Abstract
Acinetobacter baumannii is an important cause of healthcare-associated infections, and is particularly problematic among patients who undergo organ transplantation. We describe a case of fulminant sepsis caused by carbapenem-resistant A. baumannii harboring the blaOXA-23 carbapenemase gene and belonging to international clone II. This isolate led to the death of a patient 6 days after simultaneous kidney-pancreas transplantation. Autopsy findings revealed acute mitral valve endocarditis, myocarditis, splenic and renal emboli, peritonitis, and pneumonia. This case highlights the severe nature of certain A. baumannii infections and the vulnerability of transplanted patients to the increasingly intractable ‘high-risk’ clones of multidrug-resistant organisms.
Keywords: Acinetobacter baumannii, carbapenem resistance, simultaneous kidney-pancreas transplantation, endocarditis
Healthcare-associated infections are an important cause of inpatient morbidity and mortality (1). Infections with multidrug-resistant (MDR) gram-negative bacteria, especially carbapenem-resistant organisms, are of particular concern since their treatment is complicated by the paucity of active and reliable antibiotics and the limited availability of rapid and accurate diagnostics (2). In an era of organ shortages, transplant recipients, by nature of their underlying condition and anticipated clinical course, are at increased risk for infection with MDR organisms (3, 4). Given the impact of antimicrobial resistance in transplant recipients, defining fundamental strategies to prevent and manage infections will be critical for the advancement of transplantation. Here, we describe a fatal case of endocarditis and disseminated carbapenem-resistant Acinetobacter baumannii (CRAb) infection in a simultaneous pancreas-kidney transplant recipient. Our goal is to highlight the unappreciated virulence of A. baumannii and difficulties in recognizing and treating infections caused by CRAb after organ transplantation.
Case report
A 51-year-old man with type 1 diabetes mellitus underwent simultaneous pancreas and kidney transplantation after being maintained on peritoneal dialysis for 4 years, without peritonitis or other complications. He was managed with an insulin pump, and received thyroid replacement therapy. The prior surgical history was significant for a remote cholecystectomy and inguinal hernia repair, but he had not been admitted to the hospital recently. Before transplantation, the patient did not have fever or symptoms suggestive of recent or current infection.
The kidney and pancreas allografts were in good condition and the transplant procedure was uncomplicated; prophylaxis with cefazolin was administered. Immediate evidence was seen of acceptable renal allograft function, and the patient was transferred to the surgical intensive care unit (ICU), where he was extubated. Induction immunosuppression included anti-thymocyte globulin, mycophenolate mofetil, tacrolimus, and methylprednisolone. Pre-transplantation evaluation had revealed that the patient was cytomegalovirus-seronegative and he received valganciclovir, as well as trimethoprim-sulfamethoxazole and fluconazole prophylaxis. The immediate postoperative course was notable for leukocytosis and 2 episodes of hypoglycemia requiring supplemental dextrose. The patient was transferred to a regular inpatient medical unit on postoperative day (POD) 2.
On POD 4, hypothermia (34.9°C), tachycardia (115 beats per min), hypotension (90/60 mmHg), and leukopenia (1.7 white blood cells × 103/mm) were noted. During the next 24 h, the patient continued to complain of weakness, remained hypothermic, tachycardic, and hypotensive, and was transferred to the surgical ICU with significant abdominal pain. Upon examination, the abdomen was distended. Blood and urine cultures were obtained; vancomycin and piperacillin/tazobactam were started empirically, and fluconazole continued.
An exploratory laparotomy was performed on the evening of POD 5 because of concern for an intra-abdominal source of sepsis. Two liters of serosanguinous peritoneal fluid were drained; both allografts were deemed viable, and there was no evidence of anastomotic leak, bowel perforation, or necrosis. Gram stain of a sample of peritoneal fluid revealed granulocytes and mixed bacteria with predominant gram-variable coccobacilli.
The patient returned to the surgical ICU in critical condition; acidosis progressed, despite continued broad-spectrum antibiotics (with meropenem instead of piperacillin/tazobactam) and support with vasopressors. A repeat exploratory laparotomy was performed <12 h later, on the morning of POD 6. The nonviable pancreatic allograft was removed. Further deterioration and cardiopulmonary arrest ensued, and the patient died later that day without further resuscitative efforts.
