Abstract
The combination of healing anterior cruciate ligament (ACL) volume and the distributions of T2* relaxation times within it have been shown to predict the biomechanical failure properties in a porcine model. This MR-based prediction model has not yet been used to assess ligament degeneration in the aging human knee. Using a set of 15 human cadaveric knees of varying ages, we obtained in situ MR measures of volume and T2* of the intact ACL and then related these MR variables to biomechanical outcomes (maximum and yield loads, linear stiffness) obtained via ex vivo failure testing. Using volume in conjunction with the median T2* value, the multiple linear regression model did not predict maximum failure load for the intact human ACL; R2=0.23, p=0.200. Similar insignificant results were found for yield load and linear stiffness. Naturally restricted distributions of the intact ligament volume and T2* (demonstrated by the respective Z-scores) in an older cadaveric population were the likely reason for the insignificant results. These restricted distributions may negatively affect the ability to detect a correlation when one exists. Further research is necessary to understand the relationship of MRI variables and ligament degeneration. While this study failed to find a significant prediction of human biomechanical outcome using these MR variables, with further research, an MR-based approach may offer a tool to longitudinally assess changes in cruciate ligament degradation.
Keywords: T2*, relaxation time, Intact ACL, Structural Properties, Age, Degeneration
INTRODUCTION
Osteoarthritis (OA) is a degenerative condition that affects articular cartilage as well as other structures of the joint, including the anterior cruciate (ACL) and posterior cruciate ligaments (PCL) (Cross et al., 2014; Woo et al., 1991). Total knee arthroplasty (TKA) is the most common surgical intervention to treat advanced stages of OA, where it is common to remove the ACL and PCL (Bloomfield and Hozack, 2014; Buchbinder et al., 2014). The newest TKA implant designs allow retention of both ACL and PCL; thus, it would be beneficial to noninvasively assess the structural integrity of the cruciates by predicting their biomechanical properties in order to inform surgical decision making in the selection of prostheses for patients undergoing TKA.
Using MR images, it has been shown that the combination of ligament volume (amount of ligament tissue) and signal intensity (surrogate of tissue quality) is predictive of the biomechanical failure properties of a healing ligament in a porcine model (Biercevicz et al., 2013; Weiler et al., 2001). More recently, the combination of healing ligament volume and the distributions of T2* relaxation times within the ligament have been found to provide a more robust prediction model of the structural properties (Biercevicz et al., 2014a).
To date this MR-based prediction model has not yet been used to assess ligament degeneration in the aging knee. Using human cadaveric knees, the objectives of this study were to obtain in situ MR measures of volume and T2* of the intact ACL and to relate these variables to biomechanical failure outcomes (maximum and yield loads, linear stiffness) obtained ex vivo. We hypothesized that the MR values of volume and T2* of the intact ACL would predict the ligament structural properties. Such an MR-based approach would offer a valuable tool to longitudinally assess changes in cruciate ligament degeneration, and to assess cruciate ligament integrity when selecting TKA prosthesis type.
MATERIALS AND METHODS
Cadaveric Specimens
Fifteen frozen human cadaver knees (5 female and 10 male, Table 1) were thawed to room temperature and imaged in situ. The age distribution (range 24–76) was selected to capture a range of ligament degeneration states, as the structural properties of the ligament have been shown to decrease with age (Woo et al., 1991). After MR imaging the specimens were frozen until mechanical testing.
Table 1.
Human Cadaveric Demographics for 5 women and 10 male specimens
| Max | Min | Median | 25% CI | 75% CI | |
|---|---|---|---|---|---|
| Age (years) | 76 | 24 | 54 | 42 | 62 |
| Height (inches) | 72 | 62 | 68 | 67 | 71 |
| Weight (lbs) | 200 | 87 | 170 | 125 | 180 |
| BMI | 33 | 13 | 23 | 19 | 27 |
MR Imaging
All image data were acquired using a 3T Siemens Tim Trio scanner (Erlangen, Germany) using a 20cm volume extremity coil. High-resolution 3-D gradient echo (FLASH) data sets were obtained with parameters: TR=33ms, TE=4.3, 7.3, 10.2, 13.1, 16ms (6 echoes), flip angle=17°, FOV=180mm, slice thickness=0.8mm, reconstruction matrix size=512x512, slice thickness=0.8mm (contiguous slices), single average, and bandwidth=407Hz/pixel (Biercevicz et al., 2014a). The intact ACLs were manually segmented from the image stack by one examiner with 5 years of experience and 3-D models of the ligaments were created (Mimics 15.0; Materialize, Ann Arbor, MI) (Biercevicz et al., 2014a, 2013). MatLab (MathWorks, Natick, MA) was used to determine T2* maps of the ACL for each knee.
