Abstract
Pathological fear and anxiety are highly debilitating and, despite considerable advances in psychotherapy and pharmacotherapy they remain insufficiently treated in many patients with PTSD, phobias, panic and other anxiety disorders. Increasing preclinical and clinical evidence indicates that pharmacological treatments including cognitive enhancers, when given as adjuncts to psychotherapeutic approaches [cognitive behavioral therapy including extinction-based exposure therapy] enhance treatment efficacy, while using anxiolytics such as benzodiazepines as adjuncts can undermine long-term treatment success. The purpose of this review is to outline the literature showing how pharmacological interventions targeting neurotransmitter systems including serotonin, dopamine, noradrenaline, histamine, glutamate, GABA, cannabinoids, neuropeptides (oxytocin, neuropeptides Y and S, opioids) and other targets (neurotrophins BDNF and FGF2, glucocorticoids, L-type-calcium channels, epigenetic modifications) as well as their downstream signaling pathways, can augment fear extinction and strengthen extinction memory persistently in preclinical models. Particularly promising approaches are discussed in regard to their effects on specific aspects of fear extinction namely, acquisition, consolidation and retrieval, including long-term protection from return of fear (relapse) phenomena like spontaneous recovery, reinstatement and renewal of fear. We also highlight the promising translational value of the preclinial research and the clinical potential of targeting certain neurochemical systems with, for example d-cycloserine, yohimbine, cortisol, and L-DOPA. The current body of research reveals important new insights into the neurobiology and neurochemistry of fear extinction and holds significant promise for pharmacologically-augmented psychotherapy as an improved approach to treat trauma and anxiety-related disorders in a more efficient and persistent way promoting enhanced symptom remission and recovery.
Keywords: Fear extinction, Exposure therapy, Augmented relearning, Reconsolidation, Drug development, Cognitive enhancer
1. Introduction
Fear, anxiety and trauma-related disorders are associated with excessive fear reactions triggered by specific objects, situations or internal and external cues in the absence of any actual danger, and often include an inability to extinguish learned fear and to show adequate safety learning [(Jovanovic et al., 2012; Michael et al., 2007; Milad et al., 2009; Milad et al., 2013; Wessa & Flor, 2007) reviewed in (Holmes & Singewald, 2013) and (Kong et al., 2014)]. Pathological fear and anxiety occur in a range of psychiatric conditions, including various types of phobia (e.g. social phobia, agoraphobia or specific phobia), panic disorder with/without agoraphobia, obsessive–compulsive disorder (OCD), generalized anxiety (GAD) and post-traumatic stress disorder (PTSD) (DSM-5, 2013; ICD-10, 1994). These disorders comprise the most common mental disorders and are estimated to have a life-time prevalence of up to 28% among western populations (Kessler et al., 2005; Kessler et al., 2012; Wittchen et al., 2011). In addition to the personal suffering of patients, the economic burden caused by anxiety disorders is heavy (Gustavsson et al., 2011).
Available pharmacological and psychotherapeutic treatments (Bandelow et al., 2007) which aim to reduce fear and anxiety are associated with decreased symptom severity, but up to 40% of anxiety patients show only partial long-term benefit, and a majority of them fail to achieve complete remission (Bandelow et al., 2012; Hoffman & Mathew, 2008; Stein et al., 2009) clearly underlining the need for further improvement. Current pharmacological approaches either induce rapid anxiolytic effects (e.g. benzodiazepines, some antipsychotics) or require prolonged, chronic treatment (e.g. antidepressants) to attenuate symptoms of pathological fear and anxiety. Commonly employed psychotherapeutic interventions apply cognitive behavioral strategies and exposure techniques to help patients overcome the maladaptive beliefs and avoidance behaviors that reinforce the pathology related to fear-eliciting cues. Meta-analyses show that cognitive behavioral therapy (CBT) does have efficacy for several anxiety disorders, including PTSD, but patients have difficulty bearing the demanding and exhausting process of therapy and many who do manage to cope with it respond only partially and often relapse with time (Choy et al., 2007).
One strategy to improve CBT is to augment psychotherapy with ad-junctive pharmacological treatments. Early attempts at combining ‘CBT’ with anxiolytic medications [e.g. benzodiazepines (BZD)] showed that the combination was no more effective [in some instances even counterproductive (Marks et al., 1993; Wilhelm & Roth, 1997)] than psycho- or pharmacotherapy alone [for details see (Dunlop et al., 2012; Hofmann, 2012; Otto et al., 2010a,b; Rodrigues et al., 2011)]. However, at least in some cases, this failure may have reflected idiosyncratic effects of the drugs tested (especially BZDs) rather than utility of the strategy itself, and there has been an intense search to identify agents that serve as more effective adjuncts to CBT. The preclinical assay most frequently used in this search is fear extinction – the focus of this current review. Extinction of fear following Pavlovian fear learning (Pavlov, 1927) in animals is procedurally similar to exposure-based CBT (Milad & Quirk, 2012). We will briefly outline different aspects of Pavlovian fear learning [ (which is thought to be involved in the etiology and maintenance of anxiety disorders, e.g. (Amstadter et al., 2009)] and extinction highlighting the key processes that could be targeted to augment fear extinction (see Fig. 1 for an overview).
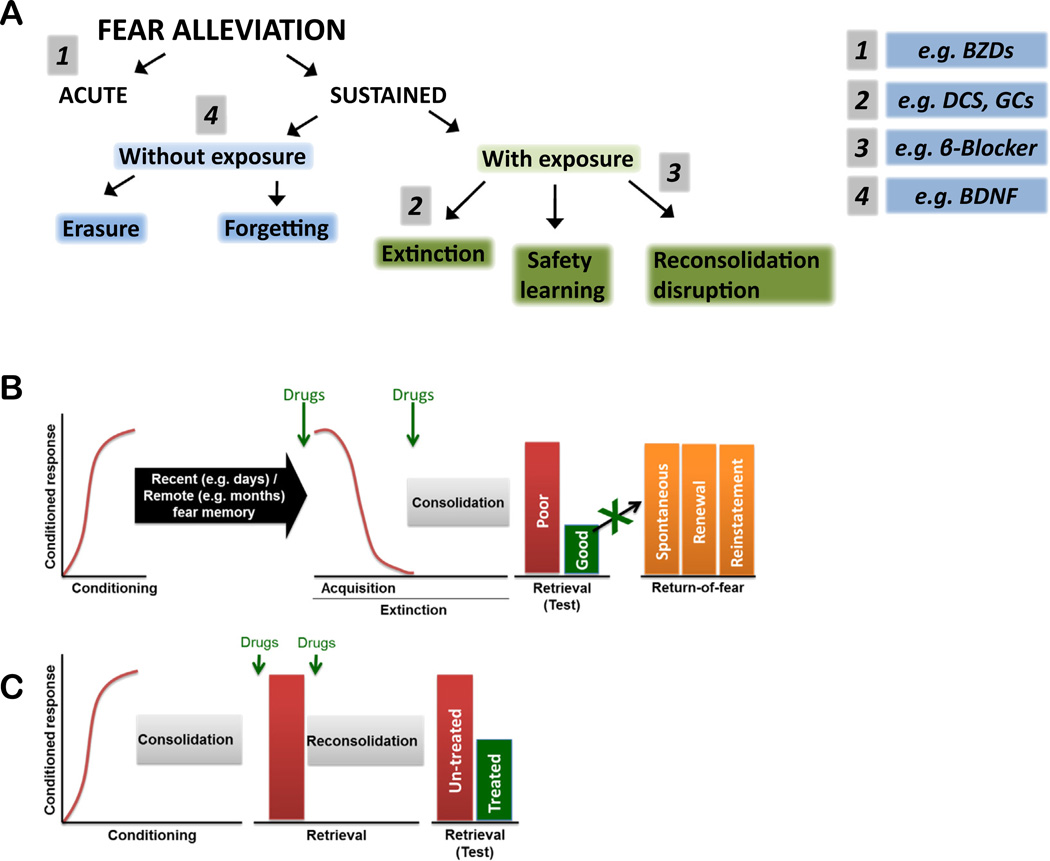
Fig. 1.
Different modes of fear alleviation and sites of possible pharmacological intervention. A, Fear alleviation is mediated via different mechanisms leading to acute or sustained fear relief. Sustained fear relief can be obtained either with exposures to the feared cues/situations or, in some instances, also without them For a detailed description of the involved mechanisms, see Riebe et al. (2012). Pharmacological interventions to boost fear relief can target different mechanisms at various levels. Examples are given with numbers 1–4, for detail see text B, Fear conditioning represents a training phase in which a novel conditioned stimulus (CS) is paired with an unconditioned stimulus (US) (redline). Throughout fear training and testing, fear is measured as a conditioned response (y-axis), typically freezing, fear-potentiated startle, increased heart rate, and other quantifiable behavioral measures of fear. Following this training period, mice undergo a consolidation phase which transfers the labile newly formed fear memory into a stable long-term memory. Fear can be extinguished by repeated presentations of the CS (without the US; red line) resulting in fear extinction. Following the extinction training session, and akin to fear learning, consolidation processes are initiated to stabilize this labile fear extinction memory into a long-term memory. Poor extinction is evidenced by high fear responding (red bar), and successful extinction retrieval is shown by reduced fear (green) during a retrieval test. Extinguished fear can recover via three main mechanisms: spontaneous recovery (recovery of extinguished fear responses occurs with the passage of time in the absence of any further training), fear renewal (when the conditioned CS is presented outside the extinction context, for example the conditioning or novel contexts), and fear reinstatement (when un-signaled presentations of the US are interposed between the completion of extinction training and a subsequent retention test). Drugs (green arrows) can be administered either immediately prior to (to induce extinction acquisition also called ‘within-session extinction’ and/or extinction consolidation) or immediately following (to rescue/ boost extinction consolidation processes) the training session to modulate extinction mechanisms. A clinical aim of drug augmentation strategies is to promote good extinction retrieval and to protect against return-of-fear phenomena to provide good ‘longterm extinction’. C, Fear memory can be reactivated (red bar) by presentation of the CS, which transfers the stabilized memory into a labile phase which requires reconsolidation (a process by which previously consolidated memories are stabilized after fear retrieval). The fear memory can then be tested during another retrieval test (red bar) to assess reconsolidation. Drugs (green arrow) can be administered either immediately prior to or after the retrieval session to modulate reconsolidation mechanisms. This effect can then be tested during subsequent retrieval tests. Evidence for successful interference with reconsolidation of the original fear memory is revealed in reduced freezing during the test session. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
1.1. Fear and fear extinction
Experimentally, fear conditioning occurs when a previously neutral stimulus [conditioned stimulus (CS) – such as a tone or light] is paired with an aversive, unconditioned stimulus (US – e.g. electric shock to the forearm in humans, mild foot shock in rodents), resulting in a CS-US association whereby the CS alone elicits a conditioned fear response (e.g. freezing in rodents or increased skin conductance in humans). Following a successful CS–US association, fear memories require consolidation, a process involving a cascade of molecular and cellular events that alter synaptic efficacy, as well as a prolonged systems level interaction between brain regions, to stabilize the memory (McGaugh, 2000). Once consolidated, fear memories, reactivated by presenting the CS, are destabilized to render the original fear memory liable to pharmacological/ behavioral interference (see overview in Fig. 1) and this is then followed by a second phase of molecular and cellular events to re-stabilize (re-consolidate) the (adapted) memory (Nader et al., 2000).
Fear memories can be attenuated by various processes and interventions (including pharmacological and psychological approaches), some producing temporary blunting of fear behaviors and others causing more long-lasting relief. Interfering with the re-consolidation by inhibiting molecular and cellular events supporting fear memory re-stabilization [Fig. 1C, (Lee et al., 2006; Nader et al., 2000)], for example with β-adrenoceptor blockers (Debiec & Ledoux, 2004; Kindt et al., 2009), has been proposed as a clinical approach to alleviating fear memories. For discussion on this and other means of reducing fear (e.g. via US habituation) we refer the reader to some excellent prior reviews (Graham et al., 2011; Schwabe et al., 2014). Other potential ways to relieve fear include safety learning (Kong et al., 2014; Rogan et al., 2005) and erasure-like mechanisms such as destruction of erasure-preventing perineuronal nets (Gogolla et al., 2009).
Alternatively, fear memories can also be extinguished. Fear extinction, a process originally described by Pavlov (Pavlov, 1927), entails repeated exposure to anxiety-provoking cues to establish a new memory that counters the original fear memory. The process is highly relevant to fear, anxiety and trauma-related disorders which are associated with negative emotional reactions triggered by specific objects, situations or internal and external cues that are excessive to the actual danger posed. Moreover, extinction in animals is procedurally similar to forms of CBT that rely on exposure to anxiety-provoking cues (see Fig. 1) (Milad & Quirk, 2012), and anxiety disorders are associated with an inability to extinguish learned fear and to respond adequately to safety signals (Jovanovic et al., 2012; Michael et al., 2007; Milad et al., 2009; Milad et al., 2013; Wessa & Flor, 2007) [reviewed in (Holmes & Singewald, 2013)]. Thus, fear extinction has considerable translational utility. The key processes that can be targeted to pharmacologically augment fear extinction are summarized in Fig. 1.
Extinction is a learning process driven by violation of the original CS=US contingency (termed ‘prediction error’ for review see (Pearce & Bouton, 2001; McNally & Westbrook, 2006) ], but it also contains other elements including habituation and desensitization some also say era-sure/destabilization (Lin et al., 2011). Some authors therefore favor the term ‘relearning’ over extinction [for discussion see (Riebe et al., 2012)]. As with fear learning, extinction occurs in two phases: extinction acquisition and extinction consolidation. The decrement in the fear response during’extinction training’ is also termed ‘within-session extinction’. Similar to fear memory, stabilization of the extinction memory requires both a cascade of overlapping, but dissociable, molecular and cellular events [for review see (Myers & Davis, 2007; Orsini & Maren, 2012) ] that alter synaptic efficacy and brain systems-level interactions (Pape & Pare, 2010). The strength of extinction memory can be assessed at some interval (usually >1 day) after extinction training in “extinction retrieval” sessions (also termed ‘extinction retention’ or ‘extinction expression’, but note that we use the term “extinction retrieval” throughout this review).
That extinction memories are prone to re-emergence (due to insufficient ‘longterm extinction’) indicates that the original fear memory is still in place and the extinction memory is weaker/more labile than the fear memory. This may be particularly true of older (‘remote’) fear memories (Tsai & Graff, 2014). The re-emergence of extinguished fear occurs under multiple circumstances: (i) renewal, when the CS is presented in a different context to that in which extinction training occurred; (ii) reinstatement, when the original US or another stress-or is given unexpectedly; and (iii) spontaneous recovery, when a significant period of time has elapsed following successful extinction training (Herry et al., 2010; Myers & Davis, 2007). The likelihood of fear re-emergence is dependent on the strength of the extinction memory which is also determined by the type of extinction protocol used see e.g. (Laborda & Miller, 2013; Li & Westbrook, 2008). Spontaneous recovery (Rowe & Craske, 1998a,b; Schiller et al., 2008), reinstatement (Schiller et al., 2008) and renewal (Effting & Kindt, 2007) are all observable in clinical settings, and can be readily exploited in the laboratory to identify drugs and other interventions that can prevent fear re-emergence in animals and relapse in humans (Vervliet et al., 2013).
Our goal in the current review is to offer a comprehensive overview of preclinical work on the possible pharmacological approaches (see Box 1 for an overview) to augment fear extinction and protect against the re-emergence of fear, and to discuss the potential translational value of these candidates as adjuncts to exposure-based CBT in anxiety patients.
Box 1 Why pharmacological augmentation of extinction?
A limitation of extinction-based exposure therapy is that patients can often relapse with the passage of time, with changes in context (out of the therapy context), or under conditions of stress or other provocations, such as experiencing trauma reminders. In the parlance of learning theory, extinction memories are labile and fragile. A key goal for pharmacotherapy, therefore, is to identify compounds that overcome this fragility, by bolstering the formation, persistence and possibly context independence of extinction memories. One approach to achieving this goal is to use drugs as adjuncts to exposure therapy (cognitive enhancers) as a way to augment the extinction learning process. In this review, we discuss the various neurochemical and molecular signaling pathways that have been targeted to this end. On the one hand, there remain significant challenges to overcome, including the selective targeting of extinction-related processes without concurrent effects on original fear memories. On the other hand, there are clearly a plethora of potentially promising avenues to pursue, and we are optimistic that real advances can be made in treating trauma-related disorders.
2. Neuronal substrates of fear extinction
Using a number of complementary techniques (including electro-physiology, immediate-early gene mapping, tracing studies, lesioning/ inactivation approaches and optogenetics) research is revealing the complex and interconnected brain circuitry mediating fear extinction. Several key brain areas including the amygdala (AMY), hippocampus (HPC), medial prefrontal cortex (mPFC), periaqueductal gray (PAG), bed nucleus of the stria terminalis and others have been implicated in extinction [for recent detailed reviews see (Duvarci & Pare, 2014; Ehrlich et al., 2009; Herry et al., 2010; Knapska et al., 2012; Myers & Davis, 2007; Orsini & Maren, 2012; Pape & Pare, 2010)]. Of these, we focus on the AMY, HPC and mPFC as major, well-defined components of the fear circuitry (see Fig. 2).
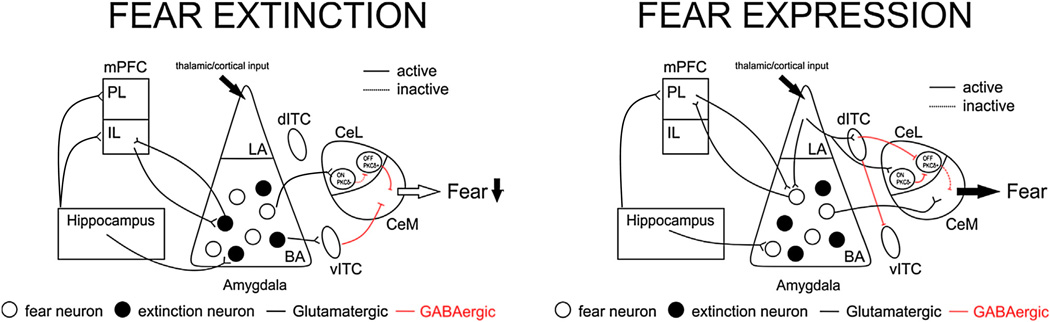
Fig. 2.
Anatomy of fear extinction and expression. Fear extinction and expression rely on neuronal processing in an anatomical circuitry centering on the AMY, mPFC, and HPC Glutamatergic and GABAergic neurons, among others, are important components of connectivity and regulation of fear. The AMY is critically involved in the expression of aversive (fear) memories. While fear neurons in the BA send excitatory projections directly to the centromedial AMY (CeM) driving expression of fear (right panel), during extinction (left panel), the infralimbic cortex (IL) inhibits CeM output by driving inhibitory ITC neurons. IL inputs might also synapse directly on “extinction” neurons within the BA. “Extinction” neurons can influence activity within the central AMY (CeA) through several routes, possibly by driving inhibitory ITC or CeL (off, PKC+) neurons that limit CeM activity. There is also a BA-CeL pathway contributing to ultimate inhibition of the CeM. The hippocampus is involved in contextual aspects of extinction via its projections to both the IL and the BA, among other brain regions. Hence, inhibitory memories built following extinction are encoded by the AMY and the mPFC and are modulated by the HPC. It is thought that extinction training and exposure therapy produce long-lasting changes in synaptic plasticity and interneuronal communication in this circuitry ultimately reducing fear responses via output stations including the CeM. For further details, the reader is refered to recent reviews of (Duvarci & Pare, 2014; Orsini & Maren, 2012).
While the AMY and the mPFC are crucial for the formation and maintenance of fear extinction memories, the HPC, linked with the mPFC and the AMY (Pape & Pare, 2010), processes contextual information linked with extinction (Orsini & Maren, 2012). Altering these hippocampal contributions to extinction memory or mimicking the hippocampal response within the extinction context (see below) may be mechanisms that render extinction context-independent. The AMY is a core hub in fear extinction processing. Data in rodents and humans suggest sustained AMY activity in extinction-impaired individuals, possibly resulting from a failure to engage pro-extinction circuits in cortical areas and subregions of the AMY [reviewed in (Holmes & Singewald, 2013)]. Different AMY subregions and neuronal populations have differential contributions to extinction. The centromedial AMY (CeM) is the major output station of the AMY that drives fear via its connections to the hypothalamus and brainstem regions (Fendt & Fanselow, 1999; LeDoux et al., 1988; Maren, 2001), and its responding is modulated following extinction via intra-amygdala and remote inputs. Extinction training has been shown to cause a rapid reduction of CS-evoked responses of lateral AMY (LA) neurons possibly via depotentiation of thalamic inputs (Duvarci & Pare, 2014). The basal AMY (BA) contains extinction-encoding neurons which drive GABAergic cells in the medial intercalated cell masses (ITC) and neurons in the centrolateral AMY (CeL) to inhibit the CeM and the expression of fear (Duvarci & Pare, 2014; Herry et al., 2008). Following from these initial findings, additional studies involving optogenetic approaches, have shown that a subpopulation of the BA pyramidal neurons which express the Thy1 gene may mark the extinction neuron subpopulation (Jasnow et al., 2013).
It is also becoming apparent that different ITCs are part of a GABAergic feed-forward relay station interconnecting AMY nuclei with distinct networks within the ITCs that are engaged in particular fear stages exerting different influences on extinction (Busti et al., 2011; Duvarci & Pare, 2014; Whittle et al., 2010). Separate neuronal populations in the CeL have been found to have contrasting roles in fear and fear extinction. CeL-OFF neurons (PKCδ+ phenotype) inhibit fear-promoting CeM neurons, while CeL-ON (PKCδ- phenotype) cells stimulate fear-promoting CeM neurons (Ciocchi et al., 2010; Haubensak et al., 2010). Additionally, a very recent finding suggests that a subpopulation of neurons within the CeM – that of the Tac2 peptide-expressing cells – is critically involved in fear learning and fear expression (Andero et al., 2014).
Another important node within the extinction circuitry is the mPFC, which shares strong interconnections with the HPC and the AMY. Fear extinction recruits the ventral segment of the mPFC, the infralimbic (IL) subdivision [the rodent correlate of the human ventromedial PFC (vmPFC)] which projects to BA extinction and ITC neurons and can thereby inhibit the activity of CeM fear output neurons [reviewed in (Amir et al., 2011; Senn et al., 2014)]. When a conditioned cue following extinction is presented in the extinction (therapy) context, the HPC activates the IL (an activation which is absent or less in a novel context), supporting subsequent CeM inhibition (Herry et al., 2010). The IL subdivision is involved in the consolidation of extinction memory and synaptic plasticity in this region is crucial for successful extinction retrieval (Mueller & Cahill, 2010). Impaired extinction has been associated with relatively low activity in IL neurons, and successful extinction with relatively high IL neuronal activity [reviewed in (Holmes & Singewald, 2013)]. The region of the mPFC neighboring the IL [the prelimbic cortex (PL) in rodents and the dorsal anterior cingulate in humans] plays an opposite role to the IL in regulating fear and extinction [reviewed in (Milad &Quirk, 2012)]. The PL interacts with BA fear neurons, augmenting output of the basolateral amygdaloid complex (BLA) and hence CeM activity (Senn et al., 2014).
There is now strong evidence from both animal and human studies that these various components of the neural circuit underlying extinction are functionally disturbed in individuals with impaired extinction (reviewed in (Holmes & Singewald, 2013) and importantly that successful extinction in animals (Whittle et al., 2010) and exposure therapy in humans (Hauner et al., 2012) can correct these abnormalities by triggering a lasting reorganization of this network.
3. Neurochemical and molecular substrates of fear extinction
Research is revealing that the activity of a number of specific intra-cellular signaling cascades [reviewed in (Orsini & Maren, 2012)] carrying biological information from the cell surface to the nucleus, is the key to successful extinction by modulating gene transcription and ultimately promoting synaptic plasticity in extinction-relevant brain regions. The consolidation of extinction memories requires new protein synthesis initiated by molecular signaling cascades within the AMY, HPC and mPFC. The extinction-related molecular signaling and gene expression modulation can differ considerably in these brain areas (Cestari et al., 2014). Key molecules in the amygdala include calcium (Ca2+) influx via NMDA-receptors [in an interaction with α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors] and VGCCs-mediated activation of Ca2+/calmodulin-dependent protein kinase II (CaMKII) and kinases such as Ca2+ /phospholipid-dependent protein kinase (PKC), and cAMP-dependent protein kinase A (PKA). Once activated, these kinases merge into a common mitogen-activated protein kinase (MAPK)/extracellular regulated kinase (ERK) signaling pathway initiating cAMP response element binding (CREB) phosphorylation and transcription of plasticity proteins [reviewed in (Orsini & Maren, 2012, see Fig. 3 for overview]. Aspects of this intracellular signaling seem to be disrupted in deficient extinction, as exemplified by the correlation of impaired extinction with reduced ERK activity in extinction-relevant brain areas (Cannich et al., 2004; Herry et al., 2006; Ishikawa et al., 2012). Similarly, aberrant expression of memory-related genes in these areas is correlated with impaired extinction (Holmes & Singewald, 2013).
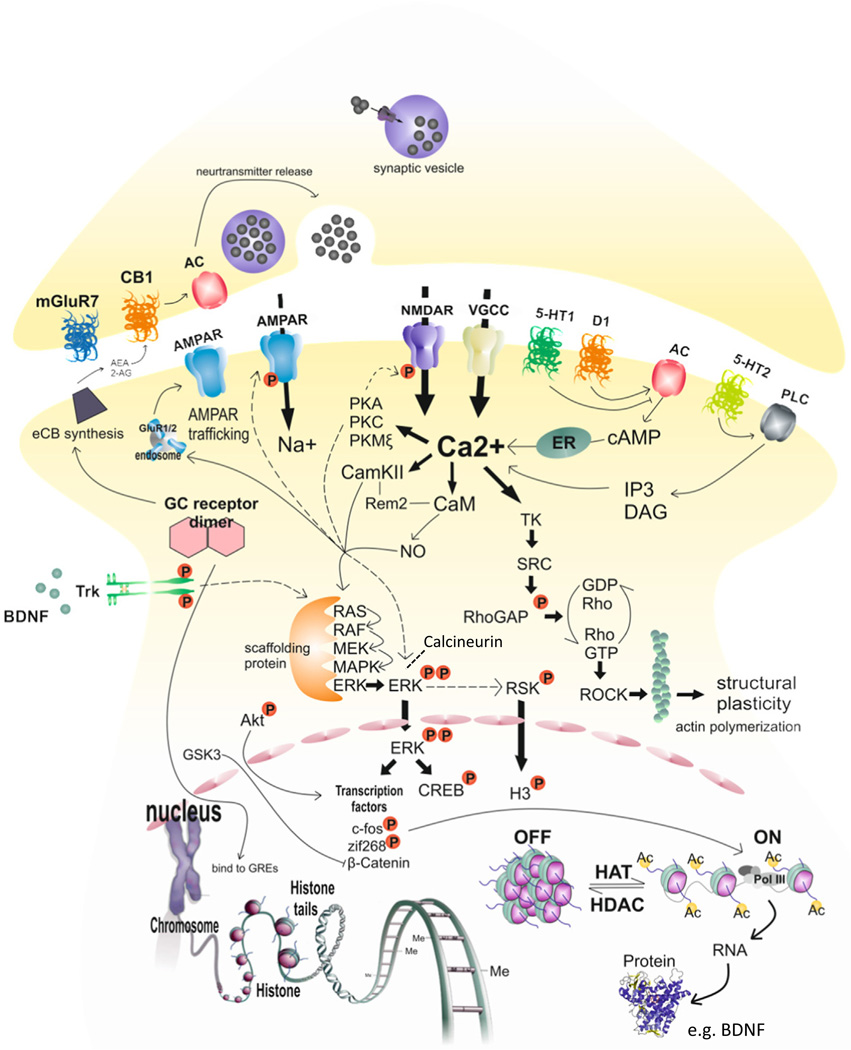
Fig. 3.
Overview of pharmacological targets and signaling cascades proposed to be important in mediating synaptic plasticity underlying extinction. The formation of extinction memories requires an intricate regulatory network of signal transduction and gene transcription and translation, leading to a complex pattern of intracellular changes and long-term structural changes. Various pre- and postsynaptic membrane receptors including ionotropic and metabotropic glutamate receptors, cannabinoid receptors, 5-HT, and dopamine receptors have been shown to be important targets. Main downstream mechanisms include calcium entry through NMDAR and VGCC in concert with AMPA receptors initiating synaptic plasticity via calcium-dependent protein kinases (e.g. PKA, PKC, PKM and CamKII, phosphatases (e.g. calcineurin) and activation of the ERK/MAPK pathway. Subsequent interaction with transcription factors, such as CREB and Zif268 within the nucleus results in a wide range of newly synthesized proteins, such as BDNF important for synaptic plasticity including LTP formation. BDNF activated TrkB receptors further regulate the ERK/MAPK pathway. Finally, epigenetic modifications are important in translating neurotransmitter/neuromodulator signaling activity generated at the synapse into activation/repression of the desired genomic response in the cell nucleus. As outlined in the text, boosting these synaptic plasticity mechanisms by pharmacological means may constitute the establishment of novel drug targets to promote fear extinction.
To enable selective therapeutic targeting, an important aim in the extinction field is to identify neural mechanisms and intracellular pathways in fear extinction that are different from those involved in fear memory formation. Many of the intracellular mechanisms are similar, however there are also a number of distinct processes and features in fear vs fear extinction learning, including differences in protein synthesis dependence, in brain area- and neuronal population-specific localization and recruitment of signaling pathways, in time course of recruitment, in distinct involvement of certain isoforms of key signaling components (e.g. ERK1 vs ERK2) and resulting gene expression (Cestari et al., 2014; Guedea et al., 2011; Lattal et al., 2006; Tronson et al., 2012). While there are examples of the direct targeting of pharmacologically important components of the aforementioned intracellular signaling, e.g. the MAPK/ERK pathway (Fischer et al., 2007; Herry et al., 2006; Lu et al., 2001) to modulate fear extinction, they were mainly targeted indirectly via membrane receptors e.g. (Cannich et al., 2004; Matsuda et al., 2010) or ion channels (Davis & Bauer, 2012; Ishikawa et al., 2012). These findings have stimulated the hypothesis that boosting specific synaptic plasticity mechanisms by pharmacological means may constitute novel drug targets to promote fear extinction. Indeed, as outlined below, systemic and brain region specific drug studies have identified a number of promising compounds which augment the behavioral and neurobiological effects of fear extinction. The results of these studies are building a framework upon which novel therapeutic interventions to facilitate the efficacy of CBT may be rationally developed and which may ultimately help to enhance symptom remission and recovery.
4. Pharmacologically enhancing extinction
4.1. Serotonergic system
The serotonin (5-HT) system is positioned to modulate the extinction circuitry via ascending 5-HT projections arising from midbrain raphe nuclei that innervate certain brain structures including the AMY (BLA > LA ≫ CeA), the HPC, the mPFC and the bed nucleus of the stria terminalis [reviewed in (Burghardt & Bauer, 2013)]. The acquisition and expression of conditioned fear increases 5-HT release in the BLA, mPFC and dPAG (Kawahara et al., 1993; Yokoyama et al., 2005; Zanoveli et al., 2009) though possible changes in 5-HT release during acquisition and consolidation of fear extinction have not been reported to the best of our knowledge.
There are 16 5-HT receptor subtypes classified into 7 receptor families (5-HT1–7) (Fig. 4). All 5-HT receptors, excluding the 5-HT3 family – a member of a superfamily of ligand-gated ion channels, are metabotropic, coupled to Gs (5-HT6 and 5-HT7), Gi/0 (5-HT1, 5-HT4 and 5-HT5) or Gq (5-HT2) proteins.
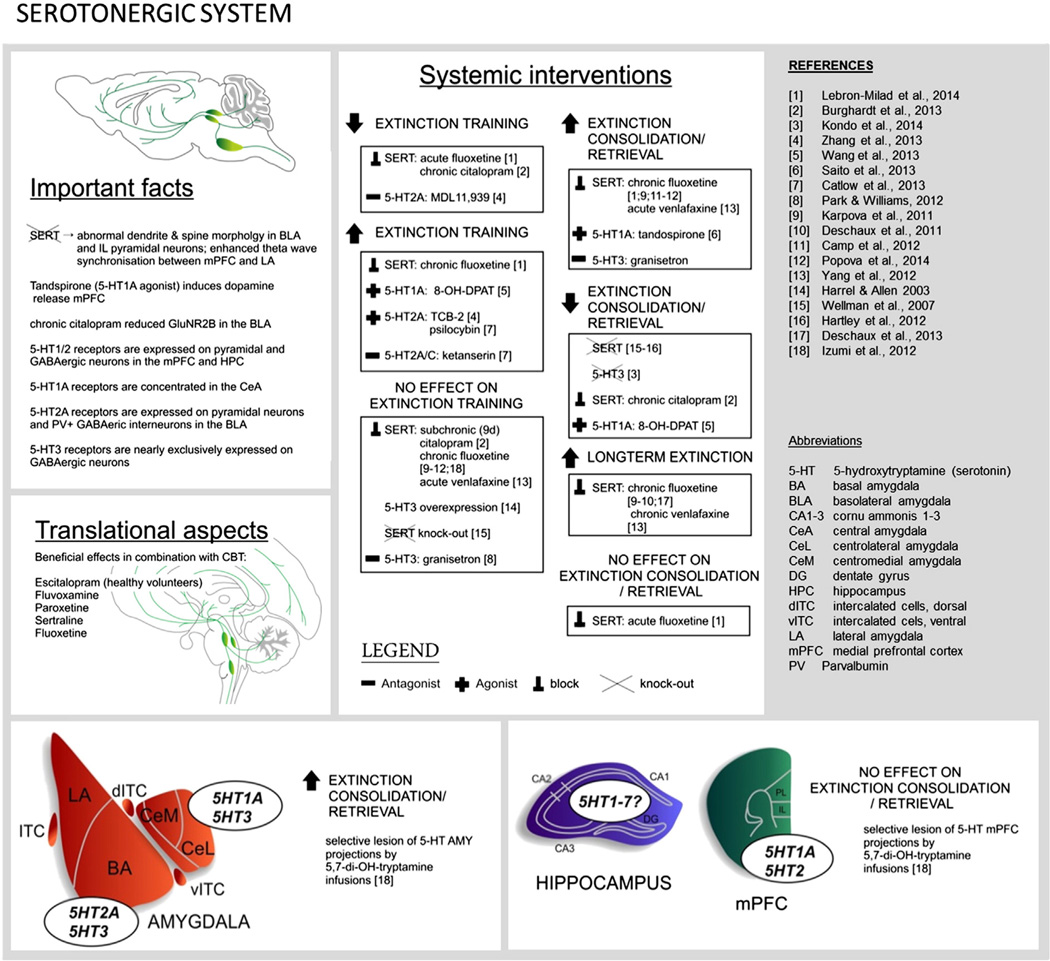
Fig. 4.
