Abstract
Excessive oxidative stress in the heart results in contractile dysfunction. While antioxidant therapies have been a disappointment clinically, exercise has shown beneficial results, in part by reducing oxidative stress. We have previously shown that neuronal nitric oxide synthase (nNOS) is essential for cardioprotective adaptations caused by exercise. We hypothesize that part of the cardioprotective role of nNOS is via the augmentation of the antioxidant defense with exercise by positively shifting the nitroso-redox balance. Our results show that nNOS is indispensable for the augmented anti-oxidant defense with exercise. Furthermore, exercise training nNOS knockout mice resulted in a negative shift in the nitroso-redox balance resulting in contractile dysfunction. Remarkably, overexpressing nNOS (conditional cardiac-specific nNOS overexpression) was able to mimic exercise by increasing VO2max. This study demonstrates that exercise results in a positive shift in the nitroso-redox balance that is nNOS-dependent. Thus, targeting nNOS signaling may mimic the beneficial effects of exercise by combating oxidative stress and may be a viable treatment strategy for heart disease.
Keywords: nitroso-redox balance, ROS, exercise, phospholamban, phosphatase
INTRODUCTION
Oxidative stress (i.e., an increase in reactive oxygen species- ROS) within ventricular myocytes can be detrimental to the heart. In fact, much of the contractile dysfunction and adverse remodeling that occurs in numerous cardiomyopathies (e.g., heart failure) involves oxidative stress [1]. Unfortunately, clinical trials investigating the effects of antioxidants in heart failure have been disappointing [2, 3]. It would appear that simply lowering ROS is not enough to alleviate disease symptoms. It has been proposed that antioxidants were not clinically effective because they failed to restore the normal nitroso-redox balance [4], which is the functional coupling between ROS, specifically the superoxide anion radical (O2·−) and nitric oxide (NO) [5]. Our hypothesis is that to effectively combat oxidative stress, one must not only decrease ROS but also simultaneously increase NO. The present study explored the idea that exercise positively shifts the nitroso-redox balance (i.e., decreasing ROS and increasing NO) which may contribute to its beneficial effects in heart failure [6].
Exercise is utilized as a therapeutic intervention for cardiovascular disease and is also beneficial to overall cardiovascular health by reducing the risk of many cardiovascular maladies [7]. The bases of the beneficial effects of exercise are still not well known, but are likely to involve activation of cellular anti-oxidant pathways and increased NO [8, 9]. Exercise is able to reduce oxidative stress in disease and improve cardiac function, and we suggest it may include a positive shift in the nitroso-redox balance. Our and others work has shown that the major regulator of the nitroso-redox balance within ventricular myocytes is the neuronal nitric oxide synthase (nNOS) isoform [4, 10]. Notably, we have also found that nNOS is necessary for many of the beneficial adaptations of exercise on the heart [11]. The purpose of this study is to determine if nNOS is responsible for the antioxidant effects of exercise in the heart. We hypothesize that nNOS plays a central role for the augmentation of the antioxidant defense with exercise. Our study demonstrated that exercise activates nNOS signaling and this prevents elevated ROS levels and shifts the nitroso-redox balance to reduce oxidative stress. Genetic deletion of nNOS caused a negative shift in the nitroso-redox balance (i.e., increasing ROS and decreasing NO) with exercise to reduce contractility (cell shortening and Ca2+ transient) via activation of protein phosphatases that decrease phospholamban (PLB) Serine16 phosphorylation. On the other hand, myocyte specific overexpression of nNOS (nNOSOE) mimicked the effects of exercise. Collectively, these studies suggest that exercise improves the hearts response to oxidative stress by increasing nNOS activity to positively shift the nitroso-redox balance.
METHODS
Murine Exercise protocol
A high intensity aerobic interval treadmill training protocol was used as previously described [11]. C57Bl/6 and nNOSKO mice (Jackson Laboratories, Bar Harbor, Maine), 5 months of age at sacrifice, underwent treadmill (Columbus Instruments, Columbus, OH) training 5 days a week for 8 weeks starting at 30 min/day and increased to 80 min. Mice were challenged at a high intensity fast pace for 4 minutes followed by 1 minute of low intensity recovery pace. This interval set was repeated until the designated time (30–80 min) was reached. An adequate warm-up of 10 minutes and cool down of 4 minutes was instituted. Exercise effects are maintained for 2 weeks [12]. Thus to avoid detraining, we isolated ventricular myocytes from Sed and Ex mice within 1 week of completing our 8 week training protocol.
nNOSOE Model
Conditional, cardiac myocyte specific nNOS overexpression mice were generated as previously described [13]. The human (α-isoform) nNOS coding sequence was used.
VO2max testing protocol
A metabolic chamber (Columbus Instruments) and Oxymax analyzer (Columbus Instruments) were used for measurements. A protocol was adopted to measure maximal O2 consumption (VO2max) [14]. Briefly, mice underwent a brief warm-up with 2 min at 10 m/min at 0° incline, 2 min at 12 m/min at 10° incline, and 2 min at 15 m/min at 20° incline. Then, the treadmill speed increased 1 m/min at 20° incline every 30 sec. VO2max was defined as the absolute maximal VO2 with a respiratory exchange ratio (RER) above 1 and exhaustion was reached (mice unwilling to run and neglecting shock).
Echocardiography
Echocardiography was performed on WT and nNOSKO mice to measure the in vivo function of the heart using the Vevo 2100 and MS-400 transducer (Visualsonics, Toronto, Ontario, Canada).
Cardiomyocyte isolation
Ventricular myocytes were isolated from Ex and age-matched Sed mice along with nNOSOE and non-induced littermates [15]. Briefly, the heart was cannulated and hung on a Langendorff apparatus. It was then perfused with Ca2+ free tyrode solution (see solutions and drugs below) for 4 min. The solution was then switched to a tyrode solution containing Liberase Blendzyme II (0.077 mg/ml) (Roche Applied Science, Indianapolis, IN). After 3–5 min, the heart was taken down, the ventricles minced, and myocytes were dissociated by trituration. Subsequently the myocytes were filtered, centrifuged, and resuspended in tyrode solution containing 200 μmol/L Ca2+. Myocytes were used within 4 hrs of isolation.
Measurement of myocyte Ca2+ transients and shortening
Ca2+ transient and shortening measurements were performed at room temperature, as previously described [15]. Briefly, myocytes were loaded at room temperature with Fluo-4 AM (10 μmol/L, Molecular Probes, Eugene, OR) for 30 min. An additional 30 min were allowed for intracellular de-esterification. The instrumentation used for cell fluorescence measurements was a Cairn Research Limited (Faversham, UK) epifluorescence system. [Ca2+]i was measured by Fluo-4 epifluorescence with excitation at 480±20 nm and emission at 535±25 nm. The illumination field was restricted to collect the emission of a single cell. Data are expressed as ΔF/F0, where F is the fluorescence intensity and F0 is the intensity at rest. Data for cell shortening were collected using a video edge detection system (Crescent Electronics). Myocytes were stimulated at 1 Hz via platinum electrodes connected to a Grass Telefactor S48 stimulator (West Warwick, RI).