Post processing: Intact Ligament T2* determination
To create the T2* map for each knee, a voxel-wise nonlinear least-squares fit of voxel signal intensity (SI) versus echo time was used. SI from all six echo times along with the SI relationship, SI (TE) = M0 e−TE/T2* + DC, where SI(TE) are the voxel specific SIs for the various echo times (TE), was used to estimate T2*. The three fit parameters M0 (equilibrium magnetization), T2* and the DC offset (DC), were used for the least squares fit estimation of T2* (Biercevicz et al., 2014b; Haacke et al., 1999). To isolate ligament specific T2* values, the voxels corresponding to the ligament were extracted from the T2* maps using the 3-D models created from the segmented images (Biercevicz et al., 2014a, 2013). Summing the total number of ACL voxels provided an estimate of the whole ligament volume (VOL) (10.2 voxels equaled one mm3).
Structural Properties
An established tensile testing protocol was used to determine the structural properties of the intact ACLs (Murray et al., 2010). The specimens were thawed to room temperature. The proximal end of the femur and the distal end of the tibia were potted in PVC pipe using a urethane resin (Smooth-On, Easton, Pennsylvania). The joint was carefully dissected, leaving only the femur-ACL-tibia complex intact. Using a servohydraulic material testing system (MTS 810; Prairie Eden, MN), tensile loads were applied at 20 mm/min to failure (Murray et al., 2010). The entire tensile load-displacement curve was recorded until a precipitous drop in load occurred. The maximum load, yield load, and linear stiffness values of the ligaments were calculated from the load-displacement data.
Statistics
Linear regression models were used to separately test the relationships between ligament: a) MR volume and structural properties and, b) T2* and structural properties. Subsequently, both ligament volume and T2* were included in a multiple linear regression model to predict the structural properties (SigmaPlot 12.0; Systat Software Inc., San Jose, CA) (Figure 1). The R-square, standard error and p-values were reported as indicators of the relationship strength and goodness of fit. Descriptive statistics such as maximum, minimum, median, 25, 75% confidence intervals and kurtosis (a variable indicating the level of data clustering) (Belle et al., 2004) were used to assess the shape and distribution of the independent variables (volume, median T2*) (Figure 2, Table 3). To visualize the shape and distribution of the independent variables, volume and median ligament T2* were transformed to Z-scores and plotted as histograms (Figure 2). Z- scores indicated the number of standard deviations from the mean of a data set a data point is (Belle et al., 2004).
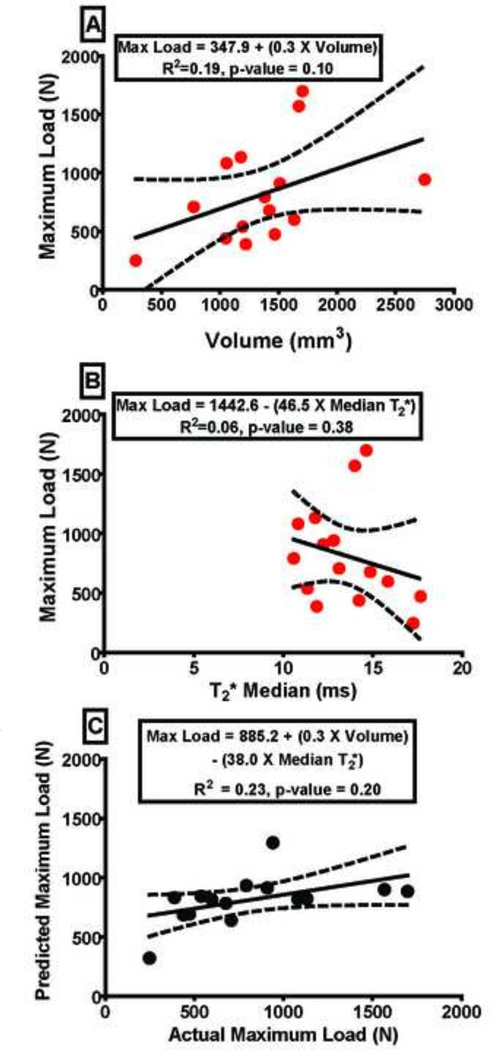
Figure 1.
