Abstract
The extracellular matrix (ECM) provides structural and biochemical signals that regulate cell function. A well-controlled balance between cells and surroundings (i.e., Dynamic Reciprocity) is crucial for regulating ECM architecture. During cancer progression, epithelial cells undergo genetic alterations, which together with stromal changes, including ECM remodeling, disturb the homeostatic dynamics of the epithelium. A parallel organization of stromal ECM fibrils is associated with tumorigenic responses. In an emerging paradigm, continuous and progressive regulation via mechanical forces and aberrant signaling are believed to be responsible for tumor-associated ECM remodeling. In this review, we discuss the discrete biomechanical and biochemical mechanisms that underlie these architectural changes and highlight their particular relevance to the regulation of the alignment of ECM in the mesenchymal stroma.
Keywords: Desmoplasia, Extracellular matrix, Rho-ROCK, Integrins, Biomechanical & Biochemical cues, Cell-ECM Dynamic Reciprocity
Deregulated ECM homeostasis alters tumorigenesis
The extracellular matrix (ECM) is the physical and biochemical framework that regulates the three-dimensional organization and function of cells in a given tissue. The unique architecture and distinct biochemical composition of the ECM directs matrix-cellular interactions mostly via cellular receptors for specific ECM proteins [1]. The ECM architecture also provides critical physical guidance during tumorigenesis, influencing cell migration, invasion and metastasis [2, 3].
The ECM exists in two biochemically and structurally distinct forms: as a basement membrane and as interstitial/stromal ECM. The basement membrane, a sheet-like structure located at the basal surface of most epithelial and endothelial monolayers, is composed mainly of laminins, collagen IV, entactin, heparin sulphate proteoglycans, and nidogen and serves as a dense barrier separating the epithelium or endothelium from the underlying mesenchyme [4]. The bulk of the mesenchymal/interstitial stromal ECM, produced by mesenchymal (i.e., fibroblastic) cells, is rich in fibrillar glycoproteins such as collagens I and III, as well as fibronectin [5]. Under normal, non-pathological conditions, the basement membrane enforces both apicobasal polarity of the epithelium as well as the quiescent phenotype of the adjacent parenchyma [6]. In certain physiological conditions, such as wound healing and development, and in pathological disorders, such as cancer and chronic fibrosis, where the homeostatic equilibrium is disrupted, the basement membrane is often thinned or degraded [7]. Under these conditions, epithelial cells are “activated” to acquire migratory features via epithelial to mesenchymal transition (EMT), traverse the basement membrane, and come into direct contact with the interstitial/stromal ECM [8]. These migrating activated epithelial cells can trigger the activation of stromal cells, directly or by means of paracrine signals, resulting in a “primed” or “activated” stroma. According to some studies, an activated stroma facilitates tumor progression [9, 10], although some other studies argue against this interpretation [11, 12]: much work remains to be done to fully understand the tumor-promoting effects of activated stroma.
In this review, we describe how loss of normal tissue homeostasis during tumorigenesis results in remodeling and realignment of stromal ECM components. We will touch upon some of the cell-ECM reciprocity mechanisms that influence the biochemical and biophysical remodeling of the ECM with specific focus on control of alignment of the matrix fibrils.
Alteration in organization and composition of ECM facilitates cell invasion
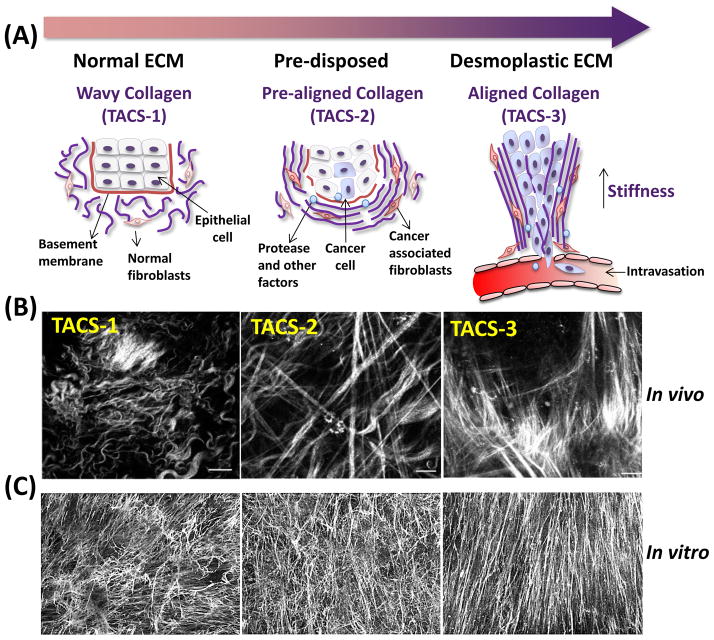
A random, isotropic arrangement of fibrillar ECM components is indicative of a “normal,” quiescent or homeostatic parenchyma, whereas an organized, anisotropic arrangement of relatively straight ECM fibers is a hallmark of a pathological microenvironment (i.e., desmoplasia) [3, 13, 14]. Such straight/anisotropic features are observed in fibrosis, and in stroma associated with epithelial tumors [15], and are indicative of poor patient prognosis [14]. Aligned stromal ECM in vivo serves as natural trails on which cancer cells migrate [13, 16]. Alterations in the alignment of structural collagen fibers associated with tumorigenesis, termed “Tumor Associated Collagen Signatures” (TACS) can be visualized in vivo using second harmonic generation of polarized light [14]. Distinct types of TACS, signifying unique stromal phenotypes, can be correlated with different stages of progressive breast cancer. Assorted TACS: the curly/anisotropic fibrils, designated as TACS-1, are found in normal, quiescent tissue (Box 1). In contrast, increasingly thickened and aligned/straight fibers (termed TACS-3) are seen in desmoplastic stroma perpendicularly oriented to the tumor boundary [14]. Hence, regions containing TACS-3 phenotypes constitute sites of focal tumor invasion [13]. Indeed, the level of TACS-3 has emerged as an independent prognostic marker in breast cancer, similar to the status of estrogen or progesterone receptors [14]. Linearization and parallel alignment of ECM fibrils has also been observed in vitro, when fibroblasts harvested from tumor tissues were used to produce cell-derived ECMs [3, 17, 18]. The tumorigenic behavior of cancer cells grown in ECMs derived from normal or tumor-associated fibroblasts is strongly regulated by the types of ECM provided, with ECM derived from normal tissue repressing the tumorigenic phenotype in cancer cells, while ECM derived from tumor-associated permissive fibroblasts activate the tumorigenic phenotype in benign cells and are permissive for tumorigenic behaviors in cancer cells [3, 18, 19].