On the morning of POD 6, cultures obtained from peritoneal fluid yielded 4+ growth of gram-negative bacilli; that afternoon (1 h after the patient died) blood cultures became positive, with gram-negative bacilli present. Urine cultures remained sterile. Results of identification and susceptibility testing (MicroScan, Siemens Healthcare) of blood and peritoneal fluid isolates became available on POD 7, indicating A. baumannii resistant to piperacillin/tazobactam, all carbapenems, all cephalosporins, fluoroquinolones, and aminoglycosides, and with intermediate susceptibility to ampicillin/sulbactam. In contrast, the isolate was susceptible to colistin, polymyxin B, and tigecycline (Table 1; 5, 6).
Table 1.
Results of antimicrobial susceptibility testing for Acinetobacter baumannii, according to Clinical Laboratory Standards Institute and FDA guidelines (5, 6)
| Antibiotic | MIC (μg/mL) | Interpretation |
|---|---|---|
| Amikacin | >32 | R |
| Aztreonam | >16 | R |
| Ampicillin/sulbactam | 16/8 | I |
| Ciprofloxacin | >2 | R |
| Colistin | 0.5 | S |
| Doripenem | >2 | R |
| Cefepime | >16 | R |
| Gentamicin | >8 | R |
| Imipenem | >8 | R |
| Levofloxacin | >8 | R |
| Meropenem | >8 | R |
| Minocycline | 8 | I |
| Piperacillin/tazobactam | >64/128 | R |
| Polymyxin B | 0.5 | S |
| Trimethoprim/sulfamethoxazole | >4/76 | R |
| Tigecycline | 1 | S |
| Tobramycin | >8 | R |
FDA, US Food & Drug Administration; MIC, minimum inhibitory concentration; R, resistant; I, intermediate; S, susceptible.
Polymerase chain reaction (PCR) was used to query the presence of acquired carbapenemase genes (blaOXA-23-like, blaOXA-24/40-like, blaOXA-58-like, blaKPC, blaVIM, blaIMP and blaNDM) in the A. baumannii blood isolate; only blaOXA-23-like was detected, and further sequencing of the amplicon identified it as blaOXA-23 (7). Genetic typing with repetitive-sequence-based PCR demonstrated that the isolate was clonally related (>95% similarity) to the predominant strain in the hospital at the time, which was associated with an outbreak at the institution (8). Further genetic typing using multilocus sequence typing with the scheme devised at the Institut Pasteur confirmed that the isolate belonged to sequence type 2 (ST2) related to international clone II (http://www.pasteur.fr/recherche/genopole/PF8/mlst/Abaumannii.html).
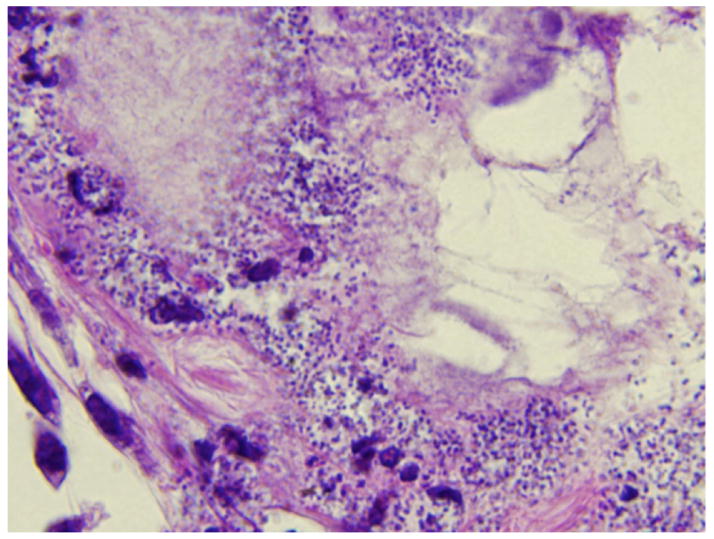
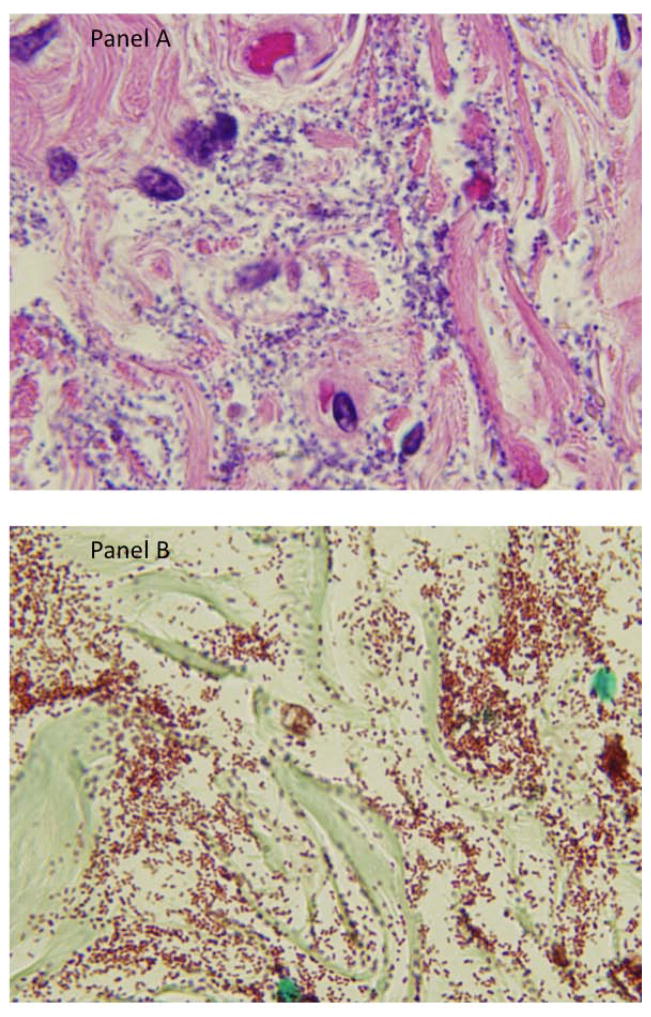