Measurement of ROS levels with CM-H2DCFDA Fluorescence
As previously described [16], isolated myocytes were loaded at room temperature with CM-H2DCFDA, (ROS-sensitive fluorescent dye - 10 μM) for 20 minutes and allowed to de-esterify for an additional 20 minutes. Fluorescence was observed on an Olympus Fluoview 1000 laser scanning confocal microscope by exciting at 488 nm line of an argon laser and emission was collected at 500–560 nm. Data was normalized to F0 and background subtracted. Myocytes were stimulated at 1 Hz.
Western blot analysis
Homogenized ventricles were used to measure specific phospholamban phosphorylation at Serine16 (Badrilla, Leeds, UK) with phospho-specific antibodies, phospholamban (Custom - Zymed, Invitrogen; Carlsbad, CA) and SERCA total (Custom - Zymed, Invitrogen; Carlsbad, CA) and normalized to GAPDH, as previously described [11].
Solutions and drugs
Normal Tyrode (NT) solution consisted of (in mmol/L): 140 NaCl, 4 KCl, 1 MgCl2, 1 CaCl2, 10 glucose, 5 HEPES, pH 7.4 adjusted with NaOH or HCl. Myocytes were superfused with MENO (5 μM), allopurinol (100 μM), apocynin (100 μM), or rotenone (0.3 μM). Myocytes were incubated with EMEPO (1 mmol/L), synthesized as previously described [4], or S-methyl-L-thiocitrulline (SMLT, 10 μM, Calbiochem, La Jolla, CA) for 30 minutes. All chemicals were from Sigma (St. Louis, MO) except where indicated.
Statistical Analysis
Data were presented as mean±SEM. Differences between 2 groups were evaluated for statistical significance (P < 0.05) by paired or unpaired Student’s t tests. To test statistical difference between multiple groups, a one-way ANOVA was used.
RESULTS
Increased antioxidant effect of exercise in the heart is nNOS dependent
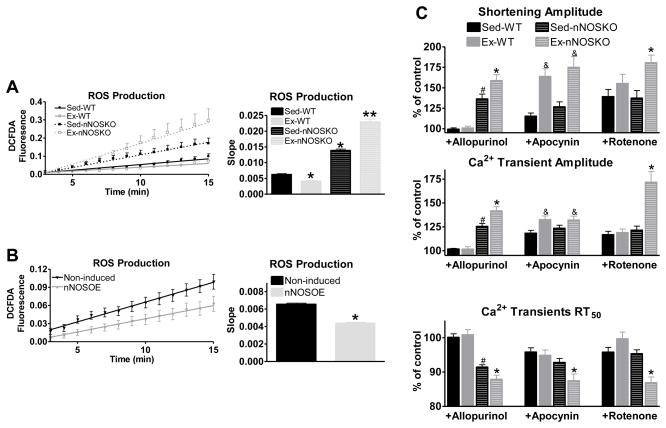
Acute periods of aerobic exercise increase ROS production within cardiac myocytes [17–23]. Therefore, the heart has to increase its anti-oxidant defenses to prevent oxidative injury. We have previously shown increased NO production specifically via nNOS is involved in the beneficial effects (contraction/relaxation and hypertrophy) of exercise in the heart [11]. In the present experiments we determined if nNOS signaling is necessary for the increased anti-oxidant defense induced by exercise. We measured ROS levels in ventricular myocytes isolated from sedentary (Sed) and exercise-trained (Ex) wildtype (WT) and nNOS knockout (nNOSKO) mice. Shown in Figure 1A, ventricular myocytes isolated from Ex-WT mice had decreased ROS levels compared to corresponding Sed-WT myocytes. Interestingly, we observed the opposite effect when we trained nNOSKO mice. That is, Ex-nNOSKO myocytes had exacerbated ROS levels compared to Sed-nNOSKO. These data suggest that nNOS is essential for the increased anti-oxidant effects of exercise in the heart.
Figure 1.
Exercise-mediated decrease in ROS levels is nNOS dependent. A) Summary data of ROS levels over a 15 minute time period (left) and calculated slopes (right) in WT and nNOSKO myocytes isolated from Sed and Ex mice (n=19–42 cells/3–4 hearts), * P<0.05 vs Sed-WT; ** P<0.05 vs all groups. B) Summary data of ROS levels in myocytes isolated from control (non-induced) and nNOSOE mice over a 15 minute time period (left) and calculated slopes (right) (n=25–33 cells/5–6 hearts), * P<0.05. C) Summary data of shortening (top), Ca2+ transient amplitude (middle) and Ca2+ transient decline (measured as time to 50% relaxation-RT50) (bottom), n=8–14 cells/3–4 hearts. # P<0.05 vs Sed-WT, & P<0.05 vs corresponding Sed, * P<0.05 vs Sed-NOS1KO.
We further tested if simply increasing nNOS expression is sufficient to enhance the antioxidant capabilities of the myocyte by using inducible, cardiac myocyte-specific nNOS transgenic mice (nNOSOE) [13]. Twenty-eight days following the removal of doxycycline (with no exercise training) to increase nNOS expression (see supplementary Figure S1), we repeated our ROS measurements in nNOSOE myocytes. Shown in Figure 1B, nNOSOE had reduced ROS levels compared to myocytes isolated from non-induced littermates. These data verify that enhanced nNOS signaling results in increased anti-oxidant effects.
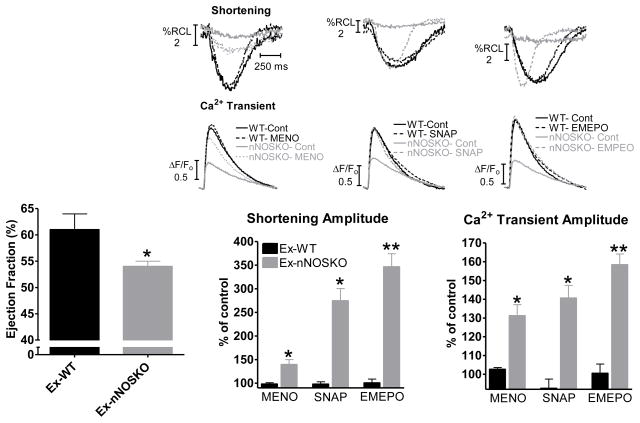
We have previously shown that there is contractile dysfunction in Ex-nNOSKO myocytes. We extended these initial observations to in vivo and demonstrate that cardiac function is depressed in Ex-nNOSKO mice as measured by left ventricular ejection fraction by echocardiography (Figure 2 and table 1). With exercise, there is increased ROS production via xanthine oxidase, NADPH oxidase and mitochondria. Since increased ROS produced via each source is able to cause contractile dysfunction, we examined which of these sources contributes to the contractile dysfunction in Ex-nNOSKO. We measured myocyte contraction (cell shortening and Ca2+ transient) and selectively inhibited each of the three major sources of ROS found in the myocyte using the xanthine oxidase inhibitor allopurinol, the NADPH oxidase inhibitor apocynin, and the mitochondria complex I inhibitor rotenone (Figure 1C). Previous work has shown that in Sed-nNOSKO myocytes the main source of the increased ROS leading to contractile dysfunction is xanthine oxidase [24, 25]. Our data are consistent with this. That is, allopurinol increased contraction in Sed-nNOSKO while the effects of apocynin and rotenone were not different between Sed-WT and Sed-nNOSKO. Interestingly, allopurinol and rotenone had the largest effect on contraction in Ex-nNOSKO; while, the contractile response to apocynin was similar between Ex-WT and Ex-nNOSKO myocytes. Therefore, ROS production via xanthine oxidase and mitochondria contributed to the contractile dysfunction in Ex-nNOSKO mice. Our data also suggests that during exercise, nNOS signaling regulates ROS production from these sources (and not NADPH oxidase). Thus, with exercise, nNOS has anti-oxidant effects by modulating xanthine oxidase and mitochondria.
