Abstract
Preterm birth (PTB) is defined as birth before 37 weeks of gestation and is a leading cause of neonatal mortality and morbidity. To date, the etiology of spontaneous PTB (sPTB) remains unclear; however, intrauterine bacterial infection-induced inflammation is considered to be one of the major triggers. Aryl hydrocarbon receptor (AhR) is a ligand-dependent transcription factor. Upon activation, AhR signaling mediates many biological processes. AhR is abundantly expressed in human placentas, primarily in trophoblasts, and several fetal organs and tissues. The activation of AhR signaling can modulate inflammatory responses via promoting production of pro-inflammatory cytokines by the placenta and fetal membranes. These cytokines could enhance expression and/or activity of cyclooxygenase-2 (COX2) in human trophoblasts and amniotic epithelia, which in turn stimulate synthesis and release of prostaglandins (PGs; e.g., PGE2 and PGF2α). Given the discovery of a number of natural and endogenous AhR ligands in human, we hypothesize that in a subset of patients with high AhR expression in placentas and fetal membranes, repeated exposure to these AhR ligands hyperactivates AhR, inducing hyperactivation of the cytokines/COX2/PGs pathway, resulting in myometrial contractions, ultimately leading to sPTB. We further hypothesize that hyperactivation of this AhR pathway can induce sPTB either directly or in synergy with the bacterial infection. Proof of this hypothesis may provide a novel mechanism underlying sPTB.
Keywords: Aryl hydrocarbon receptor, Cyclooxygenase-2, Inflammatory cytokines, Preterm birth, Prostaglandins
1. Introduction
The rate of preterm birth (PTB) defined as any birth before 37 weeks of gestation has risen recently in most developed countries, occurring in 12–13% of pregnancies in the United States and 5–9% in other developed countries [1]. By definition, PTB is associated with small birth size with increased risks of a spectrum of adult-onset diseases such as coronary heart disease, hypertension and diabetes mellitus [2]. Even worse, PTB accounts for ~70% of perinatal mortality and ~50% of long-term neurological morbidity (e.g., developmental delay, cerebral palsy, retinopathy of prematurity and hearing and vision problems) [3]. Collectively, these disorders not only cause severe emotional distress of patients and their families, but also enormous financial hardship.
PTB is clinically classified into iatrogenic PTB, spontaneous preterm labor with intact membranes, and preterm premature rupture of the membranes [1]. The latter two together comprise spontaneous PTB (sPTB), which account for ~70% of all PTB [1]. To date, although the etiology of sPTB is not fully understood, several mechanisms such as intrauterine infection/inflammation, uterine overdistension and hormonal disorders have been proposed to cause sPTB [4–6]. Among these mechanisms, intrauterine infection/inflammation is believed to be one major cause of sPTB as it contributes to at least 25–40% of sPTB [5,7].
2. Intrauterine Infection/Inflammatory Responses in sPTB
Intrauterine infection can occur within the placenta, the choriodecidual space, the amnion and chorion, the amniotic fluid, and the umbilical cord or the fetus [1,5,7]. This intrauterine infection induces inflammatory responses via promoting local production and release of a number of inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-6, IL-8, and granulocyte colony-stimulating factor [3,5,7]. These cytokines, together with bacterial endotoxins and exotoxins, potentially up-regulate the expression of cyclooxygenase 2 (COX2) in human villous trophoblasts, chorionic trophoblasts, amniotic epithelial cells and mesoderm [8,9]. Increased COX2 expression enhances synthesis and release of prostaglandins (PGs), including PGE2 and PGF2α which are considered to be key members responsible for induction of labor [10]. These cytokines produced by a variety of cells in cervix, uterus, and amnion also stimulate the release of matrix metalloproteases, which promote cervical ripening and rupture of fetal membranes [7] and comprise anteceding events in parturition [5]. Moreover, amnion is also a major intrauterine source of PGE2 and other bioactive substances, which can activate myometrial contractions and ultimately lead to sPTB [3,10].