Overview of research on the role of the serotonergic system in extinction.
To date, research has largely focused on the role of 3 of the 5-HT receptors in fear extinction; namely 5 HT1A, 5-HT2 and 5-HT3 (Table 1, Fig. 4). Selective activation of 5-HT1A (Saito et al., 2013; Wang et al., 2013) or 5-HT2A receptors (Catlow et al., 2013; Zhang et al., 2013) enhances extinction. This might occur via 5-HT1A and 5-HT2A receptors expressed in the mPFC and the LA (Chalmers & Watson, 1991; Cornea-Hebert et al., 1999; Santana et al., 2004). Both of these receptors regulate mPFC and LA excitability via direct activation of pyramidal cells and/or GABAergic interneurons (Llado-Pelfort et al., 2012; Rainnie, 1999; Stutzmann & LeDoux, 1999). 5HT2A agonists increase presynaptic glutamate release (Aghajanian & Marek, 1999) and increase NMDA receptor sensitivity (Arvanov et al., 1999) and these are mechanisms well characterized as being important in extinction (see Section 4.4 Glutamatergic system).
Table 1.
Serotonergic signaling in fear extinction (preclinical studies).
| Drug/manipulation | Extinction training | Extinction retrieval | Longterm extinction | Route | Reference |
|---|---|---|---|---|---|
| Facilitating 5-HT signaling | |||||
| SERT KO6 | ns | – | ns | No drug | (Hartley et al. 2012) |
| No effect | – | ns | No drug | (Wellman et al. 2007) | |
| Acute fluoxetine (SSRI) | (−)## | No effect | ns | ip | (Lebron-Milad et al. 2013) |
| Subchronic citalopram (SSRI, 9d) | No effect | ns | ns | ip | (Burghardt et al. 2013) |
| Chronic citalopram (SSRI, 22d) | – | (−) | ns | ip | (Burghardt et al. 2013) |
| Chronic (14d) fluoxetine (SSRI) | (+)# | (+) | ns | po | (Lebron-Milad et al. 2013) |
| Chronic fluoxetine (SSRI) | No effect | + | + (Ren-A, SR, Re-in) | ip | (Karpova et al. 2011) |
| No effect | ns | + (Re-in) | ip,po | (Deschaux et al. 2011, 2013) | |
| No effect* | +*4 | ns | po | (Camp et al. 2012) | |
| No effect | + | ns | po | (Popova et al. 2014) | |
| Acute venlafaxine (SSRI) | No effect | + | ns | ip | (Yang et al. 2012) |
| Chronic venlafaxine (SSRI) | ns | ns | + (Re-in) | ip | (Yang et al. 2012) |
| 8-OH-DPAT (5HT1 ag) | +*5 | (−)*5 | ns | ip | (Wang et al. 2013) |
| Tandospirone (5HT1 ag) | ns | +* | ns | ip | (Saito et al. 2013) |
| tcb2 (5HT2A ag) | + | ns | ns | ip | (Zhang et al. 2013) |
| Psilocybin (5HT2A ag) | (+) | ns | ns | ip | (Catlow et al. 2013) |
| 5-HT3 OE | No effect | ns | ns | No drug | Harrell and Allan (2003) |
| Inhibiting 5-HT signaling | |||||
| 5,7-Dihydroxytryptamine | (+)# | + | ns | BLA | (Izumi et al. 2012) |
| 5,7-Dihydroxytryptamine | No effect | No effect | ns | IL | (Izumi et al. 2012) |
| 5HT3 KO | ns | – | ns | No drug | (Kondo et al. 2014) |
| MDL11,939 (5HT2A ant) | – | ns | ns | ip | (Zhang et al. 2013) |
| Ketanserin (5HT2A/C ant) | (+) | ns | ns | ip | (Catlow et al. 2013) |
| Granisetron (5HT3 ant) | No effect | (+) | ns | ip | Park and Williams (2012) |
drug administration following extinction training;
SERT-KO results in increased synaptic 5-HT levels;
7 days;
protection from context over-generalization;
valproate-induced model of autism,
SERT-KO results in increased synaptic 5-HT levels.
facilitates rescue of impaired fear extinction;
reduced fear expression at the beginning of extinction training;
enhanced fear expression at the beginning of extinction training.
+, improved; -, impaired; (+) or (−), only minor effects; po, peroral administration; ip, intraperitoneal injection; ns, not studied; BLA, intra-basolateral amygdala administration; IL, infralimbic cortex; Ren-A, Fear renewal in conditioning context; SR spontaneous recovery; Re-in, reinstatement; ag, agonist; ant, antagonist, KO, knock-out; OE, over-expression.
In “Extinction training” (or ‘within-session extinction’), an effect on the reduction of the behavioral response (e.g. freezing) in response to repeated CS presentations is described.
In “Extinction retrieval”, an effect on the recall of the extinction memory 24h following extinction training is described.
In “Long-term extinction” an effect on the robustness of the extinction memory in terms of protection against spontaneous recovery of fear (passage of time), fear renewal (re-exposure to conditioning context or a novel context which is distinct to the conditioning and extinction context) and fear reinstatement (US re-exposure) is described.
In this review we aimed to summarize (druggable) systems involved in fear extinction and how pharmacological compounds have been utilized to augment fear extinction in preclinical (rodent) and small-scale clinical studies. We did not differentiate between data gained from either mice or rats as this would have gone beyond the scope of this review. However, excellent species differentiation has been provided by others (e.g. (Makkar et al. 2010, Myers et al. 2011, Bowers et al. 2012, Riaza Bermudo-Soriano et al. 2012, Burghardt and Bauer 2013, Fitzgerald et al. 2014a).
The roles in extinction of other 5-HT receptors or other components of the 5-HT system, such as melatonin ((Huang et al., 2014) have not been extensively explored. For example, recent work indicating that the 5-HT7 receptor regulates emotional memories (Eriksson et al., 2012), has not been followed up with studies on extinction. However, 5-HT3 receptors have been also associated with fear extinction mechanisms (Table 1). While 5-HT3 antagonists have been found to improve extinction (Park & Williams, 2012), constitutive deletion of 5-HT3 receptors has the opposite effect, possibly due to developmental changes in the 5-HT system (Kondo et al., 2014).
Despite the limited knowledge that we have so far regarding specific 5-HT receptor contributions to extinction, chronic treatment with SSRIs to enhance 5-HT availability is the first-line therapy for many anxiety disorders. Acute SSRI treatment induces anxiogenic effects, which can be prevented with 5-HT2C antagonists in rodents and mimicked by 5-HT2C agonists administered systemically (Bagdy et al., 2001; Salchner & Singewald, 2006) or locally into the BLA (Campbell & Merchant, 2003). Chronic treatment with some SSRIs (e.g., fluoxetine) but not all (e.g., citalopram) enhances extinction in rodents (Table 1) [for a recent review see (Burghardt & Bauer, 2013)], including models of impaired extinction [(Camp et al., 2012; Fitzgerald et al., 2014b) see Table 1]. Venlafaxine – a combined 5-HT and noradrenaline reuptake inhibitor with weaker affinity to the noradrenaline transporter (Owens et al., 1997) – also improves extinction retrieval and protects against fear reinstatement in rodents (Yang et al., 2012). Chronic fluoxetine treatment does not strengthen fear conditioning or fear expression (Camp et al., 2012) suggesting relative selectivity for extinction. Another clinically relevant attribute of chronic fluoxetine treatment is the protection it provides against the return of fear, as assayed by spontaneous recovery or fear renewal (Deschaux et al., 2011; Deschaux et al., 2013; Karpova et al., 2011). Furthermore, an important molecular mechanism through which fluoxetine produces extinction-associated reductions in fear may be the transformation of adult plasticity mechanisms to a juvenile state (Karpova et al., 2011).
These encouraging preclinical discoveries will hopefully stimulate comprehensive clinical assessment of the efficacy of SSRIs in augmenting CBT in anxiety patients (Table 1A). There have been small-scale clinical trials showing, for example, that paroxetine augments fear reductions (assessed using CAPS scores) and is associated with higher remission rates when combined with CBT in PTSD patients as compared with a placebo/CBT group. An optional treatment maintenance for an additional 12 weeks revealed that symptomatic improvements were long-lasting and consistent although no further improvements could be detected. However, this may have been due to enhanced drop-out of patients who had remitted in earlier phases of the study (Schneier et al., 2012). More evidence is available in panic disorder (Table 1). There is evidence that chronic SSRI treatment (fluvoxamine, paroxetine) increases CBT-induced fear reductions in panic disorder patients (de Beurs et al., 1995; Oehrberg et al., 1995). However results concerning the extinction-augmenting ability of SSRIs are not consistent as SSRIs, including fluvoxamine, fluoxetine, sertraline and paroxetine, have failed to demonstrate efficacy in primary outcomes [clinical global impression; (Blomhoff et al., 2001; Davidson et al., 2004; Haug et al., 2003; Koszycki et al., 2011; Stein et al., 2000). Despite their common effect of serotonin transporter inhibition, SSRIs are known to differ with regard to their pharmacological properties. Along these lines, some SSRIs (e.g. paroxetine) show additional noradrenaline transporter-inhibiting properties via their metabolites (Owens et al., 1997), while others (e.g. citalopram) show continued selectivity for the serotonergic system (Deupree et al., 2007). In this respect, differential effect sizes of SSRIs are to be expected.
Table 1A.
Human trials: serotonergic drugs combined with CBT.
| Disorder | Study design | Outcome (compared to placebo control group) | Reference |
|---|---|---|---|
| Healthy volunteers | 14 days po escitalopram pretreatment; fear conditioning paradigm |
Accelerated extinction learning (skin conductance responses) | (Bui et al., 2013) |
| Panic disorder with/without agoraphobia |
Fluvoxamine or placebo followed by exposure therapy; psychological panic management followed by exposure therapy or exposure therapy alone |
Self-reported measures All treatments effective, however fluvoxamine plus CBT superior to all other treatments |
(de Beurs et al., 1995) |
| Panic disorder with/without agoraphobia |
12 weeks paroxetine or placebo plus CBT | Reduced number of panic attacks in paroxetine/CBT group | (Oehrberg et al., 1995) |
| Panic disorder with/without agoraphobia |
10 weeks of paroxetine or placebo plus CBT in week 5 and 7 |
No significant difference in primary CGI outcome. Secondary outcome: Higher proportion of panic-free patients in paroxetine-CBT group. |
(Stein et al., 2000) |
| Panic disorder with/without agoraphobia |
12 weeks fluvoxamine or placebo with or without CBT |
All groups except placebo without CBT improved. No difference within other groups. |
(Sharp et al., 1997) |
| Panic disorder with/without agoraphobia |
12 weeks of sertraline or placebo treatment plus self-administered CBT or no CBT |
Reduced anticipatory anxiety in sertraline plus self-administered CBT No significant improvements in CGI. |
(Koszycki et al., 2011) |
| Social anxiety disorder | 24 weeks of sertraline/placebo with or without exposure therapy (8 sessions in the first 12 weeks of treatment) |
All groups improved (also placebo without CBT) Sertraline treatment (without CBT) showed higher improvement than CBT groups (placebo or sertraline). Placebo without CBT showed lowest benefits. |
(Blomhoff et al., 2001) |
| Social anxiety disorder | Follow-up study of (Blomhoff et al., 2001) Assessment of long-term effects 28 weeks after cessation of medical treatment |
Exposure therapy alone (without placebo or sertraline) showed a further improvement in CAPS 28 weeks after treatment cessation, however only reached improvement levels comparable with those of the sertraline alone group after the initial 24 weeks |
(Haug et al., 2003) |
| CAPS score in the other groups (Exposure + placebo or sertraline and placebo alone) stayed constant (no improvement compared to 24 week CAPS score) |
|||
| Social anxiety disorder | 14 weeks of fluoxetine or placebo plus weekly CBT or no CBT CBT consisted of group treatment combining in vivo exposure, cognitive restructuring and social skills training |
All treatments were superior to placebo (without CBT) but no differences between groups themselves |
(Davidson et al., 2004) |
| PTSD | 10 exposure therapy sessions (1×/week) plus paroxetine CR Optional 12 weeks of maintenance treatment |
Greater CAPS improvement in paroxetine group vs placebo after 10 weeks Higher rate of remission (61.5% vs 23.1% in placebo group) after 10 weeks No changes after additional 12 weeks. CAVE drop-out of remitters. |
(Schneier et al., 2012) |
CR…controlled release.
Nevertheless, a number of outstanding questions remain, including that concerning the specificity of SSRIs in augmenting fear extinction as opposed to fear learning (as observed with fluoxetine in preclinical models; see above). In this respect, the finding that prior fear conditioning administration of escitalopram augments extinction learning in healthy volunteers without influencing fear acquisition (Bui et al., 2013) may hint at a potential selectivity of SSRIs to preferentially engage extinction mechanisms. However, this remains to be tested.
In summary, the clinical studies performed so far have demonstrated that SSRI augmented psychotherapy holds some benefits for panic disorder patients and possibly for PTSD and SAD patients. However, further studies of different SSRIs in larger patient cohorts will be needed, as well as adequate follow-up time to reveal long-term effects, before more definite conclusions can be drawn. Until such studies, as well as studies investigating receptor selective approaches are available, it seems rational to recommend combined treatment involving exposure-based psychotherapy together with antidepressants including SSRIs such as fluoxetine, as there is more evidence supporting than disproving such a strategy at the moment.
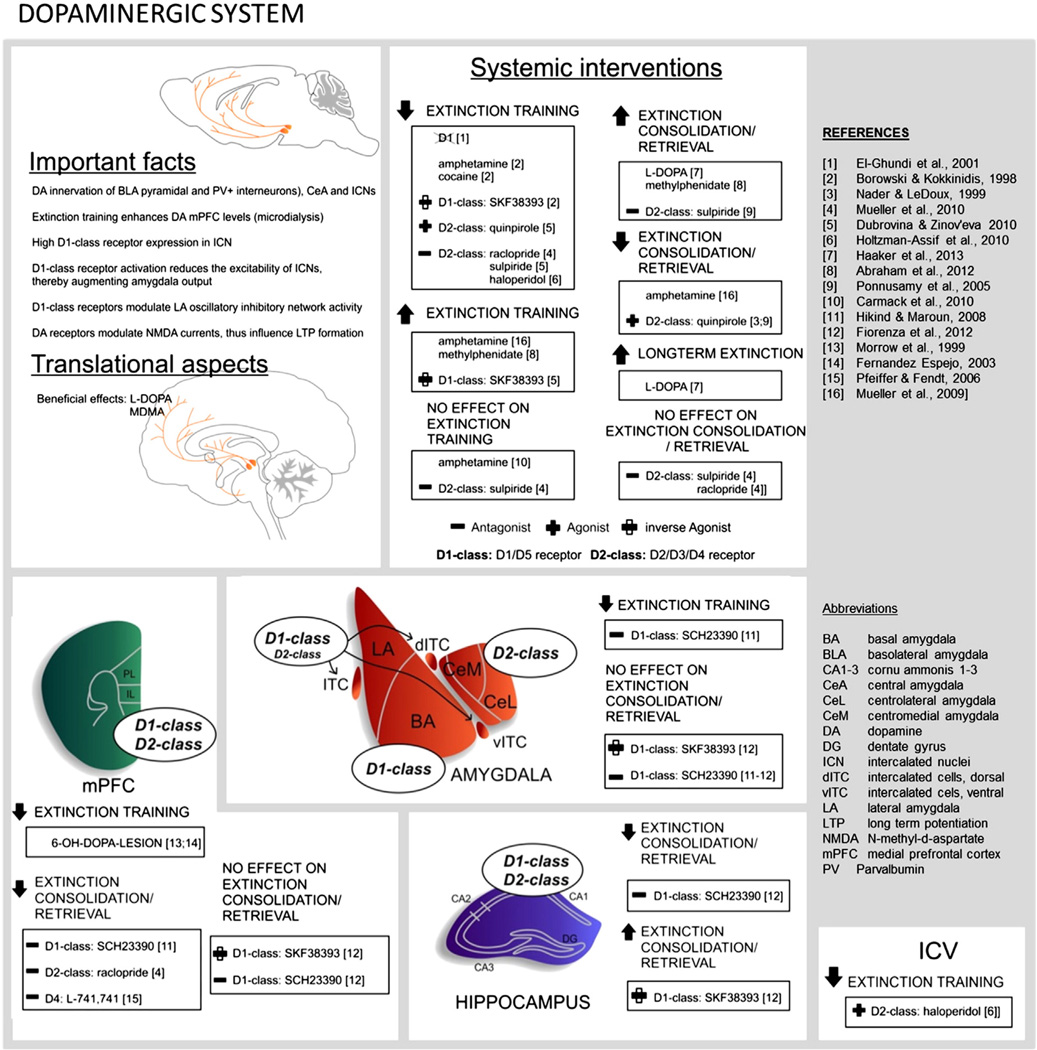
4.2. Dopaminergic system
An increasing amount of evidence suggests that dopamine (DA) signaling has an important role in extinction mechanisms (Fig. 5) [for a recent detailed review, see (Abraham et al., 2014)]. The DAergic system innervates forebrain extinction circuits through ascending mesocortical/limbic DAergic projections from the ventral tegmental area targeting certain brain regions including the mPFC, HPC and AMY (Pinard et al., 2008; Pinto & Sesack, 2008; Weiner et al., 1991). There is increased dopamine release in the mPFC during and following extinction training (Hugues et al., 2007) and boosting DAergic signaling with DA precursors or DA-releasing drugs facilitates extinction consolidation (Abraham et al., 2012; Haaker et al., 2013) and can rescue impaired fear extinction in female rats (Rey et al., 2014). Conversely, reducing DAergic transmission by lesioning mesocortical DAergic projection neurons (with 6-OH-DOPA) impairs extinction memory formation (Fernandez Espejo, 2003; Morrow et al., 1999) (see summary in Table 2). More recently, there have been further insights into the mechanisms of how and where DAergic signaling can influence fear extinction have been made. These studies (see Table 2 and below) have revealed a complex two-pronged feature of DAergic signaling that can (i) gate the expression of fear, and (ii) influence fear extinction consolidation mechanisms.
Fig. 5.
Overview of research on the role of the dopaminergic system in extinction.
Table 2.
Dopaminergic signaling in fear extinction (preclinical studies).
| Drug/manipulation | Extinction training | Extinction retrieval | Longterm extinction | Route | Reference |
|---|---|---|---|---|---|
| Facilitating DA signaling | |||||
| L-DOPA | ns | +1 | + (SR, Re-in2, Ren-A | ip | (Haaker et al., 2013) |
| Amphetamine | − | ns | ns | ip | (Borowski and Kokkinidis, 1998) |
| No effect | ns | ns | ip | (Carmack et al., 2010) | |
| (+)3 | ns | ip | (Mueller et al., 2009) | ||
| Cocaine | − | ns | ns | ip | (Borowski and Kokkinidis, 1998) |
| Methyl-phenidate | + | (+) | ns | ip | (Abraham et al., 2012) |
| ns | +1 | ns | ip | (Abraham et al., 2012) | |
| SKF38393 (pAg D1) | − | ns | ns | ip | (Borowski and Kokkinidis, 1998) |
| +*4 | ns | ns | ip | (Dubrovina and Zinov'eva, 2010) | |
| ns | +1 | ns | CA1 | (Fiorenza et al., 2012) | |
| ns | No effect1 | ns | BLA | (Fiorenza et al., 2012) | |
| ns | No effect1 | ns | IL | (Fiorenza et al., 2012) | |
| ns | +6 | ns | ip | (Rey et al., 2014) | |
| ns | −7 | ns | ip | (Rey et al., 2014) | |
| Quinpirole (D2 ag) | ns | − | ns | ip | (Ponnusamy et al., 2005) |
| ns | − | ns | ip | (Nader and LeDoux, 1999) | |
| −4 | ns | ns | ip | (Dubrovina and Zinov'eva, 2010) | |
| Inhibiting DA signaling | |||||
| 6-OH DOPA | − | ns | ns | mPFC | (Morrow et al., 1999) |
| − | (−) | ns | mPFC | (Fernandez Espejo, 2003) | |
| D1 knock-out | − | ns | ns | global KO | (El-Ghundi et al., 2001) |
| SCH23390 (D1 ant) | ns | No effect1 | ns | IL | (Fiorenza et al., 2012) |
| − | No effect | ns | BLA | (Hikind and Maroun, 2008) | |
| No effect | − | ns | IL | (Hikind and Maroun, 2008) | |
| ns | −1 | ns | CA1 | (Fiorenza et al., 2012) | |
| ns | No effect1 | ns | BLA | (Fiorenza et al., 2012) | |
| Sulpiride (D2 ant) | − | ns | ns | ip | (Dubrovina and Zinov'eva, 2010) |
| ns | + | ns | ip | (Ponnusamy et al., 2005) | |
| No effect | No effect | ns | ip | (Mueller et al., 2010) | |
| Raclopride (D2 ant) | (−) | No effect | ns | ip | (Mueller et al., 2010) |
| No effect | − | ns | IL | (Mueller et al., 2010) | |
| Haloperidol (D2/D3 ant) | − | ns | ns | ip | (Holtzman-Assif et al., 2010) |
| (−) | ns | ns | icv | (Holtzman-Assif et al., 2010) | |
| (−) | − | ns | NAcb | (Holtzman-Assif et al., 2010) | |
| L-741,741 (D4 ant) | No effect | − | ns | mPFC | (Pfeiffer and Fendt, 2006) |
Drug administration following extinction training,
37 days,
increased locomotion,
passive avoidance paradigm,
reduced locomotion with 0.3 mg/kg, no effect on locomotion, extinction training and retrieval with 0.1 mg/kg,
rescued impaired extinction retrieval in estrus/metestrus/diestrus,
impairs extinction retrieval in proestrus.
Facilitates rescue of impaired fear extinction;
reduced fear expression at the beginning of extinction training;
enhanced fear expression at the beginning of extinction training.
+, Improved; -, impaired; (+) or (−), only minor effects; ip, intraperitoneal injection; ns, not studied; BLA, intra-basolateral amygdala administration; IL, infralimbic cortex; mPFC, medial prefrontal cortex; CA1, cornu ammonis 1; NAcb, nucleus accumbens; Ren-A, Fear renewal in conditioning context; SR, spontaneous recovery; Re-in, reinstatement; ag, agonist; ant, antagonist, KO, knock-out;
Dopamine receptors are metabotropic and in the classical view, coupled to either Gs (increased cAMP, D1-like) or Gi/0 (decreased cAMP, D2-like) proteins. However, DAergic signals can also be transduced via Phospholipase C (PLC) activation (enhanced DAG, IP3) mediated by D1-like Gα proteins, by D2-like Gβγ subunits or by receptors forming D1/D2 heteromers (Felder et al., 1989; Lee et al., 2004) [for review see (Abraham et al., 2014)]. The D1-class receptor signaling modulates the excitability of BLA parvalbumin-positive interneurons (Bissiere et al., 2003; Kroner et al., 2005; Loretan et al., 2004) and ITCs (Manko et al., 2011). In concert with D2-like receptors in the CeL/CeC (Perez de la Mora et al., 2012), DAergic signaling exerts tight control over CeM-mediated expression of fear. To date, however, pharmacological manipulation of DAergic signaling in the AMY has produced conflicting results with regard to extinction (Fiorenza et al., 2012; Hikind & Maroun, 2008) (see Table 2).
Preclinical work has aimed to clarify the role of DA receptor activity in extinction. However, research has been largely complicated by the lack of receptor subtype-selective compounds, leaving many investigations reliant on drugs acting on D1-like (D1, D5) and D2-like (D2, D3, D4) receptor subfamilies. One reliable finding, however, is that DA antagonism (see Table 2) specifically in the mPFC impairs the retrieval of extinction memories (Hikind & Maroun, 2008; Mueller et al., 2010; Pfeiffer & Fendt, 2006). In this case, DAergic effects on glutamatergic NMDA receptor signaling may be involved. As discussed below (Section 4.4 Glutamatergic system), NMDA receptor-mediated burst activity of mPFC neurons is associated with stabilization of extinction memories (Burgos-Robles et al., 2007) and D1-like receptor agonists facilitate NMDA receptor currents via Gs mediated increases in adenylate cyclase (AC) activity (Snyder et al., 1998). There is also the possibility that D2 receptor mechanisms in the PFC support extinction, as (Mueller et al., 2010) showed that a pre-extinction IL injection of the D2 antagonist raclopride impaired the consolidation of extinction memory. However, following systemic administration of the (less selective) D2 receptor antagonist sulpiride, accelerated extinction was found (Ponnusamy et al., 2005).
In summary, the results of preclinical studies do not yet fully clarify the specific DA receptor or receptor class mediating the extinction augmenting effect of enhanced dopaminergic signaling. Work still needs to be done on the use of receptor-selective agonists and antagonists, as well as biased ligands (showing functional selectivity for either AC or PLC signal transduction pathways) to reveal receptor-specific functions and their modulation of downstream signaling cascades in extinction. Pharmacologically increasing the DA tone promotes extinction. L-DOPA administration following extinction training enhances fear extinction in mice and healthy humans, rendering extinction resistant to fear renewal, reinstatement and spontaneous recovery (Haaker et al., 2013). L-DOPA is a precursor for all catecholamines, but preferentially enhances dopaminergic turnover in the frontal cortex (Dayan & Finberg, 2003), eliciting D1- and D2-like receptor responses (Trugman et al., 1991). Indeed, L-DOPA augments extinction-related neural activity in the mPFC (IL) of mice and increases mPFC functional coupling in humans (Haaker et al., 2013). These findings have immediate translational potential because L-DOPA is a US Food and Drug Administration-approved compound used in the treatment of Parkinson's disease, typically in combination with carbidopa (a peripheral decarboxylase inhibitor) to maximize central availability and minimize peripheral side effects. While there are abuse related issues with any DA enhancer, this could be mitigated by supervised, acute treatment during CBT. Encouragingly, a small-scale clinical trial found improved efficacy of psychotherapy in treatment-resilient PTSD patients by administration of another DA-enhancing drug, MDMA (Mithoefer et al., 2011) and a follow-up indicated robust fear reductions for up to 74 months [(Mithoefer et al., 2013), see Table 2A]. A caveat here is that it is unclear whether these effects of MDMA are solely attributable to its effects on DA, and not also to its effect on other targets of MDMA, notably 5-HT. Nonetheless, boosting DA transmission in conjunction with CBT could have significant potential translational value.
Table 2A.
Human trials: dopaminergic enhancers combined with CBT.
| Disorder | Study design | Outcome (compared to placebo control group) | Reference |
|---|---|---|---|
| Healthy volunteers | Fear conditioning paradigm, L-DOPA po following extinction training |
Protection from renewal (skin conductance responses) | (Haaker et al., 2013) |
| PTSD (treatment-refractory) | 2 single oral MDMA prior to 8h therapy session (3–5 weeks apart, patients also received therapy prior to first MDMA exposure as well as between and in follow-up) |
MDMA augmented CBT in comparison to placebo (83% vs 25% response) (2 month follow-up); open-label MDMA use offered to patients from the placebo group after last assessment |
(Mithoefer et al., 2011) |
| PTSD (treatment-refractory) | Follow-up study from (Mithoefer et al. 2011) | MDMA augmented CBT produced long-lasting effects (17–74 months) |
(Mithoefer et al., 2013) |
4.3. Noradrenergic system
The noradrenergic system is crucial for both the formation and the maintenance of fear memories, as well as for extinction memories (reviewed in (Holmes & Quirk, 2010; Mueller & Cahill, 2010)). Noradrenaline can enhance neuronal excitability in extinction-relevant brain regions such as the IL (Mueller et al., 2008) and successful fear extinction is associated with enhanced extracellular levels of nor-adrenaline in the mPFC (Hugues et al., 2007). Boosting noradrenaline levels, via administration of either noradrenaline itself (Merlo & Izquierdo, 1967) or of compounds such as yohimbine (see below) or methylphenidate (Abraham et al., 2012) enhances fear extinction (Table 3). There is evidence from preclinical studies that activating β-adrenoceptors, one target receptor of noradrenaline, can also facilitate fear extinction (Table 3) [for recent review, see (Fitzgerald et al., 2014a)]. Conversely, depleting central noradrenaline or lesioning ascending noradrenaline projections from the locus coeruleus, impairs extinction, as does systemic alpha1 or β adrenoceptor blockade (Table 3) [reviewed in (Mueller & Cahill, 2010)].
Table 3.
Noradrenergic (NE) signaling in fear extinction (preclinical studies).
| Drug/manipulation | Extinction learning | Extinction retrieval | Longterm extinction | Route | Reference |
|---|---|---|---|---|---|
| Enhancing noradrenergic signaling | |||||
| Noradrenaline | ns | + | ns | icv | (Merlo and Izquierdo, 1967) |
| Methylphenidate | + | + | ns | ip | (Abraham et al., 2012) |
| Noradrenaline | ns | +1 | ns | BLA | (Berlau and McGaugh, 2006) |
| ns | No effect1 | ns | BLA | (Fiorenza et al., 2012) | |
| ns | No effect1 | ns | CA1 | (Fiorenza et al., 2012) | |
| ns | −1 | ns | IL | (Fiorenza et al., 2012) | |
| Atomoxetine (NE reuptake inhibitor) | ns | No effect6 | + (SR)5 | systemic | (Janak and Corbit, 2011) |
| Isoproterenol (β ag) | No effect | ns | ns | ip (subchronic) | (Do-Monte et al., 2010b) |
| ns | (+)1 | ns | ip (subchronic) | (Do-Monte et al., 2010b) | |
| ns | (+)1 | ns | mPFC | (Do-Monte et al., 2010b) | |
| Yohimbine (α2 ant)8 | + | ns | ns | sc | (Cain et al., 2004) |
| ns | No effect1 | ns | sc | (Cain et al., 2004) | |
| +#2 | +2 | ns | sc | (Cain et al., 2004) | |
| + | + | − | ip | (Morris and Bouton, 2007) | |
| No effect7 | No effect | ns | ip | (Mueller et al., 2009) | |
| ns | +* | ns | ip | (Hefner et al., 2008) | |
| ns | No effect6 | + (SR)5 | systemic | (Janak and Corbit, 2011) | |
| Atipamezole (α2 ant)8 | ns | No effect4 | ns | sc | (Davis et al., 2008) |
| Inhibiting noradrenergic signaling | |||||
| Prazosine (α1 ant) | ns | No effect1 | ns | ip (subchronic) | (Do-Monte et al., 2010a) |
| − | ns | ns | ip (subchronic) | (Do-Monte et al., 2010a) | |
| − | ns | ns | intra-mPFC | (Do-Monte et al., 2010a) | |
| Propanolol (β ant) | No effect | ns | ns | sc | (Cain et al., 2004) |
| ns | No effect1 | ns | sc | (Cain et al., 2004) | |
| No effect2 | (−)3 | ns | sc | (Cain et al., 2004) | |
| (+)# | No effect | ns | ip | (Rodriguez-Romaguera et al., 2009) | |
| − | − | ns | ip (subchronic) | (Do-Monte et al., 2010b) | |
| ns | No effect1 | ns | ip (subchronic) | (Do-Monte et al., 2010b) | |
| Atenolol (β ant) | − | ns | ns | mPFC | (Do-Monte et al., 2010b) |
| ns | No effect1 | ns | mPFC | (Do-Monte et al., 2010b) | |
| Sotalol (β ant) | No effect | No effect | ns | ip | (Rodriguez-Romaguera et al., 2009) |
| Timolol (β ant) | ns | +1 | ns | BLA | (Fiorenza et al., 2012) |
| Propanolol (β ant) | No effect | − | ns | IL | (Mueller et al., 2008) |
| ns | No effect1 | ns | IL | (Mueller et al., 2008) | |
| Timolol (β ant) | ns | +1 | ns | IL | (Fiorenza et al., 2012) |
| ns | No effect1 | ns | CA1 | (Fiorenza et al., 2012) |
Drug administration following extinction training;
spaced CS extinction, US = 0.7mA;
spaced CS extinction when US = 0.4mA; no effect when US = 0.7mA,
cocaine-conditioned place preference,
4 weeks,
instrumental lever press response,
reduced fear expression at start of extinction training, if extinction is performed in the same context as conditioning,
note that the net effect of α2 blockade is enhancement of noradrenergic signaling (see text).
Facilitates rescue of impaired fear extinction;
facilitates extinction of remote memories,
only in older animals (7 months), younger ones (2 months) are not affected
reduced fear expression at the beginning of extinction training.
