Abstract
Both elevated and blunted levels of cortisol secretion during childhood and adolescence have been linked to the subsequent onset of Major Depressive Disorder (MDD). These mixed findings may be due to developmental changes in HPA-axis functioning, which have not been previously assessed in the context of risk. In the present study, therefore, we examined whether pubertal development moderated the influence of cortisol secretion on the subsequent development of MDD. Eighty-nine never-disordered girls ages 9-15 years, many of whom were at high risk for depression by virtue of having a maternal history of the disorder, completed a laboratory stress task. To index cortisol reactivity, salivary cortisol samples were collected at baseline and 15 minutes following the onset of the stressor. Girls' levels of pubertal development were measured using Tanner staging. All participants were followed through age 18 in order to assess the subsequent development of MDD. Pubertal stage moderated the effects of cortisol stress reactivity on the development of MDD. Specifically, the onset of MDD was predicted by cortisol hyporeactivity in girls who were earlier in pubertal development (Tanner stage ≤ 2), but by cortisol hyperreactivity in girls who were later in pubertal development (Tanner stage ≥ 3.5).
Conclusions
These findings demonstrate that girls' cortisol stress reactivity predicts the subsequent onset of MDD, and further, that the nature of this effect depends on the girls' level of pubertal development. Results are discussed in the context of clarifying previous findings, and directions for future research are offered.
Keywords: Depression, adolescence, HPA-axis, cortisol, puberty
1. Introduction
Major depressive disorder (MDD) affects nearly 20% of all Americans and is the most common of all psychiatric disorders (Kessler et al., 2014). One of the most striking features of MDD is the gender difference in incidence that emerges in early adolescence, when females begin to be twice as likely as males to develop the disorder (Stroud et al., 2004; Hilt & Nolen-Hoeksema, 2014). Importantly, the age at which this gender divergence arises corresponds to the complex transition through puberty. Pubertal development involves a host of biological, psychological, and social changes that researchers posit contribute to the increase in rates of MDD in adolescent girls; in fact, investigators have found pubertal stage to be a stronger predictor of the onset of depression than is chronological age (Angold et al., 1998). Thus, puberty is a critical period during which to examine factors that increase the risk of young adolescents, particularly females, for developing MDD.
In this context, investigators have implicated stress and stress responsivity in females' pubertal development as a potential mechanism that underlies the gender difference in rates of MDD. For example, starting in early adolescence, girls report experiencing higher overall levels of stress than do boys (Brooks-Gunn, 1991; Ge et al., 2001). Moreover, sex-specific changes in the responsivity of the hypothalamic-pituitary-adrenal (HPA) axis, as assessed by levels of the stress hormone cortisol, have also been noted during puberty (Young & Altemus, 2004; Romeo, 2010; Young & Korszun, 2010). For example, in a cross-sectional study of stress reactivity across pubertal development, Gunnar and colleagues (2009) found not only that baseline cortisol levels and cortisol response to a laboratory stress-induction task (Trier Social Stress Task) increased as a function of level of pubertal development, but that around age 13, this increase in response to a stressor was more pronounced in girls than boys. Similarly, Stroud et al. (2009) found that with greater pubertal maturation, girls, but not boys, exhibited an increase in cortisol secretion following a corticotropin releasing hormone challenge. More recently, Blumenthal et al. (2014) found a positive association between cortisol response to a novel social setting and pubertal stage in adolescent girls, controlling for chronological age. Taken together, therefore, these findings document increases in cortisol responsivity in girls through puberty, which may underlie the elevated risk for depression in females that begins at this developmental period.
Consistent with this formulation, higher levels of cortisol over the transition through puberty have been found to be related to a greater vulnerability to depression in adolescence (Dahl & Gunnar, 2009; Stroud et al., 2009). For example, several investigators have found that higher levels of morning cortisol predict the subsequent onset of depression in adolescence (Adam et al., 2010; Vrshek-Schallhorn et al., 2013; Owens et al., 2014). Importantly, however, findings regarding cortisol stress responsivity as a predictor of subsequent changes in depressive symptoms are less consistent. For example, Susman et al. (1997) found that 9- to 15-year-old adolescents who had a greater cortisol response to a novel and challenging situation reported higher levels of depressive symptoms one year later. In contrast, however, Keenan et al. (2013) found that younger, 10- to 12-year-old, girls who exhibited a blunted cortisol response to a laboratory stressor and who had low absolute levels of cortisol secretion at age 12 experienced a subsequent increase in depressive symptoms. Given the changes in cortisol functioning that accompany pubertal development, it is possible that inconsistencies in previous findings are due, in part, to the different developmental stages at which participants were assessed across studies; that is, it appears that increases in depressive symptoms are predicted by blunted cortisol reactivity in younger girls, but by elevated cortisol reactivity in older girls.