Although sPTB is partly characterized by a dysregulation of the immune system, bacterial infection might not be necessary to induce sPTB [4]. An exaggeration of inflammation is sufficient to do so in the absence of bacteria [4] as intra-amniotic infusion of IL-1β can promote expression of IL-6, IL-8, and TNF-α, leading to increased myometrial activity and PTB [7].
3. Aryl hydrocarbon receptor (AhR) Signaling
AhR is a ligand-dependent transcription factor that mediates metabolism of a long list of toxicants and carcinogens including halogenated aromatic hydrocarbons such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) [11,12]. These compounds are widespread in the environment and present in the food supply [11]. Most of these compounds are resistant to metabolic breakdown, contributing to the accumulation of these compounds in the human body [11].
Upon activation by its ligands, AhR induces expression of several xenobiotic-metabolizing enzymes such as cytochrome P450 enzymes (e.g., CYP1A1 and CYP1B1) [13]. However, the activation of AhR signaling can also directly trigger a series of the downstream protein kinases and phosphatases, including MAPK, c-June N-terminal/stress-activated kinases and p38 kinases [12]. In addition, AhR directly or indirectly interacts with estrogen receptors and numerous other transcription factors (i.e., nuclear factor-B, c-Fos, and c-Jun, etc) [12,14]. These complicated interactions between AhR signaling and other signaling pathways are thought to have critical impacts in the tissue-specific control of the AhR-dependent cell functions, and are believed to mediate a number of important biological processes including immunoresponses and pregnancy [12–16].
4. AhR and Pregnancy
It is well established that repeated prenatal exposure of relatively high doses of TCDD or related chemicals in many species (e.g., mouse, rat, and monkey) causes fetotoxicity and increased prenatal mortality [15]. Such TCDD- or related chemicals-induced fetotoxicity also has been implied to occur in human [15]. Paradoxically, AhR knockout in mice also leads to difficulties in maintaining pregnancy, resulting in a decrease in litter size and an increase in neonatal death, indicating the critical physiological roles of AhR in fetal and neonatal growth and development [16]. For instance, during normal pregnancy with normal AhR expression and AhR exposure in utero, activation of AhR signaling by the AhR natural ligands may be critical to the establishment and maintenance of immunologic tolerance at the maternal-fetal interface as we proposed previously [17]. This notion is further supported by the discovery of numerous endogenous and natural AhR ligands in human, animals or plants that humans routinely eat and encounter [18]. These ligands include bilirubin, flavonoids, indole-3-carbinol, indigo, 7-ketocholesterol, kynurenine, and 2-(1’H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE) [18]. Bilirubin is the yellow breakdown product of normal heme catabolism detected in bile, urine, and circulation as well as in many plants including angiosperms, which make up ~80% of all the living plant species on Earth, including corn, wheat, rice, potato, apple, and pears [18]. Flavonoids are detected in fruits and vegetables including grape, tomato, cherry, plum, strawberry blackberry, black currant, blueberry, horseradish leaf, onion peel, garden cress, and potato peel [19]. Indole-3-carbinol is found in many cruciferous plants such as broccoli and Brussels sprouts [18]. Indigo is detected in human urine and bovine serum, and 7-ketocholesterol is a major sterol constituent of plasma in humans [18]. The tryptophan catabolite, kynurenine, is detected in the human tissue and circulation [18] and is increased in the plasma of pregnant women compared with non-pregnant women [20], suggesting that pregnant women may have greater exposure to and possibly are more susceptible to endogenous AhR ligands. ITE, first isolated from porcine lung tissues, is likely derived from tryptophan and cysteine via a condensation reaction, and has potent AhR ligand activity [21].
Information on the bioavailability and tissue distributions of these endogenous or natural AhR ligands in humans is very limited. We list five of such AhR ligands, which have been found so far, in Table 1 [22–29]. The concentrations of most of these AhR ligands in the circulation of healthy humans appear to be at nano- to micro-molar levels [22–29]. We surmise that the local levels of endogenous AhR ligands will be much higher at the sites that produce them. Thus, these endogenous AhR ligands are likely to be able to activate AhR signaling, leading to alternations in cellular functions, especially when cells are repeatedly exposed to such high levels of AhR ligands.
