Abstract
Background
Subsets of high grade endometrial cancer (EnCa) over-express HER2 (ERBB2), yet clinical trials have failed to demonstrate any anti-tumor activity utilizing trastuzumab, an approved platform for HER2 positive breast cancer (BrCa). A truncated p95HER2 variant lacking the trastuzumab binding site may confer resistance. The objective of this investigation was to characterize the expression of the p95HER2 truncated variant in EnCa.
Materials and Methods
With institutional approval, 86 high grade EnCa tumors were identified with tumor specimens from surgeries performed between 2000-2011. Clinical data were collected and all specimens underwent tumor genotyping, HER2 immunohistochemistry (IHC, HercepTest®), HER2 fluorescent in situ hybridization (FISH), along with total HER2 (H2T) and p95HER2 assessment with VeraTag® testing. Regression models were used to compare a cohort of 86 breast tumors selected for equivalent HER2 protein expression.
Results
We identified 44 high grade endometrioid and 42 uterine serous carcinomas (USC). IHC identified high HER2 expression (2+ or 3+) in 59% of the tumors. HER2 gene amplification was observed in 16 tumors (12 USC, 4 endometrioid). Both HER2 gene amplification and protein expression correlated with H2T values. High p95HER2 expression above 2.8 RF/mm2 was observed in 53% (n = 54) with significant correlation with H2T levels. When matched to a cohort of 107 breast tumors based on HercepTest HER2 expression, high grade EnCa presented with higher p95 levels (p < 0.001). Conclusions: These data demonstrate that compared to BrCa, high grade EnCa expresses higher levels of p95HER2 possibly providing rationale for the trastuzumab resistance observed in EnCa.
Keywords: High grade endometrial carcinoma, HER2 over-expression, p95HER2, trastuzumab resistance
Introduction
Endometrial cancer (EnCa) is the most common gynecologic malignancy in the United States [1]. While most women are cured, 15-20% of patients will present with aggressive subtypes histologically characterized as high grade endometrioid, uterine serous carcinoma (USC), and carcinosarcoma that present with more advanced stage disease that is commonly refractory to conventional platinum and taxane based chemotherapy[2]. While these tumors account for a minority of EnCa encountered, this high grade subset accounts for the majority of the 8,000 deaths observed annually and innovative, targeted therapies are needed to improve outcomes[3].
Amplification of the HER2 gene and over-expression of the HER2 protein have been described in many human malignancies including breast, colon, gastric, esophageal and endometrial and for some of these cancers, anti-HER2 therapies have become a mainstay of treatment[4-6]. The HER2 gene encodes a 185-kDa transmembrane tyrosine kinase receptor and is located on chromosome 17q21. HER2 is a well-characterized member of the human epidermal growth factor receptor superfamily that consists of three other tyrosine kinase receptors (HER1/EGFR, HER3 and HER4). Upon ligand binding, these receptors dimerize and induce signal transduction through the mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-phosphate (PI3K) signaling pathways. This downstream activation leads to induction of genes that promote oncogenic transformation via cell survival, proliferation, angiogenesis and metastasis[7]. For women with HER2 over-expressing breast tumors, anti-HER2 directed therapy has become a treatment platform with numerous FDA approved therapies including trastuzumab, pertuzumab and lapatinib[8, 9]. While HER2 over-expression was initially associated with the most guarded prognosis in breast cancer (BrCa), the advent of a targeted anti-HER2 therapy, has resulted in women with these HER2 positive tumors having one of the most favorable prognoses[10].
Like BrCa, high grade EnCa, including high grade endometrioid, USC and carcinosarcoma, has been shown to harbor a 10-42% rate of HER2 gene amplification, with up to 70% of tumors exhibiting HER2 protein over-expression[6, 11, 12]. Numerous studies have demonstrated HER2 over-expressing EnCa has been associated with decreased overall survival. Additionally, preclinical in vitro data has suggested that cells derived from HER2 gene amplified USC tumors are more responsive to anti-HER2 therapies compared to cells derived from non-amplified tumors[13]. Despite promising preclinical data, the two published phase II trials of anti-HER2 therapy in recurrent EnCa manifested poor responses [14, 15]. One trial evaluated single agent lapatinib, a dual HER1/HER2 (ERBB1/ERBB2) inhibitor, and found a 3% response rate, although these patients were not preselected for HER2 over-expression[15]. Another recent phase II trial pre-selected patients with HER2 over-expressing recurrent endometrial tumors and administered the HER2 monoclonal antibody trastuzumab. Unexpectedly, treatment revealed no responses [14]. Despite an extensive body of breast and gastric cancer literature suggesting HER2 over-expression to be a biomarker for response to anti-HER2 therapy, these targeted therapies failed to demonstrate any activity in EnCa, even in a preselected population enriched for HER2 over-expression. These trials suggest that single agent therapies directed against HER2, even in the setting of gene amplification and/or protein over-expression, have limited effect, possibly due to innate or drug induced resistance pathways.
