Abstract
There exists a critical need to develop strategies that promote blood vessel formation (neovascularization) in virtually all tissue engineering and regenerative medicine efforts. While research typically focuses on understanding and exploiting the role of angiogenic factors and vascular cells on new blood vessel formation, the activity of the immune system is being increasingly recognized to impact vascular formation and adaptation. This review will provide both an overview of the intersection of angiogenesis and the immune system, and how biomaterials may be designed to promote favorable interactions between these two systems to promote effective vascularization.
Keywords: Biomaterials, Immune Cells, Vascularization
INTRODUCTION
Vascularization in regenerative medicine and tissue engineering is critical to the survival of host and implanted cells, as nearly all tissues are metabolically dependent on nutrients and oxygen delivered by vasculature. Strategies for inducing vascularization are becoming increasingly reliant on biomaterials, both as scaffolds for cell growth and as delivery-devices for cells and soluble cues. These strategies include engineered vascular grafts,64 pre-vascularized scaffolds,46 and materials for delivery of pro-angiogenic factors74 and endothelial progenitor cells.79 Most of these strategies, however, have only focused on utilizing vascular cells and pro-angiogenic factors.
Inherent to all implanted biomaterials is the fact that they elicit a foreign body reaction mounted by cells of the immune system. While biomaterials research has traditionally focused on dampening the immune response to prevent host-rejection, it is becoming recognized that the immune response can be manipulated and enhanced to help promote regeneration, particularly vascularization.25 This is due to the fact that immune cells are now known to play a role in driving neovasculzarization, as first studied in wound healing20 and tumor angiogenesis.95 This review will cover the design principles for biomaterials to drive inflammatory vascularization by providing an overview of 1) biomaterials used in tissue engineering and how they interact with the immune system, 2) the role that immune cells play in promoting angiogenesis and vascular remodeling, and 3) how immunomodulatory materials have been rationally designed to drive specific immune responses.
BIOMATERIALS AND THEIR INTERACTIONS WITH THE IMMUNE SYSTEM
Biomaterials are widely used in the field of tissue engineering and regenerative medicine as scaffolds and as delivery-devices for cells and growth factors. As scaffolds, these materials act as synthetic matrices by providing structural support and architecture, as well as cues for guiding cell behavior and function. As drug delivery-materials, they enhance the efficacy of soluble cues by providing protection from degradation, sustained release, localized presentation at the injury site, and multiple factor delivery in sequence.43 As cell delivery-materials, they can enhance the viability of transplanted cells, provide cues to enhance cell function, and mechanically stabilize their environment.43 These biomaterials can be broadly categorized into synthetic and naturally-derived materials.23 Synthetic materials include hydrophobic polymers, such as polyglycolic acid (PGA), poly(L-lactide) (PLL), and poly(lactide-co-glycolide) (PLG), as well as hydrophilic polymers that form hydrogels, such as poly(ethylene oxide) (PEO), poly(vinyl alcohol) (PVA), and poly(acrylic acid) (PAA). Naturally-derived materials include extracellular matrix (ECM) derived polymers, such as collagen, fibronectin, and hyaluronic acid, as well as polymers that are not derived from mammalian sources, such as alginate, chitosan, and agar.
Chemical Composition and Modifications
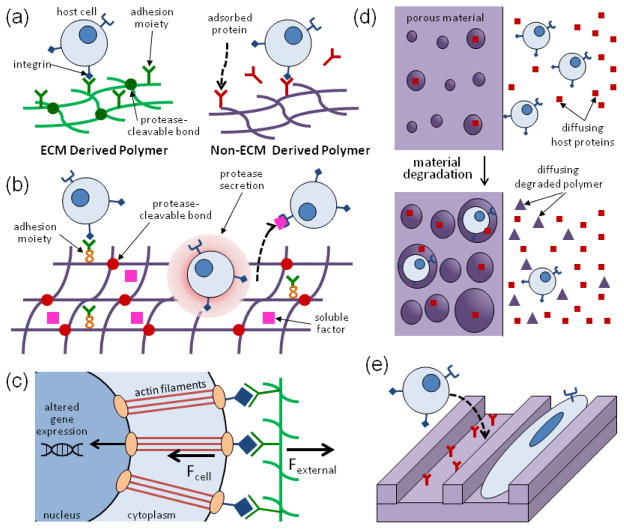
The chemical composition and modifications of a biomaterial are critical to how it interacts with surrounding cells and proteins. Polymers derived from ECM proteins can naturally engage with cells via integrins and are susceptible to cell-triggered proteolysis, whereas other materials can only indirectly interact with cells via adsorbed proteins (Fig. 1a). These materials, however, can be modified in several ways to mimic ECM-derived materials and to enhance their ability to interact with cells (Fig. 1b). Specific adhesion molecules can be included into these materials, either by surface modification or covalent attachment to the polymer backbone, to mediate specific integrin-engagement.76 Inclusion of protease cleavable bonds or cross-links into the material can also be used to enhance cell-mediated remodeling of the material.51 In addition, covalent tethering or incorporation of growth factors or other receptor ligands into the material can enhance a material’s ability to drive specific cell behavior and function.54 One example of a material that includes all three of these modifications is a synthetic PEG hydrogel formulated for bone regeneration.52 This material includes integrin-binding RGD ligands for cell adhesion, matrix metalloproteinase (MMP) cleavable linkers, and encapsulated human bone morphogenic protein-2 (BMP-2) to allow for cell infiltration and remodeling of the material into bony tissue. Rational design of these modifications into synthetic materials can allow for specific cell interactions and responses.
FIGURE 1.
The role of chemical composition, modifications, and physical properties of biomaterials on host-scaffold interactions. (A) Cells can readily interact with and remodel ECM-derived materials, whereas they typically only indirectly interact with non-ECM derived materials via adsorbed proteins. (B) Materials can be modified with adhesion moieties, protease-cleavable cross-links, and soluble factors for driving specific interactions with host cells. (C) Materials can influence the fate and function of cells via mechanotransduction, in which cells sense physical signals from their microenvironment via adhesion receptors and through their cytoskeleton. These physical signals translate to chemical signals via altered gene expression. (D) Material porosity and degradation both dictate cell influx into the material, as well as mass transport into and out of the material. (E) Material topography can be a critical regulator of cell adhesion and alignment.