Table 1.
Serum/Plasma Concentrations of AhR Ligands in Healthy Humans.
| AhR Ligands | Serum/Plasma Concentrations | References | |
|---|---|---|---|
| 1 | Bilirubin | 3.4–17.1 μM | [27] |
| 2 | Flavonoids | 0.5–1.6 μM | [25,29] |
| 3 | a) Indole-3-carbinol (I3C) | Not-detected | [22,26] (Oral doses of 400–1200 mg/d typically for periods of 1–6 months). |
| b) Diindolylmethane (formed from 2 molecules of I3C). | 0.3–2.5 μM | ||
| 4 | 7-ketocholesterol | 2–200 nM (mean value 55 nM) | [23] |
| 5 | L-kynurenine | 1.1–3 μM | [24,28] |
The AhR mRNA is expressed in various human tissues, among which placentas appear to have the highest level of AhR expression [30]. We have recently reported abundant AhR protein expression in human placentas and in several fetal organs [31,32]. These observations strongly suggest that human placentas and those fetal organs with high AhR expression are highly susceptible to AhR ligands.
5. AhR and Inflammation
AhR signaling is involved in the regulation of either pro- or anti-inflammatory responses [14,33,34, 35]. Indeed, many hematopoietic defects have been observed in AhR knockout mice, including decreased accumulation of lymphocytes in the spleen and lymph nodes [36] and prolonged prenatal extramedullary hematopoiesis [37]. The important roles of AhR signaling in regulating the immune system is further supported by studies using AhR ligands such as TCDD [14,33–35]. Specifically, AhR ligands may act on monocytes and macrophages to stimulate expression of major pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-8 [38–41], and their receptors [14,33,34], leading to potent inflammatory responses.
More importantly, it has been shown that TCDD can directly act on macrophages to increase the expression of COX2 [38]. COX2 expression can also be induced by inflammatory cytokines or by direct activation of AhR signaling in many cells and tissues including human trophoblasts [42], subsequently increasing production of PGs and enhancing inflammatory responses by infecting bacteria [43].
Both COX2 [8, 9,44,45] and AhR [31,32] are highly expressed in human placentas. In addition, COX2 is highly present in amniotic membrane [8,9,44,45]. Thus, together with the observation that AhR is present in human amniotic fluid ([46]; NCBI GEO ID # GSE25634), and amnion can actively respond to AhR ligands [47,48], activation of AhR signaling in placentas and fetuses may either induce inflammatory responses independently or enhance inflammatory responses in synergy with infecting bacteria.
6. The hypothesis: AhR Signaling and PTB
Concentrations of polycyclic aromatic hydrocarbons, most of which are exogenous AhR ligands, are higher in human placentas from the PTB compared with normal term patients [49]. Recent animal studies have also shown that developmental TCDD exposure in mice increases the incidence of premature birth in association with an increased sensitivity to inflammation [50,51]. More importantly, TCDD exposure of human placental explants from healthy pregnancies at 16–22 weeks’ gestation may enhance pro-inflammatory responses [52], further supporting a possible interaction between AhR and PTB. However, in the most cases, the chance for human exposure to high levels of TCDD or other relevant man-made chemicals is rare. Thus, given the wide presence of endogenous and natural AhR in human and animals, we hypothesize that in a subset of patients with high AhR expression in placentas and/or fetal tissues, high levels of endogenous and natural AhR ligands, derived from amino acid metabolism and/or food intake [18] will hyperactivate AhR. This hyperactivated AhR will provoke local production and release of pro-inflamatory cytokines such as IL-1, IL-6, IL-8, and TNF-α, which in turn could up-regulate COX2, increasing production and release of PGE2 and PGF2α in utero. These PGs will induce myometrial contractions, and ultimately lead to sPTB. This hypothesis awaits further systemically investigation. If proven, this hypothesis will provide a novel insight into sPTB and a possible new target to prevent and treat sPTB.
