Abstract
Objectives
To test the hypothesis that PPI use results in changes in gastric microflora which, through full column reflux, results in lung and oropharyngeal microflora changes.
Study design
We performed a prospective, cross sectional cohort study of 116 children (57 off and 59 on PPIs) undergoing simultaneous bronchoscopy and upper endoscopy for the evaluation of chronic cough. We performed 16S sequencing on gastric, bronchoalveolar lavage and oropharyngeal fluid. Fifty patients also underwent multichannel intraluminal impedance (pH-MII) testing.
Results
Streptococcus was more abundant in the gastric fluid of patients taking proton pump inhibitors (PPIs) and there was a significant correlation with PPI dose (mg/kg/day) and abundance of gastric Streptococcus (p=0.01). There was also a significant difference in the abundance of oropharyngeal Streptococcus in PPI treated patients. Eight unique bacterial genera were found in the gastric and lung fluid but not in the oropharyngeal suggesting exchange between the two sites and two of the seven (Lactococcus, Acinetobacter) were more abundant in patients with more full column reflux, suggesting direct aspiration. Principal component analysis revealed greater overlap between gastric and lung than oropharyngeal microflora.
Conclusions
PPI use was associated with differences in gastric, lung and oropharyngeal microflora. Although microflora exchange can occur between all three sites, gastric and lung microflora are more closely related and the mechanism of exchange between sites may be aspiration of full column reflux.
Keywords: Proton pump inhibitor, impedance, reflux
Proton pump inhibitors (PPIs) are commonly prescribed in children and adults despite growing evidence that these medications are associated with increased rates of pharyngitis and upper and lower respiratory tract infections. [1, 2] The mechanism behind these increased rates is not clear. We hypothesize that gastric bacterial overgrowth occurs in patients taking these medications and these changes may exert upstream effects on microflora of the oropharyngeal and lung through full column reflux. We have previously shown, using bacterial cultures, that children taking acid suppression have higher rates and concentrations of gastric bacterial growth than untreated patients. [3]We have further shown, using routine culture methods, that full column, non-acid reflux burden predicts lung culture positivity suggesting that non-acidic, bacterial laden gastric contents may be refluxed and aspirated into the lung. [4, 5] What is not known is if there are PPI-related microflora changes in the stomach and lung beyond what culture methods can detect, if the microflora changes are related to the full column reflux of bacterial laden gastric contents seen with PPI use, and what, if any, is the role of oropharyngeal microflora in seeding the stomach and lung. Because culture methods may underestimate the presence of bacteria by as much as 25%, [6] it is the goal of this study to use 16S-based community profiling to: (1) determine the presence and relative abundance of bacteria in bronchoalveolar lavage (BAL), gastric fluid and oropharyngeal swabs of patients taking PPIs relative to untreated patients; (2) determine the relationship between gastroesophageal reflux burden and oropharyngeal and lung bacterial abundance; (3) to determine the degree of microflora overlap between the stomach, lung and oropharyngeal.
Methods
We conducted a prospective cross sectional cohort study of children with cough 1-18 years of age undergoing bronchoscopy and GI endoscopy (EGD) for the evaluation of chronic cough at Boston Children's Hospital. All patients scheduled for bronchoscopy and EGD between 2006-2012 were approached to participate. Patients were considered “on therapy” if they had taken a PPI dose within 24 hours of endoscopy. Patients were considered “off therapy” if the PPI was stopped more than 48 hours prior to their procedure. Because bacterial death occurs within 10 minutes of acid exposure, 48 hours off therapy is felt to be sufficient as an “off therapy interval.” [7] Patients with gastrostomy tubes, nasogastric tubes, and a history of gastrointestinal surgery were excluded from participation. The study was approved by the Boston Children's Hospital Institutional Review Board and informed consent was obtained from all patients/parents.
Patients were recruited to participate at the time of their routinely scheduled endoscopy and bronchoscopy, which were ordered by their primary care team. At Boston Children's Hospital, an EGD is routinely performed at the time of bronchoscopy in aerodigestive patients, regardless of the underlying symptoms, as the yield of finding abnormalities is very high, independently of the presenting symptom. [8] Methods of dual sampling collection have previously been described. [3] Briefly, first, we performed brushing of the posterior tongue to obtain oropharyngeal samples. The posterior tongue was chosen because of the diversity and stability of microflora in this area, the similarity of the bacteria to saliva and greater likelihood that this area would be exposed to refluxate because of its proximity to the esophagus [9]. This is always performed before the endoscopy and bronchoscopy. Then, the bronchoscopy and BAL is performed through an endotracheal tube in distal airways of the right middle lung or the most visually inflamed lung. Finally, gastric sampling was performed during the EGD; the scope was advanced, without suctioning, immediately into the stomach where the gastric fluid is suctioned into a sterile leukitrap. The EGD was always performed last to prevent contamination of the oropharyngeal or even potentially the lungs with gastric contents as the scope was withdrawn. A minimum of 1 cc of gastric and lung fluid were collected and transferred to a −80° C freezer. The brush was then placed in TE buffer and frozen at −80° C. Each patient had a triad of samples collected, one from the oropharyngeal, one from the stomach and one from the lung.