Resistance to HER2 directed therapy is a common event in oncology, particularly in BrCa [16]. Investigators have proposed many potential resistance mechanisms including expression of a constitutively active p95HER2 variant that results from either an alternative translational start site or post-translational proteolysis that cleaves the HER2 extra-cellular domain (ECD)[17, 18] but preserves the intracellular tyrosine kinase domain. Antibodies directed towards HER2, such as trastuzumab, cannot bind in the absence of the ECD. Several retrospective analyses of HER2 positive breast cancer found that increased p95HER2 expression correlated with resistance to trastuzumab therapy and poor survival [19, 20]. Preclinical in vitro and in vivo studies demonstrated that p95HER2 exhibited kinase activity that induced tumor proliferation that was resistant to trastuzumab therapy [18, 19, 21]. As result of these observations, the p95HER2 variant is being actively investigated a biomarker for trastuzumab resistance in prospective randomized trials incorporating anti-HER2 therapies[22].
Similar to trastuzumab resistant BrCa, high grade endometrial tumors appear to manifest innate anti-HER2 resistance growth patterns. Numerous investigations have demonstrated that endometrial tumors harbor a high rate of PI3K pathway activation via PIK3CA gene mutation and PTEN inactivation which would act to uncouple anti-HER2 blockade [23, 24], but the presence of the described truncated p95HER2 variant in high grade EnCa is currently unknown. The purpose of this investigation was to evaluate p95HER2 levels utilizing the novel VeraTag™ technology [19] in a cohort of high grade EnCa and correlate these findings with total HER2 expression, HER2 gene amplification, tumor genotype and clinical outcomes. In addition, we sought to understand if the p95HER2 landscape observed in BrCa is different from that of high grade EnCa.
Materials and Methods
Patients and samples
Following internal review board approval, we identified a cohort of 86 high grade EnCa samples diagnosed between 2000-2011 with available tissue for analysis. Clinical factors were extracted from patient records, including age, grade, stage, treatment, recurrence and survival. All molecular analyses were carried out on formalin-fixed and paraffin-embedded (FFPE) diagnostic specimens. Hematoxylin and eosin-stained slides were marked for tumor location by a gynecologic oncology pathologist.
A separate set of 86 breast carcinomas was identified and matched to the endometrial samples based on HercepTest® score and used as a comparison group to understand the differences in continuous HER2 and p95HER2 protein expression generated by the VeraTag assays.
Immunohistochemistry
Paraffin embedded high grade EnCa and BrCa tissue sections of 5 μm thickness were subjected to immunohistochemistry (IHC) for HER2 using the HercepTest (Dako, Carpinteria, CA), following the manufacturer's recommendations. The intensity and pattern of the HER2 membrane immunostaining were evaluated, and all samples were scored by a pathologist on a 0 - 3+ scale with 0 representing no staining, 1+ representing weak staining in >10% of invasive tumor cells, 2+ representing moderate intensity in >10% of invasive carcinoma cells or intense staining in < 10%, and 3+ staining defined as intense circumferential membranous staining in > 10% of the invasive carcinoma. One USC sample was a technical failure for HercepTest staining and was therefore excluded from analyses incorporating HercepTest stratification.
Fluorescence in situ hybridization
To determine the HER2 gene copy number of the high grade EnCa samples, fluorescence in situ hybridization (FISH) was performed on 5 μm thick tissue sections. A PathVysion HER2 DNA Probe Kit (Abbott Laboratories, Abbott Park, IL) was used, consisting of an LSI HER2 probe with SpectrumOrange label and a CEP 17 control probe with a SpectrumGreen tag directed against the centromere region of chromosome 17. Counterstaining was carried out using Vectashield mounting medium with 4,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories). All samples were visualized and scored using the CytoVision® platform (Leica Biosystems, Buffalo Grove, IL). For each specimen, the HER2 to CEP 17 ratio was determined by counting the red (HER2) and green (CEP 17) signals in a minimum of 50 nuclei. Samples with a HER2 to CEP 17 ratio greater than 2.0 were considered amplified. A total of 2 samples (1 USC, 1 endometrioid) were technical failures of the assay following triplicate attempts.
Tumor genotyping
An adapted version of the Applied Biosystems (ABI) Prism SNaPshot multiplex system was used to genotype 3 mm core samples from >80% tumor cells from primary FFPE tumors. Total nucleic acids were extracted from each core biopsy using an automated platform based on the FormaPure System (Beckman Coulter Genomics, Danvers, MA) on a Beckman Coulter Biomek NXP workstation. This clinical mutational profiling platform screens for 130 well-characterized mutations that are distributed across 15 cancer genes including AKT1, APC, BRAF, CTNNB1, EGFR, ERBB2, IDH1, KIT, KRAS, MAP2K1, NOTCH1, NRAS, PIK3CA, PTEN, and TP53 [25].
Quantitative HER2 assay
Total HER2 protein expression (H2T) was quantified using the HERmark® assay as previously described [26, 27] in the entire 86 tumor cohort. H2T was quantified through the release of a fluorescent tag conjugated to a HER2 monoclonal antibody (mAb) via a linker that is sensitive to singlet oxygen. The antibody was paired with a biotinylated second HER2 mAb. An avidin-linked photosensitizer molecule produces singlet oxygen upon illumination with red light. Fluorescence, quantified by capillary electrophoresis, was normalized to invasive tumor area on the FFPE tissue section to give final units of Relative Fluorescence / mm2 tumor (RF/mm2). Total HER2 (H2T) measurements in FFPE BrCa tissues were compared to pre-specified cutoffs for HERmark negative (H2T<10.5 RF/mm2) and HERmark positive (H2T>17.8 RF/mm2) with equivocal defined as 10.5 RF/mm2≤H2T≤17.8 RF/mm2, derived from the <5th percentile of centrally determined HER2-positives and the >95th percentile of centrally determined HER2-negatives, respectively, within a reference database of 1,090 breast cancer patient samples. H2T > 10.5 RF/mm2 defined the primary cutoff value for elevated H2T expression in our endometrial samples.
