Abstract
Aim
We studied the use of patient/disease registries to recruit potential subjects for prospective clinical trials - describing the number, types and major benefits of using this approach.
Methods
In December 2013, we conducted a focused database search in PubMed, EMBASE, and Web of Science for studies (English language only) that used registries to recruit subjects for clinical trials published in 2004-2013. Of the 233 unique citations identified, 21 used registries to recruit subjects - 10 papers and 11 abstracts. Pearling and search for subsequent full papers of the abstracts identified 4 more papers.
Results
Our analysis, based on these 25 citations, showed 14 are related to cancer, 3 to diabetes mellitus, 1 each to stroke, asthma, and celiac disease and 5 are disease neutral. Many types of registries (population-based cancer, quality improvement, disease-specific, web-based disease-neutral registries, local general practice registers, and national health database) are used to recruit subjects for clinical trials and uncover new knowledge. Overall, 16 registries are in the US, 4 in UK, 1 each in Canada, Spain, Australia and I in many countries. Registries can identify very large number of subjects for screening for eligibility for clinical trials, especially in very large trials, rare disease trials, and trials involving minority patients.
Conclusions
Registries can retrospectively identify very large numbers of potential subjects for screening for eligibility and enrollment in prospective clinical trials. This matching can lead to more timely recruitment and help solve a major problem in conducting clinical trials.
Keywords: Patient registries, recruitment of subjects, clinical trials
Introduction
Clinical trials are needed to translate basic research findings to clinical practice. Most of these prospective trials face the major challenge of recruiting subjects (as determined by sample size calculations) needed to test the study hypothesis. Not only do trials need to recruit the number of subjects who meet study entry criteria, they must recruit them in a timely manner and within budget. In the United Kingdom (UK) fewer than one third of trials achieved their target recruitment number and half required an extension period for recruitment [1]. In the United States (US) there were enrollment delays in 90% of clinical trials with 30% under-enrolling and only 7% of sites recruited the projected number of participants [2]. More recently, of 6279 cardiovascular clinical trials in ClinicalTrials.gov 10.9% were terminated early. Of these 53.6 % occurred because of low recruitment of subjects [3]. Failure to recruit in a timely manner can have many scientific, economic and ethical consequences [4].
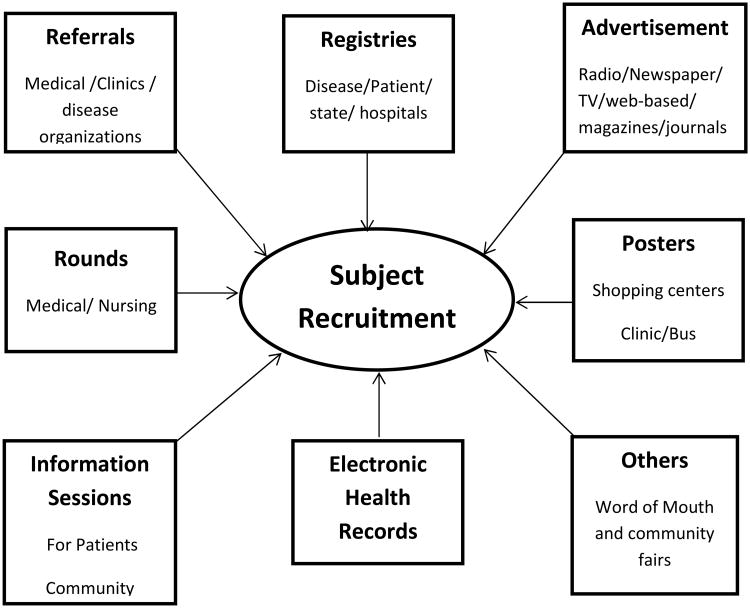
Current recruitment methods (Figure 1) include, but are not limited to, referrals (from physicians/clinics), rounds (at medical/clinic rounds), information sessions (for patients/ community groups), advertisements (in radio/television/newspapers/internet /social media), electronic health records, and registries (disease/patient) [5]. Each method has its strengths and weaknesses with success rate often depending on the study. Registries are organized databases developed for specified clinical, scientific or policy purposes [6]. Depending on the type of registry (patient, disease, product, administrative, quality improvement, etc), its data elements, structure and terminology may vary. Patient/disease registries usually contain demographic data, diagnoses and treatment details of patients with the disease, all collected in a systematic and uniform manner to serve a pre-determined purpose. They may be used to answer specific research questions, map out the natural history of a disease, assess the quality of care, track patients in medical practices, and collect post-marketing safety data, as required by regulatory agency, in patients taking new medications or using new devices. Often not developed for recruiting subjects for specific clinical trials, patient/disease registries can be used to identify potential subjects for clinical trials, especially for large, multicenter clinical trials or clinical trials involving patients with rare diseases or ethnic minorities. Such registries, when linked with health record information on study entry criteria, become “research” registries that can facilitate recruitment, making it more efficient and increasing the likelihood of success [7]. In November 2009 the National Institutes of Health (NIH) announced the first US research study recruitment registry (ResearchMatch) as one of the strategies to meet the recruitment challenge for clinical trials in the US [8, 9].
Figure 1. The many ways to recruit subjects for prospective clinical trials.
Our study examines the use of patient/disease registries to identify, screen for eligibility and recruit potential subjects for clinical trials published during the decade of 2004-2013 -describing how many registries were used, the types of registries used, the aims of the clinical trials, and the major benefits of using this approach. The focus is only clinical trials, not all clinical research.
