Abstract
HR96 is a CAR/PXR/VDR ortholog in invertebrates, and a promiscuous endo- and xenobiotic nuclear receptor involved in acclimation to toxicants. Daphnia HR96 is activated by chemicals such as atrazine and linoleic acid (LA) (n-6 fatty acid), and inhibited by triclosan and docosahexaenoic acid (DHA)(n-3 fatty acid). We hypothesized that inhibitors of HR96 may block the protective responses of HR96 based on previously performed luciferase assays. Therefore, we performed acute toxicity tests with two-chemical mixtures containing a HR96 inhibitor (DHA or triclosan) and a HR96 activator (LA or atrazine). Surprisingly, results demonstrate that triclosan and DHA are less toxic when co-treated with 20–80 μM atrazine. Atrazine provides concentration-dependent protection as lower concentrations have no effect and higher concentrations cause toxicity. LA, a weaker HR96 activator, did not provide protection from triclosan or DHA. Atrazine’s protective effects are presumably due to its ability to activate HR96 or other toxicologically relevant transcription factors and induce protective enzymes. Atrazine did not significantly induce glucosyltransferase, a crucial enzyme in triclosan detoxification. However, atrazine did increase antioxidant activities, crucial pathways in triclosan’s toxicity, as measured through GST activity and the TROLOX equivalence assay. The increase in antioxidant capacity is consistent with atrazine providing protection from a wide range of toxicants that induce ROS, including triclosan and unsaturated fatty acids predisposed to lipid peroxidation.
Keywords: nuclear receptor, HR96, mixtures, pharmaceuticals, pesticides, antioxidants
1. Introduction
NRs are transcription factors that are often activated by a diverse set of ligands, including steroid hormones, bile acids, fatty acids, and toxicants (Hernandez et al., 2009). In turn, they transcriptionally regulate the expression of specific proteins. NRs such as the PXR and CAR are involved in the regulation of phase I to III metabolic enzymes responsible for the clearance of xenobiotics and steroids (Ueda et al., 2002; Rosenfeld et al., 2003). Recent research also demonstrates the involvement of both these receptors in energy metabolism and immune response pathways (Hernandez et al., 2009).
Daphnia are widely used for toxicology testing, and the sequencing of the Daphnia pulex genome has assisted in our understanding of how the environment and genomes interact to help organisms acclimate and adapt (Colbourne et al., 2011; De Coninck et al., 2014). Both Daphnia magna and D. pulex have 26 nuclear receptors, including a HR96 (Litoff et al., 2014). HR96 is an ortholog of CAR, PXR and VDR (Litoff et al., 2014), and controls the expression of metabolic and toxicant stress response genes in invertebrates, including CYPs, carboxylesterases, GSTs, and glucosyltransferases (King-Jones et al., 2006). HR96 also plays an important role in TAG homeostasis in Drosophila melanogaster, through its regulation of CG5932 gastric lipase (Magro). Magro is also required in the intestine of Drosophila to maintain cholesterol homeostasis by increasing its clearance (Sieber and Thummel, 2012). D. pulex HR96 is a promiscuous nuclear receptor that is activated by several toxicants such as atrazine, chlorpyrifos and pyriproxyfen, and is also activated by several n-6 and n-9 (omega-6 and 9) unsaturated fatty acids (Karimullina et al., 2012). In addition, HR96 is inhibited by some chemicals including triclosan, phenobarbital and fluoxetine, and the n-3 (omega-3) unsaturated fatty acids, DHA and EPA (Karimullina et al., 2012).
The n-3 fatty acid, DHA is an efficacious HR96 inverse agonist in Daphnia (Karimullina et al., 2012) and a CAR inverse agonist in rats (Li et al., 2007), as it represses constitutive HR96 and CAR action. LA is a weak to moderate activator of HR96 in Daphnia (Karimullina et al., 2012) and CAR in mice (Finn et al., 2009). Triclosan is a widely used antimicrobial agent found in consumer products, including soap, deodorant, toothpaste, mouthwash and shampoo (Daughton and Ternes, 1999). It has been detected both in wastewater (McAvoy et al., 2002) and surface water (Kolpin et al., 2002). Atrazine is a triazine herbicide that can affect endocrine signaling pathway in vertebrates (Cooper et al., 2000). It has been reported to cause demasculinization of male frogs (Xenopus laevis) (Hayes et al., 2002) and structural disruption in testis of male goldfish (Carassius auratus) (Spanò et al., 2004). Atrazine affects endocrine signaling by increasing dopamine and reducing norepinephrine concentrations in the hypothalamus, and in turn reducing luteinizing hormone and prolactin levels. This leads to increased conversion of testosterone into 17β-estradiol (Stoker et al., 1999; Cooper et al., 2000).
Activation of CAR and PXR induces a number of detoxification enzymes (Hernandez et al., 2009). This positive regulation can be protective (Mota et al., 2010); however, CAR and PXR are both associated with drug-drug interactions because of the induction of key enzymes. For example, CAR activation increases acetaminophen toxicity (Zhang et al., 2002). PXR activation is associated with increased clearance of warfarin (Mu et al., 2006) ethinyl estradiol, (Hall et al., 2003), and immunosuppresants (Hauser et al., 2012) leading to clotting, unintended pregnancies, and rejection of organ transplants. Because HR96 is also promiscuous (Karimullina et al., 2012). and involved in the regulation of similar pathways and enzymes (King-Jones et al., 2006), we predicted that chemicals that alter HR96 activity may cause similar drug-drug or toxicant-toxicant interactions.
Previously performed transactivation assays demonstrated that triclosan and DHA repress the activation of HR96 in a dose-dependent manner (Karimullina et al., 2012), and in turn, several HR96 activators such as atrazine, LA, pyriproxyfen and estradiol either did not increase luciferase activity in their presence or their activity was significantly repressed. Because of the widespread use of atrazine and triclosan and their pervasive presence in water bodies (Gilliom et al., 2006; Halden, 2014), they were used in this study to examine the potential opposing effects of HR96 modulators on the toxicant sensing and acclimation process in Daphnia. DHA and LA were examined as HR96 also responds to dietary unsaturated fatty acids (Karimullina et al., 2012). Therefore we tested the role these fatty acid exposures may have on toxicant responses; mitigating or enhancing toxicity in D. magna. We hypothesized that HR96 inhibitors will block the protective responses of HR96 activators and in turn increase toxicity.
