Abstract
This paper explores the role visual attention plays in the recognition of objects in infancy. Research and theory on the development of infant attention and recognition memory are reviewed in three major sections. The first section reviews some of the major findings and theory emerging from a rich tradition of behavioral research utilizing preferential looking tasks to examine visual attention and recognition memory in infancy. The second section examines research utilizing neural measures of attention and object recognition in infancy as well as research on brain-behavior relations in the early development of attention and recognition memory. The third section addresses potential areas of the brain involved in infant object recognition and visual attention. An integrated synthesis of some of the existing models of the development of visual attention is presented which may account for the observed changes in behavioral and neural measures of visual attention and object recognition that occur across infancy.
Keywords: infancy, object recognition, visual attention, event-related potentials
The ability to selectively attend to objects or events in the environment shows significant development in infancy. This ability is a critical component of early cognitive functioning for the human infant and remains so throughout the lifespan. Attention is strongly related to recognition memory, another core cognitive function which is present at birth in the human infant but shows significant development throughout the infancy period (Courage & Howe, 2004; Rose & Feldman, 1997; Rose, Feldman, & Jankowski, 2004). Together these two functions account for the human infant’s responsiveness to novelty which researchers have capitalized on for decades to gain a window into the perceptual and cognitive capabilities of the non-verbal human infant. In the current paper, some of the major findings and theory on infant attention and memory which emerged from this line of research are reviewed, followed by a review of research on neural correlates of attention and object recognition in infancy, and theory on brain-behavior relations in the development of attention and recognition memory. The influence of individual differences in infant visual attention on object recognition is also discussed. The neuroanatomical basis of recognition memory is then explored followed by a section describing the development of attention systems in the brain (Reynolds, Courage, & Richards, 2013; Richards, 2001, 2008, 2010). These attention systems are associated with significant changes in stimulus processing which occur with increasing age and strongly influence recognition memory for objects and events.
Preferential Looking, Visual Attention, and Recognition Memory in Infancy
Developmental scientists have historically been interested in infant looking behavior because it provides a window into the perceptual and cognitive world of the non-verbal human infant. Being among the most altricial species, the human infant is generally incapable of complex behavior and is highly limited in range of responsiveness to environmental events. However, even in the newborn period, infants are capable of demonstrating selective attention and preferential looking for very brief periods (Fantz, 1963; Gibson, 1988; Rose, Feldman & Jankowski, 2004); and infants experience rapid gains in the voluntary control and maintenance of visual attention across the first postnatal year (for reviews, Colombo, 2001; Reynolds, Courage, & Richards, 2013). Much of the research on preferential looking has focused on responsiveness to novelty, a defining feature of infant recognition memory (Rose et al., 2004). The use of novelty preferences as an index of recognition memory in infant participants grew out of Fantz’s revolutionary studies using a preferential looking task first with chimpanzee infants (Fantz, 1956) and later with human infants (Fantz, 1964).
In his groundbreaking study published in 1964, Fantz presented 2- to 6-month-old infants with repeated pairings of photographs from magazines. One photograph was shown repeatedly to the infant for 10 presentations, but for each of the 10 presentations, this “constant” stimulus was paired with a novel photograph. Fantz found that with repeated presentations, the infants looked progressively longer toward the novel stimuli relative to the familiar (i.e., constant) stimulus. Based on this finding, he concluded that visual experience can be retained for at least a very brief period of time for infants over 2 months of age. This approach of presenting infants with a simultaneous pairing of two visual stimuli to the left and right of midline and measuring their preferential looking was modified by Fagan (1970) to include an initial familiarization phase, and is now referred to as the visual paired comparison (VPC) procedure. The majority of what we know about recognition memory in infancy has come from research utilizing some variant of this procedure.
The use of preferential looking as a measure of recognition memory requires a certain set of inferences to be made regarding what each of the possible preferences (novelty, familiarity, null) represents. The most common assumption made regarding these visual preferences is based on Sokolov’s (1963) comparator model in which he proposed that during looking, infants are actively constructing a mental representation (i.e., engram) of the fixated stimulus. If the stimulus matches an existing engram, then further looking or encoding is unnecessary and the infant will shift fixation to a different stimulus. A novelty preference is thus assumed to reflect recognition of a fully processed familiar stimulus. Longer looking toward the familiar stimulus (i.e., a familiarity preference) is assumed to reflect further encoding of stimulus that has not been fully processed. Null preferences (equal looking to each stimulus) are believed to reflect a lack of prior processing of either stimulus or equivalent levels of processing of each stimulus. Hunter and Ames (1988) proposed that infant visual preferences are influenced by multiple factors, including: the age of the infant, the amount of previous exposure to the stimuli, and task difficulty. Similar to Sokolov’s (1963) model, the infant would be expected to look longer toward a repeated (i.e., familiar) stimulus compared to novel stimuli until he/she has completely processed the familiar stimulus. Once the infant has no new information to extract from the familiar stimulus, the familiarity preference would be expected to shift to a novelty preference.
Studies in which length of familiarization was manipulated across and within age groups provide support for these inferences (e.g., Freeseman, Colombo, & Coldren, 1993; Rose, Gottfried, Mellow-Carminar, & Bridger, 1982). For example, Rose and colleagues (1982) found that with 30 s of familiarization with an object, 3.5-month-old infants demonstrated preferences for the familiar object during the VPC task. However, 4.5- and 6.5-month-olds showed novelty preferences with 30 s of exposure during familiarization. Furthermore, 6.5-month -olds allowed to accumulate 5 s of looking during familiarization with an object, subsequently demonstrated familiarity preferences in a VPC task; however, infants of the same age allowed to accumulate 15 s or longer of looking during familiarization demonstrated novelty preferences. These findings and others (e.g., Colombo, Mitchell, & Horowitz, 1988; Fagan, 1974; Freeseman, Colombo, & Coldren, 1993; Richards, 1997; Rose et al., 1982) demonstrate that longer familiarization leads to greater evidence of object recognition in infants; and with increasing age, infants require less exposure during familiarization to subsequently recognize an object. Additionally, Diamond (1990) found that with increasing age infants demonstrate evidence of recognition memory following longer delays between familiarization and testing. Four-month-old infants recognize stimuli with up to 10 s delays between testing, whereas 6-month-olds and 9-month-olds show evidence of recognition with up to 1 and 10 min of delays, respectively.
It should be clear based on this review of behavioral work in the area that visual attention and recognition memory are tightly, perhaps inseparably, coupled in research utilizing visual preference tasks with infant participants. Recognition memory is inferred based on the distribution of infant visual attention during testing. Two forms of attention, selective attention and sustained attention, are clearly involved in recognition memory tasks. Selective attention involves the selection of a specific object or spatial location as the focus of attention. This process is influenced by both external and internal factors, such as stimulus salience and the child’s goals, respectively (Nelson and Dukette, 1998). Sustained attention refers to the extended selective engagement of a behavioral or neural system that primarily enhances information processing in that system (e.g., Richards, 1997, 2003). For example, novel objects often elicit stimulus orienting (i.e., selective attention) followed by sustained attention as the infant continues to maintain visual attention toward the stimulus. Areas of the brain controlling arousal and state are involved in sustained attention (see, Richards 2008, 2010), and thus sustained attention is associated with several state-related changes that occur during periods of attention. These include behavioral changes, such as motor quieting and resistance to peripheral distracters. Thus, in the VPC task, continued looking toward the preferred stimulus in the presence of the non-preferred stimulus is a component of sustained attention. The duration of periods of sustained attention increases significantly throughout infancy (Reynolds & Richards, 2008; Ruff & Capozzolli, 2003).
Sustained attention is also associated with state-related physiological changes, most notably changes in heart rate that occur during infant attention. Richards and colleagues (e.g., Richards & Casey, 1991) identified the heart rate phases of attention, which correspond to different levels of attentional engagement that occur during the course of a single look (for review, Reynolds & Richards, 2008). The most relevant of these phases for attention are: stimulus orienting, sustained attention, and attention termination. Stimulus orienting is characterized by a large heart rate deceleration indicating the onset of attentional engagement. Sustained attention is characterized by a maintained decrease in heart rate below prestimulus levels. This is the phase of attention when the infant is actively engaged with the stimulus and the majority of information processing occurs. Infants require less time to process and subsequently recognize a stimulus if exposure occurs during sustained attention than if exposure occurs during other heart rate phases (Frick and Richards, 2001; Richards, 1997). Attention termination is characterized a return of heart rate to prestimulus levels with continued looking toward the stimulus. During this phase, the infant is no longer actively engaged in attention toward the stimulus. Infants do not demonstrate evidence of recognition memory for a visual stimulus if initial exposure occurs during attention termination (Richards, 1997). Thus, performance on recognition tasks involves both selective and sustained attention. Furthermore, state-related changes involved in attention and related to recognition memory can be measured at both the behavioral and psychophysiological level with infant participants. Table 1 shows developmental findings from both behavioral and electrophysiological research on visual attention and recognition memory across the infancy period. The potential timing of functional onset of neural systems believed to be involved in infant visual attention and recognition memory is also shown in Table 1 and discussed below.
