Abstract
Background
Latino immigrants have high rates of obesity and face barriers to weight loss.
Objective
Evaluate the effectiveness of a case-management (CM) intervention with and without community health workers (CHWs) for weight loss.
Design
Two-year, randomized controlled trial comparing two interventions to each other and to usual care (UC).
Participants/setting
Eligible participants included Latinos with a Body Mass Index of 30-60 and one or more heart disease risk factors. The 207 participants recruited from 2009-2011 had a mean age of 47 years and were mostly female (77%). At 24 months, 86% of the sample was assessed.
Intervention
The CM+CHW (n=82) and CM (n=84) interventions were compared to each other and to UC (n=41). Both included an intensive 12 month phase followed by 12 months of maintenance. The CM+CHW group received home visits.
Main outcome measures
Weight change at 24 months.
Statistical Analyses
Generalized estimating equations using intent-to-treat.
Results
At 6 months, mean weight loss in the CM+CHW arm was −2.1 kg (95% CI −2.8, −1.3) or −2% of baseline weight (−1%, −2%) compared to −1.6 kg (−2.4, −0.7; % weight change: −2%, −1%, −3%) in CM and −0.9 kg (−1.8, 0.1; % weight change: −1%, 0%, −2%) in UC. By 12 and 24 months, differences narrowed and CM+CHW was no longer statistically distinct. Men achieved greater weight loss than women in all groups at each time point (p<0.05). At 6 months, men in the CM+CHW arm lost more weight (−4.4 kg, −6.0, −2.7) compared to UC (−0.4 kg, −2.4, 1.5), but by 12 and 24 months differences were not significant.
Conclusions
Incorporation of CHWs may help promote early weight loss, especially among men, but it did not achieve weight maintenance. Social and environmental influences may need to be addressed to achieve sustained weight loss in Latino immigrant populations.
INTRODUCTION
The 51 million Latinos in the United States (US) are disproportionately represented among Americans with a high Body Mass Index (BMI), with 79% at least overweight and 39% obese.1 The high prevalence of obesity is a critical public health issue due to high costs associated with treating obesity-related diseases,2 such as type 2 diabetes mellitus (DMT2) and coronary heart disease (CHD). As the largest and fastest growing US minority group,3 efforts to address obesity in the US must deploy effective strategies for this population. Unfortunately, effective strategies for weight loss in Latinos have yet to be developed and rigorously tested.
Modest weight reductions (5 to 10% of initial weight) are sufficient to reduce the incidence of DMT2 and the risk of CHD events.4 The US Preventive Services Task Force (USPSTF) recommends individually-adapted behavioral interventions to achieve and maintain such weight loss. Intensive (12 to 26 sessions per year) case management models that integrate lifestyle interventions and multiple risk factor reduction appear to be effective.5 The Diabetes Prevention Program (DPP) study demonstrated that a case management-based intensive lifestyle intervention was effective in reducing the occurrence of DMT2 and facilitating weight loss among adults at high risk for progression to DMT2.6 Evidence for the effectiveness of these interventions among Latinos, however, is limited7-9 and there is a vital need to evaluate intensive lifestyle interventions in this population.
Latinos face social and environmental barriers to weight loss that are not adequately addressed by existing behavioral interventions. Latinos are more likely to live in poverty, lack health insurance, have limited opportunities for physical activity, and experience food insecurity compared to their non-Latino white peers.10-12 Latino immigrants may face additional barriers related to acculturation, language,13 and immigration status. Community Health Workers (CHWs), however, have proven particularly effective for other health issues among Latinos.14 This strategy is may be well-suited to overcoming the social, cultural and environmental barriers to weight loss, but has not been rigorously tested for this purpose.
We designed the Vivamos Activos Fair Oaks (VAFO) clinical trial to evaluate the impact of intensive lifestyle interventions in Latino immigrants and to determine if CHWs provide additional benefits. As a community-based program, VAFO tested a health educator case management approach to weight loss with and without added CHW support. These two lifestyle interventions were compared to each other and to a usual care control group over 24 months of follow-up. Longer than most published weight loss trials, 2 year follow-up allows for separating shorter term active weight loss from longer-term maintenance of behavior change. A secondary aim was to investigate sex differences. We hypothesized that the case management with CHW augmentation would produce greater weight loss in Latino immigrants compared to the case management alone and usual care.
METHODS
Recruitment and participants
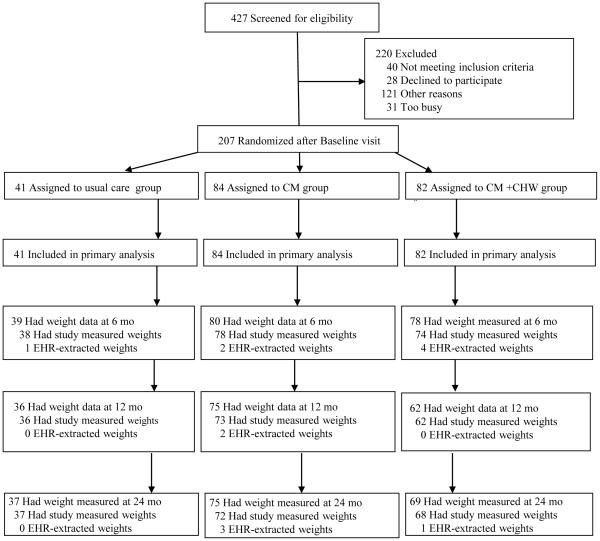
VAFO was developed as a community-based randomized controlled trial comparing two weight loss interventions to each other and to a usual care control group. The study design and methods have been published previously.15 Eligible participants were recruited from the Fair Oaks Clinic between September 2009 and October 2011. The Fair Oaks Clinic, a satellite community health center of the San Mateo County health system (SMMC), is the primary health care provider for North Fair Oaks, a 14,700-person, low-income, and largely Latino (73%) unincorporated neighborhood.16 Spanish speaking male and female patients using the clinic and residing in the neighborhood were eligible to participate if they had a BMI of 30-60 and one or more CHD risk factors (systolic blood pressure 130-200 mmHg; diastolic blood pressure 80-105 mmHg; total cholesterol greater than 180 mg/dL; LDL cholesterol greater than 120 mg/dL; HDL cholesterol less than 40 mg/dL for men and less than 50 for women; triglycerides greater than 150 mg/dL; HbA1c 6.0-11.5%; fasting plasma glucose 95-400 mg/dL; or diagnosis of DMT2). Patients unwilling to attempt weight loss, those with serious, unstable medical conditions or other circumstances that would inhibit engagement in the intervention or retention over the 2 years of follow-up (i.e., uncontrolled psychiatric disorders, advanced heart failure, uncontrolled substance abuse, pregnant, planned move, refusal of home visits) were excluded from the study. Of 427 individuals who were screened, 387 (91%) were eligible, and 207 (53%) consented to participate (Figure 1). Individuals were identified for screening by provider referral, medical record review, and through outreach in the clinic and the community.