Physical Properties
A biomaterial’s physical properties, which include its mechanical characteristics, degradation behavior, porosity, and topography, also regulate how the material interacts with the host environment. A material’s mechanical characteristics, which include its elasticity and viscoelastic behavior, can influence cell fate and function via mechanotransduction, the process by which mechanical signals from a cell’s microenvironment are translated into chemical signals (Fig. 1c).21,37 At the macroscopic level, the material’s mechanical properties will impact its ability to maintain a space and guide tissue formation, and to bear loads and resist forces that may disrupt interactions of cells and proteins with the material.48,98 The degradation behavior of the material, which include the rate and mechanism of degradation (e.g. bulk versus surface erosion), can dictate the rate of tissue formation and cell influx into the material,2 as well as the release of material and factors into the surrounding environment (Fig. 1d). Material porosity also dictates the influx of cells (in particular invasion of vascular cells forming blood vessels22), as well as diffusion of soluble cues into the material, material degradation, and bulk substrate properties (Fig. 1d). Lastly, the surface topography of the material can influence the interaction of the cells with the biomaterial and can guide cell behavior, including cell adhesion, alignment, migration, and polarization (Fig. 1e).63
Foreign Body Response
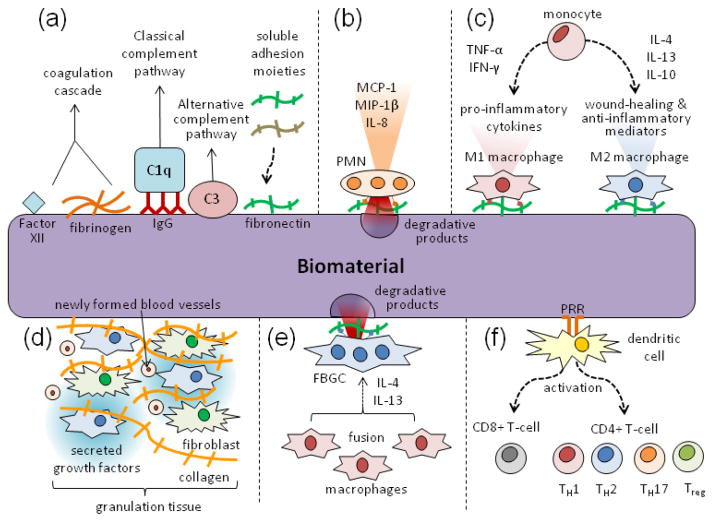
The preceding properties ultimately determine the extent of the foreign body response to a biomaterial, which follows a well known cascade of events (Fig. 2). First, proteins from the surrounding tissue and blood adsorb to the surface of the biomaterial within seconds of implantation, activating the coagulation cascade, complement system, platelets, and immune cells (Fig. 2a). The adsorbing proteins include 1) coagulation proteins such as Factor XII and fibrinogen, 2) complement proteins such as C3 and C1q, which binds via adsorbed IgG, (these proteins help activate the classical and alternative complement pathways, respectively) and 3) adhesion molecules such as fibronectin and vitronectin (Fig. 2a).25 Cells of the acute inflammatory phase, namely polymorphonuclear leukocytes (PMNs), are then recruited to the material, in part by histamine release by mast cells and fibrinogen absorption.89,97 The PMNs then release degranulation products, such as proteolytic enzymes and ROS, and secrete chemoattractants and activation factors for immune cells, such as monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1β (MIP-1β), and interleukin-8 (IL-8) (Fig. 2b).25 Cells of chronic inflammation, mainly macrophages derived from circulating monocytes, are then recruited to the material and similarly secrete inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and MCP-1, to induce further immune cell recruitment,40 and display highly phagocytic behavior. Depending on the material, these macrophages will either predominantly express one of two polarization states, the M1 or M2 phenotype (Fig. 2c).56,60 M1 macrophages, polarized by interferon-γ (IFN-γ) and TNF-α, secrete pro-inflammatory cytokines, as well as nitrogen and oxygen radicals for microbicidal activity, and are typically associated with chronic inflammation. M2 macrophages, which are polarized by interleukin-4 (IL-4), interleukin-13 (IL-3), and interleukin-10 (IL-10), secrete growth factors and regulatory cytokines and are associated with wound healing and anti-inflammatory activity. Growth factors released by macrophages are critical to the formation of granulation tissue, which is composed of recruited fibroblasts and newly formed blood vessels (Fig. 2d).3 Prolonged fibroblast activation by macrophage-secreted growth factors and excessive collagen deposition by fibroblasts can result in fibrosis.25 At the surface of the biomaterial, these macrophages may also form foreign body giant cells (FBGCs) in the presence of appropriate fusion stimuli, including IL-4 and IL-13, and appropriate adherent proteins on the material surface (Fig. 2e).3 These cells will mediate a highly degradative environment, consisting of secreted reactive oxygen radicals, proteases, and acids.3 In addition to the actions of macrophages and FBGCs, dendritic cells that are activated by pattern recognition receptor (PRR) engagement with the material will activate cytotoxic T-cells and promote helper T-cell responses (Th1, Th2, Th17, or Treg) based on their microenvironment (Fig. 2f).
FIGURE 2.
Typical sequence of events that make up the foreign body response to a biomaterial. (A) Proteins for the coagulation cascade, complement pathways, and adhesion spontaneously adsorb to the surface of the material. (B) PMNs are recruited as part of the acute inflammatory phase, secreting inflammatory cytokines and releasing degranulation products. (C) The chronic phase of inflammation consists mainly of macrophages derived from circulating monocytes. Depending on the material and microenvironment, they can polarize to either M1 or M2 macrophages. (D) Granulation tissue forms as a result of growth factors released by macrophages, resulting in newly formed blood vessels and collagen producing fibroblasts. (E) Macrophages at the surface of the material also fuse into highly degradative FBGCs in the presence of IL-4, IL-13, and specific adhesion cues. (F) Dendritic cells activate and prime the adaptive immune response by presenting antigen to cytotoxic CD8+ T-cells and helper CD4+ T-cells.
IMMUNE CELLS PROMOTE VASCULAR FORMATION
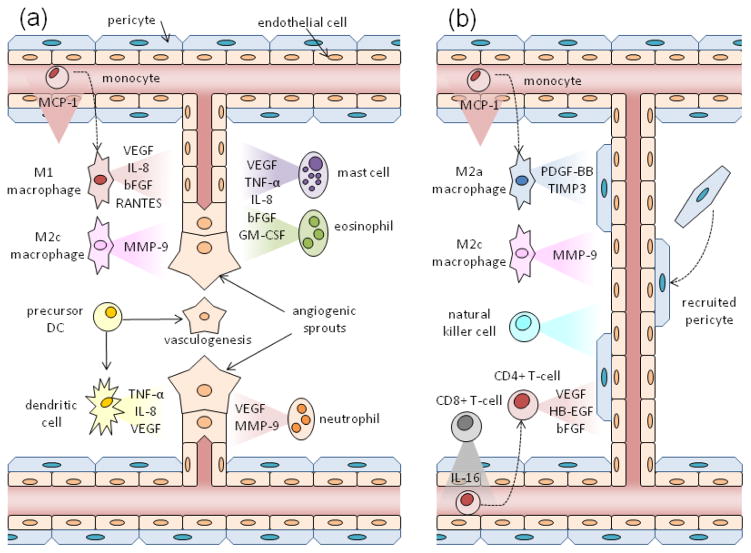
Immune cells play a critical role in regulating wound healing, particularly in controlling neovascularization. Emerging research has begun to uncover how specifically these cells promote angiogenesis80 and vascular remodeling47 in animal models of wound healing, cancer, and ischemic injury. The ability of these cells to contribute to vascular formation may be a function of their cellular microenvironment, as the pro-angiogenic activity of these cells has been shown to be strongly influenced by the inflammatory microenvironment of tumors.18 The immune cells that have been implicated in vascular formation include macrophages, dendritic cells, neutrophils, mast cells, eosinophils, T-cells, and natural killer cells. As discussed in detail below, these cells play differential roles in the early stages of angiogenesis (Fig. 3a) and vessel maturation and remodeling (Fig. 3b).
FIGURE 3.
Differential roles immune cells play in promoting (A) angiogenesis and (B) vascular remodeling. (A) M1 and M2c macrophages, derived from monocytes recruited via MCP-1, along with dendritic cells, mast cells, eosinophils, and neutrophils are believed to secrete pro-angiogenic mediators to induce new vessel formation from pre-existing vessels. Dendritic cell precursors also contribute to new vessel formation via vasculogenesis by differentiating into endothelial-like cells. (B) M2a/c macrophages, natural killer cells, and CD4+ T-cells are believed to induce arteriogenesis and vascular remodeling via secreted mediators and as cellular chaperones. These pro-arteriogenic CD4+ T-cells are recruited via IL-16 secreted by CD8+ T-cells.