Table 1.
Table 1 provides a summary of developmental findings related to infant visual attention and recognition memory on behavioral (Colombo et al., 1988, 1993; Courage et al., 2006; Rose et al., 1982) and ERP tasks (de Haan, 2007; de Haan & Nelson, 1997, Nelson & Collins, 1992; Nelson & deRegnier, 1992; Parker & Nelson, 2005; Reynolds et al., 2005, 2010; Webb et al., 2005). The potential onset of areas of the brain involved in these tasks is also shown (Colombo, 2001; Nelson, 1995; Nelson & Dukette, 1998; Posner & Petersen, 1990; Richards, 2008, 2010).
| Age | Behavioral Findings | ERP Findings | Brain Areas Involved in Attention and Recogniton Memory |
|---|---|---|---|
| Birth – 3 months | Visual attention is reflexively drawn to salient features of environment: areas of high contrast, borders of stimuli, motion. Infants may display long looks, but visual scanning and information processing are immature and inefficient. Infants require up to 60 s of prior exposure to subsequently recognize stimuli. | Latency to peak Nc: 800 – 1200 ms. By 3 months, Infants display differential Nc amplitude to oddball and standard stimuli. | Reflexive System: superior colliculus, lateral geniculate nucleus, primary visual areas Object recognition: medial temporal lobe structures, area TE |
| 3 – 6 months | Look duration decreases significantly as infants gain voluntary control of visual fixation and scanning. Infants begin to focus attention on relevant features of objects, people, and events; and require less exposure (approximately 20 s) to subsequently recognize stimuli. | Latency to peak Nc: 450 – 750 ms. Nc increases in amplitude, and infants show greater amplitude to novel stimuli, unless familiar is mother’s face or favorite toy. Differential LSW responding occurs based on familiarity and novelty. | Posterior Orienting System: frontal eye- fields, posterior parietal cortex Selective Attention Network: Pulvinar, anterior cingulate, dorsolateral prefrontal cortex. |
| 6 months – on | Infants begin to develop higher level attention, and are better able to inhibit attention to distracters and maintain sustain attention when called for. Look duration remains low to basic stimuli and increases to more complex stimuli. | Latency to peak Nc: 350 – 650 ms. Nc amplitude continues to increase up to 1 year and then begins to decrease. Infants are more likely to demonstrate differential LSW responses based on frequency of presentation of familiar stimuli. | Anterior Attention System: dorsolateral prefrontal cortex, orbitofrontal cortex, anterior cingulate. Object recognition: increasing involvement of area TE and increased dependence on hippocampus. |
Neural Measures of Visual Attention and Object Recognition in Infancy
Similar to preferential looking tasks, the event-related potential (ERP) technique has been used as a window into perceptual and cognitive processes in human infants (for review, de Haan, 2007). ERPs are voltage oscillations recorded in the electroencephalogram (EEG) which are time-locked with an event of interest (Fabiani, Gratton, & Coles, 2000; Picton, Bentin, Berg, Donchin, Hillyard, Johnson, Miller, Ritter, Ruchkin, Rugg, & Taylor, 2000). ERPs are averaged across trials by condition to increase the signal-to-noise ratio, which is inherently low in EEG. ERPs most likely reflect the summation of post-synaptic potentials produced by synchronous activation of large populations of neurons in response to the event of interest. Because the sampling rate of EEG systems is typically very high (e.g., 250 – 1000 Hz), the strength of the ERP technique lies in the high temporal resolution of ERP data. Within a 1 to 2 s time window, multiple ERP components associated with different stages of perceptual and cognitive processing can be identified in the averaged ERP waveform. This makes the ERP approach ideal for examining relations between attention and memory.
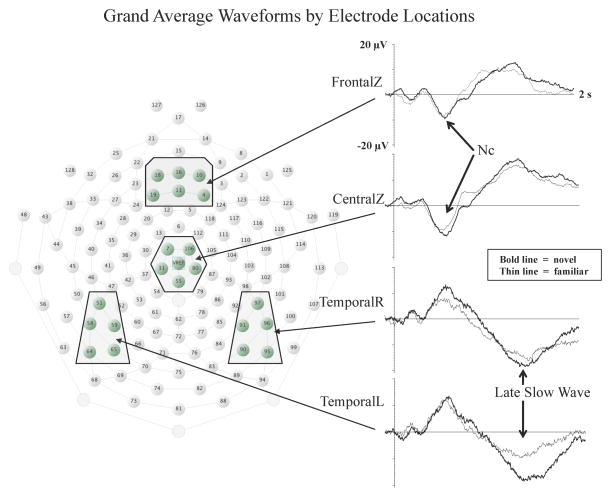
ERP components are typically defined by their morphological characteristics in the ERP waveform (e.g., latency to peak, duration, polarity, and topographical distribution), and perhaps more importantly, by their functional significance. To establish the functional significance of ERP components, experimental effects are typically analyzed across conditions by examining peak amplitude, mean amplitude, or latency to peak in the averaged ERP waveform at specific electrode sites within specific time windows that correspond with the component of interest. As discussed in the section that follows, research utilizing this approach has demonstrated that the Negative central (Nc) and the Late Slow Wave (LSW) ERP components are particularly relevant for infant attention and recognition memory, respectively. Figure 1 displays averaged waveforms from 6- and 7.5-month-old infants in response to familiar and novel objects. The Nc and LSW components are indicated on the waveforms on the right, and the topographical distribution of the electrode locations from which these averaged waveforms were recorded are shown on the left on the layout of the Electrical Geodesics Inc., (Eugene, Oregon) 128-channel recording system.
Figure 1.
Grand average waveforms showing the Nc and LSW ERP components by electrode location. The ERP waveforms are shown to the left with arrows indicating the timing and location of Nc and the LSW. The Y-axis indicates change in amplitude from baseline and the X-axis indicates time following stimulus onset. The layout of the EGI 128-channel sensor net is shown to the right with the electrode clusters for each of the averaged waveforms in boxes (figure adapted from Reynolds, Guy, & Zhang, 2011).
The Nc ERP component has been proposed to provide an index of amount of infant attentional engagement because it is often found to be greater in amplitude to novel or infrequent stimuli in comparison to familiar or frequent stimuli (e.g., Carver, Bauer, & Nelson, 2000; Courchesne, 1977; Courchesne, Ganz, & Norcia, 1981; de Haan & Nelson, 1997, 1999; Karrer & Ackles, 1987; 1988; Karrer & Monti, 1995; Nikkel & Karrer, 1994; Reynolds & Richards, 2005, 2009; Webb, Long, & Nelson, 2005). As shown in Figure 1, the Nc ERP component is a negatively polarized deflection in the waveform typically located at midline frontal and central electrodes (e.g., FrontalZ and CentralZ electrode clusters) with a peak latency usually occurring between 350 and 750 ms following stimulus onset. The Nc can be measured throughout infancy, but the latency to peak of Nc decreases from about 1000 ms in newborns (Nelson, 1996) to about 400 ms in one year olds (Nelson and deRegnier, 1992; Parker & Nelson, 2005). In contrast, Nc amplitude has been found to increase across the infancy period (Richards, 2003; Reynolds, Courage, & Richards, 2010) and then decrease in early childhood (Parker & Nelson, 2005).