Figure 1.
Flow Diagram for Vivamos Activos Fair Oaks Study (n=207)
Institutional Review Boards of both Stanford University and the San Mateo Medical Center (SMMC) approved study procedures and materials and all participants provided written informed consent. The trial is registered with clinicaltrials.gov under NCT01242683. The trial was conducted in compliance with the Health Insurance Portability and Accountability Act and was overseen by a Data Safety and Monitoring Board comprised of researchers with relevant expertise, but without ties to the study investigators and lacking conflicts of interest. Adverse events were classified as to seriousness, relationship to the study, and whether they were expected. All serious events (largely hospitalizations) were urgently reviewed by the principal investigator, including an assessment of possible relationship to the study protocol.
Randomization and blinding
Using separate blocks based on the permutations of sex, BMI (30-34.9, 35-39.9, or greater than 40), and DMT2 status (yes or no) to allocate participants to one of three study arms: Usual Care (UC); Case Management (CM); Case Management plus CHW support (CM+CHW). To maximize the proportion of participants receiving an active intervention and the statistical power available to compare the two active interventions, we randomized 40% to CM, 40% to CM+CHW, and 20% to UC. Randomization resulted in the following allocation: CM+CHW (N=82), CM (N=84) and UC (N=41). The total sample size was based on achieving a statistical power of 80% for detecting a 4.5% difference in weight loss from baseline (e.g., −2.5% weight loss in CM vs. +2.0% weight gain in UC) based on a two-tailed p-value of 0.05 accounting for the three pertinent statistical contrasts (CM vs. CM+CHW, CM vs. UC, and CM+CHW vs. UC).15 A 2.5% weight loss translates to approximately 5 lbs of weight loss for a 200 lb person. Data collection staff was blinded to treatment assignment.
Interventions
The CM and CM+CHW interventions originated from the Diabetes Prevention Program (DPP).6 The investigators’ previous Heart to Heart trial,17 based in San Mateo County and including the Fair Oaks Clinic was key to the tailoring of a weight-loss interventions to the organizational needs of SMMC and the sociocultural realities of the population. As in DPP and HTH, the interventions employed Social Cognitive Theory and the Transtheoretical Model of Behavior Change.18,19 Key interventional approaches in the case-management intervention included motivational interviewing, building self-management and goal setting skills, providing hands-on cooking and physical activity demonstrations, fostering self-efficacy, leveraging group-based social support, identifying community resources, and coordinating with primary care providers. Additional CHW approaches integrated with CM activities included building broad skills for navigating an obesogenic environment, fostering family support, enhancing participant success in food negotiations, mapping out neighborhood walking routes and engaging participants in a modified Photovoice activity. The modified photovoice activity engaged participants to take pictures of their food and physical activity and then the CHW used the pictures as triggers for goal setting and problem solving. Significant community engagement preceded our pilot testing of our adaptations of the previous interventions for the local neighborhood. Intervention staff underwent approximately 100 hours of training prior to implementation and consistency of intervention delivery was assessed by an external evaluator.
The CM and CM+CHW interventions included an intensive phase for the first 12 months (with even greater initial intensity) followed by a 12-month maintenance phase. The CM intervention included 12 groups sessions and four individual sessions in the intensive phase with three group sessions and one individual session in the maintenance phase. The session topics have been published previously.15 Each group session lasted approximately two hours and included nutrition and physical activity components, activities to promote goal setting and social support, and tools to improve implementation of skills taught in the classes. The transtheoretical model of behavior change was presented to participants as a framework to understand the long-term nature of the behavior change process. Take-home items included items such as pedometers, exercise CDs, and free weights. The individual sessions generally lasted at least 30 minutes and focused on individualized goal setting based on the patient’s stage of behavior change, problem solving, and medical and social service referrals. Participants randomized to CM+CHW received the CM intervention plus five CHW home visits in the intensive phase and two CHW home visits in the maintenance phase. Home visits were semi-structured to allow the CHW to facilitate behavioral changes relevant to the participant and his/her household, family, and neighborhood. Interventionists for the CM and CHW components were trained in health education and behavior change strategies and were all bilingual and bicultural. All participants continued to receive standard medical care throughout the study. Usual care consisted of routine primary care follow-ups with potential for referral to lifestyle counseling within a specialized diabetes clinic located within the clinic. The control group was offered a modified case management intervention at the completion of their 24 month follow-up.
Outcome measures
Bilingual, bicultural research assistants who were blinded to participant assignment collected anthropometric, clinical, behavioral, and socio-demographic information at baseline and follow-up assessment visits. The primary outcome was change in BMI from baseline to 24 months with assessments at 6 and 12 months to differentiate active weight loss from weight maintenance. Given their near identical statistical properties and for ease of comprehension we present the results as change in weight in pounds (lbs), as well as for change in BMI. We measured weight at each assessment visit in duplicate using a Detecto scale, while height was measured in duplicate using a wall-mounted stadiometer at baseline only. Anthropometric assessments were conducted without coats and shoes.
Secondary outcomes included change in obesity-related cardiovascular risk factors at 6, 12, and 24 months. Obesity-related cardiovascular risk factors included: waist circumference, systolic BP, diastolic BP, fasting blood glucose, hemoglobin A1C, total cholesterol, HDL, LDL, triglycerides, and C-reactive protein. Waist circumference was averaged from two measurements at the iliac crest at each in-clinic visit. Blood pressure was measured via automated Welch Allyn Spot Vital Signs LXi following the study protocol at each in-clinic visit. Lipids, glucose, hemoglobin A1c, and C-reactive protein were measured in a fasting blood sample.
Other measures collected at each assessment visit from the participants included depression screening,20 obesity related problems,21 food security,22 self-rated health, dietary practices, physical activity level and neighborhood resource utilization. A 7-day pedometer record was also collected in conjunction with these visits. These measures were seen as potential mediators of the intervention; that is, the intervention might produce changes in these behaviors and attitudes that would then produce the hypothesized changes in body weight. Additional information collected only at baseline included sex, date and place of birth, time in the US, years of schooling, English and Spanish fluency and literacy, language preferences and family composition. Many of these variables were conceptualized as potential moderators of the effect of the interventions; that is, baseline characteristics that might predict differential success from the interventions. Sex was chosen a priori as a key potential moderator.