Methods
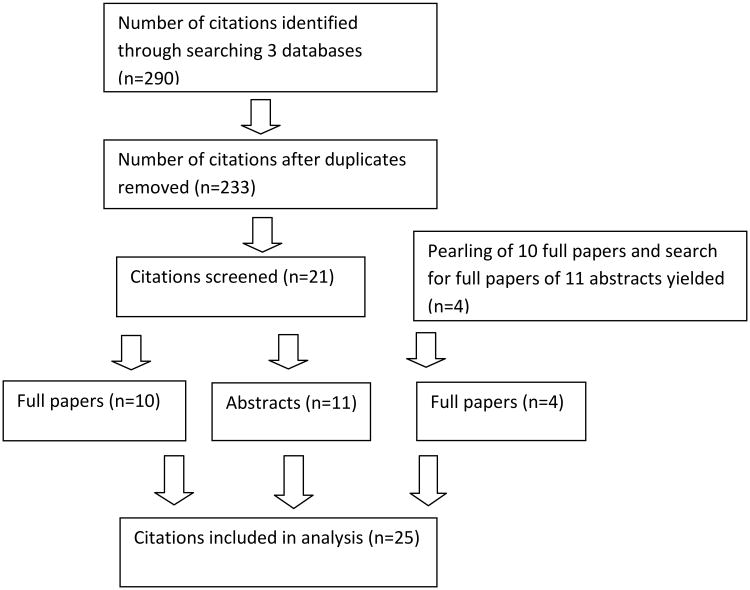
We conducted focused database searches in EMBASE, PubMed, and Web of Science for studies that acknowledged using disease/patient registries to recruit potential subjects for clinical trials. For each search, a set of ‘registry’ keywords (“registries”, “registry”, “register”) were combined with a set of ‘recruitment’ keywords (recruitment and its variations) using the Boolean operator ‘AND’. In addition to keywords, appropriate controlled vocabulary terms (registries [majr:noexp], patient selection[majr], register/exp, ‘disease registry’/exp, ‘patient selection’/exp/mj) were used in the EMBASE (EMTREE) and PubMed (MeSH) searches. All searches were restricted to English language studies and only articles published during the decade from 2004 to 2013 were considered for inclusion. Conference abstracts were captured through the Web of Science search. Search specificity was emphasized in order to eliminate the large number of false positives. The three database searches, which were originally conducted in December 2013 and rerun in July 2014, yielded a combined total of 290 citations. These citations were exported into Thomson Reuters Endnote XV, at which point 57 were identified as duplicates and therefore removed, leaving 233 unique citations (Figure 2). Two authors (MHT and MPM) reviewed each of the 233 citations and concurred on those identified as using patient/disease registries to recruit subjects for clinical trials using the criteria: (a)“registries”/ “registry” / “registers” /“database” as a source of to recruit potential subjects and (b) “clinical trial(s)” in the text of the publication as the intent of the prospective clinical trial. From the references of all citations, pearling was done to determine whether they included other citations indicating registries were used for recruiting subjects in clinical trials between 2004 and 2013. To find out whether any of the 11 abstracts had subsequently been published as full papers, we conducted a PubMed search of the authors and titles of the abstracts.
Figure 2.
Flow chart showing the processes of identification, screening, elimination and inclusion in this review.
Results
Number of citations identified
Of these 233 citations, 21 studies are identified as using patient/disease registries to recruit subjects for clinical trials - 10 papers (Table 1) and 11 abstracts (Table 2). From the references of all 10 papers, pearling identified 3 new papers - 2 were clinical trials [13, 14] and 1 disease-neutral registry [15] used to recruit subjects for clinical trials. One full paper [16] of an abstract [17] from the initial search was found. In our analysis the 10 papers from the initial search, the 3 pearling papers and 1 full paper of the abstract subsequently published are combined, giving 14 papers and 11 abstracts giving a total of 25 citations.
Table 1. Registries Used to Recruit Subjects for Clinical Trials – Papers from Search/Pearling.