Multichannel intraluminal impedance with pH (pH-MII)
A subset of 50 patients had pH-MII testing at the discretion of the patient's primary gastroenterologist. Acid reflux episodes were defined as reflux episodes detected by the impedance (MII) sensors that were associated with a drop in pH to <4. Non-acid reflux episodes were defined as a reflux episodes detected by MII sensors without an associated drop in pH to <4. The percentage of time that reflux was in the proximal/distal esophagus was calculated by dividing the sum of the bolus clearance times in the proximal/distal esophagus by the total study duration. The percentage of full column reflux events was defined as the percentage of the total reflux events that reach the proximal two impedance sensor (i.e., the proximal most impedance channel). The pH portion of the study was considered abnormal if the pH was < 4 for > 6% of the study time. [10] The MII portion of the study was considered abnormal if there were greater than 73 reflux episodes during the study time.[11, 12]
16S sequencing methods
DNA was isolated through the Harvard Digestive Disease Center Microbiome Core. 16S community profiling was performed through the Broad Institute. 180nt paired-end reads were generated on the Illumina MiSeq platform using established primers and protocols. [13] Read pairs were merged to create amplicon-spanning sequences. Sequences were filtered to require a minimum of 70% identity to any representative in the rRNA16S.gold.fasta reference set (http://drive5.com/uchime/uchime_download.html or http://microbiomeutil.sourceforge.net/) with usearch “-usearch_global -id 0.70″. [14] Filtering resulted in a dataset of 12,900,887 sequences. Further processing and OTU clustering utilized the UPARSE pipeline, software version usearch7.0.959_i86linux64. [15] The following usearch commands were used with default settings unless otherwise specified. Dereplication (-derepfulllength) resulted in 2,774,449 unique sequences. Sorting (-sortbysize -minsize 2), chimera filtering and clustering (-cluster_otus) resulted in 7,249 OTU representative sequences. Reads were mapped to OTUs (-usearch_global -strand plus -id 0.97) and an OTU table compiled (uc2otutab.py, http://drive5.com/python/).
QIIME version 1.6 was used to provide classifications of the OTU representative sequences using the gg_13_5 GreenGenes taxonomy and representative sequences established at 99% ID. [16] A phylogenetic tree was constructed using fasttree and filtered pynast alignments of the OTU representative sequences, also within the QIIME package.
Statistical Analyses
Based on the OTU (operating taxonomy unit) read count table from 16S sequencing, OTUs with < 200 total reads from all samples were filtered out as they were considered sequencing artifact. The OTU read counts were consolidated into phylum, class, order, family, genus, and species levels respectively to reduce the noise in the data due to the incomplete lineages. We focused on genus level for the downstream analyses because of its clinical relevance. Differences in abundance were determined using a Metastats analysis. [17] Relative abundance of a microbe in a sample was calculated as the read count normalized by the total reads in that sample. This measurement was adjusted for the different sequencing read yield for different samples. The microbe sample group (e.g. on vs off PPI, etc) abundance mean and standard error were calculated from the relative abundance of each genus. Detected presence or absence of each genus was scored as a 2×2 count matrix of the number of samples with presence/absence and with therapy/no therapy. Fisher's exact test was then performed on the matrix to get odds ratio (OR), 95% confidence interval (CI) and p-value. To determine differences in diversity, relative abundance was used to calculate the Shannon Index, which takes into account both the abundance and evenness of species present in the community.
To determine the relationship between reflux and microbe presence, we focused on microbes with average >1% relative abundance at the genus level only. Spearmen correlation coefficients were calculated to check the overall relationship between each reflux measurement and microbe abundance across samples. A linear regression model was further implemented to assess the significance. Calculations and statistical tests were implemented in R.
Based on a prior study using standard culture methods, we anticipated that 18% of untreated patients would have evidence of gastric bacteria compared with 46% of treated patients [3]. Based on these numbers, we calculated that we needed 56 each of treated and untreated patients to be able to reject the null hypothesis that there were no differences in bacterial presence in gastric fluid between treated and untreated with probability (power) of 0.9. The Type I error probability associated with this test of this null hypothesis is 0.05.
