Abstract
Objective
To investigate the safety of combining 6-Hz primed low-frequency repetitive transcranial magnetic stimulation (rTMS) intervention in the contralesional hemisphere with a modified constraint-induced movement therapy (mCIMT) program in children with congenital hemiparesis.
Design
Phase 1 randomized, double-blinded, placebo-controlled pretest/posttest trial.
Setting
University academic facility and a pediatric specialty hospital.
Participants
Nineteen subjects aged 8 to 17 years with congenital hemiparesis due to ischemic stroke or periventricular leukomalacia. No subject withdrew due to adverse events. All subjects included completed the study.
Interventions
Subjects were randomized to one of two groups: either rTMSreal with mCIMT (n = 10) or rTMSsham with mCIMT (n = 9).
Main Outcome Measures
Adverse events, physician assessment, ipsilateral hand function, stereognosis, cognitive function, subject report of symptoms assessment and subject questionnaire.
Results
No major adverse events occurred. Minor adverse events were found in both groups. The most common were headaches (real: 50%, sham: 89%, p=0.14) and cast irritation (real: 30%, sham: 44%, p = 0.65). No differences between groups in secondary cognitive and unaffected hand motor measures were found.
Conclusions
Primed rTMS can be used safely with mCIMT in congenital hemiparesis. We provide new information on the use of rTMS in combination with mCIMT in children. These findings could be useful in research and future clinical applications in advancing function in congenital hemiparesis.
Keywords: Hemiparesis, Transcranial Magnetic Stimulation, stroke, safety, pediatrics
The “doubly-disabled” adult brain after unilateral stroke is affected not only by the lesion itself, but also by exaggerated interhemispheric inhibition from the contralesional primary motor area (M1) acting on ipsilesional M1.1 In the child with congenital hemiparesis, a similar inhibition may occur through “developmental disuse,” in which a child predominantly uses the less affected extremities, masking potential function in the affected extremity.2 Low-frequency (inhibitory) contralesional repetitive transcranial magnetic stimulation (rTMS) has shown promising cortical effects by inhibiting the contralesional M1, thereby disinhibiting surviving neurons in ipsilesional M1.3–5 More studies are investigating the use of rTMS as an intervention to restore higher excitability in ipsilesional M1.
Iyer et al (2003) found that the effects of 1 Hz low-frequency stimulation can be enhanced through preceding the low-frequency session with a “priming” 6-Hz high-frequency session.6 The use of 6-Hz priming of low-frequency rTMS to the contralesional hemisphere in children with stroke may work by creating greater disruption of the exaggerated interhemispheric effects of the contralesional hemisphere upon the ipsilesional hemisphere. In an effort to achieve improved outcomes, 6-Hz priming rTMS employed immediately prior to the low-frequency rTMS can be used to capitalize on principles of homeostatic plasticity.7 Homeostatic plasticity encompasses several mechanisms aimed at stabilizing neuronal activity to maintain synaptic specificity and prevent unconstrained synaptic plasticity from predominating in the system.8 Importantly, homeoplastic plasticity depends on the previous history of synaptic activity. 9 Thus, excitatory priming stimulation biases the neural network to seek return to its baseline activity level. In combination then, low-frequency rTMS applied in the facilitated state yields a more pronounced inhibition compared to low-frequency rTMS that is not preceded by high-frequency rTMS.6
Distinct from rTMS, motor learning with use of constraint is an additional intervention with potent effects on brain reorganization.10–12 Modified constraint-induced movement therapy (mCIMT) is defined as <3 hours of therapy per day using the techniques of shaping, repetition and constraint.13 The combining of electrophysiologic and behavioral interventions provides a synergistic approach that may help to maximize the recovery of hand function. Both interventions are aimed at suppression of the exaggerated inhibitory interhemispheric effects, allowing increased contribution from the surviving neuronal networks within the ipsilesional hemisphere.
The important question of safety remains with such interventions. The safety of rTMS has been investigated to a much greater extent in adults with stroke than children, although understanding the risks is paramount for all ages. 14–16 Kirton et al.(2008) demonstrated that 1-Hz low-frequency contralesional M1 rTMS was safe, with no serious adverse events such as seizure, in children with stroke. 17 Although there are reports in adults with brain injury that using 6-Hz priming rTMS with one treatment 18 and multiple treatments19 is safe, primed rTMS has not been explored in children with stroke. Due to the high-frequency nature of the priming and the greater potential risk of adverse events such as seizure, investigating the safety of a 6-Hz primed, 1-Hz low-frequency application of rTMS in combination with motor learning training should be thoroughly investigated.
The purpose of this article is to report on the safety of 6-Hz primed low-frequency rTMS combined with mCIMT specific to children with hemiparesis. We defined safety by physician assessment, cognitive status, a subject report of symptoms and questionnaire. As the rTMS component of the intervention was delivered on the contralesional hemisphere, safety also included assessment of ipsilateral, unaffected, hand function.
METHODS
This study was a randomized, controlled, blinded, pretest-posttest trial comparing active and placebo rTMS in combination with mCIMT in children with congenital hemiparesis. Subjects meeting our criteria were randomized into one of two groups: rTMSreal with mCIMT and rTMSsham with mCIMT. (Figure 1) Specific exclusion and inclusion criteria guided subject enrollment and participation in stages. (Appendix A) For example, during initial screening, we excluded children with disorders of cellular migration and proliferation as exaggerated interhemispheric inhibition may not be present in these non-stroke disorders, and our intervention potentially not applicable. As children with perinatal stroke typically experience seizure within the first 48 hours after birth,20 excluding any history of seizure would yield very few children to study. We limited seizure activity to none more recent than 2 years prior to the study because the study neurologist deemed this seizure-free period as appropriate in regards to safety. At pretest, we obtained a Fluid-Attenuated Inversion Recovery (FLAIR) scan sequence for assessment of the cerebral infarction and a gradient echo scan sequence for evidence of any prior hemorrhage. The study pediatric neurologist reviewed these results for each subject enrolled. Evidence of hemorrhage, and subsequent presence of hemosiderin protein, excluded any subjects as they may have been predisposed to seizure.21,22 We allocated subjects using a random numbers table system. Researchers administering the rTMS interventions were unblinded. Testing researchers, physicians, caregivers and subjects were blinded to treatment allocation.