BOX 1.
The loss of homeostatic equilibrium initiates a stromal remodeling cascade which leads to fibroblast activation (i.e., myofibroblasts/CAFs) and production of biomechanically and biochemically altered ECM [7]. The progressive stages within ECM fiber remodeling (Figure I) correlate to metastatic progression [9, 13, 14]. In TACS-1 (normal) stage, the collagen fibers are “curly and isotropic,” indicative of normal tissue. In TACS-2 (pre-disposed) stage, the collagen fibers are straightened (taut) and arranged in parallel around the tumor, indicative of pre-invasive tissue. Finally, in TACS-3 (aligned) stage, the collagen fibers are “thickened and aligned” perpendicular to the tumor boundary, indicative of metastatic stage. Similar patterning of mesenchymal ECM fibrils has been observed in vitro in numerous CAF-derived ECM models such as in skin [17], breast [3] and pancreas [18].
Box 1-Fig I.
Stromal ECM fiber remodeling stages. (A) Schematic diagram representing progressive remodeling within collagen fibers of ECM during tumorigenesis. (B) Micrographs acquired using multiphoton laser scanning microscopy illustrating TACS in Wnt-1 mouse breast cancer model. Reproduced and modified from open access available [13]. (C) Representative reconstituted confocal images obtained from in vitro fibroblast-derived ECM associated with murine squamous cell carcinoma. Reproduced and modified with permission from [17].
Biochemical factors regulate ECM remodeling during cancer progression
Myofibroblastic cancer-associated fibroblasts (CAFs), also known as TAFs or tumor-associated fibroblasts, are the principal producers of the interstitial (i.e., desmoplastic) ECM. Tumor cells and activated stromal cells can regulate matrix alignment by releasing increasing amounts of proteases and auxiliary growth factors that trigger changes to the ECM [3, 20]. The specific ECM synthesized by CAFs typically contains high levels of collagen I, oncofetal fibronectin spliced variants, such as ED-A, and a plethora of matricellular proteins, such as periostin [21]. An increase in the levels of specific ECM components occurs in various cancer subtypes and may be used as a prognostic indicator. For instance, increased secretion of hyaluronan by activated fibroblasts is commonly observed in pancreatic cancer and is known to promote tumor growth [22]. This could be caused by increased interstitial pressure due to water molecule retention. In addition, proteoglycan molecules such as chondroitin sulfate proteoglycan (CSPG) are upregulated in glioma and play a central role in ECM organization [23]. Furthermore, there is growing understanding that the expression, composition and organization of ECM molecules, as well as ECM remodeling enzymes such as matrix metalloproteases (MMP’s), is regulated by microRNA’s [24]. Note that the possible role of microRNAs on ECM regulation has been critically reviewed elsewhere [25] and is outside the scope of this review.
ECM remodeling enzymes are essential for regulating ECM organization
MMPs are members of a large family of enzymes responsible for degrading and organizing the ECM [26]. Twenty six MMP genes in various subclasses have been identified [27], including membrane-type metalloproteinases (MT-MMPs), which are specific membrane-bound members of the MMP family. MMPs act on a variety of structural ECM components including collagens, fibronectin, laminin, tenascin, and vitronectin [28], as well as growth factor receptors present on the cell surfaces [29]. MMP-1 (collagenase-I), the first MMP discovered in 1962, is one of the proteases responsible for degrading fibrillar collagens [30]. MMP-1 mediated collagen I degradation is necessary for in vitro cell migration and wound healing [31]. High expression of MMP-1 has been linked to cell invasion in vivo; therefore, targeting MMP-1 is often used as a strategy for attenuating cell invasion [32]. Other members of the MMP family, such as the gelatinases MMP-2 and MMP-9 degrade collagen IV, which is a major component of basement membranes and also found in stromal ECMs [33]. Inhibition of both MMP-2 and MMP-3 can reduce fibroblast-mediated collagen hydrogel contraction as well as collagen production in vitro [34, 35]. Among the various members of the membrane-bound MMP family, one of the best-studied proteases is MT1-MMP (also known as MMP-14). Genetically engineered mice deficient in MT1-MMP, but not other MMPs such as MMP-2, -3, -7, -9 and -12, exhibited severe abnormalities in connective tissues due to lack of their ability to degrade and remodel collagen [36]. The activity of MT1-MMP is crucial for cleaving collagen fibers, reorganizing them into parallel bundles, and forming tube-like microtracks that facilitate collective cell migration [37].