RESULTS
Of the 116 patients recruited to participate, 59 were receiving PPI and 57 were untreated. The mean age of patients in the PPI group was 7.1±5.0 years compared with 6.5±4.3 years in the untreated group (p=0.5). The mean dose of PPIs in patients was 1.1±0.5 mg/kg/day. There were no differences in the PPI treated versus untreated groups with respect to the proportion of patients: (1) with a diagnosis of asthma (68% vs 77% respectively, p=0.3) (2) with a diagnosis of croup (8% vs 19%, p=0.1), (3) with daily cough (61% vs 74%, p=0.2), (4) with abdominal pain (40% vs 40%, p=0.9), and (5) regurgitation of food into the mouth (41% vs 39%, p=0.8). More patients had an impedance performed in the untreated patients (N=33, 61%) versus the PPI treated group (N=17, 40%, p=0.006) but there were no differences in the rate of abnormal pH-MII testing between the untreated versus treated patient groups (45% vs 40%, p=0.6) suggesting that the decision to perform impedance was related to the referring gastroenterologists’ practice rather than a higher pretest probability for reflux. Patients who had impedance were older (9.2±5 years) versus those that did not get impedance (6.1±4.6). There were no differences in the frequency of abdominal pain, dysphagia, feeling food coming up, croup and wheezing between patients who did and did not undergo pH-MII testing (p>0.07). More patients with chronic cough (58%) had an impedance compared with patients without chronic cough (26%, p=0.005).
Stomach
We observed the following 6 genera with significantly higher prevalence in the gastric fluid of treated patients: Propionibacterium (OR: 8, 95% CI: 1,371,p=0.03), Flavobacterium (OR: 2.8, 95% CI: 1.2,7.0, p=0.01), Corynebacterium ( 2.4, 95% CI: 1.06, 5.6, p=0.02), Bacteroides (OR: 2.3, 95% CI: 1.01, 5.6, p=0.03), Zoogloea (OR: 3.6, 95% CI: 1.01,16.5, p=0.03), and Janthinobacterium (OR: 3.5, 95% CI: 1.5,8.6,p=0.002). Even though failing to reach significance, Staphylococcus (OR:2.3, 95% CI: 0.93, 6.0, p=0.06) and Burkholderia (OR: 2.1, 95% CI: 0.91, 5.1, p=0.07) may also have been more prevalent in treated patients.
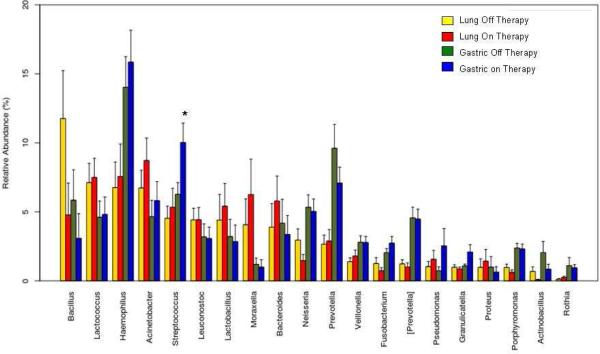
Based on our Metastats analysis, differences in absolute abundance of bacterial genera between treated and untreated patients are shown in the Table. Differences in relative abundance are shown in Figure 1; Streptococcus was more abundant in patients receiving PPI. There was no difference in the Shannon Index between treated and untreated patients.
Figure 1.
Differences in relative abundance of gastric and lung microflora in patients on and off PPIs (*=significant differences).
There was no difference in the Shannon Diversity Index of gastric fluid between PPI-treated and untreated patients (p=0.3).
Lung
We observed an increased prevalence of bacteria including Burkholderia (OR: 3.0, 95% CI: 1.1,8.3,p=0.02), Paludibacter (OR: 4.4, 95% CI: 1.2, 20.0,p=0.01), Flavobacterium (OR: 3.5, 95% CI: 1.5,8.8, p=0.003), and Tolumonas (OR: 2.4, 95% CI: 1.0,5.9, p=0.04) in treated patients.
Based on our Metastats analysis, differences in absolute abundance of bacterial genera are shown in the Table. PPI use was not associated with increased relative abundance of any genus (Figure 1). There was no difference in the Shannon Diversity Index in the lungs between treated and untreated patients (p=0.5).
