Abstract
This review explores scaffold-free methods as an additional paradigm for tissue engineering. Musculoskeletal cartilages –for example articular cartilage, meniscus, temporomandibular joint disc, and intervertebral disc – are characterized by low vascularity and cellularity, and are amenable to scaffold-free tissue engineering approaches. Scaffold-free approaches, particularly the self-assembling process, mimic elements of developmental processes underlying these tissues. Discussed are various scaffold-free approaches for musculoskeletal cartilage tissue engineering, such as cell sheet engineering, aggregation, and the self-assembling process, as well as the availability and variety of cells used. Immunological considerations are of particular importance as engineered tissues are frequently of allogeneic, if not xenogeneic, origin. Factors that enhance the matrix production and mechanical properties of these engineered cartilages are also reviewed, as the fabrication of biomimetically suitable tissues is necessary to replicate function and ensure graft survival in vivo. The concept of combining scaffold-free and scaffold-based tissue engineering methods to address clinical needs is also discussed. Inasmuch as scaffold-based musculoskeletal tissue engineering approaches have been employed as a paradigm to generate engineered cartilages with appropriate functional properties, scaffold-free approaches are emerging as promising elements of a translational pathway not only for musculoskeletal cartilages but for other tissues as well.
Keywords: Scaffoldless, Cell Sheet Engineering, Aggregate, Self-assembling Process, Self-assembly, Self-organization, Tissue Engineering Paradigm
3. INTRODUCTION
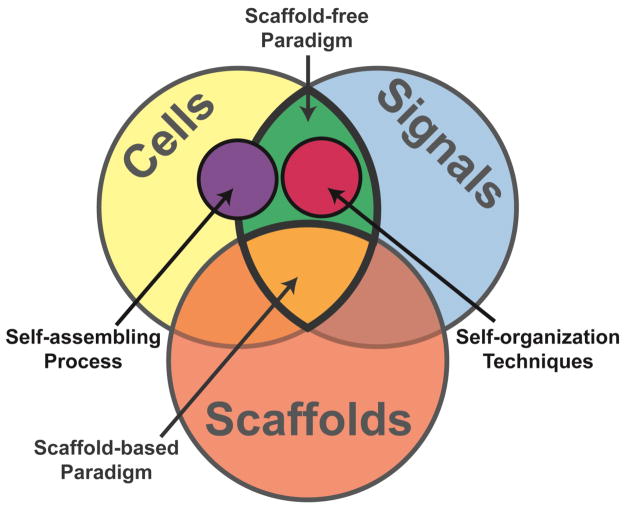
With the traditional tissue engineering (TE) paradigm of cells, signals, and scaffolds, the field of biomedical engineering has made great strides toward addressing clinical needs. More recently, approaches that do not use scaffolds have emerged as suitable modalities to engineer functional tissues (Figure 1). “Scaffoldless tissue engineering refers to any platform that does not require cell seeding or adherence within an exogenous, three-dimensional material.” 5 This term may be used interchangeably with “scaffold-free.” Scaffold-free approaches have been employed with success for musculoskeletal cartilages, such as articular cartilage, meniscus, temporomandibular joint (TMJ) disc, and intervertebral disc, as they are similar to the condensation and differentiation that occurs during native cartilage development.
Figure 1.
The traditional scaffold-based tissue engineering paradigm consists of cells, signals, and scaffolds. A new, scaffold-free paradigm consists of just cells and signals.
To make a clinically relevant scaffold-free tissue, TE considerations must include cell sourcing, stimulation of tissue-specific extracellular matrix (ECM) production, and tissue organization. As scaffold-free approaches lack the exogenous material of scaffold-based approaches, the resulting engineered cartilages (neocartilages) commonly require large cell numbers. While the use of autologous cells is ideal to enhance clinical translation, limitations associated with the number of primary cells available and donor site morbidity have led to the use of allogeneic and xenogeneic sources.71 Mirroring cartilage development, in scaffold-free culture systems, only cell-secreted ECM contributes to neotissue properties. Therefore, inspired by developmental processes, exogenous stimuli, such as growth factors, enzymes, and mechanical stimulation, are employed to enhance matrix formation and maturation to replicate native tissue structure-function relationships.34 As musculoskeletal cartilages are heterogeneous and anisotropic, achieving proper tissue morphology and organization is critical. This may be addressed by molding or confining tissue in culture, assembling tissues as building blocks and promoting their fusion to form higher order structures, and by applying mechanical stimulation used to mature the matrix in such a way that creates functional anisotropy.50–51 This review addresses types of scaffold-free tissue engineering approaches, cell sourcing, production of tissue-specific ECM, and tissue organization as they apply to the scaffold-free TE paradigm with the purpose of guiding the development of clinically useful engineered musculoskeletal cartilages.
4. SCAFFOLD-FREE APPROACHES
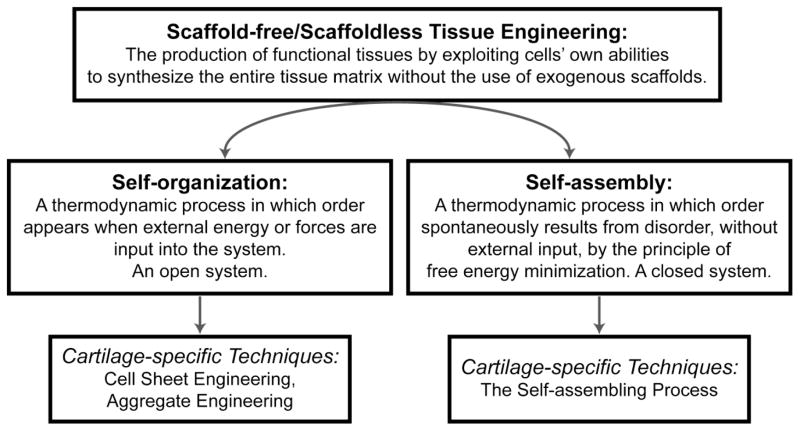
Scaffold-free approaches seek to produce tissues by mimicking developmental processes. These often follow a pattern of cell condensation, cell proliferation, cell differentiation, ECM production, and tissue maturation. Within scaffold-free approaches, two distinct categories, self-organization and self-assembly, exist and must be defined (Figure 2). Self-organization refers to “…a process in which order appears when external energy or forces are input into the system.”5 This is in contrast to self-assembly in which order spontaneously results from disorder without the use of external input by the principle of free energy minimization.5 In the TE literature, self-organization and self-assembly are used with ambiguity and incorrectly interchanged, leading to confusion and a lack of understanding of the fundamental nature of the processes driving in vitro tissue formation. These phenomena are distinct and well-defined thermodynamic processes in other scientific fields, and the correct use of these terms in TE literature allows the interdisciplinary communication and collaboration essential to the translational nature of biomedical engineering. Of scaffold-free TE approaches that produce robust musculoskeletal cartilages, three distinct methods have risen to the fore: cell sheet engineering, aggregate engineering, and self-assembling process.1, 25, 30, 54
Figure 2.
Self-organization and self-assembly represent two distinct thermodynamic processes that govern engineered tissue formation. These terms have specific meanings and should not be used interchangeably. Tissue engineering techniques that are governed by self-organization include cell sheet engineering and aggregate engineering. The self-assembling process is a tissue engineering technique governed by self-assembly.