2. Materials and Methods
2.1. Daphnia culture
D. magna were cultured in moderately hard water with a 16:8 light: dark cycle and a temperature between 21–23°C. The daphnids were fed the unicellular green algae, Pseudokirchneriella subcapitata (purchased from Aquatic Biosystems, Fort Collins, CO and cultured in the laboratory), and supplemented with TetraFin (Masterpet Corp., New South Wales, Australia) (Ginjupalli and Baldwin, 2013).
2.2. RNA extraction and qPCR
Four different age groups (2, 4, 7 and 14-day old) of D. magna were euthanized, RNA extraction was performed using an RNAeasy mini kit (Qiagen, Germantown, MD), and RNA quantified with a spectrophotometer at 260/280 nm. cDNA was synthesized from the RNA samples (2 μg RNA) with MMLV reverse transcriptase. qPCR was performed according to MIQE standards (Bustin et al., 2009) to determine the expression of HR96 in the daphnids with forward primer 5′-TCT-GCG-ACA-AGG-CTT-TAG-GTT-3′ and reverse primer 5′-AGG-GCA-TTC-CGT-CTA-AAG-AAG-GCT-3′ at an annealing temperature of 58° C. β-actin was used as the housekeeping gene with forward primer 5′-CCA-CAC-TGT-CCC-CAT-TTA-TGA-AG-3′and reverse primer 5′-CGC-GAC-CAG-CCA-AAT-CC-3′ at an annealing temperature of 52.2° C as described previously (Heckmann et al., 2006). Efficiencies of the reactions were determined based on standard curves from 1:1, 1:5, 1:25, 1:125, 1:625 and 1:3125 dilutions of cDNA mixtures taken from all samples. The efficiency of the HR96 qPCR reaction varied between 85 – 92%, and the efficiency of the β-actin qPCR varied between 88 – 102%. Samples were diluted 1:3 and quantified with 0.25X SYBR Green (Qiagen, Germantown, MD USA) using the iCycler from Bio-Rad Laboratories (Hercules, CA USA) (Mota et al., 2010). The results were normalized to the expression of β-actin. Gene expression was quantified by taking the efficiency curve of the reaction to the power of the threshold cycle (Ct) over the β-actin.
2.3. Acute toxicity assays
Forty-eight hour acute toxicity tests were conducted with LA (≥99 %), oleic acid (≥99%), α-linolenic acid (ALA) (≥99%), cholesterol (≥99%), DHA (≥98 %), triclosan (97%), and atrazine (98.9%) (Sigma-Aldrich, St. Louis, MO USA). Stock solutions of these chemicals were made in absolute ethanol (Sigma-Aldrich Chemical Co., Inc, Milwaukee, WI USA), except atrazine, which was dissolved in 99.7% dimethyl sulfoxide (Fisher Scientific, Fair Lawn, NJ USA). Toxicity was determined with individual chemicals and with two-chemical mixtures, with solutions made in moderately hard water. A variety of concentration ranges of individual chemicals were chosen to perform the first set of acute toxicity assays, in order to determine EC50 values using established protocols (USEPA, 2002) with four < 24-hour old daphnids in each 50 ml beaker and five beakers per treatment group. The EC50 values and 95% confidence interval were determined from the sigmoidal dose response curves, which were generated using GraphPad Prizm 4.0. In addition, the toxicity of chemical mixtures was determined with one HR96 activator and one HR96 inhibitor provided at different concentrations to the daphnids. LA (2, 5 and 10 μM) and atrazine (5, 10, 20, 40 and 80 μM) were used as HR96 activators and DHA (2, 5 and 8 μM) and triclosan (0.75, 1, 1.3 and 1.5 μM) were used as HR96 inhibitors in the mixture assays. In these assays four < 24-hour old daphnids were used in each 50 ml beaker with ten beakers per treatment group.
2.4. Predictive mixture modeling
The interactive hazard calculator, Computational Approach to Toxicity Assessment of Mixtures(CATAM) (http://www.ncsu.edu/project/toxresearch/model5/) (Rider and LeBlanc, 2005) was used to predict toxicity associated with the chemical mixtures using the independent join action model with experimentally determined EC50 values (Olmstead and LeBlanc, 2005). The independent joint action model was used because the mechanism of action of the individual chemicals in the mixtures is not known and toxicity caused by each chemical probably does not occur through the same mechanism. HR96 inhibitors were assigned to cassette 1 of the model and HR96 activators were assigned to cassette 2 of the model. Toxicity of the chemical mixtures as measured during toxicity tests were compared to toxicity curves determined by the CATAM model.
2.5. [14C]Testosterone glucosyltransferase assay
Less than 48-hour old neonates (n = 5 beakers per treatment group; each beaker with ten daphnids) were pre-exposed to different concentrations of atrazine (0, 10, 20, 40 μM) for 16 hours. Daphnids were exposed to the [14C]testosterone (2 mCi/mmol, 150,000 dpm/assay) for six hours. Ethyl acetate was used to extract testosterone and its lipophilic metabolites from the aqueous solution. Water fractions were collected from each tube, redissolved in 0.1 M NaAcetate, and conjugated testosterone was hydrolyzed with ten units of β-glucosidase for 2 h. Freed testosterone was extracted with ethyl acetate and the formerly glucosylated products were measured via liquid scintillation spectrophotometry and quantified according to previously published protocols (Baldwin et al., 1997).
