Abstract
The majority of autoimmune diseases have a strong gender bias, affecting mostly females. Gender-specific factors like sex-hormones, the presence or absence of a second X chromosome, and gender-specific gut microbiota may contribute to this bias. In this review we will discuss the role of the X chromosome encoded toll-like receptor 7 (TLR7) and interferon gamma (IFNγ) in the development of autoimmunity. We will also review recent data indicating how these factors may affect an immune response in a gender-dependent manner.
Keywords: gender, autoimmunity, TLR7, T-bet, IFNgamma, ABCs
About 8% of the general population suffers from one or more autoimmune disease with a strong gender bias since more than 75% of autoimmune patients are women. It is a well-documented fact that there are differences in immune responses between males and females [1]. Overall, the female immune system is known to give stronger immune responses to infections but this characteristic may also lead to stronger responses against autoantigens [2]. Female-bias in the development of autoimmune syndromes has also been reported in various murine models of autoimmunity including NZB/W F1, NOD, and others. Differences between the immune responses of males and females have been extensively studied and, as a result, a number of explanations have been suggested. Sex hormones, the presence of a second X chromosome, microchimerism, as well as influences of various environmental factors have been proposed to play a role in female-biased autoimmunity [3]. Most likely, all these factors play a role in the ability of females versus males to respond to foreign and self-antigens. However, the means by which these factors affect the immune system and create gender-dependent differences has not been established. In this review we will focus on some recent findings which suggest one phenomenon that may contribute to the differences between male and female immune and autoimmune responses.
Differences between male and female immune responses
A number of experiments suggest that infectious diseases affect males and females differently [2, 4-6]. In general the incidence and the severity of the infections are higher in males than in females [2, 5]. Studies performed in pdriatric patients indicate that with some exceptions, the severity and prevalence of viral infections (including mumps, measles, adenovirus, coxsackievirus and respiratory syncytial virus(RSV)) is generally higher in males [4]. It has also been demonstrated that, after vaccination, females generate higher titers of measles antibodies, and antibody titers that persist for a longer time, than males [7]. Thus overall, females exhibit more robust cell-mediated and humoral immune responses than males do.
The differences between the sexes are partially due to the presence of different sex hormones. Several studies have examined how male and female sex hormones influence the immune response. It has been shown that estrogen treatment enhances the antigenic response of PBMCs and induces the expression of intracellular but not surface TLRs [8]. Estrogen treatment has been recently demonstrated to enhance the expression of UNC93B1 and IRF5 – both of these proteins play critical roles in immune responses [9, 10]. In addition several studies have demonstrated that sex hormones affect the gut microbiome which in turn may affect the immune system [11].
The presence of the second X chromosome in females may also affect immune responses in a gender-dependent manner. Although one of the two X chromosomes is inactivated in females, this inactivation is not complete and about 15% of genes encoded on the second X chromosome escape inactivation in humans [12]. The escape from inactivation leads to the overexpression of some X-linked genes in females vs males. The X chromosome encodes several immune-associated genes: CD40L, CXCR3, OGT, FOXP3, TLR7, TLR8, IL2RG, BTK, and IL9R and thus their overexpression may influence the immune response in a sex-dependent manner.
Taken together these data indicate that multiple genes can be differentially regulated in males and female which in turn may lead to the differences in immune responses to vaccinations and/or infections.
ABCs, TLR7 and IFNγ in autoimmunity
The contribution of sex-hormones and the X chromosome to the development of autoimmunity has been extensively studied [3]. The influence of sex hormones has been reviewed multiple times [3, 13-15] and will not be a subject of this review. Instead we will focus on other gender-dependent differences that influence the immune system and lead to the development of autoimmunity.
In attempts to understand the origins of the female bias in autoimmunity, we and others recently identified a subset of B cells, named Age-Associated B Cells (ABCs), that are characterized by the expression of the integrin CD11c [16, 17]. As we have previously reported, ABCs accumulate in the spleens of wild type aged female but not male mice. A subset of B cells with similar characteristics appears in the spleens of autoimmune prone mice at the onset of autoimmunity. We also demonstrated that ABCs isolated from spleens of autoimmune mice are able, after stimulation, to secrete high titers of autoantibodies, predominantly of the IgG2a (IgG2c in C57BL/6 mice but termed through this review IgG2a) isotype. In the same assay other B cells, follicular, marginal zone and B1 B cells secrete relatively low levels of such autoantibodies.