+, Improved; -, impaired; (+) or (−), only minor effects; ip, intraperitoneal injection; injection; sc, subcutanous injection; icv, intracerebroventricular injection; ns, not studied; HPC, intra-hippocampal administration; BLA, intra-basolateral amygdala administration; IL, infralimbic cortex; PL, prelimbic cortex; CA1, cornu ammonis 1; Ren, Fear renewal; SR, spontaneous recovery; Re-in, reinstatement; ag, agonist; ant, antagonist, KO, knock-out; # reduced fear expression at start of extinction training.
There has been interest in the clinical utility of targeting noradrenergic mechanisms to augment extinction. Here, we focus on α2-adrenoceptors given the recent clinical findings supporting the utility of α2-adrenoceptor antagonists in improving extinction. Based on results of rodent studies (Cain et al., 2004), two small clinical studies (Powers et al., 2009; Smits et al., 2014) have shown that combining yohimbine with CBT can reduce anxiety in patients with social anxiety disorder and claustrophobic fear (Table 3A). A third study found no CBT-augmenting benefit of yohimbine in patients with fear of flying; however, CBT may have exerted a ‘ceiling effect’ that occluded the drug's effect (Meyerbroeker et al., 2012).
Table 3A.
Yohimbine combined with CBT in small-scale clinical trials.
| Disorder | Study design | Outcome (compared to placebo control group) | Reference |
|---|---|---|---|
| Social anxiety disorder (SAD) | 4 sessions consisting of yohimbine being administered prior to a CBT a session |
Yohimbine augmented CBT-induced improvement in SAD (self-report measures; 21 day follow-up) |
(Smits et al., 2014) |
| Claustrophobic fear | 1 single oral yohimbine dose prior to an exposure b session | Yohimbine augment CBT-induced reduced fear of enclosed spaces (7 day follow-up) |
(Powers et al., 2009) |
| Fear of flying | 2 single oral yohimbine prior to VRET session | No fear augmenting effect of yohimbine c | (Meyerbroeker et al., 2012) |
VRET, virtual reality exposure.
CBT involved an oral presentation on challenging topics in front of the therapists, other group members, and confederates,
behavioral approach task,
Lack of yohimbine effect might possibly have been due to the powerful influence of VRET exposure itself leading to a greater impact on the results from this than from the manipulation (drug condition) that ‘washed out’ or overrode any effect of manipulation (Meyerbroeker et al., 2012),
In rodents, blocking α2-adrenoceptors (e.g., with yohimbine) that function as autoreceptors on locus coeruleus neurons increases locus coeruleus activity and noradrenaline release in terminal regions (Singewald & Philippu, 1998). Yohimbine is anxiogenic and increases neuronal activity in widespread brain areas, including extinction-relevant areas such as the amygdala, mPFC and HPC (Singewald et al., 2003). Downstream targets of yohimbine-induced noradrenaline release include β1-adrenoceptors, and increasing activity at these receptors in locus coeruleus terminal regions (mPFC) enhances extinction (Do-Monte et al., 2010b), possibly via facilitating extinction-relevant long-term potentiation (Gelinas & Nguyen, 2005) in a BDNF-dependent manner (Furini et al., 2010). Yohimbine facilitates extinction in rodents, including extinction-impaired subjects (Hefner et al., 2008, 2008), though a more selective α2-adrenoceptor, atipamezole, does not (Table 3) [for further discussion, see (Holmes & Quirk, 2010)]. Yohimbine also protects against spontaneous fear recovery, in a manner similar to the effect of a noradrenaline uptake inhibitor, atomoxetine (Janak & Corbit,2011). However, yohimbine reduces fear only in the extinction context in which the drug was administered (Morris & Bouton, 2007), which would be a limitation of the clinical goal of achieving context-independent reductions in fear. Thus, other strategies may be needed, including combination strategies where drugs which do produce context-independent extinction, for example targeting HDACs (see Section 4.10 Epigenetics), are combined with yohimbine.
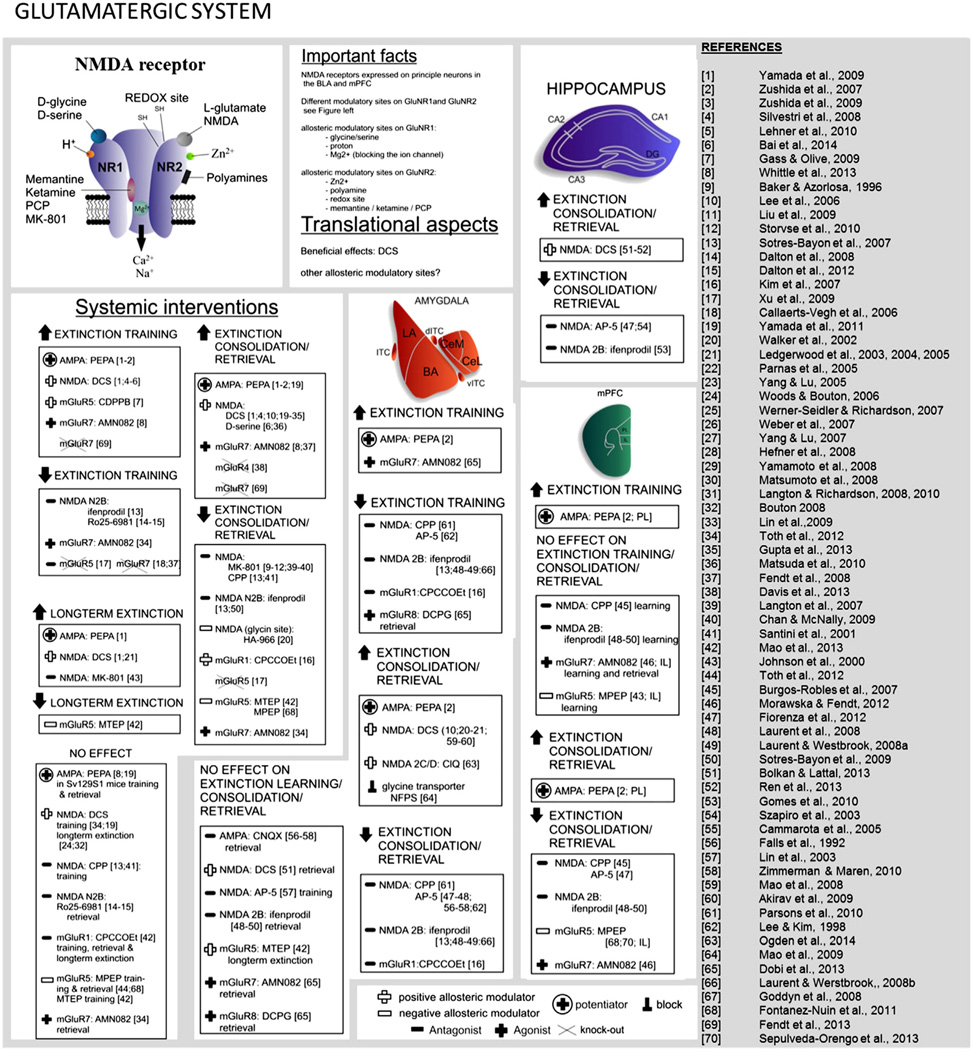
4.4. Glutamatergic system
Glutamatergic signaling plays a crucial role in synaptic plasticity and many forms of learning and memory, including fear extinction (see Fig. 6 for an overview). Fast excitatory glutamatergic signaling is mediated by ionotropic receptors (NMDA and AMPA), and slower signaling by metabotropic (mGluR1–8) receptors. Group I (mGluR1 and mGluR5) receptors are mainly expressed postsynaptically and coupled to Gq proteins enhancing phospholipase C (PLC) activity. Group II (mGluR2, mGluR3) and Group III (mGluR4, mGluR6–mGluR8) are both coupled to Gi proteins inhibiting adenylate cyclase (AC) activity and expressed on pre- as well as postsynaptic sites (Willard & Koochekpour, 2013).
Fig. 6.
Overview of research on the role of the glutamatergic system in extinction
Pharmacological potentiation of AMPA receptor activation (by PEPA) facilitates extinction learning and retrieval (Yamada et al., 2009, 2011; Zushida et al., 2007), although it is ineffective in severely extinction-impaired subjects (Whittle et al., 2013). Studies using localized infusion of AMPA potentiators (Zushida et al., 2007) and blockers (Falls et al., 1992; Milton et al., 2013; Zimmerman & Maren, 2010) coupled with electrophysiological recordings suggest that AMPA-mediated effects on extinction are localized to the mPFC [(Zushida et al., 2007) see Table 4, for recent detailed reviews (Bukalo et al., 2014; Myers et al., 2011)].
Table 4.
Glutamatergic signaling in fear extinction (preclinical studies).
| Drug/manipulation | Extinction training |
Extinction retrieval |
Longterm extinction |
Route | Reference |
|---|---|---|---|---|---|
| Facilitating AMPA signaling | |||||
| PEPA (AMPA potentiator) | No effect* | No effect* | ns | ip | (Whittle et al., 2013) |
| No effect* | +* | ns | ip | (Yamada et al., 2011) | |
| +# | + | + (Re-in) | ip | (Yamada et al., 2009) | |
| + | + | ns | ip | (Zushida et al., 2007) | |
| PEPA + NBQX (AMPA ant) | No effect | No effect | ns | ip | (Zushida et al., 2007) |
| PEPA (AMPA potentiator) | (+) | (+) | ns | PL | (Zushida et al. 2007) |
| + | + | ns | BLA/ceA | (Zushida et al., 2007) | |
| Inhibiting AMPA signaling | |||||
| CNQX (AMPA ant) | ns | No effect | ns | BLA | (Falls et al. 1992; Lin et al. 2003b; Zimmerman and Maren 2010) |
| Facilitating NMDA signaling | |||||
| D-Cycloserine | ns | + | ns | ip | (Walker et al., 2002) |
| ns | +1 | ns | Systemic | (Ledgerwood et al., 2003) | |
| ns | +1 | + (Re-in) | Systemic | (Ledgerwood et al., 2004) | |
| ns | +1 | ns | Systemic | (Ledgerwood et al., 2005) | |
| ns | +1 | ns | ip | (Parnas et al., 2005) | |
| ns | + | ns | ip | (Yang and Lu, 2005) | |
| ns | + | ns | ip | (Lee et al., 2006) | |
| ns | + | No effect | sc | (Woods and Bouton 2006) | |
| ns | + | ns | sc | (Werner-Seidler and Richardson, 2007) | |
| ns | + | ns | Systemic | (Weber et al., 2007) | |
| ns | + | ns | ip | (Yang et al., 2007) | |
| ns | + | ns | ip | (Yang et al., 2007) | |
| ns | + | ns | ip | (Hefner et al., 2008) | |
| ns | + | ns | po | (Yamamoto et al. 2008) | |
| ns | + | ns | ip | (Matsumoto et al., 2008) | |
| + | + | ns | ip | (Silvestri and Root, 2008) | |
| ns | + | ns | sc | (Langton and Richardson, 2008) | |
| ns | + | No effect | sc | (Bouton et al., 2008) | |
| ns | + | ns | ip | (Lin et al., 2010) | |
| +# | + | + (Re-in) | ip | (Yamada et al., 2009) | |
| + | ns | ns | ip | (Lehner et al., 2010) | |
| ns | +1 | ns | Systemic | (Langton and Richardson, 2010) | |
| No effect* | +* | ns | ip | (Yamada et al., 2011) | |
| No effect | + | ns | ip | (Toth et al., 2012b) | |
| ns | +1 | ns | ip | (Toth et al., 2012b) | |
| ns | + | ns | Systemic | (Gupta et al., 2013) | |
| ns | + | ns | ip | (Matsuda et al., 2010) | |
| + | + | ns | ip | (Bai et al., 2014) | |
| ns | +1* | ns | ip | (Whittle et al., 2013) | |
| ns | + | ns | BLA | (Walker et al., 2002) | |
| ns | +1 | ns | BLA | (Ledgerwood et al., 2003) | |
| ns | + | ns | BLA | (Lee et al., 2006) | |
| ns | + | ns | BLA | (Mao et al., 2008) | |
| ns | + | ns | BLA | (Lee et al., 2006) | |
| ns | + | ns | BLA | (Akirav et al., 2009) | |
| ns | No effect | ns | BLA | (Bolkan & Lattal, 2014) | |
| ns | + | ns | HPC | (Bolkan & Lattal, 2014) | |
| ns | + | ns | HPC | (Ren et al., 2013) | |
| Spermidine | ns | +1 | ns | HPC | (Gomes et al., 2010) |
| Inhibiting NMDA signaling | |||||
| MK-801 (non-competitive NMDA ant) | ns | − | ns | Systemic | (Baker & Azorlosa, 1996); (Storsve et al., 2010) |
| ns | ns | + (Re-in) | sc | (Johnson et al., 2000) | |
| ns | −1 | ns | ip | (Lee et al., 2006); (Liu et al., 2009) | |
| ns | − | ns | Systemic | (Langton et al., 2007); (Chan & McNally, 2009) | |
| CPP (competitive NMDA ant) | No effect | −5 | ns | ip | (Santini et al., 2001); (Sotres-Bayon et al., 2007) |
| No effect | − | ns | mPFC | (Burgos-Robles et al., 2007) | |
| ns | −1 | ns | mPFC | (Burgos-Robles et al., 2007) | |
| − | − | ns | BLA | (Parsons et al., 2010) | |
| AP-5 (competitive NMDA ant) | ns | − | ns | BLA | (Falls et al., 1992) |
| −## | − | ns | BLA | (Lee & Kim, 1998) | |
| ns | −1 | ns | BLA | (Lin et al., 2003a); (Laurent et al., 2008); (Fiorenza et al., 2012) | |
| No effect | − | ns | BLA | (Lin et al., 2003a); (Zimmerman & Maren, 2010) | |
| ns | − | ns | CA1 | (Szapiro et al., 2003) | |
| ns | −1 | ns | CA1 | (Fiorenza et al., 2012) | |
| ns | −1 | ns | mPFC | (Fiorenza et al., 2012) | |
| Inhibiting NMDA signaling | |||||
| ifenprodil (non-comp NR2B–NMDA ant) | − | − | ns | ip | (Sotres-Bayon et al., 2007) |
| ns | −1 | ns | Systemic | (Sotres-Bayon et al., 2009) | |
| − | − | ns | BLA | (Sotres-Bayon et al., 2007); (Laurent & Westbrook, 2008); (Laurent et al., 2008) |
|
| ns | No effect1 | ns | BLA | (Laurent et al., 2008); (Laurent & Westbrook, 2008); (Sotres-Bayon et al., 2009) | |
| No effect | − | ns | mPFC | (Laurent & Westbrook, 2008) | |
| No effect | No effect | ns | mPFC | (Sotres-Bayon et al., 2009) | |
| No effect | −1 | ns | mPFC | (Laurent & Westbrook, 2008); (Sotres-Bayon et al., 2009) | |
| ns | −1 | ns | HPC | (Gomes et al., 2010) | |
| Ro25–6981 (non-comp NR2B–NMDA ant) | − | No effect | ns | ip | (Dalton et al., 2008, 2012) |
| Facilitating mGluR signaling | |||||
| CDPPB (mGluR5 pos modulator) | +3 | ns | ns | sc | (Gass & Olive, 2009) |
| AMN082 (mGluR7 ag) | +* | +* | ns | ip | (Whittle et al., 2013) |
| ns | + | ns | po | (Fendt et al., 2008) | |
| − | No effect | ns | ip | (Toth et al., 2012b) | |
| ns | −1 | ns | ip | (Toth et al., 2012b) | |
| (+) | No effect | ns | BLA | (Dobi et al., 2013) | |
| − | − | ns | mPFC | (Morawska & Fendt, 2012) | |
| Inhibiting mGluR signaling | |||||
| CPCCOEt (non-comp mGluR1 ant) | − | − | ns | BLA | (Kim et al., 2007) |
| No effect | No effect | No effect (SR) | ip | (Mao et al., 2013) | |
| mGluR4 KO | ns | + | ns | No drug | (Davis et al., 2013) |
| mGluR5 KO | − | − | ns | No drug | (Xu et al., 2009) |
| MTEP (mGluR5 ant) | No effect | − | − (SR) | ip | (Mao et al., 2013) |
| ns | ns | − (SR) | BLA | (Mao et al., 2013) | |
| MPEP (allosteric mGluR5 ant) | No effect | No effect | ns | ip | (Toth et al., 2012b) |
| ns | No effect1 | ns | ip | (Toth et al., 2012b) | |
| No effect | − | ns | ip | (Fontanez-Nuin et al., 2011) | |
| No effect | − | ns | IL | (Fontanez-Nuin et al., 2011) | |
| − | − | ns | IL | (Sepulveda-Orengo et al., 2013) | |
| mGluR7 KO | −4 | ns | ns | No drug | (Callaerts-Vegh et al., 2006); (Goddyn et al., 2008) |
| + | + | ns | No drug | (Fendt et al., 2013) | |
| siRNA knock-down of mGluR7 | −2 | ns | ns | No drug | (Fendt et al., 2008) |
| mGluR8 KO | No effect# | No effect | ns | No drug | (Fendt et al., 2013) |
| (S)-3,4-DCPG (mGluR8 ag) | − | No effect | ns | BLA | (Dobi et al., 2013) |
Drug administration following extinction training;
conditioned taste aversion;
cocaine conditioned place preference;
food-rewarded operant conditioning,
reduced locomotion.
Facilitates rescue of impaired fear extinction;
reduced fear expression at the beginning of extinction training;
enhanced fear expression at the beginning of extinction training.
+, Improved; -, impaired; (+) or (−), only minor effects; po, peroral administration; ip, intraperitoneal injection; sc, subcutaneous injection; ns, not studied; BLA, intra-basolateral amygdala administration; HPC, hippocampus; IL, infralimbic cortex; SR, spontaneous recovery; Re-in, reinstatement; ag, agonist; ant, antagonist, KO, knock-out;
The part played in extinction by the metabotropic glutamate receptors mGluR1, mGluR5 and mGluR7 has also been evaluated. Antagonizing mGluR1 (which is expressed on ITC innervating neurons (Busti et al., 2011) with CPCCOEt (Kim et al., 2007) and antagonizing mGluR5 receptors in the IL (Sepulveda-Orengo et al., 2013) have been shown to impair extinction. Furthermore, gene mutations resulting in deficits in mGluR5 (Xu et al., 2009) or mGluR7 (Callaerts-Vegh et al., 2006; Fendt et al., 2008; Goddyn et al., 2008) impair extinction learning, while selective activation (Dobi et al., 2013; Fendt et al., 2008; Morawska & Fendt, 2012; Rodrigues et al., 2002; Siegl et al., 2008; Toth et al., 2012b; Whittle et al., 2013) improves extinction [see Table 4 for summary and (Bukalo et al., 2014; Myers et al., 2011) for more details].
The most extensively studied glutamate receptor in relation to fear extinction is the NMDA receptor (NMDAR). As shown in Table 4, systemic or local administration of NMDAR antagonists into the BLA or mPFC produces extinction deficits [reviewed in (Myers et al., 2011)]. Due to the potential for major side-effects (e.g. excitotoxicity) a general enhancement of NMDAR transmission is clinically undesirable and more subtle modulation of NMDAR signaling is required. NMDARs are specialized voltage-dependent, ligand-gated ion channels that are expressed as heterotetramers formed by GluN1 in combination with GluN2 or GluN3 subunits. To date, eight different splice variants of the GluN1 subunit, four distinctive GluN2 (GluN2A–D) subunits and two GluN3 (GluN3A–B) subunits have been identified [for review see (Paoletti et al., 2013)]. Activation of NMDARs requires L-glutamate binding to GluNR2 subunits, L-glycine or D-serine binding to GluNR1 subunits and membrane depolarization relocating the channel pore-blocking Mg2+ and Zn2+ ions.
The repertoire of NMDAR subunits permits the assembly of a range of NMDARs with varying dissociable signaling properties. Systemic (Dalton et al., 2008; Dalton et al., 2012; Leaderbrand et al., 2014; Sotres-Bayon et al., 2007) or localized BLA (Laurent & Westbrook, 2008; Sotres-Bayon et al., 2007; Sotres-Bayon et al., 2009) or mPFC (Laurent & Westbrook, 2008; Sotres-Bayon et al., 2009) inhibition of GluN2B-containing NMDARs (via ifenprodil or Ro25–6981) disrupts extinction. Conversely, GluN2B overexpression enhances extinction (Tang et al., 1999). Recently, facilitated signaling of GluN2C/D-containing NMDARs in the BLA (via CIQ infusion) was also demonstrated to enhance extinction (Ogden et al., 2014). The contribution of other subunits, such as GluN2A, remains to be determined.
The organization of NMDARs provides additional druggable pharmacological targets. GluN1 and GluN2 subunits are endowed with binding sites for positive and negative allosteric regulations that enable fine-tuning of NMDAR activity. The GluN1 subunit contains an inhibitory H+ binding site rendering GluN2B/D-containing NMDARs in particular sensitive to local pH changes, as well as to endogenous molecules with redox potential (Banke et al., 2005). The GluNR2 subunit is responsive to allosteric modification of NMDAR signaling by polyamines such as spermidine. Spermidine-induced activation of GluN2B signaling facilitates the consolidation of extinction (Gomes et al., 2010; Guerra et al., 2006), while arcaine, an antagonistic polyamine, disrupts extinction (Gomes et al., 2010). A GluN2 allosteric binding site – still uninvestigated in the field of fear extinction – enables modulation of NMDAR signaling by neurosteroids (i.a. allopregnanolone) (Irwin et al., 1994).
Nuanced pharmacological modulation of NMDARs can also be achieved by targeting the Zn2+ binding domain found on GluN2 subunits. Zn2+-binding to this modulatory site mainly inhibits GluN2A–containing NMDARs, by reducing NMDAR channel open probability (Paoletti et al., 1997). Brain Zn2+ signaling which, in addition to the NMDAR binding site, acts via other sites including the GPR39 Zn-sensing receptor (Holst et al., 2007), has been shown to exert both enhancing and attenuating effects on learning and memory via complex interactions with neurotransmitters and synaptic plasticity mechanisms (reviewed in Takeda & Tamano, 2014). Dietary Zn2+ supplementation impairs extinction (Railey et al., 2010), while dietary-induced reduction of brain Zn2+ levels rescues deficient extinction (Whittle et al., 2010). Although these effects may be mediated by Zn2+ modulation of NMDAR signaling, other actions cannot be excluded (e.g., see discussion on HDAC inhibition in Section 4.10: Epigenetics). The same is true for another NMDAR-modulating ion, Mg2+, which has also been shown to facilitate extinction when globally enhanced in the brain (Abumaria et al., 2011; Mickley et al., 2013).
The most extensively investigated allosteric modulation of NMDARs is mediated via the L-glycine binding site on the GluN1 subunit. As shown in Table 4, systemic administration of ligands on this binding site, D-serine or D-cycloserine (DCS), augments extinction consolidation [see (Bukalo et al., 2014; Myers et al., 2011) for recent detailed reviews]. There is evidence (although not consistent) that DCS may promote generalization of the extinction effect (Ledgerwood et al., 2005; Vervliet, 2008) that is, to other cues (e.g. odors, sounds, visual stimuli) that acquired fear during a more complex conditioning situation. Generalized extinction could be of potential clinical benefit, provided that cues that trigger adaptive reactions are not affected. However, DCS administration outside the consolidation window can limit the drug's effectiveness. When extinction or exposure sessions are too short or yield insufficient fear inhibition, DCS can strengthen (re-)consolidation of the original fear memory. Along these lines, the extinction augmenting effects of DCS require the subject to show at least some ability to extinguish (Bolkan & Lattal, 2014; Bouton et al., 2008; Hefner et al., 2008; Smits et al., 2013b; Tomilenko & Dubrovina, 2007; Weber et al., 2007; Whittle et al., 2013). Finally, chronic DCS treatment leads to loss of effects on extinction, possibly via NMDAR desensitization (Parnas et al., 2005; Quartermain et al., 1994). It is of note, that chronic treatment with antidepressants also disrupts the facilitating effects of DCS on extinction, possibly by interfering with NMDAR function (Werner-Seidler & Richardson, 2007).
Notwithstanding these caveats, DCS represents one of the best examples of translational research paving the way for novel anxiety treatments. DCS augmentation of CBT has been clinically studied in various anxiety disorders (Table 4A) including among pediatric patients [see (Hofmann et al., 2013a) for a recent review]. To summarize the current clinical data, DCS-augmented CBT shows advantages in PTSD (de Kleine et al., 2012; Difede et al., 2014; Scheeringa & Weems, 2014), specific phobia (Guastella et al., 2007; Nave et al., 2012; Ressler et al., 2004), SAD (Guastella et al., 2008; Hofmann et al., 2006; Hofmann et al., 2013b; Smits et al., 2013c) and OCD [(Chasson et al., 2010; Farrell et al., 2013; Kushner et al., 2007; Storch et al., 2010; Wilhelm et al., 2008) but see (Mataix-Cols et al., 2014)]. Panic disorder patients (Otto et al., 2010c; Siegmund et al., 2011) also show faster symptom alleviation after DCS-augmented CBT. Reflecting the preclinical findings, negative outcomes were associated with short CBT sessions (Litz et al., 2012), insufficient within-session fear inhibition (Smits et al., 2013b; Tart et al., 2013), chronic DCS treatment (Heresco-Levy et al., 2002), DCS administration outside the therapeutic temporal window (Storch et al., 2007) and subclinical levels of fear [(Guastella et al., 2007; Gutner et al., 2012) but see (Kuriyama et al., 2011)].
Table 4A.
Human trials: D-cycloserine (DCS) combined with CBT.
| Disorder | Study design | Outcome (compared to placebo control group) | Reference |
|---|---|---|---|
| Healthy volunteers | DCS or placebo 2–3 h prior extinction training | No effect on fear extinction or fear recovery in healthy volunteers |
(Guastella et al., 2007) |
| Healthy volunteers | DCS, placebo, valproic acid or combination of DCS and valproic acid 1.5 h prior extinction training |
Administration of DCS, valproic acid or DCS/valproic acid combination facilitates fear extinction and protects from reinstatement |
(Kuriyama et al., 2011) |
| Healthy volunteers | DCS or placebo 2 h prior extinction training | No effect on extinction learning and retention in healthy volunteers |
(Klumpers et al., 2012) |
| Acrophobia | DCS or placebo 1 h prior 2 sessions of virtual reality exposure 3 month follow-up |
Reduced fear symptoms in DCS group at all timepoints | (Ressler et al., 2004) |
| Acrophobia | 2 sessions virtual reality exposure plus DCS or placebo after the sessions 1 month follow-up Re-evaulation of (Tart et al., 2013) |
No advantage of DCS over placebo augmented VRE DCS effect depends on success of exposure sessions (within-session fear reduction) |
(Tart et al., 2013) (Smits et al., 2013a) |
| Snake phobia | DCS or placebo 1 h prior a single exposure session | Same level of improvement with DCS, but DCS group achieved fear reduction quicker |
(Nave et al., 2012) |
| Panic disorder | DCS or placebo 1 h prior CBT session 3–5 1 month follow-up |
DCS group showed greater reduction in panic symptom severity at all timepoints |
(Otto et al., 2010c) |
| Panic disorder with agoraphobia | DCS or placebo 1 h prior 3 individual exposure sessions + 8 group CBT sessions 5 months follow-up |
No benefits of DCS at all timepoints, but initial benefical effects in severely symptomatic patients? |
(Siegmund et al., 2011) |
| PTSD | DCS or placebo 1 h prior 10 weekly exposure sessions |
No overall enhancement of treatment effects. Higher symptom reduction in severe cases. |
(de Kleine et al., 2012) |
| PTSD (combat-related) |
DCS or placebo 30 min prior exposure session 2–6 | Weaker symptom reduction compared to placebo group |
(Litz et al., 2012) |
| PTSD | DCS or placebo 1.5 h prior 12 weekly VRE session 6 months follow-up |
DCS group showed earlier and greater improvement as well as higher remission rates |
(Difede et al., 2014) |
| pediatric PTSD | DCS or placebo 12 1 h prior session 5–12 | No difference in symptom reduction, DCS group showed trend for faster response and better retention in 3 month follow-up |
(Scheeringa & Weems, 2014) |
| SAD | 1× psychoeducation; DCS or placebo 1 h prior to 4 h exposure session (5×, individual or group) 1 month follow-up |
Decreased self-reported social anxiety symptoms in DCS group after 1 month |
(Hofmann et al., 2006) |
| SAD | DCS or placebo 1 h prior to 4 h exposure therapy (5×) |
Fewer social fear and avoidance in DCS group. Significant differences in DCS vs placebo following 3rd exposure session. |
(Guastella et al., 2008) |
| SAD | DCS or placebo with CBT | Greater overall rates of improvement and lower post-treatment severity |
(Smits et al., 2013b) |
| SAD | 12 weeks; DCS or placebo 1h prior 5 exposure sessions (12 sessions at all); 6-months follow-up |
Similar response and remission rates, DCS group improved quicker |
(Hofmann et al., 2013b) |
| OCD | D-Cycloserine (DCS) or placebo 4 h prior each exposure session, 12 weeks (one exposure session/week) |
No statistically significant difference. High response rates in treatment and placebo group. |
(Storch et al., 2007) |
| OCD | 2×/week DCS or placebo 2 h prior exposure and response prevention (ERP) sessions (max 10) 3 months follow-up |
Faster improvements in DCS group No difference between DCS and placebo group (no further benefit). |
(Kushner et al., 2007) |
| OCD | 2×/week DCS or placebo 1 h prior 10 ERP sessions 1 month follow-up Re-evaluation of Wilhelm et al., 2008 |
Faster improvements in DCS group No difference between DCS and placebo group (no further benefit). Specific improvements of DCS in the first 5 sessions, 6× faster than placebo group |
(Wilhelm et al., 2008) (Chasson et al., 2010) |
| Pediatric OCD | DCS or placebo 1 h prior weekly session 4–10 (psychoeduction, cognitive training and ERP) for 10 wks |
Modest reduction of obsessive symptoms | (Storch et al., 2010) |
| Pediatric OCD | DCS or placebo 1 h prior session 5–9 ERP-CBT | Significant improvements in OCD severity from posttreatment to 1-month follow-up in severe and difficult-to-treat pediatric OCD |
(Farrell et al., 2013) |
| Pediatric OCD | DCS or placebo immediately after each of 10 CBT (ERP) sessions 1 year follow-up |
both groups improved; no significant advantage of DCS at any timepoint |
(Mataix-Cols et al., 2014) |
If these factors are properly attended to [see above, reviewed in (Hofmann, 2014)], the evidence supports the adjunctive treatment of CBT with DCS for a range of anxiety disorders.
4.5. γ-Aminobutyric acid (GABA)
GABA is the main inhibitory neurotransmitter in the adult mammalian brain. Because extinction largely reflects the promotion of active inhibitory processes, it is not surprising that GABA serves as an important source of such inhibition. GABA signaling occurs largely via binding to ionotropic GABAA and metabotropic GABAB receptors (former GABAC receptors have been reclassified and are now termed GABAA rho-subclass receptors). GABAA receptors are pentameric, ligand-gated Cl− channels which, upon activation induce hyperpolarization of cells and hence reduce neuronal excitability. Seven classes of GABAA subunits (α1–6, β1–3, γ1–3, ρ1–3, δ, ε, ) have been identified so far, permitting the assembly of highly variable GABAA receptors with distinct functions and signaling properties. Furthermore, several allosteric binding sites, in addition to the GABA binding pocket located at the α/β-subunit interface, allow nuanced modifications of GABAA receptor signaling. The allosteric benzodiazepine (BZD) binding site is localized at the interface of α-and -γ-subunits and when activated, facilitates GABAergic transmission by promoting GABA binding to the receptor and increasing the opening probability of the Cl− channel. The binding sites of other allosteric modulators of GABAA signaling including barbiturates and neurosteroids among others are not yet fully characterized [see Fig. 7 and (Gunn et al., 2014; Makkar et al., 2010)].
Fig. 7.
Overview of research on the role of the GABAergic system in extinction.
In preclinical studies, the robust inhibitory effect of full GABAA receptor agonists at the GABA binding site (α/β-subunit interface) (e.g. muscimol), is often used to inactivate a brain region to clarify its participation in certain functions including extinction. Muscimol-injections into the BLA (Laurent & Westbrook, 2008; Laurent et al., 2008; Sierra-Mercado et al., 2011), mPFC/IL (Laurent & Westbrook, 2008, 2009; Sierra-Mercado et al., 2011) and HPC [(Corcoran et al., 2005; Sierra-Mercado et al., 2011), see Table 5 for a summary] disrupt extinction learning and memory, supporting the crucial involvement of these areas. However, muscimol infusion into the BLA and IL facilitates extinction in some studies (Akirav et al., 2006).
Table 5.