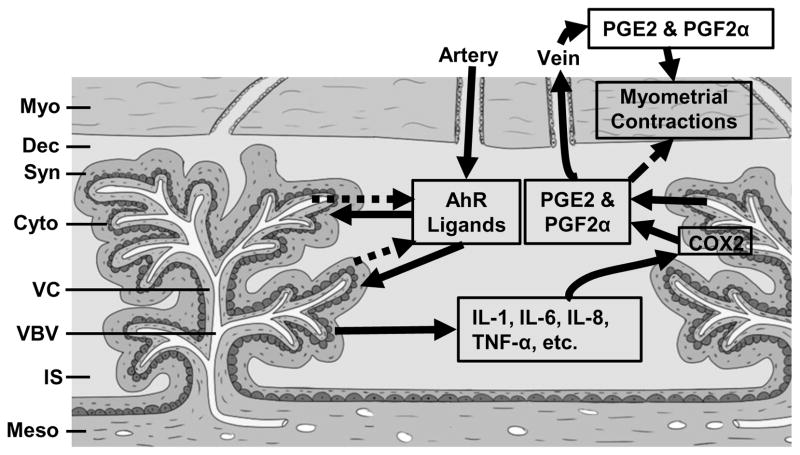
Fig. 1. A Putative Model for Placental and Fetal AhR Signaling-Induced sPTB.
In this model, AhR ligands from the maternal circulation enters the intervillous space, where they act on the placental villous trophoblasts and/or pass through the placental barrier and target fetal cells. AhR ligands could also be produced locally in the placentas and/or fetus and act on these cells in a paracrine or autocrine mechanism. The activation of AhR signaling in trophoblasts and/or fetal cells induces production of a number of pro-inflammatory cytokines (i.e., IL-1β, IL-6, IL-8, and TNF-α). These cytokines promote COX2 expression and/or activity in trophoblasts and/or fetal cells, enhancing production of PGs including PGE2 and PGF2α. PGs may reach myometrium via maternal circulation and/or via a direct diffusion mechanism from intervillous space. Upon reaching the myometrium, PGs activate myometrial contractions, inducing sPTB. Myo: Myometrium; Dec: decidua; Syn: Syncytiotrophoblast; Cyto: Cytotropblast; VC: villous core; VBV: villous blood vessels; IS: Intervillous space; Meso: Mesoderm.
Acknowledgments
Funding: This work was supported in part by the National Institutes of Health grants PO1 HD38843 to R.R.M., I.M.B, and J.Z., a Dept. of Ob/Gyn R & D Grant, University of Wisconsin-Madison to J.Z., and the National Science Foundation of China No. 81100429 and 81270703 to K.W.
The authors greatly appreciate Susanna Zheng at Kromrey Middle School, Middleton, WI, for her help drawing the schematic figure.