The LSW is commonly found at frontal, central, and temporal electrodes (see Figure 1). The LSW typically occurs from 1 to 2 s following stimulus onset and can be either positive or negative in polarity depending on several factors including stimulus type, amount of prior exposure, and electrode location. Because the polarity of the LSW has been found to vary based on several factors, it can problematic to make specific predictions regarding directional effects on the amplitude and polarity of the LSW (de Haan, 2007). However, significant changes in the amplitude of the LSW are routinely observed across repeated stimulus presentations or with increased familiarity. Thus, the LSW is believed to be associated with stimulus encoding and infant recognition memory (de Haan & Nelson, 1999; Reynolds, Guy, & Zhang, 2011; Nelson & Collins, 1991; Nelson & Collins, 1992; Snyder, 2010; Snyder, Webb, & Nelson, 2002; Snyder et al., 2010; Webb, Long, & Nelson, 2005; Wiebe et al., 2006). From 4 to 12 months, infants become increasingly more likely to demonstrate LSW correlates of recognition of a fully processed stimulus in response to previously encountered stimuli (Nelson & Collins, 1992; Nelson & deRegnier, 1992; Reynolds & Richards, 2011). Beyond 6 months of age, infants are more likely to demonstrate differential LSW to familiar stimuli based on frequency of presentation (Nelson & Collins, 1992; Reynolds & Richards, 2005).
Although the majority of studies in this area of work have used faces as stimuli, several studies have examined infant attention and recognition memory for objects using ERPs. For example, de Haan and Nelson (1999) compared 6-month-old infants ERPs to highly familiar faces compared to novel faces in one experiment and highly familiar objects compared to novel objects in a second experiment. The highly familiar stimulus was a picture of the infant’s mother in the face condition and a picture of the infant’s favorite toy in the object condition. In both conditions, infants showed greater amplitude Nc to the familiar stimulus than to the novel stimuli. This finding was highly informative regarding the functional significance of the Nc component. Earlier work familiarizing infants with previously novel stimuli in the lab had shown that Nc is greater in amplitude to novel than familiar stimuli (Courchesne, 1977, 1983; Courchesne et al, 1981). Interestingly, Carver, Meltzoff, & Dawson (2006) found a similar familiarity effect on Nc in their study when using 2-D images of a familiar toy compared to novel toys. Because an infant’s mother or favorite toy are both highly familiar but arguably very salient, attention-getting stimuli; the findings of greater amplitude Nc to familiar stimuli in this study indicate that Nc reflects level of attentional engagement as opposed to novelty detection. In support of this possibility, Reynolds and colleagues (2010) integrated VPCs into an ERP procedure and found that infants demonstrate greater amplitude Nc to their preferred stimulus regardless of novelty or familiarity.
The results of de Haan and Nelson’s (1995) analysis of the LSW went in the opposite direction of the findings of their Nc analysis with infants showing greater amplitude LSWs at midline and temporal electrodes to novel stimuli in both the faces and objects conditions. Because the LSW is associated stimulus encoding, this finding clearly indicates that the infants recognized both the familiar objects and familiar faces during testing. However, because the familiar stimuli used in the study were the mother’s faces and the favorite toy, the greater attention toward familiar stimuli indexed by greater amplitude Nc would be expected. Taken together, these findings demonstrate the utility of the ERP technique for examining multiple component processes involved in cognitive processing which may occur in a matter of 1 to 2 s. Using preferential looking measures, any novelty effects related to encoding the novel stimulus may have been masked by strong visual preferences for the mother’s face or the favorite toy.
Snyder and colleagues (Snyder, Garza, Zolot, & Kresse, 2010) investigated potential neural mechanisms underlying the LSW and infant recognition memory. Six-month-old infants were tested in a procedure which allowed for the analysis of recency and familiarity effects. Infants were shown pictures of highly familiar objects and novel objects. Each familiar and novel object was repeated once during testing. The results indicated recency effects for the LSW with infants showing reduced amplitude from the first to second presentation of a stimulus regardless of novelty or familiarity. The Nc demonstrated familiarity effects and recency effects for familiar stimuli. Nc amplitude was greater to familiar than novel stimuli, and Nc reduced in amplitude from the first to the second presentation of the familiar stimuli. The authors interpreted the recency effect in the LSW as potentially reflecting reduced neural firing of recency neurons in the medial temporal lobe to previously encountered stimuli similar to the “repetition suppression” documented in work with nonhuman primates (Baylis & Rolls, 1987; Fahy et al., 1993; Li et al., 1993; Miller, Li, & Desimone, 1991; Xiang & Brown, 1998). The Nc effects were interpreted as reflecting amount of attentional engagement (Courchesne et al., 1981, Reynolds & Richards, 2005; Richards, 2003).
Reynolds, Guy, and Zhang (2011) examined the impact of individual differences in infant visual attention on object recognition. Infants of 6 and 7.5 months of age were tested in a procedure in which they were familiarized with a single object, and then shown the familiar object on 50% of the ERP trials, and novel objects on the other 50% of trials. Look duration during familiarization was used to determine each participant’s “looker type” as an index of individual differences in infant visual attention. Colombo and colleagues (Colombo, 1993; Colombo & Mitchell, 1990; Colombo, Mitchell, Coldren, & Freeseman, 1991; Colombo, Mitchell, & Horowitz, 1988) have shown that infants who demonstrate brief visual fixations (i.e., short lookers) during familiarization are more likely to demonstrate evidence of recognition memory during subsequent stimulus exposure than infants who demonstrate long visual fixations (i.e., long lookers). Reynolds, Guy, and Zhang (2011) reported that short-looking infants demonstrated significantly greater amplitude LSWs to novel compared to familiar stimuli indicative of recognition memory for the familiar object (see Figure 2). These memory effects occurred at both frontal and temporal electrodes for short lookers. No differences in LSW amplitude were found based on stimulus type for long-looking infants.
Figure 2.
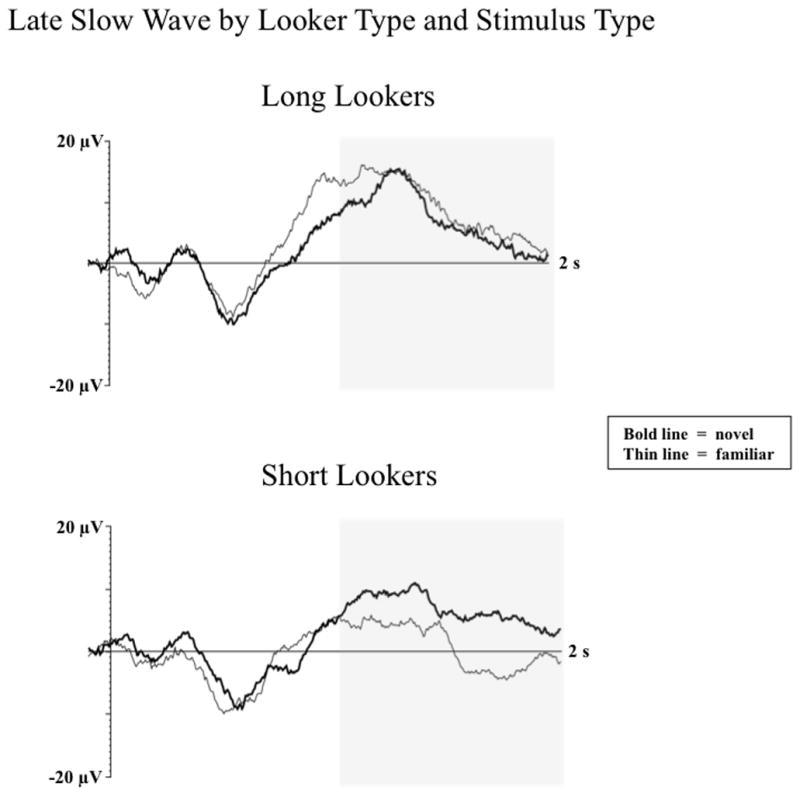
Mean amplitude of the LSW by looker type and stimulus type at frontal electrodes. The Y-axis represents change in amplitude from baseline and the X-axis represents time following stimulus onset following familiar (thin line) and novel (bold line) stimulus presentations. The shaded area indicates the portion of the waveform examined in the LSW analysis from 1 to 2 sec following stimulus onset (figure adapted from Reynolds, Guy, & Zhang, 2011).
Taken together, these findings suggest that the short-lookers fully processed the familiar stimulus with 20 s of exposure whereas the long lookers showed no evidence of recognition of the familiar object. The findings also indicate that individual differences in visual attention influence recognition memory in infancy. However, because no differences were found between the looker type groups in the Nc component, the enhanced recognition of the familiar objects for the short-looking infants does not appear to be due to greater attentional engagement. Instead of simply being more attentive, short-looking infants may utilize more efficient visual processing strategies when encoding novel stimuli than long-looking infants.