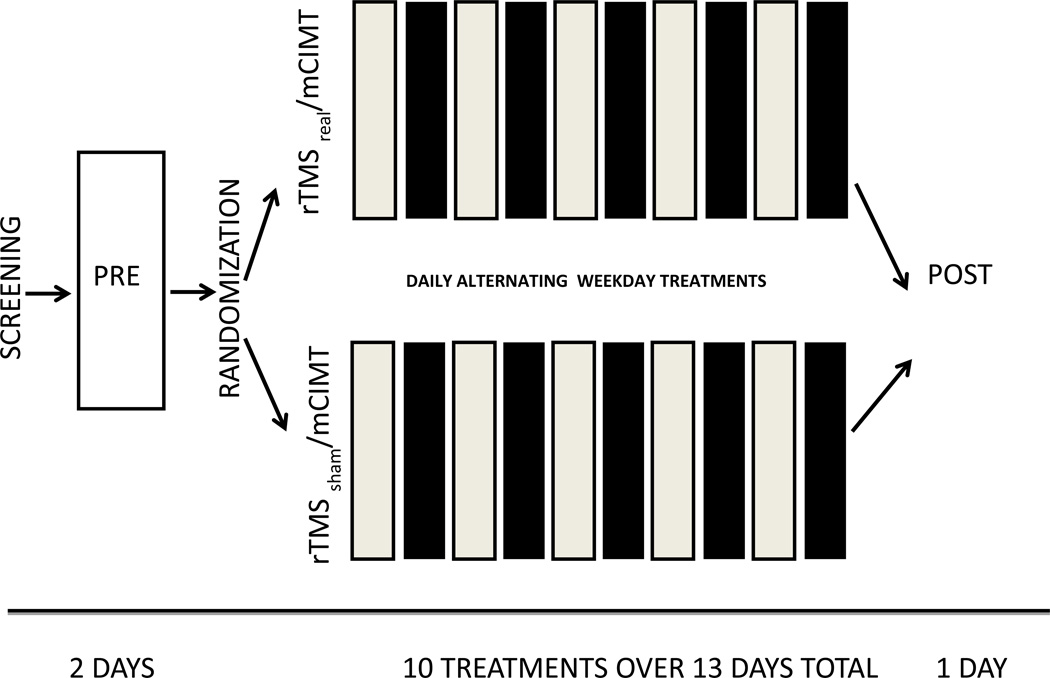
Figure 1.
Study Design. Screening was followed by a 2-day series of Pretesting: Imaging, Physician Assessment, Cognitive and Motor Testing. Repetitive transcranial magnetic stimulation sessions (light blocks) were 20 minutes total; priming for 10 minutes at 6 Hz at 90% resting motor threshold (RMT), followed by low-frequency 1-Hz rTMS for 10 minutes at 90% RMT. Modified constraint-induced movement therapy (mCIMT) (dark blocks) was performed for 2 hours with 1:1 therapist-subject treatments. The constraint cast was applied on treatment day 2, and removed on treatment day 10 (13 days total wear including weekends).
Subjects were recruited through IRB-approved mailings, community and school-based contacts, and diagnosis-specific website postings. An MRI-session with each subject included fluid attenuation inversion recovery and gradient echo sequences for identification of the location and lesion type by the study’s pediatric neurologist. After screening and approval, each child received single pulses of TMS to ipsilesional M1 to confirm the presence of a motor evoked potential, evidenced by EMG monitoring from the affected extensor digitorum muscle. If a resting motor threshold (RMT) could not be obtained, we attempted to obtain an active motor threshold (AMT). Presence of AMT was confirmed by elicitation of 30% of maximum voluntary contraction of the contralateral extensor digitorum musculature with a resultant MEP of ≥ 50uV peak to peak. If neither was found, the subject was excluded from the study to promote reasonable homogeneity across subjects and because the presence of an elicitable MEP is more favorable to higher recovery of function.23,24 The study was approved by the institutional review board of the University of Minnesota, and by Gillette Children’s Specialty Healthcare. A parent/guardian of each child gave informed consent and each child gave assent.
rTMSreal/mCIMT group. The rTMS and mCIMT interventions were therefore given in an alternating daily design, allowing the children to tolerate the intensive protocol. Long-lasting neuroplastic effects of rTMS have been found in animal studies yet such findings have not been confirmed in humans. 25,26 This group received five treatments of real rTMS and five treatments of mCIMT on alternate weekdays over two weeks. Each subject was seated in a reclining chair and wore earplugs and a swim cap for marking stimulation points. A 70-mm figure-eight TMS coil connected to a Magstim 200 stimulator a (Magstim Company Limited, Dyfed, UKa) was handheld over the approximate hotspot area for contralesional M1 tangential to the scalp and was oriented with the handle pointing posterolaterally at a 45° angle to the sagittal line. To find the hotspot the coil was moved systematically and single-pulse magnetic stimuli were delivered at approximately 0.1 Hz starting at an intensity of 50% of the stimulator maximum. This level was adjusted until the RMT or AMT was found, which was defined as the minimum intensity required to elicit MEPs greater than or equal to 50 µV peak-to-peak in at least 3 of 5 trials with the target muscle at rest. Responses from the unaffected extensor digitorum (ED) muscle were monitored using electrodes connected to a Cadwell Sierra Wedge EMG amplifierb (Cadwell Laboratories, Kennewick, WAb).
Once the hotspot and threshold were established, the child received priming rTMS followed by 1-Hz rTMS to the contralesional M1. Iyer et al found that in healthy individuals, 6-Hz priming enhanced the suppressive effect of 1-Hz rTMS6; however, similar effects in individuals with stroke are not yet confirmed. In theory, primed 1-Hz low-frequency rTMS to the contralesional M1 inhibits the contralesional M1 while simultaneously boosting disinhibition of the ipsilesional M1. The key methodological action is the pairing of priming with low-frequency rTMS. Delivery of 6-Hz priming alone to the contralesional M1 is disadvantageous according to the theory above.
Priming consisted of 10 minutes of 6-Hz rTMS at 90% of RMT delivered in 2 trains/minute with 5 seconds per train and 25-second intervals between trains (total = 600 priming pulses). Priming was followed immediately by an additional 10 minutes of 1-Hz rTMS at 90% of RMT without interruption (total = 600 low-frequency pulses). During rTMS, EMG activity monitored the ED, biceps brachii, first dorsal interosseous, and gastrocnemius muscles of the unaffected side for early signs of a possible seizure.
On alternate days, children received individualized mCIMT for the affected upper extremity. A uni-valve cast was fitted and applied to the unaffected hand and arm. The cast remained on 24 hours per day, except for one hour during the rTMS intervention. During that hour, the cast was temporarily removed to allow for range of motion checks, skin-integrity checks, neurological examination, washing, and electrode attachment for that session. mCIMT treatment was performed for two hours with a trained therapist. The mCIMT treatments consisted of shaping and repetitive activities for function, range of motion and strengthening of the affected upper extremity.27 Children continued to use their affected limb during home functional activities and a documented caregiver-supervised home program. rTMSsham/mCIMT group. These children received the same intervention as the mCIMT/real rTMS group except that a sham rTMS coil was used to mimic the sound and tactile sensation on the scalp (from the small electrical stimulator component unique to the sham coil) of the rTMS without stimulation. (Magstim Co., Whitland, Dyfed, UKb). This coil has been found to be valid for applications in naïve subjects and no subject in our study reported prior exposure to TMS.28
Safety Measures
Safety measures for this study were established by a multi-disciplinary team and consisted of the monitoring of subject responses, medical assessments and behavioral assessments including both the ipsilateral and contralateral hand function. Safety measures were administered in the same order to all subjects within the study.