Figure 2.
Contractile dysfunction in Ex-nNOSKO is reversed by restoring the nitroso-redox balance. Summary data of cardiac function (ejection fraction) evaluated by echocardiography (left) (n=10 hearts/group), * P<0.05. Summary data of the effects of MENO, SNAP, EMEPO on shortening (middle) and Ca2+ transient amplitude (right) with representative traces above (n=17–37 cells/3–4 hearts), * P<0.05 vs corresponding Ex-WT. ** P<0.05 vs MENO and SNAP.
Table 1.
LV function as assessed by echocardiography.
| Sed-WT | Ex-WT | Sed-nNOSKO | Ex-nNOSKO | |
|---|---|---|---|---|
|
| ||||
| Diastolic LV diameter, mm | 3.5±0.1 | 3.6±0.1 | 3.4±0.1 | 3.5±0.1 |
| Systolic LV diameter, mm | 2.1±0.1 | 2.3±0.1 | 2.2±0.1 | 2.4±0.1 |
| Fractional shortening, % | 38±1 | 35±1 | 35±2 | 32±1* |
| Heart rate, beats/min | 498±10 | 463±17 | 435±11 | 423±11* |
P<0.05 vs Sed-WT.
n=10 mice/group.
Contractile dysfunction with a negative shift in the nitroso-redox balance
Previous work has shown that the nitroso-redox balance is a key regulator of contraction [4, 10]. Since our Ex-nNOSKO myocytes exhibit a large negative shift in the nitroso-redox balance, we wanted to further examine the contribution of this pathway to the contractile dysfunction.
We performed experiments in isolated myocytes to determine if increased ROS contributes to the depressed contractile function in Ex-nNOSKO. Treating the myocytes with a cell permeable superoxide scavenger (MENO- methyl-ester nitroxide) reduced ROS levels in the Ex-nNOSKO myocytes to WT levels (Figure S2). Shown in Figure S3, MENO had no effect on Ex-WT. However, MENO significantly increased cell shortening, Ca2+ transient amplitude and the rate of Ca2+ transient decline in both Ex and Sed-nNOSKO (Figure S3). Additionally, MENO had a greater effect to increase contraction (Shortening: 139±10 vs 119±6 % of control, P<0.05; Ca2+ transient: 149±11 vs 114±2 % of control, P<0.05) and accelerate Ca2+ decline (−15±3 vs −7±1 % of control, P<0.05) in Ex-nNOSKO compared to Sed-nNOSKO. These data support a mechanistic link between ROS levels and the degree of contractile dysfunction (i.e., higher ROS results in greater contractile depression).
The negative shift in the nitroso-redox balance in the Ex-nNOSKO myocytes causes ROS to be increased along with a decrease in NO bioavailability. We have previously found that supplementation of NO (NO donor SNAP- S-Nitroso-N-acetyl-DL-penicillamine) can increase contraction in Sed-nNOSKO myocytes [26]. We next determined if these effects of SNAP would be present with the additional oxidative stress of exercise. Shown in Figure S4, SNAP significantly increased contraction and Ca2+ decline in Sed-WT, Sed-nNOSKO, and Ex-nNOSKO. Interestingly, SNAP did not have any effect in Ex-WT myocytes. Furthermore, SNAP had a greater effect on contraction (Shortening: 298±34 vs 207±18 % of control, P<0.05; Ca2+ transient: 143±7 vs 122±4 % of control, P<0.05) with a trend in Ca2+ transient RT50 (−26±2 vs −18±2 % of control, P=0.09) in the Ex-nNOSKO compared to Sed-nNOSKO. These results support the idea that there is a more exaggerated negative shift in the nitroso-redox balance in the Ex-nNOSKO myocytes.
Our previous work has shown that simultaneously decreasing O2·− and increasing NO (i.e., restoring the nitroso-redox balance) with a cell permeable nitrone (EMEPO) was more effective in correcting contraction than either a NO donor or an antioxidant alone [4]. EMEPO (2-(2-ethoxy-2-oxoethyl)-2-(ethoxycarbonyl)-3,4-dihydro-2H-pyrrole 1-oxide) is an ester derivative of a cyclic nitrone (DMPO) allowing for cell membrane permeation. Due to its chemical properties, EMEPO will react with O2·− and will then decompose to NO [4, 27, 28]. Thus, EMEPO is both an O2·− scavenger and NO donor. Since Ex-nNOSKO has the largest nitroso-redox imbalance, we hypothesized that EMEPO would be most beneficial in these myocytes. Our concentration of EMEPO used here was based on previous work demonstrating that EMEPO decreased ROS levels in nNOSKO myocytes to similar levels as MENO [4]. EMEPO significantly increased contraction and Ca2+ decline in both Ex-nNOSKO and Sed-nNOSKO (Figure S5). EMEPO had a greater effect to increase contraction (Shortening: 347±28 vs 250±17 % of control, P<0.05; Ca2+ transient: 157±6 vs 131±6 % of control, P<0.05) and accelerate Ca2+ decline (−17±2 vs −9±2 % of control, P<0.05) in Ex-nNOSKO compared to Sed-nNOSKO. Figure 2 shows that EMEPO increased contraction in the Ex-nNOSKO myocytes more than MENO (O2·− scavenger) or SNAP (NO donor). These data suggest that correcting the negative shift in the nitroso-redox balance is superior to restoring only one factor (i.e., decreasing ROS or increasing NO). Furthermore, EMEPO had no effect in either Sed-WT or Ex-WT likely because O2·− levels are already low and thus NO will not be produced. Similarly, MENO and SNAP had no effect in Ex-WT myocytes suggesting that exercise positively shifts the nitroso-redox balance. These data suggest that nNOS is essential for the exercise-mediated protection against oxidative stress by positively shifting the nitroso-redox balance.