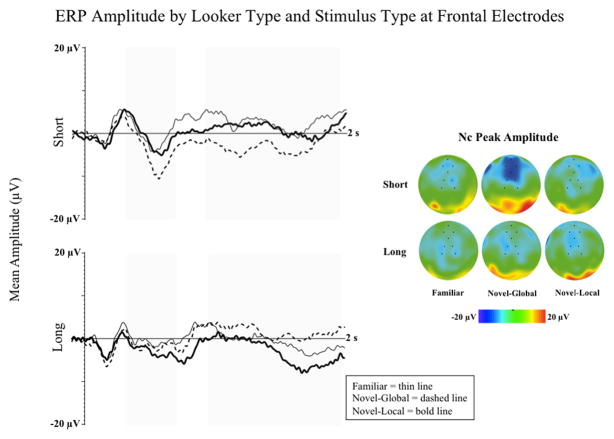
To explore this possibility, Guy, Reynolds, & Zhang (2013) utilized hierarchical patterns to examine global and local processing in 6-month-olds. Short- and long-looking infants were familiarized with a hierarchical pattern. Hierarchical patterns are patterns in which larger figures are composed of an arrangement of smaller figures. The larger figures are considered higher-level units of information and the smaller figures are considered lower-level units (Kimchi, 1992). The hierarchical patterns used consisted of a group of white, uppercase letters (i.e., local elements) arranged in a configuration that formed a larger pattern against a black background (i.e., global pattern). Following familiarization, infants were shown repeated presentations of the familiar pattern, novel-global patterns, and novel-local patterns. The novel-global patterns differed from the familiar pattern in overall shape but the pattern was composed of the same local elements (letters) as the familiar. The novel-local pattern was the same overall shape as the familiar pattern but the letters that composed the shape (local elements) were changed.
The results indicated that short- and long-looking infants utilize different processing strategies when encoding novel stimuli into memory. As can be seen in Figure 3, Short-lookers demonstrated significant differences in LSW amplitude to novel-global stimuli in comparison to familiar stimuli. In contrast, long-lookers demonstrated significant differences in LSW amplitude to novel-local stimuli in comparison to familiar stimuli. These findings indicate that short-lookers demonstrate a global-precedence effect, processing global properties of patterns (and possibly objects) prior to processing the local elements or fine details. In contrast, long-lookers process local elements prior to processing global properties. The global-precedence effect is characteristic of a mature and efficient visual processing strategy (Kimchi, 1992), and these differences in processing strategy would explain the long-lookers inability to fully process the more complex objects used as stimuli by Reynolds and colleagues (2011). Although these two sets of findings (Guy et al., 2013; Reynolds et al., 2011) indicate that an infant’s attentional style influences early recognition memory, because infants in these studies were familiarized with the repeated stimulus in a familiarization phase prior to the ERP testing the findings do not provide insight into exactly how attention influences stimulus encoding and subsequent recognition memory.
Figure 3.
ERP amplitude is presented by stimulus type at frontal electrodes. The left panel shows ERP waveforms for short and long lookers at Fz. The shaded areas on the waveform plots indicate the time-windows for the Nc (350–750 ms) and LSW (1–2 s) analyses. The right panel shows topographical plots at peak amplitude Nc by stimulus type and looker type. The black dots in the topographical plots indicate the location of the electrodes used in the midline frontal and central electrode clusters (figure adapted from Guy, Reynolds, & Zhang, 2013).
The influence of attention on stimulus encoding and recognition memory has been examined using heart rate as a psychophysiological index of infant attention during ERP tasks (Reynolds et al., 2005, 2010, 2011; Richards, 2003). Changes in HR throughout ERP testing were used to separate trials in which infants were deeply engaged in sustained attention (during looking paired with significant heart rate decelerations) from trials in which infants were inattentive and heart rate was at or above baseline levels (e.g., Casey & Richards, 1988; Richards, 1997; Richards & Casey, 1991; see review, Reynolds & Richards, 2008). In two of these studies, the authors (Reynolds et al., 2010, 2011) used a visual preference – ERP (VP – ERP) task with repeated VPC trials embedded in an ERP procedure. This was done in order to examine the progression of infant visual preferences and neural responsiveness in real-time during the process of encoding. Behavioral, HR, and ERP responses to familiar and novel or repeated and non-repeated stimuli were measured from infants of 4.5, 6, and 7.5 months of age.
Several findings from these studies provide insight into the influence of attention on stimulus encoding and subsequent recognition memory. First, differences in Nc amplitude were found based on attention (as measured with HR). Providing support for the possibility that Nc represents attentional engagement, Nc was greater in amplitude during attention than during inattention (Reynolds et al., 2010, 2011; Richards, 2003). Second, regardless of novelty or familiarity, infants demonstrated greater amplitude Nc to the stimulus they showed a visual preference for on VPC trials (Reynolds et al., 2010). Third, as shown in Figure 4, infants demonstrated significant differences in LSW amplitude based on stimulus type (i.e., familiar compared to novel, repeated compared to non-repeated) on attentive trials (as defined by HR). In contrast, no differences were found in LSW amplitude based on stimulus type on inattentive trials (Reynolds & Richards, 2005, 2011). Additionally, the LSW was greater in amplitude during attention than during inattention (Reynolds & Richards, 2005).
Figure 4.
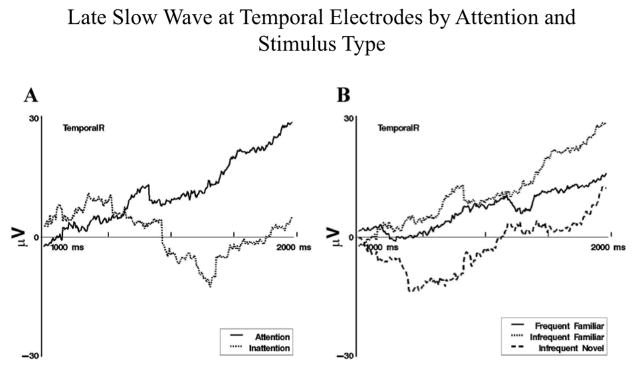
Late slow wave event-related potentials at temporal electrodes from 1 to 2 s following stimulus onset. Panel A: Display of differences in responding following infrequent-familiar stimulus presentations during attention and inattention. Panel B: Display of responses to the three memory stimulus types during attention (figure adapted from Reynolds & Richards, 2005).
The results of these studies demonstrate strong relations between infant attention during stimulus encoding and recognition memory, and these findings indicate that infants are most likely to demonstrate evidence of recognition at the neural level when behavioral and psychophysiological measures are also indicative of sustained attention. However, developmental cognitive neuroscientists are highly limited in neuroimaging techniques available for use with infant participants because of practical and ethical concerns (for further discussion, Reynolds & Richards, 2009). For example, functional magnetic resonance imaging (fMRI) requires participants to remain very still while awake for relatively extending periods of time in a highly confined space, and positron emission tomography (PET) involves exposure to ionizing radiation. Thus, both of these neuroimaging techniques are arguably inappropriate for basic research with infant participants. Given that scalp-recorded EEG does not provide a direct measure of the location of the underlying neural generators that contribute to the ERP components of interest, little is currently known about the actual areas of the brain involved in attention and recognition memory in human infants.
Neuroanatomical bases of infant visual attention and recognition memory
To date, developmental scientists have mostly relied on findings from comparative research with nonhuman primates and neuroimaging work with adult human participants for information regarding the neural bases of infant attention and recognition memory. This information can be combined with findings from the behavioral and electrophysiological work on human infants to build hypotheses regarding the contribution of specific areas of the brain to early attention and memory. Several theoretical models relating brain development to infant attention and recognition memory have been proposed. Most of these models propose that the hippocampus and surrounding areas of medial temporal lobe are associated with novelty preferences and likely play a key role in recognition memory in infancy (e.g., de Haan, 2007; Diamond, 1990; Kaldy & Sigala, 2004; Nelson, 1995; Snyder, 2010). These proposals are largely influenced by electrophysiological recordings with nonhuman primates and neuroimaging studies with adults indicating the presence of a medial temporal lobe circuit involved in recognition memory processes, which includes: the hippocampus and parahippocampal cortex; entorhinal cortex and perirhinal cortices; and additionally the visual area TE (Begleiter, Porjesz, & Wang, 1993; Brown & Aggleton, 2001; Desimone, 1996; Eichenbaum, Yonelinas, & Ranganath, 2007; Fahy et al., 1993; Li, Miller, & Desimone, 1993; Wan, Aggleton, & Brown, 1999; Wiggs & Martin, 1998; Xiang & Brown, 1998; Zhu, Brown, McCabe, & Aggleton, 1995). However, some authors have concluded that although the hippocampus and other medial temporal areas are functional in early infancy and likely play a role in early recognition memory, this early responsiveness to novelty may be a form of implicit or pre-explicit memory which does not mature into explicit memory until at least 8 – 12 months of age (e.g., Nelson, 1995; Schacter, & Moscovitch, 1984; but see, Rose et al., 2004). Thus, recognition memory may be fundamentally different in early infancy than in later development (de Haan, 2007).