An autopsy was performed. Significant findings included vegetations on a calcified native mitral valve, ascites, a splenic infarct, a hemorrhagic renal allograft, and congestion of lungs and liver. Histologic findings revealed gram-negative coccobacilli consistent with Acinetobacter species within the myocardium, in a thrombus associated with the mitral valve vegetation, in the diaphragm, native kidneys, left ureter and bladder, and thrombo-emboli laden with coccobacilli in the left upper lobe of the lung. The explanted pancreatic allograft showed ischemia, fat necrosis, with coccobacilli morphologically consistent with Acinetobacter species (Figs. 1 and 2). Postmortem cultures from the spleen, lung, and heart all yielded A. baumannii with antimicrobial susceptibilities identical to those of the peritoneal fluid and blood isolates.
Fig. 1.
High-powered (100X) microscopic image of pancreatic graft with hematoxylin and eosin stain showing coccobacilli consistent with Acinetobacter species.
Fig. 2.
High-powered (100X) microscopic image of bladder with hematoxylin and eosin (panel A) and Gram stain (panel B) demonstrating abundant coccobacilli consistent with Acinetobacter species.
Discussion
Despite significant medical and surgical advances in organ transplantation and infection prevention, bacterial infections remain a significant source of post-transplant complications (9, 10). This report alerts us to the threat posed by CRAb to transplant recipients, compounded in this case by the unpredictable nature and incredibly rapid dissemination of the infection, the challenge of timely identification and antimicrobial susceptibility results, and the absence of effective treatment options.
A. baumannii is recognized for its ability to acquire and express multiple drug-resistance determinants (11). Carbapenem resistance surged in the past decade and now occurs in the majority of A. baumannii isolates, associated with overexpression of the chromosomal OXA-51 β-lactamase and acquisition of other OXA carbapenemases and metallo-β-lactamases through mobile genetic elements; high-level cephalosporinase expression and porin mutations also may lead to carbapenem resistance (12, 13). A striking feature of CRAb is that it harbors additional resistance determinants to other important classes of antibiotics, including mutations in the chromosomal quinolone-resistance determining region, genes encoding for aminoglycoside-modifying enzymes, 16S rRNA methyltransferases, and multidrug efflux pumps (7). In our case, the isolate contained blaOXA-23 and belonged to international clone II, a successful ‘high-risk’ strain, sometimes associated with adverse clinical outcomes, and widely disseminated throughout the world (14–16).