| Reference | Registry | Clinical Trial | Study Aims | Subjects |
|---|---|---|---|---|
| 1. Cadmus-Bertram LAC et al 2011 [10] | Yale New Haven Connecticut Tumor Registry | Increasing or Maintaining Physical Activity during Cancer Treatment (IMPACT) Study | Determine whether it is feasible to recruit newly diagnosed breast cancer survivors in a randomized control trial to study the effect of exercise. | Identified 468 Eligible 405 (86.5% of identified) Enrolled 50 |
| 2. Rogerino A et al 2009 [11] | Pennsylvania and New Jersey State Cancer Registries plus 3 hospital registries. | Physical Activity and Lymphedema (PAL) trial | Recruit racially and geographically diverse breast cancer survivors for community based exercise intervention. | Invited 28921 Responded 3200 Eligible 506(1.74% of identified) Enrolled 295 |
| 3. Irwin ML et al 2008 [12] | Connecticut Tumor Registry | Yale Exercise and Survivorship (YES) Study | Comparing recruitment of breast cancer survivors from tumor registry with other methods. | Identified 1072 Eligible 763 (71.1% of identified) Enrolled 75 |
| 4. Burnell M et al 2011 [18] | UK Local health authority registers | UK Collaborative Trial on Ovarian Cancer Screening (UKCTOCS) | To reduce healthy volunteer effect in ovarian cancer screening trials. | Invited 1,243,282 Eligible 288,935(23.2% of identified) Enrolled 202,638 |
| 5. Andersen MJ et al 2005 [19] | State Cancer Registry in Washington | Recruiting women with newly diagnosed ovarian cancer for psychosocial intervention trial | Study the use of state cancer registry to rapidly recruit women with newly diagnosed late stage ovarian cancer. | Identified 441 Eligible 179 (40.6% of identified) Enrolled 60 |
| 6. Hall AE et al 2013 [20] | State cancer registry in New South Wales, Australia | Recruiting patients with hematological malignancies | Compare 2 different methods of recruitment in a randomized clinical trial | Identified 800 Eligible 732 (91.5% of identified) Responded 268 |
| 7. Ramirez AG et al 2008 [21] | South Texas cancer registry | Recruiting Hispanic patients for cancer genetics registries. | Compare 3 different recruitment methods in Hispanic cancer patients for cancer genetics registries | Identified 26100 Eligible 1145 (4.4% of identified) Enrolled 444 |
| 8.Weng CH et al 2010 [25] | New York Presbyterian Hospital Ambulatory Care Network Diabetes Registry | Trial Evaluating the Cardiovascular Outcomes for Sitagliptin (TECOS) | Compare recruitment of subjects from the clinical data warehouse with from Diabetes Registry for TECOS (n=110 at this center) | Identified 2033 Eligible 437 (21.5% of identified) Enrolled 29 |
| 9. The TRIGR Study Group 2011 [26] | Unspecified local Type 1 Diabetes Registries | Trial to reduce IDDM in genetically at risk (TRIGR) | Describes the recruitment of subjects for an international dietary intervention trial. | No details |
| 10. Harris PA et al 2012 [31] | ResearchMatch, a national electronic recruitment website | Disease-neutral clinical trials by investigators in CTSA consortium | Describes the development and utilization of this national registry for people interested in clinical trials. | No details |
| Pearling papers | ||||
| 11. Snyder DC et al 2008 [13] | Tumor registries at Duke University Medical Center and VA Medical Center, Durham | Effect of dietary intervention in breast and prostate cancer survivors (FRESH START) | Compare characteristics of subjects who are self- referred and from registries | Identified 1812 Eligible 334 (18.4% of identified) Enrolled 304 |
| 12. Vallance JKH et al 2007 [14] | Alberta, Canada, Cancer Registry | Physical activity in breast cancer survivors. | Determine effects of print materials and pedometers on physical activity and quality of life in breast cancer survivors. | Identified 1590 Enrolled 377 (23.7% of identified) |
| 13. Harris PA et al 2005 [15] | Vanderbilt University local electronic recruitment website | Disease-neutral clinical trials by investigators in Vanderbilt | Describe development and utilization of this local registry for people interested in clinical trials in Memphis. | No details |
| 14. Ashing-Giwa K, Rosales M 2012 [16] | California cancer registry, local hospital registries | Longitudinal study on health related quality of life (HRQOL) in breast cancer survivors. | Recruitment and retention strategies of minority breast cancer survivors in psycho- oncology trial. | Identified 1882 Eligible 483 |
Table 2. Registries Used to Recruit Subjects for Clinical Trials – Abstracts from Search.
| Reference | Registry | Clinical Trial | Study Aims | Subjects |
|---|---|---|---|---|
| 1.Sahota P et al 2012 [28] | One US center's stroke registry. | Safety of Intravenous Mononuclear cells for Acute Stroke (SIMVAS) | Using the registry to recruit patients with acute ischemic stroke for pilot study. | Identified 534 Eligible 25 (4.7% of identified) Enrolled 10 |
| 2. Aung T et al 2011 [27] | Centrally held registers and local GP registers in UK | A Study of Cardiovascular Events iN Diabetes (ASCEND) | Determine whether 100 mg aspirin daily vs placebo and/or 1 gm omega 3 fatty acids daily vs placebo prevents “serious vascular events”. | Invited 423,286 Eligible 26480 (6.3% of identified) Enrolled 15,481 |
| 3. Rawlings J et al 2011 [23] | St Marks Hospital's Polyposis Registry, Middlesex, UK | Trial in children with familial adenomatous polyposis (CHIP) | Study effect of Celecoxib vs placebo in children at risk for polyposis | Eligible 29 Interested 22 |
| 4. Kinney A et al 2012 [24] | Five population- based cancer registries in US. | Family CARE Trial for colorectal cancer | Recruit family members of probands with colorectal cancer for screening. | Identified 3893 Eligible 715 (18.4% of identified) Responded 48 |
| 5. Ashing- Giwa K, Rosales M 2012 [17] | California cancer registry, local hospital registries | Longitudinal study on health related quality of life (HRQOL) in breast cancer survivors. | Recruitment and retention strategies of minority breast cancer survivors in psycho-oncology trial. | Eligible 587 Enrolled 375 |
| 6. Parikh PS et al 2010 [29] | Severe Asthma Research Program Network Registry | A phase II clinical trial in asthma at Cleveland Clinic | Compare recruitment using electronic medical record and a registry. | Eligible 109 Interested 41 Enrolled 11 |
| 7. Puppa EL et al 2012 [30] | One Center's Celiac disease clinic registry | No specific clinical trials identified | Establish a research registry for celiac disease patients. | Eligible 780 Enrolled 136 |
| 8. Harris P et al 2010 [9] | ResearchMatch- a national electronic recruitment website | Disease-neutral clinical trials by investigators in CTSA consortium | Describe the development of this national electronic register who are interested in clinical trials conducted by investigators from CTSA consortium | No details |
| 9. Sullivan FM et al 2011 [32] | Scottish Health Research Register | No specific clinical trials identified | Describe the development and utilization of SHARE which has a million people | No details |
| 10. Astigarraga A et al 2011 [22] | National registry of histiocytosis patients in Spain | Intervention trials in patients with histiocytosis | Describe the number of hospitals and protocols in national and international histiocytosis trials | No details |
| 11. Atreja A et al 2010 [33] | Cleveland Clinic registry for Trial X | Disease-neutral clinical trials registry matching patients with investigators for Trial X initiative of CTSA consortium | Describe an electronic register of patients interested in clinical trials and matching them with investigators. | No details |
Diseases covered
Of these 25 citations, 14 are related to cancer (6 to breast cancer [10-14, 16,17], 2 to ovarian cancer [18-19], 1 to hematological cancer [20], 1 to prostate cancer[13], 1 to cancer genetics [21], 1 to histiocytosis [22], 1 to familial adenomatous polyposis [23], and 1 to colorectal cancer [24]), 3 to diabetes mellitus [25-27], 1 to stroke [28], 1 to asthma [29], 1 to celiac disease [30] and 5 are disease neutral [9, 15, 31-33].