Assessments at Pretest, Posttest and each rTMS Session
Physician Assessment
Using a modified Pediatric Stroke Outcome Measure,29 the physician assessed motor, cognitive, gait, spasticity, and visual field status and assigned a Gross Motor Function Classification Scale (GMFCS) level.30 Specific areas evaluated included attention, ability to follow commands, cranial nerves, muscle tone assessed with a modified Ashworth Scale31, deep tendon reflexes, presence or absence of clonus, strength, ability to perform rapid alternating movements, balance, and gait. Physician assessments occurred at each TMS session.
Vital Signs
Blood pressure and heart rate values were taken at all testing and rTMS intervention sessions.
Subject Report of Symptoms Assessment
Questions were asked about the occurrence or change of any of the following items: seizure, headache, neck pain, dental pain, hearing, nausea, abnormal muscle contractions, dizziness, abnormal sleep, difficult concentration, anxiety, memory, mood, balance, and use of unaffected hand. 18 (Appendix B) These questions were based on adverse events from rTMS that have been reported in the literature. If a symptom occurred, the child and caregiver were asked about the onset, duration and severity of the symptom. If medical care was deemed necessary, a medical team was on-site to address further issues. A follow-up within 24 hours was documented as well to assess status.
Pretest and Posttest- ipsilateral unaffected hand function and assessments
Accuracy Index
Functional magnetic imaging (fMRI) evaluated brain reorganization and will be reported in a subsequent paper. Relative to safety, subjects performed a finger movement tracking task during the fMRI. Wearing potentiometers (ETI Systems Inc., Carlsbad, CA) on the index finger at the MP joint on both hands, subjects attempted to track a displayed target waveform with finger extension/flexion movements of the designated hand. The unaffected hand performance was used to evaluate possible regression of function.
Finger strength
Subjects placed their unaffected index finger into a ring with a load cell measuring finger extension force(Interface Inc., Scottsdale, AZ, WinDaq, Akron, OH).32 Maximum finger extension force was determined as the greatest of three trials.
Stereognosis
Sensibility affects use of the involved upper extremity, including hand function and acquisition of new motor tasks.33 Many children with cerebral palsy and spastic hemiparesis have concurrent deficits in sensibility and employing stereognostic assessment has been beneficial in complete understanding of functional effects and subsequent rehabilitation treatment design. 34 The 12-Object stereognosis test evaluated subject’s ability, with vision shielded, to identify 12 different common objects individually placed in their unaffected then affected hands with vision shielded.34 Scoring was based on correct identification of the objects with fully intact functioning scoring a 12.
Cognitive Function
In order to understand the capability to follow commands, and the potential effect of the intervention on neural networks other than the motor system, cognitive testing was incorporated. These tests also allowed informal assessment of the potential needs a child may have had in the rigors of intervention participation. The TOKEN test for children (TTFC) assessed receptive language function, specifically the child’s ability to move tokens of varied sizes and shapes according to specific directions.35 Also, the digit span test, a component of the Weschler Intelligence Scale for Children-Revised, assessed the ability of the child to remember and recite a list of increasing numbers read to the child aloud.36
Subject questionnaire
This survey is a component of a larger survey developed to determine the child’s reaction to single-pulse TMS.37 Within this self-report questionnaire, rating scales for subjective report of satisfaction, willingness to repeat the study, and likelihood of recommending the study to others were assessed. (Appendix C)
Statistical analysis
This study was designed to evaluate safety and feasibility prior to initiating larger trials. At the time of study construct there were no preliminary data from our lab or from any other study involving rTMS combined with CIMT in children with which to conduct a power analysis. The literature showed a total N of between 10 and 20 subjects in the studies using rTMS in adult stroke.5,18,38 Kirton et al. recruited 10 subjects in an rTMS pediatric hemiparesis study.17 The initial sample size was 30 children. Due to recruitment challenges, the resultant sample size of 19 children with hemiparesis in this exploratory pilot was defined based on these studies.
All analyses were conducted using the as-treated study population to evaluate safety. Because all treatments both sham and real were correctly administered, this population is the same as the intent-to-treat study population. Medians and ranges are reported for continuous variables; counts and percentages are reported for categorical variables. Continuous variables were compared using a Wilcoxon rank-sum test between groups and categorical variables were compared using Fisher’s Exact Test. Changes from pretest to posttest were computed and compared using a Wilcoxon signed-rank test. In addition, an analysis of covariance (ANCOVA) was computed for the change from pretest to posttest to compare groups while controlling for baseline scores. Safety outcomes between groups were evaluated from a clinical perspective to identifying differences prior to achieving statistical significance. In this manner, we evaluated the presence of potential observed trends towards significance as a manner of minimizing any potential harmful effects for any group. Analyses were conducted using R software, version 2.15.0.
RESULTS
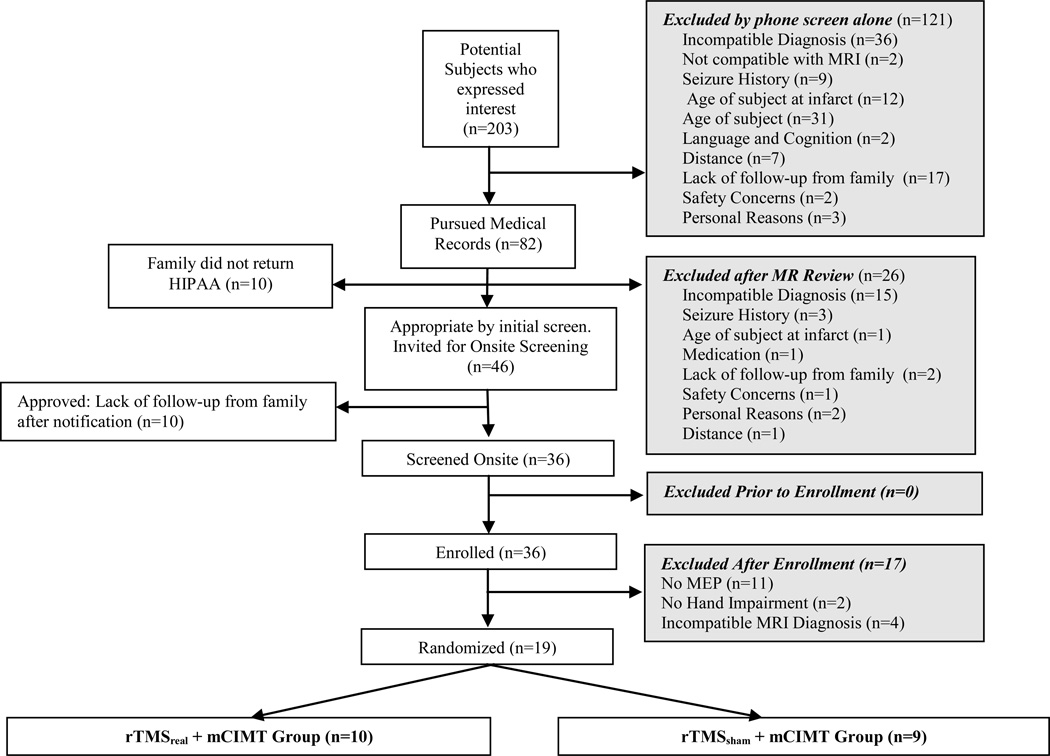
We screened 203 children and excluded 184. The remaining 19 children were randomized into two intervention groups (FIGURE 2- CONSORT). All children who entered treatment completed the study.
