Abstract
Objective
To examine cognitive function in individuals with traumatic brain injury (TBI), prior to and following participation in an aerobic exercise training program.
Design
Pre-post intervention study.
Setting
Medical research center.
Participants
Volunteer sample of individuals (n = 7; Age: 33.3 ± 7.9 years; mean ± SD) with chronic non-penetrating TBI (Injury Severity: 3 Mild, 4 Moderate; Time since most current injury: 4.0 ± 5.5 years) that were ambulatory.
Intervention
12-weeks of supervised vigorous aerobic exercise training performed 3 times a week for 30 minutes on a treadmill.
Main Outcome Measures
Cognitive function was assessed using Trail Making Test (TMT-A and B) and the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). Sleep quality and depression were measured with the Pittsburg Sleep Quality Index (PSQI) and Beck’s Depression Inventory (BDI-II). Indices of cardiorespiratory fitness were used to examine the relationship between improvements in cognitive function and cardiorespiratory fitness.
Results
After training, improvements in cognitive function were observed with greater scores on the TMT-A (+10.3 ± 6.8; P=.007), TMT-B (+9.6 ± 7.0; P=.011), and total scale RBANS (+13.3 ± 9.3; P =.009). No changes were observed in measures of PSQI and BDI-II. The magnitude of cognitive improvements was also strongly related to the gains in cardiorespiratory fitness.
Conclusion
These findings suggest that vigorous aerobic exercise training may improve specific aspects of cognitive function in individuals with TBI, and cardiorespiratory fitness gains may be a determinant of these improvements.
Keywords: Brain Injuries, Cognition, Exercise, Neuropsychological Tests, Questionnaires
INTRODUCTION
Cognitive impairments among individuals who have sustained a traumatic brain injury (TBI) and can have profound effects on quality of life,1 psychosocial outcomes,2 family functioning3 and employment status.4,5 More than 50% of patients report cognitive problems several years after a major head injury6 and long-term cognitive dysfunction may persist even in patients with mild TBI.7
In recent years, aerobic exercise has gained attention for its neuroprotective effects and has been studied as an intervention in healthy older adults,8,9 and in those with cognitive impairments10–12 and other neurological disorders.13 Aerobic exercise is associated with various physiological adaptations that have positive effects on cortical function, including angiogenesis and neurogenesis.14 Evidence from animal studies suggests that cognitive deficits associated with TBI can be improved following exercise.15–19 In rodents with experimentally-induced TBI, better performance on the Morris water maze15–18 and step-down avoidance task19 was observed when exercised compared to being sedentary. This suggested that both learning and memory had improved with exercise. The expression of the brain-derived neurotrophic factor was also found to be increased in the hippocampus,15 which is an area associated with memory and learning.20
Despite encouraging findings in animal models, exercise has not been widely studied in individuals with TBI. To our knowledge, only a few prior studies have examined cognitive changes in patients with TBI following exercise training. These studies have found cognitive performance to either improve21,22 or remain unchanged.23 However, exercise per se was not the primary focus of intervention, and vastly different components of exercise were used in these studies. This included exercise in a virtual environment,21 at home with an audiotape,23 or captured by self-reported exercise habits.22 The varied exercise interventions used by previous studies make comparison and interpretation of the findings problematic.
We have previously demonstrated that individuals with chronic non-penetrating TBI were able to perform vigorous aerobic exercise training and obtain cardiorespiratory fitness benefits with attenuated self-reported fatigue.24 In the current study, a consecutive subset of these subjects performed neuropsychological testing prior to and following a vigorous 12 week aerobic exercise program. It was hypothesized that cognitive function would be improved in individuals with TBI following aerobic exercise training.
METHODS
Written consent was obtained from all subjects prior to study participation. This study was approved by the institutional review boards of all participating institutions and registered on clinicaltrials.gov (NCT01294332).