Types and locations of registries
Of the registries related to breast cancer, 5 are state tumor registries in the US [10-13, 16, 17] and 1 in Canada [14]. One ovarian cancer trial used 13 local trust registers in the UK [18] and the other used a state cancer registry in the US [19]. The prostate cancer study used a state registry in the US [13]. The familial adenomatous polyposis trial used one hospital's registry in the UK [23]. The CARE colorectal cancer screening trial used 5 tumor registries in the US [24]. The cancer genetics clinical trial used local cancer registries in US [21], the histiocytosis registry was a national registry in Spain [22] and the hematological cancer trial used a state registry in Australia [20]. In the 3 registries related to diabetes, the first used a quality improvement registry in an US medical center [25], the second, an international study, used center specific type 1 diabetes registries (without specifying the center location) [26], and the third used central and local registers in the UK [27]. The Stroke Trial used a stroke registry in one US center [28]. The asthma study used an US severe asthma network registry [29]. The celiac disease study used one US clinic's celiac disease registry [30]. Of the 4 disease neutral registries in the US, 1 is university-based [15] and 3 national [9, 31, and 33]. Of the national registries, 1 is a national registry of the consortium of Clinical Translational Science Award (CTSA) centers in the US [9, 31], 1 is a national registry as part of the CTSA-industry partnership venture [33], and 1 is in Scotland [32]. Overall, 16 registries are in the US [9-13, 15-17, 19, 21, 24, 25, 28-31, 33], 4 in UK [18, 23, 27,32], 1 in Canada[14], 1 in Spain [22], 1 in Australia [20] and 1 involved many countries [26].
Identifying, screening and enrolling subjects
Not all citations give the same details on identifying, screening and enrolling potential subjects for each clinical trial. Tables 1 and 2 show the number of subjects identified, the number considered eligible after screening and the number enrolled in the various clinical trials. Most of the registries in our study identified large to very large number of potential subjects for a specific clinical trial. Screening reduced the number identified to a much smaller number of eligible subjects (1.74 to 91.5% of those identified). Despite the smaller number of eligible subjects, there were enough to enroll to test the study hypothesis.
Aims of clinical trials
The aims of the clinical trials covered a wide spectrum (Tables 1 and 2):
Effect of exercise in breast cancer survivors [10-12 and 14];
Psychosocial intervention in breast cancer survivors [19];
Comparing different methods of recruiting in hematological cancer patients [20], in cancer survivors for a genetics registry [21], diabetes drug trial [25] and asthma study [29];
Dietary interventions in breast and prostate cancer survivors [13] and in those genetically at risk for type 1 diabetes [26];
Drug trials - sitagliptin in patients with diabetes [25], Celecoxib in relatives of patients with familial adenomatous polyposis [23]; and aspirin and omega 3 fatty acids in patients with diabetes;
Intravenous mononuclear cells for acute ischemic strokes [28];
Unspecified Phase II asthma trial [29];
Unspecified clinical trials in patients with celiac disease [30];
Unspecified histiocytosis clinical trials [22]; and
Clinical trials using disease neutral registries [9,15,31, 32 and 33]
Benefits of using registries to recruit
(a) Large trials
Registries can be used to recruit potential subjects for small pilot studies (number of subjects recruited (n) =10) [28], mid-sized (n = 375) [16] and very large (n = 202,638) [18] trials (see Tables 1 and 2). In the UKCTOCS 1,243,282 postmenopausal women identified from the 13 registers were invited [18, 34]. Of the 288,955 (23.2% of those invited) eligible subjects after screening, 202,638 (70.1% of those eligible) were randomized. The recruitment period was only 4.3 years. In the ASCEND trial, 423,286 patients with diabetes identified from centrally held and local registers were invited to participate [27]. Of the 26,480 (6.25% of those invited) patients who entered run in, 15,480 (58.5% who entered run in) were randomized. These 2 clinical trials show that using registries which have been in existence for years allows identification of very large numbers of potential subjects and recruitment of needed subjects in a relatively short period of time [18, 27].
(b) Geographically diverse and ethnic minorities
Registries also facilitate recruitment of geographically, racially and ethnically diverse study subjects [11]. Rogerino et al recruited their subjects from 2 states. Ashing-Giwa and Rosales identified 587 potential subjects for their longitudinal psycho-oncology study in African American and Latina American breast cancer survivors [16]. Ramirez et al used local registries in Texas to identify, screen and recruit Hispanic patients with cancer to enroll in their Cancer Genetics Registry [21].