Statistical analysis
All analyses were pre-specified as per the research protocol. We compared the mean value between study arms of weight change from baseline to 24 months after randomization. We also compared the mean value of secondary outcomes at six, 12, and 24 months after randomization, between study arms. We used generalized estimating equations (GEE) for primary and secondary outcomes as intent-to-treat. GEE accounts for the correlation of repeated measures on individuals over time and produces marginal estimates of population-level changes relevant for public health recommendations.23 The effect is captured by a treatment-time interaction term. We assumed an exchangeable correlation structure and used robust variance estimation.
We tested three contrasts: 1) UC vs. CM, 2) UC vs. CM+CHW, and 3) CM vs. CM+CHW for primary and secondary outcomes. We used the Holm’s method24 to account for the three comparisons of the primary outcome at 24 months using an adjusted p-value of 0.02. The significance level for secondary outcomes was set at p<0.05. We investigated potential effect modification by sex as our first a priori subgroup by including cross products of treatment group with sex in models. All analyses were conducted using SAS (version 9.3, SAS Institute, Inc., Cary NC).
Body weight was collected from 207 participants (100%) at baseline, followed by 190 (91.8%) at 6 months, 171 (82.6%) at 12 months and 177 (85.5%) at 24 months. Participants who were lost to follow-up had significantly higher LDL and total cholesterol (p=0.01) compared to those who completed the study protocol (supplementary Table 1). Although we were able to retrieve weights from electronic medical records, no data were available within 3 months of the expected visit date. Because we did not expect the data were Missing Completely at Random (MCAR) we preformed multiple imputation for missing data under the assumption of Missing at Random (MAR). We imputed missing data using the joint modeling approach implemented in PROC MIANALYZE in SAS 9.3, with 5 imputations of the data and use Rubin's method for variance estimates.25 As a sensitivity analysis, we repeated the same analyses using a last observation carried forward approach.
RESULTS
Study participants
At baseline, participants had a mean (SD) age of 47.1 (11.1), a BMI of 35.6 (5.3) and a weight of 89.2 kg (16.2) or 196.8 lbs (35.8); 23% were men and all were foreign-born Latinos (Table 1). Participants had been in the US an average of 16.3 years (9.9), were low income with 48% reporting an annual income under $20,000, and had low educational attainment (74% less than high school). Forty-three percent of participants had a DMT2 diagnosis at baseline. A level of depressive symptoms indicating possible depression was reported by 31% of participants (CESD score >= 9) and about half (51%) were classified as being food insecure. While 41% of participants reported their current health at baseline to be good (41%), fair or poor health status was common (48%). Moderate to severe obesity-related impairments were reported by 44% of participants.
Table 1.
Demographic Characteristics of Randomized Participants in Vivamos Activos Fair Oaks Study by Randomized Arm (n=207)
| Characteristics | All (N=207) |
CMa
(N=84) |
CM+CHWb
(N=82) |
UCc
(N=41) |
p valued |
|---|---|---|---|---|---|
| Gender, n (%) | |||||
| Male | 48 (23.2) | 20 (23.8) | 19 (23.2) | 9 (22.0) | 0.97 |
| Female | 159 (76.8) | 64 (76.2) | 63 (76.8) | 32 (78.0) | |
| Age, mean (SD), (years) | 47.1 (11.1) | 47.9 (11.9) | 46.0 (10.7) | 47.6 (10.5) | 0.50 |
| Schooling, n (%) | |||||
| Eighth grade or less | 140 (67.6) | 59 (70.2) | 50 (61.0) | 31 (75.6) | 0.45 |
| Some high school | 24 (11.6) | 10 (11.9) | 10 (12.2) | 4 (9.8) | |
| High school or more | 43 (20.8) | 15 (17.9) | 22 (26.8) | 6 (14.6) | |
| Employment Status, n (%) | |||||
| Employed | 97 (46.9) | 38 (45.2) | 38 (46.3) | 21 (51.2) | 0.87 |
| Unemployed | 21 (10.1) | 7 (8.3) | 10 (12.2) | 4 (9.8) | |
| Not working | 89 (43.0) | 39 (46.4) | 34 (41.5) | 16 (39) | |
| Annual Income, n (%) | |||||
| < $10,000 | 58 (28.0) | 25 (29.8) | 22 (26.8) | 11 (26.8) | |
| $10,000 - $20,000 | 92 (44.4) | 39 (46.4) | 34 (41.5) | 19 (46.3) | 0.84 |
| > $20,000 | 56 (27.1) | 19 (22.6) | 26 (31.7) | 11 (26.8) | |
| Country of Birth, n (%) | |||||
| Mexico | 159 (76.8) | 62 (75.6) | 66 (78.6) | 31 (75.6) | 0.88 |
| Other | 48 (23.2) | 20 (24.4) | 18 (21.4) | 10 (24.4) | |
| Years in US, mean (SD) | 16.5 (9.7) | 17.2 (10.9) | 16.0 (9.5) | 15.9 (7.1) | 0.69 |
| Diabetes Mellitus Type 2, n (%) | 89 (43.0) | 37 (44.0) | 34 (41.5) | 18 (43.9) | 0.94 |
| Depressed (CESDe >9), n (%) | 65 (31.4) | 30 (35.7) | 26 (31.7) | 9 (22.0) | 0.30 |
| Obesity Related Impairment,f n (%) | |||||