The present knowledge regarding the epidemiology and clinical impact of A. baumannii is derived from institutional outbreaks and national and regional surveys (17–21). CRAb typically infects ill patients previously exposed to antibiotics and requiring critical care, including mechanical ventilation (9, 10). Thus, organ transplant recipients are especially vulnerable, acquiring CRAb from contact with the healthcare environment and personnel. In one report, 32% of organ recipients infected with CRAb were previously colonized (22). The impact of CRAb infections in the survival of transplant patients, however, is difficult to ascertain (12, 23–25). CRAb causes healthcare- and ventilator-associated pneumonia mainly after lung transplantation, and is associated with recurrent infection, allograft dysfunction, length of stay, and mortality (22, 24, 26–29).
Among recipients of abdominal organs, post-transplantation complications may predate or be unrelated to CRAb isolation (30–32). Renal transplant recipients with CRAb may suffer delayed graft function, nephrostomy placement, and renal replacement therapy (30). In general, the course of CRAb is insidious and protracted, with a median time from transplantation to infection of 20–349 days (4). Nevertheless, CRAb infections can present more immediately, as demonstrated by a case of necrotizing fasciitis that occurred 10 days after an uneventful kidney-pancreas transplant (33).
The rapid pace and fulminant nature of infection with CRAb in this case is noteworthy. Review of the autopsy findings revealed: (i) endocarditis of the mitral valve, (ii) myocarditis, (iii) splenic emboli, (iv) pneumonia, (v) peritonitis, and (vi) seeding of the native kidney and pancreatic allograft, but sparing of the renal allograft. How did overwhelming infection with A. baumannii come to occur in this case? Potential sources include healthcare-associated infection at or around the time of transplantation, prior colonization, or unrecognized pre-existent recipient infection exacerbated by immunosuppression, or donor-derived infection (34).
Acquisition of CRAb from the healthcare environment or personnel may be inferred by the similarity of the patient’s isolate to the outbreak strain of CRAb circulating in the hospital at the time (8). The concurrent outbreak of CRAb was centered in the medical ICU, however, and no cases of CRAb had occurred in the surgical ICU until that time. Detailed genomic analysis of the outbreak isolates has revealed extensive variations in their gene content, suggesting plasmid transfers and ‘microevolution’ among a complex reservoir of strains (35). Furthermore, CRAb strains belonging to international clone II and harboring blaOXA-23, such as the one responsible for this infection, also predominate among epidemiologically unrelated CRAb isolates from the same region and the rest of the United States (19, 21).
Evidence in our patient of previous colonization with CRAb is lacking, although pre-transplant screening for carriage of MDR organisms was not performed. Pre-operative colonization with MDR gram-negative organisms is infrequently associated with post-transplantation morbidity, with the exception of Burkholderia cepacia complex in lung transplantation (3, 4). It remains possible that intensive immunosuppression unmasked previously unrecognized A. baumannii acquired before his admission, resulting in the dramatic clinical presentation we witnessed. It is also possible that the infecting strain possessed characteristics, including changes in surface polysaccharide components, which altered the host response (35, 36).
The donor in this case was a young man involved in a motor vehicle accident; evidence of donor-derived infection was not found. We maintain that the presence of coccobacilli suggestive of A. baumannii in the pancreas allograft was likely the result of disseminated infection in the recipient. Even if established beforehand, bacterial infections in donors do not preclude organ procurement, because treatment of donors with broad-spectrum antibiotics and prophylaxis of recipients mitigate transmission risks (37). Donor-derived infections with MDR organisms (causing bloodstream infections and mycotic aneurysms) do occur, and present an average of 8 days post transplantation (38). Failures in timely communication of antimicrobial susceptibility results from donors’ isolates have led to ineffective initial antibiotic treatment of transplant recipients (27, 39).
Delayed initiation of effective antimicrobials for the treatment of CRAb may be associated with increased morbidity and mortality (25, 32, 40). In this case, the microbial etiology of the sepsis syndrome was not available ante mortem. Gram stain of peritoneal fluid, the only result available before the patient died, revealed coccobacilli, in retrospect indicative of A. baumannii infection. Definite identification and antimicrobial susceptibility results, however, only became available after the patient’s demise. The patient was treated empirically with piperacillin/tazobactam followed by meropenem, reasonable choices based on the local epidemiology, but ultimately ineffective. In cases such as this, the implementation in clinical practice of rapid molecular diagnostics targeted to resistance gene identification offers the potential to inform early appropriate therapy by detecting antibiotic resistance determinants (blaOXA-23, in this instance) (41, 42).