Mechanisms responsible for the ROS-induced contractile dysfunction
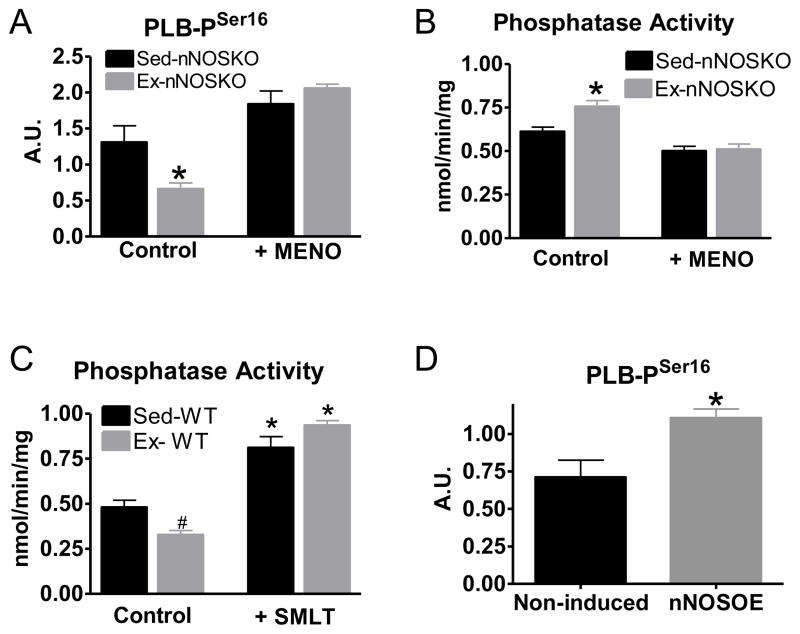
We have previously shown that nNOS signaling increases basal phospholamban Serine16 phosphorylation to enhance myocyte contraction following exercise [11]. Phospholamban Serine16 phosphorylation increases SERCA activity to enhance Ca2+ reuptake and increase sarcoplasmic reticulum Ca2+ loading. The result is a stronger contraction with faster relaxation. We determined the phosphorylation status of phospholabman Serine16 post-training in the nNOSKO (Figure 3A, representative blot shown in Figure S6). Ex-nNOSKO hearts had decreased basal phospholamban Serine16 phosphorylation compared to Sed-nNOSKO, and these changes were prevented with MENO. These data suggest that one molecular mechanism for the reduced contractility of Ex-nNOSKO hearts is a ROS mediated decrease in phospholamban Serine16 phosphorylation. Protein phosphatase 1 (PP1) and PP2a, which dephosphorylate PLB, are activated by increased oxidative stress [29]. Therefore, we measured protein phosphatase activity in the absence or presence of MENO. Ex-nNOSKO myocytes had increased protein phosphatase activity, which was reversed by scavenging ROS with MENO (Figure 3B). Interestingly, Ex-WT had significantly decreased protein phosphatase activity compared to Sed-WT (Figure 3C). We previously showed that nNOS expression/signaling is increased in Ex-WT myocyte [11]; thus we further investigated if nNOS signaling plays any role in regulating protein phosphatase activity. Acute inhibition of nNOS with S-methyl-L-thiocitrulline (SMLT) increased PP activity to the same level in both Sed and Ex myocytes. Similarly, simply overexpressing nNOS (nNOSOE) also resulted in increased basal PLB Serine16 phosphorylation (Figure 3D). These data suggest that nNOS signaling protects phosphatases from oxidation that reduces their activity.
Figure 3.
Negative shift in the nitroso-redox balance decreased phospholamban (PLB) phosphorylation via phosphatase activation. Summary data of PLB Serine16 phosphorylation (A) and phosphatase activity (B) in Sed and Ex-nNOSKO myocytes (±O2·− scavenger MENO) (n=4 hearts/group), * P<0.05 vs corresponding Sed. C) Summary data of phosphatase activity in Sed and Ex WT myocytes (±nNOS inhibitor SMLT) (n=4 hearts/group), # P<0.05 vs Sed-WT, * P<0.05 vs corresponding control. D) Summary data of PLB Serine16 phosphorylation in control (non-induced) and nNOSOE myocytes (n=6 hearts/group), * P<0.05.
nNOS overexpression produces an exercise-like effect
Exercise results in many beneficial adaptations to the heart. We tested the idea that exercise induced increases of nNOS is centrally involved in these adaptations. For this purpose, we used the nNOSOE mice (28 days with no doxycycline to induce nNOS expression with no exercise training). We tested if simply increasing nNOS expression would be sufficient to recapitulate the exercise training state in animals with a sedentary lifestyle. nNOSOE myocytes exhibited enhanced contraction and relaxation (Figure S7). These data are consistent with our previous results that Ex myocytes had a nNOS-dependent increase in Ca2+ cycling and contraction [11]. Since our Ex and nNOSOE are chronic models, we also wanted to determine if changes in Ca2+ cycling protein expression contributed to our observed phenotype. We did not observe any changes in PLB or SERCA expression, and there was no difference in PLB/SERCA ratio (Figure S8). These data suggest that the adaptive effects of exercise are due to protein post-translational modifications via the nitroso-redox signaling network.
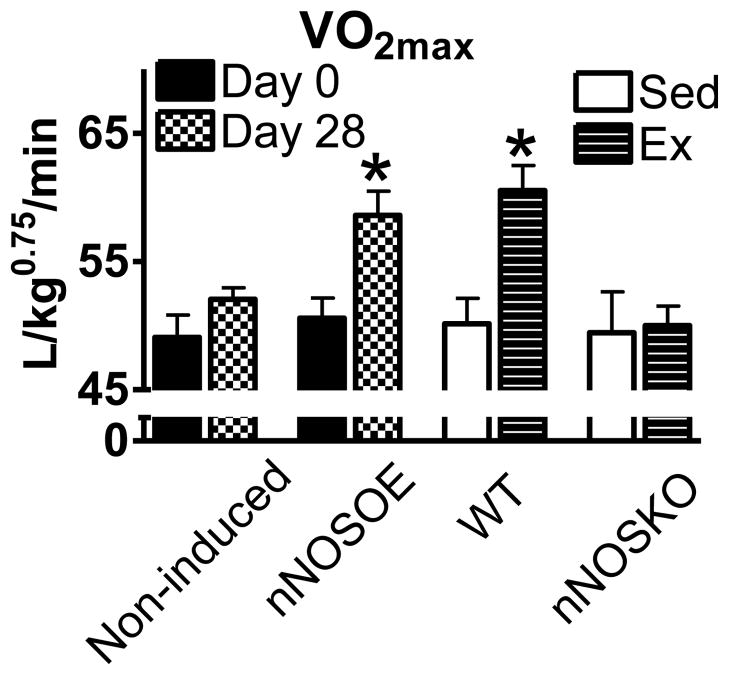
Remarkably, nNOSOE mice also obtained higher maximal oxygen consumption (VO2max), a hallmark of a fit state (Figure 4). The increase in VO2max with overexpressing nNOS was similar to the VO2max observed in exercise trained WT animals (Figure 4). Furthermore, there was no increase in VO2max in exercise trained nNOSKO mice (Figure 4). These results support the hypothesis that exercise training induces nNOS expression and this positively shifts the nitroso-redox balance (decreasing ROS levels and increasing NO levels) to enhance VO2max.