Quantitative p95HER2 assay
P95HER2 (p95) was quantified using the VeraTag platform with a proprietary mAb specific for the active M611-CTF form of p95 as previously described [19]. Briefly, the bound p95 antibody is detected by an anti-mouse secondary antibody which was conjugated a fluorescent tag via a linker that is sensitive to reduction by dithiothreitol (DTT). Following release by DTT, the fluorescence signal was quantified as described above. p95 ≥ 2.8 RF/mm2 was used to define “high” p95 protein expression as this value has been associated with trastuzumab resistance in BrCa [19].
Statistical analysis
Two-sided Fisher's exact tests and χ2 were utilized to compare proportions for univariate analysis. Correlation was assessed utilizing Pearson correlation and Spearman rank sum testing as appropriate. Continuous variables were compared utilizing t-tests and linear regression models. Kaplan–Meier survival estimates were generated from date of histological diagnosis to time of last follow up or death, across the subgroup diagnosed with invasive disease. Log-rank tests were utilized to determine statistical significance of survival curves. A Cox proportional-hazards model, incorporating significant variables on univariate survival analysis, was utilized to identify independent factors associated with overall survival (OS). An α < 0.05 defined statistical significance. Analysis was performed on STATA version 10.0 (College Station, TX).
Results
Clinical characteristics
We identified 42 USC and 44 high grade endometrioid carcinomas. The average age of the cohort was 67 with 50% of cases representing stage III and IV disease (Table 1). Median overall survival was 2.9 years. No significant differences in age, stage or OS were observed between the USC and endometrioid cohorts. Increased age at diagnosis, stage and residual disease were significantly associated with a worsened OS upon univariate analysis. A Cox proportional hazards model incorporating all these variables confirmed that stage was the only variable that associated with worsened OS with a HR 3.43 (p = 0.01, 95% CI 1.25 – 2.24).
Table 1. Cohort characteristics.
| USC (n=42) | Endometrioid (n = 44) | Total (n = 86) | ||
|---|---|---|---|---|
| Age (Ave) | 68.7 | 65.6 | 67.1 | |
| Stage | ||||
| I | 17 | 22 | 39 | |
| II | 1 | 2 | 3 | |
| III | 12 | 12 | 24 | |
| IV | 11 | 8 | 19 | |
| Progressive disease | ||||
| Yes | 9 | 6 | 15 | |
| No | 33 | 38 | 71 | |
| OS (years) | 2.2 | 4.0 | 2.9 |
Tumor genotyping
The most prevalent mutations detected in this entire cohort were in PIK3CA (16%), TP53 (14%), KRAS (7%) and PTEN (7%). No differences were observed in the mutational profile based on histologic subtype, (Fig. 1), though we did note a trend towards a higher incidence of TP53 mutations in USC (p = 0.07). HER2 over-expressing samples defined as 2+ or 3+ by Herceptest IHC, and p95HER2 over-expressing samples defined as RF/mm2 > 2.8 showed no difference in mutational profiles. While mutations in PIK3CA, KRAS and PTEN did not associate with OS, mutations in TP53 were associated with a significantly worsened OS (p = 0.02).
Figure 1.
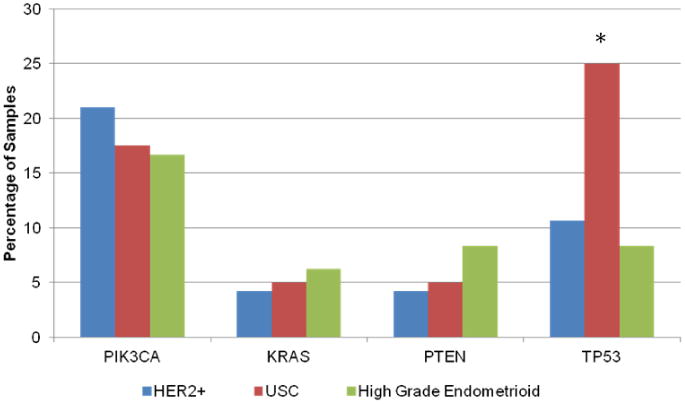
Frequency of mutations in driver genes detected in the high grade EnCa cohort. The SNaPshot® platform was used to test each high grade EnCa tumor sample. No difference in the mutational profile of USC, high grade endometrioid, HER2 or p95HER2 over-expressing samples was detected.
HER2 protein and gene expression
HercepTest IHC revealed high HER2 expression (2+ or 3+) in 59% of the cohort (n = 51) with the remainder manifesting low (0 or 1+) expression (n = 34). HER2 gene amplification was observed in 19% of the cohort (n = 16), however the USC samples accounted for a significant majority when compared to the endometrioid set of samples (n = 12, 29% vs n = 4, 9%, p = 0.03). Gene amplification was associated with a median overall survival of 1.4 years, and this was not statistically different from the 3.6 years observed in the non-amplified cohort (p = 0.07).