Accumulation of ABCs in both aged female and autoimmune mice led us to hypothesize that these cells may contribute to the gender-bias in the development of autoimmunity. Thus it is possible that the accumulation of ABCs in females leads to a predisposition for the onset of autoimmune disease. With this in mind, we explored the mechanisms leading to the appearance of ABCs.
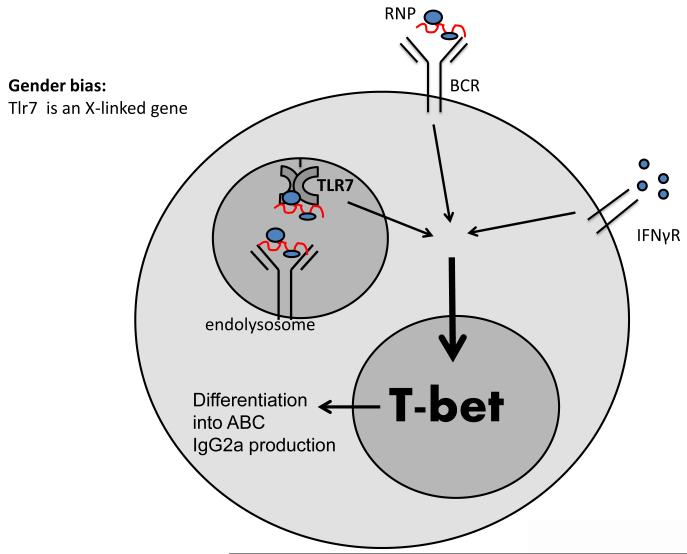
In our recent published work we established that the transcription factor, T-bet, is necessary and sufficient for the appearance of CD11c+ ABCs [18]. Moreover, we discovered the mechanism that leads to the expression of high levels of T-bet in B cells. Our data indicate that synergistic engagement of the B cell antigen receptor (BCR), TLR7, and IFNγ receptor is required for the expression of high levels of T-bet in B cells. In turn, T-bet drives expression of the ABC phenotype and a switch of antibody production to the IgG2a isotype (Figure 1) [18]. In support of this hypothesis, we also showed that intact TLR7 and IFNγR signaling is required for the accumulation of ABCs in aged female mice.
Figure 1. Model for T-bet induction in B cells.
Synergistic signaling via B cell antigen receptor (BCR), Toll-like receptor 7 (TLR7), and IFNγR in B cells leads to the induction of high levels of T-bet expression, which in turn drive the expression of an ABC phenotype and class switching to the production of IgG2a antibodies. A possible explanation for the gender bias of autoimmunity: TLR7 is an X-linked gene, which might be overexpressed in females compared to males due to incomplete X-inactivation. Overexpression of TLR7 and/or accumulation of self-antigen (RNPs) due to the ineffective clearance of apoptotic cells may lead to the accumulation of ABCs and the development of autoimmunity.
All of these three factors (BCR, TLR7 and IFNγ) have been shown to play a crucial role in the development of autoimmunity and will be further discussed in this review.
Role of TLR7 in autoimmunity
TLR7 is an intracellular toll-like receptor expressed by various cell types including B cells. TLR7 recognizes single-stranded RNA. Involvement of TLR7 in the development of autoimmunity has been demonstrated by multiple groups using various approaches. For example it has been reported that TLR7 deficiency leads to the ablation of autoimmunity in the MRLlpr mouse model of lupus [19]. In addition, treatment of MRLlpr mice with oligodeoxynucleotides with immunoregulatory sequences (IRS), that specifically block signaling via TLR7, has been reported to attenuate the disease [20]. Our group has recently demonstrated that TLR7 deficiency also abolishes the appearance of autoantibodies in B6.Nba2 and MER−/− murine models of SLE [21].