(c) Comparing methods of recruitment
Registries enable comparison of recruitment methods. In the TECOS trial, electronic medical records identified and resulted in the recruitment of more patients in a shorter time period than a quality management registry for diabetes [25]. In the asthma study, electronic medical records also identified more patients than the registry approach [29]. In both studies, the electronic records database had more patients (both out-patients and in-patients) to screen than the registries. In an Australian study a more comprehensive informed consent form was not more effective in recruitment than the standard informed consent in patients with hematological cancer [20]. Finally, 3 different ways of approaching Hispanic patients were compared in the Texas Cancer genetics study [21]. Interpersonal phone approach was the most effective way.
(d) Disease-Neutral Registries
Our study identified 5 web-based disease-neutral registries. One is local [15] and the other 4 national in scope [9, 31-33]. The US national disease-neutral registries are related to the CTSA initiative [9, 31, and 33]. The Scottish Health Research Register (SHARE) uses its national health database.
(e) Others
Recruitment of subjects for clinical trials can be accelerated by combining patient/disease registries and clinical data warehouses (which are registries). In the TECOS trial, the site that combined electronic medical record and registry approaches became the leading recruiting site in the US and the third globally [25]. Using a rare disease registry can facilitate recruitment of subjects in national and international clinical trials [22]. Finally, registries can facilitate the recruitment of relatives of registry patients for disease screening [24] and drug trials [23]. In both, the registry probands were invited to contact their relatives to consider participating in the clinical trials.
Discussion
Our study shows that many types of registries have been used to identify, screen and recruit potential subjects for many types of prospective clinical trials to uncover many aspects of new knowledge in many diseases. Types of registries included population-based cancer registries, quality improvement registry, disease-specific registries, local general practice registers, national health database, and web-based disease-neutral registries. The wide spectrum of diseases includes cancer, diabetes, asthma, celiac disease and ischemic stroke. Interventions studied in the clinical trials include drugs, diet, exercise and disease-specific print material. The clinical trials using registries to recruit potential subjects were conducted in the US, UK, Australia, Spain and Canada.
Our study shows cancer registries to be the most frequently used to recruit potential subjects for clinical trials. Of the 25 citations, 14 used cancer registries to recruit potential subjects for clinical trials. The US National Program of Cancer Registries, established by Congress in 1992, collects demographic and treatment information about cancer patients. These cancer registries cover about 96% of the US population with cancer and are a good resource for identifying, screening and recruiting potential subjects for cancer clinical trials. Cancer registries were used for such purposes as early as 1990 [35].
Registries facilitated timely recruitment of very large number of potential subjects in studies requiring such large samples [18, 27, and 34]. Ethnic minorities are under-represented in health research in the US. Despite the NIH 1993 Revitalization Act to include ethnic minorities in health research [36], this is still a problem. Because population-based cancer registries have all patients with cancer, irrespective of ethnicity, they can be used to oversample ethnic minority patients for cancer clinical trials. Both examples illustrate that multiple registries can identify more potential patients who can be contacted, screened and enrolled.
If recruiting subjects for clinical trials in high prevalence diseases is challenging, recruiting subjects for clinical trials in rare diseases (fewer than 200,000 people with the disease in the US) is much more challenging. Recognizing this, the NIH supported the development of the Rare Disease Clinical Research Network (RDCRN) Contact Registry in 2004 (37). Initially it included 40 rare diseases. In 2009 it was expanded to include 140 rare diseases (38). In 2011 the case for a global registry of rare diseases was made to better understand rare diseases and bring better treatment (39). Registries of the same rare disease in many nations can collaborate in a global clinical trial and be successful in recruitment for they increase the pool of potential patients.
Electronic medical records are increasingly used to recruit subjects for clinical trials. Our study reports comparisons of recruitment of subjects for 2 clinical trials using electronic medical records and registries, one in diabetes [25] and the other in asthma [29]. Neither registry was a research registry. One was a quality management program registry covering only ambulatory clinic diabetes patients [25]; the other was a registry of volunteer patients with severe asthma [29]. Recognizing the differences between electronic medical records and registries, each having its advantages and disadvantages, Weng et al proposed the design ELiXR (Eligibility Criteria Extraction and Representation) “to generate protocol aware research registries to facilitate electronic screening for clinical trial recruitment”. Registries with links to patient health databases have been identified as a strategy to enhance subject recruitment for clinical trials [7]. Such research registries with information on the demographics and study entry criteria can be more efficient in facilitating identification, screening and recruitment of subjects for clinical trials. Disease-neutral registries are also increasing in number, serving to match people interested in participating in clinical trials with researchers. These registries may be local or national in scope. In the US, the ResearchMatch registry was launched in November 2009 by the NIH targeting health institutions in the NIH CTSA consortium [8, 9, and 31]. People (patients with disease or conditions and healthy volunteers) interested in participating in clinical research can register using a secure web-site and give informed consent. By December 2014 [40], there were 69,735 volunteers in this national registry and 2333 researchers in 100 institutions. Matching of volunteers with researchers occurred in 411 studies. The Scottish Health Research Register (SHARE), another national disease-neutral register created to enhance recruitment to clinical trials, invites Scotland's residents who wish to participate in health related research to register on a secure website and give informed consent for their medical records to be matched with approved studies [32]. There are other web-based disease-neutral registries [31].