Figure 2.
Consolidated Standards of Reporting Trials (CONSORT) Diagram. MEP, Motor Evoked Potential. rTMS, repetitive Transcranial Magnetic Stimulation. mCIMT, modified constraint-induced movement therapy.
Subjects were enrolled over a 24-month period from 2010 to 2012. Patient characteristics are listed in Table 1, along with diagnoses of cortical ischemic lesions confirmed by MRI. All children received mCIMT. Ten children received real rTMS and had a median age of 10.5 years ( Mean 10.8 ± 2.7. Range = 8.0 – 15.0 years). Eight had a right sided hemiparesis and two had left side hemiparesis. One had a Manual Ability Classification System (MACS) level score of I, and the remaining nine children had a level II. In the sham rTMS group, the median age was 10.0 years, (Mean 10.9 ± 3.1, Range = 8.0 – 15.0 years). Four children had right hemiparesis and five had left hemiparesis. Three children had a MACS score of level I, five were at a level II, and one was at a level III. All children had a GMFCS level of 1. No statistically significant differences were found between groups at baseline for any measure.
Table 1.
Subject Demographics
| Participant | Group | Sex | Age (years) |
Stroke Location |
Side of Hemiparesis |
MACS Score |
GMFCS Score |
|---|---|---|---|---|---|---|---|
| 1 | Real | F | 15 | BG, LV | R | II | I |
| 2 | Real | M | 8 | ALIC, PLIC, T, P |
R | II | I |
| 3 | Real | M | 8 | ALIC, T, P, CS |
R | II | I |
| 4 | Real | M | 8 | MCA Frontal Parietal Lobes |
R | II | I |
| 5 | Real | F | 12 | MCA Frontal Parietal, BG, T |
R | II | I |
| 6 | Real | M | 9 | IC, T, P,LN,GP,CR, PVWM |
R | II | I |
| 7 | Real | M | 11 | Frontoparietal Cortex, IC, T, C, P, CS |
R | II | I |
| 8 | Real | F | 15 | MCA to Frontal Lobe |
L | I | I |
| 9 | Real | F | 11 | R post Frontal Lobe, CS |
L | II | I |
| 10 | Real | F | 13 | CS, T, BG | R | II | I |
| 11 | Sham | F | 15 | PVWM | L | I | I |
| 12 | Sham | M | 8 | MCA, CS | R | I | I |
| 13 | Sham | M | 8 | ALIC,T,P,BG | R | II | I |
| 14 | Sham | M | 12 | BG, T, CS | L | II | I |
| 15 | Sham | F | 8 | Posterior Frontal Lobe, CR, CS |
L | II | I |
| 16 | Sham | F | 8 | CS, PLIC,T,C,P |
R | II | I |
| 17 | Sham | M | 16 | CR, T, BG | R | II | I |
| 18 | Sham | F | 11 | MCA Frontal Temporal Parietal Lobes |
R | III | I |
| 19 | Sham | F | 15 | CS,T,BG | L | I | I |
Subjects are listed in the two groups Real: rTMSreal/mCIMT and Sham: rTMSsham/mCIMT.
Abbreviations: M, Male; F, Female; MACS, Manual Ability Classification Scale score; GMFCS, Gross Motor Function Classification Score; ALIC, Anterior Limb Internal Capsule; BG, Basal Ganglia; C, Caudate; CS,Centrum Semiovale; CR, Corona Radiata; LN, Lentiform Nucleus; LV, Lateral Ventricle; MCA, Middle Cerebral Artery; PV, Periventricular; PVWM, Periventricular White Matter; P, Putamen; PV, Periventricular; PLIC, Posterior Limb Internal Capsule; T, Thalamus; R, Right; L, Left.
Safety Measures
Seizures
No seizures were observed.
Physician Assessment
The physician found no serious adverse events, therefore, no treatments were therefore withheld.
Vital Signs
The blood pressure and heart rate measurements remained within normal values for all subjects during each rTMS session.39
Subject report of symptoms
Out of 133 TMS and rTMS testing and intervention sessions and 90 mCIMT sessions, the most common rTMS-related complaint was headache and the most common mCIMT-related complaint was irritation due to the cast on the unaffected arm. Common terminology criteria for adverse event ratings for all events were 1, indicating a mild event. All headaches subsided during the same session by clipping the elastic band of the swim cap or administering an age appropriate dose of acetaminophen or ibuprofen. Complaints of cast irritation were resolved by adjusting the cast contact position by providing additional padding or replacing the cast. All symptoms subsided within 24 hours, assessed either by phone or next-day treatment, depending upon the day of the week. No other complaints were reported. (Table 2)
Table 2.
Reported Side Effects
| rTMSreal/mCIMT Group (N=10) | rTMSsham/mCIMT Group (N=9) | P-value comparing the Number of Children with Complaints between groups |
|||
|---|---|---|---|---|---|
| Side Effect | Number of Children with Complaints |
Total Overall Number of Complaints |
Number of Children with Complaints |
Total Overall Number of Complaints |
|
| Headache | 5 | 10 | 8 | 8 | 0.141 |
| Cast Irritation | 3 | 3 | 4 | 4 | 0.650 |
| Anxiety | 3 | 4 | 1 | 1 | 0.582 |
| Dizziness | 2 | 2 | 1 | 2 | 0.999 |
| Tingling | 2 | 2 | 1 | 1 | 0.999 |
| Mood Changes | 1 | 1 | 1 | 2 | 0.999 |
| Concentration | 2 | 2 | 0 | 0 | 0.477 |
| Abnormal Muscle Contractions |
1 | 1 | 1 | 1 | 0.999 |
| Nausea | 0 | 0 | 2 | 2 | 0.744 |
| Stomachache | 1 | 1 | 1 | 1 | 0.999 |
| Fatigue | 1 | 1 | 1 | 1 | 0.999 |
Abbreviations: rTMS, repetitive Transcranial Magnetic Stimulation; mCIMT, modified Constraint-Induced Movement Therapy.