Even when antimicrobial susceptibilities are known, the treatment of CRAb infections is problematic because of the MDR nature of this pathogen and the shortcomings of available therapeutic options (e.g., polymyxins, tigecycline, and sulbactam). Therefore, attention has focused on combination therapy, where the data, derived mostly from in vitro evaluations, appear promising. Evidence from controlled clinical trials is limited, however, and the optimal combination of antimicrobials remains undefined (Table 2; 22, 29, 43–49). Only a few agents with activity against A. baumannii are in development (2, 50).
Table 2.
Therapeutic approaches to carbapenem-resistant Acinetobacter baumannii (CRAb)
| Approach | Advantages | Limitations | References |
|---|---|---|---|
| Monotherapy
| |||
| Polymyxins | Highly active in vitro Mainstay against CRAb |
Nephrotoxicity of colistin + calcineurin inhibitors Unclear pharmacokinetics and dosing regimen Emergence of resistance with monotherapy |
(43, 44) |
| Tigecycline | Highly active in vitro Large volume of distribution Tissue penetration |
Increased mortality Resistance during therapy Inadequate serum and urine concentrations |
(22, 29, 45) |
| Sulbactam | Comparable to carbapenems for susceptible strains Optimized with high dose and prolonged infusion |
Co-resistance of sulbactam in CRAb | (46) |
|
| |||
| Combination therapy
| |||
| Carbapenem + polymyxins | Favorable meta-analysis of in vitro data Successful in organ transplant recipients |
Controlled trials needed | (22, 47) |
| Polymyxins + rifampin | Randomized controlled trial: increased bacterial clearance | No difference in 30-day mortality Rifampin interacts vs. azoles, calcineurin/mTOR inhibitors |
(48) |
| Polymyxins + fosfomycin | Randomized controlled trial: favorable microbiological response | Not powered to detect differences in clinical outcomes IV fosfomycin not available in the US | (49) |
mTOR, mammalian target of rapamycin; IV, intravenous; US, United States of America.
In revisiting this case, our current appraisal is that the patient was demonstrating evidence of sepsis in the early postoperative period, although his episodes of hypoglycemia could have been related to the recent pancreas transplant. Crucially, infection with CRAb was not recognized and empirical antibiotic treatment was ineffective. As noted, few cases of CRAb among surgical patients had occurred at the facility at the time in which the patient underwent transplantation. In addition, this patient did not present with healthcare-associated pneumonia, the most common syndrome of CRAb in organ transplant recipients. While there was increased concern for an intra-abdominal source, the possibility of endocarditis was unanticipated. The patient died before microbiological diagnostic results became available and could be incorporated into clinical decision-making. Although the use of rapid molecular diagnostics may accelerate the identification of the organism and its genotype, choosing an effective antibiotic therapy against CRAb is problematic, as reviewed.
With these caveats in mind, the fulminant nature of this patient’s infection, and the degree of immunosuppression in the immediate post-transplant period, it is unclear whether the outcome would have been altered with more timely active antimicrobials or more aggressive surgical intervention. The unfortunate overlap of immune suppression and overwhelming infection likely preordained the poor outcome. The behavior of this isolate, causing valvular infection and widespread organ involvement, may also suggest unique virulence characteristics. Although the occurrence of endocarditis is a distinctly unusual complication of a gram-negative bacteremia, review of the clinical experience with Acinetobacter endocarditis involving native valves reveals this as a fulminant and aggressive infection, notable for its high organism load and the seeding of multiple organs (51).
In conclusion, this case illustrates the challenge that A. baumannii poses to organ transplantation, including the need for identification of virulence factors affecting outcome, development of novel antibiotics, and implementation of rapid diagnostic tests. Despite efforts in choosing appropriate transplant candidates and donors, the increasing prevalence of MDR organisms in our healthcare system may yet prove to be the “Achilles’ heel” of organ transplantation. Therefore, a collaborative approach between intensivists, transplant practitioners, infection preventionists, clinical microbiologists, and infectious diseases specialists remains imperative in the management of these patients. These efforts may lead to innovations in screening and early detection, insights into pharmacotherapy, and the development of passive and active immunological strategies against A. baumannii and other MDR organisms (52).
Acknowledgments
Support: This work was supported by the Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research (F.P.). In addition, this work was supported in part by the Veterans Affairs Merit Review Program (to RAB, Award 1I01BX001974), the National Institutes of Health (Grant AI072219-05 and AI063517-07 to R.A.B.), and the Geriatric Research Education and Clinical Center VISN 10 (R.A.B.).