Oropharynx
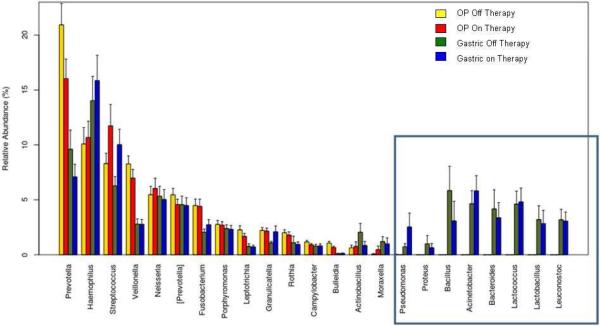
We observed an increased prevalence of Butyrivibrio (OR: 2.7, 95% CI: 1.1,7.1, p=0.02) in the oropharyngeal of treated patients which interestingly was also seen in greater abundance of the lungs of PPI treated patients. Differences in absolute abundance of bacterial genera are shown in the Table and, importantly, there was an increased abundance of Streptococcus in the oropharyngeal of treated patients. Differences in relative abundance are shown in Figure 2; there were eight bacteria that were uniquely found in the gastric fluid and not the oropharyngeal. All of these eight bacteria were also among the most prevalent in the lungs (Figure 1) suggesting that there is exchange between the gastrointestinal tract and the lungs that occurs independent of the oropharyngeal. There was no difference in the Shannon Diversity Index in the oropharyngeal between treated and untreated patients (p=0.1).
Figure 2.
Differences in relative abundance of gastric and oropharyngeal microflora in patients on and off acid suppression therapy (square highlights bacteria only found in gastric fluid).
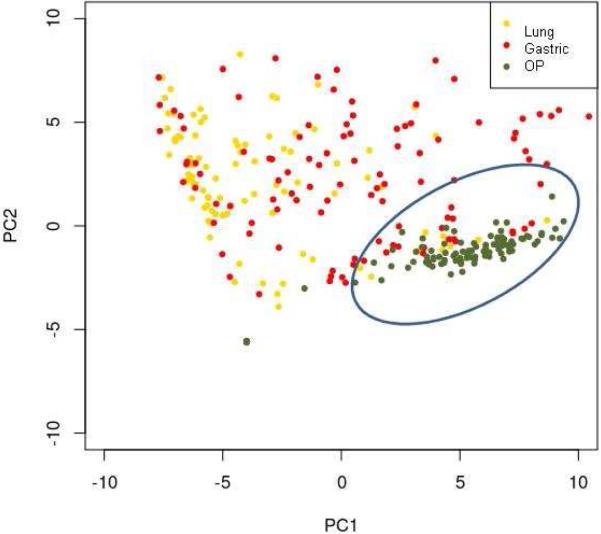
Using principal component analysis to determine if there are differences in bacterial populations between sites, we found significant overlap of gastric and lung samples with clustering of oropharyngeal samples suggesting that oropharyngeal microflora represent a distinct population (Figure 3).
Figure 3.
Principal component analysis highlighting differences in lung, gastric and oropharyngeal samples (circle highlights oropharyngeal clustering).
Impact of reflux on lung flora
Abnormal pH-MII testing was associated with a greater abundance of Mycoplasma (p=0.001), Ruminococcus (p=0.002) and Sphingobacterium (p=0.007) in the lungs. The percentage of time that non-acid reflux was in the proximal esophagus was positively correlated with the abundance of Neisseria (r= 0.4, p=0.002) and Veillonella (r= 0.38, p=0.003) in the lung. The percentage of acid reflux, either distal or proximal, did not correlate with the presence of any bacteria in the lung (p>0.2). Importantly, we also found that, of the eight bacteria found only stomach and lungs, the abundance of Acinetobacter (r=0.34, p=0.01), Lactococcus (r=0.33, p=0.01) were associated with a higher percentage of full column reflux episodes in the esophagus. There was no difference in the presence of Lactococcus or Acinetobacter in patients on and off PPIs.
Impact of reflux on oropharyngeal flora
Abnormal pH-MII testing was associated with a greater abundance of oropharyngeal Neisseria (p=0.02), Allobaculum (p=0.01), Alloiococcus (p=0.02), Chryseobacterium (p=0.01), Fusobacterium (p=0.03), Paenibacillus (p=0.04), Propionibacterium (p=0.03), Sphingobacterim (p=0.03), and Sphingomonas (p=0.006). The percentage of time that non-acid reflux was in the proximal esophagus was positively correlated with oropharyngeal Neisseria (r=0.32, p=0.047) and the percentage of full column reflux episodes was correlated with Neisseria (r=0.32, p=0.009), Fusobacterium (r=0.33, p=0.02), and Porphyromonas (r=0.32, p=0.03).
Impact of PPI dosage on bacterial concentrations
PPI dose (mg/kg/day) correlated with increased abundance of Streptococcus in the gastric fluid (p=0.01). There was no significant correlation with PPI use and any specific bacteria in the lung or oropharyngeal (p>0.05).
DISCUSSION
This study significantly advances our understanding of PPI-related infections by showing that PPI use in adults or children is associated with differences in both abundance and prevalence of gastric, oropharyngeal and lung bacteria, as determined by 16S sequencing. We found that although microflora exchange can occur between all three sites, gastric and lung microflora are more closely related and the mechanism of exchange between these sites may be aspiration of full column reflux.