A) The ligament maximum load as a function of ligament volume and B) ligament maximum load as a function of ligament median T2* in linear regression models. C) Actual ligament maximum load versus predicted ligament maximum load determined using a multiple regression model as a function of the linear combination of ligament volume and median T2*. Dashed lines represent 95% confidence interval. Maximum load prediction equations for the intact ACLs as a function of volume (VOL), median T2*, and the linear combination of VOL and median T2* are inlaid in the graphs.
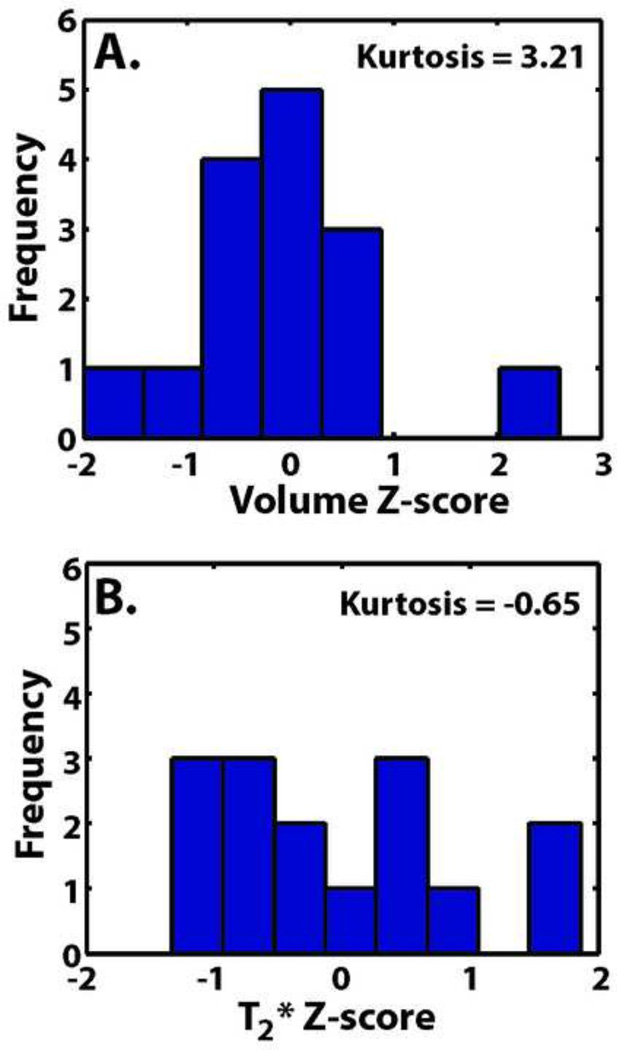
Figure 2.
Histograms of human cadaveric intact ligament Z-scores of (A) volume and (B) median T2*.
Table 3.
Summary statistics (Volume, Median T2*, Maximum Load, Yield Load, Stiffness) for the human cadaveric intact ACL.
| Variable | Max | Min | Median | 25% CI | 75% CI | Kurtosis |
|---|---|---|---|---|---|---|
| Volume (mm3) | 2750.2 | 282.3 | 1382.5 | 1087.6 | 1605.3 | 3.2 |
| Median T2* | 17.7 | 10.6 | 13.1 | 11.8 | 14.8 | −0.7 |
| Max L (N) | 1696.6 | 248 | 706.8 | 488.6 | 1046.0 | 0.3 |
| Yield L (N) | 1652.4 | 225.9 | 639.2 | 412.6 | 924.3 | 0.7 |
| Stiffness (N/mm) | 165.9 | 52.4 | 102.4 | 79.8 | 139.5 | −0.9 |
RESULTS
Regression Prediction Models
The volume of intact human cadaveric ACLs did not significantly predict maximum failure load R2=0.19, p=0.100 (Figure 1A). The median T2* value also did not predict maximum failure load, R2=0.06, p=0.380 (Figure 1B). Using volume with the median T2* value, the multiple linear regression model did not predict maximum failure load for the intact human ACL; R2=0.23, p=0.200 (Figure 1C). Similar insignificant results were found for yield load and linear stiffness (Table 2). As a more specific measure of morphology, minimum cross sectional area for each ligament was also determined but a similar insignificant ability to predict structural properties was seen (maximum load; R2=0.12, p=0.235). Sub-region analysis of T2* values was also performed. Each ligament was split into femoral insertion, mid-substance, and tibial insertion regions and sub-volumes and T2* values were determined. These sub-regions showed a similar insignificant ability to predict structural properties.