In the high grade EnCa samples, the HERmark assay characterized HER2 protein expression as a continuous variable showing a significant positive correlation between HercepTest and HERmark scores (rho = 0.42, p < 0.001). Similarly, gene amplified samples were significantly associated with high HERmark scores (p = 0.002) (Fig. 2A). An H2T ≥ 10.5 RF/mm2 score identified significantly fewer cases (n =20, 23%) when compared to the 2+ or 3+ HercepTest result (n = 50, 58%). Elevated HER2 protein expression defined as 2+ or 3+ staining showed a statistically similar rate of PIK3CA, KRAS, PTEN and TP53 mutation (p = 0.4). Both high grade endometrioid and USC presented with similar HERmark score distributions (p = 0.79) (Fig. 3A).
Figure 2.
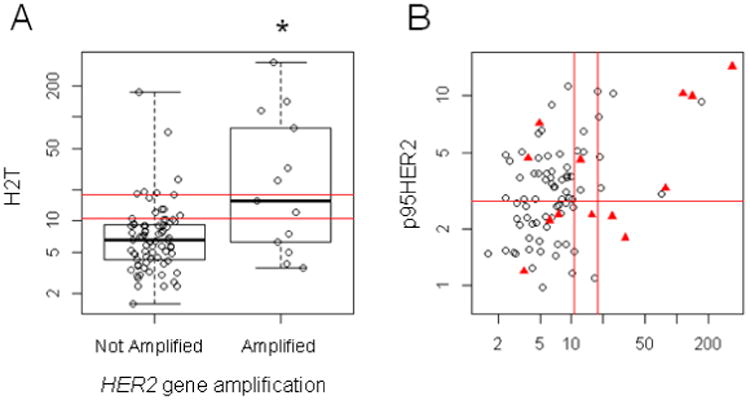
Box plots and scatterplots demonstrating relationship between HER2 gene amplification, total HER2 and p95HER2 protein expression. The double red lines delineate the HERmark negative, equivocal and positive zones which align with centrally determined HER2 status in BrCa as described in Methods. Panel A demonstrates that HER2 gene amplification was associated with a significantly higher H2T score (p < 0.002). Panel B displays the H2T and p95HER2 values, with the red triangles representing the HER2 gene amplified samples. The red line at p95HER2 = 2.8 RF/mm2 is the cutoff value associated with trastuzumab resistance in BrCa. HER2 gene amplification was not associated with p95HER2 values.
Figure 3.
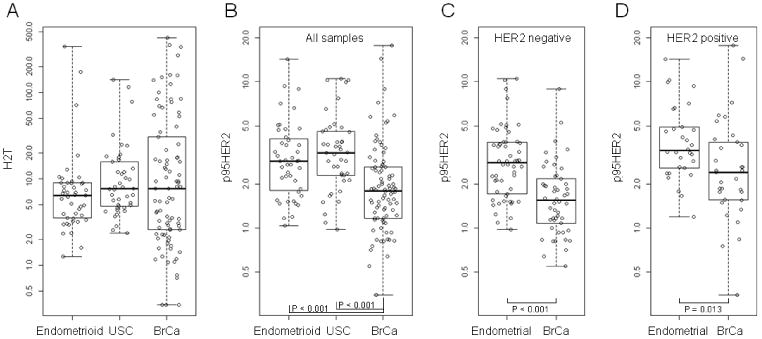
Boxplots depicting the H2T and p95HER2 scores of the high grade endometrioid, USC and BrCa samples. Panel A confirms that the HERmark H2T scores are not statistically different as they were matched by HercepTest score. In this setting of equivalent H2T scores, Panel B demonstrates how both the USC and high grade endometrioid samples harbor significantly increased levels of p95HER2 expression (both p < 0.001). Panels C and D depict the p95HER2 expression profiles of the HER2 positive and negative breast and endometrial cohorts. HER2 positive was defined as either HER2 IHC 3+ or HER2 FISH-positive. HER2 negative was defined as HER2 IHC 0 or 1+. Four cases (3 BrCa and 1 Endometrioid) were HER2 IHC 2+, FISH-unknown and were not included in 3C or 3D. One USC case had neither HER IHC nor FISH results and was not included in 3C or 3D.
p95HER2 expression in high grade EnCa
The VeraTag platform revealed that p95HER2 expression ≥ 2.8 RF/mm2 was observed in the majority of the tumor samples tested (n = 46, 53%) (Fig 2B). While p95HER2 and H2T demonstrated positive association (Spearman rho = 0.34), high p95 expression was not associated with HER2 gene amplification (Fig. 2B). Both high grade endometrioid and USC presented with similar p95HER2 score distributions (p = 0.50) (Fig. 3B). No association of p95HER2 expression and survival outcomes was observed.
Differential expression of p95HER2 and H2T in endometrial and breast carcinoma
HERmark testing of 86 breast carcinoma samples matched to the endometrial cohort by HercepTest scores revealed that H2T levels were not statistically different from the 86 endometrial samples, even when stratified by histology (Fig. 3A, Figure 4). Despite similar levels of HER2 expression as measured by HercepTest and H2T levels, however, the endometrial samples presented with significantly elevated p95HER2 levels when compared to those of the BrCa cohort (p < 0.001), and this relationship remained constant when subset analysis of cohorts with and without HER2 over-expression were performed (Figs. 3C and 3D). Scatter plots of p95HER2 vs. H2T expression confirmed that at every level of baseline H2T expression, a higher p95 expression manifests, with the most statistically robust effects seen in the USC subset (Fig. 4). To confirm a cellular localization pattern consistent with breast carcinoma p95HER2 [21], p95HER2 IHC was performed with high grade EnCa tumor samples (Fig. 5). The p95HER2 staining intensity representative of IHC 0-3+ is observed, with intense circumferential membranous staining observed with IHC 3+ samples, consistent with the staining pattern in breast carcinoma cells [19].