4.1 Cell Sheet Engineering
Cell sheet engineering falls within the self-organization category of scaffold-free approaches as it requires external manipulation to form a desired structure. To form a cell sheet, cells are expanded in monolayer for long durations to high confluence. Once sufficient ECM is produced for the culture to form a cohesive layer, the sheet is lifted from the substrate as a whole.73 For example, cell sheets are commonly lifted using temperature-responsive substrates to preserve cell-cell junctions and ECM deposition that would be degraded if the sheet were lifted through enzymatic means.45, 85 Released cell sheets are then further manipulated by rolling, layering, or draping over molds (Figure 3). Sheets then undergo tissue fusion, a process common in developmental biology. In tissue fusion, isolated cell populations make contact and adhere through cell-to-cell contact, cell-to-matrix contact, matrix-to-matrix contact, and ECM remodeling to form continuous tissues.5, 65 Variations of this technique have been used to engineer tissues including vasculature, cornea, tendon, bone, and cartilage.21
Figure 3.
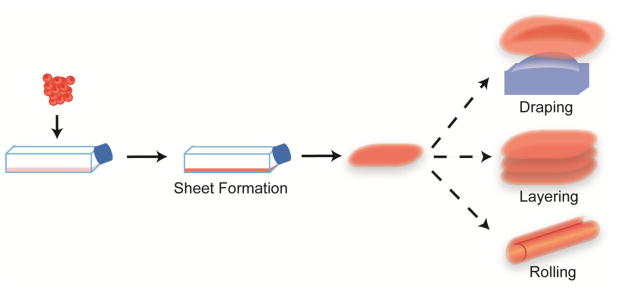
Cell sheet engineering is a scaffold-free, self-organization tissue engineering technique. Cell sheets are created by monolayer expansion of cells until a cohesive, ECM-rich sheet forms. This sheet is then lifted and assembled by, for example, rolling, layering, or draping to form shaped tissues.
Regarding musculoskeletal cartilages, cell sheet engineering processes have been used to engineer neotissues with clinically relevant dimensions and properties. Currently in Phase III clinical trials, RevaFlex (formerly DeNovo ET) (ISTO Technologies, Missouri, USA) is an articular cartilage repair technology using a sheet of expanded juvenile allogeneic chondrocytes. Repair tissue resulting from using RevaFlex to fill cartilage defects in vivo during Phase I/II clinical trials was ICRS scored as “grossly normal or nearly normal” in 6 of 9 patients with the 7th having repair level with the surrounding tissue and the remaining 2 of 9 resulting in at least 75% repair of the lesion with follow-up MRI and second-look arthroscopy indicating retention and maturation of the repair.55 Additionally, layered articular chondrocyte sheets have been used to treat full-thickness cartilage defects in miniature pigs.73 Although, implantation of the layered tissue did not fully restore the articular surface, it achieved good defect filling and integration compared to empty defect controls, indicating cell sheet engineering as a promising scaffold-free approach.17, 73
Though cell sheet engineering has been used to produce, multilayered tissues, this is not without limitations. Cell sheet engineering is initiated in monolayer, but chondrocytes are known to dedifferentiate during monolayer expansion, progressing toward a fibroblastic phenotype.15 Contraction of the cell sheet can require the use of an external support structure to retain desired sizes.57 The production of thicker tissues and defined shapes requires extensive manipulation and are difficult to achieve due to diffusion limitations. The main benefit of cell sheet engineering is the ability to expand cells and form a cell sheet in a single step. Due to the limitations in achieving sufficiently thick shaped tissues, the use of cell sheets may be best suited for repairing tissues that undergo large deformations or those that require repairs analogous to a patch, such as myocardium or bladder reconstructions.75, 80
4.2 Aggregate Tissue Engineering
Cell aggregates are commonly formed in culture by applying a rotational force to cells in a suspension or other non-adherent culture.25 Therefore, aggregate culture is categorized as a self-organization technique. Parameters like rotational speed and duration can vary from gentle swirling (60 revolutions per minute) for prolonged durations (1–21 days) (i.e., rotational culture) to high-speed centrifugation (500g) for a few minutes (5 minutes) (i.e., pellet culture).12, 33, 25 Due to this motion, rotational culture may also have improved diffusion and nutrient/gas exchange compared to static cultures, making it an appealing TE strategy.26
Aggregate culture is a common TE culture method as it is used not only to (re)differentiate cells to a chondrocytic phenotype, but also to form cartilaginous microtissues. As the phenotype of chondrocyte aggregates is similar to that of native cartilage, their formation is thought to mirror cell aggregation and matrix production that occurs in native cartilage development.79 Therefore aggregate cultures, such as pellet culture, are commonly employed to differentiate stem cells to the chondrocytic phenotype.39 Aggregate culture may also be used to redifferentiate chondrocytes that have been expanded in monolayer.25 For example, rabbit articular and meniscus cells were redifferentiated by rotational culture and used to form fibrocartilage with superior properties to tissues containing cells that did not undergo the redifferentiation step.33 Rotational culture may be used to form neocartilages in a spectrum of fibrous to hyaline cartilage.60 Aggregation may also be used to engineer cartilage. Recently, a pellet culture of human mesenchymal stem cells (MSCs) with a demineralized bone support was used to produce engineered articular cartilage with a physiologically relevant compressive Young’s modulus of greater than 800 kPa and an equilibrium friction coefficient of ~0.28.7 Commercial products using aggregate TE also exist. For example, Chondrosphere (co.don AG, Tetlow, Germany), also known as ARTOCELL 3D and 3D autologous chondrocyte transplantation (ACT3D), uses autologous cell aggregates/spheroids and is currently in phase III clinical trials in Europe. In a 1 year follow-up study, patients’ Lysholm, International Knee Documentation Committee (IKDC), SF-36, and Tegner scores significantly increased after treatment of full-thickness patellofemoral or femoral condylar defects with Chondrosphere.23 Therefore, aggregate engineering represents a highly versatile tool with which to engineer musculoskeletal cartilages. Aggregate tissue engineering represents a versatile TE tool to form musculoskeletal neocartilage, both indirectly by forming aggregates to (re)differentiate cells and then dissociating them to form tissues by other methods and directly by using aggregates to fill defects or assembling them into larger tissue structures6 (Figure 4).
Figure 4.
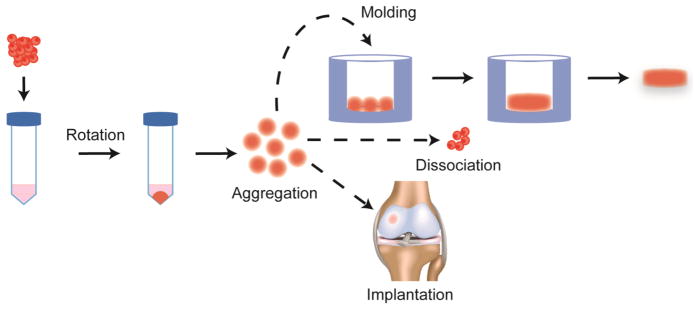
Aggregate engineering is a scaffold-free, self-organization tissue engineering technique. Aggregates are created by rotating or shaking a non-adherent suspension of cells with varying speeds and durations depending on the culture. Aggregates may then be assembled into larger tissue structures, dissociated, or implanted into defects.