Lysyl oxidase (LOX), a copper-dependent oxidative enzyme that modifies lysyl residues on numerous protein substrates, is crucial for modulating the organization of the ECM by cross-linking collagen and elastin [38]. High LOX expression has been observed in myofibroblasts proximal to the invasive front of an infiltrating breast tumor, where the stroma is aligned [39]. Interestingly, the inhibition of extracellular LOX significantly decreased both stiffening and alignment of ECM in vivo [40]. LOX-knockout mice die around the time of birth due to diaphragmatic hernia, massive aortic aneurysms and other severe defects of the cardiovascular system, which arise as a result of insufficient collagen assembly and cross-linking [41].
Fibroblast activation protein (FAP) is another key ECM modifying enzyme that plays a role in matrix remodeling, and its stromal levels of expression are indicative of infaust outcome in patients [42]. Overexpression of FAP promotes the formation of desmoplastic-like aligned matrices in vitro [18].
Additional biochemical signaling molecules that participate in regulating ECM organization
Cytosolic Rho-ROCK is a well-described signaling pathway that regulates a variety of cell functions such as cytoskeletal reorganization and gene activation [43]. The activation of Rho-ROCK is dependent on integrin, the main cell-matrix receptor, and can promote directional migration and cell motility in both tumor and stromal cells that in turn further enhance ECM alignment or organization [2, 44, 45]. As shown in experiments using an in vitro system of tumor explants embedded in collagen gels, activation of Rho-ROCK is required for contractility-dependent collagen realignment, whereas inhibition of Rho-ROCK results in a substantial reduction of contact guidance tracks [2]. Interestingly, the generation of contact guidance tracks occurred in a MMP-independent manner as the treatment of collagen gel cultures with broad spectrum MMP inhibitor did not alter the formation of tracks [2]. Furthermore, although Rho-ROCK activity is required to initiate and maintain the generation of these tracks, once the tracks are established, Rho-ROCK activity is no longer necessary for cell migration [2, 46]. Discrete signals are thus likely needed for the production of parallel oriented ECMs and the initial stimuli may differ from those provided subsequently by the altered ECM [2, 46]. Interestingly, the biophysical characteristics of both the ECM and the cells are important in ECM modulation of tumorigenicity [47]. Both ECM porosity and elasticity, as well as the degree of nuclear deformability, are key parameters that determine whether cell invasion through mesenchymal ECMs depends on MMP, integrin, and/or Rho-ROCK activities [47].
Caveolins (Cavs) belong to a family of integral membrane proteins that are present in distinct membrane rafts, i.e. caveolae, and are involved in the regulation of key signaling molecules relevant to proliferation, survival, and motility. Lack of Cav-1, as seen in Cav-1−/−mice, leads to loss of caveoli formation [48]. Cav-1 is also an important activator of Rho-ROCK [49] and, as such, plays an important role in the initiation of ECM organization in the tumor itself as well as in the tumor-associated stroma. High levels of fibroblastic/stromal Cav-1 prompts in vivo and in vitro formation of parallel organized ECMs, which in turn promote metastatic behaviours in a Rho-ROCK dependent manner [3]. Conversely, down-regulation of Cav-1 decreases Rho activity and causes altered ECM topography resulting in reduced cell contractility and a decrease in metastasis [3].
Syndecans (Synds) are heparan sulfate proteoglycan cell surface receptors that are present in both stromal and tumor cells [50]. Synds function as integrin co-receptors, binding to a wide variety of extracellular molecules, including fibronectin, vitronectin, laminins, and fibrillar collagens [51]. Synd-1 expression in breast cancer stromal fibroblasts is associated with organized parallel ECM fibrils and increased tumorigenic cell responses in vitro. This was shown in ECM from Synd-1 expressing fibroblasts, which exhibited parallel fiber architecture (Synd-1-ECM), whereas the ECM from cells lacking Synd-1 (mock-ECM) was randomly aligned. In addition, breast carcinoma cells seeded into ECMs produced by Synd-1 expressing, but not control fibroblasts, exhibited enhanced attachment, invasion, and directional movement [52].
YAP (Yes-associated protein) and TAZ (transcriptional co-activator with PDZ-binding motif) have recently emerged as a new class of mediators of mechanotransduction. YAP/TAZ are downstream signaling proteins in the Hippo signaling pathway and play an important role in stem cell renewal, organ growth, and tumorigenesis [53]. Mechanical stimuli, such as increased substrate stiffness or shear stress induced by interstitial fluid flow (IFF), can trigger nuclear localization of YAP/TAZ thus initiating their interactions with other transcription factors to control proliferation, cytoskeletal tension, and motility [54, 55]. For example, shear stress as low as 0.01 PA was sufficient to prompt the localization of TAZ to the nucleus of fibroblasts and facilitate their osteogenic differentiation [55]. Similarly, cell stretching also triggers YAP/TAZ nuclear translocation [56]. Based on these observations, nuclear localization of YAP/TAZ in stromal fibroblasts might serve as functional biomarker characteristics of desmoplastic ECM undergoing continuous remodeling.