Table 2.
Summary of the yield load and linear stiffness equations for human cadaveric intact and ACLs as a function of volume (VOL), median T2*, and the linear combination of VOL and median T2*.
| Dependant Variable |
Independent Variable | R2 | Regression p-value |
Prediction Equation |
|---|---|---|---|---|
| Yield Load (N) | VOL | 0.18 | 0.111 | Yield (N) = 296.7 + (0.3 × VOL) |
| Yield Load (N) | Median T2* | 0.05 | 0.437 | Yield (N) = 1262.9 − (39.2 × T2* Median) |
| Yield Load (N) | VOL & Median T2* | 0.21 | 0.238 | Yield (N) = 738.4 + (0.3 × VOL) − (31.2 × T2* Median) |
| Stiffness (N/mm) | VOL | 0.00 | 0.972 | Stiff (mm/N) = 106.3 + (0.00 × VOL) |
| Stiffness (N/mm) | Median T2* | 0.14 | 0.176 | Stiff (mm/N) = 188.0 − (6.0 × T2* Median) |
| Stiffness (N/mm) | VOL & Median T2* | 0.14 | 0.413 | Stiff (mm/N) = 191.4 − (0.00 × VOL) − (6.0 × T2* Median) |
Summary Statistics
The median (maximum–minimum) values for ligament volume, median T2* and maximum load were 1382.5 mm3 (2750.2-283.3 mm3), 13.1 ms (17.7-10.6 ms), and 706.8 N (1696.6-248.0 N), respectively. The 25 to 75% confidence intervals for ligament volume, median T2*, and maximum load, were 1087.6-1605.3 mm3, 11.8-14.8 ms, and 488.6-1046.0 N, respectively. The kurtosis values for ligament volume, median T2*, and maximum load were 3.2, −0.7, and 0.3, respectively (Figure 2, Table 3). Summary statistics for yield load and linear stiffness can be seen in Table 3.
DISCUSSION
The linear combination of ligament volume and median T2* value did not significantly predict the structural properties of the ACL in this human cadaveric model. This could be due to the naturally restricted variability of the data in the cadavers tested. The majority of the specimens fell in the volume range of 1087.6-1605.3 mm3 (25–75% confidence interval, Table 3) limiting the distribution of data to test the prediction. This was shown by the kurtosis value (3.2, Table 3), which indicated a high level of data clustering (Belle et al., 2004). Z-scores of the volume measure also showed a restricted variability of the data set. Twelve of the 15 ligament specimens were less than 1 standard deviation away from the mean for the volume data set (Figure 2). Distributions of the T2* Z-scores also showed restricted variability, with 10 of the 15 ligaments being less than 1 standard deviation away from the mean for the distribution. These kurtosis and Z-score statistics indicate that the volume and T2* distributions were naturally restricted, which can negatively affect the ability to detect a correlation when one exists and decrease the statistical power (Crocker and Algina, 1986; Huck, 1992).
A previous study looking at porcine transected and reconstructed ligaments found that the linear combination of volume and T2* significantly predicted the healing ligament structural properties (Biercevicz et al., 2014a, 2013; Weiler et al., 2001). In this previous study, there were two different treatment groups of actively healing ligaments that resulted in a large range of evenly distributed volume and T2* data with little to no observed clustering (Biercevicz et al., 2014a). This stands in contrast to the current human cadaveric study, where a small intact ligament volume was rare and the intact ligaments were not actively healing, leaving the volume and T2* distributions naturally restricted. Naturally restricted distributions of MR variables observed in the intact ACL of a porcine model were also found to have a similar insignificant ability to predict structural properties when compared to a proven prediction in a healing ACL porcine model (Biercevicz et al., 2013)(See Supplement). While the MR variables did not significantly predict structural properties, there was evidence that high volume and short T2* times were associated with higher structural properties. When the specimens were split into a low failure and high failure group (<700 N, n=7 and >700 N, n=8, respectively) and tested for differences in volume and median T2* using a one way ANOVA, we found the specimens in the high failure group had significantly lower T2* values in comparison to the low failure group (12.5 vs 14.7 ms, p-value=0.48). Although the high failure group tended to have larger volumes (1505 vs 1182 mm3, p-value= 0.261), the difference was not significant.