GABAergic signaling in fear extinction (preclinical studies).
| Facilitating GABA signaling | |||||
|---|---|---|---|---|---|
| Drug/manipulation | Extinction training | Extinction retrieval | Longterm extinction | Route | Reference |
| GABAA agonists GABA-binding site (αβ interface) | |||||
| Muscimol | +# | No effect | ns | BLA | (Akirav et al., 2006) |
| ns | +1,7 | ns | BLA | (Akirav et al., 2006) | |
| −# | − | ns | BLA | (Laurent et al., 2008), (Laurent & Westbrook, 2008) | |
| ns | −1 | ns | BLA | (Laurent & Westbrook, 2008) | |
| (+)# | − | ns | BLA | (Sierra-Mercado et al., 2011) | |
| − | − | ns | mPFC | (Laurent & Westbrook, 2008) | |
| ns | −1 | ns | mPFC | (Laurent & Westbrook, 2008) | |
| ns | No effect1 | ns | IL | (Akirav et al., 2006) | |
| +# | (+) | ns | IL | (Akirav et al., 2006) | |
| − | − | ns | IL | (Sierra-Mercado et al., 2011) | |
| No effect | − | ns | IL | (Laurent & Westbrook, 2009) | |
| (+)# | No effect | ns | PL | ((Laurent & Westbrook, 2009); (Sierra-Mercado et al., 2011)) | |
| ns | ns | + (Ren-A)8 | dHPC | (Corcoran et al., 2005) | |
| − | − | No effect (Ren-A) | dHPC | (Corcoran et al., 2005) | |
| (+)# | − | ns | vHPC | (Sierra-Mercado et al., 2011) | |
| GABAA: agonists BZD-binding site αγ interface) | |||||
| Chlordiazepoxide | − | ns | ns | ip | (Goldman, 1977) |
| ns | − | ns | ip | (Bouton et al., 1990) | |
| Diazepam | ns | − | ns | ip | (Pereira et al., 1989) |
| ns | − | ns | ip | (Bouton et al., 1990) | |
| Midazolam | ns | −1 | ns | ip | (Bustos et al., 2009) |
| −##6 | − | ns | ip | (Hart et al., 2009, 2010), (Hart et al., 2014) | |
| −#6 | − | ns | BLA | (Hart et al., 2009, 2010, 2014) | |
| GABAB receptor agonists | |||||
| Baclofen | ns | − | ns | ip | (Heaney et al., 2012) |
| GS39783 (positive modulator GABAB) | ns | No effect | ns | po | (Sweeney et al., 2013) |
| Inhibiting GABA signaling | |||||
| Drug/manipulation | Extinction learning | Extinction retrieval | Longterm extinction | Route | Reference |
| GAD67 KD in BLA | − | ns | ns | No drug | (Heldt et al., 2012) |
| GAD65 KO | −2 | −2 | ns | No drug | (Sangha et al., 2009) |
| α5 KD in HPC | ns | −3 | ns | No drug | (Yee et al., 2004) |
| α1 KO in CRF+ cells | − | − | ns | No drug | (Gafford et al., 2012) |
| Picrotoxin | ns | + | ns | ip | (McGaugh et al., 1990) |
| ns | +4 | ns | IL | (Thompson et al., 2010) | |
| ns | +4 | ns | PL | (Thompson et al., 2010) | |
| ns | +* | ns | (Fitzgerald et al., 2014b) | ||
| Bicuculline | ns | +1 | ns | BLA | (Berlau & McGaugh, 2006) |
| Bicuculline + propanolol | ns | No effect1 | ns | BLA | (Berlau & McGaugh, 2006) |
| FG7142 | − | − | ns | ip | (Harris & Westbrook, 1998) |
| ns | − | ns | ip | (Kim & Richardson, 2007, 2009) | |
| GABAB receptor antagonism | |||||
| GABAB(1b) KO | −5 | ns | ns | No drug | (Jacobson et al., 2006) |
| Phaclofen | ns | No effect | ns | ip | (Heaney et al., 2012) |
| CGP52432 | ns | No effect | ns | po | (Sweeney et al., 2013) |
Drug administration following extinction training;
only cued memory, contextual intact,
trace conditioning,
reconsolidation (injection prior reactivation of memory),
conditioned taste aversion,
midazolam may not impair fear extinction if initial extinction occurs drug-free [see (Hart et al., 2014)],
short extinction training (5 CS presentations),
injection prior renewal-testing.
Reduced fear expression at start of extinction training;
enhanced immobility at start of extinction training;
+, Improved; -, impaired; (+) or (−), only minor effects; ip, intraperitoneal injection; po, peroral administration; ns, not studied; HPC, intra-hippocampal administration; BLA, intra-basolateral amygdala administration; IL, infralimbic cortex; PL, prelimbic cortex; Ren-A, Fear renewal in conditioning context; ag, agonist; ant, antagonist, KO, knock-out;
There is solid evidence that fear extinction learning is associated with upregulation of GABAergic markers in the AMY, underscoring the importance of a certain level of GABAergic signaling in this brain area for the successful formation of extinction memories. For example, levels of gephyrin, a clustering protein facilitating postsynaptic scaffolding of GABAA receptors, were enhanced 2 h following extinction training along with enhanced surface expression of GABAA receptors in the BLA (Chhatwal et al., 2005a). Extinction learning also increases GABAA receptor α2- and β2 subunit mRNA and levels of the GABA-synthesizing enzyme glutamate decarboxylase (GAD) and the GABA transporter GAT1 (Heldt & Ressler, 2007). Further demonstrating the importance of GABAergic signaling to extinction, fear extinction learning is disrupted by mutation-induced deficits in the activity-dependent GAD isoform GAD65 (Sangha et al., 2009), and by viral knock-down of the constitutive GAD isoform GAD67 (Heldt et al., 2012). While these findings suggest that upregulation of GABAergic signaling supports extinction, GABAA receptor antagonists such as picrotoxin or bicuculline can enhance extinction memories when administered systemically (McGaugh et al., 1990), or when infused into the BLA (Berlau & McGaugh, 2006) or specific (fear promoting) areas (PL)of the mPFC (Fitzgerald et al., 2014b; Thompson et al., 2010). The reason for these apparent contradictions is not yet clear.
Preliminary findings from studies using genetically modified mice suggest that the development of subunit-selective GABAA receptor drugs could be a promising way to more distinctly utilize GABAergic mechanisms facilitating extinction. For example, there is reduced mRNA expression of α5 and γ1 subunits in the AMY of a mouse model of enhanced anxiety (Tasan et al., 2011) displaying impaired extinction (Yen et al., 2012). In addition, deletion of α5-containing GABAA receptors in the HPC is sufficient to impair fear extinction recall (Yee et al., 2004) while global deletion of the GABAAα3 subunit produces modest improvements in extinction learning (Fiorelli et al., 2008).
An additional possible explanation for some paradoxical findings concerning GABA regulation and AMY function in extinction is the fact that most of the prior studies have performed pharmacological or genetic manipulations that affect either the entire brain, just the AMY, or even only AMY subregions. Despite this, we now know that GABA receptors are found on most of the neuronal cell types and thus even a subregion-specific manipulation is likely to affect many different cell populations which may have opposing functional effects. One recent attempt to address this complexity involved the use of an inducible GABAa1 knockout strategy limited only to the corticotropin-releasing-hormone containing neuronal population (Gafford et al., 2012)), which revealed relatively specific effects, including enhanced anxiety and extinction deficits. It is likely that future studies aimed at cell-type specific manipulations will help address the vast complexity of the many different cell types combined with the large number of different GABA receptor populations.
As mentioned above, the multiple different binding sites found with-in GABAA receptors can be used to elicit a more nuanced modulation of GABAA receptor activity. Positive allosteric modulation of GABAA receptor activity via BZD, represents the most widely described drug-based strategy for acute treatment of anxiety states (Macaluso et al., 2010). The prevalence of BZD use among patients suffering from mental disorders is high, and is estimated to range between 40 and 70% of these patients (Clark et al., 2004) However anxiolytic treatment with BZDs is recommended only for short periods of time, due to several disadvantages including its sedative effects, the development of dependence and difficulties with discontinuing chronic treatment (Otto et al., 2002). The sedating and calming effects of BZDs might in fact interfere with extinction possibly via reduced arousal and decreased release of neurochemicals (e.g. noradrenaline) and stress hormones (e.g. glucocorticoids) that support extinction [(Bentz et al., 2010), see Section 4.8 Glucocorticoids) for details]. BZDs may also render extinction memories dependent on the drug-induced internal anxiolytic-state (state dependent learning), which upon BZD discontinuation may no longer be accessible. These concerns are borne out by preclinical data showing that systemic (Bouton et al., 1990; Bustos et al., 2009; Goldman, 1977; Hart et al., 2009, 2010, 2014; Pereira et al., 1989) or intra-BLA (Hart et al., 2009, 2010) BZD administration impairs extinction (Table 5), presumably also through state-dependent mechanisms. Systemic treatment with a BZD inverse agonist (Harris & Westbrook, 1998; Kim & Richardson, 2007, 2009) has similar effects (see Table 5 for a summary]. Hence, combining BZD with CBT needs careful consideration. As well as further development of BZDs targeting specific subunits of the GABAA receptor [reviewed in (Griebel & Holmes, 2013)], which provides some hope, there is preliminary evidence that putative novel anxiolytics that do not impair extinction learning [e.g. neuropeptide S; (Slattery et al., in press) see Section 4.7 Neuropeptides] can be developed to help reduce patients' aversion to the unpleasant and demanding procedure of recalling details of their traumatic memories, thus increasing their willingness to interact with the psychotherapist.
In contrast to the relatively large number of studies of the GABAA receptor, only a few studies have investigated the role of GABAB receptors. Functional GABAB receptors are heterodimers containing a GABAB1 and a GABAB2 subunit; the GABAB1 subunit has two isoforms – GABAB1a and GABAB1b (Fritschy et al., 1999). While functional deletion of GABAB receptors (GABAB1a/b knock-out) impairs extinction learning (Jacobson et al., 2006), pharmacological GABAB receptor antagonism does not (Heaney et al., 2012; Sweeney et al., 2013). Systemic administration of (non-selective) agonists or positive allosteric modulators of GABAB signaling also has inconsistent effects (impairing or negative) on extinction [(Heaney et al., 2012; Sweeney et al., 2013) see Table 5]. A final answer to this question regarding the role of GABAB receptors may have to await the availability of GABAB1a-and GABAB1b-selective compounds
Taken together, the available evidence indicates that GABA signaling has a major, but spatially and temporally restricted role in the formation of fear extinction memories. While GABA that is engaged during extinction is likely to support synaptic plasticity, increasing GABAergic tone globally seems to limit the efficacy of extinction-based therapies by mechanisms outlined above. Therefore, it is unlikely that drugs broadly targeting the GABA system will be useful in this respect. Pharmacological adjuncts to exposure therapy targeting the GABA system would rather need to strike a balance between driving and maintaining relevant GABA activity without over-activating the system. The evidence so far suggests that subunit-selective or allosteric (distinct from BZD) modulation of GABAA receptor signaling may hold promise in this regard.
4.6. Cannabinoids
A growing body of literature demonstrates that the endogenous cannabinoid (eCB) system modulates neuronal excitability in stressful and fearful situations (de Bitencourt et al., 2013; Gunduz-Cinar et al., 2013a). eCB signaling has been implicated in emotional and cognitive processing of threatening stimuli, for instance during the consolidation of fear and fear extinction memories (for more detailed recent reviews see (Riebe et al., 2012; Gunduz-Cinar et al., 2013b). Upon neuronal activation, an eCB (anandamide) and 2-arachidonoylglycerol (Fig. 8) are rapidly synthesized in the postsynaptic neuron (within minutes) and released into the synaptic cleft to modulate presynaptic signaling by the activation of Gi/0-coupled CB-1 receptors (CB-2 receptors are mainly expressed on immune cells, osteoblasts, osteoclasts and hematopoietic cells but also on neuroglia).
Fig. 8.
Overview of research on the role of the cannabinoid system in extinction.
Supporting a role of eCB signaling in the extinction of aversive memories (see Fig. 8 for overview), there are increased eCB levels in the mouse BLA following extinction training (Marsicano et al., 2002). Conversely, PTSD patients show low brain anandamide levels (Neumeister et al., 2013). In rodents, facilitation of eCB signaling by CB-1 receptor agonists or exogenous cannabinoids [cannabidiol, (Bitencourt et al., 2008)], reuptake inhibitors [AM404, (Bitencourt et al., 2008; Chhatwal et al., 2005b; Pamplona et al., 2008)] or anandamide degradation blockers [AM3506, URB 597, (Gunduz-Cinar et al., 2013b)] enhances the formation or consolidation of extinction memories. Blockade or knock-out of CB1-receptors (Cannich et al., 2004; Kamprath et al., 2006; Marsicano et al., 2002; Niyuhire et al., 2007; Pamplona et al., 2006; Plendl & Wotjak, 2010; Reich et al., 2008; Terzian et al., 2011) impairs extinction and blocks the pro-extinction effects of drugs that facilitate eCB transmission (Chhatwal et al., 2005b; Do Monte et al., 2013; Gunduz-Cinar et al., 2013b) (see Table 6). Collectively, these observations point to CB-1 receptor mediated eCB signaling as a powerful modulator of extinction.
Table 6.
Cannabinoid signaling in fear extinction (preclinical studies).
| Enhanced cannabinoid signaling | |||||
|---|---|---|---|---|---|
| Drug/manipulation | Extnction training |
Extinction retrieval |
Longterm extnction |
Route | Reference |
| Cannabidiol | + | + | ns | icv | (Bitencourt et al., 2008) |
| + | + | ns | IL | (Do Monte et al., 2013) | |
| Anandamide | ns | +1 | ns | dCA1 | (de Oliveira Alvares et al., 2008) |
| AM404 (eCB uptake inhibitor) | ns | + | ns | ip | (Pamplona et al., 2008) |
| ns | + | + (Re-in) | ip | (Chhatwal et al., 2005b) | |
| + | (+) | ns | icv | (Bitencourt et al., 2008) | |
| No training | + | ns | IL | (Lin et al., 2009) | |
| AM3506 (FAAH inhibitor) | ns | + | ns | ip | (Gunduz-Cinar et al., 2013b) |
| WIN 55,212-2 (low-dose CB1 Ag) | +* | No effect2 | ns | ip | (Pamplona et al., 2006) |
| WIN 55,212-2 (high-dose CB1 Ag) | (−)* | No effect2 | ns | ip | (Pamplona et al., 2006) |
| WIN 55,212-2 (CB1 Ag) | +° | +° | ns | ip | (Pamplona et al., 2006) |
| ns | + | nd | ip | (Pamplona et al., 2008) | |
| WIN 55,212-2 (CB1 Ag) | ns | +4 | ns | BLA | (Ganon-Elazar & Akirav, 2013)) |
| ns | +4 | ns | HPC | (Ganon-Elazar & Akirav, 2013) | |
| ns | No effect4 | ns | mPFC | (Ganon-Elazar & Akirav, 2013) | |
| ns | + | No effect (Re-in, SR3) | IL | (Lin et al., 2009) | |
| No training | + | ns | IL | (Lin et al., 2009) | |
| ns | No effect | ns | PL | (Kuhnert et al., 2013) | |
| HU210 | No training | + | ns | IL | (Lin et al., 2009) |
| URB597 (AEA hydrolysis inhibitor) | No training | + | ns | IL | (Lin et al., 2009) |
| AM3506 (FAAH inhibitor) + SR141716A (CB1 ant) |
ns | No effect | ns | ip + BLA | (Gunduz-Cinar et al., 2013b) |
| AM3506 (FAAH inhibitor) | ns | + | ns | BLA | (Gunduz-Cinar et al., 2013b) |
| No training | No effect | ns | BLA | (Gunduz-Cinar et al., 2013b) | |
| Cannabidiol + SR141716A (CB1 ant) | No effect | No effect | ns | IL + ip | (Do Monte et al., 2013) |
| Reduced cannabinoid signaling | |||||
| Drug/manipulation | Extnction learning |
Extinction retrieval | Longterm extinction | Administration route | Reference |
| CB1 KO | − | − | ns | No drug | (Cannich et al., 2004) |
| CB1 KO | − | ns | ns | No drug | ((Dubreucq et al., 2010); (Kamprath et al., 2006); (Marsicano et al., 2002); (Plendl & Wotjak, 2010)) |
| D1-CB1 KO | − | ns | ns | No drug | (Terzian et al., 2011) |
| SR141716A CB1 ant | ns | No effect1 | ns | sc | (Marsicano et al., 2002) |
| − | ns | ns | sc, ip | ((Kamprath et al., 2006); (Niyuhire et al., 2007); (Plendl & Wotjak, 2010) |
|
| ns | −1 | ns | ip | (Suzuki et al., 2004) | |
| − | No effect | ns | ip | (Pamplona et al., 2006, 2008) | |
| ns | − | ns | ip | (Chhatwal et al., 2005b) | |
| −## | No effect | ns | ip | (Bowers & Ressler, 2015) | |
| AM251 (CB1 inverse ag) | − | ns | ns | ip | (Reich et al., 2008) |
| ns | −1 | ns | dCA1 | (de Oliveira Alvares et al., 2008) | |
| ns | − | ns | IL | (Lin et al., 2009) | |
| No training | No effect | ns | IL | (Lin et al., 2009) | |
| ns | − | ns | PL | (Kuhnert et al., 2013) | |
Drug administration following extinction training;
drug-free retrieval of contextual fear memory;
5 days,
single-prolonged-stress model: injection immediately after trauma.
Recent memory;
remote memory;
enhanced immobility at start of extinction training;
+, Improved; -, impaired; (+) or (−), only minor effects; ip, intraperitoneal injection; ip, sc, subcutanous injection; ns, not studied; HPC, intra-hippocampal administration; BLA, intra-basolateral amygdala administration; IL, infralimbic cortex; PL, prelimbic cortex; CA1, cornu ammonis 1; Re-in, reinstatement; SR, spontaneous recovery, ag, agonist; ant, antagonist, KO, knock-out;
Following up on these studies, intra-cerebral infusions of CB1-receptor agonists and antagonists have revealed a potential role of mPFC-BLA eCB signaling in extinction [see Table 6, (Do Monte et al., 2013; Ganon-Elazar & Akirav, 2013; Gunduz-Cinar et al., 2013b)]. CB1-receptor activation inhibits both presynaptic glutamate and GABA release. The release of anandamide and concomitant CB1-receptor activation induce long-term depression of inhibitory transmission (LTDi) in the BLA (Azad et al., 2004; Gunduz-Cinar et al., 2013b). In addition, a recent study demonstrated that enhanced inhibitory input via activation of CB1 receptor expressing cholecystokinin (CCK) positive interneurons selectively reduces firing of BLA fear neurons and promotes extinction (Trouche et al., 2013). Of note is the fact that it has also been shown that one potential mechanism for the CB1 effect on extinction within the AMY is via blockade of CCK release, as an intra-AMY CCK antagonist reversed the extinction blockade of systemic CB1 antagonists, and CCK agonists block extinction (Chhatwal et al., 2009).
In summary, current preclinical data suggest that eCB signaling gates BLA function through plastic changes, including a long-lasting depression of inhibition, to modulate extinction-related AMY activity. Initial results from clinical studies in healthy volunteers suggest that exogenous cannabinoid administration strengthens extinction and protects against reinstatement [see Table 6A, (Das et al., 2013; Rabinak et al., 2013)]. Future clinical trials in extinction-impaired PTSD patients will be needed to show whether cannabinoids (or substances which increase endogenous eCB signaling, such as FAAH inhibitors) might prove useful as adjuncts to CBT-based therapy.
Table 6A.
Human trials: cannabinoids combined with CBT.
| Disorder | Study design | Outcome (compared to placebo control group) | Reference |
|---|---|---|---|
| Healthy volunteers | Fear conditioning paradigm, cannabidiol inhalation prior or following extinction |
Reduced fear in retrieval Protection against reinstatement |
(Das et al., 2013) |
| Healthy volunteers | Fear conditioning paradigm, tetrahydrocannabinol prior extinction | No effect | (Klumpers et al., 2012) |
| Healthy volunteers | Fear conditioning paradigm, Dronabinol prior extinction | Reduced fear in retrieval | (Rabinak et al., 2013) |
4.7. Neuropeptides (oxytocin, neuropeptide Y, neuropeptide S, opioids)
Various neuropeptides and their corresponding receptors are localized in brain regions mediating emotional behavior and stress response and have been considered as potential anxiolytic drug targets (Bowers et al., 2012; Ebner et al., 2009; Griebel & Holmes, 2013). While other neuroptides such as hypocretin/Orexin (Flores et al., 2014) may also be involved in fear extinction, we will here address the role of oxytocin, neuropeptide Y, neuropeptide S and opioids in extinction.
4.7.1. Oxytocin
The oxytocin (OXT) system, strongly implicated in social cognition and behavior, is posited to play a role in anxiety disorders with impaired social functioning (Meyer-Lindenberg et al., 2011; Neumann & Landgraf, 2012). OXT is synthesized in the paraventricular (PVN) and supraoptic nuclei of the hypothalamus and processed along the axonal projections to the posterior pituitary gland. Upon neuronal activation OXT is released from secretory vesicles into the peripheral circulation as neurohypophyseal hormone. The central nervous system (CNS) component of OXT is derived from dendritic release of the same cell population (Ludwig & Leng, 2006), as well as from the OXT-synthesizing parvocellular PVN cells sending projections throughout the brain [reviewed in (Gimpl & Fahrenholz, 2001)]. OXT receptors are typically Gq-protein coupled and have potential allosteric binding sites for Mg2+ and steroids like cholesterol. The distribution of OXT receptors has not been fully delineated, but OXT fibers and neurosecretory nerve endings have been described in, among other regions, the AMY [especially CeL (Knobloch et al., 2012; Veinante & Freund-Mercier, 1997)], the dorsal and ventral HPC and the main monoamine nuclei – substantia nigra (dopamine), raphe nuclei (serotonin) and the locus coeruleus (noradrenaline) [see (Gimpl & Fahrenholz, 2001) for review).
Regulatory effects of OXT on stress-induced activation of the hypothalamic–pituitary–adrenal (HPA) axis, as well as direct OXT effects on the brain and specific parts of the extinction circuitry, can affect fear extinction in various ways. Intracerebroventricular (icv) infusion of OXT prior to fear conditioning does not affect fear learning but facilitates later extinction acquisition and retrieval (Toth et al., 2012a). Conversely, OXT receptor antagonists impair extinction learning and retrieval when administered icv prior to fear acquisition (Toth et al., 2012a). More localized OXY infusions into the central CeA reduce fear expression by inhibiting CeM excitatory output to fear-eliciting brainstem structures (Huber et al., 2005) (Viviani et al., 2011), suggesting one possible mechanism for the effects of icv OXY on extinction.
Another potential mechanism might be OXT-induced dampening of stress-induced activation of the HPA axis (de Oliveira et al., 2012; Heinrichs et al., 2003; Quirin et al., 2011; Windle et al., 1997). As explained in more detail in Section 4.8 Glucocorticoids, glucocorticoid release during an actual learning process facilitates cognitive processing [reviewed in (Bentz et al., 2010)], hence the release of endogenous OXT during traumatic experiences (Zoicas et al., 2012) may interfere with the consolidation of fear memories. Along these lines, OXT-mediated reduction of HPA axis activity and subsequent lower glucocorticoid levels could interfere with fear acquisition, resulting in a weaker fear memory that is more susceptible to extinction training. In this context, OXT administration either icv or intra BLA prior to extinction training disrupts extinction learning [(Toth et al., 2012a) (Lahoud & Maroun, 2013) see Table 7 for summary]. OXT infusions into the dorsolateral septum prior to extinction training have the opposite effect and facilitate extinction learning and retrieval in a social fear conditioning paradigm that may recruit a somewhat different neural circuitry than non-social forms of fear (Zoicas et al., 2012).
Table 7.
Neuropeptide signaling in fear extinction (preclinical studies).
| Neuropeptides | |||||
|---|---|---|---|---|---|
| Drug/manipulation | Extinction training | Extinction retrieval | Longterm extinction | Route | Reference |
| Oxytocin | − | ns | ns | icv | (Toth et al., 2012a) |
| +# | + | ns | DLS | (Zoicas et al., 2012) | |
| −*5 | −*5 | ns | ip | (Eskandarian et al., 2013) | |
| Chronic oxytocin (30d) | ns | No effect6 | ns | in | (Bales et al., 2014) |
| NPY | + | + | ns | icv | (Gutman et al., 2008) |
| NPY KO | −3*** | − | ns | No drug | (Verma et al., 2012) |
| NPY Y1 KO | − | No effect | ns | No drug | (Verma et al., 2012) |
| NPY Y2KO | No effect | No effect | ns | No drug | (Verma et al., 2012) |
| NPY Y1/Y2 KO | − | − | ns | No drug | (Verma et al., 2012) |
| Leu31Pro-NPY (Y1 ag) | ns | + | ns | BLA | (Lach & de Lima, 2013) |
| BIBO 3304 (Y1 ant) | ns | − | ns | BLA | (Gutman et al., 2008) |
| NPS | (+)4 | No effect | No effect | BLA | (Jungling et al., 2008) |
| + | ns | ns | icv | (Slattery et al., in press) | |
| SHA68 (NPS-R ant) | −)4, *** | − | − (Ren-A) | BLA | (Jungling et al., 2008) |
| Naloxone | − | − | ns | Systemic | (McNally & Westbrook, 2003) |
| ns | No effect1 | ns | Systemic | (McNally & Westbrook, 2003) | |
| − | − | ns | vlPAG | (McNally et al., 2004) | |
| − | − | ns | vlPAG | (Parsons et al., 2010) | |
| No effect | No effect | ns | BLA | (Parsons et al., 2010) | |
| CTAP (MOR ant) | − | − | ns | vlPAG | (McNally et al., 2005) |
| Dynorphin KO | −3 | − | ns | No drug | (Bilkei-Gorzo et al., 2012) |
| nor-BNI (KOR ant) | ns | −1 | ns | Systemic | (Bilkei-Gorzo et al., 2012) |
Drug administration following extinction training;
social fear conditioning;
animals show accelerated fear learning;
administration 2h prior extinction training;
single prolonged stress model;
BTBR mouse autism model and C57BL6J.
Enhanced fear expression at the beginning of extinction training,
reduced fear expression at the beginning of extinction training.
+, Improved; -, impaired; (+) or (−), only minor effects; ip, intraperitoneal injection; icv, intracerebroventricular injection; ns, not studied; HPC, intra-hippocampal administration; BLA, intra-basolateral amygdala administration; DLS, dorsolateral septum; vlPAG, ventrolateral periaqueductal gray; Ren-A, fear renewal in conditioning context; ag, agonist; ant, antagonist, KO, knock-out;
Considering that systemically applied neuropeptides do not cross the blood–brain-barrier, clinical use of neuropeptides would require a direct pathway to the human brain. In the case of OXT, this may be feasible via intranasal administration (Born et al., 2002). Intranasal OXT administration has been demonstrated to reduce stress-induced cortisol release in humans (de Oliveira et al., 2012; Heinrichs et al., 2003; Quirin et al., 2011), and to attenuate AMY hyperactivity as well as AMY-hindbrain coupling in anxiety patients [(Kirsch et al., 2005; Labuschagne et al., 2010)]. In a recent study investigating the effects of intranasal OXT treatment on fear extinction in healthy volunteers, OXT increased initial fear expression during extinction training, but this effect was gone by the end of training and there was a lower level of fear in the OXT treated group in extinction retrieval (Acheson et al., 2013). These data raise the prospect of OXT-augmented CBT effectiveness.
4.7.2. Neuropeptide Y
Neuropeptide Y (NPY) contains 36 amino acids and is the most widely expressed neuropeptide in the mammalian brain. There are particularly high concentrations of NPY in the limbic system, including in the AMY, the HPC and the periaqueductal gray. Six G-protein coupled NPY receptors (Y1–6) have been identified, among which Y1, Y2, Y4, and Y5 mediate central effects. Y1 mRNA is present at a high level in brain areas involved in memory processing, namely the AMY, HPC, thalamus, hypothalamus, and the cerebral cortex. The AMY, HPC, and hypothalamus are also rich in Y2 receptors, while Y5 receptors can be detected in HPC, cingulate cortex and thalamic and hypothalamic nuclei (Parker & Herzog, 1999) [reviewed in (Holmes et al., 2003)].
Icv administration of NPY facilitates extinction learning (Gutman et al., 2008), while genetic deletion of NPY disrupts extinction acquisition and subsequent retrieval (Verma et al., 2012). Extinction deficits caused by NPY knock-out are rescued by AAV-mediated NPY re-expression in the BLA (Verma et al., 2012), where NPY co-localizes with GABAergic neurons modifying inhibitory control of BLA projection neurons (McDonald & Pearson, 1989). To elucidate which NPY receptors mediate the BLA-associated effects on extinction, the consequences of gene deletion via either deletion of Y1 or Y2 receptors or double Y1/Y2 deletion have been examined. Deletion of Y1 receptors impaired extinction learning, while Y2 receptor deletion had no effect (Verma et al., 2012). Underscoring the importance to extinction of Y1 receptors in the BLA, local infusion of the Y1 agonist Leu31Pro34-NPY prior to extinction training led to stronger extinction retrieval (Gutman et al., 2008; Lach & de Lima, 2013). Further studies are required to further delineate the underlying mechanisms of Y1-mediated facilitation of extinction, and to investigate the potential clinical use of NPY and Y1 receptor agonism as adjunctive therapy to CBT.
4.7.3. Neuropeptide S
Neuropeptide S (NPS) is synthesized in neurons located adjacent to the locus coeruleus (Xu et al., 2007) and binds to G-protein coupled NPS receptors expressed in the BLA among other areas. Intra-BLA infusion of exogenous NPS accelerates extinction learning, while BLA NPS receptor antagonism (via SHA68) impairs extinction learning and retrieval and increases fear renewal (Chauveau et al., 2012; Jungling et al., 2008) (Table 7). NPS treatment also rescues deficient extinction learning in hyperanxious rats (Slattery et al., in press), exhibiting aberrant cortico-AMY activation (Muigg et al., 2008). The activation of NPS receptors in the BLA increases excitatory input to GABAergic ITCs, thus increasing feed-forward inhibition to CeM neurons and reducing fear expression (Meis et al., 2008; Meis et al., 2011; Pape et al., 2010). Exogenous NPS administration is also associated with enhanced DA release in the mPFC (Si et al., 2010), suggesting another mechanism for strengthening extinction (see Section 4.2 Dopaminergic system).
Underscoring the translational relevance of NPS, healthy human volunteers carrying the NPS receptor polymorphism rs324981, which potentiates NPS efficacy at the receptor, show enhanced extinction in a virtual reality fear potentiated startle paradigm (Glotzbach-Schoon et al., 2013). Additional studies are required to assess whether increasing NPS signaling improves extinction in anxiety patients. This would be aided by the development of small molecule NPS receptor agonists.
4.7.4. Opioids
The opioid peptide family comprises different members including endorphins, enkephalins, dynorphins and hemorphins. The 3 classical opioid receptors [µ (MOR), κ (KOR) and δ (DOR)] show heterogeneous expression throughout the brain and are located in extinction-relevant brain areas including the AMY, mPFC and HPC. Typically, MOR, KOR and DORs are coupled to Gi-proteins, reducing (in most cases) net neuronal excitability and reducing neurotransmitter release upon activation [reviewed in (Nutt, 2014)].
There is evidence that morphine application (intended for pain management) following an acute trauma attenuates the incidence of PTSD at later timepoints (Bryant et al., 2009; Holbrook et al., 2010; Melcer et al., 2014; Szczytkowski-Thomson et al., 2013). Aside from this potential PTSD preventing property of opioids, several studies have tried to determine the importance of opioids in extinction.
Systemic administration of the opioid receptor antagonist naloxone (which has highest affinity to MOR, but also inhibits KOR and DOR) impairs extinction when given prior to extinction training (McNally & Westbrook, 2003; McNally et al., 2004) but not when applied after extinction training or prior to retrieval, see Table 7). Subsequent investigations have demonstrated that extinction-impairing effects are mediated by MORs in the PAG [as shown with the selective MOR antagonist CTAP in the PAG (McNally, 2005; Parsons et al., 2010)].
The AMY ITCs express high levels of MOR (Busti et al., 2011), and lesioning of MOR-expressing neurons in the ITCs following extinction training impairs extinction retrieval (Likhtik et al., 2008), indicating that MOR signaling in the ITCs contributes to extinction consolidates. Probably due to the high addiction potential of MOR agonists, no clinical studies have investigated their potential in CBT. However, treatment with the opioid receptor antagonist naloxone prior to exposure therapy is associated with reduced treatment success (Arntz et al., 1993; Egan et al., 1988), which is in accordance with preclinical results (Table 7).
Another opioid receptor gaining attention in the field of fear extinction is the KOR, activated by endogenous dynorphins (reviewed in Schwarzer, 2009). Human volunteers with single nucleotide polymorphisms in the prodynorphin gene showed increased skin conductance (a physiological measure of fear) during fear extinction training and blunted functional connectivity between AMY and mPFC (Bilkei-Gorzo et al., 2012). Along these lines, genetic deletion of dynorphin or systemic antagonism of KORs in rodents impairs extinction learning and reduces neuronal activity in the BLA and mPFC (Bilkei-Gorzo et al., 2012). In contrast to these extinction-impairing effects, systemic administration of KOR antagonists protects against fear renewal (Cole et al., 2011) – an effect mimicked by local infusions into the ventral HPC (Cole et al., 2013). Additional studies will help clarify these effects and their possible relevance to clinical use of opioidergic drugs in conjunction with exposure-based therapies.
4.8. Glucocorticoids
Emotionally arousing experiences activate the HPA axis and the release of glucocorticoids (GCs) into the bloodstream. GCs which include cortisol in humans, and corticosterone in rodents are lipophilic and readily cross the blood–brain-barrier to interact with glucocorticoid (GR) and mineralocorticoid receptors (MR) in the brain. GRs are widely expressed throughout the brain including the AMY, HPC and PFC (Bentz et al., 2010). Early pioneering studies reported that extinction-memory formation is disrupted by adrenalectomy (Silva, 1973), as well as, more recently, by compounds that antagonize GRs, such as mifepristone (Yang et al., 2006), or that inhibit corticosterone synthesis [metapyrone, (Barrett & Gonzalez-Lima, 2004; Blundell et al., 2011; Harrison et al., 1990; Yang et al., 2006, 2007)] (see Table 8). Conversely, systemic administration of corticosterone (Blundell et al., 2011) or the GR agonists dexamethasone or RU28362 (Ninomiya et al., 2010; Yang et al., 2006, 2007) facilitate the consolidation of aversive and appetitive extinction memories (see Table 8).
Table 8.