| Mild | 116 (56.0) | 54 (64.3) | 39 (47.6) | 23 (56.1) | 0.17 |
| Moderate | 30 (14.5) | 10 (11.9) | 12 (14.6) | 8 (19.5) | |
| Severe | 61 (29.5) | 20 (23.8) | 31 (37.8) | 10 (24.4) | |
| Self-Perceived Health,g n (%) | |||||
| Very good | 22 (10.6) | 6 (7.1) | 11 (13.4) | 5 (12.2) | 0.03* |
| Good | 85 (41.1) | 30 (35.7) | 42 (51.2) | 13 (31.7) | |
| Fair | 79 (38.2) | 42 (50.0) | 20 (24.4) | 17 (41.5) | |
| Poor | 21 (10.1) | 6 (7.1) | 9 (11) | 6 (14.6) | |
| Food Security,h n(%) | |||||
| Food Secure | 101 (48.8) | 42 (50.0) | 42 (51.2) | 17 (41.5) | 0.33 |
| Low food Security | 80 (38.6) | 32 (38.1) | 27 (32.9) | 21 (51.2) | |
| Very low food Security | 26 (12.6) | 10 (11.9) | 13 (15.9) | 3 (7.3) | |
| Clinical Characteristics | |||||
| BMI, mean (SD) | 35.6 (5.3) | 36.0 (5.7) | 35.5 (5.1) | 34.9 (4.4) | 0.50 |
| Weight, mean (SD), (lbs) | 196.8 (35.8) | 196.8 (35.1) | 196.8 (35.1) | 195.4 (33.4) | 0.95 |
| Height, mean (SD), (cm) | 62.3 (3.1) | 62.0 (3.2) | 62.5 (3.0) | 62.6 (3.1) | 0.49 |
| Systolic BP, mean (SD), (mmHg) | 115.2 (13.0) | 114.5 (13.0) | 114.8 (12.7) | 117.2 (13.9) | 0.52 |
| Diastolic BP, mean (SD),(mmHg) | 73.6 (7.6) | 73.0 (7.6) | 74.1 (7.2) | 73.8 (8.6) | 0.66 |
| LDL cholesterol, (mg/dL) | 104.9 (34.9) | 100.6 (30.8) | 107.8 (39.2) | 107.8 (33.5) | 0.36 |
| HDL cholesterol, (mg/dL) | 45.6 (10.8) | 44.3 (12.7) | 47.2 (9.4) | 44.9 (8.9) | 0.22 |
| TRG, mean (SD), (mg/dL) | 164.3 (99.5) | 175.4 (127.5) | 147.1 (70.1) | 176.2 (79.6) | 0.13 |
| Total Cholesterol, mean (SD), (mg/dL) | 181.6 (42.0) | 178.5 (38.7) | 181.6 (46) | 188.0 (40.4) | 0.50 |
| Fasting glucose, mean (SD), (mg/dL) | 113.4 (33.3) | 116.6 (37.5) | 111.9 (31.7) | 110.0 (26.5) | 0.51 |
| %HbA1c, mean (SD) | 6.5 (1.4) | 6.5 (1.3) | 6.4 (1.6) | 6.4 (1.3) | 0.89 |
| CRP,i mean (SD), (mg/dL) | 0.7 (0.5) | 0.6 (0.3) | 0.8 (0.6) | 0.6 (0.3) | 0.14 |
Significance at p < 0.05,
** Significance at p < 0.01,
*** Significance at p < 0.001
CM = Case Management arm
CM+CHW = Case Management plus Community Health Worker arm
UC = Usual Care Control arm
Fisher's exact p value as some of the cell values are < 5
CESD = Center for Epidemiologic Studies Depression Scale – Iowa 11x4
Obesity-Related Problem Scale
Self-Rated Health Item from National Health Interview Survey
Six-Item Short Form of the U.S. Department of Agriculture Food Security Survey Module (Spanish)
C-Reactive Protein
Intervention participation and Follow-up
The median number of group CM sessions attended was 12 (IQR 6-14) out of 16 possible in the CM arm and 10.5 (IQR 4-14) in the CM+CHW arm. Group sessions 1 through 4 were well attended sessions with 76% to 84% participation. Fewer participants attended sessions 5 through 8 (64% to 70%) and sessions 9 through 12 (57% to 61%). The mean attendance rate for the 3 maintenance sessions (13-15) was 33%. Of the 4 planned individual CM sessions, 96% completed one, 92% completed two, 90% completed three, and 82% completed four sessions. Of CM+CHW participants, 71% completed all 7 possible home visits.
Among the 207 participants, weight measurements were available on 197 (95%) at 6 months, 173 (84%) at 12 months and 181 (87%) at 24 months. An intensive effort was made to locate participants for the 24 month follow-up who had previously been lost to follow-up. By study arm, 24 month weight measurements were available for 89% of the CM participants, 84% of CM+CHW, and 90% of UC. There was no difference in loss to follow-up by sex. While follow-up of 24 months was planned, mean follow-up duration was 25.7 months (SD 2.6) among completers (n=181).
Weight loss
In the initial 6 month intensive intervention period weight loss in the CM+CHW arm was significantly greater at −2.1 kg (95% CI −2.8, −1.3) compared to −0.9 kg in UC (−1.9, 0.1; p=0.05), although it did not differ from −1.6 kg in CM (−2.4, −0.7; p=0.65, Table 2). At 12-months, mean changes from baseline were −1.9 kg (−2.9, −0.9) in the CM+CHW group (p=0.21 vs. UC and p=0.76 vs. CM), −1.4 kg (−2.4, −0.3) in the CM group (p=0.49 vs. UC) and −0.7 kg (−2.2, 0.8) in the UC arm. Both intervention groups experienced further recidivism by 24 months with mean changes of −1.0 kg (95% CI −2.4, 0.4) in the CM+CHW group (p=0.76 vs. UC and p=0.98 vs. CM) and −1.0 kg (−2.4, 0.5) in the CM group (p=0.78 vs. UC). Mean weight loss at 24 months in the UC arm was −0.6 kg (−2.8, 1.5). Similar results were obtained when BMI was analyzed as the outcome (Table 2). These results held in sensitivity analyses.
Table 2.
Estimated mean changes in clinical outcomes over 24 Months in the Intention-to-treat population of Vivamos Activos Fair Oaks Study using Generalized Estimating Equations (GEE) and multiple imputation to account for missing data (n=207).