2.6. Anti-oxidant and reactive oxygen species assays
Less than 48-hour old neonates (n = 4 beakers with 20 daphnids each) were exposed to individual chemicals or mixtures of chemicals (40 μM atrazine, 0.75 μM triclosan, 8 μM DHA, 40 μM atrazine + 0.75 μM triclosan and 40 μM atrazine + 8 μM DHA) for 16 hours. Daphnids were homogenized, and the supernatant collected and stored at −80°C for biochemical assays. Lipid peroxidation caused by ROS induced by chemical exposure was determined by measuring Thiobarbituric Acid Reactive Substances (TBARS) (Cayman Chemical Co., Ann Arbor, MI). Protein (10 μl) was incubated with thiobarbituric acid for 1 hour at 100°C. The reaction was stopped on ice for 10 minutes followed by centrifugation, and a 30 min incubation at room temperature before samples were read at fluorometrically at an excitation wavelength of 530 nm and an emission wavelength of 550 nm (Armstrong and Browne, 1994). Concentrations of malondialdehyde (μM) in the samples determined based on a prepared standardard curve. Glutathione S-transferase (GST) activity was determined by incubating 5 μl of protein with glutathione and 1-chloro 2, 4-dinitrobenzene (CDNB) for 10 min at room temperature. The formation of a dinitrobenzene-glutathione conjugate was measured at 340 nm (Baldwin and LeBlanc, 1996). The Trolox assay (Cayman Chemical Co., Ann Arbor, MI), which measures a number of anti-oxidant mechanisms was performed to measure the overall ability of D. magna to respond to ROS following exposure to individual chemicals or a two-chemical mixture. The anti-oxidant assay is based on the ability of the samples to inhibit metmyoglobin oxidation of 2,2′-azino-d-[3-ethylbenzthiazoline sulphonate] (ABTS) to ABTS+ (Miller and Rice-Evans, 1997). Briefly, 10 μl of protein sample was added to 10 μl of metmyoglobin, and 150 μl of chromagen. Incubations were initiated with 40 μl of hydrogen peroxide and ABTS+ was measured after a 5 min incubation at room temperature at 750 nm. The antioxidant capacity of the samples was compared to that of a Trolox (a tocopherol analog) standard and the results were quantified in Trolox equivalents (mM). All samples for each assay were performed in duplicate.
3. Results
3.1 HR96 expression
The expression of HR96, a promiscuous nuclear receptor (Karimullina et al., 2012), was examined by qPCR in < 2 to 14 day-old daphnids. qPCR demonstrates that HR96 is expressed throughout a daphnid’s lifespan at nearly equal levels at all of the ages tested; < 2, 4, 7, and 14-days old (Suppl File 1). Therefore, neonatal daphnids have the necessary HR96 to transcriptionally respond to specific toxicant stressors and neonates can be used in subsequent acute toxicity tests to determine if compounds that interact with HR96 can modify toxicity.
3.2 Single chemical toxicity tests
Forty-eight hour acute toxicity tests were performed to determine concentration ranges for subsequent mixture toxicity tests. All of the unsaturated fatty acids and cholesterol had acute toxicities ranging from 2.90 – 14.4 μM. Cholesterol was the most toxic (LC50 = 2.90 μM), followed by DHA (LC50 = 5.55 μM), LA (LC50 = 10 μM), oleic acid (LC50 = 10.2 μM) and α-linolenic acid (LC50 = 14.4 μM). Of the environmental toxicants, triclosan was the most toxic chemical tested (LC50 = 0.835 μM) and atrazine was the least toxic chemical tested (LC50 = 78 μM) (Fig. 1).
Fig. 1. Acute toxicity and dose-response curves of select xenobiotic and fatty acid HR96 modulators to D. magna.
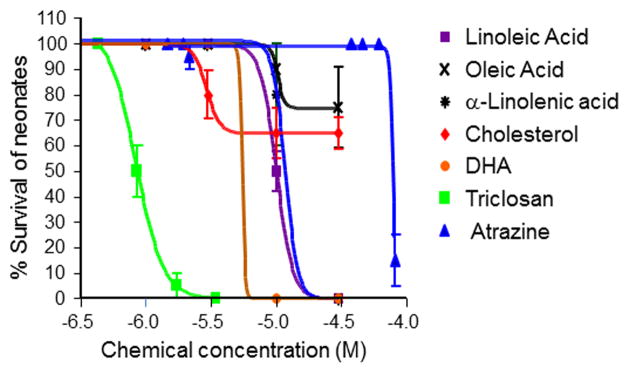
Acute toxicity assays were performed with < 24 hour old daphnids with several endobiotic and xenobiotic chemicals. Dose-response curves were determined using GraphPad Prizm 4.0 and data are provided as mean ± 95% confidence intervals (n = 5 per treatment).
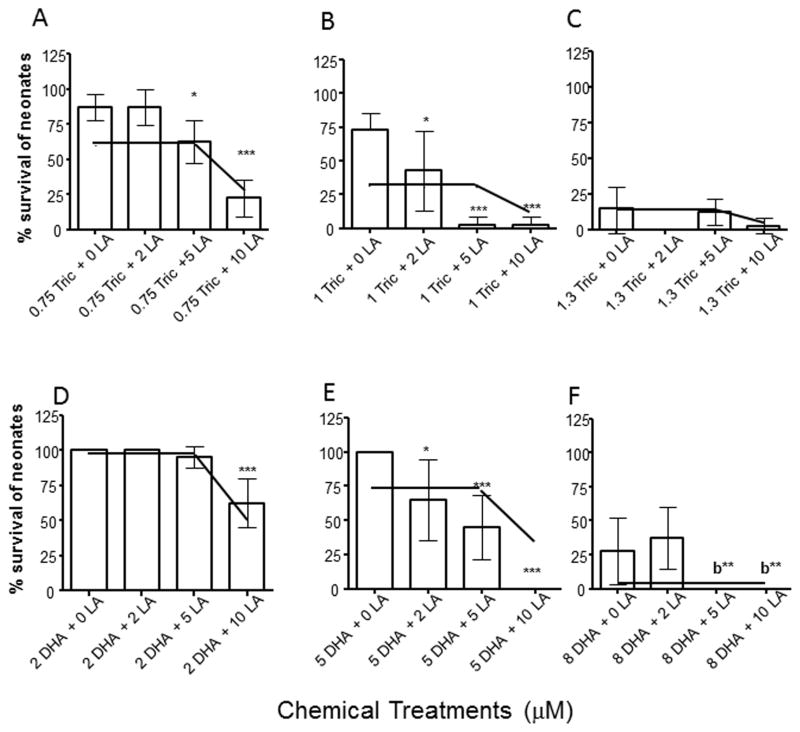
3.3 Mixtures with LA mimic independent joint action
We hypothesized that HR96 inhibitors may block HR96 activation and this would increase toxicity compared to model estimations. Therefore, daphnids were exposed to the HR96 inhibitors, triclosan or DHA concomitantly with LA (2–10 μM), a HR96 activator. The toxicity of the mixtures increased in a concentration-dependent manner for each of the chemicals tested (Fig. 2). Chemical toxicity was also compared to the CATAM model used to predict toxicity based on independent joint action (Rider and LeBlanc, 2005). Some of the lower concentrations or single chemical assays showed less toxicity than expected. However, in general, the toxicity estimated from the CATAM independent joint action model was within the 95% Confidence Intervals determined from the acute toxicity tests (Fig. 2). Originally, we hypothesized that exposure to DHA and triclosan will increase the toxicity of LA in a synergistic fashion. Therefore, our hypothesis is null as the HR96 inhibitors did not repress toxicant acclimation in vivo and cause enhanced toxicity of LA.
