF, Han MH, Dietz DM, Self DW, Hurd YL, Vialou V, Nestler EJ. ΔFosB induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. J Neurosci. 2013;33:18381–18395. doi: 10.1523/JNEUROSCI.1875-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. Regulation of gene expression and cocaine reward by CREB and ΔFosB. Nature Neurosci. 2003;11:1208–1215. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- Muschamp JW, Nemeth CL, Robison AJ, Nestler EJ, Carlezon WA., Jr ΔFosB enhances the rewarding effects of cocaine while reducing the pro-depressive effects of the kappa-opioid agonist U50488. Biol Psychiatry. 2012;71:44–50. doi: 10.1016/j.biopsych.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Transcriptional mechanisms of addiction: role of deltaFosB. Philos Trans R Soc London B Biol Sci. 2008;363:3245–3255. doi: 10.1098/rstb.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. ΔFosB: a molecular switch for reward. J Drug Alcohol Res. 2012;2:235651. [Google Scholar]
- Nestler EJ. Epigenetic mechanisms of drug addiction. Neuropharmacology. 2014;76:259–268. doi: 10.1016/j.neuropharm.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Ohnishi YN, Ohnishi YH, Hokama M, Nomaru H, Yamazaki K, Tominaga Y, Sakumi K, Nestler EJ, Nakabeppu Y. FosB Is essential for the enhancement of stress tolerance and antagonizes locomotor sensitization by ΔFosB. Biol Psychiatry. 2011;70:487–495. doi: 10.1016/j.biopsych.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi YN, Ohnishi YH, Vialou V, Mouzon E, LaPlant Q, Nishi A, Nestler EJ. Functional role of the N-terminal domain of ΔFosB in response to stress and drugs of abuse. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.10.002. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Tronson N, Neve R, Nestler EJ, Taylor JR. ΔFosB in the nucleus accumbens regulates food-reinforced instrumental behavior and motivation. J Neurosci. 2006;26:9196–9204. doi: 10.1523/JNEUROSCI.1124-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peakman MC, Colby C, Perrotti LI, Tekumalla P, Carle T, Ulery P, Chao J, Duman C, Steffen C, Monteggia L, Allen MR, Stock JL, Duman RS, McNeish JD, Barrot M, Self DW, Nestler EJ, Schaeffer E. Inducible, brain region specific expression of a dominant negative mutant of c-Jun in transgenic mice decreases sensitivity to cocaine. Brain Res. 2003;970:73–86. doi: 10.1016/s0006-8993(03)02230-3. [DOI] [PubMed] [Google Scholar]
- Perrotti LI, Hadeishi Y, Ulery P, Barrot M, Monteggia L, Duman RS, Nestler EJ. Induction of ΔFosB in reward-related brain regions after chronic stress. J Neurosci. 2004;24:10594–10602. doi: 10.1523/JNEUROSCI.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchers KK, Frohmader KS, Vialou V, Mouzon E, Nestler EJ, Lehman MN, Coolen LM. ΔFosB in the nucleus accumbens is critical for reinforcing effects of sexual reward. Genes Brain Behav. 2010;9:831–840. doi: 10.1111/j.1601-183X.2010.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchers KK, Vialou V, Nestler EJ, Lehman MN, Coolen LM. Sexual experience increases amphetamine reward and nucleus accumbens spinogenesis via dopamine D1 receptor activity and induction of deltaFosB. J Neurosci. 2013;33:3434–3442. [Google Scholar]
- Renthal W, Kumar A, Xiao GH, Wilkinson M, Covington HE, III, Maze I, Sikder D, Robison AJ, LaPlant Q, Dietz DM, Russo SJ, Vialou V, Chakravarty S, Kodadek TJ, Stack A, Kabbaj M, Nestler EJ. Genome wide analysis of chromatin regulation by cocaine reveals a novel role for sirtuins. Neuron. 2009;62:335–348. doi: 10.1016/j.neuron.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison AJ, Vialou V, Mazei-Robison M, Feng J, Kourrich S, Collins M, Wee SM, Koob G, Turecki G, Neve R, Thomas M, Nestler EJ. Behavioral and structural responses to chronic cocaine require a feed-forward loop involving ΔFosB and CaMKII in the nucleus accumbens shell. J Neurosci. 2013;33:4295–4307. doi: 10.1523/JNEUROSCI.5192-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison AJ, Vialou V, Sun HS, Labonte B, Golden S, Dias C, Turecki G, Tamminga C, Russo SJ, Mazei-Robison M, Nestler EJ. Fluoxetine epigenetically alters the CaMKII promoter in nucleus accumbens to regulate ΔFosB binding and antidepressant effects. Neuropsychopharmacology. 2014;39:1178–1186. doi: 10.1038/npp.2013.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teegarden SL, Nestler EJ, Bale TL. Delta FosB-mediated alterations in dopamine signaling are normalized by a palatable high-fat diet. Biol Psychiatry. 