Support for this possibility and for the importance of medial temporal lobe structures for early recognition memory comes from lesion studies with nonhuman primates. For example, research by Bachevalier and colleagues (e.g., Bachevalier, Brickson, & Hagger, 1993; Pascalis & Bachevalier, 1999; Zeamer, Heuer, & Bachevalier, 2010) with infant rhesus macaques has shown that lesions of the hippocampus and surrounding areas of medial temporal cortex (e.g., perirhinal and parahippocampal cortical areas) disrupt novelty preferences with even very short delays in neonate and adult macaques (Bachevalier et al., 1993; Nemanic, Alvarado, & Bachevalier, 2004; Zola, Squire, Teng, Stefanacci, Buffalo, & Clark, 2000). Zeamer and colleagues (2010) found that control macaques demonstrated significant gains in recognition memory measured in VPC tasks from 1.5 to 18 months of age. Although infant macaques with lesions limited to the hippocampus also showed significant gains in recognition memory with increasing age and showed evidence of recognition up to 18 months of age, the effects of delay between familiarization and VPC testing were much more pronounced for this group after 6 months of age than controls. The monkeys with hippocampal lesions showed more significant drops in novelty scores from 10 to 120 s of delay between familiarization and testing. However, since recognition memory was still spared to a certain extent in these infant macaques (unlike macaques with hippocampal lesions received in adulthood), the authors concluded that macaques utilize other medial temporal lobe structures for recognition in the neonatal period with the hippocampus playing an increasing role in recognition after 6 months of age. Inferotemporal areas such as TE also likely play a more significant role in recognition memory with increasing age (Nelson, 1995). The increasing role of these structures in recognition memory may be associated with a transition from pre-explicit to explicit forms of recognition memory; however, questions regarding the implicit or explicit nature of early memory are problematic for systematic testing in nonverbal infant participants and will likely remain unanswered.
Several models have been proposed for the development of neural systems involved in attention (e.g., Bronson, 1974, 1997; Maurer & Lewis, 1979, 1998; Johnson, 1990, 1995; Hood, 1995; Posner & Peterson, 1998; Reynolds, Courage, & Richards, 2013; Richards, 2008, 2010; Ruff & Rothbart, 1996). Table 1 presents a summary of key points and findings related to some of these models. Similar to models of the neural bases of recognition memory development, these attention models were highly influenced by comparative research and neuroimaging studies using adult participants. Schiller’s (1985) work on primate eye-movement systems and Posner’s model of attention systems (Posner & Peterson, 1990) have been particularly influential in the infant literature. Three attention systems are proposed to develop at different rates across infancy and early childhood, these are: the reflexive system, the posterior orienting system, and the anterior attention network. The reflexive system is believed to be the only functionally mature visual attention system available during the newborn period (i.e., birth to approximately 2 months of age). During this time, visual fixation and attention is primarily under the control of a reflexive system involving the lateral geniculate nucleus, primary visual cortex, and superior colliculus. Visual fixation is believed to be reflexively drawn to salient features of the environment, including areas of high contrast, borders of stimuli, and motion. Infants may display long looks to stimuli during this period; however, visual scanning and information processing abilities are inefficient and immature.
From 3 to 6 months of age, the posterior orienting system reaches functional maturity (Posner & Petersen, 1990) and infants begin to develop voluntary control over visual attention. There are two components which play a functional role in the posterior orienting system. The first component is involved in a spatial orienting network. This network includes areas of posterior parietal cortex, the superior colliculus, and the frontal eye fields. These areas play a significant role in voluntary disengaging and shifting of visual fixation. The second component in this system is an object recognition network. This network includes both the dorsal and ventral pathways from the primary visual cortex to the parietal cortex and the inferior temporal cortex. This component of the posterior orienting network is key for attention to object features and subsequent recognition memory for objects and other stimuli. As these systems develop across 3 to 6 months of age, look duration decreases significantly to all types of stimuli (Courage, Reynolds, and Richards, 2006), and there is a marked transition in infant attention as infants begin to focus more on the relevant features of objects, people, and events.
From six months on, the anterior attention system begins to play a role in visual attention. This attention system includes frontal areas, such as the dorsolateral prefrontal cortex, orbito-frontal cortex, and anterior cingulate. Infants develop higher-level attention when this system becomes functional and can inhibit attention to distracters while maintaining sustained attention to interesting and complex stimuli. Beyond six months of age, infants continue to display shorter looking to basic stimuli, such as black and white geometric patterns, but begin to display longer looking to more complex stimuli such as faces and objects (Courage et al., 2006). Infants also require less time to demonstrate novelty preferences across this age range (Richards, 1997; Rose et al., 1982, 2004) suggesting that the advances in infant attention based on further brain development and experience lead to more efficient processing of objects, faces, and visual patterns.
Nelson and Dukette (1998) described a selective attention network that shares common regions with both the anterior and posterior attention systems. The areas involved in the selective attention network are dorsolateral prefrontal cortex, anterior cingulate cortex, the pulvinar nucleus of the thalamus, and posterior parietal cortex. The dorsolateral prefrontal cortex, pulvinar, and posterior parietal cortex form an integrated network composed of reciprocal connections between these neural structures. This system is involved in attending to specific objects or spatial locations, and shows considerable development in infancy continuing well into childhood.
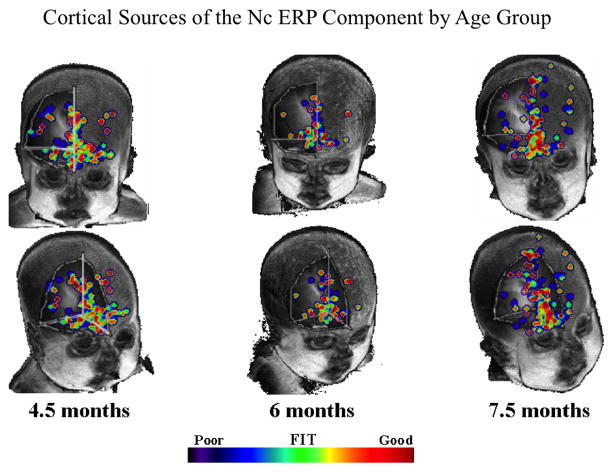
In support of the proposal that anterior areas are key for infant attention, Reynolds and colleagues (Reynolds & Richards, 2005; Reynolds, Courage, & Richards, 2010) used cortical source analysis to localize the source of the Nc ERP component. To perform the cortical source analysis, spatial independent component analysis and equivalent current dipole analysis were used to infer cortical sources of the scalp-recorded ERP (Reynolds & Richards, 2005, 2009). Figure 5 shows the equivalent current dipoles from individual participants mapped onto structural MRI images by age group. The best-fitting dipoles indicated by orange and red in the color scale in this figure represent dipole locations that were active during both the ERP task and VPC trials. The cortical source analysis localized the source of the Nc ERP component to areas of medial prefrontal cortex, inferior prefrontal cortex, and the anterior cingulate. Interestingly, the cortical sources of Nc were localized to these frontal areas across all age groups; however, the dipoles were more diffusely spread for the younger infants and more localized along the midline for the oldest infants.
Figure 5.
Common equivalent current dipoles activated across recognition memory tasks. Age groups are divided into separate columns. The best fitting areas in common between the ERP and VPC tasks are indicated using the color scale. The majority of best fitting areas were located in inferior prefrontal regions (figure adapted from Reynolds, Courage, & Richards, 2010).