Figure 4.
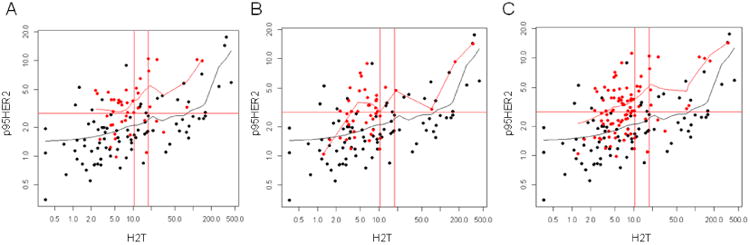
Scatterplots depicting H2T and p95HER2 expression levels observed in the USC, high grade endometrioid and total high grade EnCa cohort in comparison to a cohort of BrCa matched for HER2 protein expression using the HercepTest. Red circles represent EnCa samples with best fit line in red, black circles represent BrCa samples with best fit line in black. Panel A represents the USC samples only, with panel B depicting the high grade endometrioid only and panel C demonstrates the entire EnCa cohort simultaneously with the BrCa samples. At every H2T level of expression, the EnCa samples harbor an elevated p95HER2 expression level.
Figure 5.
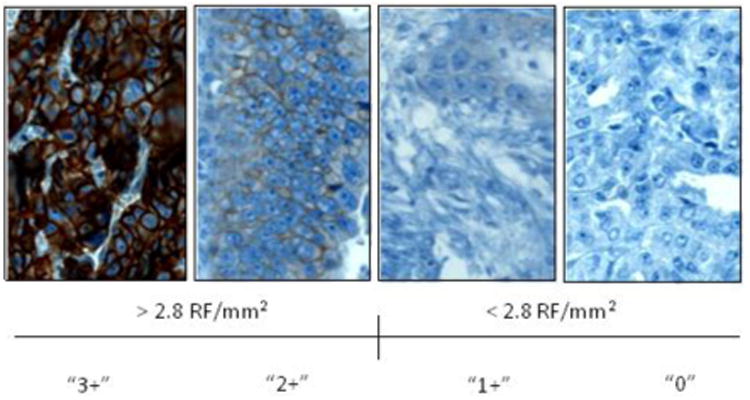
Representative p95HER2 IHC utilizing VeraTag antibody. Representative sections of 0, 1+, 2+ and 3+ IHC staining demonstrate the specificity of the membranous staining. The p95HER2 VeraTag assay utilized a continuous measurement of relative fluorescence per square mm of tumor (RF/mm2). A value of > 2.8 RF/mm2 corresponds to 2 or 3+ intensity and was used as the cutoff for elevated p95HER2 expression as this is the level that was associated with trastuzumab resistance based on a retrospective cohort of breast tumors [19].
Discussion
These data demonstrate that across all levels of total HER2 expression, endometrial tumors present with innate elevation in p95HER2 expression when compared to breast tumors. While exploratory, these data may provide rationale for the differential responses observed when women with BrCa and EnCa were treated with trastuzumab. Our genotyping results in EnCa highlight that gain of function mutations in PIK3CA and KRAS that could act to uncouple trastuzumab action, do not appear to affect a disproportionate number of the HER2 positive subset. These data highlight that in high grade EnCa, both gain of function mutation in PIK3CA and expression of the p95HER2 variant are likely to be of importance in modifying response to trastuzumab.
Trastuzumab has been a mainstay of treatment for HER2 positive (over-expressing and/or gene amplified) BrCa [10]. Numerous investigators have identified HER2 over-expression or gene amplification in other cancers, such as gastric, esophageal, ovarian and endometrial [4, 5]. Despite the hope that any HER2 positive tumor will respond to trastuzumab therapy, clinical trial results in other disease sites have failed to produce clinical benefit as robust as that demonstrated in BrCa [28-30]. EnCa is emblematic of this challenge. The clinical trial testing trastuzumab therapy in HER2 positive recurrent EnCa demonstrated no responses, suggesting that HER2 over-expression is not the sole predictor of response [14]. Investigators attempted to rationalize the negative results by suggesting the test population lacked large numbers of USC and HER2 gene amplified tumors [31]. Given the complete lack of response, one must consider that EnCa may present with innate trastuzumab resistance and that additional therapies, such as conventional cytotoxics are required in concert with HER2 blockade to induce anti-tumor effects. While any negative trial is disappointing, they do offer the opportunity to understand why therapies failed to be effective, and possible shed light on how best to overcome resistance and affect more durable responses.