Aggregate engineering, although partially able to overcome diffusion limits in producing cartilaginous tissues, still exhibits shortcomings. It is conceivable that, in the initial period of suspension culture before cell interaction and coalescence, cells may die due to lack of substrate contact. As aggregate culture is used to differentiate or restore cells to a chondrocytic phenotype, these cells proliferate minimally, except under specific culture conditions.43,26 Aggregates also have uncontrolled and nonhomogeneous shapes.26 However, larger aggregates may still experience decreased cell viability or loss of cell type homogeneity.79 Fusing small aggregates to form larger tissues or injecting aggregates into defects may be the most promising methods to use aggregates to engineer musculoskeletal cartilages.
4.3 Self-assembling Process
The self-assembling process is a TE technique that falls under the self-assembly category of scaffold-free approaches, as it does not employ external forces to form tissues. It consists of distinct phases that mirror those of native cartilage development (Figure 5). In the first phase, a high-density cell suspension is seeded into a non-adherent mold to ensure that only cellular interactions drive tissue assembly. In the second phase, spontaneous minimization of free energy drives cell coalescence, as described by the differential adhesion hypothesis.77 In the third phase, tissue-specific ECM is produced, and, in the last phase, this matrix matures to form functional tissue.5
Figure 5.
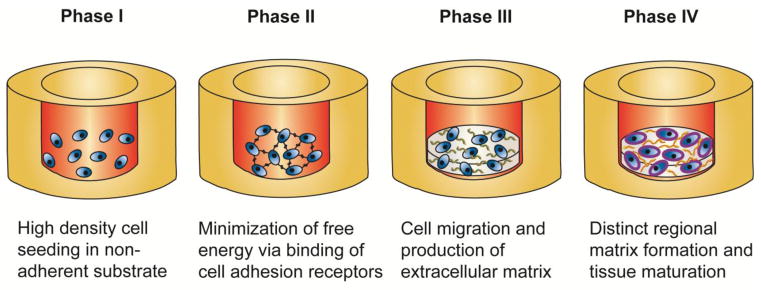
The self-assembling process is a scaffold-free tissue engineering technique governed by self-assembly. Self-assembling tissues undergo distinct phases that mirror those of native cartilage development. Control of the mold shape and matrix maturation of the neocartilage allows for the formation of shaped functional tissues. Image used with permission.5
The self-assembling process has created a variety of functional musculoskeletal cartilages. The self-assembling process differs from aggregate culture by the way the tissue forms and the properties of the resulting tissue. Specifically, self-assembling tissues 1) lack external forces during tissue formation, 2) form within non-adhesive molds, 3) have organizational and functional properties similar to native tissue, 4) have distinct dimensions and gross appearances, and 5) are easily manipulated with regard to size, thickness, and shape. Rather than using centrifugation or rotational culture to form aggregates or pellets, the non-adherent nature of the agarose mold allows cell-driven tissue formation. In contrast to aggregate cultures which often form variable and uncontrolled tissue shapes, self-assembling cartilages are of predictable and repeatable gross appearance, shape, and size as a predetermined number of cells are seeded into a mold with a defined shape. The self-assembling process can be customized to tailor neocartilages’ size and shape by modifying the agarose mold.
Articular cartilage and fibrocartilages, such as meniscus and TMJ disc, have been engineered with physiologically relevant properties, without exogenous stimuli, using the self-assembling process.16, 31 For example, primary bovine articular chondrocytes formed hyaline-like cartilage with biochemical and biomechanical values within the range of native articular cartilage.30 Another promising stem cell type, dermis-isolated adult stem (DIAS) cells, was used to produce fibrocartilage that expressed cartilage-specific genes, such as cartilage oligomeric matrix protein (COMP), collagen type I and II, and Sox9.72 Using a 50:50 co-culture of bovine articular and meniscus cells, meniscus and TMJ shape-specific neocartilages with ECM composition and organization similar to native tissue were produced.29,50 The adaptability and reproducibility of the self-assembling process to produce tissues with physiologically relevant properties makes it a highly promising TE method.
The self-assembling process, while successful in producing functional neocartilages, presents some drawbacks. Cells must be amenable to producing large amounts of ECM and survive minimal cell-substrate interactions as the ECM accumulates during the initial phase of the self-assembling process. Furthermore, the self-assembling process requires cell numbers on the order of millions of cells per construct, e.g., 10–100 million cells per mL.35 To achieve the high cell numbers required to produce the whole tissue ECM, monolayer expansion and subsequent redifferentiation via rotational culture may be necessary.35 As with other tissue formation techniques, the size of the engineered tissue is limited by diffusion.46 The self-assembling method recapitulates developmental processes while allowing for control of tissue geometry to create tissues with biologically reminiscent properties.63
5. CELLS USED FOR SCAFFOLD-FREE TISSUE ENGINEERING
As cells impart the material properties of scaffold-free cartilages through the production of the entire ECM, identifying suitable cell sources is of paramount importance. Ideally, cells used for musculoskeletal cartilage TE would be fully chondrogenically differentiated, be capable of producing cartilaginous ECM, and not provoke adverse immune responses. Additionally, scaffold-free TE typically requires large numbers of cells. To satisfy these needs, a plethora of cell sources have been explored for musculoskeletal cartilage TE, including autologous, allogeneic, and xenogeneic sources of primary, expanded, and stem/progenitor cells.
5.1 Cell Sources: Autologous, Allogeneic, Xenogeneic
Autologous cells exhibit many advantages, such as the fact that they are derived from the same individual receiving the engineered tissue and therefore do not provoke an adverse immune response. However, as these cells are scarce and their isolation is invasive and associated with donor site morbidity, these cells often require expansion before use in TE. Using autologous cells also requires that patients undergo multiple surgical procedures and time delays for the cartilage to be engineered and implanted. TE processes must also be robust to overcome patient-to-patient cellular variability. For example, systems must not be overly sensitive to factors such as gender or donor so as to produce tissues of comparable quality.49, 81, 83 Due to these limitations associated with the use of autologous cells, it is necessary to investigate other cell sources.
The use of allogeneic cells for TE mitigates cell sourcing drawbacks, such as scarcity, patient donor site morbidity, multiple patient operations, and long wait times. Because allogeneic cells are isolated from non-patient sources, a larger amount of healthy, uninjured tissue is available from which to isolate the necessary numbers of cells. Cells from non-diseased or juvenile sources may be used to more consistently form or even pre-engineer high-quality “off the shelf” replacement cartilages, therefore eliminating the need for multiple patient operations and time delays before tissue implantation.2, 66 Using allogeneic cells is common in musculoskeletal TE,10 and is the basis of several products in clinical trials (as discussed in Section 4). For example, cartilage formed by pelleting allogeneic chondrocytes was implanted into 3mm rabbit femoral condyle osteochondral defects. The implanted pellets enhanced early cartilage repair and no immune rejection was observed.12 However, allogeneic cells are still limited by the availability of donors with healthy musculoskeletal cartilages, the possibility of disease transfer from the donor to the recipient, and immunological concerns of rejection.8 The use of cells from multiple allogeneic donors also necessitates engineering processes again be robust enough to overcome cellular variability between donors. The use of allogeneic cells overcomes many restrictions associated with autologous cells; however their use is still limited by availability.