Macrophages
Of the immune cells that are known to enhance vascular formation, macrophages have been most traditionally associated with promoting wound healing and vessel growth. These cells secrete a variety of factors known to impact neovascularization, including vascular endothelial growth factor (VEGF), transforming growth factor-beta (TGF-β), basic fibroblast growth factor (bFGF), insulin-like growth factor-1 (IGF-1), IL-8, and TNF-α.86 In terms of their known contributions to vessel growth, macrophages induce angiogenesis in wounds38 and tumors,50 stimulate arteriogenesis in response to ischemia,56 and help promote vascular anastamosis.24 Both resident macrophages and macrophages derived from circulating monocytes may be critical to these processes, as both appear to induce arteriogenesis in murine ischemia models.31,44 In these ischemia models, MCP-1 is critical for recruitment of these cells to promote both the early stages of angiogenesis and arteriogenesis.32,94 In terms of their polarization state, M1 macrophages have more traditionally been associated with anti-wound healing responses, whereas M2 macrophages have been more associated with driving angiogenesis and vessel growth. The recognition that tumor-associated macrophages that drive tumor angiogenesis express an M2-like phenotype,55 and the association of M2 macrophage with arteriogenesis88 led to this impression. However, recent research suggests that both M1 and M2 macrophages, which can be further categorized into M2a/b/c subtypes, are critical for different steps of neovascularization.82 M1 macrophages produce pro-angiogenic molecules, including VEGF, bFGF, IL-8, and RANTES, which are important in early stages of angiogenesis. M2a macrophages, which are associated with Th2 responses and are induced by IL-4 and IL-13, produce platelet derived growth factor-BB (PDGF-BB) for pericyte recruitment and tissue inhibitor of matrix metalloprotease-3 (TIMP3), which inhibits the activity of matrix metallopeptidase-9 (MMP-9) and VEGF (Fig. 3b); this suggests that M2a macrophages play a role in vessel maturation and balance the pro-angiogenic effects of M1 macrophages. M2c macrophages, associated with immunotolerance and induced by interleukin-10 (IL-10), secrete MMP-9, which is a potent stimulator of angiogenesis and is believed to be important for vascular remodeling; this suggests that M2c macrophages may play a role in both stimulating the early stages of vessel growth, as well as later maturation stages.
Dendritic Cells
Dendritic cells, which share a common myeloid progenitor with macrophages, are known to be regulators of tumor angiogenesis, but have not yet been associated with vascularization in wounds. Tumor associated plasmacytoid dendritic cells (PDCs) in human ovarian cancers promote angiogenesis in vivo in humans via production of TNF-α and IL-8.19 Dendritic cell precursors also contribute to tumor vascularization in vivo in murine tumor models by undergoing endothelial-like differentiation in the presence of VEGF-A and integrating into tumor vasculature to promote vasculogenesis.16 In in vitro studies, human dendritic cells express varying pro-angiogenic activity based on their activation state73,81. Alternatively activated myeloid DCs, polarized with calcitrol, prostanglandin E2 (PGE2), or IL-10, are potent sources of VEGF compared to immature and classically activated DCs.73
Granulocytes
Granulocytes, namely neutrophils, mast cells, and eosinophils, are also believed to regulate vessel formation, and appear to mainly contribute to the early stages of angiogenesis. In murine models of ischemia, neutrophils are critical to VEGF-induced angiogenesis,29 and are potent sources of VEGF in ischemic tissue when activated with granulocyte colony stimulating factor (G-CSF).67 In addition, they secrete MMP-9 to drive tumor angiogenesis.66 Both mast cells and eosinophils are also potent sources of pro-angiogenic molecules, including TNF-α, IL-8, bFGF, and granulocyte-macrophage colony stimulating factor (GM-CSF).65,71 Secretion of tryptase by human mast cells also promotes vascular tube formation in vitro, in part by promoting endothelial cell proliferation;9 tryptase may also play a role in degrading extracellular matrix by activating metalloproteinases and plasminogen activator.28,85 In vivo, the pro-angiogenic function of mast cell secreted tryptase has not been shown directly, but it has been correlated by observing that tryptase expressing mast cells localize with newly formed vessels in tumors.7,72 Mast cells contribute to recovery in murine models of ischemia by production of VEGF under ionizing irradiation treatments.33 Soluble factors derived from human peripheral eosinophils induce endothelial cell proliferation and angiogenesis, mainly through VEGF, in a variety of in vitro assays.71
Lymphocytes
Lymphocytes, including T-cells and natural killer cells, also regulate vascular recovery in ischemic injury. Peripheral human T-cells are potent sources of VEGF26 and deficiency of T-cells in murine models of ischemia significantly impairs vessel regeneration and worsens limb necrosis.17 However, the two main T-cell subtypes, CD4+ helper T-cells and CD8+ cytotoxic T-cells, have varying pro-angiogenic ability and appear to play different roles in regulating vascular recovery in ischemia. CD4+ T-cells produce pro-angiogenic heparin binding epidermal like growth factor (HB-EGF) and bFGF, whereas CD8+ T-cells only produce bFGF.10 In murine models of ischemia, CD8+ T-cells recruit CD4+ T-cells by secretion of IL-16,84 and CD4+ T-cells help promote collateral artery development and arteriogenesis.83,93 Of the CD4+ T-cell subsets, Th1 may be more pro-angiogenic, since VEGF enhances Th1 phenotype in T-cells.59 In addition to directly promoting vessel growth, T-cells also promote the pro-angiogenic activity of monocytes via both cell-cell contact and cell secretions.34,92 Also, knock-down of natural killer cells impairs arteriogenesis and recovery in ischemia models,93 although it is unknown which factors mediate these effects.
RATIONAL DESIGN OF IMMUNOMODULATORY MATERIALS
Bioengineers have combined their knowledge of biomaterials and immune cell function and behavior to rationally design materials that can modulate immune cells. While biomaterial research has traditionally focused on reducing inflammation, bioengineers have recently begun to take advantage of the ability of immune cells to combat infectious diseases for the development of material based immunotherapies.49 Immunomodulatory materials for wound regeneration, in particular vascularization, are beginning to be explored due to our increasing knowledge of immune cell contributions to wound healing.25,58 However, these materials have thus far been limited to the manipulation of macrophages,58 due to our limited knowledge of the contribution of other immune cell types to wound healing. In designing immunomodulatory materials, bioengineers have been able to manipulate immune cell trafficking and function by controlling a material’s 1) ability to target intracellular compartments, 2) chemical composition, 3) physical properties, and 4) modifications with soluble and adhesion factors (Fig. 4). This review will not address the function of particle based materials for targeting immune related tissues and organs such as lymph nodes and mucosal tissues, as that is covered elsewhere.49
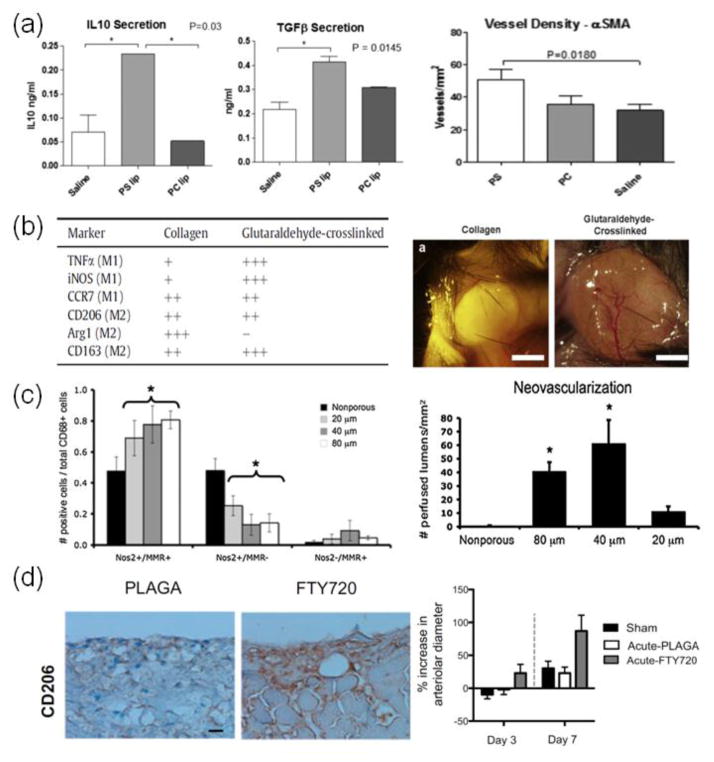
FIGURE 4.