Finger tracking
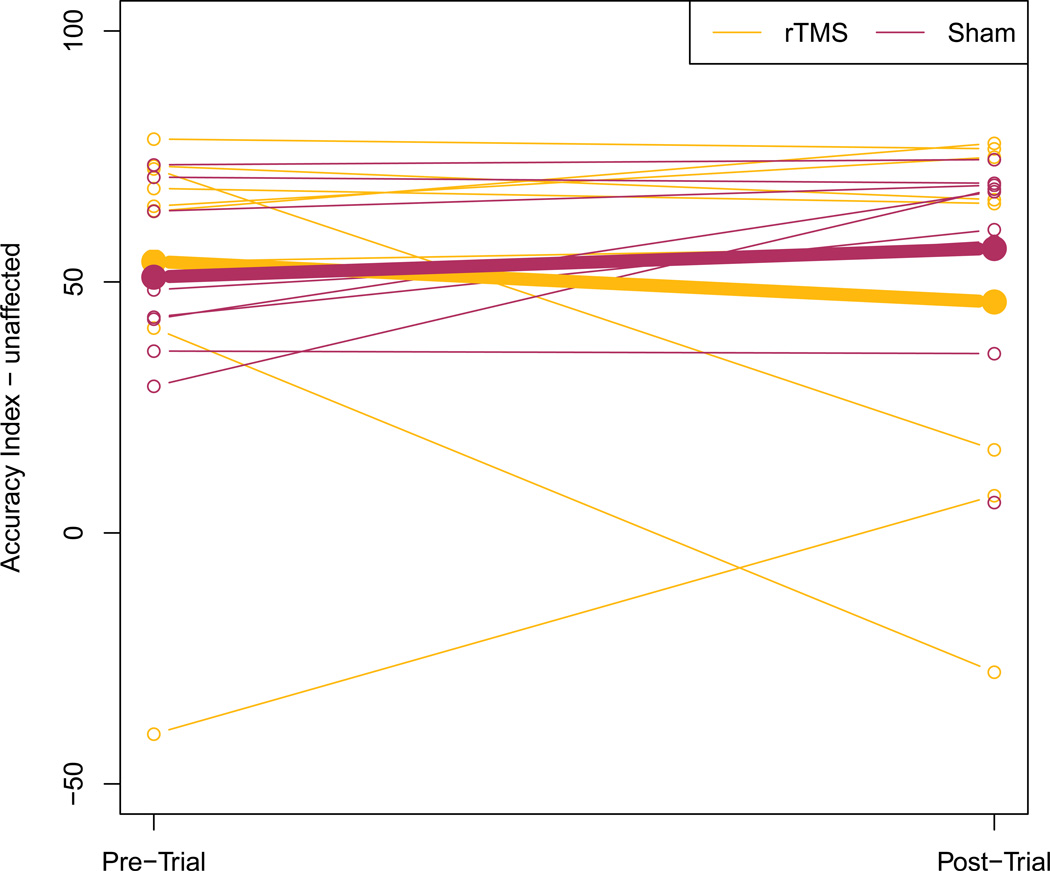
The median change in unaffected hand performance in the accuracy index was − 1.93 (Mean −6.93 ± 35.36, Range = −68.6 – 47.5) in the real group and 7.42 (Mean −12.03 ± 14.42, Range = −1.2 – 39.3) in the sham group (p = 0.15). Due to the decline in performance in the real group as compared to the sham group, we examined individual trajectories. We found that the performance of two children in the rTMS group decreased substantially in the post test. These children experienced fatigue during the MRI procedures, but performed well on other posttest measures. One participant in each group was missing finger tracking data. (FIGURE 3-ACCURACY INDEX)
Figure 3.
Accuracy Index of the Unaffected Hand. Means for rTMSreal/mCIMT group represented by bold orange line pre to posttest, and rTMSsham/mCIMT group in purple. Individuals in either group noted by thin lines of corresponding colors.
Finger strength
The median change in unaffected index finger extension force was −1.65 N (Mean −0.94 ± 4.19, Range = −6.5 − 5.6 N) in the real group and −0.63 N (Mean −1.33 ± 4.92, Range = −11.8 − 5.7 N) in the sham group (p = 0.75).
Stereognosis
All children achieved the maximum score of 12 using the unaffected hand in the pre and posttest sessions.
TOKEN Test For Children
The median change in the TTFC scores was 0.5 (Mean −1.3 ± 3.4, Range = --4.5 to 1.0) in the real group and 0.0 (Mean 0.5 ± 3.7, Range = −2.2 to 1.2) in the sham group (p = 0.70). One participant in the sham group was missing data.
Digit Span test
The median change in digit span test was 0.00 (Mean 0.0 ±1.20, Range = −.250 to 0.25 ) in the real group and 0.5 (Mean 0.38 ± 1.41, Range = −1.0 – 1.0) in the sham group (p = 0.68).
Subject questionnaire
The subject questionnaire rating satisfaction (0–10 scale, 10 most satisfied), yielded a median score for the children in the real group was 7 (IQR = 5.5 – 9.75) and 9 (IQR = 7 – 10) in the sham group (p = 0.35). When asked about repeating the study, 90% of children in the real group and 100% of children in the sham group stated they would do the study again (p = 1.0). All children stated they would recommend enrollment in the study to others.
TMS was most frequently described as a “tapping” sensation (n=11/19, 58%). The most enjoyable aspect of the TMS study was relaxing during intervention (n=8/19, 42%) and the least enjoyable was the tapping sensation (n=4/19, 21%) and the casting (n=2/19, 11%).
Importantly, an unbiased evaluative source, the Data Safety Monitoring Board (DSMB), found that the observed adverse events were minor and did not warrant altering the protocol or discontinuation of the study. As an added measure, one week after the posttest the investigators discussed, by phone, cognitive and behavioral status with the caregivers and school teachers for all children. No statements of concern in performance were raised. Also, all families and subjects were queried regarding blindedness to the study and no breech of blinding was found.