Xenogeneic cell sources overcome the limited availability of healthy donor tissue. However, TE processes are not directly applicable across species and necessitate species-specific adaptations. For example, there are interspecies differences with regard to ECM synthesis after monolayer expansion of equine, ovine, porcine, and human chondrocytes.74 Additionally disease transfer9 and mixed results with regard to adverse immune responses remain concerns.71 While there is evidence to support that cartilage offers sufficient immunoprivilege to allow the use of allogeneic cells, this may not hold true for xenogeneic cells. For example, when adult rabbit osteochondral defects were treated with pig chondrocytes, the repair tissue resulted in the production of hyaline-like tissue with the absence of inflammatory cells.68 However, implanted porcine and bovine articular cartilage in monkey suprapatellar pouches elicited an extensive humoral response to the xenografts, leading to chronic graft rejection.78 Although xenogeneic cell sources may have limited clinical use, they are critical for the in vitro development of translatable musculoskeletal TE processes.
5.2 Cell Types: Primary, Expanded, and Stem
Primary cells are non-expanded, fully differentiated, and readily produce tissue-specific ECM, especially in 3D cultures that replicate their native environment.48 Although neocartilages of primary cells can achieve biochemical and biomechanical values within the range of native tissues,30 these cells are highly limited in availability.
To obtain sufficient cell numbers and overcome limitations in the availability of healthy donor tissue, primary cells are often expanded. As expansion allows one donor to provide tissue for multiple recipients, donor variation, the risk of disease transfer, and the impact of healthy donor tissue scarcity are greatly reduced. Expansion of fully differentiated cells in monolayer causes dedifferentiation and therefore requires a redifferentiation step and phenotypic verification before TE culture (previously described in Section 4.2).33, 54, 59 It was previously thought that passaged cells would irreversibly lose their chondrogenic phenotype and form neocartilage with inferior functional properties.14 Recently however, with the advent of redifferentiation protocols, it was shown that these conditions result in neocartilage with properties as good or better than neocartilage made with primary cells.40, 47, 53 For example, the redifferentiation of leporine articular chondrocytes led to engineered neocartilages containing twice the GAG per wet weight and collagen II/collagen I ratio compared to neocartilages made with primary chondrocytes.35 Therefore, proper expansion and three-dimensional redifferentiation conditions may be applied to engineer neocartilages from passaged cells with functional properties that exceed those of primary cells. Using a system of expansion and redifferentiation may also allow the proliferation and phenotypic modulation of cartilaginous sources that do not suffer joint pathologies, such as costochondral, auricular, or nasal, to be used for musculoskeletal cartilage TE.11, 59, 64 The use of expanded cells, especially those of autologous or allogeneic origin, minimizes the limitations associated with the scarcity of healthy cells, while maintaining clinical translatability of engineered cartilage.
Embryonic stem cells (ESCs) and adult stem cells provide distinct advantages in TE. ESCs, pluripotent cells with unlimited proliferative capacity,38 may be used to create cell lines that allow for the minimization of biological variability. While this cell source has been used to produce scaffold-free cartilage, obstacles to clinical translation, such as difficult and poorly controlled chondrogenic differentiation,42 teratoma formation, and ethical concerns remain. Adult stem cells, both induced pluripotent stem cells (iPSCs) and natively residing stem cells, such as MSCs and DIAS cells, avoid ethical concerns and may be isolated from tissues such as fat or skin, minimizing donor site morbidity. However, the extensive cell manipulation required to chondrogenically differentiate iPSCs represents a significant scientific and regulatory hurdle that needs to be addressed before clinical application.4 MSCs from adipose tissue and bone marrow can also be chondrogenically differentiated and used to form neocartilage,58 but generally produce tissue of poorer quality than neocartilage made of chondrocytes.22 Progenitor cells isolated from cartilage, while not yet tested in scaffold-free culture, may also hold promise as an autologous cell source.84 While ESCs can proliferate indefinitely without losing pluripotency,56 long-term culture of adult stem cells can lead to reduction of proliferative capacity and multipotency.82 Stem cells from any source must be stably differentiated into the chondrocytic phenotype and must retain this phenotype indefinitely.37, 42 Despite limitations, stem cells represent a potentially autologous and clinically relevant population of cells with which to engineer musculoskeletal cartilages.
6. SIGNALS ENHANCING ENGINEERED CARTILAGE FUNCTIONAL PROPERTIES
Implanted scaffold-free engineered musculoskeletal cartilages must replicate the durability and function of native tissue. Without an exogenous scaffold, the material properties of TE cartilages are dependent solely on cell-produced ECM. This motivates investigation into methods to improve matrix production and quality, which ultimately improves neotissue functional properties.27 Three categories of matrix-enhancing signals shown to have significant outcomes on the functional properties of neocartilages have emerged: growth factors, matrix-remodeling enzymes, and mechanical stimulation. These types of stimuli have also been combined, resulting in additive and synergistic enhancement of neocartilage properties.18, 32
Growth factors of the transforming growth factor (TGF) superfamily are important to native cartilage tissue development and have been shown to enhance matrix properties of neocartilages.4 For example, a combination of bone morphogenetic protein 2 (BMP-2) and insulin-like growth factor 1 (IGF-1) was found to double neocartilage glycosaminoglycan (GAG) content and aggregate modulus.19 As treatment results in doubling collagen content, aggregate modulus, and tensile modulus,19 TGF-β1 is one of the most common growth factors used to enhance the functional properties of scaffold-free neocartilages.
Enzymes are also effective to enhance the functional properties of neocartilages. For example, chondroitinase-ABC (C-ABC) digests GAGs to remove the effect of their steric hindrance on collagen assembly and packing.69 A single 4 hour C-ABC treatment at 2 weeks in a 4 week culture of neocartilage resulted an increase in tensile modulus by 80%.61 Lysyl-oxidase (LOX) has also emerged as a potent matrix-remodeling enzyme that initiates fibril crosslinking.76 Hypoxia has been employed to enhance LOX expression, resulting in a 2-fold increase in the tensile modulus in neocartilage.51 Exogenous addition of LOX and C-ABC resulted in a greater than 200% increase in the tensile modulus and ultimate tensile strength (UTS) of neocartilage.52 Though TE has traditionally focused on the use of growth factors to increase ECM accumulation, enzymes may be used to mimic native tissue remodeling or activate signaling pathways that enhance matrix properties,69 making them a potent tool to enhance neocartilage functional properties.
Musculoskeletal cartilages are mechanically sensitive,44 rendering mechanical forces effective TE stimuli. Dynamic tension-compression with an axial strain of 10% at 1Hz applied to scaffold-free meniscus-shaped neotissue during days 10–14 of culture increased collagen content by 80%, compressive relaxation modulus by 66%, and radial tensile properties by 200%.32 Application of hydrostatic pressure at a static 10MPa for 1 hour at days 10–14 of culture increased aggregate modulus 1.4-fold, tensile modulus 1.9-fold, and GAG and collagen content over 2-fold.20 As native musculoskeletal cartilages experience combinations of dynamic compression, tension, shear, sliding shear, and hydrostatic pressure, commencing in utero and extending throughout life,70 the application of mechanical stimuli that parallel forces natively present can serve to enhance neocartilage functional properties.
Native musculoskeletal cartilages experience a mélange of stimuli which inform TE strategies. Combinations of signals can have additive and even synergistic effects on ECM production and composition, increasing neocartilage functional properties. For example, TGF-β1 and hydrostatic pressure increased neocartilage aggregate modulus by 1.6-fold and tensile modulus by 2.3-fold. It also synergistically increased collagen content.18 TGF-β1, C-ABC, and dynamic direct compression increased the compressive and tensile modulus 3–4-fold while also increasing collagen content nearly 4-fold of meniscus-shaped co-cultures of chondrocytes and fibrochondrocytes.31–32 Combinatorial treatments inspired by the multitude of stimuli native cartilages encounter may enable the creation of biomimetic tissues.