Several studies reported that the number of copies of the Tlr7 gene affects development of autoimmunity. Thus, duplication of Tlr7 in autoimmune-prone mice has been shown to accelerate the development of lupus [22] and overexpression of Tlr7 in non-autoimmune mouse strains leads to the development of spontaneous autoimmunity [23]. Moreover, Hwang et al., have recently demonstrated that overexpression of Tlr7 by B cells specifically, is sufficient to drive production of autoantibodies, indicating a direct effect of TLR7 signaling in B cells [24]. Looking more closely at the mechanism behind TLR7-induced autoimmunity, Walsh et al. reported that overexpression of Tlr7 leads to the development of spontaneous germinal centers and promotes differentiation of plasmablasts in a T cell dependent manner [25].
In addition to animal models, TLR7 is also involved in the development of autoimmunity in human patients. After it was demonstrated that TLRs are involved in the pathogeneses of murine lupus like disease, several studies examined the levels of the expression of these molecules in leukocytes in human SLE. A study performed on SLE patients by Komatsuda et al., demonstrated elevated expression levels of TLR7 in SLE patients [26]. Additionally, a significant increase in TLR7 gene copy number was detected in SLE patients compared to healthy controls [27]. However, Kelley et al. could not replicate this result and reported no significant difference in TLR7 copy number between SLE patients and healthy controls [28].
Together these data implicate TLR7 as a potential regulator of autoimmunity. Moreover, the copy number of the Tlr7 gene may be an important contributory factor. However, does TLR7 affect the immune response in a gender-dependent manner? Localization of Tlr7 gene to the X chromosome in both mice and humans implies that its expression might be regulated in gender-dependent manner. In mammals, females have two X chromosomes while males have only one X and one Y chromosome. In order to have equal levels of gene expression from X chromosomes in both genders, one X chromosome in female cells is inactivated. However, some genes on the X chromosome escape inactivation leading to potentially higher expression levels of several X-linked genes in females. In fact, the number of genes escaping inactivation is quite high, reaching ~15% of X-linked genes in humans [12]. Thus, localization of Tlr7 gene to the X chromosome may potentially lead to its overexpression in females. The importance of X-linked gene dosage for the development of SLE in humans has been proposed based on the fact that males with Klinefelter’s syndrome (males with an XXY genotype) have a greater risk for the development of lupus compared to the general male population [29]. In fact, these males are as susceptible to SLE as the general female population, indicating that despite differences in hormonal content, the presence of a second X chromosome is crucial for female-biased development of SLE.
In summary, the data acquired by multiple groups in both murine and human autoimmunity indicate that TLR7 is critical for the development of disease. Moreover, the level of TLR7 expression plays an important role and might differ in a gender-dependent manner due to the location of the Tlr7 gene on the X chromosome. However, none of these studies have provided a mechanism to explain how TLR7 signaling drives the production of pathogenic autoantibodies.
Interferon gamma and autoimmunity
The effect of IFNγ on the development of autoimmunity is well established. It has been demonstrated that IFNγ is essential for the development of autoimmunity in MRLlpr mice [30-32]. Deletion of IFNγ abolishes the appearance of autoantibodies in NZBxW F1 mice [33] and treatment of NZBxW F1 mice with blocking anti-IFNγ antibodies has been demonstrated to ameliorate the disease [34]. In addition it has been demonstrated by Seery et al., that IFNγ transgenic mice, mice that overexpress IFNγ, develop a lupus-like syndrome [35]. The authors demonstrated the production of high levels of anti-nucleosome, anti-histone, and anti-dsDNA antibodies by female transgenic mice. It is worth noticing that male transgenic mice did not develop a lupus-like phenotype [35]. Another piece of evidence came from Roquinsan/san mice in which a mutation of ROQUIN leads to reduced decay of IFNγ mRNA resulting in increased numbers of Tfh cells, germinal center B cells, and autoantibodies [36]. Notably, IFNγR deficiency prevents autoimmunity in Roquinsan/san mice, confirming a crucial role of IFNγ in this model of SLE.