The study of gastric, oropharyngeal and lung bacteria by 16S sequencing, and the study of its association with gastroesophageal reflux is one of the major strengths of our study. All of the prior adult and pediatric studies trying to assess the impact of PPI use on microflora have relied on culture methods, have not included the pairing of gastric, oropharyngeal and lung samples, and did not consider the impact of gastroesophageal reflux on microflora exchange. In a study of adult patients, Shindo et al cultured gastric and jejunal fluid of healthy adult controls and peptic ulcer patients after 5 weeks of 20 mg of omeprazole and found that the number of positive gastric and jejunal cultures increased significantly with PPI use and that microflora changes were identical in patients and controls exposed to PPI, suggesting that the changes occurred independent of peptic disease. [18] To assess the impact of cimetidine and omeprazole on gastric and duodenal flora, Thorens et al, using culture methods, found that the amounts of Streptococcus, Corynebacterium, E. coli, and Klebsiella in the gastric and duodenal flora increased with medication use and bacterial abundance and gastric pH were highly correlated. [19] Whether there is an upstream effect of acid suppression on the lung is not known from these studies but in a single adult study of 52 ICU patients with indwelling nasogastric tubes (which may alter microflora in itself), Segal et al found that there was significant species overlap between gastric and oropharygeal flora suggesting that gastric flora may alter upstream flora. However, in this study, only 4% of patients were taking PPIs and 33% were taking H2 antagonists so the impact of acid suppression on oropharyngeal flora is not clear and whether the oropharyngeal flora mimiced the lung flora is not known. [20] Finally, this study only included ICU patients which had medication and systemic comorbidities that could have affected microflora composition.
In addition to documenting that there are differences in the microflora in PPI-treated children, we further want to highlight three important findings: (1) PPIs results in a dose dependent increase in gastric bacterial abundance; (2) there is evidence of microflora exchange between the lung and the gastrointestinal tract that is independent of the oropharyngeal microflora; and (3) full column reflux may result both in changes in oropharyngeal and lung microflora.
First, we show that gastric Streptococcus is more abundant in a PPI-dose dependent fashion. This finding is of clinical importance because pediatric doses of PPIs are often pushed to levels as high as 3.5 mg/kg/day (of note, we found a PPI effect at much lower doses) and also because different species of Streptococcus have been implicated in upper respiratory tract infections, pharyngitis, pneumonia, otitis media, and sepsis which may explain why children taking PPIs have increased risk of these infections. [21] The increased abundance in the gastric fluid raises the possibility that gastrointestinal tract could serve as an infection source for extraesopheageal complications. When we pair this 16S data with prior culture data, we now can conclusively say that Streptococcus is more prevalent and abundant in the gastric fluid of PPI-treated children, that these gastric bacteria are actually viable, and that there are upstream oropharyngeal changes in Streptococcus abundance in PPI-treated patients which now provides a mechanism for infections such as pharyngitis in treated patients.[3]
The second important finding is that there is evidence of microflora exchange between the lung and the gastrointestinal tract that is independent of the oropharyngeal microflora. We have shown that seven of the most abundant bacteria in the gastric fluid are also abundant in the lungs but not the oropharyngeal. Of these seven, Pseudomonas, Proteus, Bacteroides, and Lactobacillus are primary gastrointestinal colonizers so the possibility that the bacteria originate from the lungs, are coughed up in sputum and swallowed is much less likely. We also show that Lactococcus, a bacterial genus found in milk, was abundant in the gastric and lung fluid and was correlated with full column reflux; this provides evidence that ingested milk can be refluxed and aspirated, independently of the oropharyngeal. Lastly, our principal component analysis, which shows that the oropharyngeal microbiome is distinct from the lung and gastric microbiome, supports the 16S mapping study from the human microbiome project which supports that the gastric and oropharyngeal flora are distinct populations. [22]
Other authors have also raised the possibility that microflora exchange occurs between gastrointestinal and extraesophageal sites in children. In a study of very low birthweight infants on day of life 0-28, Oue et al performed 16S sequencing on samples obtained during serial nasogastric and tracheal suctioning. Although there are no details on the degree of bacterial concordance between gastric and tracheal samples within individual infants, the authors did find that the presence of microbes, either in the gastric or tracheal secretions, predicted more severe lung disease. [23] In a second pediatric study, Madan et al studied, using 16S sequencing, seven infants with cystic fibrosis using stool samples and oropharyngeal swabs to map the overlap between the oropharyngeal and lower intestinal microbiome. [24] In their cohort, they found that the intestinal and oropharyngeal microflora changes often occurred simultaneously suggesting exchange between the two sites, however, the mechanism of exchange between the upper gastrointestinal tract microflora and the lung were not measured. [24] A report of 8 patients with cystic fibrosis examined the relationship between bacterial abundance in the lungs and the oropharyngeal. The authors found that the lung microflora were much less diverse that the orophynx; they found 4 dominant bacteria in the lungs versus 11 in the oropharyngeal. In contrast, in our study, we found no differences in the diveristy indices between the three sites in our patients and we identified a different panel of bacteria than in the cystic fibrosis patients[25] . Our work adds significantly to the current literature by documenting in a large cohort of older children that: (1) gastric and lung microflora may be more similar to each other than oropharyngeal microflora, as is evident by principal component analysis; (2) gastric fluid may serve as the original site of microflora exchange, as there are gastrointestinal bacteria found in the lungs; (3) the exchange between the gastric and lung sites may be through gastroesophaegal reflux; and (4) PPI use may result in unique microflora changes in all three sites.