7. FUTURE DIRECTIONS: CREATING HIGHER ORDER STRUCTURES, CONCLUSIONS
To engineer complicated musculoskeletal cartilages, design criteria based on native tissue properties must be identified, and quantitative methods must be employed to determine the outcome of TE efforts. Following structure-function relationships, musculoskeletal cartilages have distinct anatomical shape, internal structure and organization, matrix content, and biomechanical properties. Since the objective is to achieve biomimicry, the heterogeneous and anisotropic nature of musculoskeletal cartilages must be understood and then replicated.
Engineering musculoskeletal cartilages to have patient-specific anatomical shapes is necessary to ensure the implanted tissue fits correctly within the adjacent native tissue, meets mechanical demands, and that stress concentrations are minimized to avoid tissue degradation.27, 36 Using cell sheet engineering methods, shaped structures may be created by layering, rolling, or draping cell sheets (as described in Section 4.1, Figure 3). Alternatively, aggregates or cell pellets may be fused within molds to form larger shaped structures (as described in Section 4.2, Figure 4). For example, expanded annulus fibrosus cells were pelleted and cultured to form cylindrical tissues that displayed morphological characteristics similar to immature native annulus fibrosus tissue.13 To form shape-specific self-assembling tissues, cells may be seeded into shaped, non-adherent molds (as described in Section 4.3, Figure 5). Mold compliance, mold surface characteristics, and degree of confinement have been used to engineer shape-specific meniscus and TMJ disc.30, 50 Despite these advancements, further investigation should examine other methods and stimuli to enhance neocartilage fidelity, in terms of both shape and size.
Musculoskeletal cartilages exhibit cellular and matrix anisotropy and heterogeneity that must be recapitulated in engineered tissues.67 Manipulating tissue shape can be used to create the zonal GAG and collagen organization that result in functional anisotropy.50, 67 Other methods have been adapted from scaffold-based approaches. For example, primary chondrocytes originating from different zones in articular cartilage were seeded in layers in an agarose hydrogel and the resulting neocartilage replicated zonal biochemical and cellular organization.62 Applying this to scaffold-free approaches, zone-specific chondrocytes were sequentially seeded into shape-specific molds to form self-assembling neocartilage, retaining cellular zonal organization and morphology.28 Combining scaffold-free TE methods, signals that enhance ECM production and organization, and strategies to produce specific shapes and tissue organizations may achieve directional and organizational biomimicry.
Neocartilage must be quantitatively evaluated to determine if the design criteria have been achieved. Assessment modalities should measure relevant structure-function relationships with a heavy focus on biomechanical properties due to their importance in tissue function.4 While histology and immunohistochemistry can show presence or spatial distribution of specific matrix components, this technique is still semi-quantitative. Quantitative biochemical assays that evaluate structure-function include 1) assessments for matrix molecules, such as GAGs and collagen, 2) enzyme-linked immunosorbent assays (ELISAs) for collagen type I and type II content, and 3) quantitative real time polymerase chain reaction (qRT-PCR) for cartilage-associated genes, such as aggrecan, COMP, sox9, collagen type I, and collagen type II. It should be noted that expression of cartilaginous genes does not always correlate to the production of the matrix-associated proteins. Likewise matrix production does not always correlate to organization. Therefore it is equally important to assess biomechanical properties. These include tensile modulus, UTS, aggregate modulus, permeability, shear modulus, viscoelastic moduli and coefficient of viscosity, as well as coefficient of friction. Therefore, particular attention should be paid to measuring properties and characteristics that will elucidate structure-function relationships and further guide researchers in selecting appropriate stimuli to achieve design criteria.
Future success in engineering musculoskeletal cartilages and higher order structures will likely require methods that involve both scaffold-free and scaffold-based approaches. For example, to achieve satisfactory regeneration of articular cartilage, it may be necessary to achieve regeneration of the underlying subchondral bone simultaneously.24 Currently, most bone regeneration efforts employ scaffolds. Thus, toward achieving regeneration in an osteochondral defect, or the entire articulating end of a long bone, it may be necessary to combine both scaffold-free cartilage and scaffold-based bone tissues. For example, deep zone chondrocytes were seeded onto a porous calcium polyphosphate scaffold and cultured in β-glycerophosphate to induce a zone of calcified cartilage between the ceramic and hyaline-like cartilage.3 Other than zone-specific cell sources, few zone-specific factors have been exogenously applied to engineer distinct tissue zones. This remains an active area of research for both scaffold-based and scaffold-free tissue engineering approaches.41 Overall, scaffold-based and scaffold-free approaches to musculoskeletal cartilage TE present distinct advantages and outcomes; future successes will likely depend on the combination of these approaches, rather than the exclusive use of either. Inasmuch as scaffold-based approaches have been employed as a paradigm to generate engineered cartilages with appropriate functional properties, scaffold-free approaches are emerging as promising elements of a clinical translational pathway not only for musculoskeletal cartilages but for other tissues as well.
Acknowledgments
The authors would like to acknowledge support by the NIH (R01DE019666, R01DE015038, R01AR061496), the California Institute for Regenerative Medicine (TR3-05709), and the Arthritis Foundation (Postdoctoral Fellowship for G. DuRaine).
Footnotes
The authors have no conflicts of interest.
No financial support or benefits from commercial sources were received.