Fig. 2. The toxicity of Linoleic acid (LA) is accurately predicted in a two-chemical mixture containing an HR96 inhibitor (triclosan or DHA).
Acute toxicity tests were performed with low (A), medium (B) and high (C) concentrations of triclosan coupled with multiple concentrations of LA, or low (D), medium (E), and high (F) concentrations of DHA coupled with multiple concentrations of LA. Data are provided as mean ± 95% confidence intervals (n = 10). Statistical significance was determined by ANOVA followed by Tukey’s multiple comparison test (*p < 0.05, **p < 0.01) Letter ‘b’ represents a statistical difference in comparison to 2 μM LA, whereas the other statistically significant data is in comparison to the control group. The independent joint action model from CATAM was used to predict daphnid survival under mixture conditions with the average 95% confidence intervals from the toxicity tests used to predict variance in the model. The independent joint action model prediction is shown as a line in each of the graphs.
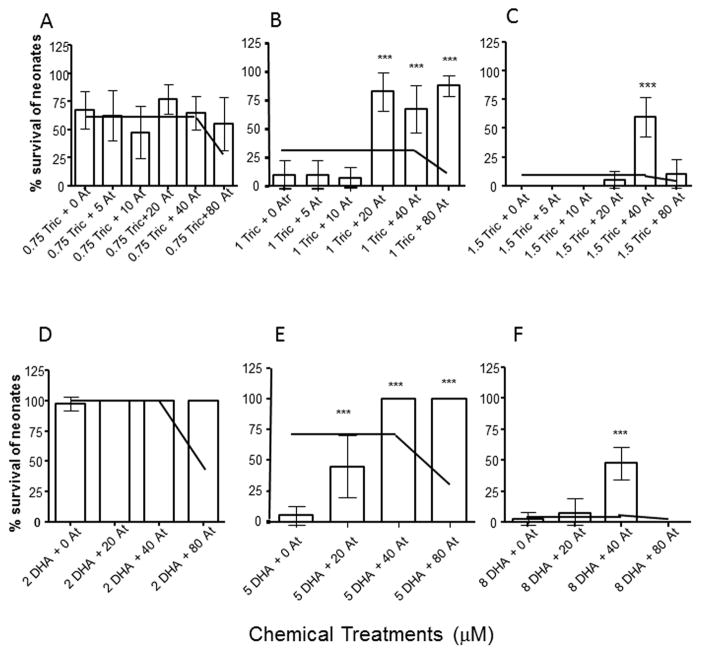
3.4 Atrazine provides protection from HR96 inhibitors
Daphnids were also exposed to the HR96 inhibitors, triclosan or DHA concomitantly with atrazine, a HR96 activator. At low concentrations of atrazine, the CATAM model accurately predicts toxicity in daphnids during co-exposures to either triclosan (Fig. 3ABC) or DHA (Fig. 3DEF). However at atrazine concentrations ranging from 20 to 80 μM with 40 μM showing the greatest protection, the toxicity of triclosan and DHA decreased. This is neither consistent with typical concentration-dependent toxicity nor consistent with the CATAM model. Instead, a concentration-dependent protective effect was observed (Fig. 3). This data is not consistent with our hypothesis that HR96 inhibitors would block the ability to adapt to toxicant stress. Conversely, significant concentrations of the HR96 activator, atrazine, are able to induce a protective effect.
Fig. 3. The HR96 activator, atrazine induces a concentration-dependent interaction that protects daphnids from toxicity induced by triclosan or DHA.
Acute toxicity tests were performed with low (A), medium (B) and high (C) concentrations of triclosan coupled with multiple concentrations of atrazine, or low (D), medium (E), and high (F) concentrations of DHA coupled with multiple concentrations of atrazine. Data are provided as mean ± 95% confidence intervals (n = 10 per treatment). Statistical significance was determined by ANOVA followed by Tukey’s multiple comparison test (*p < 0.05, **p < 0.01, ***p < 0.001.) Statistically significant data is in comparison to the control group. The independent joint action model from CATAM was used to predict daphnid survival under mixture conditions with the average 95% confidence intervals from the toxicity tests used to predict variance in the model. The independent joint action model prediction is shown as a line in each graph.
3.5 Atrazine induces anti-oxidant protection
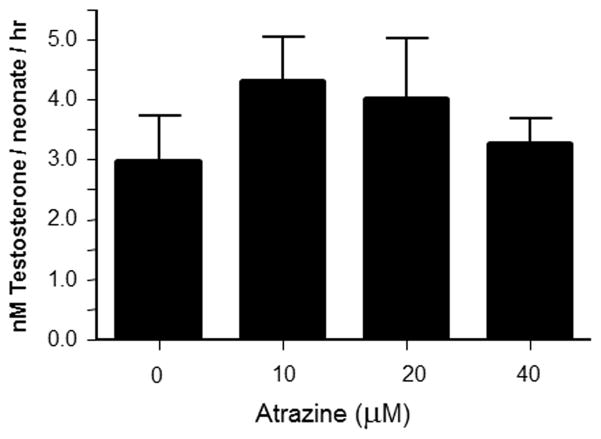
Because atrazine is a HR96 activator and HR96 regulates the expression of a suite of drug metabolism enzymes (King-Jones et al., 2006) much like CAR and PXR (Hernandez et al., 2009), we hypothesized that HR96 induced crucial phase II enzymes necessary for the detoxification of triclosan such as glucosyltransferases (Wu et al., 2010). A [14C]testosterone glucosyltransferases assay was performed to test whether atrazine could have induced this crucial triclosan detoxification pathway. However, we did not observe an increase in glucosyltransferase activity (Fig. 4).
Fig. 4. Testosterone glucosylation activity in atrazine exposed daphnids.
Data are provided as mean ± SEM (n = 5). Statistical significance was determined by ANOVA followed by Tukey’s multiple comparison test with p < 0.05 considered significant.