Footnotes
Disclaimer: Funding organizations were not involved in the design and conduct of the study; collection, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health or the Department of Veterans Affairs.
Potential conflicts of interest: R.A.B. has received research funding from Astra Zeneca, Rib-X, Merck, Steris, and Checkpoints, and has served on a Tetraphase drug safety monitoring board.
References
- 1.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boucher HW, Talbot GH, Benjamin DK, et al. 10 × ‘20 Progress—development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(12):1685–1694. doi: 10.1093/cid/cit152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Duin D, van Delden C. Multidrug-resistant gram-negative bacteria infections in solid organ transplantation. Am J Transplant. 2013;13(Suppl 4):31–41. doi: 10.1111/ajt.12096. [DOI] [PubMed] [Google Scholar]
- 4.Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol. 2013;34(1):1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 5.CLSI. 19th Informational Supplement. Wayne, PA: Clinical and Laboratory Standards Institute; 2009. Performance Standards for Antimicrobial Susceptibility Testing. [Google Scholar]
- 6.Pankey GA. Tigecycline. J Antimicrob Chemother. 2005;56(3):470–480. doi: 10.1093/jac/dki248. [DOI] [PubMed] [Google Scholar]
- 7.Hujer KM, Hujer AM, Hulten EA, et al. Analysis of antibiotic resistance genes in multidrug-resistant Acinetobacter sp. isolates from military and civilian patients treated at the Walter Reed Army Medical Center. Antimicrob Agents Chemother. 2006;50(12):4114–4123. doi: 10.1128/AAC.00778-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez F, Endimiani A, Ray AJ, et al. Carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae across a hospital system: impact of post-acute care facilities on dissemination. J Antimicrob Chemother. 2010;65(8):1807–1818. doi: 10.1093/jac/dkq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munoz-Price LS, Weinstein RA. Acinetobacter infection. N Engl J Med. 2008;358(12):1271–1281. doi: 10.1056/NEJMra070741. [DOI] [PubMed] [Google Scholar]
- 10.Pogue JM, Mann T, Barber KE, Kaye KS. Carbapenem-resistant Acinetobacter baumannii: epidemiology, surveillance and management. Expert Rev Anti Infect Ther. 2013;11(4):383–393. doi: 10.1586/eri.13.14. [DOI] [PubMed] [Google Scholar]
- 11.Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2007;51(10):3471–3484. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21(3):538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel G, Bonomo R. “Stormy waters ahead”: global emergence of carbapenemases. Front Microbiol. 2013:4. doi: 10.3389/fmicb.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zarrilli R, Pournaras S, Giannouli M, Tsakris A. Global evolution of multidrug-resistant Acinetobacter baumannii clonal lineages. Internat J Antimicrob Agents. 2013;41(1):11–19. doi: 10.1016/j.ijantimicag.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Kallen AJ, Hidron AI, Patel J, Srinivasan A. Multidrug resistance among gram-negative pathogens that caused healthcare-associated infections reported to the National Healthcare Safety Network, 2006–2008. Infect Control Hosp Epidemiol. 2010;31(5):528–531. doi: 10.1086/652152. [DOI] [PubMed] [Google Scholar]
- 16.Perez F, Hujer AM, Hulten EA, et al. Antibiotic resistance determinants in Acinetobacter spp and clinical outcomes in patients from a major military treatment facility. Am J Infect Control. 2010;38(1):63–65. doi: 10.1016/j.ajic.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villegas MV, Hartstein AI. Acinetobacter outbreaks, 1977–2000. Infect Control Hosp Epidemiol. 2003;24(4):284–295. doi: 10.1086/502205. [DOI] [PubMed] [Google Scholar]