Age negatively correlates to the structural properties of the intact cadaveric ACL (Woo et al., 1991); however, this correlation becomes insignificant in specimens over 48 years.(Noyes and Grood, 1976) Our cadaveric population was clustered around 50 years (25–75% confidence interval 42–62 years, Table 1), indicating our specimens fell in a similar age bracket where volume and T2* would not have the ability to predict structural properties. This further suggests the age of our test sample (clustered around 50) may not have been ideal to capture enough variability in the volume and T2* distributions to detect a correlation to structural properties.
Using the MR variables of volume and T2* (or SI) to detect ligament degeneration in terms of ligament strength are based on previous research showing that these same MR variables could predict ligament structural properties in animal models of ACL or graft healing. We assumed that the same trends with MR variables observed during the ligament healing process (Biercevicz et al., 2014a, 2013; Weiler et al., 2001) would be observed in reverse order with a degenerating ligament. While T2* has been used to detect degeneration in the meniscus (Williams et al., 2012), there may be other factors specifically associated with the ligament degenerative process. Unlike the healing or regenerative process, these factors may involve characteristics besides graft size and quality. For example, osteophytes could induce damage to the ligament surface, which could influence the failure properties. Additionally, while the same trends were observed with volume and T2* in the current human cadaveric prediction compared to healing porcine ligaments (larger volume and shorter T2* times were associated with higher structural properties, (Biercevicz et al., 2014a)) there could be an inherent difference with the macro anatomy of the intact ligaments that lead to the insignificant findings reported here. For instance the collagen fibers of the intact ACL twist in the intra-articular space and may cause volume-averaging artifacts that could confound the signal intensity.(Hodler et al., 1992) Further research will be needed to clarify if these additional factors may confound the results.
There were limitations to this study. To limit concerns over varying temperature effects across the samples (McRobbie et al., 2007), all knees were thawed to room temperature to standardize temperature for MR imaging. Additionally, while a carefully controlled freeze thaw cycle does not affect structural properties of ligaments (Woo et al., 1986), and our protocol controlled the number of these cycles, there is no certainty in specimen handling (number of freeze thaw cycles or delay between death and freezing) prior to receiving them. Two of the younger specimens had higher than expected T2* values and it is possible that unique circumstances of death or decay processes could have confounded these measurements. Finally, the availability and distribution of this data set may have limited our capacity to predict structural properties. With the current sample size (n=15) and considering the two independent variables in the regression model, the study was 0.80 powered to detect a multiple R2 of 0.45 or higher.(Draper and Smith, 1998) Adding additional cadaveric specimens at younger and older ages may help to better distribute the ligament volume, median T2* and biomechanical performance; and could potential help detect a relationship. Possibly 30 or more total specimens, divided evenly between a lower, middle and later age ranges, may have helped with prediction strength. (Noyes and Grood, 1976; Woo et al., 1991) Unfortunately, younger cadaveric knees are not easily obtained in high quantities for analysis.
Using a set of human cadaveric knees, we were unable to relate biomechanical outcome to MR measures of volume and T2* obtained in situ. Naturally restricted distributions of ligament volume and T2* were likely the cause of the insignificant findings. Further research is necessary to understand the relationship of MR variables and ligament degeneration in a human cadaveric model. This study failed to find a significant prediction of human cadaveric biomechanical outcome using volume and T2*. Further research geared toward capturing younger cadaveric specimens may be valuable for creating an MR-based approach to longitudinally assess changes in cruciate ligament degradation and to assess cruciate integrity when determining indications for prosthesis type with TKA procedures.
Supplementary Material
AKNOWLEDGMENTS
Funded by a seed grant awarded through the Department of Diagnostic Imaging, Division of Imaging Research, Rhode Island Medical Imaging, grants from the National Institutes of Health (RO1-AR065462 and P20-GM104937) and the Lucy Lippitt Endowment. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The authors wish to thank Gary Badger (University of Vermont), Jason Machan, Emily Robbins, Arlene Garcia (Rhode Island Hospital/Brown University) and Lynn Fanella (Brown 11 University) for their assistance. All imaging was done at Brown University Magnetic Resonance Facility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors have no financial or personal relationships that could bias this work.