With regards to human autoimmunity, increased levels of IFNγ have been reported for patients with SLE, systemic sclerosis and Sjorgen’s syndrome [37-40]. Peripheral blood monocytes (PBMCs) from patients with multiple sclerosis (MS) were demonstrated to produce more IFNγ upon in vitro stimulation with a peptide from proteolipid protein (PLP) when compared to healthy controls [41]. Moreover, the level of IFNγ production was gender-dependent. Female cells produced higher levels of IFNγ than male cells in both MS and healthy control groups. Gender-related differences in IFNγ production has also been reported in other systems. For example, female mice have been demonstrated to produce higher levels of IFNγ in response to Paracoccidioides brasiliensis infection when compared to males [42]. In this case sex hormones were shown to be responsible for the effect. Sex-determined susceptibility to Leishmania Mexicana has also been explained by higher levels of IFNγ production by females, compared to males [43].
Taken together these data indicate that IFNγ plays a critical role during the development of autoimmunity in both murine models and in human patients. Since females have been reported to produce higher levels of IFNγ under a number of circumstances, it is worth suggesting that IFNγ might be one of the factors contributing to the female bias in autoimmunity.
Role of T-bet in autoimmunity
The data we have reviewed above clearly point to a critical role for TLR7 and IFNγ in the development of autoimmunity. Moreover, gender related differences might apply to the expression of TLR7 and IFNγ production, suggesting that these factors might be involved in the female-bias of autoimmunity. However, none of the above studies have explained how IFNγ or TLR7 deficiency affects autoimmune disease. Since our data indicate that signaling via IFNγR and TLR7 are required for T-bet induction in B cells (Figure 1), we hypothesized that ablation of ABCs by TLR7 or IFNγ deficiency might lead to amelioration of autoimmunity. We have demonstrated that, at least in MER−/− mice, deletion of TLR7 prevents the accumulation of T-bet+ ABCs and this phenomenon correlates with the absence of autoantibodies [21]. However this hypothesis has to be further tested directly by depletion of T-bet+ B cells in autoimmune mice. The progression of autoimmunity in mice with B cell specific deletion of T-bet has never been studied. This is a very important experiment which we will perform in the near future. However, the role of T-bet expression during the progression of autoimmunity will be briefly reviewed here.
T-bet deficiency leads to different outcomes depending on the autoimmune model in question. T-bet deletion ameliorates disease in some models of autoimmunity including collagen antibody induced arthritis (CAIA) [44],myelin oligodendrocyte glycoprotein (MOG) induced experimental autoimmune encephalomyelitis (EAE) [45], Type 1 Diabetes (T1D) in NOD mice [46], and lupus like disease in MRLlpr animals [47]. In contrast, worsening of proteoglycal or S. aureus induced arthritis has been reported in T-bet−/− mice [48]. All these studies are complicated by the fact that the diseases in question were studied in mice in which all cell types lacked T-bet expression. Since T-bet is involved in the functions of many different cell types (T cells, NK cells, DCs, B cells), it is difficult to know which cell types are responsible for the improvement or worsening of the disease in the absence of T-bet. For this reason we believe that cell type-specific deletion of T-bet will shed more light on its role in autoimmunity.
Concluding remarks
The data reviewed above indicate that TLR7 and IFNγ might be amongst the key factors that drive gender-related differences in immune and autoimmune responses. More studies are required in order to clarify the role of T-bet expressing B cells in gender-biased autoimmunity. For instance, the role of T-bet expression in B cells during the onset of autoimmunity has to be determined. In addition, it has to be revealed whether males and females differ in their abilities to induce T-bet expression in B cells and even whether TLR7 is expressed at different levels in the lymphocytes of males and females. We think that answers to these questions might lead us to a better understanding of the mechanisms of female bias in autoimmunity and might lead ultimately to the development of better and perhaps gender specific therapies and/or diagnostics for these illnesses.
HIGHLIGHTS.