Footnotes
Declaration of Interest: The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker JL, Olsen LW, Sorensen TI. Weight at birth and all-cause mortality in adulthood. Epidemiology. 2008;19:197–203. doi: 10.1097/EDE.0b013e31816339c6. [DOI] [PubMed] [Google Scholar]
- 3.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–7. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 4.Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG. 2006;113 (Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simhan HN, Caritis SN. Prevention of preterm delivery. N Engl J Med. 2007;357:477–87. doi: 10.1056/NEJMra050435. [DOI] [PubMed] [Google Scholar]
- 6.Voltolini C, Torricelli M, Conti N, Vellucci FL, Severi FM, Petraglia F. Understanding spontaneous preterm birth: from underlying mechanisms to predictive and preventive interventions. Reprod Sci. 2013;20:1274–92. doi: 10.1177/1933719113477496. [DOI] [PubMed] [Google Scholar]
- 7.Bastek JA, Gomez LM, Elovitz MA. The role of inflammation and infection in preterm birth. Clin Perinatol. 2011;38:385–406. doi: 10.1016/j.clp.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Slater D, Dennes W, Sawdy R, Allport V, Bennett P. Expression of cyclo-oxygenase types-1 and -2 in human fetal membranes throughout pregnancy. J Mol Endocrinol. 1999;22:125–30. doi: 10.1677/jme.0.0220125. [DOI] [PubMed] [Google Scholar]
- 9.Premyslova M, Li W, Alfaidy N, et al. Differential expression and regulation of microsomal prostaglandin E(2) synthase in human fetal membranes and placenta with infection and in cultured trophoblast cells. J Clin Endocrinol Metab. 2003;88:6040–7. doi: 10.1210/jc.2003-030618. [DOI] [PubMed] [Google Scholar]
- 10.Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev. 2000;21:514–50. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- 11.Pocar P, Fischer B, Klonisch T, Hombach-Klonisch S. Molecular interactions of the aryl hydrocarbon receptor and its biological and toxicological relevance for reproduction. Reproduction. 2005;129:379–89. doi: 10.1530/rep.1.00294. [DOI] [PubMed] [Google Scholar]
- 12.Puga A, Ma C, Marlowe JL. The aryl hydrocarbon receptor cross-talks with multiple signal transduction pathways. Biochem Pharmacol. 2009;77:713–22. doi: 10.1016/j.bcp.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowlands JC, Gustafsson JA. Aryl hydrocarbon receptor-mediated signal transduction. Crit Rev Toxicol. 1997;27:109–34. doi: 10.3109/10408449709021615. [DOI] [PubMed] [Google Scholar]
- 14.Vondracek J, Umannova L, Machala M. Interactions of the aryl hydrocarbon receptor with inflammatory mediators: beyond CYP1A regulation. Curr Drug Metab. 2011;12:89–103. doi: 10.2174/138920011795016827. [DOI] [PubMed] [Google Scholar]
- 15.Peterson RE, Theobald HM, Kimmel GL. Developmental and reproductive toxicity of dioxins and related compounds: cross-species comparisons. Crit Rev Toxicol. 1993;23:283–335. doi: 10.3109/10408449309105013. [DOI] [PubMed] [Google Scholar]
- 16.Abbott BD, Schmid JE, Pitt JA, et al. Adverse reproductive outcomes in the transgenic Ah receptor-deficient mouse. Toxicol Appl Pharmacol. 1999;155:62–70. doi: 10.1006/taap.1998.8601. [DOI] [PubMed] [Google Scholar]
- 17.Hao K, Zhou Q, Chen W, Jia W, et al. Possible role of the ‘IDO-AhR axis’ in maternal-foetal tolerance. Cell Biol Int. 2013;37:105–08. doi: 10.1002/cbin.10023. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008;21:102–16. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleuriet AMJ-J. Phenolic acids in fruits and vegetables. 2. New York: Marcel Dekker Inc; 2003. [Google Scholar]
- 20.Schrocksnadel H, Baier-Bitterlich G, Dapunt O, Wachter H, Fuchs D. Decreased plasma tryptophan in pregnancy. Obstet Gynecol. 1996;88:47–50. doi: 10.1016/0029-7844(96)00084-1. [DOI] [PubMed] [Google Scholar]