REFERENCES
- Belle G van, Fisher LD, Heagerty PJ, Lumley T. Biostatistics: A Methodology For the Health Sciences. John Wiley & Sons; 2004. [Google Scholar]
- Biercevicz AM, M Murray Martha, Walsh EG, Miranda DL, Machan JT, Fleming BC. T2 * MR relaxometry and ligament volume are associated with the structural properties of the healing. ACL. J. Orthop. Res. 2014a;32:492–499. doi: 10.1002/jor.22563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biercevicz AM, Miranda DL, Machan JT, Murray MM, Fleming BC. In Situ, noninvasive, T2*-weighted MRI-derived parameters predict ex vivo structural properties of an anterior cruciate ligament reconstruction or bioenhanced primary repair in a porcine model. Am. J. Sports Med. 2013;41:560–566. doi: 10.1177/0363546512472978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biercevicz AM, Walsh EG, Murray MM, Akelman MR, Fleming BC. Improving the clinical efficiency of T2* mapping of ligament integrity. J. Biomech. 2014b;47:2522–2525. doi: 10.1016/j.jbiomech.2014.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield MR, Hozack WJ. Total hip and knee replacement in the mature athlete. Sports Health. 2014;6:78–80. doi: 10.1177/1941738113512760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchbinder R, Richards B, Harris I. Knee osteoarthritis and role for surgical intervention: lessons learned from randomized clinical trials and population-based cohorts. Curr. Opin. Rheumatol. 2014;26:138–144. doi: 10.1097/BOR.0000000000000022. [DOI] [PubMed] [Google Scholar]
- Crocker L, Algina J. Introduction to Classical and Modern Test Theory. Holt: Rinehart and Winston; 1986. [Google Scholar]
- Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, Laslett LL, Jones G, Cicuttini F, Osborne R, Vos T, Buchbinder R, Woolf A, March L. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014 doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- Draper NR, Smith H. Applied Regression Analysis. Third edition. New York: Wiley-Interscience; 1998. ed. etc. [Google Scholar]
- Haacke EM, Brown RW, Thompson MR, Venkatesan . Magnetic resonance imaging: physical principles and sequence design. New York, NY: A John Wiley and Sons; 1999. [Google Scholar]
- Hodler J, Haghighi P, Trudell D, Resnick D. The cruciate ligaments of the knee: correlation between MR appearance and gross and histologic findings in cadaveric specimens. AJR Am. J. Roentgenol. 1992;159:357–360. doi: 10.2214/ajr.159.2.1632355. [DOI] [PubMed] [Google Scholar]
- Huck SW. Group Heterogeneity And Pearson’s r. Educ. Psychol. Meas. 1992;52:253–260. [Google Scholar]
- McRobbie DW, Moore EA, Graves MJ, Prince MR. MRI from Picture to Proton. 2nd ed. Cambridge University Press; 2007. [Google Scholar]
- Murray MM, Magarian E, Zurakowski D, Fleming BC. Bone-to-Bone Fixation Enhances Functional Healing of the Porcine Anterior Cruciate Ligament Using a Collagen-Platelet Composite. Arthrosc. J. Arthrosc. Relat. Surg. 2010;26:S49–S57. doi: 10.1016/j.arthro.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes FR, Grood ES. The strength of the anterior cruciate ligament in humans and rhesus monkeys. J. Bone Jt. Surg. 1976;58:1074–1081. [PubMed] [Google Scholar]
- Weiler A, Peters G, Mäurer J, Unterhauser FN, Südkamp NP. Biomechanical properties and vascularity of an anterior cruciate ligament graft can be predicted by contrast-enhanced magnetic resonance imaging. A two-year study in sheep. Am J. Sports Med. 2001;29:751–761. doi: 10.1177/03635465010290061401. [DOI] [PubMed] [Google Scholar]
- Williams A, Qian Y, Golla S, Chu CR. UTE-T2* mapping detects sub-clinical meniscus injury after anterior cruciate ligament tear. Osteoarthritis Cartilage. 2012;20:486–494. doi: 10.1016/j.joca.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SL, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. Am. J. Sports Med. 1991;19:217–225. doi: 10.1177/036354659101900303. [DOI] [PubMed] [Google Scholar]
- Woo SL, Orlando CA, Camp JF, Akeson WH. Effects of postmortem storage by freezing on ligament tensile behavior. J Biomech. 1986;19:399–404. doi: 10.1016/0021-9290(86)90016-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.