Glucocorticoid signaling in fear extinction (preclinical studies).
| Facilitating glucocorticoid signaling | |||||
|---|---|---|---|---|---|
| Drug | Extinction training | Extinction retrieval | Long-term extinction | Route | Reference |
| Corticosterone (GR/MR ag) | ns | +1 | No effect (Re-in) | ip | (Blundell et al., 2011) |
| ns | −2 | ns | ip | (Den et al., 2014) | |
| Dexamethasone (GR ag) | ns | + | ns | ip | (Ninomiya et al., 2010); (Yang et al., 2006, 2007) |
| RU28362 (GR ag) | ns | + | ns | AMY | (Yang et al., 2006) |
| Ganoxolone (allopregnanolone analog) | +*3 | +*3 | ns | ip | (Pinna & Rasmusson, 2014) |
| Inhibiting glucocorticoid signaling | |||||
| Metyrapone (CORT/cortisol synthesis inhibitor) | No effect | − | −* | sc | (Barrett & Gonzalez-Lima, 2004); (Clay et al., 2011) |
| ns | − | ns | sc | (Blundell et al., 2011); (Yang et al., 2006, 2007) | |
| Mifepristone (GR and progesterone ant) | ns | − | ns | AMY | (Yang et al., 2006) |
Drug administration following extinction training;
in adolescent but not adult rats if exposed to CORT one week prior to (drug free) testing; adults also show impaired extinction retrieval if they were exposed to one week of CORT in their adolescence;
socially isolated mice, CAVE ganoxolone was administered following a reactivation session 24h prior extinction training.
+, Improved; -, impaired; ip, intraperitoneal injection; sc, subcutaneous injection; ns, not studied; AMY, intra-amygdala administration; Re-in, reinstatement; ag, agonist; ant, antagonist; GR, glucocorticoid receptor; MR, mineralocorticoid receptor; CORT, corticosteroid.
How can stress hormones promote the formation of extinction memories? Cytosolic GRs induce genomic responses by binding to glucocorticoid responsive elements within promoter regions of GC-responsive genes, and act as transcription factors inducing the expression of learning-related genes. In addition, membrane-bound GRs can induce non-genomic actions of relevance to extinction, including the synthesis of eCBs (see Section 4.6 Cannabinoids) from membrane phospholipids (Di et al., 2003; Hill et al., 2005, 2011). Further supporting a link between GRs and eCBs, and possibly of importance in GC-augmented extinction consolidation, adrenalectomy leads to reduced CB-1 receptor expression (Mailleux & Vanderhaeghen, 1993) and GC-mediated memory consolidation is prevented by CB-1 receptor antagonists (Campolongo et al., 2009).
GRs can also induce indirect learning-relevant genomic actions via activation of intracellular signaling cascades such as the ERK/MAPK pathway (reviewed in Reul, 2014) in cooperation with other neuro-transmitter systems or ion channels including β-adrenoceptors (Roozendaal et al., 2008), and L-type calcium channels (Karst et al., 2002). These interrelated effects are functionally relevant, given that the infusion of β-adrenoceptor antagonists into the BLA blocks the memory-facilitating effects of systemically administered glucocorticoids (Quirarte et al., 1997; Roozendaal et al., 2002).
Another important functional interaction occurs between GCs and the glutamate system. GCs potentiate glutamatergic signaling via genomic and non-genomic mechanisms, by (i) increasing the readily releasable pool of presynaptic glutamate vesicles, and (ii) enhancing NMDA and AMPA receptor surface expression through increased receptor trafficking (reviewed in Popoli et al., 2012). The concomitant activation of GC and NMDA receptors, which can occur during emotionally arousing experiences, induces downstream nuclear mechanisms that result in histone modifications (reviewed in Reul, 2014), an epigenetic mechanism implicated in extinction (see Section 4.10: Epigenetics for details). Underscoring the potential importance of emotional arousal and thus of GC release in this process, pretreatment with the anxiolytic BZD lorazepam (resulting in reduced arousal in response to the stressor) blocked epigenetic modifications on Histone H3, while application of the anxiogenic BZD inverse agonist FG7142 (Singewald et al., 2003) induced transcription facilitating acetylation and phosphorylation on histone tails (Papadopoulos et al., 2011). These epigenetic effects could contribute to the aforementioned extinction impairing effects of BZDs (see Section 4.5: γ-Aminobutyric acid (GABA) and Table 5).
In contrast to the effects of acute GC elevations, chronic high levels of corticosterone reduce cell-surface NMDA and AMPA receptor expression (Gourley et al., 2009). This loss of critical plasticity mechanisms might be one explanation as to why anxiety patients with a history of repeated traumatic events, such as combat veterans, show greater resistance to treatment. However, a considerable proportion of PTSD patients have reduced cortisol levels (Yehuda, 2004) and small case studies suggest that there are beneficial effects of CBT and adjunctive cortisol administration in PTSD patients (Yehuda et al., 2010). A number of larger studies are under way to extend this work (NCT01108146, NCT00751855, NCT01525680). In addition, it has been found that cortisol-augmented CBT has efficacy in acrophobia (de Quervain et al., 2011), arachnophobia (Soravia et al., 2006, 2014) and social phobia [(Soravia et al., 2006) see Table 8A for a summary]. Whether cortisol augmented CBT for non-phobic anxiety disorders, including also GAD, facilitates fear inhibition is currently being investigated in ongoing clinical studies (see Cain et al., 2012). Furthermore, future studies may implement more selective GC agonists than cortisol (which is also acting on MRs) to avoid nonspecific side effects.
Table 8A.
Human trials: glucocorticoids combined with CBT.
| Disorder | Study design | Outcome (compared to placebo control group) | Reference |
|---|---|---|---|
| Social phobia | Cortisone or placebo administered orally 1 h before a socio-evaluative stressor |
Reduced self-reported fear during anticipation and exposure | (Soravia et al., 2006) |
| Spider phobia | Cortisol or placebo administered orally 1 h before exposure to a spider photograph in 6 sessions distributed over 2 weeks |
Progressive reduction of stimulus-induced fear Reduction of fear was maintained also 2 days following session ending (OFF-drug) |
(Soravia et al., 2006) |
| Spider phobia | Cortisol or placebo administered orally 1 h before 2 exposure therapy sessions Follow-up after 1 month |
Significant decrease in phobic symptoms assessed with the Fear of Spiders Questionnaire Cortisol-treated patients reported significantly less anxiety during exposure to living spiders at follow-up |
(Soravia et al., 2014) |
| Acrophobia | Cortisol or placebo was administered orally 1 h before virtual reality exposure to heights Follow-up after 1 month |
Greater reduction in fear of heights measured with the acrophobia questionnaire both at post-treatment and at follow-up |
(de Quervain et al., 2011) |
| PTSD (combat-related) | Memory reactivation task followed by intravenous administration of cortisol or saline Follow-up after 1 month |
Reduced PTSD symptomatic in cortisol-treated patients No differences between cortisol and saline treated patients (initial improvement was lost after 1 month) |
(Suris et al., 2010) |
| PTSD | 10 weekly sessions of prolonged exposure therapy, cortisol or placebo 30min prior session 3–10 |
Accelerated and greater decline in PTSD symptoms Caveat – includes only 2 patients (1 cortisol/1 placebo) |
(Yehuda et al., 2010) |
4.9. Neurotrophins and miscellaneous targets
4.9.1. Fibroblast growth factor-2
Fibroblast growth factor-2 (FGF2) is a multi-functional growth factor involved in brain development and learning-related molecular signaling cascades (reviewed in Graham & Richardson, 2011a). FGF2 signaling is associated with glutamate-mediated synaptic plasticity (Numakawa et al., 2002), L-type voltage gated calcium channel expression and activation (Shitaka et al., 1996) and phosphorylation of both MAPK (Abe & Saito, 2000) and CREB (Sung et al., 2001). FGF2 also promotes LTP in the HPC (Terlau & Seifert, 1990). Hence, FGF2 interacts with the molecular tools required for the formation and consolidation of extinction memories. FGF receptors are tyrosine kinase receptors expressed widely throughout the brain, including in areas within the extinction circuitry such as the HPC and the CeA, and FGF2 expression in the HPC and the mPFC is induced under stress (Molteni et al., 2001), thus suggesting that emotionally arousing situations requiring new learning generate increased FGF2 signaling which supports the formation of emotional memories.
FGF2 has been shown to cross the blood–brain barrier (Deguchi et al., 2000) and pioneering work demonstrates that systemic administration of FGF2 prior to or following extinction training facilitates the consolidation of extinction memories (Graham & Richardson, 2009, 2010). Local infusion into the BLA replicates the extinction-facilitating effects of systemic FGF2 (Table 9), demonstrating at least one key site of this neurotrophin's action (Graham & Richardson, 2011b). Of significant importance, is the fact that FGF2-augmented extinction has been associated with enhanced protection against return of fear phenomena including renewal and reinstatement [(Graham & Richardson, 2009, 2010, 2011b), see Table 9 for details] making it an interesting candidate for future clinical applications. Clinical studies investigating the potential of FGF2 in CBT could be implemented in the near future as human trials investigating FGF2 for angiogenesis have already shown some drugs to be safe (Laham et al., 2000).
Table 9.
Neurotrophins and miscellaneous targets in fear extinction (preclinical studies).
| Neurotrophins | |||||
|---|---|---|---|---|---|
| Drug/manipulation | Extinction learning |
Extinction retrieval |
Long-term extinction |
Route | Reference |
| FGF2 | (+)# | + | ns | sc | (Graham & Richardson, 2009) |
| ns | +1 | + (Re-in) (Ren-A) | sc | (Graham & Richardson, 2009, 2010) | |
| ns | +1 | + (Ren-A) | BLA | (Graham & Richardson, 2011b) | |
| BDNF | No training | +**2 | No effect (Re-in) | IL | (Peters et al., 2010); (Rosas-Vidal et al., 2014) |
| No training | No effect2 | ns | PL | (Rosas-Vidal et al., 2014) | |
| 7.8-Dihydroxyflavone | (+)* | No effect | (+) (Re-in) | ip | (Andero et al., 2011) |
| No effect | No effect* | + (Ren-A) | ip | (Baker-Andresen et al., 2013) | |
| Lentiviral transfected dominant negative form of TrkB | No effect | − | ns | BLA | (Chhatwal et al., 2006) |
| BDNF KD in HPC | − | ns | ns | No drug | (Heldt et al., 2007) |
| Val66Met BDNF SNP | − | ns | ns | No drug | (Soliman et al., 2010) |
| BDNF +/− KO | −*** | −*** | ns | No drug | (Psotta et al., 2013) |
| BDNF antibody | − | − | ns | IL | (Rosas-Vidal et al., 2014) |
| No effect | No effect | ns | PL | (Rosas-Vidal et al., 2014) | |
| BDNF KO in forebrain | ns | No effect# | ns | PL | (Choi et al., 2010) |
| Methylene blue, nitric oxide, histamine, and LTCCs | |||||
| Methylene blue | ns | +1 | ns | ip | (Gonzalez-Lima & Bruchey, 2004) |
| ns | ns | +1* (Ren-A) | ip | (Wrubel et al., 2007) | |
| Histamine | ns | +1 | ns | CA1 | (Bonini et al., 2011) |
| Dimaprit (H2 ag) | ns | +1 | ns | CA1 | (Bonini et al., 2011) |
| Ranitidine (H2 ant) | ns | −1 | ns | CA1 | (Fiorenza et al., 2012) |
| ns | −1 | ns | BLA | (Fiorenza et al., 2012) | |
| ns | −1 | ns | PFC | (Fiorenza et al., 2012) | |
| SKF9188 (histamine methyl-transferase inhibitor) | ns | +1 | ns | CA1 | (Fiorenza et al., 2012) |
| ns | +1 | ns | BLA | (Fiorenza et al., 2012) | |
| ns | +1 | ns | PFC | (Fiorenza et al., 2012) | |
| L-NAME (nNOS inhibitor)4 | − | − | ns | ip | (Luo et al., 2014) |
| CamKII-Cre Cav1.2 KO | No effect | ns | ns | No drug | (McKinney et al., 2008) |
| Cav1.3 KO | No effect | ns | ns | No drug | (Busquet et al., 2008) |
| No effect | ns | ns | No drug | (McKinney & Murphy, 2006) | |
| Nifedipine (LTCC ant) | − | ns | ns | ip | (Cain et al., 2002) |
| No effect | ns | ns | icv | (Busquet et al., 2008) | |
| No effect | − | ns | BLA | (Davis & Bauer, 2012) | |
| ns | −1 | ns | HPC | (de Carvalho Myskiw et al., 2014) | |
| Verapamil (VGCC ant) | No effect | − | ns | BLA | (Davis & Bauer, 2012) |
Drug administration following extinction training;
drug administration 24 h prior extinction retrieval
drug administration 30 min prior extinction retrieva
ABA scheme, 40 mg/kg.
Facilitates rescue of impaired fear extinction;
facilitates extinction of remote memories,
only in older animals (7 months), younger ones (2 months) are not affected,
reduced fear expression at the beginning of extinction training.
+, improved; -, impaired; (+) or (−), only minor effects; ip, intraperitoneal injection; sc, subcutanous injection; icv, intracerebroventricular injection; ns, not studied; HPC, intra-hippocampal administration; BLA, intra-basolateral amygdala administration; IL, infralimbic cortex; PL, prelimbic cortex; CA1, cornu ammonis 1; Ren-A, Fear renewal in conditioning context; SR, spontaneous recovery; Re-in, reinstatement; ag, agonist; ant, antagonist, KO, knock-out;
4.9.2. Brain-derived neurotrophic factor
Brain-derived neurotrophic factor (BDNF) is the most abundant neurotrophin in the CNS and a key player in synaptic plasticity (reviewed in Andero & Ressler, 2012). BDNF binds with high affinity to the tropomyosin-related kinase B (TrkB) receptor and with low affinity to the p75NTR, a receptor for multiple neurotrophins (Reichardt, 2006). BDNF and TrkB are present in the HPC, AMY, PFC and the hypo-thalamus, and are integral components of fear extinction mechanisms as blunted activity of BDNF-TrkB signaling is associated with deficient extinction retrieval in rats (Kabir et al., 2013). Conversely, increased levels of BDNF mRNA are observed in the BLA (Chhatwal et al., 2006), mPFC (Bredy et al., 2007) following successful fear extinction. The potential utility of BDNF-TrkB signaling as cognitive enhancing target is underscored by the findings that stimulating TrkB signaling by administering the TrkB agonist 7,8-dihydroxyflavone (DHF) facilitates LTP induction in the AMY (Li et al., 2011) and can augment fear extinction (Peters et al., 2010; Rosas-Vidal et al., 2014).
Haploinsufficiency of BDNF leads to deficits in extinction learning and retrieval (Psotta et al., 2013), while inhibition of the BDNF-evoked signaling cascade via transfection of a dominant negative form of the TrkB receptor specifically disrupts extinction consolidation in a fear potentiated startle paradigm (Chhatwal et al., 2006). Conversely, systemic injection of the TrkB agonist DHF promoted extinction in a stress model of impaired fear extinction and protected against the reinstatement of fear (Andero et al., 2011) (for an overview, see Table 9). In mice and humans, a single nucleotide polymorphism (SNP) in the BDNF gene (Val66Met), causing inefficient BDNF trafficking and reduced activity-dependent BDNF secretion, is associated with poor extinction (Table 9) and abnormal fronto-AMY activity (Soliman et al., 2010). Moreover, human carriers of the BDNF Val66Met SNP show reduced responsivity to exposure-based therapies [reviewed in (McGuire et al., 2014)], underscoring the potential role of BDNF in deficient fear extinction.
Studies aimed at identifying the site of BDNF-induced effects on extinction showed that deletion of BDNF in the HPC, but not in the PL(Choi et al., 2010), impairs fear extinction learning (Heldt et al., 2007). In addition, injection of BDNF antibodies (anti-BDNF) in the IL prior to extinction training disrupts acquisition and retrieval of extinction memories while anti-BDNF infusions in the PL cortex do not affect extinction (Rosas-Vidal et al., 2014) (Table 9). These findings indicate that BDNF signaling in the HPC and the IL support the formation and maintenance of fear extinction memories. In fact, local BDNF injections in the IL produced fear inhibition for recent as well as remote fear memories even in the absence of extinction training (Peters et al., 2010; Rosas-Vidal et al., 2014). Given the possibility that BDNF infusions into the IL could reinstate fear by using the aforementioned extinction-independent paradigm (Peters et al., 2010), BDNF seems to induce signaling cascades crucial for the formation of extinction memories rather than decreasing the stability of the original fear memory. Overall these effects are consistent with a model in which the effects of BDNF are brain-region dependent, and BDNF appears to enhance synaptic plasticity of the most robust form of learning occurring in a time- and region-dependent manner.
Various targets described in this review may mediate their effects on extinction via interactions with BDNF. For example, chronic treatment with the SSRI fluoxetine (Section 4.1: Serotonergic system) facilitates extinction and enhances BDNF levels in the AMY and the HPC (Karpova et al., 2011). Valproate, an extinction-promoting inhibitor of histone deacetylation (see Section 4.10: Epigenetics), increases BDNF mRNA in the mPFC (Bredy et al., 2007). Furthermore, TrkB activation triggers the release of eCBs (Lemtiri-Chlieh & Levine, 2010) and increases the expression of CB1 receptors (Maison et al., 2009), hence potentially contributing to extinction memory formation (see Section 4.6: Cannabinoids). Finally, BDNF Met66 knock-in mice exhibit dysfunctional NMDA receptor-dependent synaptic plasticity in addition to reduced extinction acquisition (Ninan et al., 2010) that is rescued by systemic administration of D-cycloserine (Yu et al., 2009), underscoring an additional strong interaction between BDNF and the glutamatergic system [see (Andero & Ressler, 2012) for more details].
These findings suggest that BDNF signaling may be an important common signaling pathway for various systems involved in extinction. Although poor blood–brain-barrier permeability (Wu, 2005) limits the therapeutic potential of BDNF itself, TrkB receptor agonists such as DHF (see above) or LM22A-4 (Massa et al., 2010) may hold promise for CBT-adjunctive therapy in anxiety.
4.9.3. Nitric oxide and methylene blue
Nitric oxide (NO) signaling has received considerable attention in terms of its effect on fear learning and has been shown, for example, to be implicated in the formation of LTP at thalamic input synapses in the LA (Shin et al., 2013). One recent study did demonstrate that reducing NO signaling via inhibition of nitric oxide synthase (e.g. via L-NAME) impairs extinction learning and retrieval (Luo et al., 2014), indicating that NO signaling may also be involved in the formation and maintenance of fear extinction memories.
More work has been done on the brain penetrant methylene blue, which is an inhibitor of NO synthase, as well as a central monoamine oxidase (MAO) inhibitor and a cerebral metabolic enhancer (reviewed in Rojas et al., 2012). A contribution of nitric oxide to the behavioral effects of this and related compounds was suggested in relation to their observed antidepressant-like activity (Harvey et al., 2010).
In rodents, subchronic administration of methylene blue facilitates extinction retrieval (Gonzalez-Lima & Bruchey, 2004) (Table 9) and increases metabolic activity in the IL subdivision of the mPFC. Methylene blue administration also rendered extinction memories resistant to fear renewal when administered following extinction training in a learned helplessness model exhibiting impaired extinction (Wrubel et al., 2007).). Because methylene blue is used to treat metemoglobinemia and has a well-described safety profile (Ohlow & Moosmann, 2011), these preclinical data could be quickly moved forward to clinical investigations. Indeed, the first clinical study has recently reported that methylene blue treatment during CBT led to greater fear inhibition that was persistent for at least one month [(Telch et al., 2014) see Table 9 A for details]. However, similar to the profile of DCS discussed earlier, only those patients capable of showing some extinction benefited from the methylene blue treatment. A clinical trial investigating the potential of methylene blue in PTSD (NCT01188694) is now under way.
4.9.4. Histamine
The tuberomammillary nucleus is the main source of histamine in the brain. Histaminergic projections innervate main monoaminergic nuclei, such as raphe nuclei and locus coeruleus (Lee et al., 2005), as well as extinction relevant brain areas, including the AMY, mPFC and hippocampus (Kohler et al., 1985). To date, 4 histamine receptor subtypes (H1–4) have been identified – H1–3 are expressed in the CNS, while H4 is involved in chemotactic processes of the immune system. Local histamine infusions into the CA1 region of the HPC have been associated with improved extinction consolidation (Bonini et al., 2011). This effect is H2 receptor mediated, since the H2 agonist dimaprit facilitates extinction consolidation while the histamine H2 antagonist ranitidine impairs it, when injected into the CA1, BLA or IL following extinction training (Bonini et al., 2011; Fiorenza et al., 2012). Furthermore, both histamine and the H2 agonist dimaprit facilitate extinction-induced phosphorylation of Erk1, suggesting a possible molecular pathway mediating the augmenting effects of H2 receptor agonism on extinction consolidation (Bonini et al., 2011). However, although the H1 antagonist hydroxyzine has been used in some anxiety therapies (Guaiana et al., 2010), the potential clinical use of histaminergic drugs in particular targeting the H2 receptor for CBT augmentation has not been investigated.
4.9.5. Voltage gated calcium channels
Voltage-gated calcium channels (VGCCs) are classified into L-, P/Q-, R-, N- and T-type VGCCs (for a review see Catterall et al., 2005). A rise in intracellular calcium via NMDA receptor or VGCC activation initiates various second messenger signaling cascades that play a role in LTP formation. Due to their neuronal expression as well as their association with gene transcription the L-type calcium channels (LTCCs) Cav1.2 and Cav1.3 (Striessnig et al., 2006, 2014) have attracted most attention in the fear conditioning field.
Early studies have shown that systemic injections of LTCC blockers, e.g. nifedipine (that inhibits Cav1.2 and Cav1.3 channels), impair fear extinction acquisition (Cain et al., 2002). However, whole-brain gene knockout of Cav1.3 channels (Busquet et al., 2008; McKinney & Murphy, 2006) as well as conditional knockout of Cav1.2 on CaMKII-expressing principal neurons (McKinney et al., 2008) failed to replicate deleterious effects of LTCC blockers on extinction training. Pre-training icv infusion of nifedipine has revealed no effect on extinction learning (Busquet et al., 2008), suggesting that the extinction-impairing effects of systemic nifedipine may be due to peripheral actions (Waltereit et al., 2008).
However, pre-training intra-BLA or intra-HPC infusion of nifedipine, or of another LTCC blocker, verapamil, impairs extinction retrieval 24h later (Davis & Bauer, 2012; de Carvalho Myskiw et al., 2014), indicating a role for LTCCs within these regions in the consolidation of extinction. This effect could be related to Cav1.3 mediation of synaptic plasticity and neuronal excitability in the AMY (McKinney et al., 2009) or to altered neuronal firing in the CA1 region of the HPC (Gamelli et al., 2011). However, the effect on extinction consolidation of selective Cav1.3 activation has not been assessed so far.
While activation of Cav1.2 channels produces severe neurological effects and germline gain of Cav1.3 function is deleterious (Scholl et al., 2013), selective Cav1.3 activation in adulthood has been shown to be tolerated at least in mice (Hetzenauer et al., 2006; Sinnegger-Brauns et al., 2004). Hence, short term use of such compounds, as would typically be required in combination with exposure therapy, should be possible. Taken together, these preclinical findings raise the prospect of targeting LTCCs, and Cav1.3 in particular, for the treatment of anxiety.
4.10. Epigenetics
The formation of extinction memories requires an intricate regulatory network of signal transduction, gene transcription and translation. A key step in this process involves the coordinated transcription of specific genes coding for learning-associated transcription factors, synaptic plasticity factors, neurotransmitter receptors, cytoskeletal proteins and other cellular substrates [reviewed in (Monti, 2013; Whittle & Singewald, 2014)]. Gene transcription is in turn tightly controlled by epigenetic mechanisms (Callinan & Feinberg, 2006). Epigenetic mechanisms regulate the accessibility of deoxyribonucleic acid (DNA) to transcription-initiating machinery via certain molecular events including histone tail post-translational modifications and DNA methylation. In this section we will focus on histone acetylation and DNA methylation, with emphasis on their dynamic interactions within the chromatin environment that orchestrate the regulation of genes at the molecular level, and review accumulating evidence that modulation of extinction-relevant receptors at the synapse can initiate epigenetic programs within the nucleus and modulate extinction in a persistent and context-independent manner. We will also review data showing that drugs targeting epigenetic mechanisms may serve to strengthen extinction.
4.10.1. Histone modifications
To date, a main focus of research is on understanding the interaction between histone modifications and the strengthening of fear extinction memories. In eukaryotic cells, DNA is organized in a highly conserved structural polymer, termed chromatin. The basic building block of chromatin is the nucleosome, which consists of 146 base pairs (bp) of DNA wrapped around an octamer consisting of (dimers of) core histone proteins (H2A, H2B, H3, and H4) held together by a H1 linker protein (Kornberg & Lorch, 1999). Histones contain a globular domain with a covalently modifiable N-terminal tail consisting of lysine and/or arginine residues. Histone modifications constitute a signal that is read alone or, in combination with other modifications and interactions with neighboring histones – a ‘histone code’ (Kouzarides, 2007). At least 9 different posttranslational modifications, including acetylation, phosphorylation, methylation, biotinylation, SUMOylation, ADP ribosylation, and ubiquitination, influence chromatin condensation, resulting in gene transcription by coordinating protein and enzyme complex accessibility to DNA (Ruthenburg et al., 2007). Here, we focus primarily on the most well-studied histone modification in extinction – acetylation of histone proteins.
Histone acetylation on lysine (K) residues leads to transcriptional activation by neutralizing the positive charge of the K ε-amino group of histone tails, which in-turn relaxes chromatin by reducing the electrostatic bonding of histone with negatively charged DNA (Grayson et al., 2010). The enzymes involved in regulating this process are 1) histone acetyltransferases (HATs), also known as lysine acetyltransferases (KATs) (Allis et al., 2007) or ‘writers’ (Monti, 2013) and 2) histone deacetylases (HDACs), also known as lysine deacetylases (KDACs) (Choudhary et al., 2009) or ‘erasers’ (Monti, 2013). HATs acetylate K residues to promote gene transcription, whereas HDACs, which are present in co-repressor complexes, remove K residues to impair gene transcription (see Fig. 9.) [for greater detail, see (Fischer et al., 2010)].
Fig. 9.
Schematic representation depicting the epigenetic regulation of fear extinction. DNA methylation is associated with suppression of gene transcription. The precise molecular processes through which this occurs are complex. In brief, methylation of cytosines at CpG dinucleotides recruits methyl-DNA binding proteins, at specific sites in the genome. Proteins that bind to methylated DNA, including methyl CpG-binding protein 2(MeCP2) and methyl CpG-binding domain protein1 (MBD1), have both a methyl-DNA binding domain and a transcription-regulatory domain. The transcription-regulatory domain recruits adapter/scaffolding proteins, which in turn recruit HDACs, including HDAC2, to repress gene transcription [reviewed in (Sweatt, 2009). Following extinction related neuronal activity a number of molecular mechanisms are invoked, ultimately leading to facilitated histone acetylation and subsequent enhancement of gene transcription. These mechanisms include enhancement of HAT activity, such as PCAF (see text) and a reduction in HDAC activity by mechanisms which include nitrosylation (NO) of HDACs, including HDAC2, which facilitates dissociation of HDAC from the chromatin. Moreover, enhanced histone acetylation may also be mediated by the conversion of DNA demethylation which is mediated by the Tet family of methylsytosine dioxygenases. Emerging pharmaceutical evidence is showing that HDAC inhibitors can facilitate gene transcription by enhancing histone acetylation in the promoter region of extinction-relevant genes (see text).
There is some evidence that extinction is associated with alterations in HAT activity and that drugs increasing HAT activity could constitute a novel approach to augmenting extinction (Fig. 9.). Following extinction, there is increased expression of the HAT p300/CBP-associated factor (PCAF) in the rodent IL, and intra-IL infusion of the PCAF activator, SPV106, facilitates extinction and protects against fear renewal (Wei et al., 2012). However, inhibiting, rather than activating, the activity of another HAT, p300, in the IL also strengthens extinction (Marek et al., 2011). One possible explanation of this finding is that rather than strengthening extinction, p300 may bolster fear memory reconsolidation via transcription of genes including nuclear factor κB (NF-κB) (Furia et al., 2002; Perkins et al., 1997) and yin-yang 1 (YY1) (Yao et al., 2001), known to be involved in reconsolidation (Gao et al., 2010; Lubin & Sweatt, 2007). These two studies that demonstrate how targeting HATs affects fear in different ways indicate the need for additional work to determine the optimal approach to facilitate extinction with HAT modulators.
There is an increasing amount of evidence to suggest that histone acetylation is an important mechanism promoting successful fear extinction (reviewed in (Whittle & Singewald, 2014)] (Fig. 9.). An important early study found that histone extinction is associated with increased acetylation of histone H4 in the promoter region of bdnf exon IV (Bredy et al., 2007). Subsequent work showed that administration of various HDAC inhibitors including TSA (trichostatin A), sodium butyrate, entinostat (MS-275), vorinostat (SAHA, suberoylanilide hydroxamic acid), VPA (valproic acid) and Cl-944 can augment fear extinction (Table 10). The potential clinical utility of using HDAC inhibitors has been further strengthened by showing that Cl-944, MS-275 and SAHA rescue extinction deficits in various rodent models (Graff et al., 2014; Hait et al., 2014; Matsumoto et al., 2013; Whittle et al., 2013) (see Table 10).
Table 10.
Studies showing that HDAC inhibitors augment exposure-based fear extinction and rescue extinction learning deficits.
| Drug/manipulat | Extinction training | Extinction retrieval | Longterm extinction | Route | Reference |
|---|---|---|---|---|---|
| HDAC1 KO (HPC) | ns | −# | ns | No drug | (Bahari-Javan et al., 2012) |
| HDAC2 KO in forebrain CamKII neurons | ns | +*# | ns | No drug | (Morris et al., 2013) |
| Vorinostat/SAHA | ns | + | ns | ip | (Fujita et al., 2012) |
| ns | + ** | ns | ip | (Matsumoto et al., 2013) | |
| ns | + ** | ns | ip | (Hait et al., 2014) | |
| TSA (trichostatin A) | ns | +* | ns | ip, HPC | (Lattal et al., 2007) |
| Sodium butyrate | − | + | + (SR) | ip, HPC | (Stafford et al., 2012) |
| ns | + | ns | ip | (Itzhak et al., 2012) | |
| ns | + | ns | ip, HPC | (Lattal et al., 2007) | |
| Valproic acid | − | +* | ns | ip | (Bredy et al., 2007) |
| +* | + (Ren-A) | ip | (Bredy & Barad, 2008) | ||
| + ** | + ** | + (SR, Ren-N) | ip | (Whittle et al., 2013) | |
| MS-275 (entinostat) | − | + ** | + (SR, Ren-N) | ip | (Whittle et al., 2013) |
| Cl-994 | ns | + *** | + (SR) | ip | (Graff et al., 2014) |
| FTY720 (fingolimod) | − | + ** | ns | ip | (Hait et al., 2014) |
Partial extinction training: reduction of fear during the extinction training session was not to pre-conditioning levels,
facilitates rescue of impaired fear extinction;
facilitate remote memories combined with memory re-consolidation update paradigm;
extinction protocol based on a re-consolidation/update paradigm
+, Improved;-, impaired; ip, intraperitoneal injection; ns, not studied; HPC, intra-hippocampal administration; Ren-A, Fear renewal in the conditioning context; Ren-N, Fear renewal in a novel context; SR, spontaneous recovery; # reduced freezing levels at the beginning of extinction training
An additional attribute of HDAC inhibitors is their ability to extinguish remote (aged) fear memories that are normally resistant to extinction. Extinction of recent fear memories (1 day after conditioning), using a memory update-consolidation paradigm, increases HDAC2 nitrosylation (Graff et al., 2014), which facilitates the dissociation of HDAC2 from chromatin (Nott et al., 2008), leading to histone hyper-acetylation and HPC expression of extinction-relevant genes including c-Fos. By contrast, nitrosylation of HDAC2 and c-Fos expression do not increase after extinction training of remote (1 month post conditioning) fear memories (Graff et al., 2014) (Fig. 9.). Systemic administration of the HDAC inhibitor Cl-994 was able to rescue remote extinction and the associated gene expression deficits. Rescue of impaired remote extinction has been observed with dietary zinc restriction, which inhibits HDAC in addition to affecting other systems (Holmes & Singewald, 2013; Whittle et al., 2010). Collectively, these two studies support the potential clinical utility of HDAC inhibitors as adjuncts to CBT treatment of fearful memories that started long after the exposure to trauma.
Further supporting a role of HDAC activity in the modulation of fear extinction mechanisms is the observation that reduced HDAC activity is associated with improvements in fear extinction (Hait et al., 2014). At present 18 different mammalian HDAC isoforms have been identified, and they are divided into four classes (Classes I–IV). Targeting individual HDAC isoforms is an important aim of drug development (Grayson et al., 2010). The majority of currently available HDAC inhibitors target multiple isoforms of the HDAC family (e.g. classes I, II, and IV), while some of the HDAC inhibitors that have proved to be successful in augmenting fear extinction (Table 10) exhibit preferential selectivity towards class-I HDACs (encompassing HDAC1, HDAC2, HDAC3 and HDAC8) (Haggarty & Tsai, 2011).
Studies using gene knockdown or over-expression suggest that targeting specific HDAC isoforms could indeed be a promising approach to modulating extinction [reviewed in (Whittle & Singewald, 2014)]. This is exemplified by the recent finding that gene silencing of HDAC2, but not HDAC1, in forebrain neurons promotes extinction (Morris et al., 2013). However, the effect of specifically inhibiting other HDAC isoforms, including HDAC3 [recently shown to facilitate the extinction of drug-seeking memories (Malvaez et al., 2013)], remains to be tested.