Subjects
The inclusion/exclusion criteria for subject enrollment have been reported previously.24 Briefly, subjects had a non-penetrating head injury of 6 months or greater, were ambulatory and had no present contraindications to exercise. Subjects were also sedentary prior to enrollment and had not participated in regular moderate to vigorous exercise. A physician specializing in TBI performed the history and physical to determine subject eligibility and classification of TBI severity using VA/DoD guidelines.25
Study Design
Subjects completed neuropsychological assessments and self-report questionnaires prior to performing a treadmill cardiopulmonary exercise test (CPET) to volitional exhaustion. The CPET was performed on a treadmill as described previously.24 Following baseline assessments, subjects participated in a supervised aerobic exercise training program for 12 weeks.24 Sessions were conducted on a treadmill 3 times a week, with subjects exercising at their target training heart rate range (70 – 80% heart rate reserve) for 30 minutes. All baseline assessments were repeated following completion of the training program.
Measures of cardiorespiratory fitness such as peak O2 consumption (VO2), peak work rate (WR) and VO2 at the anaerobic threshold (AT-VO2) were collected during the CPET as described elsewhere.24 The neuropsychological assessments performed included the Trail Making Test (TMT)26 and the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS).27 The TMT Part A (TMT-A) measures speed of information processing and Part B (TMT-B) measures select aspects of executive functioning.28 Demographically corrected T-scores were obtained and adjusted based on age, gender and education level.29 The RBANS is a brief screening test that evaluates abilities across 5-specific cognitive domains. A total scale index score is derived from the domain-specific index scores and higher scores are indicative of better performance. The Pittsburg Sleep Quality Index (PSQI)30 was used to measure sleep quality and a score greater than 5 was used as a cut-off to indicate significant sleep disturbance.30 The Beck Depression Inventory, version 2 (BDI-II)31 was used to measure the severity of symptoms related to depression. The presence of depression was indicated by cut-off scores of 19 for those with mild TBI, and 35 for moderate to severe TBI.32
Statistical Analysis
Data were analyzed using SPSS version 21a. The neuropsychological assessments, cardiorespiratory fitness measures and questionnaires were compared using a paired sample t-test. A Pearson correlation was also performed between the main outcomes of the CPET and neuropsychological assessments. Significance was set at P < .05. Data are presented as mean ± one standard deviation (SD).
RESULTS
Nine subjects enrolled in the study and performed baseline neuropsychological testing prior to beginning the exercise program. As previously reported,24 two of the subjects were administratively withdrawn from the study due to pregnancy (n=1) and for non-compliance (n=1). Complete data was available and analyzed for the 7 remaining subjects.
Characteristics of the subjects that completed participation are shown in Table 1. Subjects demonstrated high adherence to the 12 week training program (93% ± 5% attendance) and were able to average between 74% and 80% of their target training heart rate (78% ± 3%). Exercise was performed at or above their target heart rate range for 99% ± 2% of the aerobic exercise time. Due to personal schedules and highly flexible session availabilities, subjects rarely exercised together with other subjects in this study. The supervised nature of the program resulted in significant interaction with research personnel. There were no serious adverse events reported during this study, however minor lower limb musculoskeletal injuries were observed, which was expected in sedentary individuals participating in an exercise program.33 Cardiorespiratory fitness was improved following the aerobic exercise training program, indicated by greater peak VO2 (+2.2 ± 2.3 ml/min/kg; P = .044), peak work rate (+72 ± 47 W; P = .007) and VO2 at AT (+2.8 ± 1.8 ml/min/kg; P = .006).
Table 1.