The present study was designed to examine this formulation by assessing whether cortisol stress reactivity measured across puberty predicts the subsequent onset of MDD. We recruited 9- to 15-year-old never-disordered girls who spanned the full range of pubertal development, examined their patterns of cortisol stress reactivity, and followed them regularly through age 18 to assess the subsequent onset of depression. Specifically, we tested whether pubertal status at the time of cortisol assessment moderated the relation between cortisol reactivity and the subsequent development of MDD. To maximize the likelihood that a significant proportion of the girls would develop an episode of depression, we included in our sample girls at familial risk for the disorder by virtue of having a mother with a history of depressive episodes. Given the documented increase in cortisol secretion in girls across the transition through puberty (Gunnar et al., 2009; Blumenthal et al., 2014), combined with the increase in females' rates of depression during and after this period, we hypothesized that girls at later stages of pubertal development who exhibit greater cortisol reactivity to a laboratory stressor would be more likely to experience a subsequent depressive episode than would comparable girls at earlier stages of pubertal development. Thus, we tested the prediction that cortisol reactivity would be a more sensitive marker of risk for the onset of MDD at later than at earlier stages of puberty.
2. Method
2.1. Participants
Participants were 89 girls who, at entry into the study at Time 1 (T1), were between 9 and 15 years of age and had no past or current Axis I disorder. Forty-seven girls had mothers who also had no past or current Axis I disorder (low risk for depression; CTL), and 42 girls were at high risk for developing depression (RSK) by virtue of having mothers who had recurrent episodes of MDD during their daughters' lifetime. As we noted above, given that maternal history of depression is a strong predictor of depression in adolescence (Gotlib & Colich, 2014), we included daughters of depressed mothers in our sample to ensure that a meaningful proportion of our study participants would experience a depressive episode at follow-up. As part of a larger study examining the intergenerational transmission of depression, participants were recruited through local community outreach. A telephone screening interview established that both the participants and their mothers were fluent in English. Girls were excluded from participating in the study if they had experienced severe head trauma or learning disabilities. Neither the participants nor their mothers had current or past substance abuse and, consistent with the absence of diagnosed depression in the daughters, no girls in the study were taking antidepressant medications.1 Mothers and daughters who were potentially eligible to participate in the study were then brought to the laboratory where they were administered structured diagnostic interviews. This study was approved by the institutional review board (IRB) at Stanford University. All mothers gave informed consent and daughters gave assent. Data from this study are part of a larger study of 190 mother-daughter pairs exploring the intergenerational transmission of risk for depression. After attrition, we are continuing to follow 175 pairs, 89 of whom have either developed an episode of MDD or been followed through age 18. Participants in the current study sample and participants who are no longer in the study do not differ with respect to either cortisol reactivity or cortisol recovery (both ps >.50).
2.2. Clinical Assessment
2.2.1. Interviews at T1
At the first laboratory session, trained interviewers assessed the diagnostic status of participants by administering the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime version (KSADS-PL; Kaufman et al., 1997) to both the girls and their mothers (about the daughters) to ensure that no girl had experienced any current or past Axis I disorder. A different interviewer administered the Structured Clinical Interview for DSM (SCID; First et al., 1996) to the mothers. Interviewers for the K-SADS-PL and SCID had previous experience administering structured clinical interviews and had excellent interrater reliability (K=1.00). Mothers in the RSK group reported experiencing at least two depressive episodes in their daughter's lifetime; mothers in the CTL group reported an absence of any current or lifetime Axis I disorders.
2.2.2. Questionnaires
At T1, all girls completed the Child Depression Inventory - Short Form (CDI-S; Kovacs, 1992) to assess severity of any subclinical depressive symptoms, and the Tanner Staging questionnaire to assess self-reported pubertal status (Marshall & Tanner, 1968). The CDI-S is a 10-item self- report measure of depressive symptoms experienced in the past two weeks. The internal consistency for this measure in the current sample was α = .74. On the Tanner Staging questionnaire, each girl reported her own developmental stage using schematic drawings of two secondary sex characteristics (breast and pubic hair); a composite score of these two ratings was created to create a single measure of girls' pubertal status at T1. A Tanner staging of 1 signifies that no pubertal development has begun, and a staging of 5 signifies that adult levels of pubertal maturation have been achieved. Although the Tanner Staging questionnaire is a self- report measure, scores from this assessment have been found to correlate with physicians' physical examinations of pubertal development (see Coleman & Coleman, 2002 for a review; see also Shirtcliff et al., 2009).
2.3. Cortisol Collection and Stress Task
After completing the structured interviews, participants returned to the laboratory for a second visit within two weeks of the interview session in which their HPA-axis function was assessed. Participants were instructed to refrain from eating or drinking for one hour prior to this session, during which they participated in a 15-minute long social stress task. This began with a 3-minute serial subtraction task, in which girls were instructed to count backwards, starting from 400 and counting backward by 7′s as quickly and accurately as possible. If a participant made a mistake, the experimenter told her that she was incorrect and should begin again at 400. In the event that a participant showed no difficulty with this task, she was instructed to start again, this time, at 4000 and count backward by 17′s. Immediately following this 3-minute subtraction task, the girls participated in a 12-minute Ewart Social Competence Interview (Ewart, & Kolodnar, 1991; Ewart et al., 2002) to induce emotional arousal. In this interview, the participants were encouraged to recount the details of a stressful life situation that they had previously rated as extremely stressful. The experimenter encouraged the participants to recount vivid details of this stressful event to promote an emotional response similar to reliving the emotional event. Following the stressor, participants watched a neutral video about the Denali National Forest for 30-minutes before being debriefed about the stress task.