Mechanical factors regulate remodeling of ECM during cancer progression
Onset and progression of tumorigenesis are characterized by increases in matrix stiffness, matrix strain, and elevated interstitial fluid flow and/or pressure [57]. Mechanical forces can align the ECM in two ways: by directly aligning the matrix due to increase in physical force, or indirectly by first aligning the cells which will then remodel and vectorially re-deposit the ECM. This latter mechanism reflects the fact that stromal cells exposed to mechanical forces produced by adjacent tumor respond by adjusting their cytoskeletal contractility, thus creating a tensional imbalance, which in turn results in a field effect that further alters the stromal ECM architecture [7]. For example, interstitial fluid movement at flow rates of 5–13 μm/s (equivalent to a shear stress of 0.1–0.3 dynes/cm2) through a collagen matrix seeded with lung and dermal fibroblasts causes the direct physical alignment of collagen fibrils [58]. In turn, the pre-aligned collagen fibers provide contact guidance cues for the alignment of the cells along the newly oriented collagen fibers, further enhancing the extent of remodeling and alignment in this ECM.
Increase in contractility of fibroblasts in response to mechanical stimuli and their transition from quiescent fibroblasts to myofibroblasts are two important parameters driving ECM alignment [59]. Growth factors such as transforming growth factor beta (TGF-β), which also needed to evade immune surveillance [60], and platelet-derived growth factor (PDGF) are necessary in myofibroblastic activation [61]. Contractile myofibroblasts are often characterized by the expression of alpha smooth muscle actin (α-SMA) and its localization to stress fibers [62]. For example, application of a static tensile force of 0.65 pN/μm2 via collagen-coated magnetic beads increased the expression of α-SMA in fibroblasts up to 2-fold within just 4 hrs [63]. Mechanical stress can activate latent MMPs [64], increase the production of collagen [65], and modify cellular responses via integrin/focal adhesion kinase (FAK) engagement [40]. Moreover, the ECM acts as a reservoir for growth factors such as TGF-β, fibroblast growth factor (FGF), platelet derived growth factor-BB (PDGF-BB), vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), and insulin like growth factor-1 (IGF-1) [66]. Hence, augmented mechanical stimuli, such as mechanical load, can result in the local release or activation of the above-mentioned factors stored in the ECM [67]. At the same time, application of physical forces can expose cryptic sites present on particular ECM molecules, which can facilitate binding to other proteins. For example, cell-traction forces encountered within a three-dimensional human fibroblast matrix can induce unfolding of fibronectin from its compact-to-extended conformation [68]. The unfolding of fibronectin reveals binding sites for tenascin which are inaccessible when fibronectin is in the folded conformation [69]. The biomechanical properties of the ECM also play a crucial role in regulating stem cell responses. For example, Engler and co-workers reported that naïve mesenchymal stem cells can acquire specific phenotypes and lineages in response to different viscoelastic properties or stiffnesses of the three-dimensional ECMs [70]. In fact, ECM signals influencing stem cell behavior may intersect or share pathways with signals that regulate cancer cells and thus may be useful in understanding signals that regulate cancer ECM remodeling [40].
Dynamic reciprocity between biochemistry and mechanics affecting ECM remodeling
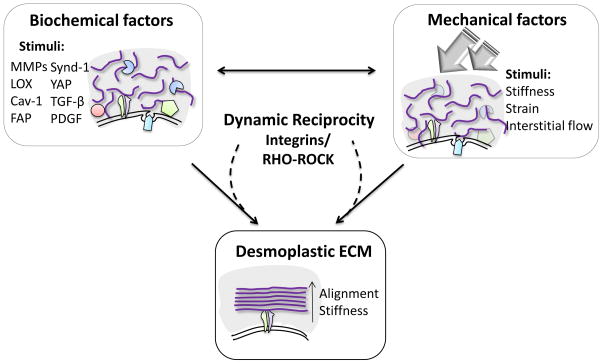
The interplay between mechanical and biochemical factors can trigger ECM remodeling [71]. For example, the secretion of ECM modifying enzymes and expression of numerous proteins (Table S1) by activated CAFs and tumor cells triggers alterations in the biomechanical properties of the ECM. Conversely, the altered biomechanical changes in the ECM can in turn affect cell responses, eventually leading to persistent, bidirectional signaling events between cells and ECM termed as “dynamic reciprocity” that further promote ECM remodeling/alignment and tumorigenesis [71, 72, 73].
Central to the dynamic reciprocity feedback signaling cascades, the integrin dependent Rho-ROCK pathway plays a crucial role in governing ECM remodeling and tumor progression. By means of integrins, cells are able to “sense” concomitant physical and biochemical cues, which via downstream molecules such as FAK and Rho-ROCK [74, 75] both react to ECM and further prompt additional ECM changes [71, 72, 73]. Interestingly, Rho-ROCK activation is known to be regulated by many of the same CAFs expressed stromal signaling proteins such as Cav-1 [76], FAP [77], and YAP [78], known to promote matrix alignment. Moreover, Rho-ROCK activation is also triggered by mechanical forces, such as the ones imparted by desmoplastic ECMs, and transmitted to cells via integrins [79]. For instance, an increase in matrix stiffness disturbs the tensional status quo between a given mammary epithelial cell and its environment. This in turn activates Rho-ROCK inside the cell promoting EMT [7]. Taken together, the activation of Rho-ROCK pathway is critical in the mechanotransduction dynamic reciprocity loop between cells and ECM that promote desmoplastic ECM alignment (Box 2).