References
- 1.Adkisson HD, Gillis MP, Davis EC, Maloney W, Hruska KA. In vitro generation of scaffold independent neocartilage. Clinical orthopaedics and related research. 2001:S280–294. doi: 10.1097/00003086-200110001-00026. [DOI] [PubMed] [Google Scholar]
- 2.Adkisson HDt, Martin JA, Amendola RL, Milliman C, Mauch KA, Katwal AB, Seyedin M, Amendola A, Streeter PR, Buckwalter JA. The potential of human allogeneic juvenile chondrocytes for restoration of articular cartilage. Am J Sports Med. 2010;38:1324–1333. doi: 10.1177/0363546510361950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allan KS, Pilliar RM, Wang J, Grynpas MD, Kandel RA. Formation of biphasic constructs containing cartilage with a calcified zone interface. Tissue engineering. 2007;13:167–177. doi: 10.1089/ten.2006.0081. [DOI] [PubMed] [Google Scholar]
- 4.Athanasiou KA. Articular cartilage. Boca Raton, FL: CRC Press/Taylor & Francis; 2013. [Google Scholar]
- 5.Athanasiou KA, Eswaramoorthy R, Hadidi P, Hu JC. Self-organization and the self-assembling process in tissue engineering. Annu Rev Biomed Eng. 2013;15:115–136. doi: 10.1146/annurev-bioeng-071812-152423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babur BK, Ghanavi P, Levett P, Lott WB, Klein T, Cooper-White JJ, Crawford R, Doran MR. The interplay between chondrocyte redifferentiation pellet size and oxygen concentration. PloS one. 2013;8:e58865. doi: 10.1371/journal.pone.0058865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhumiratana S, Eton RE, Oungoulian SR, Wan LQ, Ateshian GA, Vunjak-Novakovic G. Large, stratified, and mechanically functional human cartilage grown in vitro by mesenchymal condensation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:6940–6945. doi: 10.1073/pnas.1324050111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolano L, Kopta JA. The immunology of bone and cartilage transplantation. Orthopedics. 1991;14:987–996. doi: 10.3928/0147-7447-19910901-10. [DOI] [PubMed] [Google Scholar]
- 9.Boneva RS, Folks TM. Xenotransplantation and risks of zoonotic infections. Ann Med. 2004;36:504–517. doi: 10.1080/07853890410018826. [DOI] [PubMed] [Google Scholar]
- 10.Brenner JM, Ventura NM, Tse MY, Winterborn A, Bardana DD, Pang SC, Hurtig MB, Waldman SD. Implantation of scaffold-free engineered cartilage constructs in a rabbit model for chondral resurfacing. Artificial organs. 2014;38:E21–32. doi: 10.1111/aor.12199. [DOI] [PubMed] [Google Scholar]
- 11.Candrian C, Vonwil D, Barbero A, Bonacina E, Miot S, Farhadi J, Wirz D, Dickinson S, Hollander A, Jakob M, Li Z, Alini M, Heberer M, Martin I. Engineered cartilage generated by nasal chondrocytes is responsive to physical forces resembling joint loading. Arthritis Rheum. 2008;58:197–208. doi: 10.1002/art.23155. [DOI] [PubMed] [Google Scholar]
- 12.Cheuk YC, Wong MW, Lee KM, Fu SC. Use of allogeneic scaffold-free chondrocyte pellet in repair of osteochondral defect in a rabbit model. J Orthop Res. 2011;29:1343–1350. doi: 10.1002/jor.21339. [DOI] [PubMed] [Google Scholar]
- 13.Cho H, Park SH, Park K, Shim JW, Huang J, Smith R, Elder S, Min BH, Hasty KA. Construction of a tissue-engineered annulus fibrosus. Artificial organs. 2013;37:E131–138. doi: 10.1111/aor.12066. [DOI] [PubMed] [Google Scholar]
- 14.Darling EM, Athanasiou KA. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J Orthop Res. 2005;23:425–432. doi: 10.1016/j.orthres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Darling EM, Pritchett PE, Evans BA, Superfine R, Zauscher S, Guilak F. Mechanical properties and gene expression of chondrocytes on micropatterned substrates following dedifferentiation in monolayer. Cell Mol Bioeng. 2009;2:395–404. doi: 10.1007/s12195-009-0077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Detamore MS, Athanasiou KA. Evaluation of three growth factors for tmj disc tissue engineering. Annals of biomedical engineering. 2005;33:383–390. doi: 10.1007/s10439-005-1741-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebihara G, Sato M, Yamato M, Mitani G, Kutsuna T, Nagai T, Ito S, Ukai T, Kobayashi M, Kokubo M, Okano T, Mochida J. Cartilage repair in transplanted scaffold-free chondrocyte sheets using a minipig model. Biomaterials. 2012;33:3846–3851. doi: 10.1016/j.biomaterials.2012.01.056. [DOI] [PubMed] [Google Scholar]
- 18.Elder BD, Athanasiou KA. Synergistic and additive effects of hydrostatic pressure and growth factors on tissue formation. PloS one. 2008;3:e2341. doi: 10.1371/journal.pone.0002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elder BD, Athanasiou KA. Osteoarthritis and cartilage/OARS. Osteoarthritis Research Society; 2008. Systematic assessment of growth factor treatment on biochemical and biomechanical properties of engineered articular cartilage constructs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elder BD, Athanasiou KA. Effects of temporal hydrostatic pressure on tissue-engineered bovine articular cartilage constructs. Tissue Eng Part A. 2009;15:1151–1158. doi: 10.1089/ten.tea.2008.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elloumi-Hannachi I, Yamato M, Okano T. Cell sheet engineering: A unique nanotechnology for scaffold-free tissue reconstruction with clinical applications in regenerative medicine. Journal of Internal Medicine. 2010;267:54–70. doi: 10.1111/j.1365-2796.2009.02185.x. [DOI] [PubMed] [Google Scholar]
- 22.Erickson IE, Huang AH, Chung C, Li RT, Burdick JA, Mauck RL. Differential maturation and structure-function relationships in mesenchymal stem cell- and chondrocyte-seeded hydrogels. Tissue Eng Part A. 2009;15:1041–1052. doi: 10.1089/ten.tea.2008.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fickert S, Gerwien P, Helmert B, Schattenberg T, Weckbach S, Kaszkin-Bettag M, Lehmann L. One-year clinical and radiological results of a prospective, investigator-initiated trial examining a novel, purely autologous 3-dimensional autologous chondrocyte transplantation product in the knee. Cartilage. 2012;3 doi: 10.1177/1947603511417616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frenkel SR, Bradica G, Brekke JH, Goldman SM, Ieska K, Issack P, Bong MR, Tian H, Gokhale J, Coutts RD, Kronengold RT. Regeneration of articular cartilage--evaluation of osteochondral defect repair in the rabbit using multiphasic implants. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2005;13:798–807. doi: 10.1016/j.joca.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 25.Furukawa KS, Suenaga H, Toita K, Numata A, Tanaka J, Ushida T, Sakai Y, Tateishi T. Rapid and large-scale formation of chondrocyte aggregates by rotational culture. Cell transplantation. 2003;12:475–479. doi: 10.3727/000000003108747037. [DOI] [PubMed] [Google Scholar]
- 26.Gigout A, Buschmann MD, Jolicoeur M. Chondrocytes cultured in stirred suspension with serum-free medium containing pluronic-68 aggregate and proliferate while maintaining their differentiated phenotype. Tissue engineering. Part A. 2009;15:2237–2248. doi: 10.1089/ten.tea.2008.0256. [DOI] [PubMed] [Google Scholar]