Although the areas the cortical sources of Nc were localized to in these studies are associated with the anterior attention network (Posner & Petersen, 1990), Nc most likely does not exclusively reflect activation of the anterior attention network. The anterior attention network is associated with more mature, executive forms of attention that are believed to only begin to reach functional onset in later infancy. Instead, increases in Nc amplitude likely reflect activation of a general arousal system Richards and colleagues (Reynolds et al., 2013; Richards 2008, 2010) propose is involved in infant attention. This general arousal system initiates the decreased heart rate associated with sustained attention through parasympathetic influence of the brainstem on the heart. Additionally, this general arousal system enhances processing throughout the cortex through the ascending influence of the noradrenergic and cholinergic neurochemical systems (Richards, 2008, 2010; Robbins & Everitt, 1995; Sarter, Givens, & Bruno, 2001). Cortical areas involved in more specific attention systems will demonstrate enhanced activity when the infant is attentive and this general arousal system is engaged. Thus, the results of the cortical source analyses of Nc on these recognition memory tasks may reflect enhanced cortical activation based on the ascending influence of thalamo-cortical projections on frontal areas involved in the selective attention network (Nelson and Dukette, 1998). Given this framework for interpreting Nc, it is also possible that the topographical distribution of cortical sources of Nc may vary based on age and type of attention involved in the experimental task; for example, Nc has been found to be most prominent over midline parietal electrodes as opposed to midline central and frontal electrodes when infants are exposed to multimodal (i.e., audio-visual) stimuli (Reynolds, Bahrick, Lickliter, & Guy, 2014).
Because activation of the general arousal system leads to several state-related changes that foster stimulus processing (e.g., extended looking, decreased HR, decreased distractibility, and enhanced cortical processing), infants are more likely to recognize stimuli they are exposed to during periods of sustained attention than if exposure occurs during periods of inattention (Frick and Richards, 2001; Richards, 1997). However, relations between infant attention and recognition memory are clearly bidirectional. Recognition of a familiar stimulus, such as the mother’s face, a familiar pattern, or a favorite toy, influences the infant’s level of attentional engagement and may elicit sustained attention (e.g., de Haan & Nelson, 1997, 1999; Reynolds & Richards, 2005). In order to impact the amplitude of the Nc ERP component, the influence of the general arousal system on attention must occur by at least a few hundred milliseconds after stimulus onset in older infants and up to a second after stimulus onset in newborns (Nelson and deRegnier, 1992; Parker & Nelson, 2005; Reynolds et al., 2005, 2010; Richards, 2003).
Conclusion
The development of the voluntary control of visual attention demonstrates substantial overlap with the development of reaching and object manipulation, and as these cognitive and motor skills develop the infant becomes increasingly object oriented (Gibson, 1998; Ruff & Rothbart, 1996). Infant attention and object recognition are thus tightly coupled. Infants demonstrate greater memory for objects and events if they are in an attentive state during initial exposure than if they are in an inattentive state during initial exposure (Frick & Richards, 2001; Richards, 1997). Furthermore, infants are more likely to demonstrate evidence of recognition at both the behavioral and neural levels during attention than during inattention (Reynolds et al., 2005, 2010, 2011; Richards, 2003). The infancy period is characterized by substantial gains in both the voluntary control of visual attention (Colombo, 2001, Courage et al., 2006), and in the ability to recognize objects with increasingly shorter prior exposure (Courage & Howe, 2004; Diamond, 1990; Richards, 1997) and increasingly longer delays between familiarization and testing (Rose et al., 1983, 2004).
Areas of the brain that show further development throughout infancy and are likely involved in these developmental gains in voluntary control of attention and stimulus encoding include posterior parietal cortex, frontal eye-fields, inferior prefrontal cortex, and the anterior cingulate (Colombo, 2001; Posner & Peterson, 1990; Reynolds et al., 2005, 2009, 2010, 2013). Cortical areas most likely involved in early recognition memory include a medial temporal lobe circuit, with the hippocampus and area TE playing an increasingly important role with increasing age (Bachevalier, Brickson, & Hagger, 1993; Diamond, 1990; Kaldy & Sigala, 2004; Pascalis & Bachevalier, 1999; Nelson, 1995; Zeamer, Heuer, & Bachevalier, 2010). With increasing age, infants demonstrate increased arousal responses associated with attention, and infants are able to maintain sustained attention for longer periods of time (e.g., Richards & Casey, 1992). These developmental changes in attention linked to arousal likely play a key role in the increased efficiency of stimulus encoding and gains in recognition memory occurring across this age range.
Based on the past 50 years of research on early cognitive development, much is known about the characteristics of early visual attention and recognition memory. However, there is still much to learn about the neural basis of the observed patterns of development in these two core cognitive functions. The field has been historically limited in this area due to a lack of non-invasive neuroimaging tools for use with infant participants. However, with the increased sophistication of tools available for use in this area, such as cortical source analysis and near infrared spectroscopy, the near future holds great promise for significant gains in our understanding of relations between developing neural systems involved in infant visual attention and object recognition.
Research Highlights.
Infant visual attention and object recognition are closely related.
The bulk of research on infant attention and recognition memory is based on preferential looking tasks.
Infants demonstrate increased voluntary control of attention across infancy as well as major gains in recognition memory.
The Nc and late slow ERP components serve as neural correlates of infant attention and object recognition.
Acknowledgments
Research reported in this article and the writing of this article were supported by the National Institute of Child Health and Human Development Grant R03-HD05600, and the National Science Foundation Developmental and Learning Sciences Division Grant 1226646 to Greg D. Reynolds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bachevalier J, Brickson M, Hagger C. Limbic-dependent recognition memory in monkeys develops early in infancy. Neuroreport. 1993;4:77–80. doi: 10.1097/00001756-199301000-00020. [DOI] [PubMed] [Google Scholar]
- Baylis LL, Rolls ET. Responses of neurons in the inferior temporal cortex in short term and serial recognition memory tasks. Experimental Brain Research. 1987;65:614–622. doi: 10.1007/BF00235984. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Wang W. A neurophysiologic correlate of visual short-term memory in humans. Electroencephalography and Clinical Neurophysiology. 1993;87:46–53. doi: 10.1016/0013-4694(93)90173-s. [DOI] [PubMed] [Google Scholar]
- Bronson GW. The postnatal growth of visual capacity. Child Development. 1974;45:873–890. [PubMed] [Google Scholar]
- Bronson GW. The growth of visual capacity: Evidence from infant scanning patterns. In: Rovee-Collier C, Lipsitt LP, editors. Advances in infancy research. Vol. 11. Greenwich, CT: Ablex; 1997. pp. 109–141. [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nature Reviews Neuroscience. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Richards JE. Sustained visual attention measured with an adapted version of the visual preference paradigm. Child Development. 1988;59:1514–1521. [PubMed] [Google Scholar]
- Carver LJ, Bauer PJ, Nelson CA. Associations between infant brain activity and recall memory. Developmental Science. 2000;3:234–246. [Google Scholar]
- Colombo J. Infant cognition: Predicting later intellectual functioning. Newbury Park, CA: Sage Production, Inc; 1993. [Google Scholar]
- Colombo J. The development of visual attention in infancy. Annual Review of Psychology. 2001;52:337–367. doi: 10.1146/annurev.psych.52.1.337. [DOI] [PubMed] [Google Scholar]
- Colombo J, Mitchell DW. Individual and developmental differences in infant visual attention. In: Colombo J, Fagan JW, editors. Individual differences in infancy. Hillsdale, NJ: Erlbaum; 1990. pp. 193–227. [Google Scholar]
- Colombo J, Mitchell DW, Coldren JT, Freeseman LJ. Individual differences in infant visual attention: Are short lookers faster processors or feature processors? Child Development. 1991;62:1247–1257. [PubMed] [Google Scholar]