Examples showing modulation of inflammation-induced angiogenesis and arteriogenesis by controlling (A) intracellular targeting with nanoparticles, (B) material composition, (C) material physical properties, and (D) presentation of immunomodulatory factors. (A) Phosphatidylserine presenting liposomes (PS) increased secretion of anti-inflammatory cytokines, IL-10 and TGF-β, in cardiac macrophages after myocardial infarction compared to PS-lacking liposomes (PC) and saline treatment (saline). Treatment with PS also increased vessel formation in ischemic heart over PC and saline.30 (B) Unmodified collagen gels only showed recruitment of M2 macrophages, whereas gluteraldehyde cross-linked gels showed both M1 and M2 macrophage recruitment. Recruitment of both macrophage phenotypes correlated with enhanced vessel growth in the gluteraldehyde cross-linked gels.82 (C) Myocardial implantation of acellular scaffolds showed greater shift in M2 polarization with pore size of 40 μm, as exemplified by the M1 marker nitric oxide synthase 2 (NOS2) and the M2 marker MΦ mannose receptor (MMR). This correlated with enhanced neovascularization.53 (D) Presentation of FTY720 from PLAGA films led to increased numbers of M2 macrophages compared to PLAGA films alone, as represented by staining with the M2 marker, CD206. This correlated with increased arteriogenesis as determined by arterial diameter.4
Targeting Intracellular Compartments
Materials in the form of micelles and nanoparticles can modulate immune cell function by targeting intracellular compartments; this has been explored to enhance immune cell response to antigens and danger signals, as well as to control antigen presentation by immune cells. Certain PRRs in antigen presenting cells (APCs), such as toll-like receptor 7 (TLR7) for single-stranded RNA and toll-like receptor 9 (TLR9) for bacterial CpG DNA, are only present within the endolysosome and thus must be intracellularly targeted. Nanoparticles for delivering antigens to intracellular compartments in APCs have been effective for immunotherapy, particularly with triggered antigen release in the environment of phagosomes. In one example, antigen peptides were conjugated to poly(propylene sulfide) nanoparticles via a reducible disulfide bond; these peptides were rapidly released from the particle in the reductive environment of the phagasome in dendritic cells, inducing enhanced antigen cross-presentation and T-cell activation compared to presentation via soluble antigen.36 To enhance wound regeneration, nanoparticle uptake by macrophages has been used to induce anti-inflammatory responses and help promote angiogenesis. Cardiac macrophages in an acute rat model of myocardial infarction were shown to engulf liposomes that present the anti-inflammatory, apoptotic signal phosphatidylserine (PS). Macrophages that engulfed these particles showed enhanced secretion of anti-inflammatory TGF-β (a potent inducer of regulatory T-cells15) and IL-10, and this was linked with the ability of these nanoparticles to enhance angiogenesis and ventricular remodeling in cardiac tissue (Fig. 4a).30 A similar study was also done in a rat model of myocardial infarction, where liposomes with encapsulated phenytoin (PHT), a non-selective voltage gated sodium channel inhibitor, suppressed expansion of CD43+ pro-inflammatory monocytes, which correlated with attenuated ischemia injury and improved ventricular remodeling.99
Chemical Composition
The choice of the base material is critical in designing an immunomodulatory material, as the material’s chemistry alone can be important in determining the immune response. Hydrogels that are not derived from ECM, such as alginate, often induce a low inflammatory response since cellular receptors cannot directly bind and proteins do not readily adsorb to these gels. Materials derived from ECM, such as collagen, can be pro-inflammatory as cells can directly engage via integrins. Synthetic, hydrophobic materials such as PLG, can also be inflammatory because cells can bind to them indirectly via adsorbed proteins. Bioengineers have manipulated these inherent inflammatory properties in the design of materials for immunotherapy, as well as inflammation-driven vascularization. For immunotherapy, PLG was used to help program dendritic cells for cancer immunotherapy,1 as the material enhances maturation and cytokine production of murine dendritic cells.96 For vascularization, PLG scaffolds have been shown to induce greater vessel formation than collagen-chitosan-hydroxyapatite hydrogels, likely due to different immune responses. PLG scaffolds were shown to induce moderate inflammation, with greater macrophage recruitment than PMN recruitment. The hydrogel, however, had a less controlled inflammatory response that ultimately led to the apoptosis of surrounding immune cells.77 The cross-linking chemistry can also change immune responses to a biomaterial. Calcium used to cross-link alginate can influence the phenotype of encapsulated dendritic cells, in particular the upregulation of expression of IL-1β compared to alginate gels cross-linked with barium; this effect is observed with free, soluble calcium as well.12 Gluteraldehyde cross-linked collagen gels have been shown to induce a greater amount of vascularization than unmodified collagen, likely due to differential macrophage recruitment (Fig. 4b); gluteraldehyde cross-linked collagen gels showed greater macrophage infiltration compared to unmodified collagen gels. Also, cells in gluteraldehyde cross-linked collagen gels showed both M1 and M2 macrophage markers, whereas cells in unmodified gels only expressed M2 markers.82 The surface properties of a material have also been shown to be critical to immune cell activity, as they can regulate macrophage and FBGC adherence and cytokine production,40 as well as complement activation.90 Hydrophobicity, as well, has been proposed as a universal danger signal-associated molecular pattern (DAMP), as exposed hydrophobic regions in aqueous solutions tend to form toxic and non-productive aggregates; many immunostimulators are hydrophobic or contain hydrophobic regions.78 Furthermore, cytokine gene expression by splenocytes increases with the hydrophobicity of head groups presented on the surface of gold nanoparticles.62
Physical Properties
The physical structure and properties of a material can also be engineered to illicit specific immune responses. Topographical cues, in the form of different sized gratings, can influence macrophage adhesion, morphology, and secretion of factors such as TNF-α and VEGF.14 In addition, topographical cues have shown the potential to influence the polarization of human and mouse macrophages in vitro,6,11 and this correlated with altered tissue formation and foreign body response in vivo.11,13 These cues can also control cell shape, which has also been shown to alter macrophage polarization; macrophages with elongated shapes showed more M2 polarization, and this was dependent on actin and actin/myosin contractility.57 The pore structure in conjunction with mechanical properties of macroporous PLGA scaffolds has been shown to alter enrichment of DCs for immunotherapy.45 For cardiac tissue engineering, collagen-modified poly(2-hydroyethylmethacrylate-co-methacrylic acid) pHEMA-co-MAA) hydrogels with pore sizes of 30–40 microns showed enhanced angiogenesis and reduced fibrosis compared to other pore sizes, and this correlated with increased expression of M2 markers in scaffold-associated macrophages (Fig. 4c).53 Other studies using pHEMA hydrogels that support neovascularization,8 however, revealed that enhanced vascularization in different pore size gels correlated instead with increased M1 macrophage polarization inside the porous structures.87 These seemingly conflicting results may be due to the fact that both M1 and M2 macrophages may be critical to angiogenesis.82 Mechanical cues delivered by a material can also influence immune cells, as the polarization of human peripheral blood mononuclear cells into the M1 and M2 phenotypes was modulated by the amount of cyclic strain applied to their adhesion substrate.5