Figure 4.
Simply overexpressing myocyte nNOS (nNOSOE) mimics exercise. Summary data of maximal oxygen consumption (VO2max) in non-induced (control) littermates and nNOSOE mice (left, n=6 mice/group), sedentary (sed) and exercise trained (Ex) WT and nNOSKO mice (left, n=12 mice/group). *P< 0.05.
DISCUSSION
Within ventricular myocytes, ROS levels are tightly regulated so that enhanced ROS production, as occurs with aerobic exercise, is offset by enhanced ROS degradation. In many diseases (heart failure, infarct, ischemia/reperfusion, etc.), the increase in ROS production does not appear to be offset by sufficient increases in ROS degradation; and ROS related contractile disturbances are present. Supporting this idea is the finding that increasing ROS in normal myocytes results in contractile dysfunction like that observed in heart failure [30, 31]. Our data support the hypothesis that oxidative stress results in contractile dysfunction (Figure 2). Indeed, scavenging O2·− with MENO increased contraction in nNOSKO myocytes. Furthermore, consistent with high levels of ROS resulting in contractile dysfunction, MENO had a greater effect on contraction in Ex-nNOSKO (Figure S3). MENO had minimal effect in WT (Ex and Sed) myocytes consistent with the robust ROS buffering in healthy myocytes. Moreover, our current data, as well as previously published data [24, 32], suggest that oxidative stress leading to contractile dysfunction involves reversible protein modifications since acute MENO treatment partially increased contraction. Thus, these data suggests the increased ROS levels contributed to the depressed contractility in Ex-nNOSKO hearts.
Anti-oxidant therapies have been tested in heart failure with disappointing results [2, 3]. Exercise has been shown to be an effective therapy for heart failure, and this is thought to involve a reduction in ROS levels. Previous studies have demonstrated that the adaptive antioxidant response and cardioprotection with exercise training involves manganese superoxide dismutase (MnSOD) [33, 34]. However, the elevated ROS levels observed in Ex-nNOSKO (Figure 1) suggest that nNOS is necessary for the augmented anti-oxidant adaptation of exercise. Our studies suggest that increasing nNOS activity in heart failure could have beneficial effects similar to those of exercise training. Indeed, myocyte nNOS overexpression is cardioprotective after ischemia/reperfusion injury [35].
nNOS plays a critical role in regulating the nitroso-redox balance in response to exercise
Increased nNOS activity enhances contraction and physiological hypertrophy with exercise [11]. In the present study, we extend these results to show that nNOS is also involved in the protective antioxidant effects of exercise. Since simply lowering ROS levels are not clinically beneficial, we believe the positive outcome brought about by exercise is via a positive shift in the nitroso-redox balance (i.e., increasing NO and decreasing O2·−). In fact, our data with EMEPO demonstrates that restoring the nitroso-redox balance in Ex-nNOSKO completely restores contraction, while only lowering ROS (MENO) partially restored contraction.
The interaction between NO and ROS influences myocyte contraction. Together, in correct balance, the NO and O2·− tandem maintains physiological signaling pathways that control cardiac contractility [36]. One direct positive outcome of this interaction is that NO can act to “buffer” ROS. In fact, in the absence of nNOS (acute inhibition or knockout), ROS levels increase and negatively shift the nitroso-redox balance (Fig 1) [4, 37–39]. The current study suggests that one mechanism responsible for these effects is that the negative shift in the nitroso-redox balance activates phosphatases to decrease PLB Serine16 phosphorylation (Figure 3) and these results in alterations in the contractile Ca2+ transient. Thus, the nitroso-redox signaling network leads to reversible modifications of phosphatases to modulate contraction. However, since only lowering ROS levels is not beneficial for cardiac patients, we propose that decreasing phosphatase activity alone is also insufficient. That is, to restore the proper kinase/phosphatase balance, one must also increase kinase activity. Interestingly, nNOS signaling can activate PKA by S-nitrosylation via peroxynitrite [40–43]. Hence, the nNOS-mediated positive shift in the nitroso-redox balance with exercise will shift the kinase/phosphatase balance towards normal and promote normal levels of contractility even when ROS production is increased.
The most consistent observed change with exercise is increased PLB phosphorylation [11, 44, 45], and our previous work demonstrated that nNOS signaling is responsible for the increased PLB phosphorylation with exercise [11]. While we did observe a decrease in PLB Serine16 phosphorylation in the Ex-nNOSKO myocytes, it is likely there other mechanisms beyond PLB and altered kinase/phosphatase balance. These mice have a negative shift of the nitroso-redox balance, and it’s known that the nitroso-redox signaling network modulates multiple protein targets (e.g., SERCA, RyR2, etc.) involved with contraction [46]. In addition to modulating contractile proteins, there may also be cardiac structural abnormalities. While chronic, ultra-high endurance athletes (human and animals) exhibit signs of increased fibrosis [47, 48], our training protocol is not considered ultra-high endurance. Furthermore, there are no signs of fibrosis in nNOSKO hearts [49, 50], so we do not believe fibrosis is an issue.
Increasing NO levels could improve contractile properties in disease. Our previous work has shown that exogenous NO (SNAP) increases myocyte contraction in Sed-nNOSKO myocytes [26]. Since Ex-nNOS myocytes had an even greater oxidative stress, we tested if supplementing these myocytes with NO would improve their contractility. While NO increased contraction in the Ex-nNOS myocytes (Figure S4), the effect was not equal to EMEPO even though NO should be acting as an anti-oxidant itself (Figure 2). We surmise that this is due to localized signaling [51]. We believe that the SNAP concentration used here resulted in S-nitrosylation of proteins [26] and positive inotropy, but did not reach high enough concentration to limit ROS via inhibition of xanthine oxidase/mitochondria. If we would use a higher concentration of SNAP to increase localized NO concentration; this will result in activation of the cGMP pathway and negative inotropy [52]. Furthermore, treating cardiac patients with NO donors alone has been found to lead to tolerance and increased ROS levels [53, 54]. Therefore, during oxidative stress, nitrones such as EMEPO may be a more effective treatment [55] since they will only produce localized NO at the site of the increased ROS.