Throughout the session, four saliva samples were collected from each participant using Sarstedt Salivettes (Sarstedt, Numbrecht, Germany). A saliva sample was collected from the daughters upon their arrival at the laboratory, again immediately following the stressor (15 minutes after the onset of the stressor), and again at 30 and 45 minutes following the onset of the stressor, during which time the participants watched the neutral video described above. On average, the first saliva sample was collected at 1308h; importantly, neither the RSK and CTL groups, nor the girls who subsequently developed MDD and the girls who did not develop MDD, differed with respect to the time of the first saliva collection, t(87) = 0.41, p = .67; t(87) = 1.13, p = .26, respectively. Nevertheless, given normative diurnal variation in salivary cortisol levels, we included time of cortisol collection (operationalized as the number minutes from midnight to the time of the first sample) as a covariate in all analyses.
Saliva samples were stored in a freezer chest until they could be transferred to a -20° freezer located the General Clinical Research Center at Stanford University, where they were maintained until radioimmunoassay. Cortisol levels were assayed by luminescence immunoassay reagents using a commercial kit from Immuno-Biological Laboratories Inc. (Hamburg, Germany), and the assay sensitivity was set at 0.015 mg/dl. Samples were assayed together in large batches to control for interassay error, and control samples were included to evaluate variability. The intraassay variation on three saliva pools of the low, medium, and high controls were averaged 2.78%, 10.45%, and 4.79%, respectively. The mean values of the low, medium, and high controls were .054, .228, and .863 mg/dL, respectively. The interassay coefficients of the variations of the low, medium, and high controls were 10.9%, 10.5%, and 5.5%, respectively.
2.4. Follow-up Interviews
To monitor diagnostic status, mothers and daughters returned to the laboratory every 18 months to participate in a follow-up clinical interview until the daughters had developed a depressive episode through age 18 or had passed 18 years of age without developing any DSM Axis I disorder. They were administered the KSADS-PL (if they were under 18) and the SCID (if they were 18 years of age) to assess the presence of MDD and the occurrence of MDD in the daughter since their last visit. Daughters who were found to have met DSM-IV criteria for MDD at any follow-up assessment before age 19 were categorized in the “MDD outcome” group. Similarly, daughters who did not meet DSM-IV criteria for MDD at any follow-up assessment before age 19 were categorized in the “no-MDD outcome” group.
2.5. Data Analysis
As in previous studies (Chen et al., 2010), we examined the cortisol data for outliers and winsorized values 2 SD above and below the mean to the 2-SD value based on methods described by Tukey (1977). Because intra-individual change in cortisol levels has been found to be a stronger indicator of the stress response than are absolute levels of cortisol output (Susman et al., 1997), we calculated two variables: girls' initial cortisol stress response, or cortisol reactivity; and their subsequent cortisol recovery following the social stress task. Visual inspection of the data indicated that the peak cortisol response occurred at the second cortisol collection (15 minutes after the onset of the stressor, Figure 1); thus, following Luby et al. (2003), we operationalized cortisol reactivity as the percent change in cortisol levels between the first and the second cortisol collections ([cortisol 2 – cortisol 1]/cortisol 1 * 100) and cortisol recovery as the percent change in cortisol level between its peak 15 minutes after the onset of the stressor and the final cortisol collection 45 minutes after the onset of the stressor ([cortisol 4 – cortisol 2]/cortisol 2 * 100).
Figure 1. Cortisol patterns across the Social Stress Task (SST).
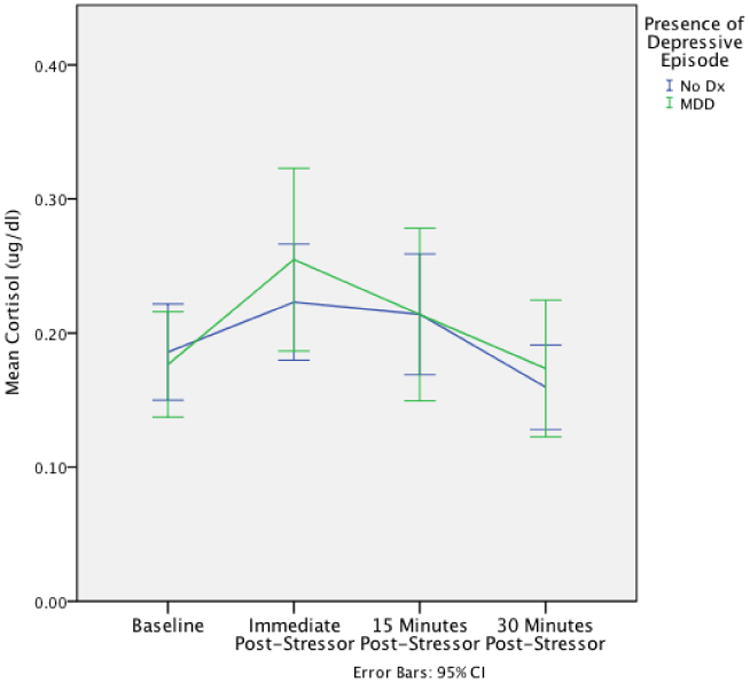
Mean cortisol levels are displayed on the y-axis for each point of cortisol collection for both individuals who developed a depressive episode prior to age 19 and those who did not.