In BrCa, trastuzumab resistance has been linked to loss of PTEN function and gain of function mutations in downstream signaling proteins, such as PIK3CA[16]. Endometrial cancer harbors the most frequent alterations in the PI3K pathway of any solid tumor, making this an attractive candidate to explain the observed clinical resistance [24]. Echoing more comprehensive genomic analyses[24], our investigation confirmed that HER2 expression did not correlate with the presence of gain of function mutations suggesting that additional factors contribute the observed resistance. One promising factor characterized in breast tumors that led to a plausible explanation of treatment failure was expression of the p95HER2 truncated variant that lacks the ECD required for trastuzumab binding. Expression of p95HER2 in BrCa diminished sensitivity to trastuzumab in vitro, and was associated with reduced clinical response to trastuzumab with a subsequently decreased survival [19, 21]. Using the VeraTag technology, this investigation demonstrates that a significant proportion of high grade EnCa exhibited elevated p95HER2 as defined by a threshold associated with trastuzumab resistance in BrCa[20]. While this is the first report to characterize p95HER2 expression in high grade EnCa, these data are consistent with another investigation that demonstrated that HER2 over-expressing USC cell lines shed HER2 ECD in vitro leading the authors to conclude that response to therapy could be associated with the degree of cleaved ECD [32]. When the p95HER2 and full length HER2 expression profiles in high grade EnCa were compared to breast tumors matched by the most widely used HER2 IHC test in the clinical setting (HercepTest), the p95HER2 levels in endometrial tumors were significantly higher. These data suggest the HER2 landscape is fundamentally different in EnCa compared to BrCa and possibly predisposes these tumors to innate trastuzumab resistance.
Although HER2 over-expressing endometrial tumors, particularly USC, have been associated with aggressive disease and worsened survival in numerous investigations [6, 33], expression of the p95HER2 variant did not correlate with important clinical factors, such as stage and survival. In the breast literature, p95HER2 status has been shown to have prognostic value because those patients who had been treated with trastuzumab in the metastatic setting had been shown to be more refractory [19]. In our cohort of endometrioid and USC tumors, no patients received trastuzumab or any anti-HER2 therapies making conclusions about the p95HER2 contribution to survival or response to therapy difficult to assess. Given that p95HER2 expression is likely to be a factor conferring resistance to anti-HER2 therapies that target the ECD of HER2, one could hypothesize that the presence or absence of elevated p95HER2 expression would not be related to clinical response or OS unless trastuzumab therapy was a major part of the therapeutic strategy.
This investigation confirms a significant body of literature that has found HER2 over-expression and gene amplification to be prevalent amongst high grade EnCa [34]. While the rate of HER2 gene amplification was significantly higher in USC compared to the endometrioid tumors, both histologies exhibited similar HERmark total HER2 expression. Numerous studies have supported that HER2 is a promising target for the treatment of endometrial tumors not curable with surgery or radiation[35], the majority of which are high grade EnCa. The general conclusion of these studies is that tumors harboring HER2 protein over-expression with concurrent gene amplification will likely be the most responsive to anti-HER2 therapies[36]. Unfortunately, the available clinical data do not yet provide support for this rational position. The addition of this p95HER2 data offers new insight into the HER2 landscape of these high grade endometrial tumors and provides rationale for a therapeutic strategy that utilizes dual anti-HER2 therapy with two agents so that both the ECD and intracellular domain (ICD) can be targeted[37]. This concept has gained traction in the BrCa literature leading some investigators to hypothesize dual anti-HER2 therapies could replace cytotoxics chemotherapy for a significant subset of patients [38, 39]. Currently, this concept is untested in EnCa, but pre-clinical models utilizing USC non-immortalized cell lines and patient derived xenografts supported an approach of combining trastuzumab with lapatinib[40].
This molecular investigation of HER2 expression supports that a significant subset of high grade EnCa presents with elevated expression of the p95HER2 variant when compared to breast carcinomas matched for equivalent HER2 protein expression. As p95HER2 has been associated with trastuzumab resistance, this alteration may contribute to the trastuzumab resistance observed in pre-clinical studies, as well as in the clinical trial setting [20]. Several breast carcinoma trials are actively validating p95HER2 levels as a biomarker associated with resistance to anti-HER2 therapies. Early reports have described that the addition of conventional cytotoxic chemotherapy can act to stabilize full length HER2 and re-sensitize tumors to trastuzumab [20, 22]. These findings validate the ongoing randomized phase II trial in EnCa of conventional carboplatin and paclitaxel therapy with or without trastuzumab (NCT01367002). Given the elevated p95HER2 levels in high grade EnCa compared to BrCa, p95HER2 expression may be of equal or greater importance as a biomarker as investigators design and conduct future trials in high grade EnCa that test the anti-HER2 therapies.
Research Highlights.
A majority of high grade endometrial cancers exhibited high levels of p95HER2 associated with trastuzumab resistance in breast tumors
Matched for total HER2 expression, endometrial tumors expressed a higher level of p95HER2 compared to breast tumors
Acknowledgments
Financial Support: This research was funded by an institutional K12 Proton Share NCI Grant C06 CA059267 (WBG), and funding from the Advanced Medical Research Foundation (BRR) and Vincent Department of Obstetrics and Gynecology Research Funds (BRR).
Footnotes
Conflict of Interest Statement: Whitfield B. Growdon: No conflicts of interest to report
Jolijn Groeneweg: No conflicts of interest to report
Virginia Byron: No conflicts of interest to report
Celeste DiGloria: No conflicts of interest to report
Darrell R. Borger: No conflicts of interest to report
Rosemary Tambouret: No conflicts of interest to report
Rosemary Foster: No conflicts of interest to report
Ahmed Chenna: Employee of Monogram Biosciences.