- 27.Guilak F, Butler DL, Goldstein SA, Baaijens FP. Biomechanics and mechanobiology in functional tissue engineering. J Biomech. 2014;47:1933–1940. doi: 10.1016/j.jbiomech.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han EH, Bae WC, Hsieh-Bonassera ND, Wong VW, Schumacher BL, Gortz S, Masuda K, Bugbee WD, Sah RL. Shaped, stratified, scaffold-free grafts for articular cartilage defects. Clinical orthopaedics and related research. 2008;466:1912–1920. doi: 10.1007/s11999-008-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoben GM, Hu JC, James RA, Athanasiou KA. Self-assembly of fibrochondrocytes and chondrocytes for tissue engineering of the knee meniscus. Tissue engineering. 2007;13:939–946. doi: 10.1089/ten.2006.0116. [DOI] [PubMed] [Google Scholar]
- 30.Hu JC, Athanasiou KA. A self-assembling process in articular cartilage tissue engineering. Tissue engineering. 2006;12:969–979. doi: 10.1089/ten.2006.12.969. [DOI] [PubMed] [Google Scholar]
- 31.Huey DJ, Athanasiou KA. Maturational growth of self-assembled, functional menisci as a result of tgf-beta1 and enzymatic chondroitinase-abc stimulation. Biomaterials. 2011;32:2052–2058. doi: 10.1016/j.biomaterials.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huey DJ, Athanasiou KA. Tension-compression loading with chemical stimulation results in additive increases to functional properties of anatomic meniscal constructs. PloS one. 2011;6:e27857. doi: 10.1371/journal.pone.0027857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huey DJ, Athanasiou KA. Alteration of the fibrocartilaginous nature of scaffoldless constructs formed from leporine meniscus cells and chondrocytes through manipulation of culture and processing conditions. Cells, tissues, organs. 2013;197:360–371. doi: 10.1159/000346252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338:917–921. doi: 10.1126/science.1222454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huey DJ, Hu JC, Athanasiou KA. Chondrogenically tuned expansion enhances the cartilaginous matrix-forming capabilities of primary, adult, leporine chondrocytes. Cell transplantation. 2013;22:331–340. doi: 10.3727/096368912X657648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hung CT, Mauck RL, Wang CC, Lima EG, Ateshian GA. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Annals of biomedical engineering. 2004;32:35–49. doi: 10.1023/b:abme.0000007789.99565.42. [DOI] [PubMed] [Google Scholar]
- 37.Hwang NS, Elisseeff J. Application of stem cells for articular cartilage regeneration. The journal of knee surgery. 2009;22:60–71. doi: 10.1055/s-0030-1247728. [DOI] [PubMed] [Google Scholar]
- 38.Hwang NS, Varghese S, Elisseeff J. Derivation of chondrogenically-committed cells from human embryonic cells for cartilage tissue regeneration. PLoS One. 2008;3:e2498. doi: 10.1371/journal.pone.0002498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imabayashi H, Mori T, Gojo S, Kiyono T, Sugiyama T, Irie R, Isogai T, Hata J, Toyama Y, Umezawa A. Redifferentiation of dedifferentiated chondrocytes and chondrogenesis of human bone marrow stromal cells via chondrosphere formation with expression profiling by large-scale cdna analysis. Exp Cell Res. 2003;288:35–50. doi: 10.1016/s0014-4827(03)00130-7. [DOI] [PubMed] [Google Scholar]
- 40.Jakob M, Demarteau O, Schafer D, Hintermann B, Dick W, Heberer M, Martin I. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J Cell Biochem. 2001;81:368–377. doi: 10.1002/1097-4644(20010501)81:2<368::aid-jcb1051>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 41.Klein TJ, Malda J, Sah RL, Hutmacher DW. Tissue engineering of articular cartilage with biomimetic zones. Tissue Eng Part B Rev. 2009;15:143–157. doi: 10.1089/ten.teb.2008.0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koay EJ, Hoben GM, Athanasiou KA. Tissue engineering with chondrogenically differentiated human embryonic stem cells. Stem Cells. 2007;25:2183–2190. doi: 10.1634/stemcells.2007-0105. [DOI] [PubMed] [Google Scholar]
- 43.Kolettas E, Buluwela L, Bayliss MT, Muir HI. Expression of cartilage-specific molecules is retained on long-term culture of human articular chondrocytes. J Cell Sci. 1995;108(Pt 5):1991–1999. doi: 10.1242/jcs.108.5.1991. [DOI] [PubMed] [Google Scholar]
- 44.Koo S, Andriacchi TP. A comparison of the influence of global functional loads vs. Local contact anatomy on articular cartilage thickness at the knee. J Biomech. 2007;40:2961–2966. doi: 10.1016/j.jbiomech.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kushida A, Yamato M, Konno C, Kikuchi A, Sakurai Y, Okano T. Decrease in culture temperature releases monolayer endothelial cell sheets together with deposited fibronectin matrix from temperature-responsive culture surfaces. Journal of biomedical materials research. 1999;45:355–362. doi: 10.1002/(sici)1097-4636(19990615)45:4<355::aid-jbm10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 46.Leddy HA, Awad HA, Guilak F. Molecular diffusion in tissue-engineered cartilage constructs: Effects of scaffold material, time, and culture conditions. Journal of biomedical materials research. Part B, Applied biomaterials. 2004;70:397–406. doi: 10.1002/jbm.b.30053. [DOI] [PubMed] [Google Scholar]
- 47.Lin L, Zhou C, Wei X, Hou Y, Zhao L, Fu X, Zhang J, Yu C. Articular cartilage repair using dedifferentiated articular chondrocytes and bone morphogenetic protein 4 in a rabbit model of articular cartilage defects. Arthritis Rheum. 2008;58:1067–1075. doi: 10.1002/art.23380. [DOI] [PubMed] [Google Scholar]
- 48.Lin Z, Willers C, Xu J, Zheng MH. The chondrocyte: Biology and clinical application. Tissue engineering. 2006;12:1971–1984. doi: 10.1089/ten.2006.12.1971. [DOI] [PubMed] [Google Scholar]
- 49.Lotz M, Loeser RF. Effects of aging on articular cartilage homeostasis. Bone. 2012;51:241–248. doi: 10.1016/j.bone.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.MacBarb RF, Chen AL, Hu JC, Athanasiou KA. Engineering functional anisotropy in fibrocartilage neotissues. Biomaterials. 2013;34:9980–9989. doi: 10.1016/j.biomaterials.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Makris EA, Hu JC, Athanasiou KA. Hypoxia-induced collagen crosslinking as a mechanism for enhancing mechanical properties of engineered articular cartilage. Osteoarthritis Cartilage. 2013;21:634–641. doi: 10.1016/j.joca.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Makris EA, MacBarb RF, Paschos NK, Hu JC, Athanasiou KA. Combined use of chondroitinase-abc, tgf-beta1, and collagen crosslinking agent lysyl oxidase to engineer functional neotissues for fibrocartilage repair. Biomaterials. 2014;35:6787–6796. doi: 10.1016/j.biomaterials.2014.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin I, Suetterlin R, Baschong W, Heberer M, Vunjak-Novakovic G, Freed LE. Enhanced cartilage tissue engineering by sequential exposure of chondrocytes to fgf-2 during 2d expansion and bmp-2 during 3d cultivation. J Cell Biochem. 2001;83:121–128. doi: 10.1002/jcb.1203. [DOI] [PubMed] [Google Scholar]
- 54.Masuda K, Sah RL, Hejna MJ, Thonar EJ. A novel two-step method for the formation of tissue-engineered cartilage by mature bovine chondrocytes: The alginate-recovered-chondrocyte (arc) method. J Orthop Res. 2003;21:139–148. doi: 10.1016/S0736-0266(02)00109-2. [DOI] [PubMed] [Google Scholar]
- 55.McCormick F, Cole BJ, Nwachukwu B, Harris JD, Adkisson HDIV, Farr J. Treatment of focal cartilage defects with a juvenile allogeneic 3-dimensional articular cartilage graft. Operative Techniques in Sports Medicine. 21:95–99. [Google Scholar]