It is likely that the extinction-facilitating effects of HDAC inhibitors are due to activation of extinction-related gene transcription programs. The HDAC inhibitors SAHA and VPA increase, respectively histone acetylation in the promoter region of grin2b (NMDA receptor subunit 2B) (Fujita et al., 2012), and histone H4 acetylation in promoter IV of bdnf, leading to increased BDNF exon IV mRNA expression (Bredy et al., 2007). In addition, the HDAC inhibitor Cl-994 increases histone H3 acetylation in the promoter region of plasticity associated genes including igf2, adcy6, c-Fos, npas4, and arc (Graff et al., 2014). Indeed, a range of neurotransmitter/neuromodulator systems that are known to mediate extinction, induce transcription of relevant genes via enhancement of histone acetylation, including histone H3 acetylation in the promoter region of bdnf, camk2a, creb and the NMDA receptor subunit genes grin2a and grin2b (Shibasaki et al., 2011; Tian et al., 2009, 2010; Tsankova et al., 2006). Concerning a possible convergent downstream mechanism for these effects, serotonin via activation of PKA signaling pathways (Guan et al., 2002), L-type calcium channel activation (Dolmetsch et al., 2001) and BDNF/TrkB activation (Correa et al., 2012) all activate the MAPK/ERK signaling pathway, which is crucial for fear extinction (Pape & Pare, 2010) (see also Fig. 3). MAPK/ERK signaling to the nucleus could increase histone acetylation by enhancing activity of the HAT CREB-binding protein (CBP) (Chrivia et al., 1993).
4.10.2. DNA methylation
DNA methylation is a crucial mechanism for controlling chromatin remodeling in the adult mammalian nervous system (Levenson et al., 2006; Nelson et al., 2008) and can occur at multiple sites within a gene. DNA methylation is a direct chemical modification of a cytosine side-chain that adds a methyl (–CH3) group through a covalent bond, catalyzed by a class of enzymes known as DNA methyltransferases (DNMTs)(Okano et al., 1998). The DNMTs transfer methyl groups to cytosine residues within a continuous stretch of DNA, specifically at the 5′-position of the pyrimidine ring (5-methylcytosine) (Chen et al., 1991). Not all cytosines can be methylated; typically, cytosines are followed by a guanine in order to be methylated (Day & Sweatt, 2011) (Fig. 9.). However, given the recent finding that methylation can occur outside CpG islands and in CpH (where H = adenine/cytosine/thymine) islands and that these CpH islands can repress (Guo et al., 2014) substantially expands and adds further complexity to how methylation can regulate gene transcription in the central nervous system. Furthermore, this finding raises the open question of whether CpH methylation is a molecular mechanism regulating fear extinction.
In a pioneering study, linking DNA methylation and extinction, extinction-resistant female mice were found to exhibit increased DNA methylation of Bdnf exon IV and a concomitant decrease in BDNF mRNA expression in the PFC (Baker-Andresen et al., 2013). The fact that DNA methylation is reversible suggests that enhancement of gene transcription by DNA demethylation could represent a novel mechanism for strengthening extinction that has the potential to be pharmaceutically exploited. Along these lines, the ten-eleven translocation (Tet) family of methylcytosine dioxygenases, which includes Tet1, Tet2, and Tet3 enzymes, catalyze oxidation of 5-methylcytosine to 5-hydroxymethylcytosine and thereby promotes DNA demethylation (Ito et al., 2011; Tahiliani et al., 2009).
Two recent studies demonstrate the importance of these Tet enzymes in extinction. Extinction leads to a genome-wide redistribution of 5-hydroxymethylcytosine and Tet3 occupancy to extinction-relevant genes including the GABAA clustering protein, gephyrin (see Section 4.5: γ-aminobutyric acid (GABA)), and to enhanced gephyrin mRNA expression in the IL (Li et al., 2014). Furthermore, gene knockdown of Tet1 impairs extinction (Rudenko et al., 2013). While agents targeting Tet enzymes are not currently available, these emerging pre-clinical findings may provide the impetus to develop such compounds.
4.10.3. Non-coding RNA
In addition to histone modifications and DNA methylation, gene expression is also regulated by non-coding RNAs including micro-RNAs (miRNAs) (Spadaro & Bredy, 2012). miRNAs represent classes of non-coding RNAs that mediate post-transcriptional regulation of gene expression, including genes associated with synaptic plasticity, learning and memory (Barry, 2014). Although miRNAs are usually associated with preventing mRNA translation and thereby with suppressing new protein synthesis, a specific miRNA was recently shown to augment fear extinction. Fear extinction enhanced the expression of miR-128b in the IL associated with down-regulation of plasticity-related genes including creb1 and sp1 in tandem with inhibition of the original fear memory (Lin et al., 2011). Clearly, further clarification of the role of non-coding RNAs during fear extinction is warranted, including through the development of tools by which specific non-coding RNAs can be modulated.
5. Discussion and final conclusions
In this review we aimed to summarize (druggable) systems involved in fear extinction and how pharmacological compounds have been utilized to augment fear extinction in preclinical and small-scale clinical studies. It is anticipated that these findings are paving the way for clinical use of such approaches given that extinction training is procedurally similar to exposure-based psychotherapy and the underlying fear/extinction circuitries and molecular mechanisms are well conserved across species (Milad & Quirk, 2012). As mentioned in the introduction, the main problems with current exposure-based CBT treatment of fear, anxiety and trauma-related disorders are that a significant proportion of patients either: 1) have difficulties bearing the demanding and exhausting process of this therapy, 2) do not, or not sufficiently, respond to the therapy – failing to substantially reduce their fear response, or 3) responds initially, but suffer from return of fear phenomena hampering remission and full recovery. In this review we summarize the pharmacological approaches that have been investigated and utilized to improve these shortcomings. Initial attempts to combine exposure-based CBT with pharmacotherapy included benzodiazepines that dampened anxiety but also reduced the learning effects of this procedure hence provoking return of fear symptoms upon discontinuation (Hofmann, 2012; Otto et al., 2002, 2010a). A complicating issue is the observation that the interoceptive state induced by such anxiolytics can form part of a “context”, and consequently may promote fear in subsequent testing in an “off drug state”, leading to state-dependent learning due to a change in interoceptive context (Maren et al., 2013). However, there is evidence that targeting novel anxiolytic systems such as the NPS system or fibroblast growth factor-2 may elicit acute anxiolytic effects without producing sedation, state-dependency and/or interference with the formation of extinction memories. These findings are particularly exciting as they are beginning to address one of the important problems associated with CBT (i.e. intolerability to CBT exposure). However, more research is necessary to prove the utility of such approaches. As well as improving tolerability to exposure, improvements concerning onset, magnitude and duration of the therapeutic effect of exposure-based CBT are also in the main focus of drug development in this field. As outlined in this review, the augmentation of fear extinction/exposure therapy with a range of different drugs acting as cognitive enhancers to strengthen extinction memories represents an important approach to enhancing therapy efficacy. This has been made possible by revealing the neurochemical/neurobiological responses within the neural circuitry whose activity is required for fear extinction and, by extension, to rescue fear extinction deficits.
Considerable progress has been made in defining the neural basis of fear extinction and fear extinction deficits (for recent reviews see (Milad & Quirk, 2012; Holmes & Singewald, 2013; Parsons & Ressler, 2013). The steadily growing knowledge concerning where and how fear extinction is processed and how it can be pharmacologically augmented is an essential platform from which to identify promising candidates for extinction-promoting therapeutics. Indeed, by successfully translating findings from animal models, progress has been made in enhancing the outcomes of exposure-based CBT using compounds such as DCS, yohimbine and glucocorticoids as cognitive enhancers in the treatment of patients with anxiety disorders, PTSD and OCD (Hofmann & Smits, 2008; McGuire et al., 2014; Norberg et al., 2008).
As outlined in this review, an impressive number of additional extinction augmenting drugs, acting on a wide variety of pharmacological targets, have indeed been identified. Data arising from the use of animal models displaying deficient fear extinction, reflecting patients resistant to exposure therapy, indicate that different neurotransmitter/neurobiological signaling pathways are involved in different temporal phases of extinction. Hence, it is likely that different pharmacological approaches will be required to either induce fear reductions during extinction training or rescue/boost the consolidation of fear extinction (reviewed in (Holmes & Singewald, 2013). The important finding that within-session fear reduction/habituation favors drug-augmented extinction rather than facilitating reconsolidation of existing fear memories (Graham et al., 2011; Merlo et al., 2014) has also been demonstrated in clinical trials using DCS ((Hofmann, 2014) and yohimbine (Smits et al., 2014). Moreover, multi-target approaches that simultaneously modulate the activity of diverse neurotransmitter/neurobiological systems seem to be an important option to pursue, in particular for individuals with severe extinction deficit/resistance to exposure therapy. Interestingly, in the much more well established medical fields of infectious disease, hypertension, oncology, and similar areas, targeting multiple different mechanisms of action is also the norm, not the exception and it is also considered promising in other fields of psychiatry (Millan, 2014).
One of the most important aspects of extinction-promoting drugs is their ability to bolster extinction by enhancing memory consolidation. It is likely that this extinction enhancement occurs via the enhancement of cortico-AMY synaptic plasticity through multiple mechanisms (reviewed in (Myers & Davis, 2007; Sierra-Mercado et al., 2011; Orsini & Maren, 2012; Duvarci & Pare, 2014), ultimately providing lasting effects and better protection from return of fear phenomena. If translated into clinical use, these effects would support sustained symptom remission and recovery. Some of the drug targets outlined in this review [e.g. epigenetic targets including histone modification, BDNF and facilitated dopaminergic and fibroblast-growth-factor-2 signaling) and also cholinergic targets – see (Zelikowsky et al., 2013)] seem to be promising in this respect, as they promote long-term and context-independent fear inhibition when combined with fear extinction. Context-independent extinction learning (extinction generalization) offering enhanced protection against fear return phenomena such as renewal, may be supported not only by targeting specific pharmacological mechanisms, but also by behavioral approaches (see below). Fortunately, some of the promising substances in that respect are approved for human use (e.g. Valproate, L-DOPA, FGF2) and thus, their extinction-augmenting capacity could be immediately examined in anxiety and trauma-focused clinical trials. Although some of the discussed agents may share important common downstream signaling pathways, it seems clear that there are a number of different pharmacological pathways to access the circuits that underlie fear extinction learning and memory (see Fig. 3), which will enable even more specific targeting of the essential extinction-promoting mechanisms in the future.
Despite this clear progress there remain major challenges for the development of medications to improve the effectiveness of exposure-based therapies, including challenges related to the design of safe, brain-penetrant molecules with limited adverse side-effects. Individual differences in treatment tolerability and efficacy, caused by genetic variation, and prior medication history are further complicating concerns. For example, the careful dissection of the brain regions mediating fear extinction including the AMY, PFC, HPC and other regions has also shown that some systems can promote both extinction-facilitating and extinction-impairing effects depending on the brain area/cellular substrate they are acting on. An example is the β-adrenoceptor, blockade of which interferes with extinction when restricted to the IL, but promotes extinction when the BLA is targeted – hence it is difficult to predict the net effect of medicating a patient with a β-adrenoceptor blocker or agonist during exposure therapy. The targeting of specific intracellular signaling cascades might be a solution for future, more selective approaches in this direction. Research in the last few years has revealed that the specific ligands can bias the signal output of G-protein coupled receptors by stabilizing the receptor structure in a distinct way to the natural ligand. By providing a means to produce functional selectivity, biased agonists and antagonists may be utilized to activate preferential, even unnatural, signaling cascades mediating the therapeutically favored downstream biological consequences [for a recent review see (Luttrell, 2014)].
Another important aspect for future clinical implementation of drug-induced facilitation of exposure therapy outcome concerns the time course of drug administration. While current drug treatment for anxiety and trauma-related disorders involves mainly chronic treatment (e.g. with SSRIs, SNRIs, TCA, MAOI) the compounds discussed in this review are (with the exception of antidepressants) recommended to be applied acutely in combination with psychotherapeutic interventions for a limited number of exposure trials. This strategy includes several advantages: First, chronic application of drugs can induce very different biological effects from those observed with acute administration. It is extremely challenging to predict how and at which stage (recall of fear memory, learning, reconsolidation, consolidation, retrieval of extinction memory) chronically applied drugs will enhance/interfere with fear extinction. For example, it is likely that facilitation of extinction and disruption of fear reconsolidation, both leading to fear reduction, depend on opposing molecular processes, such that chronic administration of the same drug may lead to very different therapeutic outcomes depending on which process (i.e. extinction or reconsolidation) it enhances (Graham et al., 2011). Second, acute administration implies a reduced side effect potential. One example is the addiction potential of some of the compounds currently examined for extinction augmentation (e.g. MDMA). Indeed, first clinical trials reported that none of the involved patients developed a substance abuse problem when MDMA was administered acutely to assist psychotherapy (Mithoefer et al., 2013).
In addition to pharmacological parameters, the efficacy and duration of exposure therapy in humans may also depend (as shown in animal studies) on manipulation of specific behavioral extinction training parameters [see also e.g. (de Carvalho Myskiw et al., 2014; Schiller et al., 2012)] such as providing sufficient extinction training, enhancing the number of contexts for such training, trial spacing, and other approaches (reviewed in Herry et al., 2010; Fitzgerald et al., 2014a). It will be an important future line of research to test the optimal combinations between such behavioral interventions and the outlined pharmacological approaches in order to maximize treatment outcome. It is also important to note that thought needs to be given to a variety of factors concerning how to optimally utilize adjunctive and novel treatments. Such factors include consideration of monotherapy vs. add-on adjunctive treatment; use of medications in drug naïve vs. already medicated subjects; combined use of medication explicitly with exposure/extinction psychotherapy vs. medication taken without explicit psychotherapy; timing of medication – e.g. at night to aid memory consolidation during sleep vs. immediately before or after exposure; and use of medications in countries with well-developed vs. poorly developed health systems. As the field of psychopharmacotherapy matures beyond the models that it has followed for decades, all of these considerations are important for new drug development. For example some medications may not be as efficacious in subjects with a history of antidepressant use, as has been suggested for D-cycloserine (Werner-Seidler & Richardson, 2007). Furthermore, as sleep is increasingly thought to be associated with extinction learning, targeting medication to enhance the memory consolidation process during sleep may be important (Pace-Schott et al., 2012). Finally, medications which are available readily in generic form and that do not require specialized adjunctive psychotherapy may be more readily disseminated to locations with less well-developed mental health care systems than those that are novel, or more expensive, or that may require specialized combined therapy. Taken together, these considerations remind us that identifying molecular mechanisms and novel drug approaches to fear regulation is only part of the challenge, and that the context of the dissemination of such new therapeutics is also of critical concern.
In summary, based on the evidence outlined in this review, the next few years promise to produce several novel clinical approaches to treating trauma- and anxiety-related disorders in a more efficient and persistent way that will most likely lead to enhanced symptom remission. Progress will depend on translational research approaches and a close interaction between preclinical and clinical researchers. Fig. 10 outlines how the different levels of understanding, from genetics to epigenetics to neural circuits, can inform each other as well as how they can be informed across species due to the high level of convergence of shared fear-related processing across mammals. This research and the considerable number of compounds to choose from in the growing pharmacological tool box in this field, many of which display minimal side effects, may enable psychiatrists/psychologists to individualize drug treatment according to specific symptoms of anxiety disorders, PTSD and OCD. Such options are expected to further improve treatment outcome and to reduce the amount of psychotherapy sessions needed, or the duration or intensity that is required for long-lasting treatment success. As outlined above, evidence is beginning to shape the hypothesis that specific classes of compounds and cognitive enhancers may be superior to others in their ability to support long-term fear-inhibiting effects. There is evidence that the efficacy of different classes of drugs may be distinctly influenced by a number of factors including age, gender and other examples of individual differences. Hence, future research is warranted in order to investigate whether specific drugs used as cognitive enhancers in this field are more or less appropriate for specific individuals, conditions and treatment durations. In conclusion, cognitive enhancers present a promising option to safely augment extinction-based anxiety- and trauma therapy and to reduce treatment duration, thereby facilitating treatment coherence and outcomes.
Fig. 10.
Strategy for translating research from humans to mice and back, focusing on human disorders of fear dysregulation. Rodent models of enhanced fear and impaired extinction (induced by genetic or environmental manipulations) closely resemble human symptomatology, particularly the fear dysregulation that occurs in PTSD, panic, and phobic disorders. Using these models increases the chances of identifying candidate genes for human anxiety disorders by reducing the problems of genetic heterogeneity and a variable environment. It is also important to study the involvement of these candidate genes in patients, as well as using unbiased genome-wide association studies and genome-wide epigenetic approaches to identify previously unknown genetic pathways contributing to risk in humans. Subsequently, elucidating the function of the identified gene and its epigenetic regulation using rodent models as well as the human population increases our knowledge of the neurobiology of fear disorders, which, at the same time, is necessary to improve the models. It is also necessary to use rodent models to test the ability to pharmacologically enhance extinction of fear and diminish fear expression prior to clinical test phases. Arrows demonstrate how the different levels of understanding, from genetics to epigenetics to neural circuits, can inform each other, as well as how they can be informed across species due to the high level of convergence of shared fear-related processing across mammals.
Acknowledgments
We acknowledge the Austrian Science Fund [FWF grant numbers SFB F4410 and P25375-B24] for funding our research related to this review (NS).
Abbreviations
- 5-HT
5-hydroxytryptamine = serotonin
- AC
adenylate cyclase
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AMY
amygdala
- BA
basal amygdala
- BDNF
brain-derived neurotrophic factor
- BLA
basolateral amygdaloid complex
- BZD
benzodiazepine
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- cAMP
3′-5′-cyclicadenosine monophosphate
- Cav 1.2
L-type calcium channel alpha 1C isoform
- Cav 1.3
L-type calcium channel alpha 1D isoform
- CBT
cognitive behavioral therapy
- CCK
cholecystokinin
- CeA
central amygdala
- CeM
centromedial amygdala
- CeL
centrolateral amygdala
- CNS
central nervous system
- CREB
cAMP response element binding
- CS
conditioned stimulus
- DA
dopamine
- DCS
d-cycloserine
- DNA
deoxyribonucleic acid
- eCB
endogenous cannabinoiod
- ERK
extracellular regulated kinase
- ERP
exposure and prevention CBT
- FGF2
fibroblast growth factor-2
- GABA
γ-aminobutyric acid
- GABAA
GABAA receptor
- GAD
generalized anxiety disorder
- GAD (65/67)
glutamate decarboxylase 65/67
- GluN
NMDA receptor subtype
- GR
glucocorticoid receptor
- H3
histone H3 protein
- H4
histone H4 protein
- HAT
histone acetyltransferase
- HDAC
histone deacetylase
- HDAC2
histone deacetylase 2
- HPA
hypothalamic-pituitary-adrenal axis
- HPC
hippocampus
- icv
intracerebroventricular
- IL
infralimbic cortex
- ITC
intercalated cell masses
- K
lysine
- KOR
kappa opioid receptor
- LA
lateral amygdala
- L-DOPA
levodopa
- MAPK
mitogen-activated protein kinase
- MDMA
3,4-methylenedioxy-N-methylamphetamine
- Mg2+
magnesium ion
- mGluR
metabotropic glutamate receptor
- miRNA
micro-RNA
- mPFC
medial prefrontal cortex
- mRNA
messenger ribonucleic acid
- MS-275
entinostat (Pyridin-3-ylmethyl N-[[4-[(2-aminophenyl)carbamoyl] phenyl]methyl]carbamate)
- NMDA
N-methyl-d-aspartate
- NMDAR
NMDA receptor
- ns
not studied
- NO
nitric oxide
- NPS
neuropeptide S
- NPY
neuropeptide Y
- OCD
obsessive-compulsive disorder
- OXT
oxytocin
- PAG
periaqueductal gray
- PEPA
4-[2-(Phenylsulphonylamino)ethylthio]-2,6-difluorophenoxyacetamide
- PKC
as Ca2+ /phospholipid-dependent protein kinase C
- PTSD
post-traumatic stress disorder
- PL
prelimbic cortex
- SAD
social anxiety disorder
- SAHA
vorinostat(N-hydroxy-N’-phenyl-octanediamide)
- SNP
single nucleotide polymorphism
- SNRI
selective noradrenaline reuptake inhibitor
- SSRI
selective serotonin reuptake inhibitor
- TrkB
tropomyosin-related kinase B
- US
unconditioned stimulus
- VGCC
voltage-gated calcium channel
- VRET
virtual reality exposure
- Zn2+
zinc ion
Footnotes
Conflict of interest statement
Drs. Singewald, Schmuckermair, Whittle and Holmes declare no conflict of interest. Dr. Ressler is a founding member of Extinction Pharmaceuticals/Therapade Technologies. He has received no equity or income from this relationship within the last 5 years. The terms of these arrangements have been reviewed and approved by Emory University in accordance with its conflict of interest policies.
References
- Abe K, Saito H. Neurotrophic effect of basic fibroblast growth factor is mediated by the p42/p44 mitogen-activated protein kinase cascade in cultured rat cortical neurons. Brain Res Dev Brain Res. 2000;122:81–85. doi: 10.1016/s0165-3806(00)00054-7. [DOI] [PubMed] [Google Scholar]
- Abraham AD, Cunningham CL, Lattal KM. Methylphenidate enhances extinction of contextual fear. Learn Mem. 2012;19:67–72. doi: 10.1101/lm.024752.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham AD, Neve KA, Lattal KM. Dopamine and extinction: a convergence of theory with fear and reward circuitry. Neurobiol Learn Mem. 2014;108:65–77. doi: 10.1016/j.nlm.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abumaria N, Yin B, Zhang L, Li XY, Chen T, Descalzi G, et al. Effects of elevation of brain magnesium on fear conditioning, fear extinction, and synaptic plasticity in the infralimbic prefrontal cortex and lateral amygdala. J Neurosci. 2011;31:14871–14881. doi: 10.1523/JNEUROSCI.3782-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson D, Feifel D, de Wilde S, McKinney R, Lohr J, Risbrough V. The effect of intranasal oxytocin treatment on conditioned fear extinction and recall in a healthy human sample. Psychopharmacology (Berl) 2013;229:199–208. doi: 10.1007/s00213-013-3099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ. Serotonin, via 5-HT2A receptors, increases EPSCs in layer V pyramidal cells of prefrontal cortex by an asynchronous mode of glutamate release. Brain Res. 1999;825:161–171. doi: 10.1016/s0006-8993(99)01224-x. [DOI] [PubMed] [Google Scholar]
- Akirav I, Raizel H, Maroun M. Enhancement of conditioned fear extinction by infusion of the GABA(A) agonist muscimol into the rat prefrontal cortex and amygdala. Eur J Neurosci. 2006;23:758–764. doi: 10.1111/j.1460-9568.2006.04603.x. [DOI] [PubMed] [Google Scholar]
- Akirav I, Segev A, Motanis H, Maroun M. D-cycloserine into the BLA reverses the impairing effects of exposure to stress on the extinction of contextual fear, but not conditioned taste aversion. Learn Mem. 2009;16:682–686. doi: 10.1101/lm.1565109. [DOI] [PubMed] [Google Scholar]
- Allis CD, Berger SL, Cote J, Dent S, Jenuwien T, Kouzarides T, et al. New nomenclature for chromatin-modifying enzymes. Cell. 2007;131:633–636. doi: 10.1016/j.cell.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Amir A, Amano T, Pare D. Physiological identification and infralimbic responsiveness of rat intercalated amygdala neurons. J Neurophysiol. 2011;105:3054–3066. doi: 10.1152/jn.00136.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstadter AB, Nugent NR, Koenen KC. Genetics of PTSD: Fear conditioning as a model for future research. Psychiatr Ann. 2009;39:358–367. doi: 10.3928/00485713-20090526-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andero R, Dias BG, Ressler KJ. A role for Tac2, NkB, and Nk3 receptor in normal and dysregulated fear memory consolidation. Neuron. 2014;83:444–454. doi: 10.1016/j.neuron.2014.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andero R, Heldt SA, Ye K, Liu X, Armario A, Ressler KJ. Effect of 7,8-dihydroxyflavone, a small-molecule TrkB agonist, on emotional learning. Am J Psychiatry. 2011;168:163–172. doi: 10.1176/appi.ajp.2010.10030326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andero R, Ressler KJ. Fear extinction and BDNF: translating animal models of PTSD to the clinic. Genes Brain Behav. 2012;11:503–512. doi: 10.1111/j.1601-183X.2012.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arntz A, Merckelbach H, de Jong P. Opioid antagonist affects behavioral effects of exposure in vivo. J Consult Clin Psychol. 1993;61:865–870. doi: 10.1037//0022-006x.61.5.865. [DOI] [PubMed] [Google Scholar]
- Arvanov VL, Liang X, Magro P, Roberts R, Wang RY. A pre- and postsynaptic modulatory action of 5-HT and the 5-HT2A, 2C receptor agonist DOB on NMDA-evoked responses in the rat medial prefrontal cortex. Eur J Neurosci. 1999;11:2917–2934. doi: 10.1046/j.1460-9568.1999.00708.x. [DOI] [PubMed] [Google Scholar]
- Azad SC, Monory K, Marsicano G, Cravatt BF, Lutz B, Zieglgansberger W, et al. Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. J Neurosci. 2004;24:9953–9961. doi: 10.1523/JNEUROSCI.2134-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdy G, Graf M, Anheuer ZE, Modos EA, Kantor S. Anxiety-like effects induced by acute fluoxetine, sertraline or m-CPP treatment are reversed by pretreatment with the 5-HT2C receptor antagonist SB-242084 but not the 5-HT1A receptor antagonist WAY-100635. Int J Neuropsychopharmacol. 2001;4:399–408. doi: 10.1017/S1461145701002632. [DOI] [PubMed] [Google Scholar]
- Bahari-Javan S, Maddalena A, Kerimoglu C, Wittnam J, Held T, Bahr M, et al. HDAC1 regulates fear extinction in mice. J Neurosci. 2012;32:5062–5073. doi: 10.1523/JNEUROSCI.0079-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Zhou L, Wu X, Dong Z. d-Serine enhances fear extinction by increasing GluA2-containing AMPA receptor endocytosis. Behav Brain Res. 2014;270:223–227. doi: 10.1016/j.bbr.2014.05.025. [DOI] [PubMed] [Google Scholar]
- Baker JD, Azorlosa JL. The NMDA antagonist MK-801 blocks the extinction of Pavlovian fear conditioning. Behav Neurosci. 1996;110:618–620. doi: 10.1037//0735-7044.110.3.618. [DOI] [PubMed] [Google Scholar]
- Baker-Andresen D, Flavell CR, Li X, Bredy TW. Activation of BDNF signaling prevents the return of fear in female mice. Learn Mem. 2013;20:237–240. doi: 10.1101/lm.029520.112. [DOI] [PubMed] [Google Scholar]
- Bales KL, Solomon M, Jacob S, Crawley JN, Silverman JL, Larke RH, et al. Long-term exposure to intranasal oxytocin in a mouse autism model. Transl Psychiatry. 2014;4:e480. doi: 10.1038/tp.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandelow B, Seidler-Brandler U, Becker A, Wedekind D, Ruther E. Meta-analysis of randomized controlled comparisons of psychopharmacological and psychological treatments for anxiety disorders. World J Biol Psychiatry. 2007;8:175–187. doi: 10.1080/15622970601110273. [DOI] [PubMed] [Google Scholar]
- Bandelow B, Sher L, Bunevicius R, Hollander E, Kasper S, Zohar J, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder inprimary care. Int J Psychiatry Clin Pract. 2012;16:77–84. doi: 10.3109/13651501.2012.667114. [DOI] [PubMed] [Google Scholar]
- Banke TG, Dravid SM, Traynelis SF. Protons trap NR1/NR2B NMDA receptors in a nonconducting state. J Neurosci. 2005;25:42–51. doi: 10.1523/JNEUROSCI.3154-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett D, Gonzalez-Lima F. Behavioral effects of metyrapone on Pavlovian extinction. Neurosci Lett. 2004;371:91–96. doi: 10.1016/j.neulet.2004.08.046. [DOI] [PubMed] [Google Scholar]
- Barry G. Integrating the roles of long and small non-coding RNA in brain function and disease. Mol Psychiatry. 2014;19:410–416. doi: 10.1038/mp.2013.196. [DOI] [PubMed] [Google Scholar]
- Bentz D, Michael T, de Quervain DJ, Wilhelm FH. Enhancing exposure therapy for anxiety disorders with glucocorticoids: from basic mechanisms of emotional learning to clinical applications. J Anxiety Disord. 2010;24:223–230. doi: 10.1016/j.janxdis.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Berlau DJ, McGaugh JL. Enhancement of extinction memory consolidation: the role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiol Learn Mem. 2006;86:123–132. doi: 10.1016/j.nlm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Erk S, Schurmann B, Mauer D, Michel K, Boecker H, et al. Dynorphins regulate fear memory: from mice to men. J Neurosci. 2012;32:9335–9343. doi: 10.1523/JNEUROSCI.1034-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissiere S, Humeau Y, Luthi A. Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat Neurosci. 2003;6:587–592. doi: 10.1038/nn1058. [DOI] [PubMed] [Google Scholar]
- Bitencourt RM, Pamplona FA, Takahashi RN. Facilitation of contextual fear memory extinction and anti-anxiogenic effects of AM404 and cannabidiol in conditioned rats. Eur Neuropsychopharmacol. 2008;18:849–859. doi: 10.1016/j.euroneuro.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Blomhoff S, Haug TT, Hellstrom K, Holme I, Humble M, Madsbu HP, et al. Randomised controlled general practice trial of sertraline, exposure therapy and combined treatment in generalised social phobia. Br J Psychiatry. 2001;179:23–30. doi: 10.1192/bjp.179.1.23. [DOI] [PubMed] [Google Scholar]
- Blundell J, Blaiss CA, Lagace DC, Eisch AJ, Powell CM. Block of glucocorticoid synthesis during re-activation inhibits extinction of an established fear memory. Neurobiol Learn Mem. 2011;95:453–460. doi: 10.1016/j.nlm.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolkan SS, Lattal KM. Opposing effects of d-cycloserine on fear despite a common extinction duration: Interactions between brain regions and behavior. Neurobiol Learn Mem. 2014;113:25–34. doi: 10.1016/j.nlm.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini JS, Da Silva WC, Da Silveira CK, Kohler CA, Izquierdo I, Cammarota M. Histamine facilitates consolidation of fear extinction. Int J Neuropsychopharmacol. 2011;14:1209–1217. doi: 10.1017/S1461145710001501. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Borowski TB, Kokkinidis L. The effects of cocaine, amphetamine, and the dopamine D1 receptor agonist SKF 38393 on fear extinction as measured with potentiated startle: implications for psychomotor stimulant psychosis. Behav Neurosci. 1998;112:952–965. doi: 10.1037//0735-7044.112.4.952. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Kenney FA, Rosengard C. State-dependent fear extinction with two benzodiazepine tranquilizers. Behav Neurosci. 1990;104:44–55. doi: 10.1037//0735-7044.104.1.44. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Vurbic D, Woods AM. D-cycloserine facilitates context-specific fear extinction learning. Neurobiol Learn Mem. 2008;90:504–510. doi: 10.1016/j.nlm.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers ME, Choi DC, Ressler KJ. Neuropeptide regulation of fear and anxiety: Implications of cholecystokinin, endogenous opioids, and neuropeptide Y. Physiol Behav. 2012;107:699–710. doi: 10.1016/j.physbeh.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers ME, Ressler KJ. Interaction between the cholecystokinin and endogenous cannabinoid systems in cued fear expression and extinction retention. Neuropsychopharmacology. 2015;40:688–700. doi: 10.1038/npp.2014.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredy TW, Barad M. The histone deacetylase inhibitor valproic acid enhances acquisition, extinction, and reconsolidation of conditioned fear. Learn Mem. 2008;15:39–45. doi: 10.1101/lm.801108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, Barad M. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn Mem. 2007;14:268–276. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RA, Creamer M, O'Donnell M, Silove D, Clark CR, McFarlane AC. Post-traumatic amnesia and the nature of post-traumatic stress disorder after mild traumatic brain injury. J Int Neuropsychol Soc. 2009;15:862–867. doi: 10.1017/S1355617709990671. [DOI] [PubMed] [Google Scholar]