- 18.Villar M, Cano ME, Gato E, et al. Epidemiologic and clinical impact of Acinetobacter baumannii colonization and infection: a reappraisal. Medicine. 2014;93(5):202–210. doi: 10.1097/MD.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Decker BK, Perez F, Hujer AM, et al. Longitudinal analysis of the temporal evolution of Acinetobacter baumannii strains in Ohio, USA, by using rapid automated typing methods. PLoS One. 2012;7(4):e33443. doi: 10.1371/journal.pone.0033443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thom KA, Maragakis LL, Richards K, et al. Assessing the burden of Acinetobacter baumannii in Maryland: a statewide cross-sectional period prevalence survey. Infect Control Hosp Epidemiol. 2012;33(9):883–888. doi: 10.1086/667376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams-Haduch JM, Onuoha EO, Bogdanovich T, et al. Molecular epidemiology of carbapenem-nonsusceptible Acinetobacter baumannii in the United States. J Clin Microbiol. 2011;49(11):3849–3854. doi: 10.1128/JCM.00619-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shields RK, Clancy CJ, Gillis LM, et al. Epidemiology, clinical characteristics and outcomes of extensively drug-resistant Acinetobacter baumannii infections among solid organ transplant recipients. PLoS One. 2012;7(12):e52349. doi: 10.1371/journal.pone.0052349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trottier V, Namias N, Pust DG, et al. Outcomes of Acinetobacter baumannii infection in critically ill surgical patients. Surg Infect (Larchmt) 2007;8(4):437–443. doi: 10.1089/sur.2006.029. [DOI] [PubMed] [Google Scholar]
- 24.Nunley DR, Bauldoff GS, Mangino JE, Pope-Harman AL. Mortality associated with Acinetobacter baumannii infections experienced by lung transplant recipients. Lung. 2010;188(5):381–385. doi: 10.1007/s00408-010-9250-7. [DOI] [PubMed] [Google Scholar]
- 25.de Gouvea EF, Martins IS, Halpern M, et al. The influence of carbapenem resistance on mortality in solid organ transplant recipients with Acinetobacter baumannii infection. BMC Infect Dis. 2012;12:351. doi: 10.1186/1471-2334-12-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doi Y, Husain S, Potoski BA, McCurry KR, Paterson DL. Extensively drug-resistant Acinetobacter baumannii. Emerg Infect Dis. 2009;15(6):980–982. doi: 10.3201/eid1506.081006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martins N, Martins IS, de Freitas WV, et al. Severe infection in a lung transplant recipient caused by donor-transmitted carbapenem-resistant Acinetobacter baumannii. Transpl Infect Dis. 2012;14(3):316–320. doi: 10.1111/j.1399-3062.2011.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sopirala MM, Pope-Harman A, Nunley DR, Moffatt-Bruce S, Ross P, Martin SI. Multidrug-resistant Acinetobacter baumannii pneumonia in lung transplant recipients. J Heart Lung Transplant. 2008;27(7):804–807. doi: 10.1016/j.healun.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 29.Shields RK, Kwak EJ, Potoski BA, et al. High mortality rates among solid organ transplant recipients infected with extensively drug-resistant Acinetobacter baumannii: using in vitro antibiotic combination testing to identify the combination of a carbapenem and colistin as an effective treatment regimen. Diagn Microbiol Infect Dis. 2011;70(2):246–252. doi: 10.1016/j.diagmicrobio.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 30.Reddy P, Zembower TR, Ison MG, Baker TA, Stosor V. Carbapenem-resistant Acinetobacter baumannii infections after organ transplantation. Transpl Infect Dis. 2010;12(1):87–93. doi: 10.1111/j.1399-3062.2009.00445.x. [DOI] [PubMed] [Google Scholar]
- 31.Shi SH, Kong HS, Xu J, et al. Multidrug resistant gram-negative bacilli as predominant bacteremic pathogens in liver transplant recipients. Transpl Infect Dis. 2009;11(5):405–412. doi: 10.1111/j.1399-3062.2009.00421.x. [DOI] [PubMed] [Google Scholar]
- 32.Kim YJ, Yoon JH, Kim SI, et al. High mortality associated with Acinetobacter species infection in liver transplant patients. Transplant Proc. 2011;43(6):2397–2399. doi: 10.1016/j.transproceed.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Clemente WT, Sanches MD, Coutinho RL, et al. Multidrug-resistant Acinetobacter baumannii causing necrotizing fasciitis in a pancreas-kidney transplant recipient: a case report. Transplantation. 2012;94(6):e37–38. doi: 10.1097/TP.0b013e318265083b. [DOI] [PubMed] [Google Scholar]