- TLR7, IFNγ and BCR synergistically induce expression of T-bet in B cells
- T-bet expressing B cells acquire distinct phenotype and become ABCs
- accumulation of ABCs in females may lead to a predisposition for the autoimmunity
- TLR7 and IFNγ may drive gender-related differences in (auto)immune response
Acknowledgments
The work was supported in part by USPHS grant AI-18785.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Oertelt-Prigione S. The influence of sex and gender on the immune response. Autoimmunity reviews. 2012;11:A479–485. doi: 10.1016/j.autrev.2011.11.022. [DOI] [PubMed] [Google Scholar]
- [2].Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nature reviews. Immunology. 2008;8:737–744. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rubtsov AV, Rubtsova K, Kappler JW, Marrack P. Genetic and hormonal factors in female-biased autoimmunity. Autoimmunity reviews. 2010;9:494–498. doi: 10.1016/j.autrev.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Muenchhoff M, Goulder PJ. Sex differences in pediatric infectious diseases. The Journal of infectious diseases. 2014;209(Suppl 3):S120–126. doi: 10.1093/infdis/jiu232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].van Eijk LT, Dorresteijn MJ, Smits P, van der Hoeven JG, Netea MG, Pickkers P. Gender differences in the innate immune response and vascular reactivity following the administration of endotoxin to human volunteers. Critical care medicine. 2007;35:1464–1469. doi: 10.1097/01.CCM.0000266534.14262.E8. [DOI] [PubMed] [Google Scholar]
- [6].Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Human reproduction update. 2005;11:411–423. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- [7].Mossong J, O’Callaghan CJ, Ratnam S. Modelling antibody response to measles vaccine and subsequent waning of immunity in a low exposure population. Vaccine. 2000;19:523–529. doi: 10.1016/s0264-410x(00)00175-4. [DOI] [PubMed] [Google Scholar]
- [8].Young NA, Wu LC, Burd CJ, Friedman AK, Kaffenberger BH, Rajaram MV, Schlesinger LS, James H, Shupnik MA, Jarjour WN. Estrogen modulation of endosome-associated toll-like receptor 8: an IFNalpha-independent mechanism of sex-bias in systemic lupus erythematosus. Clinical immunology. 2014;151:66–77. doi: 10.1016/j.clim.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Panchanathan R, Liu H, Choubey D. Expression of murine Unc93b1 is up-regulated by interferon and estrogen signaling: implications for sex bias in the development of autoimmunity. International immunology. 2013;25:521–529. doi: 10.1093/intimm/dxt015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shen H, Panchanathan R, Rajavelu P, Duan X, Gould KA, Choubey D. Gender-dependent expression of murine Irf5 gene: implications for sex bias in autoimmunity. Journal of molecular cell biology. 2010;2:284–290. doi: 10.1093/jmcb/mjq023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, Antonopoulos D, Umesaki Y, Chervonsky AV. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39:400–412. doi: 10.1016/j.immuni.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- [13].Gonzalez DA, Diaz BB, Mdel C. Rodriguez Perez, Hernandez AG, Chico BN, de Leon AC. Sex hormones and autoimmunity. Immunology letters. 2010;133:6–13. doi: 10.1016/j.imlet.2010.07.001. [DOI] [PubMed] [Google Scholar]
- [14].Hughes GC, Choubey D. Modulation of autoimmune rheumatic diseases by oestrogen and progesterone. Nature reviews. Rheumatology. 2014 doi: 10.1038/nrrheum.2014.144. [DOI] [PubMed] [Google Scholar]
- [15].Cutolo M, Sulli A, Capellino S, Villaggio B, Montagna P, Seriolo B, Straub RH. Sex hormones influence on the immune system: basic and clinical aspects in autoimmunity. Lupus. 2004;13:635–638. doi: 10.1191/0961203304lu1094oa. [DOI] [PubMed] [Google Scholar]