In this study, by performing pH-MII testing in children, in addition to 16S sequencing, we provide evidence that full column reflux episodes may exert an impact on oropharyngeal and lung microflora. We have previously shown that full column, non-acid reflux has been associated with increased BAL culture positivity in our clinical microbiology lab as well as increased Pseudomonas positivity in BAL cultures of cystic fibrosis patients. [4, 5] We add to our prior studies by using 16S sequencing and by clarifying the role of the oropharyngeal relative to the gastric and lung microbiome. We have now addressed the critical question: does reflux alter the oropharyngeal microflora, the latter of which is then aspirated, or can the gastric fluid exert an independent effect on the lung through the direct aspiration of full column reflux. Our data suggest that both mechanisms can occur. Although full column non-acid reflux was associated with greater abundances of both oropharyngeal and lung Neisseria, suggesting that some lung microflora changes may result from aspiration of oropharyngeal contents, we also show that direct aspiration can occur independently of oropharyngeal microflora changes as we saw that seven of the most abundant genera in the gastrointestinal tract were also abundant in the lung, but not in the oropharyngeal.
We recognize that not all of the microflora changes that we identified represent changes towards recognized pathogenic bacteria but rather indicate changes in commensal microflora which we hypothesize may also alter infection risk. An example of this is the documented relationship between Streptococcus pneumonae and respiratory syncytial virus (RSV); although S. pneumonae is a common colonizer of the nasopharynx, its presence at the time of RSV infection increases the risk of lower respiratory tract infections and a more severe clinical course. [26-28] Similar interactions are seen with colonizers such as Haemophilus, Moraxella, Neisseria, Rothia and Actinomyces and the risk for the development of otitis media from concurrent viral infections. [28-30] A logical next study would be, using a longitudinal study design, to determine which changes in commensal or pathogenic bacteria after the start of PPIs predict clinical infection risk.
There are several limitations to this study. First, the bacteria identified in this study represent a single point in time rather than longitudinal changes within individuals so definitively proving causality between PPI use and gastric and lung microflora changes is difficult. To obtain serial data for the gastric and lung fluid would require serial nasogastric and BAL sampling which is not ethical or feasible in ambulatory children. We do feel, however, that this study represents a significant contribution to the literature by mapping the interrelationship between gastric, lung and oropharyngeal microflora and the associated PPI changes in a difficult-to-study population. A second limitation to this study is that all of the patients had chronic cough that precipitated the need for bronchoscopy. Therefore, the results may be biased towards no PPI effect because any PPI effect may be dwarfed by the microflora perturbations related to the underlying respiratory pathology. However, these chronic cough patients are frequently give therapeutic trials with PPIs so we feel that these results are clinically relevant. [31] Even if there is a bias towards no PPI effect, we still observed changes in PPI-treated patients, suggesting the true PPI effect may be even greater.
In conclusion, PPIs exert an effect on gastric, oropharyngeal and lung microflora. The significance, in terms of clinical infection risk, is not known, but this study raises the possibility that gastric microflora changes in children taking PPIs may have effects beyond the gastrointestinal tract and additional studies are needed to determine if these changes in microflora predict which patients will develop clinical infections.
Table 1.