We have synthesized a novel, cell-permeable nitrone (EMEPO) that acts as a O2·− scavenger which releases NO as a byproduct [4]. This compound only produces NO when there is oxidative stress. Supporting these ideas are the findings that EMEPO had no effect in WT (Sed and Ex) myocytes (Figure S5) unlike SNAP (Figure S4). EMEPO increased the Ca2+ transient amplitude and accelerated the slowed [Ca2+]i decline in Sed-nNOSKO and Ex-nNOSKO myocytes (Figure 5). Moreover, EMEPO elicited the greatest effect in Ex-nNOSKO and enhanced myocyte contraction to Ex-WT levels. These data suggest that exercise positively shifts the nitroso-redox balance and that knockout of nNOS results in a negative shift in the nitroso-redox balance to depress cardiac function. EMEPO had the greatest effect on contraction compared to the other sole redox treatments (e.g., MENO or SNAP) (Figure 2), suggesting that the nitroso-redox balance is critical in modulating contraction.
nNOS overexpression emulates exercise adaptation
Our previous results demonstrated that Ex resulted in increased myocyte expression of nNOS, which was necessary for the beneficial heart adaptations (contraction, physiological hypertrophy) [11]. Our current results also indicate that nNOS is indispensable for the augmented anti-oxidant effect of exercise. However, there are numerous changes in the heart with exercise (e.g., increased AKT, SERCA, and MnSOD expression). Hence, we tested if simply increasing myocyte nNOS expression can replicate aspects of the exercise effects. nNOSOE myocytes had decreased levels of ROS (Figure 1- consistent with previous studies demonstrating that nNOS is an antioxidant [56]) and increased PLB Serine16 phosphorylation (Figure 3). We further tested the effects of nNOSOE in vivo by measuring VO2max. Increasing myocyte nNOS expression resulted in a greater VO2max, emulating the adaptations of exercise training (Figure 4). While there are many contributing factors to VO2max, it is mainly limited by maximal stroke volume [57, 58]. Therefore, our finding that nNOSOE increases VO2max without exercise training suggests that nNOS derived NO enhances heart function and increases VO2max. A previous study did indeed demonstrate that endogenous NO can increase stroke volume [59].
Regrettably many patients do not exercise (>70%) or cannot reach the intensity needed to elicit the associated beneficial effects. Our current results illustrate that nNOS signaling is responsible for the anti-oxidant effects of exercise. Thus, targeting nNOS signaling may mimic the beneficial effects of exercise by combating oxidative stress and maybe a viable treatment strategy to treat cardiac patients.
Supplementary Material
Highlights.
nNOS is indispensable for the hearts augmented anti-oxidant defense with exercise
Exercise positively shifts the heart’s nitroso-redox balance
nNOS overexpression mimics exercise by reducing ROS and increasing VO2max
Combating oxidative stress with nNOS may be a novel treatment that mimics exercise
Acknowledgments
Funding: This work was supported by the National Institutes of Health (K02HL094692, MTZ; SRH) and INSERM, ANR and AFM (CH).
Footnotes
DISCLOSURES
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sag CM, Wagner S, Maier LS. Role of oxidants on calcium and sodium movement in healthy and diseased cardiac myocytes. Free Radic Biol Med. 2013;63:338–49. doi: 10.1016/j.freeradbiomed.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 2.Hare JM, Mangal B, Brown J, Fisher C, Jr, Freudenberger R, Colucci WS, et al. Impact of oxypurinol in patients with symptomatic heart failure. Results of the OPT-CHF study. J Am Coll Cardiol. 2008;51:2301–9. doi: 10.1016/j.jacc.2008.01.068. [DOI] [PubMed] [Google Scholar]
- 3.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. Jama. 2005;293:1338–47. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 4.Traynham CJ, Roof SR, Wang H, Prosak RA, Tang L, Viatchenko-Karpinski S, et al. Diesterified Nitrone Rescues Nitroso-Redox Levels and Increases Myocyte Contraction Via Increased SR Ca(2+) Handling. PLoS One. 2012;7:e52005. doi: 10.1371/journal.pone.0052005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hare JM. Nitroso-redox balance in the cardiovascular system. N Engl J Med. 2004;351:2112–4. doi: 10.1056/NEJMe048269. [DOI] [PubMed] [Google Scholar]
- 6.Crimi E, Ignarro LJ, Cacciatore F, Napoli C. Mechanisms by which exercise training benefits patients with heart failure. Nat Rev Cardiol. 2009;6:292–300. doi: 10.1038/nrcardio.2009.8. [DOI] [PubMed] [Google Scholar]
- 7.Manson JE, Greenland P, LaCroix AZ, Stefanick ML, Mouton CP, Oberman A, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347:716–25. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 8.Calvert JW, Condit ME, Aragon JP, Nicholson CK, Moody BF, Hood RL, et al. Exercise protects against myocardial ischemia-reperfusion injury via stimulation of beta(3)-adrenergic receptors and increased nitric oxide signaling: role of nitrite and nitrosothiols. Circ Res. 2011;108:1448–58. doi: 10.1161/CIRCRESAHA.111.241117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powers SK, Sollanek KJ, Wiggs MP, Demirel HA, Smuder AJ. Exercise-induced improvements in myocardial antioxidant capacity: the antioxidant players and cardioprotection. Free Radic Res. 2013:7. doi: 10.3109/10715762.2013.825371. [DOI] [PubMed] [Google Scholar]
- 10.Dulce RA, Yiginer O, Gonzalez DR, Goss G, Feng N, Zheng M, et al. Hydralazine and organic nitrates restore impaired excitation-contraction coupling by reducing calcium leak associated with nitroso-redox imbalance. J Biol Chem. 2013;288:6522–33. doi: 10.1074/jbc.M112.412130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roof SR, Tang L, Ostler JE, Periasamy M, Gyorke S, Billman GE, et al. Neuronal nitric oxide synthase is indispensable for the cardiac adaptive effects of exercise. Basic Res Cardiol. 2013;108:332. doi: 10.1007/s00395-013-0332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kemi OJ, Haram PM, Wisloff U, Ellingsen O. Aerobic fitness is associated with cardiomyocyte contractile capacity and endothelial function in exercise training and detraining. Circulation. 2004;109:2897–904. doi: 10.1161/01.CIR.0000129308.04757.72. [DOI] [PubMed] [Google Scholar]
- 13.Loyer X, Gomez AM, Milliez P, Fernandez-Velasco M, Vangheluwe P, Vinet L, et al. Cardiomyocyte overexpression of neuronal nitric oxide synthase delays transition toward heart failure in response to pressure overload by preserving calcium cycling. Circulation. 2008;117:3187–98. doi: 10.1161/CIRCULATIONAHA.107.741702. [DOI] [PubMed] [Google Scholar]