| CMa (n=84) | CM+CHWb
(n=82) |
UCc (n=41) | p values | |||
|---|---|---|---|---|---|---|
|
|
||||||
| Change in Outcome Measures |
Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | CM vs. UC |
CM+ CHW vs.UC |
CM+ CHW vs. CM |
| Weight (kg) | ||||||
| 6 months | −1.6 (−2.4, −0.7) | −2.1 (−2.8, −1.3) | −0.9 (−1.9, 0.1) | 0.28 | 0.05 | 0.65 |
| 12 months | −1.4 (−2.4, −0.3) | −1.9 (−2.9, −0.9) | −0.7 (−2.2, 0.8) | 0.49 | 0.21 | 0.76 |
| 24 months | −1.0 (−2.4, 1.0) | −1.0 (−2.4, 0.4) | −0.6 (−2.8, 1.5) | 0.78 | 0.76 | 0.98 |
| BMI | ||||||
| 6 months | −0.6 (−1.0, −0.3) | −0.8 (−1.1, −0.5) | −0.4 (−0.7, 0.0) | 0.27 | 0.07 | 0.49 |
| 12 months | −0.6 (−1.0, −0.1) | −0.7 (−1.1, −0.3) | −0.3 (−0.8, 0.3) | 0.39 | 0.20 | 0.60 |
| 24 months | −0.4 (−1.0, 0.2) | −0.4 (−0.9, 0.2) | −0.2 (−1.1, 0.7) | 0.67 | 0.72 | 0.93 |
| Percentage Weight Change (%) | ||||||
| 6 months | −0.02 (−0.02, −0.01) | −0.02 (−0.03, −0.01) | −0.01 (−0.02, 0) | 0.50 | 0.24 | 0.54 |
| 12 months | −0.01 (−0.03, 0) | −0.02 (−0.04, −0.01) | −0.01 (−0.03, 0.01) | 0.96 | 0.92 | 0.95 |
| 24 months | −0.01 (−0.02, 0.01) | −0.02 (−0.03, 0) | 0 (−0.03, 0.02) | 0.92 | 0.72 | 0.76 |
| Waist circumference | ||||||
| 6 months | −0.7 (−1.3, −0.2) | −0.6 (−1, −0.2) | 0.0 (−0.7, 0.7) | 0.11 | 0.14 | 0.89 |
| 12 months | −1.5 (−2.2, −0.8) | −0.6 (−1.4, 0.2) | −1.3 (−2.2, −0.4) | 0.76 | 0.26 | 0.36 |
| 24 months | −1.4 (−2.1, −0.7) | −0.8 (−1.5, −0.1) | −0.7 (−1.7, 0.2) | 0.24 | 0.95 | 0.52 |
| Systolic BP | ||||||
| 6 months | −0.1 (−2.9, 2.7) | −1.8 (−4.2, 0.6) | −2.2 (−6.4, 2) | 0.41 | 0.87 | 0.71 |
| 12 months | −2.2 (−5.1, 0.6) | −1.3 (−4.3, 1.6) | −3.0 (−7.8, 1.7) | 0.77 | 0.57 | 0.86 |
| 24 months | 0.6 (−2.3, 3.5) | 0.5 (−2.9, 3.8) | −0.2 (−4.3, 3.8) | 0.74 | 0.79 | 0.98 |
| Diastolic BP | ||||||
| 6 months | −0.1 (−1.5, 1.3) | −0.2 (−1.6, 1.2) | 0.3 (−1.9, 2.6) | 0.73 | 0.72 | 0.98 |
| 12 months | −0.6 (−2.3, 1.1) | 0.3 (−1.7, 2.2) | −1.3 (−4.1, 1.5) | 0.62 | 0.40 | 0.78 |
| 24 months | 1.2 (−0.5, 2.9) | 0.9 (−0.9, 2.7) | −0.2 (−2.2, 1.8) | 0.33 | 0.46 | 0.90 |
| Total Cholesterol | ||||||
| 6 months | 1.7 (−4.6, 7.9) | −0.6 (−10.2, 9.1) | 2.8 (−7.6, 13.2) | 0.86 | 0.64 | 0.85 |
| 12 months | 1.8 (−5.2, 8.8) | 8.7 (1.2, 16.2) | 1.5 (−9.3, 12.3) | 0.96 | 0.30 | 0.55 |
| 24 months | 7.0 (−1.8, 15.8) | 10.8 (0.7, 20.8) | 5.6 (−4.5, 15.6) | 0.82 | 0.48 | 0.76 |
| Fasting Blood Glucose | ||||||
| 6 months | 0.7 (−5.9, 7.2) | 0.4 (−5.4, 6.2) | 4.0 (−3.7, 11.7) | 0.52 | 0.47 | 0.98 |
| 12 months | −1.6 (−9.8, 6.7) | −2.9 (−8.8, 3.0) | 6.3 (−1.5, 14.1) | 0.18 | 0.07 | 0.88 |
| 24 months | 2.7 (−8.5, 13.9) | −1.9 (−11.9, 8.2) | 4.6 (−6.3, 15.5) | 0.81 | 0.34 | 0.72 |
| Hemoglobin A1C | ||||||
| 6 months | 0.0 (−0.1, 0.2) | −0.2 (−0.4, 0.0) | −0.1 (−0.3, 0.1) | 0.30 | 0.50 | 0.33 |
| 12 months | 0.0 (−0.2, 0.2) | −0.2 (−0.5, 0.0) | −0.1 (−0.5, 0.3) | 0.57 | 0.73 | 0.61 |
| 24 months | −0.1 (−0.3, 0.2) | −0.3 (−0.6, −0.1) | −0.3 (−0.5, 0.0) | 0.19 | 0.75 | 0.33 |
| HDL Cholesterol | ||||||
| 6 months | −1.4 (−3.3, 0.5) | −0.4 (−1.8, 1.1) | 0.0 (−1.6, 1.6) | 0.29 | 0.76 | 0.61 |
| 12 months | 0.6 (−1.9, 3.1) | 1.6 (−0.8, 4.0) | 1.7 (−1.2, 4.5) | 0.59 | 0.98 | 0.75 |
| 24 months | −0.2 (−3.7, 3.3) | 0.3 (−3.2, 3.8) | 1.4 (−1.4, 4.3) | 0.46 | 0.62 | 0.89 |
| LDL Cholesterol | ||||||
| 6 months | 16.3 (−6.4, 39) | 5.5 (−5.6, 16.6) | 12.9 (0.6, 25.3) | 0.79 | 0.39 | 0.54 |
| 12 months | 2.9 (−2.9, 8.7) | 4.0 (−2.6, 10.7) | 1.9 (−7.3, 11.1) | 0.86 | 0.72 | 0.91 |
| 24 months | 5.8 (−1.3, 12.8) | 4.8 (−3.8, 13.4) | 4.0 (−6.5, 14.4) | 0.77 | 0.91 | 0.93 |
| Trigylcerides | ||||||
| 6 months | −7.4 (−30.5, 15.7) | −3.2 (−19.4, 13.0) | −17.2 (−38.1, 3.7) | 0.53 | 0.29 | 0.87 |
| 12 months | −12.3 (−37.2, 12.6) | 1.5 (−14.0, 17.0) | −19.0 (−40.8, 2.8) | 0.69 | 0.13 | 0.58 |
| 24 months | 5.0 (−25.5, 35.5) | 15.1 (−14.4, 44.6) | −1.3 (−36.8, 34.2) | 0.79 | 0.48 | 0.80 |
| C-Reactive Protein (not measured at 6 months) | ||||||
| At 12 months | 0.1 (0.0, 0.2) | 0.0 (−0.2, 0.2) | 0.2 (−0.1, 0.5) | 0.51 | 0.35 | 0.54 |
| At 24 months | 0.1 (0.0, 0.3) | 0.0 (−0.2, 0.1) | 0.3 (0.0, 0.7) | 0.32 | 0.07 | 0.12 |
* Significance at p < 0.05,
** Significance at p < 0.01,
*** Significance at p < 0.001
CM = Case Management arm
CM+CHW = Case Management plus Community Health Worker arm
UC = Usual Care Control arm
Sex differences
There were significant differences by treatment arm according to sex (p<0.05), with men achieving greater weight loss than women in all groups at each time point (Supplemental Table 2). The mean weight loss for men at 6 months in CM+CHW was −4.4 kg (95% CI −6.0, −2.7) (p<0.01 vs. UC and p=0.37 vs. CM), in CM was −2.4 kg (−3.9, −0.8) (p=0.12 vs. UC) and in UC was −0.4 kg (−2.4, 1.5). In contrast, mean weight loss for women at 6 months in CM+CHW was −1.4 kg (95% CI −2.2, −0.5) (p=0.57 vs. UC and p=0.96 vs. CM), in CM was −1.3 kg (−2.4, −0.3) (p=0.65 vs. UC) and in UC was −1.0 kg (−2.1, 0.1). Both sexes regained weight by 24 months and the two intervention arms did not significantly differ from UC. The same results were obtained when BMI was analyzed as the outcome (Table 2).