2008;64:941–950. doi: 10.1016/j.biopsych.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulery PG, Rudenko G, Nestler EJ. Regulation of ΔFosB stability by phosphorylation. J Neurosci. 2006;26:5131–5142. doi: 10.1523/JNEUROSCI.4970-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulery-Reynolds PG, Castillo MA, Vialou V, Russo SJ, Nestler EJ. Phosphorylation of ΔFosB mediates its stability in vivo. Neuroscience. 2009;158:369–372. doi: 10.1016/j.neuroscience.2008.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Robison AJ, LaPlant QC, Covington HE, III, Dietz DM, Ohnishi YN, Mouzon E, Rush AJ, III, Watts EL, Wallace DL, Iñiguez SD, Ohnishi YH, Steiner MA, Warren B, Krishnan V, Neve RL, Ghose S, Berton O, Tamminga CA, Nestler EJ. ΔFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nature Neurosci. 2010a;13:745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Maze I, Renthal W, LaPlant QC, Watts EL, Mouzon E, Ghose S, Tamminga CA, Nestler EJ. Serum response factor promotes resilience to chronic social stress through the induction of ΔFosB. J Neurosci. 2010b;30:14585–14592. doi: 10.1523/JNEUROSCI.2496-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Feng J, Robison AJ, Ku SM, Ferguson D, Scobie KN, Mazei-Robison MA, Mouzon E, Nestler EJ. Serum response factor and cAMP response element binding protein are both required for cocaine induction of ΔFosB. J Neurosci. 2012;32:7577–7584. doi: 10.1523/JNEUROSCI.1381-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Bagot RC, Cahill ME, Ferguson D, Robison AJ, Dietz DM, Fallon B, Mazei-Robison M, Ku SM, Harrigan E, Winstanley CA, Joshi T, Feng J, Berton O, Nestler EJ. Prefrontal cortical circuit for depression- and anxiety-related behaviors mediated by cholecystokinin: role of ΔFosB. J Neurosci. 2014a;34:3878–3887. doi: 10.1523/JNEUROSCI.1787-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Thibault M, Kaska S, Gajewski P, Eagle A, Mazei-Robison M, Nestler EJ, Robison AJ. Differential induction of FosB isoforms throughout the brain by fluoxetine and chronic stress. Neuropharmacology. 2014b doi: 10.1016/j.neuropharm.2015.07.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DL, Vialou V, Rios L, Carle-Florence TL, Chakravarty S, Kumar A, Graham DL, Green TA, Kirk A, Iniguez SD, Perrotti LI, Barrot M, DiLeone RJ, Nestler EJ, Bolaños CA. The influence of ΔFosB in the nucleus accumbens on natural reward-related behavior. J Neurosci. 2008;28:10272–10277. doi: 10.1523/JNEUROSCI.1531-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cesena T, Ohnishi Y, Burger-Caplan R, Vivian L, Kirchhoff P, Scott L, Larsen M, Nestler EJ, Rudenko G. Small molecule screening identifies regulators of the transcription factor DeltaFosB. ACS Chem Neurosci. 2012;3:546–556. doi: 10.1021/cn3000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werme M, Messer C, Olson L, Gilden L, Thorén P, Nestler EJ, Brené S. ΔFosB regulates wheel running. J Neurosci. 2002;22:8133–8138. doi: 10.1523/JNEUROSCI.22-18-08133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson MB, Xiao GH, Kumar A, LaPlant Q, Renthal W, Sikder D, Kodadek TJ, Nestler EJ. Imipramine treatment and resiliency exhibit similar chromatin regulation in a key brain reward region. J Neurosci. 2009;29:7820–7832. doi: 10.1523/JNEUROSCI.0932-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, LaPlant Q, Theobald DEH, Green TA, Bachtell RK, Perrotti LI, DiLeone RJ, Russo SJ, Garth WJ, Self DW, Nestler EJ. ΔFosB induction in orbitofrontal cortex mediates tolerance to cocaine-induced cognitive dysfunction. J Neurosci. 2007;27:10497–10507. doi: 10.1523/JNEUROSCI.2566-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Bolanos CA, Selley DE, Theobald D, Cassidy MP, Kelz MB, Shaw-Lutchmann T, Berton O, Sim-Selley LJ, DiLeone RJ, Kumar A, Nestler EJ. ΔFosB: An essential role for ΔFosB in the nucleus accumbens in morphine action. Nature Neurosci. 2006;9:205–211. doi: 10.1038/nn1636. [DOI] [PubMed] [Google Scholar]
- Zhang YF, Crofton EJ, Li D, Lobo MK, Fan XZ, Nestler EJ, Green TA. Overexpression of DeltaFosB in nucleus accumbens mimics the protective addiction phenotype, but not the protective depression phenotype, of environmental enrichment. Front Behav Neurosci. 2014 doi: 10.3389/fnbeh.2014.00297. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]