- 21.Song J, Clagett-Dame M, Peterson RE, et al. A ligand for the aryl hydrocarbon receptor isolated from lung. Proc Natl Acad Sci USA. 2002;99:14694–9. doi: 10.1073/pnas.232562899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paganga G, Rice-Evans CA. The identification of flavonoids as glycosides in human plasma. FEBS Lett. 1997;401:78–82. doi: 10.1016/s0014-5793(96)01442-1. [DOI] [PubMed] [Google Scholar]
- 23.Savouret JF, Antenos M, Quesne M, Xu J, Milgrom E, Casper RF. 7-ketocholesterol is an endogenous modulator for the arylhydrocarbon receptor. J Biol Chem. 2001;276:3054–9. doi: 10.1074/jbc.M005988200. [DOI] [PubMed] [Google Scholar]
- 24.Van Hoydonck PG, Temme EH, Schouten EG. Serum bilirubin concentration in a Belgian population: the association with smoking status and type of cigarettes. Int J Epidemiol. 2001;30:1465–72. doi: 10.1093/ije/30.6.1465. [DOI] [PubMed] [Google Scholar]
- 25.Macheix AFaJ-J. Flavonoids in Health and Disease (Antioxidants in Health and Disease) 2. New York, NY: Marcel Dekker, Inc; 2003. [Google Scholar]
- 26.Reed GA, Arneson DW, Putnam WC, et al. Single-dose and multiple-dose administration of indole-3-carbinol to women: pharmacokinetics based on 3,3′-diindolylmethane. Cancer Epidemiol Biomarkers Prev. 2006;15:2477–81. doi: 10.1158/1055-9965.EPI-06-0396. [DOI] [PubMed] [Google Scholar]
- 27.Howells LM, Moiseeva EP, Neal CP, et al. Predicting the physiological relevance of in vitro cancer preventive activities of phytochemicals. Acta Pharmacol Sin. 2007;28:1274–304. doi: 10.1111/j.1745-7254.2007.00690.x. [DOI] [PubMed] [Google Scholar]
- 28.Yoshikawa T, Hara T, Tsurumi H, et al. Serum concentration of L-kynurenine predicts the clinical outcome of patients with diffuse large B-cell lymphoma treated with R-CHOP. Eur J Haematol. 2010;84:304–9. doi: 10.1111/j.1600-0609.2009.01393.x. [DOI] [PubMed] [Google Scholar]
- 29.Opitz CA, Litzenburger UM, Sahm F, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 30.Dolwick KM, Schmidt JV, Carver LA, Swanson HI, Bradfield CA. Cloning and expression of a human Ah receptor cDNA. Mol Pharmacol. 1993;44:911–7. [PubMed] [Google Scholar]
- 31.Jiang YZ, Wang K, Fang R, Zheng J. Expression of aryl hydrocarbon receptor in human placentas and fetal tissues. J Histochem Cytochem. 2010;58:679–85. doi: 10.1369/jhc.2010.955955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hao K, He Q, Zheng J, Wang K. Protein expression of aryl hydrocarbon receptors in human placentas from mild preeclamptic and early pregnancies. In: Zheng J, editor. Recent advances in research on the human placenta. 1. Rijeka, Croatia: In Tech-Open Access Pbulisher; 2012. pp. 119–26. [Google Scholar]
- 33.Stevens EA, Mezrich JD, Bradfield CA. The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology. 2009;127:299–311. doi: 10.1111/j.1365-2567.2009.03054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fardel O, Kolasa E, Le Vee M. Environmental chemicals as substrates, inhibitors or inducers of drug transporters: implication for toxicokinetics, toxicity and pharmacokinetics. Expert Opin Drug Metab Toxicol. 2012;8:29–46. doi: 10.1517/17425255.2012.637918. [DOI] [PubMed] [Google Scholar]
- 35.Stockinger B, Di Meglio P, Gialitakis M, Duarte JH. The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev Immunol. 2014;32:403–32. doi: 10.1146/annurev-immunol-032713-120245. [DOI] [PubMed] [Google Scholar]
- 36.Fernandez-Salguero P, Pineau T, Hilbert DM, et al. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–6. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci USA. 1996;93:6731–6. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogel CF, Sciullo E, Matsumura F. Activation of inflammatory mediators and potential role of ah-receptor ligands in foam cell formation. Cardiovasc Toxicol. 2004;4:363–73. doi: 10.1385/ct:4:4:363. [DOI] [PubMed] [Google Scholar]