Genera of bacteria more abundant in the gastric, lung, and OP of patients taking PPIs.
| Gastric Treated | Lung Treated | OP Treated |
|---|---|---|
| Blautia (p=0.02) | Butyrivibrio (p=0.0004) | Allobaculum (p=0.005) |
| Brevibacterium (p=0.001) | Ruminococcus (p=0.01) | Bifidobacterium (p<0.0001) |
| Coprococcus (p=0.004) | Vogesella (p=0.01) | Cloacibacterium (p<0.0001) |
| Granulicatella (p=0.03) | Janthinobacterium (p=0.002) | |
| Streptococcus (p=0.0009) | Ralstonia (p=0.02) | |
| Rhodobacter (p=0.007) | ||
| Rhodoferax (p=0.007) | ||
| Streptococcus (p=0.04) | ||
| Yersinia (p<0.0001) | ||
| Zoogloea (p=0.0001) |
Acknowledgments
Supported by the National Institutes of Health (K23 DK073713 and R03DK089146 [to R.R.]) and Boston Children's Hospital Translational Research Program Junior Investigator Award (to R.R.). The authors declare no conflicts of interest.
Abbreviations
- BAL
bronchoalveolar lavage
- EGD
esophagogastroduodenoscopy
- pH-MII
multichannel intraluminal impedance
- PPI
proton pump inhibitor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of the study were presented at the North American Society for Pediatric Gastroenterology, Heapatology, and Nutrition meeting <>, <>, <>, 2013.
References
- 1.Holbrook JT, Wise RA, Gold BD, Blake K, Brown ED, Castro M, et al. Lansoprazole for children with poorly controlled asthma: a randomized controlled trial. JAMA. 2012;307:373–81. doi: 10.1001/jama.2011.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canani RB, Cirillo P, Roggero P, Romano C, Malamisura B, Terrin G, et al. Therapy with gastric acidity inhibitors increases the risk of acute gastroenteritis and community-acquired pneumonia in children. Pediatrics. 2006;117:e817–20. doi: 10.1542/peds.2005-1655. [DOI] [PubMed] [Google Scholar]
- 3.Rosen R, Amirault J, Liu H, Mitchell P, Hu L, Khatwa U, et al. Changes in gastric and lung microflora with Acid suppression: Acid suppression and bacterial growth. JAMA Pediatr. 2014;168:932–7. doi: 10.1001/jamapediatrics.2014.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palm K, Sawicki G, Rosen R. The impact of reflux burden on Pseudomonas positivity in children with cystic fibrosis. Pediatr Pulmonol. 2012;47:582–7. doi: 10.1002/ppul.21598. [DOI] [PubMed] [Google Scholar]
- 5.Rosen R, Johnston N, Hart K, Khatwa U, Katz E, Nurko S. Higher rate of bronchoalveolar lavage culture positivity in children with nonacid reflux and respiratory disorders. J Pediatr. 2011;159:504–6. doi: 10.1016/j.jpeds.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris KA, Hartley JC. Development of broad-range 16S rDNA PCR for use in the routine diagnostic clinical microbiology service. J Med Microbiol. 2003;52:685–91. doi: 10.1099/jmm.0.05213-0. [DOI] [PubMed] [Google Scholar]
- 7.Hill M. Normal and pathological microbial flora of the upper gastrointestinal tract. Scand J Gastroenterol Suppl. 1985;111:1–6. doi: 10.3109/00365528509093747. [DOI] [PubMed] [Google Scholar]
- 8.Rosen R, Amirault J, Johnston N, Haver K, Khatwa U, Rubinstein E, et al. The utility of endoscopy and multichannel intraluminal impedance testing in children with cough and wheezing. Pediatr Pulmonol. 2014;49:1090–6. doi: 10.1002/ppul.22949. [DOI] [PubMed] [Google Scholar]
- 9.Eren AM, Borisy GG, Huse SM, Mark Welch JL. Oligotyping analysis of the human oral microbiome. Proc Natl Acad Sci U S A. 2014;111:E2875–84. doi: 10.1073/pnas.1409644111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudolph CD, Mazur LJ, Liptak GS, Baker RD, Boyle JT, Colletti RB, et al. Guidelines for evaluation and treatment of gastroesophageal reflux in infants and children: recommendations of the North American Society for Pediatric Gastroenterology and Nutrition. J Pediatr Gastroenterol Nutr. 2001;32(Suppl 2):S1–31. doi: 10.1097/00005176-200100002-00001. [DOI] [PubMed] [Google Scholar]
- 11.Shay S, Tutuian R, Sifrim D, Vela M, Wise J, Balaji N, et al. Twenty-four hour ambulatory simultaneous impedance and pH monitoring: a multicenter report of normal values from 60 healthy volunteers. Am J Gastroenterol. 2004;99:1037–43. doi: 10.1111/j.1572-0241.2004.04172.x. [DOI] [PubMed] [Google Scholar]