Statistical analyses were conducted using SPSS statistical software (version 22.00). We used hierarchical multiple logistic regression to test our prediction that puberty would moderate the association between cortisol secretion and the subsequent onset of MDD, modeling T1 cortisol reactivity and recovery, pubertal stage, and their interactions as predictor variables (all variables centered; mean = 0), and the onset or non-onset of MDD between T1 and age 18 (dummy coded: 0=non-onset, 1=onset) as the outcome variable. To ensure that the findings were not due to confounding variables, we included T1 risk-group status (dummy coded: 0=CTL, 1=RSK), age, CDI-S scores, and time of the first sample (minutes from midnight) as predictors in the initial step of the analysis.
3. Results
3.1. Participant Characteristics
Demographic and clinical characteristics of the MDD and no-MDD outcome groups are presented in Table 1. Girls who developed MDD did not differ from girls who did not develop MDD on most T1 variables, including time of day of cortisol collection, t(87) = 1.129, p = 0.262; pubertal stage, t(87) = 1.524, p = 0.146; ethnicity, χ2(1, N = 89) = 2.236, p = .135; medication use, x2(1,89) = 0.425, p = .608; and body mass index (BMI), t(75) = .668, p = 0.506. Compared with girls who did not develop MDD, however, girls who developed MDD were significantly younger at T1, t(87) = 3.110, p = .003, and had significantly higher CDI-S scores at T1, t(87) = 2.020, p = .046; therefore, we controlled for T1 age and CDI-S scores, in addition to time of cortisol collection, in all subsequent analyses.2
Table 1. Demographic Information and Clinical Characteristics.
| Variable | No MDD outcome (n = 58) | MDD outcome (n = 31) | Statistics | p value |
|---|---|---|---|---|
| Age at T1, M (SD) | 13.37 (1.37) | 12.39 (1.48) | t(87) = 3.110 | 0.003 |
| CDI-S at T1, M (SD) | 1.52 (1.54) | 2.45 (2.84) | t(87) = 2.020 | 0.046 |
| Number of Rsk girls | 18/58 | 24/31 | χ2(1, N = 89) = 17.442 | < 0.001 |
| Ethnicity (% caucasian) | 65.5 | 80.6 | x2(1,89) = 2.236 | 0.135 |
| Age of menstration, M (SD) | 11.87 (1.12) | 11.33 (0.78) | t(41) = 1.524 | 0.135 |
| Tanner stage at T1 | 3.45 (0.91) | 3.15 (0.96) | t(87) = 1.468 | 0.146 |
| BMI, M (SD) | 19.68 (2.87) | 20.14 (2.82) | t(75) = .668 | 0.506 |
| Time of first cortisol collection (minutes from midnight), M (SD) | 773.53 (168.70) | 815.81 (167.60) | t(87) = 1.129 | 0.262 |
| Medication usage at T1, % | 4 | 6.50 | x2(1,89) = .425 | 0.608 |
| Cortisol reactivity, M (SD) | 30.21 (78.83) | 51.96 (100.05) | t(87) = 1.127 | 0.263 |
| Cortisol recovery, M (SD) | -22.52 (32.03) | -19.84 (71.09) | t(87) = .245 | 0.807 |
Note: Standard deviations are presented in parentheses. MDD = Major Depressive Disorder; No MDD outcome = participants who did develop major depressive disorder prior to age 21; MDD outcome = participants who did develop major depressive disorder prior to age 21; T1 = 1st visit to the laboratory (baseline); CDI = Children's Depression Inventory - Short Form.
3.2. Predicting the Onset of Depression
Results of the multiple logistic regression analysis predicting the onset of depression are presented in Table 2. In model 1, we examined the main effects of risk group, age, CDI-S score at T1, and time of cortisol collection. This first model yielded a significant effect, χ2(4) = 33.422, p <.001, Nagelkerke R2 = .423. Risk group was a significant predictor of the subsequent onset of MDD: not surprisingly, a greater proportion of RSK (57%) than CTL (15%) girls developed a subsequent episode of MDD. Age was also a significant predictor of the occurrence of MDD: younger age at T1 predicted a more likely occurrence of MDD. Neither CDI-S score at T1 nor time of cortisol collection predicted the occurrence of MDD.
Table 2.
Multiple logistic regression of associations among cortisol reactivity and pubertal development (Tanner stage) at T1 and the development of MDD prior to age 19.