Triclosan toxicity is often associated with the formation of reactive oxygen species (Binelli et al., 2009; Riva et al., 2012). Therefore, we also examined the potential for increased antioxidant defense or decreased ROS after atrazine exposure with three different assays (Fig. 5). TBARS is a measure for monitoring lipid peroxidation (Armstrong and Browne, 1994). TBARS/daphnid was increased in the atrazine + DHA group relative to the untreated (control), triclosan, and triclosan plus atrazine groups (Fig. 5A). Atrazine (40 μM) co-exposed with DHA, which is thought to be protective, increased TBARs, indicating that DHA at 8 μM increases lipid peroxidation in the presence of atrazine. Interestingly, chemical treatments with triclosan did not produce significant increases in lipid peroxidation when compared to the DHA + atrazine co-treatments and lipid peroxidation was at UT levels in the presence of 40 μM atrazine + 0.75 μM triclosan.
Fig. 5. Measurement of GST activity, antioxidant capacity and ROS (TBARS) induced by chemicals and chemical mixtures.
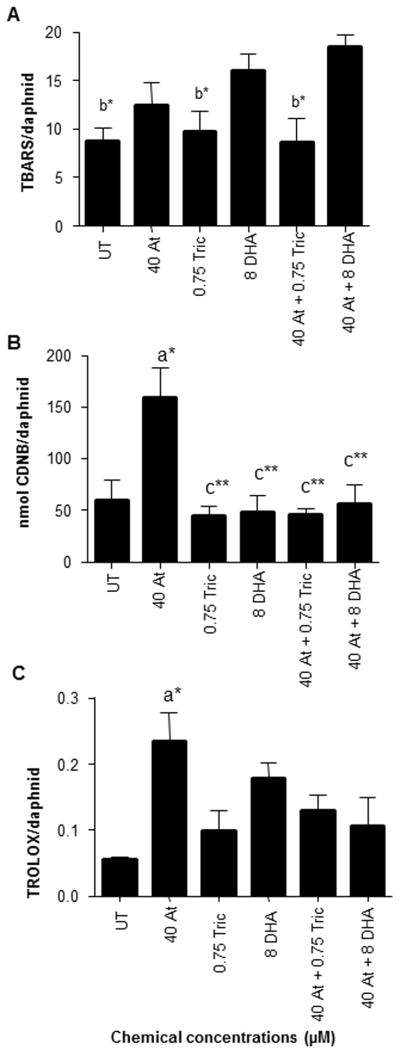
Alteration of ROS and protective enzymes by HR96 modulators were determined using the following measurements (A) TBARS assay for lipid peroxidation (B) GST activity determined by CDNB and (C) TROLOX assay for antioxidant mechanism. Data are provided as mean ± SEM (n = 4). Statistical significance was determined by ANOVA followed by Tukey’s multiple comparison test with an ‘a’ refering to groups that are different from UT, ‘b’ refering to groups that are different from DHA+Atrazine, and ‘c’ referring to groups different from atrazine treatments. Asterisks indicate the level of statistical significance (*p < 0.05, **p < 0.01).
Atrazine did increase GST activity in D. magna with the other treatment groups showing significantly lower CDNB activity (Fig. 5B). In addition, the TROLOX antioxidant assay kit that measures the cooperativity of several different antioxidant systems, including glutathione peroxidase, superoxide dismutase, catalase, α-tocopherol, ascorbic acid, and glutathione and converts them to TROLOX equivalents demonstrates that atrazine can induce the overall antioxidant defense system of the daphnid (Fig. 5C). None of the other treatment groups increased TROLOX equivalents. These results are consistent with atrazine providing protection from a wide range of toxicants that induce ROS, including triclosan and unsaturated fatty acids predisposed to lipid peroxidation, through increased antioxidant defenses (Fig. 5BC).
4. Discussion
We hypothesized that HR96 inverse agonists such as triclosan or DHA would block HR96-mediated transcription by atrazine or LA and increase toxicity by blocking detoxification responses. Neither triclosan nor DHA showed any increased mixture toxicity relative to the CATAM model. This indicates that neither triclosan nor DHA significantly perturbs detoxification responses, or the responses blocked by these chemicals are not involved in the detoxification of atrazine or LA. Instead our data demonstrates that the HR96 activator atrazine provides concentration-dependent protection from other chemicals such as the HR96 inhibitors DHA and triclosan (Fig. 3). Atrazine’s protection is consistent with CAR- or PXR-like induction of detoxification enzymes and transporters that mediate protection from drugs and environmental toxicants (Wei et al., 2000; Mota et al., 2010).
Triclosan contains a hydroxyl group at the 2′ position. Therefore, in mammals, glucuronidation is the most common pathway for triclosan detoxification (Wu et al., 2010). We hypothesized that atrazine was decreasing triclosan toxicity trough induction of glucosidation. However, we did not observe increased testosterone glucosidation (Fig. 4), indicating that increased glucosidation was not responsible for atrazine’s concentration-dependent protection. We attempted to measure triclosan glucosidation directly through HPLC (Wu et al., 2010), but were unsuccessful in the neonatal daphnids. Next, we considered that triclosan and DHA may be increasing the production of reactive oxygen species (ROS). Triclosan’s mode of action is primarily through increasing ROS and causing DNA damage (Binelli et al., 2009). Atrazine did induce GST activity and overall anti-oxidant defenses as shown by the TROLOX and CDNB assays (Fig. 5). Taken together, our data suggests that atrazine acts to protect daphnids from DHA and triclosan, two chemicals that can induce ROS, by inducing anti-oxidant defenses.
Anti-oxidant defenses are regulated by a number of transcription factors such as CAR, PXR, HR96, NF-kB, and Nrf2 (King-Jones et al., 2006; Hernandez et al., 2009; Zhao et al., 2010). Several GST members are regulated by CAR and PXR in vertebrates, and HR96 induces GSTs and carboxylesterases in D. melanogaster in response to xenobiotic stress, including phenobarbital (King-Jones et al., 2006). The transcription factors involved in the regulation of GSTs in D. magna have not been investigated. However phenobarbital has been shown to increase GST activity and protein levels in D. magna in previous studies (Baldwin and LeBlanc, 1996), indicating a potential role of HR96.