Jeff Sperinde: Employee of Monogram Biosciences.
John Winslow: Employee of Monogram Biosciences.
Bo R. Rueda: No conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366:491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton CA, Cheung MK, Osann K, Chen L, Teng NN, Longacre TA, et al. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer. 2006;94:642–6. doi: 10.1038/sj.bjc.6603012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan DS, Twine CP, Lewis WG. Systematic review and meta-analysis of the influence of HER2 expression and amplification in operable oesophageal cancer. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2012;16:1821–9. doi: 10.1007/s11605-012-1979-2. [DOI] [PubMed] [Google Scholar]
- 5.Hechtman JF, Polydorides AD. HER2/neu gene amplification and protein overexpression in gastric and gastroesophageal junction adenocarcinoma: a review of histopathology, diagnostic testing, and clinical implications. Archives of pathology & laboratory medicine. 2012;136:691–7. doi: 10.5858/arpa.2011-0168-RS. [DOI] [PubMed] [Google Scholar]
- 6.Slomovitz BM, Broaddus RR, Burke TW, Sneige N, Soliman PT, Wu W, et al. Her-2/neu overexpression and amplification in uterine papillary serous carcinoma. J Clin Oncol. 2004;22:3126–32. doi: 10.1200/JCO.2004.11.154. [DOI] [PubMed] [Google Scholar]
- 7.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nature reviews Molecular cell biology. 2001;2:127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 8.Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–19. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blackwell KL, Burstein HJ, Storniolo AM, Rugo HS, Sledge G, Aktan G, et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J Clin Oncol. 2012;30:2585–92. doi: 10.1200/JCO.2011.35.6725. [DOI] [PubMed] [Google Scholar]
- 10.Baselga J. Treatment of HER2-overexpressing breast cancer. Ann Oncol. 2010;21(Suppl 7):vii36–40. doi: 10.1093/annonc/mdq421. [DOI] [PubMed] [Google Scholar]
- 11.Santin AD, Bellone S, Van Stedum S, Bushen W, De Las Casas LE, Korourian S, et al. Determination of HER2/neu status in uterine serous papillary carcinoma: Comparative analysis of immunohistochemistry and fluorescence in situ hybridization. Gynecol Oncol. 2005;98:24–30. doi: 10.1016/j.ygyno.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 12.Livasy CA, Reading FC, Moore DT, Boggess JF, Lininger RA. EGFR expression and HER2/neu overexpression/amplification in endometrial carcinosarcoma. Gynecol Oncol. 2006;100:101–6. doi: 10.1016/j.ygyno.2005.07.124. [DOI] [PubMed] [Google Scholar]
- 13.Konecny GE, Venkatesan N, Yang G, Dering J, Ginther C, Finn R, et al. Activity of lapatinib a novel HER2 and EGFR dual kinase inhibitor in human endometrial cancer cells. Br J Cancer. 2008;98:1076–84. doi: 10.1038/sj.bjc.6604278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleming GF, Sill MW, Darcy KM, McMeekin DS, Thigpen JT, Adler LM, et al. Phase II trial of trastuzumab in women with advanced or recurrent, HER2-positive endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2010;116:15–20. doi: 10.1016/j.ygyno.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leslie KK, Sill MW, Lankes HA, Fischer EG, Godwin AK, Gray H, et al. Lapatinib and potential prognostic value of EGFR mutations in a Gynecologic Oncology Group phase II trial of persistent or recurrent endometrial cancer. Gynecol Oncol. 2012;127:345–50. doi: 10.1016/j.ygyno.2012.07.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pohlmann PR, Mayer IA, Mernaugh R. Resistance to Trastuzumab in Breast Cancer. Clin Cancer Res. 2009;15:7479–91. doi: 10.1158/1078-0432.CCR-09-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christianson TA, Doherty JK, Lin YJ, Ramsey EE, Holmes R, Keenan EJ, et al. NH2-terminally truncated HER-2/neu protein: relationship with shedding of the extracellular domain and with prognostic factors in breast cancer. Cancer Res. 1998;58:5123–9. [PubMed] [Google Scholar]
- 18.Anido J, Scaltriti M, Bech Serra JJ, Santiago Josefat B, Todo FR, Baselga J, et al. Biosynthesis of tumorigenic HER2 C-terminal fragments by alternative initiation of translation. The EMBO journal. 2006;25:3234–44. doi: 10.1038/sj.emboj.7601191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sperinde J, Jin X, Banerjee J, Penuel E, Saha A, Diedrich G, et al. Quantitation of p95HER2 in paraffin sections by using a p95-specific antibody and correlation with outcome in a cohort of trastuzumab-treated breast cancer patients. Clin Cancer Res. 2010;16:4226–35. doi: 10.1158/1078-0432.CCR-10-0410. [DOI] [PubMed] [Google Scholar]
- 20.Duchnowska R, Sperinde J, Chenna A, Haddad M, Paquet A, Lie Y, et al. Quantitative measurements of tumoral p95HER2 protein expression in metastatic breast cancer patients treated with trastuzumab: independent validation of the p95HER2 clinical cutoff. Clin Cancer Res. 2014;20:2805–13. doi: 10.1158/1078-0432.CCR-13-2782. [DOI] [PubMed] [Google Scholar]