| Exp (B) (Odds ratio) | 95% C.I. Lower | 95% C.I. Upper | p-value | |
|---|---|---|---|---|
| Model 1 | ||||
| Risk Group | 8.363 | 2.455 | 28.492 | 0.001 |
| Age | 0.499 | 0.328 | 0.759 | 0.001 |
| CDI | 1.258 | 0.935 | 1.693 | 0.129 |
| Time of cortisol collection | 1.002 | 0.999 | 1.006 | 0.170 |
| Model 2 | ||||
| Risk Group | 8.637 | 2.379 | 31.357 | 0.001 |
| Age | 0.483 | 0.273 | 0.854 | 0.012 |
| CDI | 1.270 | 0.937 | 1.723 | 0.124 |
| Time of cortisol collection | 1.003 | 0.999 | 1.006 | 0.134 |
| Tanner Staging | 1.123 | 0.501 | 2.518 | 0.779 |
| Cortisol Reactivity | 1.002 | 0.996 | 1.008 | 0.586 |
| Cortisol Recovery | 1.005 | 0.994 | 1.016 | 0.386 |
| Model 3 | ||||
| Risk Group | 8.125 | 1.965 | 33.599 | 0.004 |
| Age | 0.492 | 0.258 | 0.940 | 0.032 |
| CDI | 1.442 | 1.017 | 2.045 | 0.040 |
| Time of cortisol collection | 1.003 | 0.999 | 1.007 | 0.090 |
| Tanner Staging | 0.931 | 0.345 | 2.517 | 0.888 |
| Cortisol Reactivity | 1.004 | 0.997 | 1.011 | 0.319 |
| Cortisol Recovery | 0.996 | 0.977 | 1.016 | 0.699 |
| Tanner × Cortisol Reactivity | 1.013 | 1.003 | 1.023 | 0.011 |
| Tanner × Cortisol Recovery | 0.984 | 0.960 | 1.008 | 0.196 |
Note: MDD = Major Depressive Disorder; CDI-S = Children's Depression Inventory - Short Form; Continuous variables were centered (mean=0) to aid in interpretation. Categorical variables were kept in their original form (Risk Group: 0 = CTL, 1 = RSK; Outcome: 0 = no MDD, 1 = MDD)
In model 2, we added the main effects of pubertal stage, cortisol reactivity, and cortisol recovery, in addition to the covariates that were included in model 1, in predicting the onset of MDD. This analysis did not significantly improve the model's prediction of MDD (χ2(3) = 1.184, p = .757, Nagelkerke R2 = .444) and did not yield main effects of any of these variables in predicting the development of MDD.
In model 3, we added the interactions of pubertal stage and cortisol reactivity, and of pubertal stage and cortisol recovery. Adding these interaction terms significantly improved the model's prediction of MDD, χ2(2) = 11.366, p <.01, Nagelkerke R2 = .556. As we hypothesized, there was a significant interaction of pubertal stage and cortisol reactivity in predicting the development of MDD (see Figure 2). Tests of the simple slopes indicated that, for those girls who were relatively later in pubertal development at the time of assessment (1 SD above the mean, corresponding to Tanner Stage 4.28, and above), greater cortisol reactivity to the laboratory stressor predicted the subsequent development of depression. In contrast, for those girls who were less sexually mature at the time of initial assessment (1 SD below the mean, corresponding to Tanner Stage 2.40, and below), cortisol reactivity to the laboratory stressor was only a marginally significant predictor of the development of depression. Finally, for girls at the mean value of Tanner Stage (corresponding to Tanner Stage 3.34), cortisol reactivity did not predict the development of depression. In addition to these tests of simple slopes, we conducted a Region of Significance analysis (Hayes & Matthews, 2009) to determine more precisely the points along the continuum of pubertal development at which cortisol reactivity significantly predicted the occurrence of MDD. The results of this analysis indicated that whereas lower cortisol reactivity was a significant predictor of developing MDD at Tanner stages 2.12 and below, greater cortisol reactivity was a significant predictor of MDD at Tanner stages 3.63 and above.2 Finally, pubertal stage did not interact significantly with cortisol recovery to predict the onset of MDD.
Figure 2. Likelihood of the subsequent onset of MDD as a function of cortisol reactivity and pubertal development.
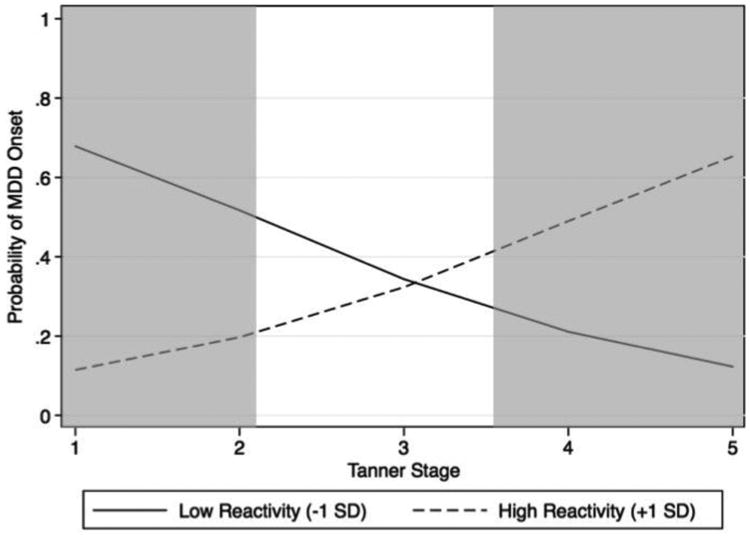
Results from the multiple logistic regression revealed a significant interaction between cortisol reactivity and pubertal development on subsequent diagnoses of MDD. Whereas cortisol hyporeactivity in girls earlier in pubertal development predicted the subsequent onset of MDD, cortisol hyperreactivity in girls later in pubertal development predicted the subsequent onset of MDD. The shaded areas represent the points along the Tanner staging continuum in which there are significant differences in probability of MDD between those who score 1 SD above the mean of cortisol reactivity and those who score 1 SD below the mean of cortisol reactivity.