The role of HR96 in the regulation of xenobiotic metabolism has been demonstrated in fruitfly (D. melanogaster) (King-Jones et al., 2006) and in part in D. pulex (Karimullina et al., 2012). D. magna’s HR96 is 96% identical to D. pulex in the LBD and 98% in the DBD suggesting that D. magna’s responses are similar to those of D. pulex (Litoff et al., 2014). However, we do not have HR96-null Daphnia and the long-term utilization of siRNAs with free swimming D. magna has not been examined thoroughly (Kato et al., 2011; Hiruta et al., 2013). Therefore, we do not have proof that the daphnids are responding to atrazine through HR96 in vivo. The transcription factor Nrf2 is also known to exert protective roles against injuries from oxidative stress (Chen and Kong, 2005; Zhao et al., 2010) as its regulates the expression of several antioxidant and cytoprotective genes (Itoh et al., 1999; Ishii et al., 2000). Nrf2 has been shown to regulate the expression of NQO and GST in mice (Itoh et al., 1997; McMahon et al., 2001) and humans (Venugopal and Jaiswal, 1996). A Nrf2 ortholog has been found within the D. melanogaster genome (CG43286; cnc), and recent research indicates that it is crucial in response to ROS (Pickering et al., 2013). A BLAST search of the D. pulex genome indicates the presence of a Nrf2 ortholog (jgi|Dappu1|312770|NCBI_GNO_0700043). Therefore, atrazine could be inducing its protective mechanisms through Nrf2 or in conjunction with HR96 as data indicates that both transcription factors regulate antioxidant defenses.
Our results demonstrated that at higher concentrations of atrazine, toxicity due to HR96 inhibitors decreased. This concentration-dependent protection failed to fit the CATAM model. This protective effect was consistent at concentrations of approximately 20 – 80 μM, indicating that atrazine must reach levels that are high enough to activate a specific biochemical process. These concentrations of atrazine are not environmentally relevant; however, the purpose of our research was to investigate D. magna responses to antagonistic mixtures of chemicals; in this case HR96 modulators (Karimullina et al., 2012). Atrazine may prove to be a key positive control for activation of HR96 similar to high doses of phenobarbital, TCPOBOP, or dexamethasone that are often used reference compounds or positive controls for activation of CAR or PXR (Kawamoto et al., 1999; Pascussi et al., 2000; Tzameli et al., 2000). The expression of HR96, a NR orthologous to CAR, PXR, and VDR may explain D. magna’s ability to respond to toxicants in a similar manner to mammals (King-Jones et al., 2006; Hernandez et al., 2009).
A mixture of chemicals that act additively or synergistically may reach the necessary concentrations to induce similar measured stress response pathways. For example, propazine, simazine and other triazine herbicides may act alone or additively with atrazine and other dietary and anthropogenic chemicals to activate HR96 (Baldwin and Roling, 2009) The subsequent transcriptional responses may lead to perturbed regulation of detoxification enzymes or other processes. In turn, unintended consequences may occur, not just mixture effects such as response addition, but also antagonistic effects as observed here, or potentially synergistic or potentiation as well.
In summary, HR96 inhibitors did not block the ability of atrazine, an HR96 activator, to help daphnids respond to toxicant or fatty acid stress. Instead, atrazine appears to have activated detoxification responses, probably through HR96 but also potentially through other transcription factors, which induced a concentration-dependent protective response. This work demonstrates that atrazine and other chemicals may have unique mixture effects on aquatic invertebrates such as daphnids if their concentrations or responses are strong enough leading to drug-toxicant and toxicant-toxicant interactions (Delgoda and Westlake, 2004; Hernandez et al., 2009).
Supplementary Material
Highlights.
Surprisingly, atrazine provides protection from the toxicity of triclosan and DHA
Atrazine’s protective effects are concentration-dependent
Atrazine’s protection may be elicited through HR96
Atrazine induces antioxidant activity at the concentrations tested
Acknowledgments
Funds for this project were provided by NIH grant ES017321 to WSB.
Abbreviations
- NR
Nuclear Receptor
- PXR
Pregnane-X-Receptor
- CAR
Constitutive Androstane Receptor
- TAG
triacylglycerol
- GST
glutathione S-transferase
- EPA
eicosapentaenoic acid
- DHA
docosahexaenoic acid
- LA
linoleic acid
- qPCR
quantitative real-time Polymerase Chain Reaction
- CATAM
Computational Approach to the Toxicity Assessment of Mixtures
- TBARS
thiobarbituric acid reactive substances
- ROS
reactive oxygen species
- ABTS
2,2′-Azin0-di-[3-ethylbenthiazoline sulphonate]
- TROLOX
6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid
- UT
untreated
- CDNB
1,2 chloro-dinitrobenzene
- HPLC
high performance liquid chromatography
- Nrf2
NF-E2-related factor 2
- DBD
DNA binding domain
- LBD
ligand binding domain
- NQO
NAD(P)H: quinone oxidoreductase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armstrong D, Browne R. The analyis of free radicals, lipid peroxides, antioxidant enzymes and compounds to oxidative stress as applied to the clinical chemistry laboratory. Adv Exp Med Biol. 1994;366:43–58. doi: 10.1007/978-1-4615-1833-4_4. [DOI] [PubMed] [Google Scholar]
- Baldwin WS, Graham SE, Shea D, LeBlanc GA. Metabolic androgenization of female Daphnia magna by the xenoestrogen 4-nonylphenol. Environ Toxicol Chem. 1997;16:1905–1911. [Google Scholar]
- Baldwin WS, LeBlanc GA. Expression and induction of an immunochemically related class of glutathione S-transferases in Daphnia magna. Comp Biochem Physiol. 1996;113B:261–267. doi: 10.1016/0305-0491(95)02021-7. [DOI] [PubMed] [Google Scholar]