- Colombo J, Mitchell DW, Horowitz FD. Infant visual attention in the paired-comparison paradigm: Test–retest and attention-performance relations. Child Development. 1988;59:1198–1210. doi: 10.1111/j.1467-8624.1988.tb01489.x. [DOI] [PubMed] [Google Scholar]
- Courage ML, Howe ML. Advances in early memory development research: Insights about the dark side of the moon. Developmental Review. 2004;24:6– 32. [Google Scholar]
- Courage ML, Reynolds GD, Richards JE. Infants’ attention to patterned stimuli: Developmental change from 3 to 12 months of age. Child Development. 2006;77:680–695. doi: 10.1111/j.1467-8624.2006.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E. Event-related brain potentials: Comparison between children and adults. Science. 1977;197:589–592. doi: 10.1126/science.877575. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Cognitive components of the event-related brain potential: Changes associated with development. Advances in Psychology. 1983;10:329–344. [Google Scholar]
- Courchesne E, Ganz L, Norcia AM. Event-related brain potentials to human faces in infants. Child Development. 1981;52:804–811. [PubMed] [Google Scholar]
- de Haan M. Visual attention and recognition memory in infancy. In: de Haan M, editor. Infant EEG and event-related potentials. New York: Psychology Press; 2007. pp. 101–144. [Google Scholar]
- de Haan M, Nelson CA. Recognition of the mother’s face by six-month-old infants: A neurobehavioral study. Child Development. 1997;68:187–210. [PubMed] [Google Scholar]
- de Haan M, Nelson CA. Brain activity differentiates face and object processing in 6-month-old infants. Developmental Psychology. 1999;35:1113–1121. doi: 10.1037//0012-1649.35.4.1113. [DOI] [PubMed] [Google Scholar]
- Desimone R. Neural mechanisms for visual memory and their role in attention. PNAS. 1996;93:13494–13499. doi: 10.1073/pnas.93.24.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Rate of maturation of the hippocampus and the developmental progression of children’s performance on the delayed non-matching to sample and visual paired comparison tasks. In: Diamond A, editor. Development and neural bases of higher cognitive functions. New York: New York Academy of Sciences Press; 1990. pp. 394–426. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas A, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiani M, Gratton G, Coles MGH. Event-related brain potentials: Methods, theory, and applications. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. New York: Cambridge University Press; 2000. pp. 53–84. [Google Scholar]
- Fagan JF. Memory in the infant. Journal of Experimental Child Psychology. 1970;9:217–226. doi: 10.1016/0022-0965(70)90087-1. [DOI] [PubMed] [Google Scholar]
- Fagan JF. Infant recognition memory: The effects of length of familiarization and type of discrimination task. Child Development. 1974;45:351–356. doi: 10.1111/j.1467-8624.1974.tb00603.x. [DOI] [PubMed] [Google Scholar]
- Fahy FL, Riches IP, Brown MW. Neuronal activity related to visual recognition memory: Long-term memory and the encoding of recency and familiarity information in the primate anterior and medial inferior rhinal cortex. Experimental Brain Research. 1993;96:457–472. doi: 10.1007/BF00234113. [DOI] [PubMed] [Google Scholar]
- Fantz RL. A method for studying early visual development. Perceptual and Motor Skills. 1956;6:13–15. [Google Scholar]
- Fantz RL. Pattern vision in newborn infants. Science. 1963;140:296–97. doi: 10.1126/science.140.3564.296. [DOI] [PubMed] [Google Scholar]
- Fantz JF. Visual experience in infants: Decreased attention to familiar patterns relative to novel ones. Science. 1964;146:668–670. doi: 10.1126/science.146.3644.668. [DOI] [PubMed] [Google Scholar]
- Freeseman LJ, Colombo J, Coldren JT. Individual differences in infant visual attention: Four-month-olds’ discrimination and generalization of global and local stimulus properties. Child Development. 1993;64:1191–1203. [PubMed] [Google Scholar]
- Frick JE, Richards JE. Individual differences in infants’ recognition of briefly presented visual stimuli. Infancy. 2001;2:331–352. doi: 10.1207/S15327078IN0203_3. [DOI] [PubMed] [Google Scholar]
- Gibson EJ. Exploratory behavior in the development of perceiving, acting, and the acquiring of knowledge. Annual Review of Psychology. 1988;39:1–41. [Google Scholar]
- Guy MW, Reynolds GD, Zhang D. Visual attention to global and local stimulus properties in six-month-old infants: Individual differences and event-related potentials. Child Development. 2013;84:1392–1406. doi: 10.1111/cdev.12053. [DOI] [PubMed] [Google Scholar]
- Hood BM. Shifts of visual attention in the human infant: A neuroscientific approach. Advances in Infancy Research. 1995;10:163–216. [Google Scholar]
- Hunter M, Ames E. A multifactor model of infant preferences for novel and familiar stimuli. In: Rovee-Collier C, Lipsitt LP, editors. Advances in infancy research. Vol. 5. Norwood, NJ: Ablex; 1988. pp. 69–95. [Google Scholar]
- Johnson MH. Cortical maturation and the development of visual attention in early infancy. Journal of Cognitive Neuroscience. 1990;2:81–95. doi: 10.1162/jocn.1990.2.2.81. [DOI] [PubMed] [Google Scholar]
- Johnson MH. The development of visual attention: A cognitive neuroscience perspective. In: Gazzaniga MS, editor. The cognitive neurosciences. Cambridge, MA: MIT Press; 1995. pp. 735–747. [Google Scholar]
- Kaldy Z, Sigala N. The neural mechanisms of object working memory: What is where in the infant brain? Neuroscience and Biobehavioral Reviews. 2004;28:113–121. doi: 10.1016/j.neubiorev.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Karrer R, Ackles PK. Visual event-related potentials of infants during a modified oddball procedure. In: Johnson R, Rohrbaugh JW, Parasuraman R, editors. Current trends in event-related potential research. Amsterdam: Elsevier Science Publishers; 1987. pp. 603–608. [Google Scholar]
- Karrer R, Ackles PK. Brain organization and perceptual/cognitive development in normal and Down syndrome infants: A research program. In: Vietze P, Vaughan HG Jr, editors. The early identification of infants with developmental disabilities. Philadelphia: Grune & Stratton; 1988. pp. 210–234. [Google Scholar]
- Karrer R, Monti LA. Event-related potentials of 4–7 week-old infants in a visual recognition memory task. Electroencephalography and Clinical Neurophysiology. 1995;94:414–424. doi: 10.1016/0013-4694(94)00313-a. [DOI] [PubMed] [Google Scholar]
- Kimchi R. Primacy of wholistic processing and global/local paradigm: A critical review. Psychological Bulletin. 1992;112:24–38. doi: 10.1037/0033-2909.112.1.24. [DOI] [PubMed] [Google Scholar]
- Li L, Miller EK, Desimone R. The representation of stimulus familiarity in anterior inferior temporal cortex. Journal of Neurophysiology. 1993;69:1918–1929. doi: 10.1152/jn.1993.69.6.1918. [DOI] [PubMed] [Google Scholar]
- Maurer D, Lewis TL. A physiological explanation of infants’ early visual development. Canadian Journal of Psychology. 1979;33:232–252. doi: 10.1037/h0081723. [DOI] [PubMed] [Google Scholar]
- Maurer D, Lewis TL. Overt orienting toward peripheral stimuli: Normal development and underlying mechanisms. In: Richards JE, editor. Cognitive neuroscience of attention: A developmental perspective. Hillsdale, NJ: Lawrence Erlbaum; 1998. pp. 51–102. [Google Scholar]
- Miller EK, Li L, Desimone R. A verbal mechanism for working and recognition memory in inferior temporal cortex. Neuroscience Abstracts. 1991;2:1377–1379. doi: 10.1126/science.1962197. [DOI] [PubMed] [Google Scholar]
- Nelson CA. Neural correlates of recognition memory in the first postnatal year of life. In: Dawson G, Fischer K, editors. Human behavior and the developing brain. New York, NY: Gilford Press; 1994. pp. 269–313. [Google Scholar]
- Nelson CA. The ontogeny of human memory: A cognitive neuroscience perspective. Developmental Psychology. 1995;5:723–738. [Google Scholar]
- Nelson CA. Electrophysiological correlates of early memory development. In: Reese HW, Franzen MD, editors. Thirteenth West virginia University Conference on Life Span Developmental Psychology: Biological and Neuropsychological Mechanisms. Hillsdale, NJ: Erlbaum; 1996. pp. 95–131. [Google Scholar]
- Nelson CA, deRegnier RA. Neural correlates of attention and memory in the first year of life. Developmental Neuropsychology. 1992;8(2–3):119–134. [Google Scholar]
- Nelson CA, Dukette D. A cognitive neuroscience perspective on the relation between attention and memory development. In: Richards J, editor. Cognitive neuroscience of attention: A developmental perspective. Hillsdale, NJ: Erlbaum; 1998. pp. 327–362. [Google Scholar]