DISCUSSION
This study evaluated the safety of 6-Hz primed low-frequency rTMS combined with mCIMT in 19 children with stroke. Comprehensive safety evaluation tools were employed in the domains of behavior, cognition, physician clinical assessment, subject symptom report and satisfaction questionnaire, plus DSMB assessment. Attempts to establish homogeneity across subjects was established by inclusion of children who had both ipsilesional and contralesional motor evoked potentials through TMS assessment. The results of this comprehensive assessment suggest that five treatments of 600 pulses of intermittent 6-Hz rTMS followed by 600 pulses of continuous 1Hz rTMS given at 90% of RMT to contralesional M1, combined with mCIMT, was safe for the children in this study.
Numerous studies have shown low-frequency rTMS to contralesional M1 to be safe (i.e. no serious adverse events) in stroke, most recently reported by Kakuda et al.19 And several studies have demonstrated safety with high-frequency priming of low-frequency rTMS to contralesional M1 in adults with stroke.18,40 Due to the potential risk for seizure with high-frequency rTMS intervention, the current study is the first to show that high-frequency priming before low-frequency rTMS can be applied without serious adverse events in children with stroke. The use of 6-Hz priming of low-frequency rTMS to the contralesional hemisphere in children with hemiparesis was employed to theoretically may prove to achieve greater disruption of interhemispheric inhibition than low-frequency settings alone. With safety verified in this study, further studies comparable to the work in healthy subjects by Iyer et al (2003) are now needed in stroke research comparing the efficacy of priming while continuing to monitor safety along with efficacy.6
Rarely, serious adverse events have occurred in children with stroke during low-frequency rTMS to contralesional M1. Kirton et al. reported that two children experienced neurocardiogenic syncope.41 Further investigation disclosed that both subjects had positive histories of presyncope and fainting. We integrated this information in our study by screening for a history of fainting to minimize this potential adverse event. In addition, on-site vital signs were assessed at each rTMS visit and subjects were positioned semi-recumbent. We requested that all children have a regular meal and maintain adequate hydration during all sessions.
Minor adverse events (MAE) did occur in this study in both the real and sham groups. However small, all symptoms were noted as recommended by Machii et al.42 Headache was the most common complaint, followed by nausea. This is consistent with other reports where 113 healthy adult participants in 1270 experimental sessions were monitored for MAEs.43 MAE’s were found to be higher in initial TMS sessions compared to continuing sessions. Other rTMS studies have reported MAE’s such as headache43, and nausea as well.44 Many of the same symptoms were shared by both the real and sham groups in our study, suggesting that the source of the symptoms was similar. Anxiety was found to be present during six interventions, five in the real rTMS group. Further analysis showed that three anxiety reports were from one individual in the real rTMS group, who also reported headache, cast irritation, difficulty concentrating, transient unaffected arm tingling. During this subject’s participation in the study, the DSMB and consultants undertook to assess the subject’s continuation in the study. As the reports were assigned a CTCAE rating of 1(mild), the decision was made to continue. As indicated on the posttest questionnaire, this subject would repeat the study, recommend it to family and friends, and rated the experience a seven out of ten overall. The subject’s main complaints were related to becoming comfortable and familiar with wearing the cast.
The most prevalent casting MAEs were skin irritation, redness, and impaired performance of activities of daily living, education, play, leisure and social participation. However no child resisted application of the cast, and all children completed the study without untimely cast removal. Similar reports have also been noted regarding adjustment to casting during mCIMT, with similar modifications to enable continued participation.45
While safety is of paramount importance when creating a study design that addresses the complex needs of children with congenital hemiparesis, the feasibility of running such a study is also central to determine the value of future clinical interventions. We contend that our study has demonstrated feasibility of combining rTMS with mCIMT in children with stroke by the observation that all children who entered the study and received treatment completed the study. Also, when queried, all nineteen children stated that they would recommend enrollment to a friend or family member. Detailed understanding of the intricacies of working with the pediatric population as well as understanding the perception of the child’s experience is central to the success of such a study. 46
Study Limitations
The small sample size is a limitation in this study and generalizing results to a larger population, especially outside of the 8–17 year age range, should be made with caution. To promote safety and avoid fatigue, rTMS and mCIMT interventions were given on alternating days. With this alternate-day paradigm, it is possible that the optimal time period for synergizing rTMS effects with mCIMT effects was missed.
With safety now demonstrated, it would seem reasonable in future work to apply the behavioral training the same day as the rTMS with an appropriate intervening break to avoid fatigue. Only with continued studies and diligent reporting of all adverse events will the full impact of rTMS alone and in combination with behavioral interventions in children with hemiparesis be known. Future investigation of not only the dosing parameters but also inter-individual variability in physiologic response to intervention, as well as hormonal, genetic, developmental and other influences may play key roles in the determination of the safety and optimal response to use of rTMS.
CONCLUSIONS
This study demonstrated that 6-Hz primed low-frequency rTMS can be applied safely in the understudied population with pediatric hemiparesis. Key components to assess safety include 1) thorough screening prior to inclusion for history of conditions such as syncope and seizures, 2) review of medical records and imaging results, and 3) behavioral, cognitive and subject tolerance reports. In future studies, considerations for safety remain paramount but allow,, at the same time, for pursuit of optimal rTMS stimulation intensity, frequency and duration as well as optimal behavioral intervention parameters.
Study Inclusion and exclusion criteria.
Inclusion criteria
Congenital hemiparesis due to arterial ischemic stroke that occurred before, during or within one year of birth or periventricular leukomalacia confirmed by Magnetic Resonance Imaging
Current age between 8–17 years
Presence of ten degrees of active metacarpophalangeal joint flexion and extension on the affected hand
Presence of a resting or active ipsilesional motor evoked potential as assessed using Transcranial Magnetic Stimulation
Exclusion criteria
Multiple Strokes
Metabolic disorders
Neoplasm
Seizure within the previous two years
Disorders of cellular migration and proliferation
Hemorrhage
Receptive aphasia
Pregnancy
Indwelling metal or medical devices incompatible with Magnetic Resonance Imaging or Transcranial Magnetic Stimulation
Claustrophobia
Gross visual field cuts that would interfere with tasks performed during MRI
Acknowledgments
Acknowledgment of financial support: This study was funded by NIH grant #1 RC1HD063838-01. This publication [or project] was also supported by Grant Number 1UL1RR033183-01from the National Center for Research Resources (NCRR) and by Grant Number 8 UL1 TR000114-02 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) to the University of Minnesota Clinical and Translational Science Institute (CTSI). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the CTSI or the NIH. The University of Minnesota CTSI is part of a national Clinical and Translational Science Award (CTSA) consortium created to accelerate laboratory discoveries into treatments for patients. University of Minnesota Center for Magnetic Resonance Research funding supported the imaging work #P41 EB015894. Dr. Gillick was supported by the Foundation for Physical Therapy Promotion of Doctoral Studies during this thesis work.