- Bui E, Orr SP, Jacoby RJ, Keshaviah A, LeBlanc NJ, Milad MR, et al. Two weeks of pretreatment with escitalopram facilitates extinction learning in healthy individuals. Hum Psychopharmacol. 2013;28:447–456. doi: 10.1002/hup.2330. [DOI] [PubMed] [Google Scholar]
- Bukalo O, Pinard C, Holmes A. Mechanisms to medicines: elucidating neural and molecular substrates of fear extinction to identify novel treatments for anxiety disorders. Br J Pharmacol. 2014;171:4690–4718. doi: 10.1111/bph.12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt NS, Bauer EP. Acute and chronic effects of selective serotonin reuptake inhibitor treatment on fear conditioning: implications for underlying fear circuits. Neuroscience. 2013;247:253–272. doi: 10.1016/j.neuroscience.2013.05.050. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Sigurdsson T, Gorman JM, McEwen BS, LeDoux JE. Chronic antidepressant treatment impairs the acquisition of fear extinction. Biol Psychiatry. 2013;73:1078–1086. doi: 10.1016/j.biopsych.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Busquet P, Hetzenauer A, Sinnegger-Brauns MJ, Striessnig J, Singewald N. Role of L-type Ca2+ channel isoforms in the extinction of conditioned fear. Learn Mem. 2008;15:378–386. doi: 10.1101/lm.886208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busti D, Geracitano R, Whittle N, Dalezios Y, Manko M, Kaufmann W, et al. Different fear states engage distinct networks within the intercalated cell clusters of the amygdala. J Neurosci. 2011;31:5131–5144. doi: 10.1523/JNEUROSCI.6100-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustos SG, Maldonado H, Molina VA. Disruptive effect of midazolam on fear memory reconsolidation: decisive influence of reactivation time span and memory age. Neuropsychopharmacology. 2009;34:446–457. doi: 10.1038/npp.2008.75. [DOI] [PubMed] [Google Scholar]
- Cain CK, Blouin AM, Barad M. L-type voltage-gated calcium channels are required for extinction, but not for acquisition or expression, of conditional fear in mice. J Neurosci. 2002;22:9113–9121. doi: 10.1523/JNEUROSCI.22-20-09113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain CK, Blouin AM, Barad M. Adrenergic transmission facilitates extinction of conditional fear in mice. Learn Mem. 2004;11:179–187. doi: 10.1101/lm.71504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain CK, Maynard GD, Kehne JH. Targeting memory processes with drugs to prevent or cure PTSD. Expert Opin Investig Drugs. 2012;21:1323–1350. doi: 10.1517/13543784.2012.704020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaerts-Vegh Z, Beckers T, Ball SM, Baeyens F, Callaerts PF, Cryan JF, et al. Concomitant deficits in working memory and fear extinction are functionally dissociated from reduced anxiety in metabotropic glutamate receptor 7-deficient mice. J Neurosci. 2006;26:6573–6582. doi: 10.1523/JNEUROSCI.1497-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callinan PA, Feinberg AP. The emerging science of epigenomics. Hum Mol Genet. 2006;15:R95–R101. doi: 10.1093/hmg/ddl095. (Spec No 1) [DOI] [PubMed] [Google Scholar]
- Camp MC, Macpherson KP, Lederle L, Graybeal C, Gaburro S, Debrouse LM, et al. Genetic strain differences in learned fear inhibition associated with variation in neuroendocrine, autonomic, and amygdala dendritic phenotypes. Neuropsychopharmacology. 2012;37:1534–1547. doi: 10.1038/npp.2011.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell BM, Merchant KM. Serotonin 2C receptors within the basolateral amygdala induce acute fear-like responses in an open-field environment. Brain Res. 2003;993:1–9. doi: 10.1016/s0006-8993(03)03384-5. [DOI] [PubMed] [Google Scholar]
- Campolongo P, Roozendaal B, Trezza V, Hauer D, Schelling G, McGaugh JL, et al. Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proc Natl Acad Sci U S A. 2009;106:4888–4893. doi: 10.1073/pnas.0900835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannich A, Wotjak CT, Kamprath K, Hermann H, Lutz B, Marsicano G. CB1 cannabinoid receptors modulate kinase and phosphatase activity during extinction of conditioned fear in mice. Learn Mem. 2004;11:625–632. doi: 10.1101/lm.77904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmack SA, Wood SC, Anagnostaras SG. Amphetamine and extinction of cued fear. Neurosci Lett. 2010;468:18–22. doi: 10.1016/j.neulet.2009.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlow BJ, Song S, Paredes DA, Kirstein CL, Sanchez-Ramos J. Effects of psilocybin on hippocampal neurogenesis and extinction of trace fear conditioning. Exp Brain Res. 2013;228:481–491. doi: 10.1007/s00221-013-3579-0. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- Cestari V, Rossi-Arnaud C, Saraulli D, Costanzi M. The MAP(K) of fear: from memory consolidation to memory extinction. Brain Res Bull. 2014;105:8–16. doi: 10.1016/j.brainresbull.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Watson SJ. Comparative anatomical distribution of 5-HT1A receptor mRNA and 5-HT1A binding in rat brain-A combined in situ hybridisation/ in vitro receptor autoradiographic study. Brain Res. 1991;561:51–60. doi: 10.1016/0006-8993(91)90748-k. [DOI] [PubMed] [Google Scholar]
- Chan WY, McNally GP. Conditioned stimulus familiarity determines effects of MK-801 on fear extinction. Behav Neurosci. 2009;123:303–314. doi: 10.1037/a0014988. [DOI] [PubMed] [Google Scholar]
- Chasson GS, Buhlmann U, Tolin DF, Rao SR, Reese HE, Rowley T, et al. Need for speed: Evaluating slopes of OCD recovery in behavior therapy enhanced with d-cycloserine. Behav Res Ther. 2010;48:675–679. doi: 10.1016/j.brat.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Chauveau F, Lange MD, Jungling K, Lesting J, Seidenbecher T, Pape HC. Prevention of stress-impaired fear extinction through neuropeptide s action in the lateral amygdala. Neuropsychopharmacology. 2012;37:1588–1599. doi: 10.1038/npp.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, MacMillan AM, Chang W, Ezaz-Nikpay K, Lane WS, Verdine GL. Direct identification of the active-site nucleophile in a DNA (cytosine-5)-methyl-transferase. Biochemistry. 1991;30:11018–11025. doi: 10.1021/bi00110a002. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Davis M, Maguschak KA, Ressler KJ. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology. 2005a;30:516–524. doi: 10.1038/sj.npp.1300655. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Gutman AR, Maguschak KA, Bowser ME, Yang Y, Davis M, et al. Functional interactions between endocannabinoid and CCK neurotransmitter systems may be critical for extinction learning. Neuropsychopharmacology. 2009;34:509–521. doi: 10.1038/npp.2008.97. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Myers KM, Ressler KJ, Davis M. Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. J Neurosci. 2005b;25:502–506. doi: 10.1523/JNEUROSCI.3301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nat Neurosci. 2006;9:870–872. doi: 10.1038/nn1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Maguschak KA, Ye K, Jang SW, Myers KM, Ressler KJ. Prelimbic cortical BDNF is required for memory of learned fear but not extinction or innate fear. Proc Natl Acad Sci U S A. 2010;107:2675–2680. doi: 10.1073/pnas.0909359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- Choy Y, Fyer AJ, Lipsitz JD. Treatment of specific phobia in adults. Clin Psychol Rev. 2007;27:266–286. doi: 10.1016/j.cpr.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- Clark RE, Xie H, Brunette MF. Benzodiazepine prescription practices and substance abuse in persons with severe mental illness. J Clin Psychiatry. 2004;65:151–155. doi: 10.4088/jcp.v65n0202. [DOI] [PubMed] [Google Scholar]
- Clay R, Hebert M, Gill G, Stapleton LA, Pridham A, Coady M, et al. Glucocorticoids are required for extinction of predator stress-induced hyperarousal. Neurobiol Learn Mem. 2011;96:367–377. doi: 10.1016/j.nlm.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Cole S, Richardson R, McNally GP. Kappa opioid receptors mediate where fear is expressed following extinction training. Learn Mem. 2011;18:88–95. doi: 10.1101/lm.2049511. [DOI] [PubMed] [Google Scholar]
- Cole S, Richardson R, McNally GP. Ventral hippocampal kappa opioid receptors mediate the renewal of fear following extinction in the rat. PLoS One. 2013;8:e58701. doi: 10.1371/journal.pone.0058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci. 2005;25:8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornea-Hebert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Correa SA, Hunter CJ, Palygin O, Wauters SC, Martin KJ, McKenzie C, et al. MSK1 regulates homeostatic and experience-dependent synaptic plasticity. J Neurosci. 2012;32:13039–13051. doi: 10.1523/JNEUROSCI.0930-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton GL, Wang YT, Floresco SB, Phillips AG. Disruption of AMPA receptor endocytosis impairs the extinction, but not acquisition of learned fear. Neuropsychopharmacology. 2008;33:2416–2426. doi: 10.1038/sj.npp.1301642. [DOI] [PubMed] [Google Scholar]
- Dalton GL, Wu DC, Wang YT, Floresco SB, Phillips AG. NMDA GluN2A and GluN2B receptors play separate roles in the induction of LTP and LTD in the amygdala and in the acquisition and extinction of conditioned fear. Neuropharmacology. 2012;62:797–806. doi: 10.1016/j.neuropharm.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Das RK, Kamboj SK, Ramadas M, Yogan K, Gupta V, Redman E, et al. Cannabidiol enhances consolidation of explicit fear extinction in humans. Psychopharmacology (Berl) 2013;226:781–792. doi: 10.1007/s00213-012-2955-y. [DOI] [PubMed] [Google Scholar]
- Davidson JR, Foa EB, Huppert JD, Keefe FJ, Franklin ME, Compton JS, et al. Fluoxetine, comprehensive cognitive behavioral therapy, and placebo in generalized social phobia. Arch Gen Psychiatry. 2004;61:1005–1013. doi: 10.1001/archpsyc.61.10.1005. [DOI] [PubMed] [Google Scholar]
- Davis SE, Bauer EP. L-type voltage-gated calcium channels in the basolateral amygdala are necessary for fear extinction. J Neurosci. 2012;32:13582–13586. doi: 10.1523/JNEUROSCI.0809-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, Iancu OD, Acher FC, Stewart BM, Eiwaz MA, Duvoisin RM, et al. Role of mGluR4 in acquisition of fear learning and memory. Neuropharmacology. 2013;66:365–372. doi: 10.1016/j.neuropharm.2012.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AR, Shields AD, Brigman JL, Norcross M, McElligott ZA, Holmes A, et al. Yohimbine impairs extinction of cocaine-conditioned place preference in an alpha2-adrenergic receptor independent process. Learn Mem. 2008;15:667–676. doi: 10.1101/lm.1079308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Sweatt JD. Epigenetic mechanisms in cognition. Neuron. 2011;70:813–829. doi: 10.1016/j.neuron.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan L, Finberg JP. L-DOPA increases noradrenaline turnover in central and peripheral nervous systems. Neuropharmacology. 2003;45:524–533. doi: 10.1016/s0028-3908(03)00190-4. [DOI] [PubMed] [Google Scholar]
- de Beurs E, van Balkom AJ, Lange A, Koele P, van Dyck R. Treatment of panic disorder with agoraphobia: Comparison of fluvoxamine, placebo, and psychological panic management combined with exposure and of exposure in vivo alone. Am J Psychiatry. 1995;152:683–691. doi: 10.1176/ajp.152.5.683. [DOI] [PubMed] [Google Scholar]
- de Bitencourt RM, Pamplona FA, Takahashi RN. A current overview of cannabinoids and glucocorticoids in facilitating extinction of aversive memories: Potential extinction enhancers. Neuropharmacology. 2013;64:389–395. doi: 10.1016/j.neuropharm.2012.05.039. [DOI] [PubMed] [Google Scholar]
- de Carvalho Myskiw J, Furini CR, Benetti F, Izquierdo I. Hippocampal molecular mechanisms involved in the enhancement of fear extinction caused by exposure to novelty. Proc Natl Acad Sci U S A. 2014;111:4572–4577. doi: 10.1073/pnas.1400423111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kleine RA, Hendriks GJ, Kusters WJ, Broekman TG, van Minnen A. A randomized placebo-controlled trial of D-cycloserine to enhance exposure therapy for posttraumatic stress disorder. Biol Psychiatry. 2012;71:962–968. doi: 10.1016/j.biopsych.2012.02.033. [DOI] [PubMed] [Google Scholar]
- de Oliveira Alvares L, Pasqualini Genro B, Diehl F, Molina VA, Quillfeldt JA. Opposite action of hippocampal CB1 receptors in memory reconsolidation and extinction. Neuroscience. 2008;154:1648–1655. doi: 10.1016/j.neuroscience.2008.05.005. [DOI] [PubMed] [Google Scholar]
- de Oliveira DC, Zuardi AW, Graeff FG, Queiroz RH, Crippa JA. Anxiolytic-like effect of oxytocin in the simulated public speaking test. J Psychopharmacol. 2012;26:497–504. doi: 10.1177/0269881111400642. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Bentz D, Michael T, Bolt OC, Wiederhold BK, Margraf J, et al. Glucocorticoids enhance extinction-based psychotherapy. Proc Natl Acad Sci U S A. 2011;108:6621–6625. doi: 10.1073/pnas.1018214108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, Ledoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Deguchi Y, Naito T, Yuge T, Furukawa A, Yamada S, Pardridge WM, et al. Blood-brain barrier transport of 125I–labeled basic fibroblast growth factor. Pharm Res. 2000;17:63–69. doi: 10.1023/a:1007570509232. [DOI] [PubMed] [Google Scholar]
- Den ML, Altmann SR, Richardson R. A comparison of the short- and long-term effects of corticosterone exposure on extinction in adolescence versus adulthood. Behav Neurosci. 2014;128:722–735. doi: 10.1037/bne0000022. [DOI] [PubMed] [Google Scholar]
- Deschaux O, Spennato G, Moreau JL, Garcia R. Chronic treatment with fluoxetine prevents the return of extinguished auditory-cued conditioned fear. Psychopharmacology (Berl) 2011;215:231–237. doi: 10.1007/s00213-010-2134-y. [DOI] [PubMed] [Google Scholar]
- Deschaux O, Zheng X, Lavigne J, Nachon O, Cleren C, Moreau JL, et al. Post-extinction fluoxetine treatment prevents stress-induced reemergence of extinguished fear. Psychopharmacology (Berl) 2013;225:209–216. doi: 10.1007/s00213-012-2806-x. [DOI] [PubMed] [Google Scholar]
- Deupree JD, Montgomery MD, Bylund DB. Pharmacological properties of the active metabolites of the antidepressants desipramine and citalopram. Eur J Pharmacol. 2007;576:55–60. doi: 10.1016/j.ejphar.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: A fast feedback mechanism. J Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Difede J, Cukor J, Wyka K, Olden M, Hoffman H, Lee FS, et al. D-cycloserine augmentation of exposure therapy for post-traumatic stress disorder: A pilot randomized clinical trial. Neuropsychopharmacology. 2014;39:1052–1058. doi: 10.1038/npp.2013.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Monte FH, Souza RR, Bitencourt RM, Kroon JA, Takahashi RN. Infusion of cannabidiol into infralimbic cortex facilitates fear extinction via CB1 receptors. Behav Brain Res. 2013;250:23–27. doi: 10.1016/j.bbr.2013.04.045. [DOI] [PubMed] [Google Scholar]
- Dobi A, Sartori SB, Busti D, Van der Putten H, Singewald N, Shigemoto R, et al. Neural substrates for the distinct effects of presynaptic group III metabotropic glutamate receptors on extinction of contextual fear conditioning in mice. Neuropharmacology. 2013;66:274–289. doi: 10.1016/j.neuropharm.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294:333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- Do-Monte FH, Allensworth M, Carobrez AP. Impairment of contextual conditioned fear extinction after microinjection of alpha-1-adrenergic blocker prazosin into the medial prefrontal cortex. Behav Brain Res. 2010a;211:89–95. doi: 10.1016/j.bbr.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Do-Monte FH, Kincheski GC, Pavesi E, Sordi R, Assreuy J, Carobrez AP. Role of beta-adrenergic receptors in the ventromedial prefrontal cortex during contextual fear extinction in rats. Neurobiol Learn Mem. 2010b;94:318–328. doi: 10.1016/j.nlm.2010.07.004. [DOI] [PubMed] [Google Scholar]
- DSM-5. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: APA Press; 2013. [Google Scholar]
- Dubreucq S, Koehl M, Abrous DN, Marsicano G, Chaouloff F. CB1 receptor deficiency decreases wheel-running activity: Consequences on emotional behaviours and hippocampal neurogenesis. Exp Neurol. 2010;224:106–113. doi: 10.1016/j.expneurol.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Dubrovina NI, Zinov'eva DV. Effects of activation and blockade of dopamine receptors on the extinction of a passive avoidance reaction in mice with a depressive-like state. Neurosci Behav Physiol. 2010;40:55–59. doi: 10.1007/s11055-009-9226-3. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Mansson E, Gerardi M. Pharmacological innovations for post-traumatic stress disorder and medication-enhanced psychotherapy. Curr Pharm Des. 2012;18:5645–5658. doi: 10.2174/138161212803530899. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Pare D. Amygdala microcircuits controlling learned fear. Neuron. 2014;82:966–980. doi: 10.1016/j.neuron.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner K, Sartori SB, Singewald N. Tachykinin receptors as therapeutic targets in stress-related disorders. Curr Pharm Des. 2009;15:1647–1674. doi: 10.2174/138161209788168074. [DOI] [PubMed] [Google Scholar]
- Effting M, Kindt M. Contextual control of human fear associations in a renewal paradigm. Behav Res Ther. 2007;45:2002–2018. doi: 10.1016/j.brat.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Egan KJ, Carr JE, Hunt DD, Adamson R. Endogenous opiate system and systematic desensitization. J Consult Clin Psychol. 1988;56:287–291. doi: 10.1037//0022-006x.56.2.287. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- El-Ghundi M, O'Dowd BF, George SR. Prolonged fear responses in mice lacking dopamine D1 receptor. Brain Res. 2001;892:86–93. doi: 10.1016/s0006-8993(00)03234-0. [DOI] [PubMed] [Google Scholar]
- Eriksson TM, Holst S, Stan TL, Hager T, Sjogren B, Ogren SO, et al. 5-HT1A and 5-HT7 receptor crosstalk in the regulation of emotional memory: Implications for effects of selective serotonin reuptake inhibitors. Neuropharmacology. 2012;63:1150–1160. doi: 10.1016/j.neuropharm.2012.06.061. [DOI] [PubMed] [Google Scholar]
- Eskandarian S, Vafaei AA, Vaezi GH, Taherian F, Kashefi A, Rashidy-Pour A. Effects of systemic administration of oxytocin on contextual fear extinction in a rat model of post-traumatic stress disorder. Basic Clin Neurosci. 2013;4:315–322. [PMC free article] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: Blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell LJ, Waters AM, Boschen MJ, Hattingh L, McConnell H, Milliner EL, et al. Difficult-to-treat pediatric obsessive-compulsive disorder: Feasibility and preliminary results of a randomized pilot trial of D-cycloserine-augmented behavior therapy. Depress Anxiety. 2013;30:723–731. doi: 10.1002/da.22132. [DOI] [PubMed] [Google Scholar]
- Felder CC, Jose PA, Axelrod J. The dopamine-1 agonist, SKF 82526, stimulates phospholipase-C activity independent of adenylate cyclase. J Pharmacol Exp Ther. 1989;248:171–175. [PubMed] [Google Scholar]
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Fendt M, Imobersteg S, Peterlik D, Chaperon F, Mattes C, Wittmann C, et al. Differential roles of mGlu(7) and mGlu(8) in amygdala-dependent behavior and physiology. Neuropharmacology. 2013;72:215–223. doi: 10.1016/j.neuropharm.2013.04.052. [DOI] [PubMed] [Google Scholar]
- Fendt M, Schmid S, Thakker DR, Jacobson LH, Yamamoto R, Mitsukawa K, et al. mGluR7 facilitates extinction of aversive memories and controls amygdala plasticity. Mol Psychiatry. 2008;13:970–979. doi: 10.1038/sj.mp.4002073. [DOI] [PubMed] [Google Scholar]
- Fernandez Espejo E. Prefrontocortical dopamine loss in rats delays long-term extinction of contextual conditioned fear, and reduces social interaction without affecting short-term social interaction memory. Neuropsychopharmacology. 2003;28:490–498. doi: 10.1038/sj.npp.1300066. [DOI] [PubMed] [Google Scholar]
- Fiorelli R, Rudolph U, Straub CJ, Feldon J, Yee BK. Affective and cognitive effects of global deletion of alpha3-containing gamma-aminobutyric acid-A receptors. Behav Pharmacol. 2008;19:582–596. doi: 10.1097/FBP.0b013e32830dc0c7. [DOI] [PubMed] [Google Scholar]
- Fiorenza NG, Rosa J, Izquierdo I, Myskiw JC. Modulation of the extinction of two different fear-motivated tasks in three distinct brain areas. Behav Brain Res. 2012;232:210–216. doi: 10.1016/j.bbr.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Fischer A, Radulovic M, Schrick C, Sananbenesi F, Godovac-Zimmermann J, Radulovic J. Hippocampal Mek/Erk signaling mediates extinction of contextual freezing behavior. Neurobiol Learn Mem. 2007;87:149–158. doi: 10.1016/j.nlm.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Mungenast A, Tsai LH. Targeting the correct HDAC(s) to treat cognitive disorders. Trends Pharmacol Sci. 2010;31:605–617. doi: 10.1016/j.tips.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PJ, Seemann JR, Maren S. Can fear extinction be enhanced? A review of pharmacological and behavioral findings. Brain Res Bull. 2014a;105:46–60. doi: 10.1016/j.brainresbull.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PJ, Whittle N, Flynn SM, Graybeal C, Pinard CR, Gunduz-Cinar O, et al. Prefrontal single-unit firing associated with deficient extinction in mice. Neurobiol Learn Mem. 2014b;113:69–81. doi: 10.1016/j.nlm.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores A, Valls-Comamala V, Costa G, Saravia R, Maldonado R, Berrendero F. The hypocretin/orexin system mediates the extinction of fear memories. Neuropsychopharmacology. 2014;39:2732–2741. doi: 10.1038/npp.2014.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanez-Nuin DE, Santini E, Quirk GJ, Porter JT. Memory for fear extinction requires mGluR5-mediated activation of infralimbic neurons. Cereb Cortex. 2011;21:727–735. doi: 10.1093/cercor/bhq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Meskenaite V, Weinmann O, Honer M, Benke D, Mohler H. GABAB-receptor splice variants GB1a and GB1b in rat brain: Developmental regulation, cellular distribution and extrasynaptic localization. Eur J Neurosci. 1999;11:761–768. doi: 10.1046/j.1460-9568.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Morinobu S, Takei S, Fuchikami M, Matsumoto T, Yamamoto S, et al. Vorinostat, a histone deacetylase inhibitor, facilitates fear extinction and enhances expression of the hippocampal NR2B–containing NMDA receptor gene. J Psychiatr Res. 2012;46:635–643. doi: 10.1016/j.jpsychires.2012.01.026. [DOI] [PubMed] [Google Scholar]
- Furia B, Deng L, Wu K, Baylor S, Kehn K, Li H, et al. Enhancement of nuclear factor-kappa B acetylation by coactivator p300 and HIV-1 Tat proteins. J Biol Chem. 2002;277:4973–4980. doi: 10.1074/jbc.M107848200. [DOI] [PubMed] [Google Scholar]
- Furini CR, Rossato JI, Bitencourt LL, Medina JH, Izquierdo I, Cammarota M. Beta-adrenergic receptors link NO/sGC/PKG signaling to BDNF expression during the consolidation of object recognition long-term memory. Hippocampus. 2010;20:672–683. doi: 10.1002/hipo.20656. [DOI] [PubMed] [Google Scholar]
- Gafford GM, Guo JD, Flandreau EI, Hazra R, Rainnie DG, Ressler KJ. Cell-type specific deletion of GABA(A)alpha1 in corticotropin-releasing factor-containing neurons enhances anxiety and disrupts fear extinction. Proc Natl Acad Sci U S A. 2012;109:16330–16335. doi: 10.1073/pnas.1119261109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamelli AE, McKinney BC, White JA, Murphy GG. Deletion of the L-type calcium channel Ca(V) 1.3 but not Ca(V) 1.2 results in a diminished sAHP in mouse CA1 pyramidal neurons. Hippocampus. 2011;21:133–141. doi: 10.1002/hipo.20728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganon-Elazar E, Akirav I. Cannabinoids and traumatic stress modulation of contextual fear extinction and GR expression in the amygdala-hippocampal-prefrontal circuit. Psychoneuroendocrinology. 2013;38:1675–1687. doi: 10.1016/j.psyneuen.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Gao J, Wang WY, Mao YW, Graff J, Guan JS, Pan L, et al. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466:1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Positive allosteric modulation of mGluR5 receptors facilitates extinction of a cocaine contextual memory. Biol Psychiatry. 2009;65:717–720. doi: 10.1016/j.biopsych.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas JN, Nguyen PV. Beta-adrenergic receptor activation facilitates induction of a protein synthesis-dependent late phase of long-term potentiation. J Neurosci. 2005;25:3294–3303. doi: 10.1523/JNEUROSCI.4175-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Glotzbach-Schoon E, Andreatta M, Reif A, Ewald H, Troger C, Baumann C, et al. Contextual fear conditioning in virtual reality is affected by 5HTTLPR and NPSR1 polymorphisms: Effects on fear-potentiated startle. Front Behav Neurosci. 2013;7:31. doi: 10.3389/fnbeh.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddyn H, Callaerts-Vegh Z, Stroobants S, Dirikx T, Vansteenwegen D, Hermans D, et al. Deficits in acquisition and extinction of conditioned responses in mGluR7 knockout mice. Neurobiol Learn Mem. 2008;90:103–111. doi: 10.1016/j.nlm.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Caroni P, Luthi A, Herry C. Perineuronal nets protect fear memories from erasure. Science. 2009;325:1258–1261. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- Goldman MS. Effect of chlordiazepoxide administered early in extinction on subsequent extinction of a conditioned emotional response in rats: Implications for human clinical use. Psychol Rep. 1977;40:783–786. doi: 10.2466/pr0.1977.40.3.783. [DOI] [PubMed] [Google Scholar]
- Gomes GM, Mello CF, da Rosa MM, Bochi GV, Ferreira J, Barron S, et al. Polyaminergic agents modulate contextual fear extinction in rats. Neurobiol Learn Mem. 2010;93:589–595. doi: 10.1016/j.nlm.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Bruchey AK. Extinction memory improvement by the metabolic enhancer methylene blue. Learn Mem. 2004;11:633–640. doi: 10.1101/lm.82404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Kedves AT, Olausson P, Taylor JR. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology. 2009;34:707–716. doi: 10.1038/npp.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J, Joseph NF, Horn ME, Samiei A, Meng J, Seo J, et al. Epigenetic priming of memory updating during reconsolidation to attenuate remote fear memories. Cell. 2014;156:261–276. doi: 10.1016/j.cell.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Langton JM, Richardson R. Pharmacological enhancement of fear reduction: Preclinical models. Br J Pharmacol. 2011;164:1230–1247. doi: 10.1111/j.1476-5381.2010.01175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Richardson R. Acute systemic fibroblast growth factor-2 enhances long-term extinction of fear and reduces reinstatement in rats. Neuropsychopharmacology. 2009;34:1875–1882. doi: 10.1038/npp.2009.14. [DOI] [PubMed] [Google Scholar]
- Graham BM, Richardson R. Fibroblast growth factor-2 enhances extinction and reduces renewal of conditioned fear. Neuropsychopharmacology. 2010;35:1348–1355. doi: 10.1038/npp.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Richardson R. Memory of fearful events: The role of fibroblast growth factor-2 in fear acquisition and extinction. Neuroscience. 2011a;189:156–169. doi: 10.1016/j.neuroscience.2011.05.041. [DOI] [PubMed] [Google Scholar]
- Graham BM, Richardson R. Intraamygdala infusion of fibroblast growth factor 2 enhances extinction and reduces renewal and reinstatement in adult rats. J Neurosci. 2011b;31:14151–14157. doi: 10.1523/JNEUROSCI.3014-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson DR, Kundakovic M, Sharma RP. Is there a future for histone deacetylase inhibitors in the pharmacotherapy of psychiatric disorders? Mol Pharmacol. 2010;77:126–135. doi: 10.1124/mol.109.061333. [DOI] [PubMed] [Google Scholar]
- Griebel G, Holmes A. 50 years of hurdles and hope in anxiolytic drug discovery. Nat Rev Drug Discov. 2013;12:667–687. doi: 10.1038/nrd4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaiana G, Barbui C, Cipriani A. Hydroxyzine for generalised anxiety disorder. Cochrane Database Syst Rev. 2010:CD006815. doi: 10.1002/14651858.CD006815.pub2. [DOI] [PubMed] [Google Scholar]
- Guan Z, Giustetto M, Lomvardas S, Kim JH, Miniaci MC, Schwartz JH, et al. Integration of long-term-memory-related synaptic plasticity involves bidirectional regulation of gene expression and chromatin structure. Cell. 2002;111:483–493. doi: 10.1016/s0092-8674(02)01074-7. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Lovibond PF, Dadds MR, Mitchell P, Richardson R. A randomized controlled trial of the effect of D-cycloserine on extinction and fear conditioning in humans. Behav Res Ther. 2007;45:663–672. doi: 10.1016/j.brat.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Richardson R, Lovibond PF, Rapee RM, Gaston JE, Mitchell P, et al. A randomized controlled trial of D-cycloserine enhancement of exposure therapy for social anxiety disorder. Biol Psychiatry. 2008;63:544–549. doi: 10.1016/j.biopsych.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Guedea AL, Schrick C, Guzman YF, Leaderbrand K, Jovasevic V, Corcoran KA, et al. ERK-associated changes of AP-1 proteins during fear extinction. Mol Cell Neurosci. 2011;47:137–144. doi: 10.1016/j.mcn.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra GP, Mello CF, Sauzem PD, Berlese DB, Furian AF, Tabarelli Z, et al. Nitric oxide is involved in the memory facilitation induced by spermidine in rats. Psychopharmacology (Berl) 2006;186:150–158. doi: 10.1007/s00213-006-0376-5. [DOI] [PubMed] [Google Scholar]
- Gunduz-Cinar O, Hill MN, McEwen BS, Holmes A. Amygdala FAAH and anandamide: Mediating protection and recovery from stress. Trends Pharmacol Sci. 2013a;34:637–644. doi: 10.1016/j.tips.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Cinar O, MacPherson KP, Cinar R, Gamble-George J, Sugden K, Williams B, et al. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol Psychiatry. 2013b;18:813–823. doi: 10.1038/mp.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn BG, Cunningham L, Mitchell SG, Swinny JD, Lambert JJ, Belelli D. GABA receptor-acting neurosteroids: A role in the development and regulation of the stress response. Front Neuroendocrinol. 2014 Jun 12; doi: 10.1016/j.yfrne.2014.06.001. pii: S0091-3022(14)00055-7 http://dx.doi.org/10.1016/j.yfrne.2014.06.001 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Guo JU, Su Y, Shin JH, Shin J, Li H, Xie B, et al. Distribution, recognition and regulation of non-CpG methylation in the adult mammalian brain. Nat Neurosci. 2014;17:215–222. doi: 10.1038/nn.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SC, Hillman BG, Prakash A, Ugale RR, Stairs DJ, Dravid SM. Effect of D-cycloserine in conjunction with fear extinction training on extracellular signal-regulated kinase activation in the medial prefrontal cortex and amygdala in rat. Eur J Neurosci. 2013;37:1811–1822. doi: 10.1111/ejn.12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsson A, Svensson M, Jacobi F, Allgulander C, Alonso J, Beghi E, et al. Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:718–779. doi: 10.1016/j.euroneuro.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Gutman AR, Yang Y, Ressler KJ, Davis M. The role of neuropeptide Y in the expression and extinction of fear-potentiated startle. J Neurosci. 2008;28:12682–12690. doi: 10.1523/JNEUROSCI.2305-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutner CA, Weinberger J, Hofmann SG. The effect of D-cycloserine on subliminal cue exposure in spider fearful individuals. Cogn Behav Ther. 2012;41:335–344. doi: 10.1080/16506073.2012.711770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaker J, Gaburro S, Sah A, Gartmann N, Lonsdorf TB, Meier K, et al. Single dose of L-dopa makes extinction memories context-independent and prevents the return of fear. Proc Natl Acad Sci U S A. 2013;110:E2428–E2436. doi: 10.1073/pnas.1303061110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggarty SJ, Tsai LH. Probing the role of HDACs and mechanisms of chromatin-mediated neuroplasticity. Neurobiol Learn Mem. 2011;96:41–52. doi: 10.1016/j.nlm.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait NC, Wise LE, Allegood JC, O'Brien M, Avni D, Reeves TM, et al. Active, phosphorylated fingolimod inhibits histone deacetylases and facilitates fear extinction memory. Nat Neurosci. 2014;17:971–980. doi: 10.1038/nn.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell AV, Allan AM. Improvements in hippocampal-dependent learning and decremental attention in 5-HT(3) receptor overexpressing mice. Learn Mem. 2003;10:410–419. doi: 10.1101/lm.56103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Westbrook RF. Evidence that GABA transmission mediates context-specific extinction of learned fear. Psychopharmacology (Berl) 1998;140:105–115. doi: 10.1007/s002130050745. [DOI] [PubMed] [Google Scholar]
- Harrison SA, Buxton JM, Clancy BM, Czech MP. Insulin regulation of hexose transport in mouse 3T3-L1 cells expressing the human HepG2 glucose transporter. J Biol Chem. 1990;265:20106–20116. [PubMed] [Google Scholar]
- Hart G, Harris JA, Westbrook RF. Systemic or intra-amygdala injection of a benzodiazepine (midazolam) impairs extinction but spares re-extinction of conditioned fear responses. Learn Mem. 2009;16:53–61. doi: 10.1101/lm.1154409. [DOI] [PubMed] [Google Scholar]
- Hart G, Harris JA, Westbrook RF. Systemic or intra-amygdala infusion of the benzodiazepine, midazolam, impairs learning, but facilitates re-learning to inhibit fear responses in extinction. Learn Mem. 2010;17:210–220. doi: 10.1101/lm.1682410. [DOI] [PubMed] [Google Scholar]