- 56.Mitalipov S, Wolf D. Totipotency, pluripotency and nuclear reprogramming. Advances in biochemical engineering/biotechnology. 2009;114:185–199. doi: 10.1007/10_2008_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitani G, Sato M, Lee JI, Kaneshiro N, Ishihara M, Ota N, Kokubo M, Sakai H, Kikuchi T, Mochida J. The properties of bioengineered chondrocyte sheets for cartilage regeneration. BMC Biotechnol. 2009;9:17. doi: 10.1186/1472-6750-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mueller MB, Tuan RS. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 2008;58:1377–1388. doi: 10.1002/art.23370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murphy MK, DuRaine GD, Reddi A, Hu JC, Athanasiou KA. Inducing articular cartilage phenotype in costochondral cells. Arthritis research & therapy. 2013;15:R214. doi: 10.1186/ar4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murphy MK, Masters TE, Hu JC, Athanasiou KA. Engineering a fibrocartilage spectrum through modulation of aggregate redifferentiation. Cell transplantation. 2013 doi: 10.3727/096368913X676204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Natoli RM, Revell CM, Athanasiou KA. Chondroitinase abc treatment results in greater tensile properties of self-assembled tissue-engineered articular cartilage. Tissue Eng Part A. 2009;15:3119–3128. doi: 10.1089/ten.tea.2008.0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ng KW, Ateshian GA, Hung CT. Zonal chondrocytes seeded in a layered agarose hydrogel create engineered cartilage with depth-dependent cellular and mechanical inhomogeneity. Tissue engineering. Part A. 2009;15:2315–2324. doi: 10.1089/ten.tea.2008.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ofek G, Revell CM, Hu JC, Allison DD, Grande-Allen KJ, Athanasiou KA. Matrix development in self-assembly of articular cartilage. PloS one. 2008;3:e2795. doi: 10.1371/journal.pone.0002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park SS, Jin HR, Chi DH, Taylor RS. Characteristics of tissue-engineered cartilage from human auricular chondrocytes. Biomaterials. 2004;25:2363–2369. doi: 10.1016/j.biomaterials.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 65.Perez-Pomares JM, Foty RA. Tissue fusion and cell sorting in embryonic development and disease: Biomedical implications. Bioessays. 2006;28:809–821. doi: 10.1002/bies.20442. [DOI] [PubMed] [Google Scholar]
- 66.Pestka JM, Schmal H, Salzmann G, Hecky J, Sudkamp NP, Niemeyer P. In vitro cell quality of articular chondrocytes assigned for autologous implantation in dependence of specific patient characteristics. Archives of orthopaedic and trauma surgery. 2011;131:779–789. doi: 10.1007/s00402-010-1219-8. [DOI] [PubMed] [Google Scholar]
- 67.Poole AR, Kojima T, Yasuda T, Mwale F, Kobayashi M, Laverty S. Composition and structure of articular cartilage: A template for tissue repair. Clinical orthopaedics and related research. 2001:S26–33. doi: 10.1097/00003086-200110001-00004. [DOI] [PubMed] [Google Scholar]
- 68.Ramallal M, Maneiro E, Lopez E, Fuentes-Boquete I, Lopez-Armada MJ, Fernandez-Sueiro JL, Galdo F, De Toro FJ, Blanco FJ. Xeno-implantation of pig chondrocytes into rabbit to treat localized articular cartilage defects: An animal model. Wound Repair Regen. 2004;12:337–345. doi: 10.1111/j.1067-1927.2004.012309.x. [DOI] [PubMed] [Google Scholar]
- 69.Responte DJ, Arzi B, Natoli RM, Hu JC, Athanasiou KA. Mechanisms underlying the synergistic enhancement of self-assembled neocartilage treated with chondroitinase-abc and tgf-beta1. Biomaterials. 2012;33:3187–3194. doi: 10.1016/j.biomaterials.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Responte DJ, Lee JK, Hu JC, Athanasiou KA. Biomechanics-driven chondrogenesis: From embryo to adult. FASEB J. 2012;26:3614–3624. doi: 10.1096/fj.12-207241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Revell CM, Athanasiou KA. Success rates and immunologic responses of autogenic, allogenic, and xenogenic treatments to repair articular cartilage defects. Tissue Eng Part B Rev. 2009;15:1–15. doi: 10.1089/ten.teb.2008.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sanchez-Adams J, Athanasiou KA. Dermis isolated adult stem cells for cartilage tissue engineering. Biomaterials. 2012;33:109–119. doi: 10.1016/j.biomaterials.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 73.Sato M, Yamato M, Hamahashi K, Okano T, Mochida J. Articular cartilage regeneration using cell sheet technology. Anat Rec (Hoboken) 2014;297:36–43. doi: 10.1002/ar.22829. [DOI] [PubMed] [Google Scholar]
- 74.Schulze-Tanzil G, Muller RD, Kohl B, Schneider N, Ertel W, Ipaktchi K, Hunigen H, Gemeinhardt O, Stark R, John T. Differing in vitro biology of equine, ovine, porcine and human articular chondrocytes derived from the knee joint: An immunomorphological study. Histochem Cell Biol. 2009;131:219–229. doi: 10.1007/s00418-008-0516-6. [DOI] [PubMed] [Google Scholar]
- 75.Shimizu T, Sekine H, Yamato M, Okano T. Cell sheet-based myocardial tissue engineering: New hope for damaged heart rescue. Curr Pharm Des. 2009;15:2807–2814. doi: 10.2174/138161209788923822. [DOI] [PubMed] [Google Scholar]
- 76.Siegel RC, Pinnell SR, Martin GR. Cross-linking of collagen and elastin. Properties of lysyl oxidase. Biochemistry. 1970;9:4486–4492. doi: 10.1021/bi00825a004. [DOI] [PubMed] [Google Scholar]
- 77.Steinberg MS. Differential adhesion in morphogenesis: A modern view. Curr Opin Genet Dev. 2007;17:281–286. doi: 10.1016/j.gde.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 78.Stone KR, Walgenbach AW, Abrams JT, Nelson J, Gillett N, Galili U. Porcine and bovine cartilage transplants in cynomolgus monkey: I. A model for chronic xenograft rejection. Transplantation. 1997;63:640–645. doi: 10.1097/00007890-199703150-00005. [DOI] [PubMed] [Google Scholar]
- 79.Tacchetti C, Quarto R, Nitsch L, Hartmann DJ, Cancedda R. In vitro morphogenesis of chick embryo hypertrophic cartilage. The Journal of cell biology. 1987;105:999–1006. doi: 10.1083/jcb.105.2.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Talab SS, Kajbafzadeh A-M, Elmi A, Tourchi A, Sabetkish S, Sabetkish N, Monajemzadeh M. Bladder reconstruction using scaffold-less autologous smooth muscle cell sheet engineering: Early histological outcomes for autoaugmentation cystoplasty. BJU International. 2014 doi: 10.1111/bju.12685. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 81.Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: Associations with degenerative changes. Arthritis Rheum. 2001;44:585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 82.Vacanti V, Kong E, Suzuki G, Sato K, Canty JM, Lee T. Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture. Journal of cellular physiology. 2005;205:194–201. doi: 10.1002/jcp.20376. [DOI] [PubMed] [Google Scholar]
- 83.van der Kraan PM, Goumans MJ, Blaney Davidson E, ten Dijke P. Age-dependent alteration of tgf-beta signalling in osteoarthritis. Cell Tissue Res. 2012;347:257–265. doi: 10.1007/s00441-011-1194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Williams R, Khan IM, Richardson K, Nelson L, McCarthy HE, Analbelsi T, Singhrao SK, Dowthwaite GP, Jones RE, Baird DM, Lewis H, Roberts S, Shaw HM, Dudhia J, Fairclough J, Briggs T, Archer CW. Identification and clonal characterisation of a progenitor cell sub-population in normal human articular cartilage. PLoS One. 2010;5:e13246. doi: 10.1371/journal.pone.0013246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yamada N, Okano T, Sakai H, Karikusa F, Sawasaki Y, Sakurai Y. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Die Makromolekulare Chemie. Rapid Communications. 1990;11:571–576. [Google Scholar]