List of Abbreviations
- rTMS
repetitive transcranial magnetic stimulation
- mCIMT
modified constraint-induced movement therapy
- M1
primary motor area
- RMT
resting motor threshold
- AMT
active motor threshold
- CONSORT
Consolidated Standards of Reporting Trials
- ED
extensor digitorum
- GMFCS
Gross Motor Function Classification Scale
- fMRI
Functional magnetic imaging
- TTFC
TOKEN test for children
- ANCOVA
analysis of covariance
- MACS
Manual Ability Classification System
- DSMB
Data Safety Monitoring Board
- MAE
Minor adverse events
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presentation of Work:
Gillick BT, Krach LE, Moberg K, Rich T, Carey JR. Pediatric Hemiparesis: Repetitive Transcranial Magnetic Stimulation and Constraint-Induced Therapy. AACPDM Annual Conference, September 13, 2012, Toronto, Canada.
Gillick BT, Ellsworth ST, Elmajri L, Henneman ES, Carey JR. Pediatric Hemiparesis: Synergistic Treatment Using Repetitive Transcranial Magnetic Stimulation and Constraint-Induced Therapy. APTA Combined Sections, 2012, Chicago, IL, Student co-authors: Ellsworth, Elmajri, Henneman.
No author has a conflict of interest.
The study is registered on clinicaltrials.gov (# NCT01104064) of the United States National Institutes of Health.
Suppliers
Cadwell Sierra Wedge EMG amplifier (Cadwell Laboratories, Kennewick, WA, Cadwell Laboratories, Inc, 909 N. Kellogg Street, Kennewick, WA 99336 USA)b
Magstim 200 stimulator (Magstim Company Limited, Dyfed, UK The Magstim Company Limited, Spring Gardens, Whitland, Carmarthenshire, SA34 0HR, UK)a
REFERENCES
- 1.Khedr EM, Abdel-Fadeil MR, Farghali A, Qaid M. Role of 1 and 3 hz repetitive transcranial magnetic stimulation on motor function recovery after acute ischaemic stroke. Eur J Neurol. 2009;16(12):1323–1330. doi: 10.1111/j.1468-1331.2009.02746.x. [DOI] [PubMed] [Google Scholar]
- 2.Charles J, Gordon AM. A critical review of constraint-induced movement therapy and forced use in children with hemiplegia. Neural Plast. 2005;12(2–3):245–61. doi: 10.1155/NP.2005.245. discussion 263-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55(3):400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- 4.Conforto A, Anjos S, Saposnik G, Mello E, Nagaya E. Transcranial magnetic stimulation in mild to severe hemiparesis early after stroke: A proof of principle and novel approach to improve motor function. J Neurol. 2012;259(7):1399–1405. doi: 10.1007/s00415-011-6364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fregni F, Boggio PS, Valle AC, et al. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke. 2006;37(8):2115–2122. doi: 10.1161/01.STR.0000231390.58967.6b. [DOI] [PubMed] [Google Scholar]
- 6.Iyer MB, Schleper N, Wassermann EM. Priming stimulation enhances the depressant effect of low-frequency repetitive transcranial magnetic stimulation. J Neurosci. 2003;23(34):10867–10872. doi: 10.1523/JNEUROSCI.23-34-10867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassidy Jessica Gillick, Bernadette Carey James. Priming the brain to capitalize on metaplasticity in stroke rehabilitation. Phys Ther. 2014;94(1):139–150. doi: 10.2522/ptj.20130027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turrigiano, Leslie GG, Desai KR, Rutherford NS, Nelson LC, SB Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391(6670):892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- 9.Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: Orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2(1):32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleim JA, Barbay S, Cooper NR, et al. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex* 1. Neurobiol Learn Mem. 2002;77(1):63–77. doi: 10.1006/nlme.2000.4004. [DOI] [PubMed] [Google Scholar]
- 11.Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: The EXCITE randomized clinical trial. JAMA. 2006;296(17):2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 12.Liepert J, Uhde I, Graf S, Leidner O, Weiller C. Motor cortex plasticity during forced-use therapy in stroke patients: A preliminary study. J Neurol. 2001;248(4):315–321. doi: 10.1007/s004150170207. [DOI] [PubMed] [Google Scholar]
- 13.Hoare BJ, Wasiak J, Imms C, Carey L. Constraint-induced movement therapy in the treatment of the upper limb in children with hemiplegic cerebral palsy. Cochrane Database of Systematic Reviews. 2007;(2):004149. doi: 10.1002/14651858.CD004149.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Lin KL, Pascual-Leone A. Transcranial magnetic stimulation and its applications in children. Chang Gung Med J. 2002;25(7):424–436. [PubMed] [Google Scholar]
- 15.Frye RE, Rotenberg A, Ousley M, Pascual-Leone A. Transcranial magnetic stimulation in child neurology: Current and future directions. J Child Neurol. 2008;23(1):79–96. doi: 10.1177/0883073807307972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirton A, Deveber G, Gunraj C, Chen R. Cortical excitability and interhemispheric inhibition after subcortical pediatric stroke: Plastic organization and effects of rTMS. Clin Neurophysiol. 2010;121(11):1922–1929. doi: 10.1016/j.clinph.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 17.Kirton A, Chen R, Friefeld S, Gunraj C, Pontigon AM, Deveber G. Contralesional repetitive transcranial magnetic stimulation for chronic hemiparesis in subcortical paediatric stroke: A randomised trial. Lancet Neurology. 2008;7(6):507–513. doi: 10.1016/S1474-4422(08)70096-6. [DOI] [PubMed] [Google Scholar]
- 18.Carey JR, Evans CD, Anderson DC, et al. Safety of 6-hz primed low-frequency rTMS in stroke. Neurorehabil Neural Repair. 2008;22(2):185–192. doi: 10.1177/1545968307305458. [DOI] [PubMed] [Google Scholar]
- 19.Kakuda W, Abo M, Kobayashi K, et al. Application of combined 6-hz primed low-frequency rTMS and intensive occupational therapy for upper limb hemiparesis after stroke. NeuroRehabilitation. 2011;29(4):365–371. doi: 10.3233/NRE-2011-0714. [DOI] [PubMed] [Google Scholar]
- 20.Kirton A. Can noninvasive brain stimulation measure and modulate developmental plasticity to improve function in stroke-induced cerebral palsy? Semin Pediatr Neurol. 2013;20(2):116–126. doi: 10.1016/j.spen.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Roelcke, Ulrich Boxheimer, Larissa Fathi, Ali Schwyzer, Lucia Ortega, Marcos Berberat, Jatta Remonda, Luca Cortical hemosiderin is associated with seizures in patients with newly diagnosed malignant brain tumors. J Neurooncol. 2013;115(3):463–468. doi: 10.1007/s11060-013-1247-7. [DOI] [PubMed] [Google Scholar]