Changes in secondary outcomes
Secondary clinical outcomes including waist circumference, blood pressure, lipid levels, and indicators of glucose tolerance did not significantly differ between active treatment arms or compared to UC control at 6, 12 or 24 months (Table 2). Among men, however, those in CM+CHW achieved a greater reduction in waist circumference at 6 months and fasting blood glucose at 12 months compared to UC and systolic and diastolic blood pressure, LDL cholesterol, and C-Reactive Protein compared to CM at 24 months (p< 0.05) (Supplemental Table 1).
Adverse events
Overall, 11 hospitalizations (5 in CM+CHW, 5 in CM, and 1 in UC) and 69 visits to the emergency room (29 in CM+CHW, 23 in CM, and 17 in UC) occurred over the 24 months of the study. None of the events were determined to be related to the study. Although adverse events in the UC arm were lower, there were fewer participants in this arm (n=41) compared to CM (n=84) and CM+CHW (n=82). One UC participant and one CM participant became pregnant during the course of the study; their data were included in the analysis in accordance with an intent-to-treat approach. There were no deaths.
DISCUSSION
Using a design with strong internal and external validity, Vivamos Activos Fair Oaks (VAFO) showed that two interventions aimed at facilitating long-term weight loss among obese low-income Latino immigrants with one or more CHD risk factors were no more effective than usual care over 2 years. Weight loss was observed in the CM+CHW participants during the intensive, initial 6 months, a finding that was particularly pronounced for males, although likely not clinically significant. Unfortunately, the interventions were unsuccessful at preventing weight regain during the last 18 months of the trial. These findings suggest the promise of the VAFO approach for early adoption of weight-loss behaviors while indicating the need for more effective weight maintenance strategies.
Low-income Latino immigrants have been underrepresented in primary care-based weight loss trials despite their high risk for obesity-related comorbidities and socioeconomic and environmental disadvantage.26 Racial/ethnic minorities demonstrate poorer weight loss outcomes compared to non-Latino whites in trials with at least 12 months of follow-up.7 Trials with 24 months of follow-up among racial/ethnic minorities have been rare.7,27 The Be Fit, Be Well trial (n=365) was designed to test the effectiveness of a primary-care based lifestyle intervention in a racial/ethnic minority (71% African American, 13% Hispanic) population of obese adults on one or more antihypertensive medications over 24 months.28 At 24 months, intervention participants lost approximately 3.4 lbs, which was greater than the weight loss in VAFO of 2.1 lbs (0.9 kg) in the CM arm and 2.3 lbs ((1.0 kg) in the CM+CHW arm. The VAFO population was of similar income level but lower educational level compared to Be Fit, Be Well. Education level possibly played a role in the intervention effectiveness; over two-thirds (68%) of the participants in VAFO had an eighth grade education or less.
The mean weight loss was greatest in the CM+CHW arm at each time point although the difference was only significant at 6 months. A recent trial of a community-based translation of the Diabetes Prevention Program incorporating community health workers (n=301) demonstrated 12-month weight loss of 7.1 kg compared to 1.4 kg among controls. This study’s participants were primarily non-Latino whites (74%) with greater than a high school education (80%),29 making it difficult to compare to our results. Still, given the low cost of incorporating a CHW approach, and the results from this trial, using CHWs to promote lifestyle changes may be beneficial, particularly in low-income populations.
We succeeded in recruiting and retaining a sample with nearly one-quarter men (23%) and they appeared to respond more favorably to both interventions in comparison to women. Ethnic minority men make up less than 2% of participants in US weight loss trials. The limited evidence suggests mixed results with men doing better in some trials and women in others.30 It is possible that culturally specific gender roles contributed to the differential effect within this low-income Latino immigrant population. Compared to women, it may be easier for Latino men to make diet and activity pattern changes that affect the rest of the family. Other potential explanations include the higher income and education among men than women and their greater level of physical activity. Given the limited existing evidence on the effectiveness of lifestyle interventions among racial/ethnic minority men, future trials focusing on men may provide particularly useful information.
VAFO participants may have faced medical and psychosocial barriers to weight loss. A significant proportion of participants had a diagnosis of diabetes (43%) at baseline, which may have made it difficult for them to lose weight. These participants’ mean hemoglobin A1c level was 7.2% (SD 1.6), suggestive of tight glucose control through medications such as sulfonylureas and insulin, which are associated with weight gain. Approximately one-third of participants were on at least one these medications at baseline. Participants also faced significant psychosocial barriers including depression, fair to poor perceived health, food insecurity, and lack of perceived neighborhood safety. Integration of additional strategies that address psychosocial and environmental barriers to weight loss may be needed. Promising strategies identified by intervention staff included the inclusion of additional family members, direct provision of mental health services, and enhanced resources for healthful eating and physical activity.