- 34.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357(25):2601–2614. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 35.Wright MS, Haft DH, Harkins DM, et al. New insights into dissemination and variation of the health care-associated pathogen Acinetobacter baumannii from genomic analysis. MBio. 2014;5(1):e00963–00913. doi: 10.1128/mBio.00963-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruhn KW, Pantapalangkoor P, Nielsen T, et al. Host fate is rapidly determined by innate effector-microbial interactions during Acinetobacter baumannii bacteremia. J Infect Dis. 2014 Nov 5; doi: 10.1093/infdis/jiu593. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lumbreras C, Sanz F, González A, et al. Clinical significance of donor-unrecognized bacteremia in the outcome of solid-organ transplant recipients. Clin Infect Dis. 2001;33(5):722–726. doi: 10.1086/322599. [DOI] [PubMed] [Google Scholar]
- 38.Sifri CD, Ison MG. Highly resistant bacteria and donor-derived infections: treading in uncharted territory. Transpl Infect Dis. 2012;14(3):223–228. doi: 10.1111/j.1399-3062.2012.00752.x. [DOI] [PubMed] [Google Scholar]
- 39.Ison MG, Grossi P. Donor-derived infections in solid organ transplantation. Am J Transplant. 2013;13(Suppl 4):22–30. doi: 10.1111/ajt.12095. [DOI] [PubMed] [Google Scholar]
- 40.Lemos EV, de la Hoz FP, Einarson TR, et al. Carbapenem resistance and mortality in patients with Acinetobacter baumannii infection: systematic review and meta-analysis. Clin Microbiol Infect. 2014;20(5):416–423. doi: 10.1111/1469-0691.12363. [DOI] [PubMed] [Google Scholar]
- 41.Ecker DJ, Massire C, Blyn LB, et al. Molecular genotyping of microbes by multilocus PCR and mass spectrometry: a new tool for hospital infection control and public health surveillance. Methods Molec Biol. 2009;551:71–87. doi: 10.1007/978-1-60327-999-4_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin YC, Sheng WH, Chang SC, et al. Application of a microsphere-based array for rapid identification of Acinetobacter spp. with distinct antimicrobial susceptibilities. J Clin Microbiol. 2008;46(2):612–617. doi: 10.1128/JCM.01798-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garonzik SM, Li J, Thamlikitkul V, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother. 2011;55(7):3284–3294. doi: 10.1128/AAC.01733-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rao GG, Ly NS, Haas CE, et al. New dosing strategies for an old antibiotic: pharmacodynamics of front-loaded regimens of colistin at simulated pharmacokinetics in patients with kidney or liver disease. Antimicrob Agents Chemother. 2014;58(3):1381–1388. doi: 10.1128/AAC.00327-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dixit D, Madduri RP, Sharma R. The role of tigecycline in the treatment of infections in light of the new black box warning. Expert Rev Anti Infect Ther. 2014;12(4):397–400. doi: 10.1586/14787210.2014.894882. [DOI] [PubMed] [Google Scholar]
- 46.Adnan S, Paterson DL, Lipman J, Roberts JA. Ampicillin/sulbactam: its potential use in treating infections in critically ill patients. Internat J Antimicrob Agents. 2013;42(5):384–389. doi: 10.1016/j.ijantimicag.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 47.Zusman O, Avni T, Leibovici L, et al. Systematic review and meta-analysis of in vitro synergy of polymyxins and carbapenems. Antimicrob Agents Chemother. 2013;57(10):5104–5111. doi: 10.1128/AAC.01230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Durante-Mangoni E, Signoriello G, Andini R, et al. Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant Acinetobacter baumannii: a multicenter, randomized clinical trial. Clin Infect Dis. 2013;57(3):349–358. doi: 10.1093/cid/cit253. [DOI] [PubMed] [Google Scholar]
- 49.Sirijatuphat R, Thamlikitkul V. Preliminary study of colistin versus colistin plus fosfomycin for treatment of carbapenem-resistant Acinetobacter baumannii infections. Antimicrob Agents Chemother. 2014;58(9):5598–5601. doi: 10.1128/AAC.02435-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Viehman JA, Nguyen MH, Doi Y. Treatment options for carbapenem-resistant and extensively drug-resistant Acinetobacter baumannii infections. Drugs. 2014;74(12):1315–1333. doi: 10.1007/s40265-014-0267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gradon JD, Chapnick EK, Lutwick LI. Infective endocarditis of a native valve due to Acinetobacter: case report and review. Clin Infect Dis. 1992;14(5):1145–1148. doi: 10.1093/clinids/14.5.1145. [DOI] [PubMed] [Google Scholar]
- 52.Perez F, Bonomo RA. Vaccines for Acinetobacter baumannii: thinking “out of the box”. Vaccine. 2014;32(22):2537–2539. doi: 10.1016/j.vaccine.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]