- 39.Vogel CF, Sciullo E, Wong P, Kuzmicky P, Kado N, Matsumura F. Induction of proinflammatory cytokines and C-reactive protein in human macrophage cell line U937 exposed to air pollution particulates. Environ Health Perspect. 2005;113:1536–41. doi: 10.1289/ehp.8094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheon H, Woo YS, Lee JY, et al. Signaling pathway for 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced TNF-alpha production in differentiated THP-1 human macrophages. Exp Mol Med. 2007;39:524–34. doi: 10.1038/emm.2007.58. [DOI] [PubMed] [Google Scholar]
- 41.Sparfel L, Pinel-Marie ML, Boize M, et al. Transcriptional signature of human macrophages exposed to the environmental contaminant benzo(a)pyrene. Toxicol Sci. 2010;114:247–59. doi: 10.1093/toxsci/kfq007. [DOI] [PubMed] [Google Scholar]
- 42.Dominguez-Lopez P, Diaz-Cueto L, Olivares A, Ulloa-Aguirre A, Arechavaleta-Velasco F. Differential effect of DDT, DDE, and DDD on COX-2 expression in the human trophoblast derived HTR-8/SVneo cells. J Biochem Mol Toxicol. 2012;26:454–60. doi: 10.1002/jbt.21444. [DOI] [PubMed] [Google Scholar]
- 43.Dubois RN, Abramson SB, Crofford L, et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–73. [PubMed] [Google Scholar]
- 44.Fuentes A, Spaziani EP, O’Brien WF. The expression of cyclooxygenase-2 (COX-2) in amnion and decidua following spontaneous labor. Prostaglandins. 1996;52:261–7. doi: 10.1016/s0090-6980(96)00088-3. [DOI] [PubMed] [Google Scholar]
- 45.Sawdy RJ, Slater DM, Dennes WJ, Sullivan MH, Bennett PR. The roles of the cyclo-oxygenases types one and two in prostaglandin synthesis in human fetal membranes at term. Placenta. 2000;21:54–7. doi: 10.1053/plac.1999.0438. [DOI] [PubMed] [Google Scholar]
- 46.Koide K, Slonim DK, Johnson KL, Tantravahi U, Cowan JM, Bianchi DW. Transcriptomic analysis of cell-free fetal RNA suggests a specific molecular phenotype in trisomy 18. Hum Genet. 2011;129:295–305. doi: 10.1007/s00439-010-0923-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirako M, Aoki M, Kimura K, Hanafusa Y, Ishizaki H, Kariya Y. Comparison of the concentrations of polychlorinated dibenzo-p-dioxins, dibenzofurans, and dioxin-like polychlorinated biphenyls in maternal and fetal blood, amniotic and allantoic fluids in cattle. Reprod Toxicol. 2005;20:247–54. doi: 10.1016/j.reprotox.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki G, Nakano M, Nakano S. Distribution of PCDDs/PCDFs and Co-PCBs in human maternal blood, cord blood, placenta, milk, and adipose tissue: dioxins showing high toxic equivalency factor accumulate in the placenta. Biosci Biotechnol Biochem. 2005;69:1836–47. doi: 10.1271/bbb.69.1836. [DOI] [PubMed] [Google Scholar]
- 49.Singh VK, Singh J, Anand M, et al. Comparison of polycyclic aromatic hydrocarbon levels in placental tissues of Indian women with full- and preterm deliveries. Int J Hyg Environ Health. 2008;211:639–47. doi: 10.1016/j.ijheh.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Bruner-Tran KL, Osteen KG. Developmental exposure to TCDD reduces fertility and negatively affects pregnancy outcomes across multiple generations. Reprod Toxicol. 2011;31:344–50. doi: 10.1016/j.reprotox.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ding T, McConaha M, Boyd KL, Osteen KG, Bruner-Tran KL. Developmental dioxin exposure of either parent is associated with an increased risk of preterm birth in adult mice. Reprod Toxicol. 2011;31:351–8. doi: 10.1016/j.reprotox.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peltier MR, Arita Y, Klimova NG, et al. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) enhances placental inflammation. J Reprod Immunol. 2013;98:10–20. doi: 10.1016/j.jri.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]