4. Discussion
This study was designed to examine the utility of cortisol response to stress and pubertal development, both alone and in interaction, in predicting the onset of MDD in young girls. We assessed cortisol reactivity and pubertal stage in a sample of 9- to 15-year-old girls who spanned the range of pubertal development and found a significant interaction between cortisol reactivity and pubertal stage in predicting the development of depression. For girls at the mean Tanner stage of the sample, level of cortisol reactivity did not predict onset of depression; it is plausible that at this midpoint in pubertal development, cortisol reactivity is not as reliable a marker for the onset of MDD because of the substantial reorganization of the HPA axis that is occurring during this period. As we hypothesized, however, for girls who were more advanced in puberty, those who produced more cortisol in response to the social stress task at baseline were more likely to develop MDD by follow-up. Moreover, while unexpected, we found that within the sample of girls who were at an earlier pubertal stage, those who produced less cortisol in response to the social stress task were also more likely to develop MDD. Thus, both elevated stress reactivity later in pubertal development and blunted cortisol secretion earlier in pubertal development appear to be risk factors for the development of MDD. This aberrant responding of the HPA-axis may reflect difficulty in regulating emotional responses in a stressful context, which might in turn place individuals at risk for developing MDD (Nemeroff, 1996; Lam et al., 2009). Given that adolescence, and in particular the transition through puberty, can involve physical, social, and psychological challenges, girls' psychological and physiological responses to such stressors are likely to have important implications for their mental health.
Although we did not hypothesize that an attenuated cortisol response to the social stress task in less mature girls would predict the subsequent onset of a depressive episode, this finding is consistent with reports that younger children who exhibit depressive symptoms demonstrate blunted cortisol responses to a laboratory stressor (Luby et al., 2003; Badanes et al, 2011). For example, Hankin et al. (2010) found that in contrast to older dysthymic adolescents, who exhibit greater cortisol reactivity to a laboratory stressor than do healthy age- matched controls, younger dysthymic children (under age 12) show a blunted cortisol response to the stressor. Given that Tanner stage 2 signifies the beginning of pubertal development, our results in prepubertal girls provide further support that cortisol has a different relation with psychopathology prior to than following the onset of puberty. Such findings, taken in conjunction with our results in post-pubertal girls, demonstrate that cortisol reactivity has different effects over the transition through puberty, and may help to resolve discrepancies in the literature concerning findings that both hypo- and hyperreactivity of cortisol is related to the development of MDD (Susman et al., 1997; Keenan et al., 2013). Thus, our findings underscore the need for future studies to consider pubertal stage when examining the association between HPA-axis responsivity and mental health outcome.
In addition to finding that pubertal stage and stress reactivity interact to predict the subsequent onset of MDD, we found that both risk status (defined as maternal history of depression) and participant's age at T1 are also significant predictors of the onset of MDD. It is important to note, however, that at T1 risk status was not related to age, pubertal status, or cortisol reactivity and recovery. Given the well-documented intergenerational transmission of risk for depression (Gotlib & Colich, 2014), it is not surprising that daughters of mothers with a history of depression were more likely to have developed a depressive episode than were daughters of never-depressed mothers. The finding that younger age at entry into the study was a significant predictor of depression, however, was not predicted. It is possible that younger girls had a longer interval between their entry into the study and the follow-up period through age 18, with consequently more time during which to develop MDD.
4.1. Limitations and future directions
Our study provides empirical support for the formulation that risk for the onset of MDD is associated with unique biomarkers at specific pubertal stages. This specificity may be due to the reorganization of the HPA axis that occurs during puberty (Romeo, 2010), although longitudinal studies examining the effects of gonadal hormones on this reorganization are necessary to test this hypothesis. In this context, one limitation of this study is the use of cross-sectional data to model pubertal development; a related limitation is the use of a self-report measure of pubertal development, although we should note here that this measure has been found to be related to physicians' physical examinations of pubertal development (e.g., Coleman & Coleman, 2002). Finally, it is important to note that we did not explicitly assess the physical health of our participants at the time of the stress task. Given evidence that physical illness can affect stress cortisol levels (Nickels & Moore, 1989), it is important that future studies of cortisol reactivity explicitly assess the physical health of participants. Ideally, future work will examine within- subject changes in cortisol stress reactivity as individuals progress from childhood through pubertal development. It will also be important to broaden the age range of participants in future studies to understand more clearly the developmental trajectory of cortisol reactivity from early childhood through puberty and into adulthood, and how aberrations in HPA-axis functioning may contribute to the onset of depression at various stages of development. Finally, future studies should examine whether cortisol reactivity and pubertal status predict not only the onset of depression, but also the severity and recurrence of depressive symptoms.
5. Conclusions
In conclusion, the findings of this study contribute to a literature documenting anomalous HPA- axis functioning in MDD. Importantly, the results reported here indicate that altered cortisol functioning precedes the onset of MDD and, therefore, may be a risk factor for this disorder. The present findings also underscore the importance of considering pubertal development when examining biological processes throughout adolescence and risk for psychopathology. Indeed, the association between girls' cortisol reactivity and the subsequent onset of depression effectively switched directions depending on whether cortisol was assessed during early or late pubertal development. Understanding the neurobiological changes that occur throughout puberty is critical if we are to understand and, ultimately, to prevent the increase in depression in adolescence.
Highlights.