Modifications with Soluble and Adhesion Cues
Delivery and presentation of soluble and adhesion cues can greatly enhance the specificity and potency of the immune response to a material. Traditional immunodulatory materials have focused on delivery of anti-inflammatory drugs, such as dexamethasome69 and nitric oxide from biomaterial-coatings,35 to reduce inflammation. Conversely, the inflammatory response can be enhanced and modulated by presentation of adhesion factors, such as RGD and PHSRN, which impact macrophage adhesion and FBGC formation.41,42 Encapsulation within and functionalization of materials with cytokines, pathogen associated molecular patterns (PAMPs), and antigens have been used to activate immune cells for immunotherapy. Controlled delivery of TGF-β inhibitor and IL-2 from nanoscale liposomal polymeric gels in murine melanoma models was shown to enhance CD8+ T-cell activation, along with increased activity and number of natural killer cells, and resulted in potent anti-tumor immune responses.68 Scaffold based materials that presented GM-CSF, CpG, and tumor antigen to recruit and reprogram dendritic cells in situ also induced very potent immune responses from CD8+ T-cells for combating melanoma.1 Similar methods of presenting these cues from materials have shown efficacy in driving immune cell mediated neovascularzation. Release of MCP-1 from vascular grafts was shown to recruit monocytes to remodel the grafts into mature blood vessels.75 Delivery of FTY720, an agonist for sphingosine 1-phosphate receptor 3, from PLGA thin films increased recruitment of anti-inflammatory monocytes (AMs) from the circulation, in part by upregulating SDF-1 release from endothelial cells and enhancing SDF-1 mediated chemotaxis of AMs; increased recruitment of AMs correlated with enhanced arteriolar diameter expansion and length density in soft tissues (Fig. 4d).4 Presentation and delivery of TLR agonists may also help induce inflammatory angiogenesis, as these can regulate the angiogenic activity of immune cells.70
SUMMARY AND FUTURE DIRECTIONS
In summary, a material’s chemical and physical properties, composition, and modifications with soluble and adhesive cues play a critical role in defining its foreign body response. These properties can be rationally designed and modulated to drive vascular formation by altering the recruitment, function, and phenotype of immune cells at the material site. M1/M2c macrophages, dendritic cells, neutrophils, eosinophils, and mast cells appear to promote new vessel formation via angiogenesis, whereas M2a/c macrophages, CD4+ T-cells, and natural killer cells appear to induce remodeling of existing collaterals or maturation of newly formed vessels.
Moving forward, the design and utilization of immunomodulatory materials to enhance neovascularization for tissue engineering and regenerative medicine will require a better understanding of the specific mechanisms by which immune cells contribute to vessel formation, and how this function is regulated by the cells’ chemical and physical microenvironment. Most work to date provides correlative data, but demonstration of cause and effect is often lacking. The combined effects of soluble cues (cytokines, growth factors, and chemokines), adhesion cues, topographical cues, and physical signals, such as substrate rigidity and viscoelasticity, presented from a biomaterial will also need to be explored. Our current understanding of this topic is largely limited to the roles of macrophages and the M1/M2 paradigm. The role(s) of other immune cell phenotypes, such as Th1, Th2, Th17, and Treg CD4+ T-cells in vascularization is not as well defined. Our incomplete understanding of immune cell phenotypic and functional plasticity27 currently limits our ability to explore these relationships.
The interplay of immunology and angiogenesis with biomaterials is still a new topic of research, and critical to this growing area of research is the design of materials that have greater control over immune cell trafficking and their local microenvironment. Materials that can provide improved spatiotemporal presentation of immunomodulatory factors will likely be required. Biomaterials capable of delivering factors on demand, for example, may be particularly useful for exploring how the specific timing of cytokine availability impacts the transition from a pro-inflammatory environment to an anti-inflammatory, pro-wound healing environment.43 These systems could allow one to explore how each stage of angiogenesis is impacted by different immune cell phenotypes.82 One concern with current immunomodulatory materials is unwanted or off-target inflammatory responses. The development of materials of increased complexity that can combine various chemical, mechanical, and structural stimuli may provide greater control over guiding immune cell function. Conversely, materials that are “invisible” to the immune system by avoiding PRR recognition or interactions with surrounding cells can provide a clean slate for selectively targeting immune cell populations with distinct cues.
One potential challenge in utilizing materials to promote angiogenesis via manipulation of inflammation is that many injury sites may already be inflamed, and this could impair the ability of the material to modulate immune cell function. Several types of tissue injuries that require revascularization, such as ischemic, bone, and muscle injury, have inflammatory responses characterized by specific immune cell recruitment and cytokine profiles. 39,61,91 In addition, microbial infections associated with these injuries, and surgical procedures also can regulate cytokine production and immune cell recruitment and phenotype by the presentation of PAMPs. These signals may induce an inflammatory profile that is deleterious or conflicting with the desired response to the immunomodulatory material. It may be necessary to first alter the pre-existing inflammatory response. Alternatively, one may need to time the delivery of these materials after an acute inflammatory response resulting from an injury or infection has subsided. It may be necessary to customize the design of these materials for specific types of injuries.
A long-term objective of the field is to promote vascularization solely with an appropriately designed material, without including exogenous cytokines or growth factors. This potentially could be accomplished by choosing or designing the material to act as a distinct PRR, or combination of PRRs, in order to invoke a particular and desirable immune response.