BOX 2.
Dynamic reciprocal interactions between cell and ECM (Figure I) lead, maintain and further promote biochemical and biomechanical re-arrangements in the stroma (i.e. aligned ECM) [71, 72, 73]. During the onset of tumorigenesis, transformed stromal/cancer cells express biochemical factors such as matrix modifying enzymes (i.e., MMPs, LOX, and FAP) and growth factors (i.e., TGF-β and PDGF) that assist in the remodeling and patterning of the matrix. Reciprocally, mechanical changes such as elevated interstitial flow and increase in stiffness generated within an evolving tumor also facilitate ECM remodeling. Both these mechanical and biochemical stimuli trigger and promote the activation of integrin-dependent Rho-ROCK resulting in increased fibroblast contractility, thus feeding forward a dynamic reciprocal loop promoting the formation and expansion of the desmoplastic ECM
Box 2-Fig. I.
Schematic diagram illustrating the “Dynamic Reciprocity” leading, maintaining and further expanding desmoplastic ECM alignment.
Desmoplastic ECM “normalization” as potential therapeutic strategy intervention
The vast majority of literature suggests that tumor stroma promotes cancer progression and aids in tumor growth and invasion [9]. However, some recent studies suggest that inhibition of the stroma or desmoplasia through drugs or genetic engineering accelerates tumor growth and decreases survival, implying that tumor stroma may actually be restrictive, rather than supportive of tumor growth [11, 12]. Even in transgenic mice with the ability to delete α-SMA myofibroblasts, the subsequent depletion of CAFs and omission of fibrosis resulted in increased immunosuppression thus accelerating pancreatic cancer and reducing survival [11]. Similarly, when the effects of genetically deleting activated stroma (i.e., via sonic hedgehog deletion) in pancreatic cancer were examined, it was found that reduced stromal desmoplasia enhances tumor growth and leads to reduced survival [12]. These studies strongly suggest that instead of aiming to eliminate desmoplasia, a more effective approach would be trying to “normalize” the tumor-associated stromal ECM by reducing matrix stiffness or reversing the cell-ECM alignment in the tumor stroma. Although the concept of using normal stroma to restrain cancer progression had been suggested a long time ago, only recently has the idea of restoring normal stroma been viewed as a potential therapeutic strategy to treat cancer. For instance, activating the stromal Vitamin D receptor, using the ligand calcipotriol, can reprogram this microenvironment by reducing fibrosis, enhancing angiogenesis, and increasing the efficacy of gemcitabine treatment in pancreatic cancer [10]. We surmise that additional studies capitalizing on these ideas will lead to a more comprehensive understanding of this promising type of approach. In fact, acute or chronic stroma-targeted treatments may be responsible, respectively, for protective or deleterious treatment outcomes [80].
Concluding Remarks and Future Perspectives
In this brief review, we have discussed various biochemical and biomechanical factors that lead to the remodeling of tumor-associated stromal ECM. Under normal physiological conditions, the interactions between cells and their surrounding microenvironment are tightly controlled in order to maintain quiescent yet dynamic tissue homeostasis. If either the cellular or ECM components are perturbed, this delicate cell-ECM balance will be reorganized to restore a new homeostatic equilibrium. Stromal rearrangement is a common event in wounding or development, in which activated myofibroblasts undergo apoptosis after the tissue has fully healed [15]. In cancer, however, activated myofibroblasts are not eliminated, and the malignancy progresses to a stage at which the stroma itself is altered and eventually facilitates cancer progression. To fulfill this supportive role, the normal stromal ECM must undergo considerable physicochemical remodeling. Nonetheless, the stated tumoral and stromal changes further aggravate each other, both through cell autonomous and non-cell autonomous mechanisms, which in turn will proceed to promote a dynamic reciprocity cycle that has yet to be fully appreciated.
For example, certain biomechanical properties of the matrix, such as the alignment of certain ECM constituents (i.e., collagen I), have been correlated with cell invasion and poor prognosis [3, 13, 14, 15]. However, there are a number of other known molecules (Table S1), which participate in the observed changes in stromal ECM architecture, but their functions in this process have not been completely elucidated. Future studies using appropriate 3D tumor assemblies in vitro and animal models in vivo, together with conditions that better recapitulate proposed clinical trial conditions (i.e., acute vs. chronic treatments), should be directed towards identifying additional participants and uncovering more detailed mechanisms responsible for the unique realignment of tumor associated ECM.
Supplementary Material
Highlights.