- Baldwin WS, Roling JA. A concentration addition model for the activation of the constitutive androstane receptor by xenobiotic mixtures. Toxicol Sci. 2009;107:93–105. doi: 10.1093/toxsci/kfn206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binelli A, Cogni D, Parolini M, Riva C, Provini A. In vivo experiments for the evaluation of genotoxic and cytotoxic effects of Triclosan in Zebra mussel hemocytes. Aquat Toxicol. 2009;91:238–244. doi: 10.1016/j.aquatox.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubesta M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE Guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Chen C, Kong AN. Dietary cancer-chemopreventive compounds: From signaling and gene expression to pharmacological effects. Trends Pharmacol Sci. 2005;26:318–326. doi: 10.1016/j.tips.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Colbourne JK, Pfrender ME, Gilbert D, Thomas WK, Tucker A, Oakley TH, Tokishita S, Aerts A, Arnold GJ, Basu MK, Bauer DJ, Caceres CE, Carmel L, Casola C, Choi JH, Detter JC, Dong Q, Dusheyko S, Eads BD, Frohlich T, Geiler-Samerotte KA, Gerlach D, Hatcher P, Jogdeo S, Krijgsveld J, Kriventseva EV, Kultz D, Laforsch C, Lindquist E, Lopez J, Manak R, Muller J, Pangilinan J, Patwardhan RP, Pitluck S, Pritham EJ, Rechtsteiner A, Rho M, Rogozin IB, Sakarya O, Salamov A, Schaack S, Shapiro H, Shiga Y, Skalitzky C, Smith Z, Souvorov A, Sung W, Tang Z, Tsuchiya D, Tu H, Vos H, Wang M, Wolf YI, Yamagata H, Yamada T, Ye Y, Shaw JR, Andrews J, Crease TJ, Tang H, Lucas SM, Robertson HM, Bork P, Zdobnov EM, Grigoriev IV, Lynch M, Boore JL. The ecoresponsive genome of Daphnia pulex. Science. 2011;331:555–561. doi: 10.1126/science.1197761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper RL, Stoker TE, Tyrey L, Goldman JM, McElroy WK. Atrazine disrupts the hypothalamic control of pituitary-ovarian function. Toxicol Sci. 2000;53:297–307. doi: 10.1093/toxsci/53.2.297. [DOI] [PubMed] [Google Scholar]
- Daughton CG, Ternes T. Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ Health Perspect. 1999;107:907–937. doi: 10.1289/ehp.99107s6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Coninck DI, Asselman J, Glaholt S, Janssen CR, Colbourne JK, Shaw JR, De Schamphelaere KA. Genome-wide transcription profiles reveal genotype-dependent responses of biological pathways and gene-families in Daphnia exposed to single and mixed stressors. Environ Sci Technol. 2014;48:3513–3522. doi: 10.1021/es4053363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgoda R, Westlake AC. Herbal interactions involving cytochrome P450 enzymes: a mini-review. Toxicol Rev. 2004;23:239–249. doi: 10.2165/00139709-200423040-00004. [DOI] [PubMed] [Google Scholar]
- Finn RD, Henderson CJ, Scott CL, Wolf CR. Unsaturated fatty acid regulation of cytochrome P450 expression via a CAR-dependent pathway. Biochem J. 2009;417:43–54. doi: 10.1042/BJ20080740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliom RJ, Barbash JE, Crawford CG, Hamilton PA, Martin JD, Nakagaki N, Nowell LH, Scott JC, Stackelberg PE, Thelin GP, Wolock DM. US Department of the Interior and US Geological Survey. 172 Vol. 1291. Reston, Virginia: 2006. The Quality of Our Nation’s Waters—Pesticides in the Nation’s Streams and Ground Water, 1992–2001. [Google Scholar]
- Ginjupalli GK, Baldwin WS. The time- and age-dependent effects of the juvenile hormone analog pesticide, pyriproxyfen on Daphnia magna reproduction. Chemosphere. 2013;92:1260–1266. doi: 10.1016/j.chemosphere.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halden RU. On the need and speed of regulating triclosan and triclocarban in the United States. Environ Sci Technol. 2014;48:3603–3611. doi: 10.1021/es500495p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SD, Wang Z, Huang SM, Hamman MA, Vasavada N, Adigun AQ, Hilligoss JK, Miller M, Gorski JC. The interaction between St. John’s wort and an oral contraceptive. Clin Pharmacol Ther. 2003;74:525–535. doi: 10.1016/j.clpt.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Hauser IA, Kruck S, Gauer S, Nies AT, Winter S, Bedke J, Geiger H, Hoefeld H, Kleemann J, Asbe-Vollkopf A, Engel J, Burk O, Schwab M, Schaeffeler E. Human pregnane X receptor genotype of the donor but not of the recipient is a risk factor for delayed graft function after renal transplantation. Clin Pharmacol Ther. 2012;91:905–916. doi: 10.1038/clpt.2011.346. [DOI] [PubMed] [Google Scholar]
- Hayes TB, Collins A, Lee M, Mendoza M, Noriega N, Stuart AA, Vonk A. Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proc Nat Acad Sci USA. 2002;99:5476–5480. doi: 10.1073/pnas.082121499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann LH, Connon R, Hutchinson TH, Maund SJ, Sibly RM, Callaghan A. Expression of target and reference genes in Daphnia magna exposed to ibuprofen. BMC Genomics. 2006;7:175. doi: 10.1186/1471-2164-7-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JP, Mota LC, Baldwin WS. Activation of CAR and PXR by dietary, environmental and occupational chemicals alters drug metabolism, intermediary metabolism, and cell proliferation. Curr Pharmacog Personal Med. 2009;7:81–105. doi: 10.2174/187569209788654005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiruta C, Toyota K, Miyakawa H, Ogino Y, Miyagawa S, Tatarazako N, Shaw JR, Iguchi T. Development of a microinjection system for RNA interference in the water flea Daphnia pulex. BMC Biotechnology. 2013;13:96. doi: 10.1186/1472-6750-13-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem. 2000;275:16023–16029. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimullina E, Li Y, Gingupalli GK, Baldwin WS. Daphnia HR96 is a promiscuous xenobiotic and endobiotic nuclear receptor. Aquat Toxicol. 2012;116–117:69–78. doi: 10.1016/j.aquatox.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Shiga Y, Kobayashi K, Tokishita S, Yamagata H, Iguchi T, Watanabe H. Development of an RNA interference method in the cladoceran crustacean Daphnia magna. Dev Genes Evol. 2011;220:337–345. doi: 10.1007/s00427-011-0353-9. [DOI] [PubMed] [Google Scholar]