- 14.Wisloff U, Helgerud J, Kemi OJ, Ellingsen O. Intensity-controlled treadmill running in rats: VO(2 max) and cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2001;280:H1301–10. doi: 10.1152/ajpheart.2001.280.3.H1301. [DOI] [PubMed] [Google Scholar]
- 15.Kohr MJ, Wang H, Wheeler DG, Velayutham M, Zweier JL, Ziolo MT. Biphasic effect of SIN-1 is reliant upon cardiomyocyte contractile state. Free Radic Biol Med. 2008;45:73–80. doi: 10.1016/j.freeradbiomed.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho HT, Liu B, Snyder JS, Lou Q, Brundage EA, Velez-Cortes F, et al. Ryanodine receptor phosphorylation by oxidized CaMKII contributes to the cardiotoxic effects of cardiac glycosides. Cardiovasc Res. 2014;101:165–74. doi: 10.1093/cvr/cvt233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muthusamy VR, Kannan S, Sadhaasivam K, Gounder SS, Davidson CJ, Boeheme C, et al. Acute exercise stress activates Nrf2/ARE signaling and promotes antioxidant mechanisms in the myocardium. Free Radic Biol Med. 2012;52:366–76. doi: 10.1016/j.freeradbiomed.2011.10.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vina J, Gimeno A, Sastre J, Desco C, Asensi M, Pallardo FV, et al. Mechanism of free radical production in exhaustive exercise in humans and rats; role of xanthine oxidase and protection by allopurinol. IUBMB Life. 2000;49:539–44. doi: 10.1080/15216540050167098. [DOI] [PubMed] [Google Scholar]
- 19.Lin WT, Yang SC, Tsai SC, Huang CC, Lee NY. L-Arginine attenuates xanthine oxidase and myeloperoxidase activities in hearts of rats during exhaustive exercise. Br J Nutr. 2006;95:67–75. doi: 10.1079/bjn20051602. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Cabrera MC, Domenech E, Vina J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med. 2008;44:126–31. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Gomez-Cabrera MC, Pallardo FV, Sastre J, Vina J, Garcia-del-Moral L. Allopurinol and markers of muscle damage among participants in the Tour de France. Jama. 2003;289:2503–4. doi: 10.1001/jama.289.19.2503-b. [DOI] [PubMed] [Google Scholar]
- 22.Leeuwenburgh C, Hansen PA, Holloszy JO, Heinecke JW. Hydroxyl radical generation during exercise increases mitochondrial protein oxidation and levels of urinary dityrosine. Free Radic Biol Med. 1999;27:186–92. doi: 10.1016/s0891-5849(99)00071-4. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez G, Escobar M, Pedrozo Z, Macho P, Domenech R, Hartel S, et al. Exercise and tachycardia increase NADPH oxidase and ryanodine receptor-2 activity: possible role in cardioprotection. Cardiovasc Res. 2008;77:380–6. doi: 10.1093/cvr/cvm011. [DOI] [PubMed] [Google Scholar]
- 24.Khan SA, Lee K, Minhas KM, Gonzalez DR, Raju SV, Tejani AD, et al. Neuronal nitric oxide synthase negatively regulates xanthine oxidoreductase inhibition of cardiac excitation-contraction coupling. Proc Natl Acad Sci U S A. 2004;101:15944–8. doi: 10.1073/pnas.0404136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinugawa S, Huang H, Wang Z, Kaminski PM, Wolin MS, Hintze TH. A defect of neuronal nitric oxide synthase increases xanthine oxidase-derived superoxide anion and attenuates the control of myocardial oxygen consumption by nitric oxide derived from endothelial nitric oxide synthase. Circ Res. 2005;96:355–62. doi: 10.1161/01.RES.0000155331.09458.A7. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Viatchenko-Karpinski S, Sun J, Gyorke I, Benkusky NA, Kohr MJ, et al. Regulation of myocyte contraction via neuronal nitric oxide synthase: role of ryanodine receptor S-nitrosylation. J Physiol. 2010;588:2905–17. doi: 10.1113/jphysiol.2010.192617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villamena FA. Superoxide radical anion adduct of 5,5-dimethyl-1-pyrroline N-oxide. 5. Thermodynamics and kinetics of unimolecular decomposition. J Phys Chem A. 2009;113:6398–403. doi: 10.1021/jp902269t. [DOI] [PubMed] [Google Scholar]
- 28.Locigno EJ, Zweier JL, Villamena FA. Nitric oxide release from the unimolecular decomposition of the superoxide radical anion adduct of cyclic nitrones in aqueous medium. Org Biomol Chem. 2005;3:3220–7. doi: 10.1039/b507530k. [DOI] [PubMed] [Google Scholar]
- 29.Kohr MJ, Davis JP, Ziolo MT. Peroxynitrite increases protein phosphatase activity and promotes the interaction of phospholamban with protein phosphatase 2a in the myocardium. Niric Oxide. 2009;20:217–21. doi: 10.1016/j.niox.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohr MJ, Kaludercic N, Tocchetti CG, Dong Gao W, Kass DA, Janssen PM, et al. Nitroxyl enhances myocyte Ca2+ transients by exclusively targeting SR Ca2+-cycling. Front Biosci. 2010;2:614–26. doi: 10.2741/e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuster GM, Lancel S, Zhang J, Communal C, Trucillo MP, Lim CC, et al. Redox-mediated reciprocal regulation of SERCA and Na+-Ca2+ exchanger contributes to sarcoplasmic reticulum Ca2+ depletion in cardiac myocytes. Free Radic Biol Med. 2010;48:1182–7. doi: 10.1016/j.freeradbiomed.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez DR, Beigi F, Treuer AV, Hare JM. Deficient ryanodine receptor S-nitrosylation increases sarcoplasmic reticulum calcium leak and arrhythmogenesis in cardiomyocytes. Proc Natl Acad Sci U S A. 2007;104:20612–7. doi: 10.1073/pnas.0706796104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown DA, Jew KN, Sparagna GC, Musch TI, Moore RL. Exercise training preserves coronary flow and reduces infarct size after ischemia-reperfusion in rat heart. J Appl Physiol. 2003;95:2510–8. doi: 10.1152/japplphysiol.00487.2003. [DOI] [PubMed] [Google Scholar]
- 34.Demirel HA, Powers SK, Zergeroglu MA, Shanely RA, Hamilton K, Coombes J, et al. Short-term exercise improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. J Appl Physiol. 2001;91:2205–12. doi: 10.1152/jappl.2001.91.5.2205. [DOI] [PubMed] [Google Scholar]
- 35.Burkard N, Williams T, Czolbe M, Blomer N, Panther F, Link M, et al. Conditional overexpression of neuronal nitric oxide synthase is cardioprotective in ischemia/reperfusion. Circulation. 2010;122:1588–603. doi: 10.1161/CIRCULATIONAHA.109.933630. [DOI] [PubMed] [Google Scholar]