Acknowledgments
The authors acknowledge support from the NIH (R01 DE013349 and R01 HL069957), the NSF GRFP (Grant No. DGE1144152), and the Wyss Institute.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ. Infection-mimicking materials to program dendritic cells in situ. Nat Mater. 2009;8:151–158. doi: 10.1038/nmat2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alsberg E, Kong HJ, Hirano Y, Smith MK, Albeiruti A, Mooney DJ. Regulating bone formation via controlled scaffold degradation. J Dent Res. 2003;82:903–908. doi: 10.1177/154405910308201111. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Awojoodu AO, Ogle ME, Sefcik LS, Bowers DT, Martin K, Brayman KL, Lynch KR, Peirce-Cottler SM, Botchwey E. Sphingosine 1-phosphate receptor 3 regulates recruitment of anti-inflammatory monocytes to microvessels during implant arteriogenesis. Proc Natl Acad Sci U S A. 2013;110:13785–13790. doi: 10.1073/pnas.1221309110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballotta V, Driessen-Mol A, Bouten CV, Baaijens FP. Strain-dependent modulation of macrophage polarization within scaffolds. Biomaterials. 2014;35:4919–4928. doi: 10.1016/j.biomaterials.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Barth KA, Waterfield JD, Brunette DM. The effect of surface roughness on raw 264.7 macrophage phenotype. J Biomed Mater Res A. 2013;101:2679–2688. doi: 10.1002/jbm.a.34562. [DOI] [PubMed] [Google Scholar]
- 7.Benitez-Bribiesca L, Wong A, Utrera D, Castellanos E. The role of mast cell tryptase in neoangiogenesis of premalignant and malignant lesions of the uterine cervix. J Histochem Cytochem. 2001;49:1061–1062. doi: 10.1177/002215540104900816. [DOI] [PubMed] [Google Scholar]
- 8.Bhrany AD, Irvin CA, Fujitani K, Liu Z, Ratner BD. Evaluation of a sphere-templated polymeric scaffold as a subcutaneous implant. JAMA Facial Plast Surg. 2013;15:29–33. doi: 10.1001/2013.jamafacial.4. [DOI] [PubMed] [Google Scholar]
- 9.Blair RJ, Meng H, Marchese MJ, Ren S, Schwartz LB, Tonnesen MG, Gruber BL. Human mast cells stimulate vascular tube formation. Tryptase is a novel, potent angiogenic factor. J Clin Invest. 1997;99:2691–2700. doi: 10.1172/JCI119458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blotnick S, Peoples GE, Freeman MR, Eberlein TJ, Klagsbrun M. T lymphocytes synthesize and export heparin-binding epidermal growth factor-like growth factor and basic fibroblast growth factor, mitogens for vascular cells and fibroblasts: Differential production and release by cd4+ and cd8+ t cells. Proc Natl Acad Sci U S A. 1994;91:2890–2894. doi: 10.1073/pnas.91.8.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bota PC, Collie AM, Puolakkainen P, Vernon RB, Sage EH, Ratner BD, Stayton PS. Biomaterial topography alters healing in vivo and monocyte/macrophage activation in vitro. J Biomed Mater Res A. 2010;95:649–657. doi: 10.1002/jbm.a.32893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan G, Mooney DJ. Ca(2+) released from calcium alginate gels can promote inflammatory responses in vitro and in vivo. Acta Biomater. 2013;9:9281–9291. doi: 10.1016/j.actbio.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chehroudi B, Ghrebi S, Murakami H, Waterfield JD, Owen G, Brunette DM. Bone formation on rough, but not polished, subcutaneously implanted ti surfaces is preceded by macrophage accumulation. J Biomed Mater Res A. 2010;93:724–737. doi: 10.1002/jbm.a.32587. [DOI] [PubMed] [Google Scholar]
- 14.Chen S, Jones JA, Xu Y, Low HY, Anderson JM, Leong KW. Characterization of topographical effects on macrophage behavior in a foreign body response model. Biomaterials. 2010;31:3479–3491. doi: 10.1016/j.biomaterials.2010.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral cd4+cd25− naive t cells to cd4+cd25+ regulatory t cells by tgf-beta induction of transcription factor foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conejo-Garcia JR, Benencia F, Courreges MC, Kang E, Mohamed-Hadley A, Buckanovich RJ, Holtz DO, Jenkins A, Na H, Zhang L, Wagner DS, Katsaros D, Caroll R, Coukos G. Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of vegf-a. Nat Med. 2004;10:950–958. doi: 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- 17.Couffinhal T, Silver M, Kearney M, Sullivan A, Witzenbichler B, Magner M, Annex B, Peters K, Isner JM. Impaired collateral vessel development associated with reduced expression of vascular endothelial growth factor in apoe−/− mice. Circulation. 1999;99:3188–3198. doi: 10.1161/01.cir.99.24.3188. [DOI] [PubMed] [Google Scholar]
- 18.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curiel TJ, Cheng P, Mottram P, Alvarez X, Moons L, Evdemon-Hogan M, Wei S, Zou L, Kryczek I, Hoyle G, Lackner A, Carmeliet P, Zou W. Dendritic cell subsets differentially regulate angiogenesis in human ovarian cancer. Cancer Res. 2004;64:5535–5538. doi: 10.1158/0008-5472.CAN-04-1272. [DOI] [PubMed] [Google Scholar]
- 20.DiPietro LA. Wound healing: The role of the macrophage and other immune cells. Shock. 1995;4:233–240. [PubMed] [Google Scholar]
- 21.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 22.Druecke D, Langer S, Lamme E, Pieper J, Ugarkovic M, Steinau HU, Homann HH. Neovascularization of poly(ether ester) block-copolymer scaffolds in vivo: Long-term investigations using intravital fluorescent microscopy. J Biomed Mater Res A. 2004;68:10–18. doi: 10.1002/jbm.a.20016. [DOI] [PubMed] [Google Scholar]
- 23.Drury JL, Mooney DJ. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 24.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of vegf-mediated endothelial tip cell induction. Blood. 2010;116:829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franz S, Rammelt S, Scharnweber D, Simon JC. Immune responses to implants - a review of the implications for the design of immunomodulatory biomaterials. Biomaterials. 2011;32:6692–6709. doi: 10.1016/j.biomaterials.2011.05.078. [DOI] [PubMed] [Google Scholar]
- 26.Freeman MR, Schneck FX, Gagnon ML, Corless C, Soker S, Niknejad K, Peoples GE, Klagsbrun M. Peripheral blood t lymphocytes and lymphocytes infiltrating human cancers express vascular endothelial growth factor: A potential role for t cells in angiogenesis. Cancer Res. 1995;55:4140–4145. [PubMed] [Google Scholar]
- 27.Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: Macrophages, mast cells and neutrophils. Nat Immunol. 2011;12:1035–1044. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gruber BL, Marchese MJ, Suzuki K, Schwartz LB, Okada Y, Nagase H, Ramamurthy NS. Synovial procollagenase activation by human mast cell tryptase dependence upon matrix metalloproteinase 3 activation. J Clin Invest. 1989;84:1657–1662. doi: 10.1172/JCI114344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hao Q, Chen Y, Zhu Y, Fan Y, Palmer D, Su H, Young WL, Yang GY. Neutrophil depletion decreases vegf-induced focal angiogenesis in the mature mouse brain. J Cereb Blood Flow Metab. 2007;27:1853–1860. doi: 10.1038/sj.jcbfm.9600485. [DOI] [PubMed] [Google Scholar]
- 30.Harel-Adar T, Ben Mordechai T, Amsalem Y, Feinberg MS, Leor J, Cohen S. Modulation of cardiac macrophages by phosphatidylserine-presenting liposomes improves infarct repair. Proc Natl Acad Sci U S A. 2011;108:1827–1832. doi: 10.1073/pnas.1015623108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heil M, Ziegelhoeffer T, Pipp F, Kostin S, Martin S, Clauss M, Schaper W. Blood monocyte concentration is critical for enhancement of collateral artery growth. Am J Physiol Heart Circ Physiol. 2002;283:H2411–2419. doi: 10.1152/ajpheart.01098.2001. [DOI] [PubMed] [Google Scholar]
- 32.Heil M, Ziegelhoeffer T, Wagner S, Fernandez B, Helisch A, Martin S, Tribulova S, Kuziel WA, Bachmann G, Schaper W. Collateral artery growth (arteriogenesis) after experimental arterial occlusion is impaired in mice lacking cc-chemokine receptor-2. Circ Res. 2004;94:671–677. doi: 10.1161/01.RES.0000122041.73808.B5. [DOI] [PubMed] [Google Scholar]