- [16].Hao Y, O’Neill P, Naradikian MS, Scholz JL, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118:1294–1304. doi: 10.1182/blood-2011-01-330530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, Marrack P. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c(+) B-cell population is important for the development of autoimmunity. Blood. 2011;118:1305–1315. doi: 10.1182/blood-2011-01-331462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rubtsova K, Rubtsov AV, van Dyk LF, Kappler JW, Marrack P. T-box transcription factor T-bet, a key player in a unique type of B-cell activation essential for effective viral clearance. Proceedings of the National Academy of Sciences of the United States of America. 2013 doi: 10.1073/pnas.1312348110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- [20].Pawar RD, Ramanjaneyulu A, Kulkarni OP, Lech M, Segerer S, Anders HJ. Inhibition of Toll-like receptor-7 (TLR-7) or TLR-7 plus TLR-9 attenuates glomerulonephritis and lung injury in experimental lupus. Journal of the American Society of Nephrology : JASN. 2007;18:1721–1731. doi: 10.1681/ASN.2006101162. [DOI] [PubMed] [Google Scholar]
- [21].Rubtsov AV, Rubtsova K, Kappler JW, Marrack P. TLR7 drives accumulation of ABCs and autoantibody production in autoimmune-prone mice. Immunologic research. 2012 doi: 10.1007/s12026-012-8365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- [23].Deane JA, Pisitkun P, Barrett RS, Feigenbaum L, Town T, Ward JM, Flavell RA, Bolland S. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27:801–810. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hwang SH, Lee H, Yamamoto M, Jones LA, Dayalan J, Hopkins R, Zhou XJ, Yarovinsky F, Connolly JE, de Lafaille M.A. Curotto, Wakeland EK, Fairhurst AM. B cell TLR7 expression drives anti-RNA autoantibody production and exacerbates disease in systemic lupus erythematosus-prone mice. Journal of immunology. 2012;189:5786–5796. doi: 10.4049/jimmunol.1202195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Walsh ER, Pisitkun P, Voynova E, Deane JA, Scott BL, Caspi RR, Bolland S. Dual signaling by innate and adaptive immune receptors is required for TLR7-induced B-cell-mediated autoimmunity. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:16276–16281. doi: 10.1073/pnas.1209372109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Komatsuda A, Wakui H, Iwamoto K, Ozawa M, Togashi M, Masai R, Maki N, Hatakeyama T, Sawada K. Up-regulated expression of Toll-like receptors mRNAs in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Clinical and experimental immunology. 2008;152:482–487. doi: 10.1111/j.1365-2249.2008.03646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Garcia-Ortiz H, Velazquez-Cruz R, Espinosa-Rosales F, Jimenez-Morales S, Baca V, Orozco L. Association of TLR7 copy number variation with susceptibility to childhood-onset systemic lupus erythematosus in Mexican population. Annals of the rheumatic diseases. 2010;69:1861–1865. doi: 10.1136/ard.2009.124313. [DOI] [PubMed] [Google Scholar]
- [28].Kelley J, Johnson MR, Alarcon GS, Kimberly RP, Edberg JC. Variation in the relative copy number of the TLR7 gene in patients with systemic lupus erythematosus and healthy control subjects. Arthritis and rheumatism. 2007;56:3375–3378. doi: 10.1002/art.22916. [DOI] [PubMed] [Google Scholar]
- [29].Scofield RH, Bruner GR, Namjou B, Kimberly RP, Ramsey-Goldman R, Petri M, Reveille JD, Alarcon GS, Vila LM, Reid J, Harris B, Li S, Kelly JA, Harley JB. Klinefelter’s syndrome (47,XXY) in male systemic lupus erythematosus patients: support for the notion of a gene-dose effect from the X chromosome. Arthritis and rheumatism. 2008;58:2511–2517. doi: 10.1002/art.23701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Haas C, Ryffel B, Le Hir M. IFN-gamma is essential for the development of autoimmune glomerulonephritis in MRL/Ipr mice. Journal of immunology. 1997;158:5484–5491. [PubMed] [Google Scholar]
- [31].Schwarting A, Wada T, Kinoshita K, Tesch G, Kelley VR. IFN-gamma receptor signaling is essential for the initiation, acceleration, and destruction of autoimmune kidney disease in MRL-Fas(lpr) mice. Journal of immunology. 1998;161:494–503. [PubMed] [Google Scholar]
- [32].Balomenos D, Rumold R, Theofilopoulos AN. Interferon-gamma is required for lupus-like disease and lymphoaccumulation in MRL-lpr mice. The Journal of clinical investigation. 1998;101:364–371. doi: 10.1172/JCI750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Haas C, Ryffel B, Le Hir M. IFN-gamma receptor deletion prevents autoantibody production and glomerulonephritis in lupus-prone (NZB x NZW)F1 mice. Journal of immunology. 1998;160:3713–3718. [PubMed] [Google Scholar]