Subject Characteristics
| Subject | Gender | Age (years) | BMI (kg/m2) | TBI Severity* | Time Since Most Current Injury (years) | Education (years) |
|---|---|---|---|---|---|---|
| 1 | M | 35 | 23 | Moderate | 1.0 | 14 |
| 2 | F | 42 | 25 | Moderate | 1.1 | 16 |
| 3 | F | 29 | 31 | Moderate | 13.8 | 18 |
| 4 | F | 34 | 24 | Mild | 9.9 | 16 |
| 5 | M | 23 | 22 | Mild | 0.3 | 18 |
| 6 | F | 44 | 19 | Mild | 1.1 | 20 |
| 7 | F | 26 | 21 | Mild | 0.6 | 16 |
|
| ||||||
| Mean (±SD) |
33.3 (7.9) |
23.6 (3.8) |
4.0 (5.5) |
16.9 (2.0) |
||
M, male; F, Female; BMI, Body Mass Index;
TBI severity as defined by VA/DoD (Department of Veterans Affairs/Department of Defense) Clinical Practice Guidelines.25
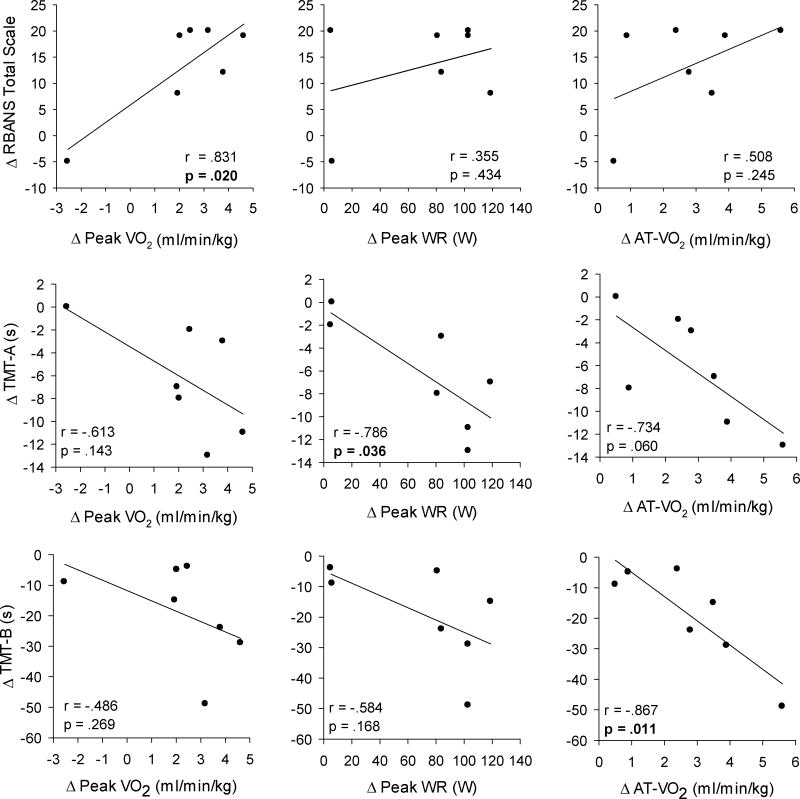
Table 2 displays results of the neuropsychological assessments and self-report questionnaires, with correlations for changes in cognitive outcomes and indices of cardiorespiratory fitness shown in Figure 1. The standardized t-scores for the TMT was significantly higher after exercise training, with improvements of 25% ± 18% (P = .007) and 26% ± 24% (P=.001) observed in TMT-A and TMT-B, respectively. Compared to baseline, an increase of 15% ± 10% (P =.009) in the total RBANS scale was observed after training indicating significant improvement in overall cognitive function. A significant improvement in 3 of the 5 specific domains related to visuospatial/constructional, language and delayed memory were also observed after exercise training. There were no changes in the PSQI and BDI-II total scores following exercise training. Significant correlations were observed for the change in peak VO2 and RBANS total scale (r = .831, P = .020), change in peak WR and TMT-A (r = −.786, P = .036), and change in AT-VO2 and TMT-B (r = −.867, P = 0.011).
Table 2.