- 33.Heissig B, Rafii S, Akiyama H, Ohki Y, Sato Y, Rafael T, Zhu Z, Hicklin DJ, Okumura K, Ogawa H, Werb Z, Hattori K. Low-dose irradiation promotes tissue revascularization through vegf release from mast cells and mmp-9-mediated progenitor cell mobilization. J Exp Med. 2005;202:739–750. doi: 10.1084/jem.20050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hellingman AA, Zwaginga JJ, van Beem RT, Te RMSMC, Hamming JF, Fibbe WE, Quax PH, Geutskens SB. T-cell-pre-stimulated monocytes promote neovascularisation in a murine hind limb ischaemia model. Eur J Vasc Endovasc Surg. 2011;41:418–428. doi: 10.1016/j.ejvs.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 35.Hetrick EM, Prichard HL, Klitzman B, Schoenfisch MH. Reduced foreign body response at nitric oxide-releasing subcutaneous implants. Biomaterials. 2007;28:4571–4580. doi: 10.1016/j.biomaterials.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirosue S, Kourtis IC, van der Vlies AJ, Hubbell JA, Swartz MA. Antigen delivery to dendritic cells by poly(propylene sulfide) nanoparticles with disulfide conjugated peptides: Cross-presentation and t cell activation. Vaccine. 2010;28:7897–7906. doi: 10.1016/j.vaccine.2010.09.077. [DOI] [PubMed] [Google Scholar]
- 37.Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010;9:518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunt TK, Knighton DR, Thakral KK, Goodson WH, 3rd, Andrews WS. Studies on inflammation and wound healing: Angiogenesis and collagen synthesis stimulated in vivo by resident and activated wound macrophages. Surgery. 1984;96:48–54. [PubMed] [Google Scholar]
- 39.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: Role of inflammatory cells. J Leukoc Biol. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones JA, Chang DT, Meyerson H, Colton E, Kwon IK, Matsuda T, Anderson JM. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells. J Biomed Mater Res A. 2007;83:585–596. doi: 10.1002/jbm.a.31221. [DOI] [PubMed] [Google Scholar]
- 41.Kao WJ, Lee D. In vivo modulation of host response and macrophage behavior by polymer networks grafted with fibronectin-derived biomimetic oligopeptides: The role of rgd and phsrn domains. Biomaterials. 2001;22:2901–2909. doi: 10.1016/s0142-9612(01)00037-0. [DOI] [PubMed] [Google Scholar]
- 42.Kao WJ, Lee D, Schense JC, Hubbell JA. Fibronectin modulates macrophage adhesion and fbgc formation: The role of rgd, phsrn, and prrarv domains. J Biomed Mater Res. 2001;55:79–88. doi: 10.1002/1097-4636(200104)55:1<79::aid-jbm110>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 43.Kearney CJ, Mooney DJ. Macroscale delivery systems for molecular and cellular payloads. Nat Mater. 2013;12:1004–1017. doi: 10.1038/nmat3758. [DOI] [PubMed] [Google Scholar]
- 44.Khmelewski E, Becker A, Meinertz T, Ito WD. Tissue resident cells play a dominant role in arteriogenesis and concomitant macrophage accumulation. Circ Res. 2004;95:E56–64. doi: 10.1161/01.RES.0000143013.04985.E7. [DOI] [PubMed] [Google Scholar]
- 45.Kim J, Li WA, Sands W, Mooney DJ. Effect of pore structure of macroporous poly(lactide-co-glycolide) scaffolds on the in vivo enrichment of dendritic cells. ACS Appl Mater Interfaces. 2014;6:8505–8512. doi: 10.1021/am501376n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koffler J, Kaufman-Francis K, Shandalov Y, Egozi D, Pavlov DA, Landesberg A, Levenberg S. Improved vascular organization enhances functional integration of engineered skeletal muscle grafts. Proc Natl Acad Sci U S A. 2011;108:14789–14794. doi: 10.1073/pnas.1017825108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.la Sala A, Pontecorvo L, Agresta A, Rosano G, Stabile E. Regulation of collateral blood vessel development by the innate and adaptive immune system. Trends Mol Med. 2012;18:494–501. doi: 10.1016/j.molmed.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 48.Lee KY, Peters MC, Anderson KW, Mooney DJ. Controlled growth factor release from synthetic extracellular matrices. Nature. 2000;408:998–1000. doi: 10.1038/35050141. [DOI] [PubMed] [Google Scholar]
- 49.Li WA, Mooney DJ. Materials based tumor immunotherapy vaccines. Curr Opin Immunol. 2013;25:238–245. doi: 10.1016/j.coi.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN, Pollard JW. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 51.Lutolf MP, Raeber GP, Zisch AH, Tirelli N, Hubbell JA. Cell-responsive synthetic hydrogels. Advanced Materials. 2003;15:888–892. [Google Scholar]
- 52.Lutolf MP, Weber FE, Schmoekel HG, Schense JC, Kohler T, Muller R, Hubbell JA. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol. 2003;21:513–518. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 53.Madden LR, Mortisen DJ, Sussman EM, Dupras SK, Fugate JA, Cuy JL, Hauch KD, Laflamme MA, Murry CE, Ratner BD. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc Natl Acad Sci U S A. 2010;107:15211–15216. doi: 10.1073/pnas.1006442107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mann BK, Schmedlen RH, West JL. Tethered-tgf-< i> β</i> increases extracellular matrix production of vascular smooth muscle cells. Biomaterials. 2001 doi: 10.1016/s0142-9612(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 55.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized m2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 56.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 57.McWhorter FY, Wang T, Nguyen P, Chung T, Liu WF. Modulation of macrophage phenotype by cell shape. Proc Natl Acad Sci U S A. 2013;110:17253–17258. doi: 10.1073/pnas.1308887110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mokarram N, Bellamkonda RV. A perspective on immunomodulation and tissue repair. Ann Biomed Eng. 2014;42:338–351. doi: 10.1007/s10439-013-0941-0. [DOI] [PubMed] [Google Scholar]
- 59.Mor F, Quintana FJ, Cohen IR. Angiogenesis-inflammation cross-talk: Vascular endothelial growth factor is secreted by activated t cells and induces th1 polarization. J Immunol. 2004;172:4618–4623. doi: 10.4049/jimmunol.172.7.4618. [DOI] [PubMed] [Google Scholar]
- 60.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mountziaris PM, Mikos AG. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng Part B Rev. 2008;14:179–186. doi: 10.1089/ten.teb.2008.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moyano DF, Goldsmith M, Solfiell DJ, Landesman-Milo D, Miranda OR, Peer D, Rotello VM. Nanoparticle hydrophobicity dictates immune response. J Am Chem Soc. 2012;134:3965–3967. doi: 10.1021/ja2108905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nikkhah M, Edalat F, Manoucheri S, Khademhosseini A. Engineering microscale topographies to control the cell-substrate interface. Biomaterials. 2012;33:5230–5246. doi: 10.1016/j.biomaterials.2012.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284:489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 65.Norrby K. Mast cells and angiogenesis. APMIS. 2002;110:355–371. doi: 10.1034/j.1600-0463.2002.100501.x. [DOI] [PubMed] [Google Scholar]
- 66.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ohki Y, Heissig B, Sato Y, Akiyama H, Zhu Z, Hicklin DJ, Shimada K, Ogawa H, Daida H, Hattori K, Ohsaka A. Granulocyte colony-stimulating factor promotes neovascularization by releasing vascular endothelial growth factor from neutrophils. FASEB J. 2005;19:2005–2007. doi: 10.1096/fj.04-3496fje. [DOI] [PubMed] [Google Scholar]