We expected UC participants to gain weight over the 24-month follow-up, but observed weight loss in this group (-1.3 lbs at 24 months). Other weight loss studies have reported weight loss among control participants.31 Because we recruited participants from a clinical setting, it is possible that the “usual care” of the control group was better than that accessed by the majority of low-income Hispanic populations. Weight loss among UC participants limited our ability to detect an effect of the interventions.
The VAFO trial had several strengths supporting the internal and external validity of the design, such as a focus on a high-risk minority population, 87% 24-month follow-up, and evidence-based, innovative and practical intervention strategies integrated into a primary care clinic. Nevertheless, several considerations affect the interpretation of our study results. The participants in our trial were significantly more socioeconomically vulnerable than participants in comparable trials.28,32,33 In addition to low income and education, VAFO participants were all foreign born. Although we avoided inquiring about immigration status, it is likely that undocumented participants faced barriers to weight loss resources and experienced additional life stressors. For example, undocumented participants could not access the Supplemental Nutrition Assistance Program (SNAP) despite their low incomes. These factors were compounded because the trial took place during the worst of our recent economic recession.
CONCLUSIONS
Case management alone and in combination with a community health worker were no more effective than usual care at maintaining weight loss over 24 months. The intensive, initial 6 month interventions appeared promising and may reflect the ability to facilitate active adoption of behavior change. Research to identify additional strategies, however, is needed to address psychosocial and environmental barriers weight maintenance among low-income Latino immigrants.
Supplementary Material
ACKNOWLEDGEMENTS
The authors are indebted to Gloria Flores-Garcia, Jeannette Quintana, Jonathan Messinger, and Drs. Wes Alles, Jeanette Aviles, Christopher Gardner, William Haskell, Abby King, Donna Matheson, Marcia Stefanick, and Marilyn Winkleby for expert guidance on project development; to Ernesto Ceja, Rosa Gill, Elidia Contreras, and Gabriela Spencer for services as intervention staff; to Oralia Espinoza, Alexis Fields, Olivia Tigre, Jessica Ng Luna, Sophia Colombari Figueroa, Angeles Ramans, Aracely Mondragon, Monica Leyva, and Ulysses Rosas for data collection and research support; to Dr. Dave Ahn for data management; to Diane Castle and June Shu for assistance with management of project funds; to the Data and Safety Monitoring Board (Dr. Douglas Bauer [Chair], Dr. Bud Gerstman, and David Han [Executive Secretary (formerly Sandra Bravo]); to El Concilio of San Mateo County and the San Mateo Medical Center for collaboration and support; and to patients and their families for contribution to the research.
FUNDING/SUPPORT DISCLOSURE
This study was funded by National Heart, Lung, and Blood Institute (NHLBI) Grant R01 HL089448, K24 HL086703, and the REDCap data management tools were supported by the National Institutes of Health grant UL1 RR025744.
Contributor Information
Lisa Goldman Rosas, Stanford University, Program on Prevention Outcomes and Practices, Stanford Prevention Research Center, 1070 Arastradero Road, Suite 100, Palo Alto, CA 94304, Phone: 650-575-9519, Fax: 650-725-6906.
Sreedevi Thiyagarajan, 1420 Harbor Way Parkway, Alameda, CA, 94502; she was the former Data Analyst/Manager, Program on Prevention Outcomes and Practices, Stanford Prevention Research Center), Stanford University, 1070 Arastradero Rd. Suite 315, Palo Alto, CA).
Benjamin Alan Goldstein, Stanford University, Quantitative Sciences Unit, Department of Medicine, 1070 Arastradero Road, Suite 300, Palo Alto, CA, 94304.
Rebecca Lucia Drieling, University of Washington School of Public Health, Department of Epidemiology, Department of Epidemiology, NCI Biobehavioral Cancer Prevention and Control Program Affiliate, Fred Hutchinson Cancer Research Center Seattle, WA; she was the former Research Director, Program on Prevention Outcomes and Practices, Stanford Prevention Research Center, Stanford University, Palo Alto, CA).
Priscilla Padilla Romero, Fair Oaks Clinic a part of San Mateo Medical Center, 2710 Middlefield Rd, Redwood City, CA, 94063.
Jun Ma, Palo Alto Medical Foundation Research Institute, 795 El Camino Real, Ames Building, Palo Alto, CA, 94301; Consulting Assistant Professor, Stanford Prevention Research Center, Stanford University, School of Medicine CA.
Veronica Yank, General Medical Disciplines, Stanford School of Medicine, Medical School Office Building, 1265 Welch Road, Room 216, Stanford, CA, 94305; At the time of the research, she was also a joint fellow at the Stanford Prevention Research Center and the Palo Alto Medical Foundation Research Institute, 795 El Camino Real, Ames Building, Palo Alto, CA, 94301.
Randall Scott Stafford, Stanford University, Stanford Prevention Research Center, Medical School Office Building, 1265 Welch Road, Stanford University, Stanford, CA, 94305.