- 22.Wang, Xiaoyu Tao, Zhang You, Chao Li, Qiang Liu, Yi Extended resection of hemosiderin fringe is better for seizure outcome: A study in patients with cavernous malformation associated with refractory epilepsy. Neurol India. 2013;61(3):288–292. doi: 10.4103/0028-3886.115070. [DOI] [PubMed] [Google Scholar]
- 23.Talelli, Greenwood P, Rothwell RJ, JC Arm function after stroke: Neurophysiological correlates and recovery mechanisms assessed by transcranial magnetic stimulation. Clinical neurophysiology. 2006;117(8):1641–1659. doi: 10.1016/j.clinph.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Dimyan MC, Leonardo Contribution of transcranial magnetic stimulation to the understanding of functional recovery mechanisms after stroke. Neurorehabil Neural Repair. 2010;24(2):125–135. doi: 10.1177/1545968309345270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gersner, Roman Kravetz, Elena Feil, Jodie Pell, Gaby Zangen, Abraham Long-term effects of repetitive transcranial magnetic stimulation on markers for neuroplasticity: Differential outcomes in anesthetized and awake animals. The Journal of neuroscience. 2011;31(20):7521–7526. doi: 10.1523/JNEUROSCI.6751-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290(5491):533. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- 27.Taub E, Uswatte G, King DK, Morris D, Crago JE, Chatterjee A. A placebo-controlled trial of constraint-induced movement therapy for upper extremity after stroke. Stroke. 2006;37(4):1045–1049. doi: 10.1161/01.STR.0000206463.66461.97. [DOI] [PubMed] [Google Scholar]
- 28.Mennemeier, Triggs M, Chelette W, Woods Kc, Kimbrell Aj, Dornhoffer T, Sham J. transcranial magnetic stimulation using electrical stimulation of the scalp. Brain stimulation. 2009;2(3):168–173. doi: 10.1016/j.brs.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.deVeber GA, MacGregor D, Curtis R, Mayank S. Neurologic outcome in survivors of childhood arterial ischemic stroke and sinovenous thrombosis. J Child Neurol. 2000;15(5):316–324. doi: 10.1177/088307380001500508. [DOI] [PubMed] [Google Scholar]
- 30.Carnahan KD, Arner M, Hagglund G. Association between gross motor function (GMFCS) and manual ability (MACS) in children with cerebral palsy. A population-based study of 359 children. BMC Musculoskeletal Disorders. 2007;8:50. doi: 10.1186/1471-2474-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bohannon RW, Smith MB. Interrater reliability of a modified ashworth scale of muscle spasticity. Phys Ther. 1987;67(2):206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 32.Carey JR. Manual stretch: Effect on finger movement control and force control in stroke subjects with spastic extrinsic finger flexor muscles. Arch Phys Med Rehabil. 1990;71(11):888–894. [PubMed] [Google Scholar]
- 33.Krumlinde-Sundholm L, Eliasson AC. Comparing tests of tactile sensibility: Aspects relevant to testing children with spastic hemiplegia. Developmental Medicine & Child Neurology. 2002;44(9):604–612. doi: 10.1017/s001216220100264x. [DOI] [PubMed] [Google Scholar]
- 34.Van Heest AE, House J, Putnam M. Sensibility deficiencies in the hands of children with spastic hemiplegia. J Hand Surg Am. 1993;18(2):278–281. doi: 10.1016/0363-5023(93)90361-6. [DOI] [PubMed] [Google Scholar]
- 35.DiSimoni F. The token test for children, manual. Pro-Ed, Austin. 1978 [Google Scholar]
- 36.Loughan A, Perna R, Hertza J. The value of the wechsler intelligence scale for children-fourth edition digit span as an embedded measure of effort: An investigation into children with dual diagnoses. Archives of clinical neuropsychology. 2012 doi: 10.1093/arclin/acs072. [DOI] [PubMed] [Google Scholar]
- 37.Garvey MA, Kaczynski KJ, Becker DA, Bartko JJ. Subjective reactions of children to single-pulse transcranial magnetic stimulation. J Child Neurol. 2001;16(12):891–894. doi: 10.1177/088307380101601205. [DOI] [PubMed] [Google Scholar]
- 38.Kim YH, You SH, Ko MH, et al. Repetitive transcranial magnetic stimulation-induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke. 2006;37(6):1471–1476. doi: 10.1161/01.STR.0000221233.55497.51. [DOI] [PubMed] [Google Scholar]
- 39.Deckmann RBD. In: Pediatric education for prehospital professionals. American Academy of Pediatrics, editor. Jones & Bartlett; 2000. [Google Scholar]
- 40.Kakuda W, Abo M, Kobayashi K, et al. Low-frequency rTMS combined with intensive occupational therapy for upper limb hemiparesis after brain tumour resection. Brain Injury. 2010;24(12):1505–1510. doi: 10.3109/02699052.2010.523040. [DOI] [PubMed] [Google Scholar]
- 41.Kirton A, Deveber G, Gunraj C, Chen R. Neurocardiogenic syncope complicating pediatric transcranial magnetic stimulation. Pediatr Neurol. 2008;39(3):196–197. doi: 10.1016/j.pediatrneurol.2008.06.004. [DOI] [PubMed] [Google Scholar]; 43 Machii K, Cohen D, Ramos-Estebanez C, Pascual-Leone A. Safety of rTMS to non-motor cortical areas in healthy participants and patients. Clin Neurophysiol. 2006;117(2):455–471. doi: 10.1016/j.clinph.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 42.Machii K, Cohen D, Ramos-Estebanez C, et al. Safety of rTMS to non-motor cortical areas in healthy participants and patients. Clin Neurophysiol. 2006;117(2):455–471. doi: 10.1016/j.clinph.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 43.Maizey L, Allen CPG, Dervinis M, et al. Comparative incidence rates of mild adverse effects to transcranial magnetic stimulation. Clinical neurophysiology. 2012 doi: 10.1016/j.clinph.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 44.Satow T, Mima T, Hara H, et al. Nausea as a complication of low-frequency repetitive transcranial magnetic stimulation of the posterior fossa. Clinical neurophysiology. 2002;113(9):1441–1443. doi: 10.1016/s1388-2457(02)00187-6. [DOI] [PubMed] [Google Scholar]
- 45.Taub E, Ramey S, DeLuca S, Echols K. Efficacy of constraint-induced movement therapy for children with cerebral palsy with asymmetric motor impairment. Pediatrics. 2004;113(2):305–312. doi: 10.1542/peds.113.2.305. [DOI] [PubMed] [Google Scholar]
- 46.Garvey M, Mall V. Transcranial magnetic stimulation in children. Clinical neurophysiology. 2008;119(5):973–984. doi: 10.1016/j.clinph.2007.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]