- [34].Jacob CO, van der Meide PH, McDevitt HO. In vivo treatment of (NZB X NZW)F1 lupus-like nephritis with monoclonal antibody to gamma interferon. The Journal of experimental medicine. 1987;166:798–803. doi: 10.1084/jem.166.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Seery JP, Carroll JM, Cattell V, Watt FM. Antinuclear autoantibodies and lupus nephritis in transgenic mice expressing interferon gamma in the epidermis. The Journal of experimental medicine. 1997;186:1451–1459. doi: 10.1084/jem.186.9.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lee SK, Silva DG, Martin JL, Pratama A, Hu X, Chang PP, Walters G, Vinuesa CG. Interferon-gamma excess leads to pathogenic accumulation of follicular helper T cells and germinal centers. Immunity. 2012;37:880–892. doi: 10.1016/j.immuni.2012.10.010. [DOI] [PubMed] [Google Scholar]
- [37].Theofilopoulos AN, Koundouris S, Kono DH, Lawson BR. The role of IFN-gamma in systemic lupus erythematosus: a challenge to the Th1/Th2 paradigm in autoimmunity. Arthritis research. 2001;3:136–141. doi: 10.1186/ar290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Csiszar A, Nagy G, Gergely P, Pozsonyi T, Pocsik E. Increased interferon-gamma (IFN-gamma), IL-10 and decreased IL-4 mRNA expression in peripheral blood mononuclear cells (PBMC) from patients with systemic lupus erythematosus (SLE) Clinical and experimental immunology. 2000;122:464–470. doi: 10.1046/j.1365-2249.2000.01369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Radstake TR, van Bon L, Broen J, Hussiani A, Hesselstrand R, Wuttge DM, Deng Y, Simms R, Lubberts E, Lafyatis R. The pronounced Th17 profile in systemic sclerosis (SSc) together with intracellular expression of TGFbeta and IFNgamma distinguishes SSc phenotypes. PloS one. 2009;4:e5903. doi: 10.1371/journal.pone.0005903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Willeke P, Schluter B, Schotte H, Domschke W, Gaubitz M, Becker H. Interferon-gamma is increased in patients with primary Sjogren’s syndrome and Raynaud’s phenomenon. Seminars in arthritis and rheumatism. 2009;39:197–202. doi: 10.1016/j.semarthrit.2008.04.002. [DOI] [PubMed] [Google Scholar]
- [41].Pelfrey CM, Cotleur AC, Lee JC, Rudick RA. Sex differences in cytokine responses to myelin peptides in multiple sclerosis. Journal of neuroimmunology. 2002;130:211–223. doi: 10.1016/s0165-5728(02)00224-2. [DOI] [PubMed] [Google Scholar]
- [42].Pinzan CF, Ruas LP, Casabona-Fortunato AS, Carvalho FC, Roque-Barreira MC. Immunological basis for the gender differences in murine Paracoccidioides brasiliensis infection. PloS one. 2010;5:e10757. doi: 10.1371/journal.pone.0010757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Satoskar A, Alexander J. Sex-determined susceptibility and differential IFN-gamma and TNF-alpha mRNA expression in DBA/2 mice infected with Leishmania mexicana. Immunology. 1995;84:1–4. [PMC free article] [PubMed] [Google Scholar]
- [44].Wang J, Fathman JW, Lugo-Villarino G, Scimone L, von Andrian U, Dorfman DM, Glimcher LH. Transcription factor T-bet regulates inflammatory arthritis through its function in dendritic cells. The Journal of clinical investigation. 2006;116:414–421. doi: 10.1172/JCI26631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bettelli E, Sullivan B, Szabo SJ, Sobel RA, Glimcher LH, Kuchroo VK. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. The Journal of experimental medicine. 2004;200:79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Esensten JH, Lee MR, Glimcher LH, Bluestone JA. T-bet-deficient NOD mice are protected from diabetes due to defects in both T cell and innate immune system function. Journal of immunology. 2009;183:75–82. doi: 10.4049/jimmunol.0804154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Peng SL, Szabo SJ, Glimcher LH. T-bet regulates IgG class switching and pathogenic autoantibody production. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:5545–5550. doi: 10.1073/pnas.082114899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hultgren OH, Verdrengh M, Tarkowski A. T-box transcription-factor-deficient mice display increased joint pathology and failure of infection control during staphylococcal arthritis. Microbes and infection / Institut Pasteur. 2004;6:529–535. doi: 10.1016/j.micinf.2004.02.005. [DOI] [PubMed] [Google Scholar]