We measured cortisol response to a laboratory stressor in adolescent girls
Participants were followed to assess the development of Major Depressive Disorder
Cortisol hyporeactivity in early pubertal girls predicted subsequent onset of MDD
Cortisol hyperreactivity in later pubertal girls predicted subsequent onset of MDD
Association between cortisol reactivity and onset of MDD depends on pubertal development
Acknowledgments
We thank Hannah Burley, Brooke Gilbert and Maria Lemus for their help in recruiting and running the participants.
Funding: This work was supported by NIMH Grants MH074849 to IHG, F32 MH096385 to KK, and F32 MH090617 to LCFR, by NARSAD Young Investigator Awards 20814 to KK and 19018 to LCFR, and by a National Science Foundation Award to NLC.
Footnotes
In addition to the fact that none of the participants was taking antidepressant medication at the time of cortisol collection, none of the participants endorsed smoking cigarettes. One participant endorsed taking birth control pills and four participants endorsed taking allergy medication. Given these low numbers, we did not include these variables as covariates in the analyses.
Girls in the RSK and CTL groups did not differ on any of the variables assessed at T1 (time of day of cortisol collection, cortisol reactivity, cortisol recovery, age, pubertal stage, ethnicity, use of medication; all ps > .05) except for CDI-S scores: RSK girls obtained slightly but significantly higher CDI-S scores than did CTL girls, t(87) = 3.718, p < .001. As we noted above, we controlled for CDI-S scores in all subsequent analyses.
We also conducted a three-way hierarchical multiple logistic regression including risk group, pubertal stage, and cortisol reactivity and found no significant interaction of these three variables in predicting the development of MDD (OR = 1.013, 95% CI = 0.990 – 1.036, p = .282). In addition, we examined whether total cortisol output during the stress task, as measured by area under the curve with respect to ground (AUCg), predicted the onset of MDD. There was no significant main effect of AUCg (OR = 1.026, 95% CI = 0.959 – 1.097, p = .458) and no significant interaction of AUCg and pubertal stage (OR = 1.048, 95 % CI = 0.966 – 1.136, p = .259) in predicting the onset of MDD. Finally, given the effects of BMI and the experience of stressful life events on both cortisol reactivity and risk for depression, we conducted the multiple logistic regression analyses controlling for these variables in the subset of participants for whom we had these data. Neither including BMI (n = 77), nor including number of negative life events from the child-administered Life Events Checklist (LEC; Johnson & McCutcheon, 1980) (n = 59) changed the significant interaction between cortisol reactivity and pubertal stage in predicting the onset of MDD (BMI: OR = 1.014, 95% CI = 1.001 – 1.028, p = .036; LEC: OR = 1.015, 95 % CI = 1.001 = 1.029, p = .033).
Conflicts of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam EK, Doane LD, Zinbarg RE, Mineka S, Craske MG, Griffith JW. Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology. 2010;35(6):921–931. doi: 10.1016/j.psyneuen.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Worthman CM. Puberty and depression: the roles of age, pubertal status, and pubertal timing. Psychol Med. 1998;28:51–61. doi: 10.1017/s003329179700593x. [DOI] [PubMed] [Google Scholar]
- Badanese LS, Watamura SE, Hankin BL. Hypocortisolism as a potential marker of allostatic load in children: Associations with family risk and internalizing disorders. Dev Psychopathol. 2011;23:881–896. doi: 10.1017/S095457941100037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal H, Leen-Feldner EW, Badour CL, Trainor CD, Babson KA. Pubertal maturation and cortisol level in response to a novel social environment among female adolescents. J Adolesc. 2014;37:893–900. doi: 10.1016/j.adolescence.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J. How stressful is the transition to adolescence for girls? In: Colten ME, Gore S, editors. Adolescent stress: Causes and consequences. Aldine de Gruyter; New York: 1991. pp. 131–149. [Google Scholar]
- Chen M, Joormann J, Hallmeyer J, Gotlib I. Serotonin transporter polymorphism predicts waking cortisol in young girls. Psychoneuroendocrinology. 2010;34:681, 686. doi: 10.1016/j.psyneuen.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman L, Coleman J. The measurement of puberty: A review. J Adolesc. 2002;25:535–550. doi: 10.1006/jado.2002.0494. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Gunnar MR. Heightened stress responsiveness and emotional reactivity during pubertal maturation: implications for psychopathology. Dev Psychopathol. 2009;21:1–6. doi: 10.1017/S0954579409000017. [DOI] [PubMed] [Google Scholar]
- Ewart CK, Jorgensen RS, Suchday S, Chen E, Matthews KA. Measuring stress resilience and coping in vulnerable youth: the social competence interview. Psychol Assess. 2002;14:339–352. doi: 10.1037//1040-3590.14.3.339. [DOI] [PubMed] [Google Scholar]
- Ewart CK, Kolodnar KB. Social competence interview for assessing physiological reactivity in adolescents. Psychosom Med. 1991;53:289–304. doi: 10.1097/00006842-199105000-00003. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. Structured clinical interview for DSM-IV axis I disorders: clinician version. American Psychiatric Press; Washington, D.C: 1996. [Google Scholar]