Results of the Neuropsychological Assessments and Self-Reported Questionnaires at Pre- and Post-Aerobic Exercise Training
| Pre | Post | Δ | p-value | |
|---|---|---|---|---|
| Neuropsychological Assessments | ||||
| TMT t-score | ||||
| Part A | 46.9 (15.9) | 57.1 (14.4) | +10.3 (6.8) | 0.007 |
| Part B | 49.6 (16.7) | 59.1 (11.4) | +9.6 (7.0) | 0.011 |
| RBANS Index Score | ||||
| Immediate Memory | 94.9 (18.7) | 103.6 (13.9) | +8.7 (20.2) | 0.298 |
| Visuospatial/Constructional | 85.0 (16.6) | 97.0 (16.8) | +12.0 (7.2) | 0.004 |
| Language | 95.3 (17.3) | 107.7 (15.6) | +12.4 (12.9) | 0.043 |
| Attention | 101.0 (17.5) | 104.3 (21.1) | +3.3 (12.1) | 0.499 |
| Delayed Memory | 93.0 (10.6) | 103.4 (11.8) | +10.4 (5.7) | 0.003 |
| Total Scale | 92.0 (17.3) | 105.3 (19.7) | +13.3 (9.3) | 0.009 |
| Self-report Questionnaires | ||||
| PSQI total score | 4.6 (1.4) | 3.7 (2.5) | −0.9 (1.7) | 0.225 |
| BDI-II total score | 7.7 (7.3) | 4.6 (4.3) | −3.1 (4.9) | 0.139 |
Figure 1.
The relationship between changes in cognitive function (TMT-A, TMT-B, RBANS) and cardiorespiratory fitness (peak VO2, peak WR, AT-VO2) following aerobic exercise training are shown for all subjects. TMT Part A and B are shown in seconds, where better performance is indicated by less time taken to complete the task. Bold values indicate significant correlations (P <.05).
DISCUSSION
Following participation in a supervised vigorous aerobic exercise training program, individuals with TBI showed significant improvements in cognitive function. These improvements were observed in the domains of processing speed, aspects of executive functioning, as well as overall cognitive function. The lack of change in self-reported measures of sleep quality and symptoms of depression suggest that improvements in cognition were primarily related to aerobic exercise training. Indicators of cardiorespiratory fitness were strongly related to aspects of cognitive function. Overall, the results of this study suggest that in these individuals with TBI, exercise-induced improvements in cognitive function could be gained from participation in vigorous aerobic exercise training. The magnitude of these improvements may also be influenced by the degree to which the cardiorespiratory system adapts to training.
The effect of aerobic exercise training on cognitive function in individuals with TBI has not been sufficiently studied, with only 3 previous studies on this topic identified.21–23 Unfortunately, all three studies used different methods of exercise for the training intervention, thus precluding an accurate comparison of their results. Nevertheless, two21,22 of the three studies reported improved cognitive function following participation in light to moderate intensity exercise, thus supporting the concept that cognition was modifiable with exercise in individuals with TBI. The current study utilized vigorous exercise intensity with a longer program length than previous studies, finding not only improvements in cognitive function but also a strong association between these improvements and increases in cardiorespiratory fitness. Thus, these results support the existence of physiologically adaptable mediators or moderators of cognitive improvement in individuals who have TBI.
In individuals with TBI, sleep disturbance and depression can negatively affect cognitive performance.34,35 Pharmacological treatment of depression appears to improve cognitive performance.36 The subjects in this study did not report significant sleep abnormalities or symptoms of depression at baseline, and changes in sleep quality or depression scores were not observed following exercise training. This supports the contention that improvements in cognitive function in this study were not related to changes in sleep quality or depressive symptoms.
The strong association between the improvements in cardiorespiratory fitness and cognitive function was similar to previous observations in older adults37 and patients with stroke.38 Since gains in cardiorespiratory fitness are directly related to training intensity and frequency,39 more vigorous exercise training may offer greater improvements in cognition. There are currently no specific exercise prescription criteria for eliciting optimal cognitive improvements. The exercise regimen used in this study followed recommendations for the general population for improving cardiorespiratory fitness40 and was targeted at the middle to upper limit of the recommended range. The exercise-training regimen was well tolerated by the participants, with no serious adverse events.24
Study Limitations
First, the absence of a control arm and lack of randomization makes it unclear as to the extent spontaneous recovery may have contributed to improvements in cognition. The rate of cognitive recovery in individuals with TBI is largely dependent on the severity of their injury. For example, recovery generally occurs within 3 to 6 months in those with mild TBI, but may take up to 5 years for more severe injuries.7 The subjects in this study had mild to moderate TBI but the window of time since injury was between 4 months and 13 years. Of note, the two subjects that were several years past their injuries appeared to gain as much cognitive improvement as those who were less than one year.