Deregulation of ECM (i.e., desmoplastic ECM alignment) alters tumorigenesis
Both biomechanical and biochemical factors promote desmoplastic ECM formation
Cell-ECM “Dynamic Reciprocity” is regulated by integrins/Rho-ROCK
Acknowledgments
We would first like to acknowledge and apologize to our numerous colleagues whose excellent work we could not cite due to space restrictions. We also thank Dr. Erica Golemis for critical comments and Ms. Ellen Ragan for proofreading. We gratefully acknowledge support by the Temple-FCCC Nodal Multi-PI Grant (PIL/EC), NIH/NCI’s R01 CA113451 (EC) as well as the Greenberg Family (EC) and Bucks County Board of Associates (EC) Funds in support of FCCC’s Pancreatic Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Geiger B, Yamada KM. Molecular architecture and function of matrix adhesions. Cold Spring Harbor perspectives in biology. 2011;3:a005033. doi: 10.1101/cshperspect.a005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Provenzano PP, Inman DR, Eliceiri KW, Trier SM, Keely PJ. Contact guidance mediated three-dimensional cell migration is regulated by Rho/ROCK-dependent matrix reorganization. Biophysical journal. 2008;95:5374–5384. doi: 10.1529/biophysj.108.133116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goetz JG, Minguet S, Navarro-Lerida I, Lazcano JJ, Samaniego R, Calvo E, Del Pozo MA. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell. 2011;146:148–163. doi: 10.1016/j.cell.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nature Reviews Cancer. 2003;3:422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 5.Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: structure and function. Acta biomaterialia. 2009;5:1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Royer C, Lu X. Epithelial cell polarity: a major gatekeeper against cancer? Cell Death & Differentiation. 2011;18:1470–1477. doi: 10.1038/cdd.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.PaszeK MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Boettiger D. Tensional homeostasis and the malignant phenotype. Cancer cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. The Journal of clinical investigation. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beacham DA, Cukierman E. Seminars in cancer biology. Elsevier; 2005. Stromagenesis: the changing face of fibroblastic microenvironments during tumor progression; pp. 329–341. [DOI] [PubMed] [Google Scholar]
- 10.Sherman MH, Ruth TY, Engle DD, Ding N, Atkins AR, Tiriac H, Kozlov S. Vitamin D Receptor-Mediated Stromal Reprogramming Suppresses Pancreatitis and Enhances Pancreatic Cancer Therapy. Cell. 2014;159:80–93. doi: 10.1016/j.cell.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Özdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu C-C, Simpson TR, Novitskiy SV. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer cell. 2014 doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Stanger BZ. Stromal Elements Act to Restrain, Rather Than Support, Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC medicine. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, Keely PJ. Aligned collagen is a prognostic signature for survival in human breast carcinoma. The American journal of pathology. 2011;178:1221–1232. doi: 10.1016/j.ajpath.2010.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rybinski B, Franco-Barraza J, Cukierman E. The wound healing, chronic fibrosis, and cancer progression triad. Physiological genomics. 2014;46:223–244. doi: 10.1152/physiolgenomics.00158.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Condeelis J, Segall JE. Intravital imaging of cell movement in tumours. Nature Reviews Cancer. 2003;3:921–930. doi: 10.1038/nrc1231. [DOI] [PubMed] [Google Scholar]
- 17.Amatangelo MD, Bassi DE, Klein-Szanto AJ, Cukierman E. Stroma-derived three-dimensional matrices are necessary and sufficient to promote desmoplastic differentiation of normal fibroblasts. The American journal of pathology. 2005;167:475–488. doi: 10.1016/S0002-9440(10)62991-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HO, Mullins SR, Franco-Barraza J, Valianou M, Cukierman E, Cheng JD. FAP-overexpressing fibroblasts produce an extracellular matrix that enhances invasive velocity and directionality of pancreatic cancer cells. BMC cancer. 2011;11:245. doi: 10.1186/1471-2407-11-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castelló-Cros R, Khan DR, Simons J, Valianou M, Cukierman E. Staged stromal extracellular 3D matrices differentially regulate breast cancer cell responses through PI3K and beta1-integrins. Bmc Cancer. 2009;9:94. doi: 10.1186/1471-2407-9-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cukierman E, Bassi DE. Seminars in cancer biology. Elsevier; 2010. Physico-mechanical aspects of extracellular matrix influences on tumorigenic behaviors; pp. 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cirri P, Chiarugi P. Cancer associated fibroblasts: the dark side of the coin. American journal of cancer research. 2011;1:482. [PMC free article] [PubMed] [Google Scholar]