REFERENCES
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA : the journal of the American Medical Association. 2012 Feb 1;307(5):491–497. doi: 10.1001/jama.2012.39. [PMID:22253363] [DOI] [PubMed] [Google Scholar]
- 2.Tsai AG, Williamson DF, Glick HA. Direct medical cost of overweight and obesity in the USA: a quantitative systematic review. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2011 Jan;12(1):50–61. doi: 10.1111/j.1467-789X.2009.00708.x. [PMID:20059703] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Census Bureau Hispanic Heritage Month. 2012 [Google Scholar]
- 4.Blackburn G. Effect of degree of weight loss on health benefits. Obesity research. 1995 Sep;3(Suppl 2):211s–216s. doi: 10.1002/j.1550-8528.1995.tb00466.x. [PMID:8581779] [DOI] [PubMed] [Google Scholar]
- 5.US Preventive Services Task Force The USPSTF recommends screening all adults for obesity. Clinicians should offer or refer patients with a body mass index (BMI) of 30 kg/m2 or higher to intensive, multicomponent behavioral interventions. 2012 [Google Scholar]
- 6.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002 Feb 7;346(6):393–403. doi: 10.1056/NEJMoa012512. [PMID:11832527] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumanyika S. Ethnic minorities and weight control research priorities: where are we now and where do we need to be? Preventive medicine. 2008 Dec;47(6):583–586. doi: 10.1016/j.ypmed.2008.09.012. [PMID:18955076] [DOI] [PubMed] [Google Scholar]
- 8.Kumanyika SK, Fassbender JE, Sarwer DB, et al. One-year results of the Think Health! study of weight management in primary care practices. Obesity (Silver Spring) 2012 Jun;20(6):1249–1257. doi: 10.1038/oby.2011.329. [PMID:22051940] [DOI] [PubMed] [Google Scholar]
- 9.Corsino L, Rocha-Goldberg MP, Batch BC, Ortiz-Melo DI, Bosworth HB, Svetkey LP. The Latino Health Project: pilot testing a culturally adapted behavioral weight loss intervention in obese and overweight Latino adults. Ethnicity & disease. 2012 Winter;22(1):51–57. [PMID:22774309] [PMC free article] [PubMed] [Google Scholar]
- 10.DeNavas-Walt C, Proctor BD, Smith JC. Income, poverty, and health insurance coverage in the United States: 2011. Published September. 2012 [Google Scholar]
- 11.Rutledge MS, McLaughlin CG. Hispanics and health insurance coverage: the rising disparity. Medical care. 2008 Oct;46(10):1086–1092. doi: 10.1097/MLR.0b013e31818828e3. [PMID:18815531] [DOI] [PubMed] [Google Scholar]
- 12.Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low-income NHANES participants. The Journal of nutrition. 2010 Feb;140(2):304–310. doi: 10.3945/jn.109.112573. [PMID:20032485] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Timmins CL. The impact of language barriers on the health care of Latinos in the United States: a review of the literature and guidelines for practice. Journal of Midwifery & Women’s Health. 2002;47(2):80–96. doi: 10.1016/s1526-9523(02)00218-0. [DOI] [PubMed] [Google Scholar]
- 14.Spencer MS, Rosland AM, Kieffer EC, et al. Effectiveness of a community health worker intervention among African American and Latino adults with type 2 diabetes: a randomized controlled trial. American journal of public health. 2011 Dec;101(12):2253–2260. doi: 10.2105/AJPH.2010.300106. [PMID:21680932] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drieling RL, Ma J, Stafford RS. Evaluating clinic and community-based lifestyle interventions for obesity reduction in a low-income Latino neighborhood: Vivamos Activos Fair Oaks Program. BMC Public Health. 2011;11:98. doi: 10.1186/1471-2458-11-98. [PMID:21320331] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Profile of General Population and Housing Characteristics. 2010 [Internet]. 2010 [cited November 17, 2013]. Available from: http://factfinder2.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=DEC_10_DP_DPDP1.
- 17.Ma J, Berra K, Haskell WL, et al. Case management to reduce risk of cardiovascular disease in a county health care system. Archives of internal medicine. 2009 Nov 23;169(21):1988–1995. doi: 10.1001/archinternmed.2009.381. [PMID:19933961] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bandura A. Health promotion from the perspective of social cognitive theory. Psychology and Health. 1998;13(4):623–649. [Google Scholar]
- 19.Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. American journal of health promotion. 1997;12(1):38–48. doi: 10.4278/0890-1171-12.1.38. [DOI] [PubMed] [Google Scholar]
- 20.Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 21.Karlsson J, Taft C, Sjostrom L, Torgerson JS, Sullivan M. Psychosocial functioning in the obese before and after weight reduction: construct validity and responsiveness of the Obesity-related Problems scale. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2003 May;27(5):617–630. doi: 10.1038/sj.ijo.0802272. [PMID:12704406] [DOI] [PubMed] [Google Scholar]
- 22.Agriculture USDo . U.S. HOUSEHOLD FOOD SECURITY SURVEY MODULE—SPANISH: THREE-STAGE DESIGN, WITH SCREENERS. Economic Research Service; USDA: 2012. [Google Scholar]
- 23.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 24.Proschan MA. A multiple comparison procedure for three- and four-armed controlled clinical trials. Statistics in medicine. 1999 Apr 15;18(7):787–798. doi: 10.1002/(sici)1097-0258(19990415)18:7<787::aid-sim77>3.0.co;2-m. [PMID:10327527] [DOI] [PubMed] [Google Scholar]
- 25.Little RJA, Rubin DB. In: Statistical analysis with missing data. 2nd Hoboken NJ, editor. Wiley; 2002. [Google Scholar]
- 26.Adam Gilden Tsai M., MD Treatment of obesity in primary care practice in the United States: a systematic review. Journal of general internal medicine. 2009;24(9):1073–1079. doi: 10.1007/s11606-009-1042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seo DC, Sa J. A meta-analysis of psycho-behavioral obesity interventions among US multiethnic and minority adults. Preventive medicine. 2008 Dec;47(6):573–582. doi: 10.1016/j.ypmed.2007.12.010. [PMID:18201758] [DOI] [PubMed] [Google Scholar]
- 28.Bennett GG, Warner ET, Glasgow RE, et al. Obesity treatment for socioeconomically disadvantaged patients in primary care practice. Archives of internal medicine. 2012 Apr 9;172(7):565–574. doi: 10.1001/archinternmed.2012.1. [PMID:22412073] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katula JA, Vitolins MZ, Rosenberger EL, et al. One-year results of a community-based translation of the Diabetes Prevention Program: Healthy-Living Partnerships to Prevent Diabetes (HELP PD) Project. Diabetes care. 2011 Jul;34(7):1451–1457. doi: 10.2337/dc10-2115. [PMID:21593290] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pagoto SL, Schneider KL, Oleski JL, Luciani JM, Bodenlos JS, Whited MC. Male inclusion in randomized controlled trials of lifestyle weight loss interventions. Obesity (Silver Spring) 2012 Jun;20(6):1234–1239. doi: 10.1038/oby.2011.140. [PMID:21633403] [DOI] [PubMed] [Google Scholar]
- 31.Ma J, Yank V, Xiao L, et al. Translating the Diabetes Prevention Program lifestyle intervention for weight loss into primary care: a randomized trial. JAMA internal medicine. 2013 Jan 28;173(2):113–121. doi: 10.1001/2013.jamainternmed.987. [PMID:23229846] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai AG, Wadden TA. Treatment of obesity in primary care practice in the United States: a systematic review. Journal of general internal medicine. 2009 Sep;24(9):1073–1079. doi: 10.1007/s11606-009-1042-5. [PMID:19562419] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osei-Assibey G, Kyrou I, Adi Y, Kumar S, Matyka K. Dietary and lifestyle interventions for weight management in adults from minority ethnic/non-White groups: a systematic review. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2010 Nov;11(11):769–776. doi: 10.1111/j.1467-789X.2009.00695.x. [PMID:20059708] [DOI] [PubMed] [Google Scholar]
- 34.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [PMID:18929686] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.