Secondly, the number of subjects enrolled was small and this limits our ability to generalize our results. Our subjects had generally fewer depressive symptoms and sleep disturbance than might ordinarily be observed in a typical cohort of individuals with TBI. This may have prevented measurable changes from being further observed (i.e., floor effects). Given that depression and sleep dysfunction adversely affects cognitive performance,34,35 more symptomatic subjects might respond even more favorably to exercise training.
Thirdly, the social interaction that occurs with participation in an exercise program was not controlled or accounted for in this study. This may also have contributed towards the observed cognitive improvements. Previous studies have shown that greater social engagement in the form of support and relationships are associated with better cognitive function in older adults and may be protective against cognitive decline.41 However, the strong correlation between cognitive performance and cardiorespiratory fitness suggest that the role of cardiorespiratory fitness gains were a major contributor to cognitive improvements, explaining for as much as 75% of the variance.
Lastly, it is hard to speculate how more cognitively impaired subjects might respond to exercise intervention. Subjects in the present study were highly educated (average education level of 17 years), with 4 of the 7 subjects scoring average or better on cognitive assessments at baseline. Therefore, the ceiling effect of the cognitive testing instruments,42 together with the subject’s relatively high level of baseline functioning may have limited the ability to fully detect the magnitude of change as a result of the training regimen, and subsequent impact on the individual’s functional performance.
CONCLUSION
Aerobic exercise training was found to improve cognitive function in this small group of individuals with non-penetrating TBI. Exercise-induced improvements were observed in aspects of executive functioning, processing speed and overall cognition. Improvements in cognitive function were related to increases in cardiorespiratory fitness. These findings are encouraging and demonstrate the need for larger randomized trials aimed at further characterizing the relationship between cardiorespiratory fitness and cognitive function in individuals who have TBI, and the degree to which cognitive deficits are modifiable through an exercise intervention.
Highlights.
Cognitive function was examined in persons with TBI before and after exercise training
Supervised aerobic exercise training was performed for 12 weeks on a treadmill
Improved cognitive function was observed following exercise training
Improvements in cognition were related to changes in physical performance measures
Acknowledgments
This study was supported by the Center for Neuroscience and Regenerative Medicine [G192HI-H] and the National Institutes of Health Clinical Center [Rehabilitation Medicine Department Intramural Funds]. All authors have no conflict of interest to declare. We would like to thank Eric Christensen, Bart Drinkard, Anne Quinn and Joshua Woolstenhulme for their assistance with the exercise training sessions and data collection. We also appreciate the assistance provided by Dr. Christian Shenouda with the subjects and patient enrollment.
List of Abbreviations
- AT
Anaerobic Threshold
- AT-VO2
O2 consumption at the anaerobic threshold
- BDI-II
Beck’s Depression Inventory Version 2
- CPET
Cardiopulmonary Exercise Test
- PSQI
Pittsburg Sleep Quality Index
- RBANS
Repeatable Battery for the Assessment of Neuropsychological Status
- TBI
Traumatic Brain Injury
- TMT
Trail Making Test
- TMT-A
Trail Making Test Part A
- TMT-B
Trail Making Test Part B
- VO2
O2 consumption
- WR
Work rate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Suppliers List
a. SPSS Version 21; IBM Corp., 1 New Orchard Road, Armonk, New York, 10504
References
- 1.Klonoff PS, Costa LD, Snow WG. Predictors and indicators of quality of life in patients with Closed-Head injury. J Clin Exp Neuropsychol. 1986;8(5):469–485. doi: 10.1080/01688638608405171. [DOI] [PubMed] [Google Scholar]