- 12.Rosen R, Furuta G, Fritz J, Donovan K, Nurko S. Role of acid and nonacid reflux in children with eosinophilic esophagitis compared with patients with gastroesophageal reflux and control patients. J Pediatr Gastroenterol Nutr. 2008;46:520–3. doi: 10.1097/MPG.0b013e318158600c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–4. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–1. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 15.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10:996–8. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 16.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–6. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White JR, Nagarajan N, Pop M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput Biol. 2009;5:e1000352. doi: 10.1371/journal.pcbi.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shindo K, Machida M, Fukumura M, Koide K, Yamazaki R. Omeprazole induces altered bile acid metabolism. Gut. 1998;42:266–71. doi: 10.1136/gut.42.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thorens J, Froehlich F, Schwizer W, Saraga E, Bille J, Gyr K, et al. Bacterial overgrowth during treatment with omeprazole compared with cimetidine: a prospective randomised double blind study. Gut. 1996;39:54–9. doi: 10.1136/gut.39.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Segal R, Pogoreliuk I, Dan M, Baumoehl Y, Leibovitz A. Gastric microbiota in elderly patients fed via nasogastric tubes for prolonged periods. J Hosp Infect. 2006;63:79–83. doi: 10.1016/j.jhin.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Vandenplas Y, Rudolph CD, Di Lorenzo C, Hassall E, Liptak G, Mazur L, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr. 2009;49:498–547. doi: 10.1097/MPG.0b013e3181b7f563. [DOI] [PubMed] [Google Scholar]
- 22.Delgado S, Cabrera-Rubio R, Mira A, Suarez A, Mayo B. Microbiological survey of the human gastric ecosystem using culturing and pyrosequencing methods. Microb Ecol. 2013;65:763–72. doi: 10.1007/s00248-013-0192-5. [DOI] [PubMed] [Google Scholar]
- 23.Oue S, Hiroi M, Ogawa S, Hira S, Hasegawa M, Yamaoka S, et al. Association of gastric fluid microbes at birth with severe bronchopulmonary dysplasia. Arch Dis Child Fetal Neonatal Ed. 2009;94:F17–22. doi: 10.1136/adc.2008.138321. [DOI] [PubMed] [Google Scholar]
- 24.Madan JC, Koestler DC, Stanton BA, Davidson L, Moulton LA, Housman ML, et al. Serial analysis of the gut and respiratory microbiome in cystic fibrosis in infancy: interaction between intestinal and respiratory tracts and impact of nutritional exposures. MBio. 2012:3. doi: 10.1128/mBio.00251-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goddard AF, Staudinger BJ, Dowd SE, Joshi-Datar A, Wolcott RD, Aitken ML, et al. Direct sampling of cystic fibrosis lungs indicates that DNA-based analyses of upper-airway specimens can misrepresent lung microbiota. Proc Natl Acad Sci U S A. 2012;109:13769–74. doi: 10.1073/pnas.1107435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esposito S, Zampiero A, Terranova L, Ierardi V, Ascolese B, Daleno C, et al. Pneumococcal bacterial load colonization as a marker of mixed infection in children with alveolar community-acquired pneumonia and respiratory syncytial virus or rhinovirus infection. Pediatr Infect Dis J. 2013;32:1199–204. doi: 10.1097/INF.0b013e31829ec274. [DOI] [PubMed] [Google Scholar]
- 27.Hament JM, Aerts PC, Fleer A, van Dijk H, Harmsen T, Kimpen JL, et al. Direct binding of respiratory syncytial virus to pneumococci: a phenomenon that enhances both pneumococcal adherence to human epithelial cells and pneumococcal invasiveness in a murine model. Pediatr Res. 2005;58:1198–203. doi: 10.1203/01.pdr.0000188699.55279.1b. [DOI] [PubMed] [Google Scholar]
- 28.Pettigrew MM, Gent JF, Pyles RB, Miller AL, Nokso-Koivisto J, Chonmaitree T. Viral-bacterial interactions and risk of acute otitis media complicating upper respiratory tract infection. J Clin Microbiol. 2011;49:3750–5. doi: 10.1128/JCM.01186-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruohola A, Pettigrew MM, Lindholm L, Jalava J, Raisanen KS, Vainionpaa R, et al. Bacterial and viral interactions within the nasopharynx contribute to the risk of acute otitis media. J Infect. 2013;66:247–54. doi: 10.1016/j.jinf.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laufer AS, Metlay JP, Gent JF, Fennie KP, Kong Y, Pettigrew MM. Microbial communities of the upper respiratory tract and otitis media in children. MBio. 2011;2:e00245–10. doi: 10.1128/mBio.00245-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson SP, Kothari S, Wu EQ, Beaulieu N, McHale JM, Dabbous OH. Pediatric gastroesophageal reflux disease and acid-related conditions: trends in incidence of diagnosis and acid suppression therapy. J Med Econ. 2009;12:348–55. doi: 10.3111/13696990903378680. [DOI] [PubMed] [Google Scholar]