There is a recruitment phenomenon (Lasagna's Law or “Recruitment Funnel”) which can be paraphrased to state only 10% of the number of patients originally thought available for a clinical trial were eligible for enrollment after going through the various filters (contact, screening, run-in and enrollment) of recruitment (41). In 1979, Lasagna (42) described his involvement in recruiting for an analgesic medication efficacy trial in post-surgical patients. Of the 8027 patients available in the surgical wards during the study period, only 4928 were available for screening. After screening only 649 were left for interview. After the interview processes and considering other study criteria, only 338 remained. Of these 246 gave informed consent (3.1%). Of these 146 never received the analgesic medication, leaving only 100 subjects for the clinical trial.
There are a few reports on the reasons why eligible participants do not wish to participate in cancer clinical trials. In Weinstein et al (43) survey of cancer patients eligible for clinical trials, the top 3 reasons for not participating were fear of adverse events, concern of random assignment of treatment, and cost. In the Penberthy et al survey (44), the top 3 reasons given for not wanting to participate in cancer clinical trials were extra cost and logistical problems, lack of interest in the trials, and avoidance of specific treatment. In the Meropol et al survey (45), the top 3 reasons for not wanting to participate in cancer clinical trials were fear of side effects of clinical trial treatment, uncomfortable with randomization to treatment, and fear of receiving a placebo. In the Cooley et al survey (46) on quality of life in female lung cancer patients, the top 3 reasons for refusing to participate were health limitation, not interested and no time/inconvenience.
Patient registries may be able to identify very large numbers of potential patients for clinical trials. In so doing, it increases the chances of having many more patients screened, studied in the run-in period and eventually enrolled. In the UKCTOCS, only 23.2% of those invited were considered eligible after screening (18, 34) and 70.1% of those eligible were randomized. In the ASCEND trial only 6.25% of those invited entered run in (27) and 58.5% of those entering run-in were randomized.
Registries used for clinical research must meet local, regional, and national regulatory guidelines [47]. The registry must be stored in a secured computerized database. Use of the registry data must meet the requirements of the specific database. All these are intended to keep patients' protected health information confidential. Websites used for registering patients must be secured to ensure subject confidentiality.
This study is not a systematic review of published clinical trials that used registries as sources to recruit subjects. It is a descriptive review that used targeted search strategies in 3 databases (PubMed, EMBASE and Science Web) to capture citations that clearly acknowledged use of a patient/disease registry for subject identification, screening and recruitment for published clinical trials in 2004-2013. That we found 3 relevant papers during pearling and 1 full paper of an abstract indicates our search missed a number of clinical trials that recruited from registries. From a search perspective, the difficulty in retrieving all studies that acknowledge use of registries for subject recruitment to clinical trials is that the reporting and discussion of such recruitment methods is often relegated to the full-text method section, which PubMed and other key citation databases do not search. Consequently, keyword searches for registry and recruitment terms in citation titles and abstracts are rendered partially incomplete at capturing relevant studies. This partially explains why our searches only retrieved 21 relevant citations. Of the 233 unique citations, 129 used registries for retrospective studies which were not prospective clinical trials. We do not discuss them in this paper as our focus is on the use of registries for recruitment of subjects for clinical trials.
Conclusion
Our findings demonstrated that many types of registries have been used to identify, screen and recruit potential subjects for many clinical trials. Registries, being large databases, can very quickly and efficiently identify large number of potential subjects for a clinical trial. They have been and should be used more often to identify subjects for prospective clinical trials leading to more timely recruitment of subjects. In so doing, they can help solve the most critical problem of clinical trials which is recruitment. The costs, benefits and limitations of registries, as compared to electronic medical records, warrant further study.
Acknowledgments
We wish to acknowledge Michelle Bass, Taubman Health Sciences Library, University of Michigan, for her data collection efforts. We also wish to thank Dr. William Herman of University of Michigan for reviewing the manuscript.
The project described was supported by Grant Number P30DK020572 (MDRC) and Matthew Thomas' studentship was supported by T32DK007245 both from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Conflict of interest: The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McDonald AM, Knight RC, Campbell MK, et al. What influences recruitment to randomized controlled trials? A review of trials funded by two UK funding agencies. Trials. 2006;7(9):1–8. doi: 10.1186/1745-6215-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strasser JE, Cola P, Rosenblum D. Evaluating various areas of process improvement in an effort to improve clinical research: Discussions from the 2012 Clinical Translational Science Award (CTSA) Clinical Research Management Workshop. Clin Trans Sci. 2013;6:317–320. doi: 10.1111/cts.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernardez-Pereira S, Lopez RD, Carrion MJM, et al. Prevalence, characteristics, and predictors of early termination of cardiovascular clinical trials due to low recruitment: Insights from the ClinicalTrials.gov registry. Am Heart J. 2014;168:213–219 e1. doi: 10.1016/j.ahj.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Lovato LC, Hill K, Hertert S, Hunninghake DB, Probstfield JL. Recruitment for Controlled Clinical Trials: Literature Summary and Annotated Bibliography. Controlled Clinical Trials. 1997;18:328–357. doi: 10.1016/s0197-2456(96)00236-x. [DOI] [PubMed] [Google Scholar]
- 5.Lamberti MJ, Mathias A, Myles JE, Howe D, Getz K. Evaluating the Impact of Patient Recruitment and Retention Practices. Drug Information Journal. 2012;46:573–580. [Google Scholar]
- 6.Gliklich RE, Dreyer NA, editors. Registries for Evaluating Patient Outcomes: A User's Guide. 2nd. Rockville, MD: Sep, 2010. AHRQ Publication No. 10-EHC049. [PubMed] [Google Scholar]
- 7.Treweek S. Recruitment to trials - why is it hard and how might we make it less so? Trials. 2011;12(Suppl 1):A110. [Google Scholar]
- 8.NIH Press Release November 10, 2011 NIH Announces First National Research Study Recruitment Registry. [Accessed on June 6, 2014]; www.nih.gov/news/health/nov2009/index.htm.