- 2.Ross SR, Millis SR, Rosenthal M. Neuropsychological prediction of psychosocial outcome after traumatic brain injury. Appl Neuropsychol. 1997;4(3):165–170. doi: 10.1207/s15324826an0403_4. [DOI] [PubMed] [Google Scholar]
- 3.Anderson MI, Parmenter TR, Mok M. The relationship between neurobehavioural problems of severe traumatic brain injury (TBI), family functioning and the psychological well-being of the spouse/caregiver: path model analysis. Brain Inj. 2002;16(9):743–757. doi: 10.1080/02699050210128906. [DOI] [PubMed] [Google Scholar]
- 4.Sherer M, Novack TA, Sander AM, Struchen MA, Alderson A, Thompson RN. Neuropsychological assessment and employment outcome after traumatic brain injury: A review. Clin Neuropsychol. 2002;16(2):157–178. doi: 10.1076/clin.16.2.157.13238. [DOI] [PubMed] [Google Scholar]
- 5.Benedictus MR, Spikman JM, van der Naalt J. Cognitive and behavioral impairment in traumatic brain injury related to outcome and return to work. Arch Phys Med Rehabil. 2010;91(9):1436–1441. doi: 10.1016/j.apmr.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 6.Van Balen HGG, Mulder T, Keyser A. Towards a disability-oriented epidemiology of traumatic brain injury. Disabil Rehabil. 1996;18(4):181–190. doi: 10.3109/09638289609166298. [DOI] [PubMed] [Google Scholar]
- 7.Rabinowitz AR, Levin HS. Cognitive sequelae of traumatic brain injury. Psychiatr Clin North Am. 2014;37(1):1–11. doi: 10.1016/j.psc.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith PJ, Blumenthal JA, Hoffman BM, et al. Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hindin SB, Zelinski EM. Extended practice and aerobic exercise interventions benefit untrained cognitive outcomes in older adults: a meta-analysis. J Am Geriatr Soc. 2012 Jan;60(1):136–141. doi: 10.1111/j.1532-5415.2011.03761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86(9):876–884. doi: 10.4065/mcp.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gates N, Fiatarone Singh MA, Sachdev PS, Valenzuela M. The effect of exercise training on cognitive function in older adults with mild cognitive impairment: a meta-analysis of randomized controlled trials. Am J Geriatr Psychiatry. 2013;21(11):1086–1097. doi: 10.1016/j.jagp.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 12.Farina N, Rusted J, Tabet N. The effect of exercise interventions on cognitive outcome in Alzheimer’s disease: a systematic review. Int Psychogeriatr. 2014;26(1):9–18. doi: 10.1017/S1041610213001385. [DOI] [PubMed] [Google Scholar]
- 13.McDonnell MN, Smith AE, Mackintosh SF. Aerobic exercise to improve cognitive function in adults with neurological disorders: A systematic review. Arch Phys Med Rehabil. 2011;92(7):1044–1052. doi: 10.1016/j.apmr.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 14.Cotman CW, Berchtold NC, Christie L-A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Griesbach GS, Hovda DA, Molteni R, Wu A, Gomez-Pinilla F. Voluntary exercise following traumatic brain injury: Brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004;125(1):129–139. doi: 10.1016/j.neuroscience.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 16.Griesbach GS, Hovda DA, Gomez-Pinilla F. Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNF activation. Brain Res. 2009;1288:105–115. doi: 10.1016/j.brainres.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu A, Ying Z, Gomez-Pinilla F. Exercise facilitates the action of dietary DHA on functional recovery after brain trauma. Neuroscience. 2013;248:655–663. doi: 10.1016/j.neuroscience.2013.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itoh T, Imano M, Nishida S, et al. Exercise inhibits neuronal apoptosis and improves cerebral function following rat traumatic brain injury. J Neural Transm. 2011;118(9):1263–1272. doi: 10.1007/s00702-011-0629-2. [DOI] [PubMed] [Google Scholar]
- 19.Kim D-H, Ko I-G, Kim B-K, et al. Treadmill exercise inhibits traumatic brain injury-induced hippocampal apoptosis. Physiol Behav. 2010;101(5):660–665. doi: 10.1016/j.physbeh.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 20.Stuchlik A. Dynamic learning and memory, synaptic plasticity and neurogenesis: an update. Front Behav Neurosci. 2014;8:106. doi: 10.3389/fnbeh.2014.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grealy MA, Johnson DA, Rushton SK. Improving cognitive function after brain injury: The use of exercise and virtual reality. Arch Phys Med Rehabil. 1999;80(6):661–667. doi: 10.1016/s0003-9993(99)90169-7. [DOI] [PubMed] [Google Scholar]