- Ge Z, Conger RD, Elder GH., Jr Pubertal transition, stressful life events and the emergence of gender differences in adolescent depressive symptoms. Dev Psychol. 2001;37:404–417. doi: 10.1037//0012-1649.37.3.404. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Colich NL. Children of depressed parents. In: Gotlib IH, Hammen CL, editors. Handbook of Depression. Third. Guildford Press; New York: 2014. pp. 240–258. [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: normative changes and associations with puberty. Dev Psychopathol. 2009;21:69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Badanes LS, Abela JRZ, Watamura SE. Hypothalamic pituitary adrenal axis dysregulation in dysphoric children and adolescents: Cortisol reactivity to psychosocial stress from preschool through middle adolescence. Biol Psychiatry. 2010;68:484–490. doi: 10.1016/j.biopsych.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behav Res Methods. 2009;41:924–936. doi: 10.3758/BRM.41.3.924. [DOI] [PubMed] [Google Scholar]
- Hilt LM, Nolen-Hoeksema S. Gender differences in depression. In: Gotlib IH, Hammen CL, editors. Handbook of Depression. Third. The Guildford Press; New York: 2014. pp. 355–373. [Google Scholar]
- Johnson JH, McCutcheon SM. Assessing life stress in older children and adolescents: Preliminary findings with the Life Events Checklist. In: Sarason IG, Spielberger CD, editors. Stress and Anxiety. Vol. 7. Hemisphere; Washington, D.C.: 1980. pp. 111–125. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. K-SADS-PL: Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;39:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Keenan K, Hipwell A, Babinski D, Bortner J, Henneberger A, Hinze A, Klostermann S, Rischall M, Sapotichne B. Examining the development interface of cortisol and depression symptoms in young adolescent girls. Psychoneuroendocrinology. 2013;38:2291–2299. doi: 10.1016/j.psyneuen.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, de Jonge P, Shahly V, van Loo HM, Wang PS, Wilcox MA. The epidemiology of depression. In: Gotlib IH, Hammen CL, editors. Handbook of Depression. Third. Guilford Press; New York: 2014. pp. 7–24. [Google Scholar]
- Kovacs M. The Children's Depression Inventory manual. Multi-Health Systems; New York: 1992. [Google Scholar]
- Lam S, Dickerson SS, Zoccola PM, Zaldivar F. Emotion regulation and cortisol reactivity to a social-evaluative speech task. Psychoneuroendocrinology. 2009;34:1355–62. doi: 10.1016/j.psyneuen.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Luby JL, Heffelfinger A, Mrakotsky C, Brown K, Hessler M, Spitznagel E. Alterations in stress cortisol reactivity in depressed preschoolers relative to psychiatric and no-disorder comparison groups. Arch Gen Psychiatry. 2003;60:1248–1255. doi: 10.1001/archpsyc.60.12.1248. [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Growth and physiological development during adolescence. Ann Rev Med. 1968;19:283–300. doi: 10.1146/annurev.me.19.020168.001435. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. The corticotropin-releasing factor (CRF) hypothesis of depression: new findings and new directions. Mol Psychiatry. 1996;1:336–342. [PubMed] [Google Scholar]
- Nickels DA, Moore DC. Serum cortisol responses in febrile children. Pediatr Infect Dis J. 1989;8:16–20. doi: 10.1097/00006454-198901000-00005. [DOI] [PubMed] [Google Scholar]
- Owens M, Herbert J, Jones PB, Sahakian BJ, Wilkinson PO, Dunn VJ, Croudace TJ, Goodyer IM. Elevated morning cortisol is a stratified population-level biomarker for major depression in boys only with high depressive symptoms. Proc Natl Acad Sci, USA. 2014;111:3638–43. doi: 10.1073/pnas.1318786111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD. Pubertal maturation and programming of hypothalamic-pituitary-adrenal reactivity. Front Neuroendocrinol. 2010;31:232–240. doi: 10.1016/j.yfrne.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: correspondence between hormonal and physical development. Child Dev. 2009;80:327–37. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Papandonatos GD, Williamson DE, Dahl RE. Sex differences in the effects of pubertal development on responses to a corticotropic-releasing hormone challenge: the Pittsburgh psychobiologic studies. Ann N Y Acad Sci. 2004;1021:348–351. doi: 10.1196/annals.1308.043. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: performance versus peer rejection stressors. Dev Psychopathol. 2009;21:47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susman EJ, Dorn LD, Inoff-Germain G, Nottelmann ED, Chrousos GP. Cortisol reactivity, distress behavior and behavioral and psychological problems in young adolescents: a longitudinal perspective. J Res Adoles. 1997;7:81–105. [Google Scholar]
- Tukey JW. Addison-Wesley series in behavioral science: Quantitative methods. Reading, MA: Addison-Wesley; 1977. p. 231. [Google Scholar]
- Vrshek-Schallhorn S, Doane LD, Mineka S, Zinbarg RE, Craske MG, Adam EK. The cortisol awakening response predicts major depression: predictive stability over a 4- year follow-up and effect of depression history. Psychol Med. 2013;43:483–493. doi: 10.1017/S0033291712001213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA, Altemus M. Puberty, ovarian steroids, and stress. Ann N Y Acad Sci. 2004;1021:124–133. doi: 10.1196/annals.1308.013. [DOI] [PubMed] [Google Scholar]
- Young EA, Korszun A. Feature review: sex, trauma, stress hormones and depression. Mol Psychiatry. 2010;15:23–28. doi: 10.1038/mp.2009.94. [DOI] [PubMed] [Google Scholar]