- 36.Santos CX, Anilkumar N, Zhang M, Brewer AC, Shah AM. Redox signaling in cardiac myocytes. Free Radic Biol Med. 2011;50:777–93. doi: 10.1016/j.freeradbiomed.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang YH, Dingle L, Hall R, Casadei B. The role of nitric oxide and reactive oxygen species in the positive inotropic response to mechanical stretch in the mammalian myocardium. Biochim Biophys Acta. 2009;1787:811–7. doi: 10.1016/j.bbabio.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan SA, Lee K, Minhas KM, Gonzalez DR, Raju SV, Tejani AD, et al. Neuronal nitric oxide synthase negatively regulates xanthine oxidoreductase inhibition of cardiac excitation-contraction coupling. Proc Natl Acad Sci U S A. 2004;101:15944–8. doi: 10.1073/pnas.0404136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kinugawa S, Huang H, Wang Z, Kaminski PM, Wolin MS, Hintze TH. A defect of neuronal nitric oxide synthase increases xanthine oxidase-derived superoxide anion and attenuates the control of myocardial oxygen consumption by nitric oxide derived from endothelial nitric oxide synthase. Circ Res. 2005;96:355–62. doi: 10.1161/01.RES.0000155331.09458.A7. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Kohr MJ, Traynham CJ, Wheeler DG, Janssen PM, Ziolo MT. Neuronal nitric oxide synthase signaling within cardiac myocytes targets phospholamban. Am J Physiol Cell Physiol. 2008;294:C1566–75. doi: 10.1152/ajpcell.00367.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kohr MJ, Traynham CJ, Roof SR, Davis JP, Ziolo MT. cAMP-independent activation of protein kinase A by the peroxynitrite generator SIN-1 elicits positive inotropic effects in cardiomyocytes. J Mol Cell Cardiol. 2010:18. doi: 10.1016/j.yjmcc.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang YH, Zhang MH, Sears CE, Emanuel K, Redwood C, El-Armouche A, et al. Reduced phospholamban phosphorylation is associated with impaired relaxation in left ventricular myocytes from neuronal NO synthase-deficient mice. Circ Res. 2008;102:242–9. doi: 10.1161/CIRCRESAHA.107.164798. [DOI] [PubMed] [Google Scholar]
- 43.Burgoyne JR, Eaton P. Transnitrosylating nitric oxide species directly activate type I protein kinase A, providing a novel adenylate cyclase-independent cross-talk to beta-adrenergic-like signaling. J Biol Chem. 2009;284:29260–8. doi: 10.1074/jbc.M109.046722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacDonnell SM, Kubo H, Crabbe DL, Renna BF, Reger PO, Mohara J, et al. Improved myocardial beta-adrenergic responsiveness and signaling with exercise training in hypertension. Circulation. 2005;111:3420–8. doi: 10.1161/CIRCULATIONAHA.104.505784. [DOI] [PubMed] [Google Scholar]
- 45.Medeiros A, Rolim NP, Oliveira RS, Rosa KT, Mattos KC, Casarini DE, et al. Exercise training delays cardiac dysfunction and prevents calcium handling abnormalities in sympathetic hyperactivity-induced heart failure mice. J Appl Physiol. 2008;104:103–9. doi: 10.1152/japplphysiol.00493.2007. [DOI] [PubMed] [Google Scholar]
- 46.Ziolo MT, Houser SR. Abnormal Ca2+ cycling in failing ventricular myocytes: role of NOS1-mediated nitroso-redox balance. Antioxidants & Redox Signaling. 2014;21:2044–59. doi: 10.1089/ars.2014.5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benito B, Gay-Jordi G, Serrano-Mollar A, Guasch E, Shi Y, Tardif JC, et al. Cardiac arrhythmogenic remodeling in a rat model of long-term intensive exercise training. Circulation. 2011;123:13–22. doi: 10.1161/CIRCULATIONAHA.110.938282. [DOI] [PubMed] [Google Scholar]
- 48.La Gerche A, Burns AT, Mooney DJ, Inder WJ, Taylor AJ, Bogaert J, et al. Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J. 2012;33:998–1006. doi: 10.1093/eurheartj/ehr397. [DOI] [PubMed] [Google Scholar]
- 49.Dawson D, Lygate CA, Zhang MH, Hulbert K, Neubauer S, Casadei B. nNOS gene deletion exacerbates pathological left ventricular remodeling and functional deterioration after myocardial infarction. Circulation. 2005;112:3729–37. doi: 10.1161/CIRCULATIONAHA.105.539437. [DOI] [PubMed] [Google Scholar]
- 50.Saraiva RM, Minhas KM, Raju SV, Barouch LA, Pitz E, Schuleri KH, et al. Deficiency of neuronal nitric oxide synthase increases mortality and cardiac remodeling after myocardial infarction: role of nitroso-redox equilibrium. Circulation. 2005;112:3415–22. doi: 10.1161/CIRCULATIONAHA.105.557892. [DOI] [PubMed] [Google Scholar]
- 51.Ziolo MT, Bers DM. The real estate of NOS signaling: location, location, location. Circ Res. 2003;92:1279–81. doi: 10.1161/01.RES.0000080783.34092.AF. [DOI] [PubMed] [Google Scholar]
- 52.Gonzalez DR, Fernandez IC, Ordenes PP, Treuer AV, Eller G, Boric MP. Differential role of S-nitrosylation and the NO-cGMP-PKG pathway in cardiac contractility. Nitric Oxide. 2008;18:157–67. doi: 10.1016/j.niox.2007.09.086. [DOI] [PubMed] [Google Scholar]
- 53.Szocs K, Lassegue B, Wenzel P, Wendt M, Daiber A, Oelze M, et al. Increased superoxide production in nitrate tolerance is associated with NAD(P)H oxidase and aldehyde dehydrogenase 2 downregulation. J Mol Cell Cardiol. 2007;42:1111–8. doi: 10.1016/j.yjmcc.2007.03.904. [DOI] [PubMed] [Google Scholar]
- 54.Dikalov S, Fink B, Skatchkov M, Bassenge E. Comparison of glyceryl trinitrate-induced with pentaerythrityl tetranitrate-induced in vivo formation of superoxide radicals: effect of vitamin C. Free Radic Biol Med. 1999;27:170–6. doi: 10.1016/s0891-5849(99)00066-0. [DOI] [PubMed] [Google Scholar]
- 55.Villamena FA, Das A, Nash KM. Potential implication of the chemical properties and bioactivity of nitrone spin traps for therapeutics. Future Med Chem. 2012;4:1171–207. doi: 10.4155/fmc.12.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watts VL, Sepulveda FM, Cingolani OH, Ho AS, Niu X, Kim R, et al. Anti-hypertrophic and anti-oxidant effect of beta3-adrenergic stimulation in myocytes requires differential neuronal NOS phosphorylation. J Mol Cell Cardiol. 2013;62:8–17. doi: 10.1016/j.yjmcc.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bassett DR, Jr, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc. 2000;32:70–84. doi: 10.1097/00005768-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 58.Levine BD. VO2max: what do we know, and what do we still need to know? J Physiol. 2008;586:25–34. doi: 10.1113/jphysiol.2007.147629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rassaf T, Poll LW, Brouzos P, Lauer T, Totzeck M, Kleinbongard P, et al. Positive effects of nitric oxide on left ventricular function in humans. Eur Heart J. 2006;27:1699–705. doi: 10.1093/eurheartj/ehl096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.