- 22.Gordon WA, Sliwinski M, Echo J, McLoughlin M, Sheerer MS, Meili TE. The benefits of exercise in individuals with traumatic brain injury: a retrospective study. J Head Trauma Rehabil. 1998;13(4):58–67. doi: 10.1097/00001199-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 23.McMillan T, Robertson IH, Brock D, Chorlton L. Brief mindfulness training for attentional problems after traumatic brain injury: A randomised control treatment trial. Neuropsychol Rehabil. 2002;12(2):117–125. [Google Scholar]
- 24.Chin LMK, Chan L, Woolstenhulme JG, Christensen EJ, Shenouda CN, Keyser RE. Improved cardiorespiratory fitness with aerobic exercise training in individuals with traumatic brain injury. J Head Trauma Rehabil. 2014 doi: 10.1097/HTR.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury. J Rehabil Res Dev. 2009;46(6):Cp1–68. [PubMed] [Google Scholar]
- 26.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Tucson, AZ: Neuropsychology Press; 1993. [Google Scholar]
- 27.Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 28.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 29.Heaton RK, Miller SW, Taylor MJ, Grant I. Revised Comprehensive Norms for An Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults: Professional Manual. Lutz, FL: Psychological Assessment Resources; 2004. [Google Scholar]
- 30.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 31.Beck A, Steer R, Brown G. Manual for the Beck Depression Inventory II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 32.Homaifar BY, Brenner LA, Gutierrez PM, et al. Sensitivity and specificity of the Beck Depression Inventory-II in persons with traumatic brain injury. Arch Phys Med Rehabil. 2009;90(4):652–656. doi: 10.1016/j.apmr.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hootman JM, Macera CA, Ainsworth BE, Addy CL, Martin M, Blair SN. Epidemiology of musculoskeletal injuries among sedentary and physically active adults. Med Sci Sports Exerc. 2002;34(5):838–844. doi: 10.1097/00005768-200205000-00017. [DOI] [PubMed] [Google Scholar]
- 34.Mahmood O, Rapport LJ, Hanks RA, Fichtenberg NL. Neuropsychological performance and sleep disturbance following traumatic brain injury. J Head Trauma Rehabil. 2004;19(5):378–390. doi: 10.1097/00001199-200409000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Rapoport MJ, McCullagh S, Shammi P, Feinstein A. Cognitive impairment associated with major depression following mild and moderate traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2005;17(1):61–65. doi: 10.1176/jnp.17.1.61. [DOI] [PubMed] [Google Scholar]
- 36.Fann JR, Uomoto JM, Katon WJ. Cognitive improvement with treatment of depression following mild traumatic brain injury. Psychosomatics. 2001;42(1):48–54. doi: 10.1176/appi.psy.42.1.48. [DOI] [PubMed] [Google Scholar]
- 37.Voss MW, Heo S, Prakash RS, et al. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: Results of a one-year exercise intervention. Hum Brain Mapp. 2013;34(11):2972–2985. doi: 10.1002/hbm.22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kluding PM, Tseng BY, Billinger SA. Exercise and executive function in individuals with chronic stroke: a pilot study. J Neurol Phys Ther. 2011;35(1):11–17. doi: 10.1097/NPT.0b013e318208ee6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wenger HA, Bell GJ. The interactions of intensity, frequency and duration of exercise training in altering cardiorespiratory fitness. Sports Med. 1986;3(5):346–356. doi: 10.2165/00007256-198603050-00004. [DOI] [PubMed] [Google Scholar]
- 40.Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 41.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3(6):343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 42.Banks S. Ceiling Effect. In: Kreutzer J, DeLuca J, Caplan B, editors. Encyclopedia of Clinical Neuropsychology. New York, NY: Springer New York; 2011. pp. 506–507. [Google Scholar]