- Nemanic S, Alvarado MC, Bachevalier J. The hippocampal/parahippocampal regions and recognition memory: insights from visual paired comparison versus object-delayed nonmatching in monkeys. Journal of Neuroscience. 2004;24:2013–2026. doi: 10.1523/JNEUROSCI.3763-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikkel L, Karrer R. Differential effects of experience on the ERP and behavior of 6-month-old infants: Trends during repeated stimulus presentation. Developmental Neuropsychology. 1994;10:1–11. [Google Scholar]
- Parker SW, Nelson CA. The Impact of Early Institutional Rearing on the Ability to Discriminate Facial Expressions of Emotion: An Event-Related Potential Study. Child Development. 2005;76(1):54–72. doi: 10.1111/j.1467-8624.2005.00829.x. [DOI] [PubMed] [Google Scholar]
- Pascalis O, Bachevalier J. Neonatal aspiration lesions of the hippocampal formation impair visual recognition memory when assessed by paired-comparison task but not by delayed nonmatching to sample task. Hippocampus. 1999;9:609–616. doi: 10.1002/(SICI)1098-1063(1999)9:6<609::AID-HIPO1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Jr, Miller GA, Ritter W, Ruchkin DS, Rugg MD, Taylor MJ. Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- Posner MI, Peterson S. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Reynolds GD, Bahrick LE, Lickliter R, Guy MW. Neural correlates of intersensory processing in 5-month-old infants. Developmental Psychobiology. 2014 doi: 10.1002/dev.21104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GD, Courage ML, Richards JE. Infant attention and visual preferences: Converging evidence from behavior, event-related potentials, and cortical source localization. Developmental Psychology. 2010;46:886–904. doi: 10.1037/a0019670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GD, Courage ML, Richards JE. The development of attention. In: Reisberg D, editor. Oxford Handbook of Cognitive Psychology. Oxford University Press; New York, NY: 2013. pp. 1000–1013. [Google Scholar]
- Reynolds GD, Guy MW. Brain–behavior relations in infancy: Integrative approaches to examining infant looking behavior and event-related potentials. Developmental Neuropsychology. 2012;37(3):210–225. doi: 10.1080/87565641.2011.629703. [DOI] [PubMed] [Google Scholar]
- Reynolds GD, Guy MW, Zhang D. Neural correlates of individual differences in infant visual attention and recognition memory. Infancy. 2011;16(4):368–391. doi: 10.1111/j.1532-7078.2010.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GD, Richards JE. Familiarization, attention, and recognition memory in infancy: An ERP and cortical source localization study. Developmental Psychology. 2005;41:598–615. doi: 10.1037/0012-1649.41.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GD, Richards JE. Infant heart rate: A developmental psychophysiological perspective. In: Schmidt LA, Segalowitz SJ, editors. Developmental Psychophysiology: Theory, Systems, and Applications. Cambridge University Press; 2008. pp. 173–212. [Google Scholar]
- Reynolds GD, Richards JE. Cortical source localization of infant cognition. Developmental Neuropsychology. 2009;34(3):312–329. doi: 10.1080/87565640902801890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GD, Richards JE. Processing of repeated and non-repeated visual stimuli in infancy: visual preferences and event-related potentials. Presented at the biennial meeting of the Society for Research in Child Development; Montreal, Quebec. 2011. [Google Scholar]
- Richards JE. Effects of attention on infants’ preference for briefly exposed visual stimuli in the paired-comparison recognition-memory paradigm. Developmental Psychology. 1997;33:22–31. doi: 10.1037//0012-1649.33.1.22. [DOI] [PubMed] [Google Scholar]
- Richards JE. Attention affects the recognition of briefly presented visual stimuli in infants: An ERP study. Developmental Science. 2003;6:312–328. doi: 10.1111/1467-7687.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JE. Attention in young infants: A developmental psychophysiological perspective. In: Nelson CA, Luciana M, editors. Handbook of developmental cognitive neuroscience. Cambridge, MA, US: MIT Press; 2008. pp. 479–497. [Google Scholar]
- Richards JE. Attention in the brain and early infancy. In: Johnson SP, editor. Neoconstructivism: The new science of cognitive development. New York, NY: Oxford University Press; 2010. pp. 3–31. [Google Scholar]
- Richards JE, Casey BJ. Heart rate variability during attention phases in young infants. Psychophysiology. 1991;28:43–53. doi: 10.1111/j.1469-8986.1991.tb03385.x. [DOI] [PubMed] [Google Scholar]
- Richards JE, Casey BJ. Development of sustained visual attention in the human infant. In: Campbell BA, Hayne H, editors. Attention and Information Processing in Infants and Adults: Perspectives from Human and Animal Research. Hillsdale, NJ: Erlbaum Publishing; 1992. pp. 30–60. [Google Scholar]
- Robbins TW, Everitt BJ. Arousal systems and attention. In: Gazzaniga MS, editor. Cognitive neurosciences. Cambridge, MA: MIT; 1995. pp. 703–720. [Google Scholar]
- Rose SA. Differential rates of visual information processing in fullterm and preterm infants. Child Development. 1983;54:1189–1198. [PubMed] [Google Scholar]
- Rose SA, Feldman JF. Memory and speed: Their role in the relation of infant information processing to later IQ. Child Development. 1997;68:630– 641. [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Infant visual recognition memory. Developmental Review. 2004;24:74– 100. doi: 10.1037/0012-1649.39.3.563. [DOI] [PubMed] [Google Scholar]
- Rose SA, Gottfried AW, Melloy-Carminar PM, Bridger WH. Familiarity and novelty preferences in infant recognition memory: Implications for information processing. Developmental Psychology. 1982;18:704–713. [Google Scholar]
- Ruff HA, Capozzoli MC. Development of attention and distractibility in the first 4 years of life. Developmental Psychology. 2003;39:877–890. doi: 10.1037/0012-1649.39.5.877. [DOI] [PubMed] [Google Scholar]
- Ruff HA, Rothbart MK. Attention in early development. New York: Oxford University Press; 1996. [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain research reviews. 2001;35(2):146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Moscovitch M. Infant memory. Springer; US: 1984. Infants, amnesics, and dissociable memory systems; pp. 173–216. [Google Scholar]
- Schiller PH. A model for the generation of visually guided saccadic eye movements. In: Rose D, Dobson VG, editors. Models of the visual cortex. New York: Wiley; 1985. pp. 62–70. [Google Scholar]
- Snyder K. Neural correlates of encoding predict infants’ memory in the paired-comparison procedure. Infancy. 2010;15:487–516. doi: 10.1111/j.1532-7078.2009.00015.x. [DOI] [PubMed] [Google Scholar]
- Snyder K, Garza J, Zolot L, Kresse A. Electrophysiological signals of familiarity and recency in the infant brain. Infancy. 2010;15:487–516. doi: 10.1111/j.1532-7078.2009.00021.x. [DOI] [PubMed] [Google Scholar]
- Snyder K, Webb SJ, Nelson CA. Theoretical and methodological implications of variability in infant brain response during a recognition memory paradigm. Infant Behavior and Development. 2002;25:466–494. [Google Scholar]
- Sokolov EN. Perception and the conditioned reflex. Oxford: Pergamon Press; 1963. [Google Scholar]
- Wan H, Aggleton JP, Brown MW. Different contributions of the hippocampus and perirhinal cortex to recognition memory. Journal of Neuroscience. 1999;19:1142–1148. doi: 10.1523/JNEUROSCI.19-03-01142.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Long JD, Nelson CA. A longitudinal investigation of visual event-related potentials in the first year of life. Developmental Science. 2005;8:605–616. doi: 10.1111/j.1467-7687.2005.00452.x. [DOI] [PubMed] [Google Scholar]
- Wiebe SA, Cheatham CL, Lukowski AF, Haight JC, Muehleck AJ, Bauer PJ. Infants’ ERP responses to novel and familiar stimuli change over time: Implications for novelty detection and memory. Infancy. 2006;9:21–44. [Google Scholar]
- Wiggs CL, Martin A. Properties and mechanisms of perceptual priming. Current opinion in neurobiology. 1998;8(2):227–233. doi: 10.1016/s0959-4388(98)80144-x. [DOI] [PubMed] [Google Scholar]
- Xiang JZ, Brown MW. Differential neuronal encoding of novelty, familiarity and recency in regions of the anterior temporal lobe. Neuropharmacology. 1998;37:657–676. doi: 10.1016/s0028-3908(98)00030-6. [DOI] [PubMed] [Google Scholar]
- Zeamer A, Heuer E, Bachevalier J. Developmental trajectory of object recognition in infant rhesus macaques with and without neonatal hippocampal lesions. The Journal of Neuroscience. 2010;30:9157– 9165. doi: 10.1523/JNEUROSCI.0022-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XO, Brown MW, McCabe BJ, Aggleton JP. Effects of the novelty or familiarity of visual stimuli on the expression of the intermediate early gene c-fos in the rat brain. Neuroscience. 1995;69:821–829. doi: 10.1016/0306-4522(95)00320-i. [DOI] [PubMed] [Google Scholar]
- Zola SM, Squire LR, Teng E, Stefanucci L, Buffalo EA, Clark RE. Impaired recognition memory in monkeys after damage limited to the hippocampal region. The Journal of Neuroscience. 2000;20(1):451–463. doi: 10.1523/JNEUROSCI.20-01-00451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]