- 22.Kultti A, Zhao C, Singha NC, Zimmerman S, Osgood RJ, Symons R, Infante JR. Accumulation of extracellular hyaluronan by hyaluronan synthase 3 promotes tumor growth and modulates the pancreatic cancer microenvironment. BioMed research international. 2014 doi: 10.1155/2014/817613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silver DJ, Siebzehnrubl FA, Schildts MJ, Yachnis AT, Smith GM, Smith AA, Steindler DA. Chondroitin sulfate proteoglycans potently inhibit invasion and serve as a central organizer of the brain tumor microenvironment. The Journal of Neuroscience. 2013;33:15603–15617. doi: 10.1523/JNEUROSCI.3004-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rutnam ZJ, Wight TN, Yang BB. miRNAs regulate expression and function of extracellular matrix molecules. Matrix Biology. 2013;32:74–85. doi: 10.1016/j.matbio.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piccinini AM, Midwood KS. Illustrating the interplay between the extracellular matrix and microRNAs. International journal of experimental pathology. 2014;95:158–180. doi: 10.1111/iep.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. The Journal of pathology. 2003;200:448–464. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- 27.Birkedal-Hansen H, Moore W, Bodden M, Windsor L, Birkedal-Hansen B, DeCarlo A, Engler J. Matrix metalloproteinases: a review. Critical Reviews in Oral Biology & Medicine. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 28.McCawley LJ, Matrisian LM. Matrix metalloproteinases: they’re not just for matrix anymore! Current opinion in cell biology. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 29.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nature Reviews Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 30.Gross J, Lapiere CM. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proceedings of the National Academy of Sciences of the United States of America. 1962;48:1014. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pilcher BK, Dumin JA, Sudbeck BD, Krane SM, Welgus HG, Parks WC. The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. The Journal of cell biology. 1997;137:1445–1457. doi: 10.1083/jcb.137.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Botta GP, Reginato MJ, Reichert M, Rustgi AK, Lelkes PI. Constitutive K-RasG12D activation of ERK2 specifically regulates 3D invasion of human pancreatic cancer cells via MMP-1. Molecular Cancer Research. 2012;10:183–196. doi: 10.1158/1541-7786.MCR-11-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng ZS, Cohen AM, Guillem JG. Loss of basement membrane type IV collagen is associated with increased expression of metalloproteinases 2 and 9 (MMP-2 and MMP-9) during human colorectal tumorigenesis. Carcinogenesis. 1999;20:749–755. doi: 10.1093/carcin/20.5.749. [DOI] [PubMed] [Google Scholar]
- 34.Margulis A, Nocka KH, Wood NL, Wolf SF, Goldman SJ, Kasaian MT. MMP dependence of fibroblast contraction and collagen production induced by human mast cell activation in a three-dimensional collagen lattice. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2009;296:L236–L247. doi: 10.1152/ajplung.90462.2008. [DOI] [PubMed] [Google Scholar]
- 35.Daniels JT, Cambrey AD, Occleston NL, Garrett Q, Tarnuzzer RW, Schultz GS, Khaw PT. Matrix metalloproteinase inhibition modulates fibroblast-mediated matrix contraction and collagen production in vitro. Investigative ophthalmology & visual science. 2003;44:1104–1110. doi: 10.1167/iovs.02-0412. [DOI] [PubMed] [Google Scholar]
- 36.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Pidoux I. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 37.Friedl P, Wolf K. Tube travel: the role of proteases in individual and collective cancer cell invasion. Cancer research. 2008;68:7247–7249. doi: 10.1158/0008-5472.CAN-08-0784. [DOI] [PubMed] [Google Scholar]
- 38.Lucero H, Kagan H. Lysyl oxidase: an oxidative enzyme and effector of cell function. Cellular and Molecular Life Sciences CMLS. 2006;63:2304–2316. doi: 10.1007/s00018-006-6149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peyrol S, Raccurt M, Gerard F, Gleyzal C, Grimaud JA, Sommer P. Lysyl oxidase gene expression in the stromal reaction to in situ and invasive ductal breast carcinoma. The American journal of pathology. 1997;150:497. [PMC free article] [PubMed] [Google Scholar]
- 40.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Weninger W. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mäki JM, Räsänen J, Tikkanen H, Sormunen R, Mäkikallio K, Kivirikko KI, Soininen R. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106:2503–2509. doi: 10.1161/01.cir.0000038109.84500.1e. [DOI] [PubMed] [Google Scholar]
- 42.Cohen SJ, Alpaugh RK, Palazzo I, Meropol NJ, Rogatko A, Xu Z, Cheng JD. Fibroblast activation protein and its relationship to clinical outcome in pancreatic adenocarcinoma. Pancreas. 2008;37:154–158. doi: 10.1097/MPA.0b013e31816618ce. [DOI] [PubMed] [Google Scholar]
- 43.Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- 44.Provenzano PP, Keely PJ. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. Journal of cell science. 2011;124:1195–1205. doi: 10.1242/jcs.067009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Developmental biology. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 46.Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nature cell biology. 2007;9:1392–1400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 47.Wolf K, te Lindert M, Krause M, Alexander S, te Riet J, Willis AL, Friedl P. Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. The Journal of cell biology. 2013;201:1069–1084. doi: 10.1083/jcb.201210152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Luft FC. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 49.Joshi B, Strugnell SS, Goetz JG, Kojic LD, Cox ME, Griffith OL, Masoudi H. Phosphorylated caveolin-1 regulates Rho/ROCK-dependent focal adhesion dynamics and tumor cell migration and invasion. Cancer Research. 2008;68:8210–8220. doi: 10.1158/0008-5472.CAN-08-0343. [DOI] [PubMed] [Google Scholar]
- 50.Xian X, Gopal S, Couchman JR. Syndecans as receptors and organizers of the extracellular matrix. Cell and tissue research. 2010;339:31–46. doi: 10.1007/s00441-009-0829-3. [DOI] [PubMed] [Google Scholar]