- Kawamoto T, Sueyoshi T, Zelko I, Moore R, Washburn K, Negishi M. Phenobarbital-responsive nuclear translocation of the receptor CAR in induction of the CYP2B gene. Mol Cell Biol. 1999;19:6318–6322. doi: 10.1128/mcb.19.9.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Jones K, Horner MA, Lam G, Thummel CS. The DHR96 nuclear receptor regulates xenobiotic responses in Drosophila. Cell Metab. 2006;4:37–48. doi: 10.1016/j.cmet.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999–2000: A National Reconnaissance. Environ Sci Technol. 2002;36:1202–1211. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- Li CC, Lii CK, Liu KL, Yang JJ, Chen HW. DHA down-regulates phenobarbital-induced cytochrome P450 2B1 gene expression in rat primary hepatocytes by attenuating CAR translocation. Toxicol Appl Pharmacol. 2007;225:329–336. doi: 10.1016/j.taap.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Litoff EJ, Garriott TE, Ginjupalli GK, Butler L, Gay C, Scott K, Baldwin WS. Annotation of the Daphnia magna nuclear receptors: Comparison to Daphnia pulex. Gene. 2014;552:116–125. doi: 10.1016/j.gene.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvoy DC, Schatowitz B, Jacob M, Hauk A, Eckhoff WS. Measurement of triclosan in wastewater treatment systems. Environ Toxicol Chem. 2002;21:1323–1329. [PubMed] [Google Scholar]
- McMahon M, Itoh K, Yamamoto M, Chanas SA, Henderson CJ, McLellan LI, Wolf CR, Cavin C, Hayes JD. The Cap’n’Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 2001;61:3299–3307. [PubMed] [Google Scholar]
- Miller N, Rice-Evans C. Factors influencing the antioxidant activity determined by the ABTS.+ radical cation assay. Free Radic Res. 1997;26:195–199. doi: 10.3109/10715769709097799. [DOI] [PubMed] [Google Scholar]
- Mota LC, Hernandez JP, Baldwin WS. CAR-null mice are sensitive to the toxic effects of parathion: Association with reduced cytochrome P450-mediated parathion metabolism. Drug Metab Dispos. 2010;38:1582–1588. doi: 10.1124/dmd.110.032961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Zhang J, Zhang S, Zhou HH, Toma D, Ren S, Huang L, Yaramus M, Baum A, Venkataramanan R, Xie W. Traditional Chinese medicines Wu Wei Zi (Schisandra chinensis Baill) and Gan Cao (Glycyrrhiza uralensis Fisch) activate PXR and increase warfarin clearance in rats. J Pharmacol Exp Ther. 2006;316:1369–1377. doi: 10.1124/jpet.105.094342. [DOI] [PubMed] [Google Scholar]
- Olmstead AW, LeBlanc GA. Toxicity assessment of environmentally relevant pollutant mixtures using a heuristic model. Int Environ Assess Management. 2005;1:114–122. doi: 10.1897/ieam_2004-005r.1. [DOI] [PubMed] [Google Scholar]
- Pascussi JM, Gerbal-Chaloin S, Fabre JM, Maurel P, Vilarem MJ. Dexamethasone enhances constitutive androstane receptor expression in human hepatocytes: consequences on cytochrome P450 gene regulation. Mol Pharmacol. 2000;58:1441–1450. doi: 10.1124/mol.58.6.1441. [DOI] [PubMed] [Google Scholar]
- Pickering AM, Staab TA, Tower J, Sieburth D, Davies KJ. A conserved role for the 20S proteasome and Nrf2 transcription factor in oxidative stress adaptation in mammals, Caenorhabditis elegans and Drosophila melanogaster. J Exp Biol. 2013;216:543–553. doi: 10.1242/jeb.074757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider CV, LeBlanc GA. An integrated addition and interaction model for assessing toxicity of chemical mixtures. Toxicol Sci. 2005;87:520–528. doi: 10.1093/toxsci/kfi247. [DOI] [PubMed] [Google Scholar]
- Riva C, Cristoni S, Binelli A. Effects of triclosan in the freshwater mussel Dreissena polymorpha: a proteomic investigation. Aquat Toxicol. 2012;118–119:62–71. doi: 10.1016/j.aquatox.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Rosenfeld JM, Vargas R, Xie W, Evans RM. Genetic profiling defines the xenobitic gene network controlled by the nuclear receptor pregnane X receptor. Mol Endocrinol. 2003;17:1268–1282. doi: 10.1210/me.2002-0421. [DOI] [PubMed] [Google Scholar]
- Sieber MH, Thummel CS. Coordination of triacylglycerol and cholesterol homeostasis by DHR96 and the Drosophila LipA homolog magro. Cell Metab. 2012;15:122–127. doi: 10.1016/j.cmet.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanò L, Tyler CR, van Aerle R, Devos P, Mandiki SN, Silvestre F, Thomé JP, Kestemont P. Effects of atrazine on sex steroid dynamics, plasma vitellogenin concentration and gonad development in adult goldfish (Carassius auratus) Aquat Toxicol. 2004;66:369–379. doi: 10.1016/j.aquatox.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Stoker TE, Robinette CL, Cooper RL. Maternal exposure to atrazine during lactation suppresses suckling-induced prolactin release and results in prostatitis in the adult offspring. Toxicol Sci. 1999;52:68–79. doi: 10.1093/toxsci/52.1.68. [DOI] [PubMed] [Google Scholar]
- Tzameli I, Pissios P, Schuetz EG, Moore DD. The xenobiotic compound 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene is an agonist ligand for the nuclear receptor CAR. Mol Cell Biol. 2000;20:2951–2958. doi: 10.1128/mcb.20.9.2951-2958.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda A, Hamadeh HK, Webb HK, Yamamoto Y, Sueyoshi T, Afshari CA, Lehmann JM, Negishi M. Diverse roles of the nuclear orphan receptor CAR in regulating hepatic genes in response to phenobarbital. Mol Pharmacol. 2002;61:1–6. doi: 10.1124/mol.61.1.1. [DOI] [PubMed] [Google Scholar]
- USEPA. Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms. 5. 2002. EPA-821-R-02-012. [Google Scholar]
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci USA. 1996;93:14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei P, Zhang J, Egan-Hafley M, Liang S, Moore DD. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature. 2000;407:920–923. doi: 10.1038/35038112. [DOI] [PubMed] [Google Scholar]
- Wu J-l, Liu J, Cai Z. Determination of triclosan metabolites by using in-source fragmentation from high-performance liquid chromatography/negative atmospheric pressure chemical ionization ion trap mass spectrometry. Rapid Comm in Mass Spectrometry. 2010;24:1828–1834. doi: 10.1002/rcm.4558. [DOI] [PubMed] [Google Scholar]
- Zhang J, Huang W, Chua SS, Wei P, Moore DD. Modulation of acetaminophen-induced hepatotoxicity by the xenobiotic receptor CAR. Science. 2002;298:422–424. doi: 10.1126/science.1073502. [DOI] [PubMed] [Google Scholar]
- Zhao CR, Gao ZH, Qu XJ. NRF2-ARE signaling pathway and natural products for cancer chemoprevention. Cancer Epidemiol. 2010;34:523–533. doi: 10.1016/j.canep.2010.06.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.