- 9.Harris PA, Scott KW, Lightner C, et al. ResearchMatch.org – A National Research Recruitment. Clin and Trans Sci. 2010;3:S6. [Google Scholar]
- 10.Cadmus-Bertram LA, Chung G, Yu H, Salovey P, Irwin M. Feasibility of Institutional Registry-Based Recruitment for Enrolling Newly diagnosed Breast Cancer Patients in an Exercise Trial. J Physical Activity and Health. 2011;8:955–963. doi: 10.1123/jpah.8.7.955. [DOI] [PubMed] [Google Scholar]
- 11.Rogerino A, Grant LL, Wilcox H, III, Schmitz KH. Geographic Recruitment of Breast Cancer Survivors into Community–Based Exercise Interventions. Med Sci Sports Exerc. 2009;41:1413–1420. doi: 10.1249/MSS.0b013e31819af871. [DOI] [PubMed] [Google Scholar]
- 12.Irwin ML, Cadmus L, Alvarez-Reeves M, et al. Recruiting and Retaining Breast Cancer Survivors into a Randomized Controlled Exercise Trial. The Yale Exercise and Survivorship Study Cancer. 2008;112(Suppl):2593–2606. doi: 10.1002/cncr.23446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snyder DC, Sloane R, Lobach D. Differences in Baseline Characteristics and Outcomes at 1- and 2-Year Follow-up of Cancer Survivors Accrued via Self-Referred versus Cancer Registry in the FRESH START Diet and Exercise Trial. Cancer Epidemiol Biomarkers Prev. 2008;17:1288–1294. doi: 10.1158/1055-9965.EPI-07-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vallance JKH, Courneya KS, Plotnikoff RC, Yasui Y, MacKey JR. Randomized Controlled Trial of the effects of Print Materials and Step Pedometers on Physical Activity and Quality of Life in Breast Cancer Survivors. J Clin Oncol. 2007;25:2352–2359. doi: 10.1200/JCO.2006.07.9988. [DOI] [PubMed] [Google Scholar]
- 15.Harris PA, Lane L, Biaggioni I. Clinical Research subject Recruitment: The Volunteer for Vanderbilt Research Program. J Am Med Inform Assoc. 2005;12:608–613. doi: 10.1197/jamia.M1722. www.volunteer.mc.vanderbilt.edu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashing-Giwa K, Rosales K. Recruitment and Retention Strategies of African American and Latina American Breast Cancer Survivors in a Longitudinal Psycho-Oncology Study. Oncology Nursing Forum. 2012;39:E434–442. doi: 10.1188/12.ONF.E434-E442. [DOI] [PubMed] [Google Scholar]
- 17.Ashing-Giwa K, Rosales M, Fernandez A. Recruitment and Retention of African-American and Latina-American Breast Cancer Survivors into a Psychoeducation Trial. Psycho-Oncology. 2011;20:63. [Google Scholar]
- 18.Burnell M, Gentry-Maharaj A, Ryan A, et al. Impact on Mortality and Cancer Incidence Rates of using random invitation from population registers for recruitment to trials. Trials. 2011;61:1–10. doi: 10.1186/1745-6215-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersen MR, Schroeder T, Gaul M, Moinpour C, Urban N. Using a population-based cancer registry for recruitment of newly diagnosed patients with ovarian cancer. Am J Clin Oncol. 2005;28:17–20. doi: 10.1097/01.coc.0000138967.62532.2e. [DOI] [PubMed] [Google Scholar]
- 20.Hall AE, Sanson-Fisher RW, Lynagh MC, Threlfall T, D'Este CA. Format and readability of an enhanced invitation letter did not affect participation rates in a cancer registry-based study: a randomized controlled trial. J Clin Epid. 2013;66:85–94. doi: 10.1016/j.jclinepi.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 21.Ramirez AG, Miller AR, Gallon K, de Majors SSM, Chalela P, Aramburo SG. Testing three different cancer genetics registry recruitment methods with Hispanic cancer patients and their family members previously registered in local cancer registries in Texas. Community Genet. 2008;11:215–223. doi: 10.1159/000116882. [DOI] [PubMed] [Google Scholar]
- 22.Astigarraga A, Sastre S, Garci'a-Obrego'n JM, Melo CM, Pardo N. Recruitment and participation of Spanish hospitals in national and HS protocols in the last 15 years. Ped Blood Cancer. 2011;56:689. [Google Scholar]
- 23.Rawlings J, Hyer W, Neale KF, Philips RKS, Clark SK. Feasibility of recruiting children with polyposis to a clinical trial. Familial Cancer. 10:S34–S35. [Google Scholar]
- 24.Kinney AY, Simmons RG, Lee YCA, et al. Finding a needle in a haystack: Population-based approaches to recruiting relatives of crc patients into a behavioral intervention trial. Asia-Pacific Journal of Clinical Oncology. 8:285. [Google Scholar]
- 25.Weng CH, Bigger JT, Busacca L, Wilcox A, Getaneh A. Comparing the Effectiveness of a Clinical Registry and a Clinical Data Warehouse for Supporting clinical Trial Recruitment: a Case Study. American Medical Informatics Association 2010 Symposium Proceedings. :867–871. [PMC free article] [PubMed] [Google Scholar]