- 51.Carey D. Syndecans: multifunctional cell-surface co-receptors. Biochem J. 1997;327:1–16. doi: 10.1042/bj3270001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang N, Mosher R, Seo S, Beebe D, Friedl A. Syndecan-1 in breast cancer stroma fibroblasts regulates extracellular matrix fiber organization and carcinoma cell motility. The American journal of pathology. 2011;178:325–335. doi: 10.1016/j.ajpath.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Low BC, Pan CQ, Shivashankar G, Bershadsky A, Sudol M, Sheetz M. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS letters. 2014 doi: 10.1016/j.febslet.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 54.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 55.Kim KM, Choi YJ, Hwang JH, Kim AR, Cho HJ, Hwang ES, Hong JH. Shear Stress Induced by an Interstitial Level of Slow Flow Increases the Osteogenic Differentiation of Mesenchymal Stem Cells through TAZ Activation. PloS one. 2014;9:e92427. doi: 10.1371/journal.pone.0092427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Piccolo S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 57.Graziano L, Preziosi L. Modeling of biological materials. Springer; 2007. Mechanics in tumor growth; pp. 263–321. [Google Scholar]
- 58.Ng CP, Swartz MA. Mechanisms of interstitial flow-induced remodeling of fibroblast–collagen cultures. Annals of biomedical engineering. 2006;34:446–454. doi: 10.1007/s10439-005-9067-3. [DOI] [PubMed] [Google Scholar]
- 59.Thomopoulos S, Fomovsky GM, Holmes JW. The development of structural and mechanical anisotropy in fibroblast populated collagen gels. Journal of biomechanical engineering. 2005;127:742–750. doi: 10.1115/1.1992525. [DOI] [PubMed] [Google Scholar]
- 60.Liu VC, Wong LY, Jang T, Shah AH, Park I, Yang X, Lee C. Tumor evasion of the immune system by converting CD4+CD25- T cells into CD4+CD25+ T regulatory cells: role of tumor-derived TGF-beta. Journal of immunology. 2007;178:2883–2892. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
- 61.Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Molecular biology of the cell. 2001;12:2730–2741. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang J, Chen H, Seth A, McCulloch CA. Mechanical force regulation of myofibroblast differentiation in cardiac fibroblasts. American Journal of Physiology-Heart and Circulatory Physiology. 2003;285:H1871–H1881. doi: 10.1152/ajpheart.00387.2003. [DOI] [PubMed] [Google Scholar]
- 64.Seliktar D, Nerem RM, Galis ZS. The role of matrix metalloproteinase-2 in the remodeling of cell-seeded vascular constructs subjected to cyclic strain. Annals of biomedical engineering. 2001;29:923–934. doi: 10.1114/1.1415522. [DOI] [PubMed] [Google Scholar]
- 65.Breen EC. Mechanical strain increases type I collagen expression in pulmonary fibroblasts in vitro. Journal of applied physiology. 2000;88:203–209. doi: 10.1152/jappl.2000.88.1.203. [DOI] [PubMed] [Google Scholar]
- 66.Kanematsu A, Marui A, Yamamoto S, Ozeki M, Hirano Y, Yamamoto M, Tabata Y. Type I collagen can function as a reservoir of basic fibroblast growth factor. Journal of controlled release. 2004;99:281–292. doi: 10.1016/j.jconrel.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 67.Hinz B. Tissue stiffness, latent TGF-β1 activation, and mechanical signal transduction: implications for the pathogenesis and treatment of fibrosis. Current rheumatology reports. 2009;11:120–126. doi: 10.1007/s11926-009-0017-1. [DOI] [PubMed] [Google Scholar]
- 68.Smith ML, Gourdon D, Little WC, Kubow KE, Eguiluz RA, Luna-Morris S, Vogel V. Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLoS biology. 2007;5:e268. doi: 10.1371/journal.pbio.0050268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ingham KC, Brew SA, Erickson HP. Localization of a cryptic binding site for tenascin on fibronectin. Journal of Biological Chemistry. 2004;279:28132–28135. doi: 10.1074/jbc.M312785200. [DOI] [PubMed] [Google Scholar]
- 70.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 71.Xu R, Boudreau A, Bissell MJ. Tissue architecture and function: dynamic reciprocity via extra-and intra-cellular matrices. Cancer and metastasis reviews. 2009;28:167–176. doi: 10.1007/s10555-008-9178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annual review of cell and developmental biology. 2006;22:287. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maller O, Martinson H, Schedin P. Extracellular matrix composition reveals complex and dynamic stromal-epithelial interactions in the mammary gland. Journal of mammary gland biology and neoplasia. 2010;15:301–318. doi: 10.1007/s10911-010-9189-6. [DOI] [PubMed] [Google Scholar]
- 74.Mitra SK, Schlaepfer DD. Integrin-regulated FAK–Src signaling in normal and cancer cells. Current opinion in cell biology. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 75.Huveneers S, Danen EH. Adhesion signaling–crosstalk between integrins, Src and Rho. Journal of cell science. 2009;122:1059–1069. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- 76.Grande-García A, Echarri A, de Rooij J, Alderson NB, Waterman-Storer CM, Valdivielso JM, del Pozo MA. Caveolin–1 regulates cell polarization and directional migration through Src kinase and Rho GTPases. The Journal of cell biology. 2007;177:683–694. doi: 10.1083/jcb.200701006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chung KM, Hsu SC, Chu YR, Lin MY, Jiaang WT, Chen RH, Chen X. Fibroblast Activation Protein (FAP) Is Essential for the Migration of Bone Marrow Mesenchymal Stem Cells through RhoA Activation. PloS one. 2014;9:e88772. doi: 10.1371/journal.pone.0088772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nature reviews Molecular cell biology. 2012;13:591–600. doi: 10.1038/nrm3416. [DOI] [PubMed] [Google Scholar]
- 79.Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Bershadsky AD. Focal contacts as mechanosensors externally applied local mechanical force induces growth of focal contacts by an mdia1-dependent and rock-independent mechanism. The Journal of cell biology. 2001;153:1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bijlsma MF, van Laarhoven HW. The conflicting roles of tumor stroma in pancreatic cancer and their contribution to the failure of clinical trials: a systematic review and critical appraisal. Cancer metastasis reviews. 2015 doi: 10.1007/s10555-014-9541-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.