- 21.Scaltriti M, Rojo F, Ocana A, Anido J, Guzman M, Cortes J, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99:628–38. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- 22.Parra-Palau JL, Morancho B, Peg V, Escorihuela M, Scaltriti M, Vicario R, et al. Effect of p95HER2/611CTF on the Response to Trastuzumab and Chemotherapy. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dedes KJ, Wetterskog D, Ashworth A, Kaye SB, Reis-Filho JS. Emerging therapeutic targets in endometrial cancer. Nat Rev Clin Oncol. 2011 doi: 10.1038/nrclinonc.2010.216. [DOI] [PubMed] [Google Scholar]
- 24.Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dias-Santagata D, Akhavanfard S, David SS, Vernovsky K, Kuhlmann G, Boisvert SL, et al. Rapid targeted mutational analysis of human tumours: a clinical platform to guide personalized cancer medicine. EMBO Mol Med. 2010;2:146–58. doi: 10.1002/emmm.201000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang W, Reinholz M, Weidler J, Yolanda L, Paquet A, Whitcomb J, et al. Comparison of central HER2 testing with quantitative total HER2 expression and HER2 homodimer measurements using a novel proximity-based assay. American journal of clinical pathology. 2010;134:303–11. doi: 10.1309/AJCP3BZY4YAFNTRG. [DOI] [PubMed] [Google Scholar]
- 27.Shi Y, Huang W, Tan Y, Jin X, Dua R, Penuel E, et al. A novel proximity assay for the detection of proteins and protein complexes: quantitation of HER1 and HER2 total protein expression and homodimerization in formalin-fixed, paraffin-embedded cell lines and breast cancer tissue. Diagn Mol Pathol. 2009;18:11–21. doi: 10.1097/PDM.0b013e31818cbdb2. [DOI] [PubMed] [Google Scholar]
- 28.Bookman MA, Darcy KM, Clarke-Pearson D, Boothby RA, Horowitz IR. Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a phase II trial of the Gynecologic Oncology Group. J Clin Oncol. 2003;21:283–90. doi: 10.1200/JCO.2003.10.104. [DOI] [PubMed] [Google Scholar]
- 29.Thibault C, Khodari W, Lequoy M, Gligorov J, Belkacemi Y. HER2 status for prognosis and prediction of treatment efficacy in adenocarcinomas: a review. Critical reviews in oncology/hematology. 2013;88:123–33. doi: 10.1016/j.critrevonc.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 31.Santin AD. Letter to the Editor referring to the manuscript entitled: “Phase II trial of trastuzumab in women with advanced or recurrent HER-positive endometrial carcinoma: a Gynecologic Oncology Group study” recently reported by Fleming et al., (Gynecol Oncol., 116;15-20;2010) Gynecol Oncol. 2010;118:95–6. doi: 10.1016/j.ygyno.2010.01.043. author reply 6-7. [DOI] [PubMed] [Google Scholar]
- 32.Todeschini P, Cocco E, Bellone S, Varughese J, Lin K, Carrara L, et al. Her2/neu extracellular domain shedding in uterine serous carcinoma: implications for immunotherapy with trastuzumab. Br J Cancer. 2011;105:1176–82. doi: 10.1038/bjc.2011.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santin AD, Bellone S, Van Stedum S, Bushen W, Palmieri M, Siegel ER, et al. Amplification of c-erbB2 oncogene: a major prognostic indicator in uterine serous papillary carcinoma. Cancer. 2005;104:1391–7. doi: 10.1002/cncr.21308. [DOI] [PubMed] [Google Scholar]
- 34.English DP, Roque DM, Santin AD. HER2 Expression Beyond Breast Cancer: Therapeutic Implications for Gynecologic Malignancies. Mol Diagn Ther. 2013;17:85–99. doi: 10.1007/s40291-013-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roque DM, Santin AD. Updates in therapy for uterine serous carcinoma. Current opinion in obstetrics & gynecology. 2013;25:29–37. doi: 10.1097/GCO.0b013e32835af98d. [DOI] [PubMed] [Google Scholar]
- 36.Buza N, Roque DM, Santin AD. HER2/neu in Endometrial Cancer: A Promising Therapeutic Target With Diagnostic Challenges. Archives of pathology & laboratory medicine. 2014;138:343–50. doi: 10.5858/arpa.2012-0416-RA. [DOI] [PubMed] [Google Scholar]
- 37.Kumler I, Tuxen MK, Nielsen DL. A systematic review of dual targeting in HER2-positive breast cancer. Cancer treatment reviews. 2014;40:259–70. doi: 10.1016/j.ctrv.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Prat A, Baselga J. Dual human epidermal growth factor receptor 2 (HER2) blockade and hormonal therapy for the treatment of primary HER2-positive breast cancer: one more step toward chemotherapy-free therapy. J Clin Oncol. 2013;31:1703–6. doi: 10.1200/JCO.2012.48.4998. [DOI] [PubMed] [Google Scholar]
- 39.Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633–40. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Groeneweg JW, Hernandez SF, Byron VF, DiGloria CM, Lopez H, Scialabba V, et al. Dual HER2 Targeting Impedes Growth of HER2 Gene Amplified Uterine Serous Carcinoma Xenografts. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-14-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]