- 26.The TRIGR Study Group. The Trial to Reduce IDDM in the Genetically at Risk (TRIGR) study: recruitment, intervention and follow-up. Diabetologia. 2011;54:627–633. doi: 10.1007/s00125-010-1964-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aung T, Cowan H, Haynes R, Bowman L, Armitage J. Recruiting patients cost-effectively by mail. Trials. 2011;12(Suppl 1):A117. [Google Scholar]
- 28.Sahota P, Vahidy F, Hovis JP, et al. Challenges to recruiting stroke patients into cell therapy clinical trials. Stroke. 43(2) [Google Scholar]
- 29.Parikh PS, Ratanamaneechat S, George D, et al. Recruitment and Enrollment of Asthmatics in a Phase II Clinical Trial. Am J Respir Crit Care Med. 2010;181:A2735. [Google Scholar]
- 30.Puppa EL, Fasano A. Facilitating retrospective and prospective research recruitment through development of a research registry. Gastroenterology. 2012;142:S712–S713. [Google Scholar]
- 31.Harris PA, Scott KW, Lebo L, Hassan N, Lightner C, Pulley J. ResearchMatch: A National Registry to Recruit Volunteers for clinical Research. Acad Med. 2012;87:66–73. doi: 10.1097/ACM.0b013e31823ab7d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sullivan FM, Treweek S, Grant A, et al. Improving recruitment to clinical trials with a register of a million patients who agree to the use of their clinical records for research in the Scottish Health Research Register(SHARE) Trails. 2011;12(Suppl 1):A115. [Google Scholar]
- 33.Atreja A, Patel CO, Trunick C, Garg V, Serpil E. TRIALX: A web-based patient recruitment registry and platform. Clin Trans Sci. 2010;3:S16. [Google Scholar]
- 34.Menon U, Ryan A, Sharma A, et al. Recruitment to multicenter trials – lessons from UKCTOCS: descriptive study. BMJ. 2008;337:a2079. doi: 10.1136/bmj.a2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newcomb PA, Love RR, Phillips JL, Buckmaster BJ. Using a population –based cancer registry for recruitment in a pilot cancer control study. Pre Med. 1990;19:61. doi: 10.1016/0091-7435(90)90008-8. [DOI] [PubMed] [Google Scholar]
- 36.National Institutes of Health. NIH policy and guidelines on the inclusion of women and minorities as subjects in clinical research. [accessed September 20, 2014];2001 Retrieved from http://grants.nih.gov/grants/funding/women_min/guidelines_amended_10_2001.htm.
- 37.Richeeson RL, Lee HS, Cuthbertson D, Llyod J, Young K, Krischer JP. An automated communication system in a contact registry for persons with rare diseases: Scalable tools for identifying and recruiting for clinical research participants. Contemporary Clinical Trials. 2009;30:55–62. doi: 10.1016/j.cct.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richeeson RL, SAutphen R, Sheroff D, Krischer JP. The Rare Disease Clinical Research Network Contact Registry Update: Features and Functionality. Contemporary clinical Trials. 2012;33:647–656. doi: 10.1016/j.cct.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forrest CB, Bartek RJ, Rubinstein Y, Groft SC. The case for a global rare-disease registry. The Lancet. 2011;377:1057–1059. doi: 10.1016/S0140-6736(10)60680-0. [DOI] [PubMed] [Google Scholar]
- 40.www.ResearchMatch.org (accessed December 13, 2014)
- 41.Gul R, Ali PA. Clinical trials: the challenge of recruitment and retention of participants. J Clin Nursing. 2010;19:227–233. doi: 10.1111/j.1365-2702.2009.03041.x. [DOI] [PubMed] [Google Scholar]
- 42.Lasagna L. Problems in publication of clinical trial methodology. Clin Pharmacol Ther. 1979;25:751–753. doi: 10.1002/cpt1979255part2751. [DOI] [PubMed] [Google Scholar]
- 43.Weinstein DJ, Thomas CA, Emery IF, et al. Assessment of Perceived Cost to the Patient and Other Barriers to Clinical Trial Participation. J Oncology Practice. 2011;7:330–333. doi: 10.1200/JOP.2011.000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Penberthy LA, Brown R, Wilson-Genderson M, Dahman B, Ginder G, Siminoff L. Barriers to the therapeutic clinical trial enrollment: Differences between African-Americans and White cancer patients identified at the time of eligibility assessment. Clinical Trials. 2012;9:788–797. doi: 10.1177/1740774512458992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meropol NJ, Buzaglo JS, Millard J, et al. Barriers to Clinical Trial Participation as Perceived by Oncologists and Patients. J National Comprehensive Cancer Network. 2007;5:753–762. doi: 10.6004/jnccn.2007.0067. [DOI] [PubMed] [Google Scholar]
- 46.Cooley ME, Sarna L, Brown JK, et al. Challenges of Recruitment and Retention in Multisite Clinical Research. Cancer Nursing. 2003;26:376–384. doi: 10.1097/00002820-200310000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Levesque E, Leclerc D, Puymirat J, Knoppers BM. Developing registries of volunteers: key principles to manage issues regarding personal information protection. J Med Ethics. 2010;36:712–714. doi: 10.1136/jme.2010.036715. [DOI] [PubMed] [Google Scholar]