- 68.Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R, Jay SM, Demento SL, Agawu A, Licona Limon P, Ferrandino AF, Gonzalez D, Habermann A, Flavell RA, Fahmy TM. Combination delivery of tgf-beta inhibitor and il-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat Mater. 2012;11:895–905. doi: 10.1038/nmat3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patil SD, Papadimitrakopoulos F, Burgess DJ. Dexamethasone-loaded poly(lactic-co-glycolic) acid microspheres/poly(vinyl alcohol) hydrogel composite coatings for inflammation control. Diabetes Technol Ther. 2004;6:887–897. doi: 10.1089/dia.2004.6.887. [DOI] [PubMed] [Google Scholar]
- 70.Pinhal-Enfield G, Ramanathan M, Hasko G, Vogel SN, Salzman AL, Boons GJ, Leibovich SJ. An angiogenic switch in macrophages involving synergy between toll-like receptors 2, 4, 7, and 9 and adenosine a(2a) receptors. Am J Pathol. 2003;163:711–721. doi: 10.1016/S0002-9440(10)63698-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Puxeddu I, Alian A, Piliponsky AM, Ribatti D, Panet A, Levi-Schaffer F. Human peripheral blood eosinophils induce angiogenesis. Int J Biochem Cell Biol. 2005;37:628–636. doi: 10.1016/j.biocel.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 72.Ribatti D, Vacca A, Marzullo A, Nico B, Ria R, Roncali L, Dammacco F. Angiogenesis and mast cell density with tryptase activity increase simultaneously with pathological progression in b-cell non-hodgkin’s lymphomas. Int J Cancer. 2000;85:171–175. [PubMed] [Google Scholar]
- 73.Riboldi E, Musso T, Moroni E, Urbinati C, Bernasconi S, Rusnati M, Adorini L, Presta M, Sozzani S. Cutting edge: Proangiogenic properties of alternatively activated dendritic cells. J Immunol. 2005;175:2788–2792. doi: 10.4049/jimmunol.175.5.2788. [DOI] [PubMed] [Google Scholar]
- 74.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 75.Roh JD, Sawh-Martinez R, Brennan MP, Jay SM, Devine L, Rao DA, Yi T, Mirensky TL, Nalbandian A, Udelsman B, Hibino N, Shinoka T, Saltzman WM, Snyder E, Kyriakides TR, Pober JS, Breuer CK. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci U S A. 2010;107:4669–4674. doi: 10.1073/pnas.0911465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 77.Rucker M, Laschke MW, Junker D, Carvalho C, Schramm A, Mulhaupt R, Gellrich NC, Menger MD. Angiogenic and inflammatory response to biodegradable scaffolds in dorsal skinfold chambers of mice. Biomaterials. 2006;27:5027–5038. doi: 10.1016/j.biomaterials.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 78.Seong SY, Matzinger P. Hydrophobicity: An ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 79.Silva EA, Kim ES, Kong HJ, Mooney DJ. Material-based deployment enhances efficacy of endothelial progenitor cells. Proc Natl Acad Sci U S A. 2008;105:14347–14352. doi: 10.1073/pnas.0803873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Silvestre JS, Mallat Z, Tedgui A, Levy BI. Post-ischaemic neovascularization and inflammation. Cardiovasc Res. 2008;78:242–249. doi: 10.1093/cvr/cvn027. [DOI] [PubMed] [Google Scholar]
- 81.Sozzani S, Rusnati M, Riboldi E, Mitola S, Presta M. Dendritic cell-endothelial cell cross-talk in angiogenesis. Trends Immunol. 2007;28:385–392. doi: 10.1016/j.it.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 82.Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, Vunjak-Novakovic G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35:4477–4488. doi: 10.1016/j.biomaterials.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stabile E, Burnett MS, Watkins C, Kinnaird T, Bachis A, la Sala A, Miller JM, Shou M, Epstein SE, Fuchs S. Impaired arteriogenic response to acute hindlimb ischemia in cd4-knockout mice. Circulation. 2003;108:205–210. doi: 10.1161/01.CIR.0000079225.50817.71. [DOI] [PubMed] [Google Scholar]
- 84.Stabile E, Kinnaird T, la Sala A, Hanson SK, Watkins C, Campia U, Shou M, Zbinden S, Fuchs S, Kornfeld H, Epstein SE, Burnett MS. Cd8+ t lymphocytes regulate the arteriogenic response to ischemia by infiltrating the site of collateral vessel development and recruiting cd4+ mononuclear cells through the expression of interleukin-16. Circulation. 2006;113:118–124. doi: 10.1161/CIRCULATIONAHA.105.576702. [DOI] [PubMed] [Google Scholar]
- 85.Stack MS, Johnson DA. Human mast cell tryptase activates single-chain urinary-type plasminogen activator (pro-urokinase) J Biol Chem. 1994;269:9416–9419. [PubMed] [Google Scholar]
- 86.Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. J Leukoc Biol. 1994;55:410–422. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- 87.Sussman EM, Halpin MC, Muster J, Moon RT, Ratner BD. Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction. Ann Biomed Eng. 2014;42:1508–1516. doi: 10.1007/s10439-013-0933-0. [DOI] [PubMed] [Google Scholar]
- 88.Takeda Y, Costa S, Delamarre E, Roncal C, Leite de Oliveira R, Squadrito ML, Finisguerra V, Deschoemaeker S, Bruyere F, Wenes M, Hamm A, Serneels J, Magat J, Bhattacharyya T, Anisimov A, Jordan BF, Alitalo K, Maxwell P, Gallez B, Zhuang ZW, Saito Y, Simons M, De Palma M, Mazzone M. Macrophage skewing by phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis. Nature. 2011;479:122–126. doi: 10.1038/nature10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tang L, Jennings TA, Eaton JW. Mast cells mediate acute inflammatory responses to implanted biomaterials. Proc Natl Acad Sci U S A. 1998;95:8841–8846. doi: 10.1073/pnas.95.15.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thomas SN, van der Vlies AJ, O’Neil CP, Reddy ST, Yu SS, Giorgio TD, Swartz MA, Hubbell JA. Engineering complement activation on polypropylene sulfide vaccine nanoparticles. Biomaterials. 2011;32:2194–2203. doi: 10.1016/j.biomaterials.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 91.Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 2005;288:R345–353. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- 92.van Beem RT, Noort WA, Voermans C, Kleijer M, ten Brinke A, van Ham SM, van der Schoot CE, Zwaginga JJ. The presence of activated cd4(+) t cells is essential for the formation of colony-forming unit-endothelial cells by cd14(+) cells. J Immunol. 2008;180:5141–5148. doi: 10.4049/jimmunol.180.7.5141. [DOI] [PubMed] [Google Scholar]
- 93.van Weel V, Toes RE, Seghers L, Deckers MM, de Vries MR, Eilers PH, Sipkens J, Schepers A, Eefting D, van Hinsbergh VW, van Bockel JH, Quax PH. Natural killer cells and cd4+ t-cells modulate collateral artery development. Arterioscler Thromb Vasc Biol. 2007;27:2310–2318. doi: 10.1161/ATVBAHA.107.151407. [DOI] [PubMed] [Google Scholar]
- 94.Willenborg S, Lucas T, van Loo G, Knipper JA, Krieg T, Haase I, Brachvogel B, Hammerschmidt M, Nagy A, Ferrara N, Pasparakis M, Eming SA. Ccr2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood. 2012;120:613–625. doi: 10.1182/blood-2012-01-403386. [DOI] [PubMed] [Google Scholar]
- 95.Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor gr+cd11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 96.Yoshida M, Mata J, Babensee JE. Effect of poly(lactic-co-glycolic acid) contact on maturation of murine bone marrow-derived dendritic cells. J Biomed Mater Res A. 2007;80:7–12. doi: 10.1002/jbm.a.30832. [DOI] [PubMed] [Google Scholar]
- 97.Zdolsek J, Eaton JW, Tang L. Histamine release and fibrinogen adsorption mediate acute inflammatory responses to biomaterial implants in humans. J Transl Med. 2007;5:31. doi: 10.1186/1479-5876-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao X, Kim J, Cezar CA, Huebsch N, Lee K, Bouhadir K, Mooney DJ. Active scaffolds for on-demand drug and cell delivery. Proc Natl Acad Sci U S A. 2011;108:67–72. doi: 10.1073/pnas.1007862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou X, Luo YC, Ji WJ, Zhang L, Dong Y, Ge L, Lu RY, Sun HY, Guo ZZ, Yang GH, Jiang TM, Li YM. Modulation of mononuclear phagocyte inflammatory response by liposome-encapsulated voltage gated sodium channel inhibitor ameliorates myocardial ischemia/reperfusion injury in rats. PloS one. 2013;8:e74390. doi: 10.1371/journal.pone.0074390. [DOI] [PMC free article] [PubMed] [Google Scholar]