- Hart G, Panayi MC, Harris JA, Westbrook RF. Benzodiazepine treatment can impair or spare extinction, depending on when it is given. Behav Res Ther. 2014;56:22–29. doi: 10.1016/j.brat.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Hartley CA, McKenna MC, Salman R, Holmes A, Casey BJ, Phelps EA, et al. Serotonin transporter polyadenylation polymorphism modulates the retention of fear extinction memory. Proc Natl Acad Sci U S A. 2012;109:5493–5498. doi: 10.1073/pnas.1202044109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey BH, Duvenhage I, Viljoen F, Scheepers N, Malan SF, Wegener G, et al. Role of monoamine oxidase, nitric oxide synthase and regional brain mono-amines in the antidepressant-like effects of methylene blue and selected structural analogues. Biochem Pharmacol. 2010;80:1580–1591. doi: 10.1016/j.bcp.2010.07.037. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug TT, Blomhoff S, Hellstrom K, Holme I, Humble M, Madsbu HP, et al. Exposure therapy and sertraline in social phobia: I-year follow-up of a randomised controlled trial. Br J Psychiatry. 2003;182:312–318. doi: 10.1192/bjp.182.4.312. [DOI] [PubMed] [Google Scholar]
- Hauner KK, Mineka S, Voss JL, Paller KA. Exposure therapy triggers lasting reorganization of neural fear processing. Proc Natl Acad Sci U S A. 2012;109:9203–9208. doi: 10.1073/pnas.1205242109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney CF, Bolton MM, Murtishaw AS, Sabbagh JJ, Magcalas CM, Kinney JW. Baclofen administration alters fear extinction and GABAergic protein levels. Neurobiol Learn Mem. 2012;98:261–271. doi: 10.1016/j.nlm.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson RM, Saksida LM, et al. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci. 2008;28:8074–8085. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Mou L, Ressler KJ. In vivo knockdown of GAD67 in the amygdala disrupts fear extinction and the anxiolytic-like effect of diazepam in mice. Transl Psychiatry. 2012;2:e181. doi: 10.1038/tp.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, Ressler KJ. Training-induced changes in the expression of GABAA-associated genes in the amygdala after the acquisition and extinction of Pavlovian fear. Eur J Neurosci. 2007;26:3631–3644. doi: 10.1111/j.1460-9568.2007.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heresco-Levy U, Kremer I, Javitt DC, Goichman R, Reshef A, Blanaru M, et al. Pilot-controlled trial of D-cycloserine for the treatment of post-traumatic stress disorder. Int J Neuropsychopharmacol. 2002;5:301–307. doi: 10.1017/S1461145702003061. [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Luthi A. Neuronal circuits of fear extinction. Eur J Neurosci. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- Herry C, Trifilieff P, Micheau J, Luthi A, Mons N. Extinction of auditory fear conditioning requires MAPK/ERK activation in the basolateral amygdala. Eur J Neurosci. 2006;24:261–269. doi: 10.1111/j.1460-9568.2006.04893.x. [DOI] [PubMed] [Google Scholar]
- Hetzenauer A, Sinnegger-Brauns MJ, Striessnig J, Singewald N. Brain activation pattern induced by stimulation of L-type Ca2+-channels: Contribution of Ca(V) 1.3 and Ca(V)1.2 isoforms. Neuroscience. 2006;139:1005–1015. doi: 10.1016/j.neuroscience.2006.01.059. [DOI] [PubMed] [Google Scholar]
- Hikind N, Maroun M. Microinfusion of the D1 receptor antagonist, SCH23390 into the IL but not the BLA impairs consolidation of extinction of auditory fear conditioning. Neurobiol Learn Mem. 2008;90:217–222. doi: 10.1016/j.nlm.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Hill MN, Ho WS, Meier SE, Gorzalka BB, Hillard CJ. Chronic corticosterone treatment increases the endocannabinoid 2-arachidonylglycerol in the rat amygdala. Eur J Pharmacol. 2005;528:99–102. doi: 10.1016/j.ejphar.2005.10.058. [DOI] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Pan B, Fitzgerald ML, Roberts CJ, Lee TT, et al. Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J Neurosci. 2011;31:10506–10515. doi: 10.1523/JNEUROSCI.0496-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EJ, Mathew SJ. Anxiety disorders: A comprehensive review of pharmacotherapies. Mt Sinai J Med. 2008;75:248–262. doi: 10.1002/msj.20041. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, editor. Psychobiological approaches for anxiety disorders: treatment combination strategies. John Wiley & Sons; 2012. [Google Scholar]
- Hofmann SG. D-cycloserine for treating anxiety disorders: Making good exposures better and bad exposures worse. Depress Anxiety. 2014;31:175–177. doi: 10.1002/da.22257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K, et al. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry. 2006;63:298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Smits JA. Cognitive-behavioral therapy for adult anxiety disorders: A meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69:621–632. doi: 10.4088/jcp.v69n0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Smits JA, Rosenfield D, Simon N, Otto MW, Meuret AE, et al. D-Cycloserine as an augmentation strategy with cognitive-behavioral therapy for social anxiety disorder. Am J Psychiatry. 2013a;170:751–758. doi: 10.1176/appi.ajp.2013.12070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Wu JQ, Boettcher H. D-Cycloserine as an augmentation strategy for cognitive behavioral therapy of anxiety disorders. Biol Mood Anxiety Disord. 2013b;3:11. doi: 10.1186/2045-5380-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook TL, Galarneau MR, Dye JL, Quinn K, Dougherty AL. Morphine use after combat injury in Iraq and post-traumatic stress disorder. N Engl J Med. 2010;362:110–117. doi: 10.1056/NEJMoa0903326. [DOI] [PubMed] [Google Scholar]
- Holmes A, Heilig M, Rupniak NM, Steckler T, Griebel G. Neuropeptide systems as novel therapeutic targets for depression and anxiety disorders. Trends Pharmacol Sci. 2003;24:580–588. doi: 10.1016/j.tips.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Holmes A, Quirk GJ. Pharmacological facilitation of fear extinction and the search for adjunct treatments for anxiety disorders—The case of yohimbine. Trends Pharmacol Sci. 2010;31:2–7. doi: 10.1016/j.tips.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Singewald N. Individual differences in recovery from traumatic fear. Trends Neurosci. 2013;36:23–31. doi: 10.1016/j.tins.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst B, Egerod KL, Schild E, Vickers SP, Cheetham S, Gerlach LO, et al. GPR39 signaling is stimulated by zinc ions but not by obestatin. Endocrinology. 2007;148:13–20. doi: 10.1210/en.2006-0933. [DOI] [PubMed] [Google Scholar]
- Holtzman-Assif O, Laurent V, Westbrook RF. Blockade of dopamine activity in the nucleus accumbens impairs learning extinction of conditioned fear. Learn Mem. 2010;17:71–75. doi: 10.1101/lm.1668310. [DOI] [PubMed] [Google Scholar]
- Huang F, Yang Z, Liu X, Li CQ. Melatonin facilitates extinction, but not acquisition or expression, of conditional cued fear in rats. BMC Neurosci. 2014;15:86. doi: 10.1186/1471-2202-15-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Hugues S, Garcia R, Lena I. Time course of extracellular catecholamine and glutamate levels in the rat medial prefrontal cortex during and after extinction of conditioned fear. Synapse. 2007;61:933–937. doi: 10.1002/syn.20448. [DOI] [PubMed] [Google Scholar]
- ICD-10. World Health Organisation International classification of diseases. 1994
- Irwin RP, Lin SZ, Rogawski MA, Purdy RH, Paul SM. Steroid potentiation and inhibition of N-methyl-D-aspartate receptor-mediated intracellular Ca++ responses: Structure-activity studies. J Pharmacol Exp Ther. 1994;271:677–682. [PubMed] [Google Scholar]
- Ishikawa S, Saito Y, Yanagawa Y, Otani S, Hiraide S, Shimamura K, et al. Early postnatal stress alters extracellular signal-regulated kinase signaling in the corticolimbic system modulating emotional circuitry in adult rats. Eur J Neurosci. 2012;35:135–145. doi: 10.1111/j.1460-9568.2011.07921.x. [DOI] [PubMed] [Google Scholar]
- Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg JA, et al. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. 2011;333:1300–1303. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhak Y, Anderson KL, Kelley JB, Petkov M. Histone acetylation rescues contextual fear conditioning in nNOS KO mice and accelerates extinction of cued fear conditioning in wild type mice. Neurobiol Learn Mem. 2012;97:409–417. doi: 10.1016/j.nlm.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T, Ohmura Y, Futami Y, Matsuzaki H, Kubo Y, Yoshida T, et al. Effects of serotonergic terminal lesion in the amygdala on conditioned fear and innate fear in rats. Eur J Pharmacol. 2012;696:89–95. doi: 10.1016/j.ejphar.2012.09.028. [DOI] [PubMed] [Google Scholar]
- Jacobson LH, Kelly PH, Bettler B, Kaupmann K, Cryan JF. GABA(B(1)) receptor isoforms differentially mediate the acquisition and extinction of aversive taste memories. J Neurosci. 2006;26:8800–8803. doi: 10.1523/JNEUROSCI.2076-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak PH, Corbit LH. Deepened extinction following compound stimulus presentation: Noradrenergic modulation. Learn Mem. 2011;18:1–10. doi: 10.1101/lm.1923211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasnow AM, Ehrlich DE, Choi DC, Dabrowska J, Bowers ME, McCullough KM, et al. Thy1-expressing neurons in the basolateral amygdala may mediate fear inhibition. J Neurosci. 2013;33:10396–10404. doi: 10.1523/JNEUROSCI.5539-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DM, Baker JD, Azorlosa JL. Acquisition, extinction, and reinstatement of Pavlovian fear conditioning: The roles of the NMDA receptor and nitric oxide. Brain Res. 2000;857:66–70. doi: 10.1016/s0006-8993(99)02388-4. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Kazama A, Bachevalier J, Davis M. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology. 2012;62:695–704. doi: 10.1016/j.neuropharm.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungling K, Seidenbecher T, Sosulina L, Lesting J, Sangha S, Clark SD, et al. Neuropeptide S-mediated control of fear expression and extinction: Role of intercalated GABAergic neurons in the amygdala. Neuron. 2008;59:298–310. doi: 10.1016/j.neuron.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir ZD, Katzman AC, Kosofsky BE. Molecular mechanisms mediating a deficit in recall of fear extinction in adult mice exposed to cocaine in utero. PLoS One. 2013;8:e84165. doi: 10.1371/journal.pone.0084165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamprath K, Marsicano G, Tang J, Monory K, Bisogno T, Di Marzo V, et al. Cannabinoid CB1 receptor mediates fear extinction via habituation-like processes. J Neurosci. 2006;26:6677–6686. doi: 10.1523/JNEUROSCI.0153-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova NN, Pickenhagen A, Lindholm J, Tiraboschi E, Kulesskaya N, Agustsdottir A, et al. Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science. 2011;334:1731–1734. doi: 10.1126/science.1214592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst H, Nair S, Velzing E, Rumpff-van Essen L, Slagter E, Shinnick-Gallagher P, et al. Glucocorticoids alter calcium conductances and calcium channel subunit expression in basolateral amygdala neurons. Eur J Neurosci. 2002;16:1083–1089. doi: 10.1046/j.1460-9568.2002.02172.x. [DOI] [PubMed] [Google Scholar]
- Kawahara H, Yoshida M, Yokoo H, Nishi M, Tanaka M. Psychological stress increases serotonin release in the rat amygdala and prefrontal cortex assessed by in vivo microdialysis. Neurosci Lett. 1993;162:81–84. doi: 10.1016/0304-3940(93)90565-3. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, McLaughlin KA, Green JG, Lakoma MD, Petukhova M, et al. Lifetime co-morbidity of DSM-IV disorders in the US National Comorbidity Survey Replication Adolescent Supplement (NCS-A) Psychol Med. 2012;42:1997–2010. doi: 10.1017/S0033291712000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kim J, Lee S, Park H, Song B, Hong I, Geum D, et al. Blockade of amygdala metabotropic glutamate receptor subtype 1 impairs fear extinction. Biochem Biophys Res Commun. 2007;355:188–193. doi: 10.1016/j.bbrc.2007.01.125. [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R. A developmental dissociation of context and GABA effects on extinguished fear in rats. Behav Neurosci. 2007;121:131–139. doi: 10.1037/0735-7044.121.1.131. [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R. Expression of renewal is dependent on the extinction-test interval rather than the acquisition-extinction interval. Behav Neurosci. 2009;123:641–649. doi: 10.1037/a0015237. [DOI] [PubMed] [Google Scholar]
- Kindt M, Soeter M, Vervliet B. Beyond extinction: Erasing human fear responses and preventing the return of fear. Nat Neurosci. 2009;12:256–258. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpers F, Denys D, Kenemans JL, Grillon C, van der Aart J, Baas JM. Testing the effects of Delta9-THC and D-cycloserine on extinction of conditioned fear in humans. J Psychopharmacol. 2012;26:471–478. doi: 10.1177/0269881111431624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, Macias M, Mikosz M, Nowak A, Owczarek D, Wawrzyniak M, et al. Functional anatomy of neural circuits regulating fear and extinction. Proc Natl Acad Sci U S A. 2012;109:17093–17098. doi: 10.1073/pnas.1202087109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Kohler C, Swanson LW, Haglund L, Wu JY. The cytoarchitecture, histochemistry and projections of the tuberomammillary nucleus in the rat. Neuroscience. 1985;16:85–110. doi: 10.1016/0306-4522(85)90049-1. [DOI] [PubMed] [Google Scholar]
- Kondo M, Nakamura Y, Ishida Y, Yamada T, Shimada S. The 5-HT3A receptor is essential for fear extinction. Learn Mem. 2014;21:1–4. doi: 10.1101/lm.032193.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong E, Monje FJ, Hirsch J, Pollak DD. Learning not to fear: Neural correlates of learned safety. Neuropsychopharmacology. 2014;39:515–527. doi: 10.1038/npp.2013.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg RD, Lorch Y. Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell. 1999;98:285–294. doi: 10.1016/s0092-8674(00)81958-3. [DOI] [PubMed] [Google Scholar]
- Koszycki D, Taljaard M, Segal Z, Bradwejn J. A randomized trial of sertraline, self-administered cognitive behavior therapy, and their combination for panic disorder. Psychol Med. 2011;41:373–383. doi: 10.1017/S0033291710000930. [DOI] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Kroner S, Rosenkranz JA, Grace AA, Barrionuevo G. Dopamine modulates excitability of basolateral amygdala neurons in vitro. J Neurophysiol. 2005;93:1598–1610. doi: 10.1152/jn.00843.2004. [DOI] [PubMed] [Google Scholar]
- Kuhnert S, Meyer C, Koch M. Involvement of cannabinoid receptors in the amygdala and prefrontal cortex of rats in fear learning, consolidation, retrieval and extinction. Behav Brain Res. 2013;250:274–284. doi: 10.1016/j.bbr.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Kuriyama K, Honma M, Soshi T, Fujii T, Kim Y. Effect of D-cycloserine and valproic acid on the extinction of reinstated fear-conditioned responses and habituation of fear conditioning in healthy humans: A randomized controlled trial. Psychopharmacology (Berl) 2011;218:589–597. doi: 10.1007/s00213-011-2353-x. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Kim SW, Donahue C, Thuras P, Adson D, Kotlyar M, et al. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry. 2007;62:835–838. doi: 10.1016/j.biopsych.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Laborda MA, Miller RR. Preventing return of fear in an animal model of anxiety: Additive effects of massive extinction and extinction in multiple contexts. Behav Ther. 2013;44:249–261. doi: 10.1016/j.beth.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, et al. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology. 2010;35:2403–2413. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lach G, de Lima TC. Role of NPY Y1 receptor on acquisition, consolidation and extinction on contextual fear conditioning: Dissociation between anxiety, locomotion and non-emotional memory behavior. Neurobiol Learn Mem. 2013;103:26–33. doi: 10.1016/j.nlm.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Laham RJ, Chronos NA, Pike M, Leimbach ME, Udelson JE, Pearlman JD, et al. Intracoronary basic fibroblast growth factor (FGF-2) in patients with severe ischemic heart disease: Results of a phase I open-label dose escalation study. J Am Coll Cardiol. 2000;36:2132–2139. doi: 10.1016/s0735-1097(00)00988-8. [DOI] [PubMed] [Google Scholar]
- Lahoud N, Maroun M. Oxytocinergic manipulations in corticolimbic circuit differentially affect fear acquisition and extinction. Psychoneuroendocrinology. 2013;38:2184–2195. doi: 10.1016/j.psyneuen.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Langton JM, Kim JH, Nicholas J, Richardson R. The effect of the NMDA receptor antagonist MK-801 on the acquisition and extinction of learned fear in the developing rat. Learn Mem. 2007;14:665–668. doi: 10.1101/lm.692407. [DOI] [PubMed] [Google Scholar]
- Langton JM, Richardson R. D-cycloserine facilitates extinction the first time but not the second time: Anexamination of the role of NMDA across the course of repeated extinction sessions. Neuropsychopharmacology. 2008;33:3096–3102. doi: 10.1038/npp.2008.32. [DOI] [PubMed] [Google Scholar]
- Langton JM, Richardson R. The effect of D-cycloserine on immediate vs. delayed extinction of learned fear. Learn Mem. 2010;17:547–551. doi: 10.1101/lm.1927310. [DOI] [PubMed] [Google Scholar]
- Lattal KM, Barrett RM, Wood MA. Systemic or intra hippocampal delivery of histone deacetylase inhibitors facilitates fear extinction. Behav Neurosci. 2007;121:1125–1131. doi: 10.1037/0735-7044.121.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattal KM, Radulovic J, Lukowiak K. Extinction: [corrected] does it or doesn't it? The requirement of altered gene activity and new protein synthesis. Biol Psychiatry. 2006;60:344–351. doi: 10.1016/j.biopsych.2006.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V, Marchand AR, Westbrook RF. The basolateral amygdala is necessary for learning but not relearning extinction of context conditioned fear. Learn Mem. 2008;15:304–314. doi: 10.1101/lm.928208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V, Westbrook RF. Distinct contributions of the basolateral amygdala and the medial prefrontal cortex to learning and relearning extinction of context conditioned fear. Learn Mem. 2008;15:657–666. doi: 10.1101/lm.1080108. [DOI] [PubMed] [Google Scholar]
- Laurent V, Westbrook RF. Inactivation of the infralimbic but not the prelimbic cortex impairs consolidation and retrieval of fear extinction. Learn Mem. 2009;16:520–529. doi: 10.1101/lm.1474609. [DOI] [PubMed] [Google Scholar]
- Leaderbrand K, Corcoran KA, Radulovic J. Co-activation of NR2A and NR2B subunits induces resistance to fear extinction. Neurobiol Learn Mem. 2014;113:35–40. doi: 10.1016/j.nlm.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebron-Milad K, Tsareva A, Ahmed N, Milad MR. Sex differences and estrous cycle in female rats interact with the effects of fluoxetine treatment on fear extinction. Behav Brain Res. 2013;253:217–222. doi: 10.1016/j.bbr.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. Effects of D-cycloserine on extinction of conditioned freezing. Behav Neurosci. 2003;117:341–349. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. D-cycloserine and the facilitation of extinction of conditioned fear: Consequences for reinstatement. Behav Neurosci. 2004;118:505–513. doi: 10.1037/0735-7044.118.3.505. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. D-cycloserine facilitates extinction of learned fear: Effects on reacquisition and generalized extinction. Biol Psychiatry. 2005;57:841–847. doi: 10.1016/j.biopsych.2005.01.023. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Kim JJ. Amygdalar NMDA receptors are critical for new fear learning in previously fear-conditioned rats. J Neurosci. 1998;18:8444–8454. doi: 10.1523/JNEUROSCI.18-20-08444.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Lee BY, Waterhouse BD. Retrograde study of projections from the tuberomammillary nucleus to the dorsal raphe and the locus coeruleus in the rat. Brain Res. 2005;1043:65–75. doi: 10.1016/j.brainres.2005.02.050. [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: Inhibition and potentiation. J Neurosci. 2006;26:10051–10056. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SP, So CH, Rashid AJ, Varghese G, Cheng R, Lanca AJ, et al. Dopamine D1 and D2 receptor Co-activation generates a novel phospholipase C-mediated calcium signal. J Biol Chem. 2004;279:35671–35678. doi: 10.1074/jbc.M401923200. [DOI] [PubMed] [Google Scholar]
- Lehner M, Wislowska-Stanek A, Taracha E, Maciejak P, Szyndler J, Skorzewska A. The effects of midazolam and D-cycloserine on the release of glutamate and GABA in the basolateral amygdala of low and high anxiety rats during extinction trial of a conditioned fear test. Neurobiol Learn Mem. 2010;94:468–480. doi: 10.1016/j.nlm.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, Levine ES. BDNF evokes release of endogenous cannabinoids at layer 2/3 inhibitory synapses in the neocortex. J Neurophysiol. 2010;104:1923–1932. doi: 10.1152/jn.00472.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, Roth TL, Lubin FD, Miller CA, Huang IC, Desai P, et al. Evidence that DNA (cytosine-5) methyltransferase regulates synaptic plasticity in the hippocampus. J Biol Chem. 2006;281:15763–15773. doi: 10.1074/jbc.M511767200. [DOI] [PubMed] [Google Scholar]
- Li C, Dabrowska J, Hazra R, Rainnie DG. Synergistic activation of dopamine D1 and TrkB receptors mediate gain control of synaptic plasticity in the basolateral amygdala. PLoS One. 2011;6:e26065. doi: 10.1371/journal.pone.0026065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wei W, Zhao QY, Widagdo J, Baker-Andresen D, Flavell CR, et al. Neo-corticalTet3-mediated accumulation of 5-hydroxymethylcytosine promotes rapid behavioral adaptation. Proc Natl Acad Sci USA. 2014;111:7120–7125. doi: 10.1073/pnas.1318906111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SH, Westbrook RF. Massed extinction trials produce better short-term but worse long-term loss of context conditioned fear responses than spaced trials. J Exp Psychol Anim Behav Process. 2008;34:336–351. doi: 10.1037/0097-7403.34.3.336. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Lee CC, Gean PW. Involvement of a calcineurin cascade in amygdala depotentiation and quenching of fear memory. Mol Pharmacol. 2003a;63:44–52. doi: 10.1124/mol.63.1.44. [DOI] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Su CL, Gean PW. The role of prefrontal cortex CB1 receptors in the modulation of fear memory. Cereb Cortex. 2009;19:165–175. doi: 10.1093/cercor/bhn075. [DOI] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Su CL, Gean PW. Alterations of excitatory transmission in the lateral amygdala during expression and extinction of fear memory. Int J Neuropsychopharmacol. 2010;13:335–345. doi: 10.1017/S1461145709990678. [DOI] [PubMed] [Google Scholar]
- Lin Q, Wei W, Coelho CM, Li X, Baker-Andresen D, Dudley K, et al. The brain-specific microRNA miR-128b regulates the formation of fear-extinction memory. Nat Neurosci. 2011;14:1115–1117. doi: 10.1038/nn.2891. [DOI] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Lu HY, Gean PW. The similarities and diversities of signal pathways leading to consolidation of conditioning and consolidation of extinction of fear memory. J Neurosci. 2003b;23:8310–8317. doi: 10.1523/JNEUROSCI.23-23-08310.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litz BT, Salters-Pedneault K, Steenkamp MM, Hermos JA, Bryant RA, Otto MW, et al. A randomized placebo-controlled trial of D-cycloserine and exposure therapy for posttraumatic stress disorder. J Psychiatr Res. 2012;46:1184–1190. doi: 10.1016/j.jpsychires.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Liu JL, Li M, Dang XR, Wang ZH, Rao ZR, Wu SX, et al. A NMDA receptor antagonist, MK-801 impairs consolidating extinction of auditory conditioned fear responses in a Pavlovian model. PLoS One. 2009;4:e7548. doi: 10.1371/journal.pone.0007548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llado-Pelfort L, Santana N, Ghisi V, Artigas F, Celada P. 5-HT1A receptor agonists enhance pyramidal cell firing in prefrontal cortex through a preferential action on GABA interneurons. Cereb Cortex. 2012;22:1487–1497. doi: 10.1093/cercor/bhr220. [DOI] [PubMed] [Google Scholar]
- Loretan K, Bissiere S, Luthi A. Dopaminergic modulation of spontaneous inhibitory network activity in the lateral amygdala. Neuropharmacology. 2004;47:631–639. doi: 10.1016/j.neuropharm.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Lu KT, Walker DL, Davis M. Mitogen-activated protein kinase cascade in the basolateral nucleus of amygdala is involved in extinction of fear-potentiated startle. J Neurosci. 2001;21:RC162. doi: 10.1523/JNEUROSCI.21-16-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin FD, Sweatt JD. The IkappaB kinase regulates chromatin structure during reconsolidation of conditioned fear memories. Neuron. 2007;55:942–957. doi: 10.1016/j.neuron.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Luo H, Han L, Tian S. Effect of nitric oxide synthase inhibitor l-NAME on fear extinction in rats: A task-dependent effect. Neurosci Lett. 2014;572:13–18. doi: 10.1016/j.neulet.2014.04.031. [DOI] [PubMed] [Google Scholar]
- Luttrell LM. Minireview: More than just a hammer: Ligand “bias” and pharmaceutical discovery. Mol Endocrinol. 2014;28:281–294. doi: 10.1210/me.2013-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso M, Kalia R, Ali F, Khan AY. The role of benzodiazepines in the treatment of anxiety disorders: A clinical review. Psychiatr Ann. 2010;40:605–610. [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Glucocorticoid regulation of cannabinoid receptor messenger RNA levels in the rat caudate-putamen. An in situ hybridization study. Neurosci Lett. 1993;156:51–53. doi: 10.1016/0304-3940(93)90437-p. [DOI] [PubMed] [Google Scholar]
- Maison P, Walker DJ, Walsh FS, Williams G, Doherty P. BDNF regulates neuronal sensitivity to endocannabinoids. Neurosci Lett. 2009;467:90–94. doi: 10.1016/j.neulet.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Makkar SR, Zhang SQ, Cranney J. Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory. Neuropsychopharmacology. 2010;35:1625–1652. doi: 10.1038/npp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvaez M, McQuown SC, Rogge GA, Astarabadi M, Jacques V, Carreiro S, et al. HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner. Proc Natl Acad Sci USA. 2013;110:2647–2652. doi: 10.1073/pnas.1213364110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manko M, Geracitano R, Capogna M. Functional connectivity of the main intercalated nucleus of the mouse amygdala. J Physiol. 2011;589:1911–1925. doi: 10.1113/jphysiol.2010.201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao SC, Chang CH, Wu CC, Orejarena MJ, Manzoni OJ, Gean PW. Inhibition of spontaneous recovery of fear by mGluR5 after prolonged extinction training. PLoS One. 2013;8:e59580. doi: 10.1371/journal.pone.0059580. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mao SC, Lin HC, Gean PW. Augmentation of fear extinction by D-cycloserine is blocked by proteasome inhibitors. Neuropsychopharmacology. 2008;33:3085–3095. doi: 10.1038/npp.2008.30. [DOI] [PubMed] [Google Scholar]
- Marek R, Coelho CM, Sullivan RK, Baker-Andresen D, Li X, Ratnu V, et al. Paradoxical enhancement of fear extinction memory and synaptic plasticity by inhibition of the histone acetyltransferase p300. J Neurosci. 2011;31:7486–7491. doi: 10.1523/JNEUROSCI.0133-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I. The contextual brain: Implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks IM, Swinson RP, Basoglu M, Kuch K, Noshirvani H, O'Sullivan G, et al. Alprazolam and exposure alone and combined in panic disorder with agoraphobia. A controlled study in London and Toronto. Br J Psychiatry. 1993;162:776–787. doi: 10.1192/bjp.162.6.776. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Massa SM, Yang T, Xie Y, Shi J, Bilgen M, Joyce JN, et al. Small molecule BDNF mimetics activate TrkB signaling and prevent neuronal degeneration in rodents. J Clin Invest. 2010;120:1774–1785. doi: 10.1172/JCI41356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataix-Cols D, Turner C, Monzani B, Isomura K, Murphy C, Krebs G, et al. Cognitive-behavioural therapy with post-session D-cycloserine augmentation for paediatric obsessive-compulsive disorder: Pilot randomised controlled trial. Br J Psychiatry. 2014;204:77–78. doi: 10.1192/bjp.bp.113.126284. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Matsuzawa D, Nakazawa K, Sutoh C, Ohtsuka H, Ishii D, et al. d-serine enhances extinction of auditory cued fear conditioning via ERK1/2 phosphorylation in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:895–902. doi: 10.1016/j.pnpbp.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Morinobu S, Yamamoto S, Matsumoto T, Takei S, Fujita Y, et al. Vorinostat ameliorates impaired fear extinction possibly via the hippocampal NMDA-CaMKII pathway in an animal model of posttraumatic stress disorder. Psychopharmacology (Berl) 2013;229:51–62. doi: 10.1007/s00213-013-3078-9. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Togashi H, Konno K, Koseki H, Hirata R, Izumi T, et al. Early postnatal stress alters the extinction of context-dependent conditioned fear in adult rats. Pharmacol Biochem Behav. 2008;89:247–252. doi: 10.1016/j.pbb.2007.12.017. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Pearson JC. Coexistence of GABA and peptide immunoreactivity in non-pyramidal neurons of the basolateral amygdala. Neurosci Lett. 1989;100:53–58. doi: 10.1016/0304-3940(89)90659-9. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory-a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Castellano C, Brioni J. Picrotoxin enhances latent extinction of conditioned fear. Behav Neurosci. 1990;104:264–267. doi: 10.1037//0735-7044.104.2.264. [DOI] [PubMed] [Google Scholar]
- McGuire JF, Lewin AB, Storch EA. Enhancing exposure therapy for anxiety disorders, obsessive-compulsive disorder and post-traumatic stress disorder. Expert Rev Neurother. 2014:1–18. doi: 10.1586/14737175.2014.934677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney BC, Murphy GG. The L-Type voltage-gated calcium channel Cav1.3 mediates consolidation, but not extinction, of contextually conditioned fear in mice. Learn Mem. 2006;13:584–589. doi: 10.1101/lm.279006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney BC, Sze W, Lee B, Murphy GG. Impaired long-term potentiation and enhanced neuronal excitability in the amygdala of Ca(V)1.3 knockout mice. Neurobiol Learn Mem. 2009;92:519–528. doi: 10.1016/j.nlm.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney BC, Sze W, White JA, Murphy GG. L-type voltage-gated calcium channels in conditioned fear: A genetic and pharmacological analysis. Learn Mem. 2008;15:326–334. doi: 10.1101/lm.893808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally GP. Facilitation of fear extinction by midbrain periaqueductal gray infusions of RB101(S), an inhibitor of enkephalin-degrading enzymes. Behav Neurosci. 2005;119:1672–1677. doi: 10.1037/0735-7044.119.6.1672. [DOI] [PubMed] [Google Scholar]
- McNally GP, Lee BW, Chiem JY, Choi EA. The midbrain periaqueductal gray and fear extinction: Opioid receptor subtype and roles of cyclic AMP, protein kinase A, and mitogen-activated protein kinase. Behav Neurosci. 2005;119:1023–1033. doi: 10.1037/0735-7044.119.4.1023. [DOI] [PubMed] [Google Scholar]
- McNally GP, Pigg M, Weidemann G. Opioid receptors in the midbrain periaqueductal gray regulate extinction of pavlovian fear conditioning. J Neurosci. 2004;24:6912–6919. doi: 10.1523/JNEUROSCI.1828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally GP, Westbrook RF. Opioid receptors regulate the extinction of Pavlovian fear conditioning. Behav Neurosci. 2003;117:1292–1301. doi: 10.1037/0735-7044.117.6.1292. [DOI] [PubMed] [Google Scholar]
- McNally GP, Westbrook RF. Predicting danger: The nature, consequences, and neural mechanisms of predictive fear learning. Learn Mem. 2006;13:245–253. doi: 10.1101/lm.196606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meis S, Bergado-Acosta JR, Yanagawa Y, Obata K, Stork O, Munsch T. Identification of a neuropeptide S responsive circuitry shaping amygdala activity via the endopiriform nucleus. PLoS One. 2008;3:e2695. doi: 10.1371/journal.pone.0002695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meis S, Stork O, Munsch T. Neuropeptide S-mediated facilitation of synaptic transmission enforces subthreshold theta oscillations within the lateral amygdala. PLoS One. 2011;6:e18020. doi: 10.1371/journal.pone.0018020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcer T, Walker J, Sechriest VF, II, Lebedda M, Quinn K, Galarneau M. Glasgow Coma Scores, early opioids, and posttraumatic stress disorder among combat amputees. J Trauma Stress. 2014;27:152–159. doi: 10.1002/jts.21909. [DOI] [PubMed] [Google Scholar]
- Merlo AB, Izquierdo I. The effect of catecholamines on learning in rats. Med Pharmacol Exp Int J Exp Med. 1967;16:343–349. doi: 10.1159/000137009. [DOI] [PubMed] [Google Scholar]
- Merlo E, Milton AL, Goozee ZY, Theobald DE, Everitt BJ. Reconsolidation and extinction are dissociable and mutually exclusive processes: Behavioral and molecular evidence. J Neurosci. 2014;34:2422–2431. doi: 10.1523/JNEUROSCI.4001-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerbroeker K, Powers MB, van Stegeren A, Emmelkamp PM. Does yohimbine hydrochloride facilitate fear extinction in virtual reality treatment of fear of flying? A randomized placebo-controlled trial. Psychother Psychosom. 2012;81:29–37. doi: 10.1159/000329454. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Michael T, Blechert J, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in panic disorder: Enhanced resistance to extinction. J Abnorm Psychol. 2007;116:612–617. doi: 10.1037/0021-843X.116.3.612. [DOI] [PubMed] [Google Scholar]
- Mickley GA, Hoxha N, Luchsinger JL, Rogers MM, Wiles NR. Chronic dietary magnesium-L-threonate speeds extinction and reduces spontaneous recovery of a conditioned taste aversion. Pharmacol Biochem Behav. 2013;106:16–26. doi: 10.1016/j.pbb.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Furtak SC, Greenberg JL, Keshaviah A, Im JJ, Falkenstein MJ, et al. Deficits in conditioned fear extinction in obsessive-compulsive disorder and neurobiological changes in the fear circuit. JAMA Psychiatry. 2013;70:608–618. doi: 10.1001/jamapsychiatry.2013.914. quiz 554. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: Ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. On ‘polypharmacy’ and multi-target agents, complementary strategies for improving the treatment of depression: A comparative appraisal. Int J Neuropsychopharmacol. 2014;17:1009–1037. doi: 10.1017/S1461145712001496. [DOI] [PubMed] [Google Scholar]
- Milton AL, Merlo E, Ratano P, Gregory BL, Dumbreck JK, Everitt BJ. Double dissociation of the requirement for GluN2B- and GluN2A–containing NMDA receptors in the destabilization and restabilization of are consolidating memory. J Neurosci. 2013;33:1109–1115. doi: 10.1523/JNEUROSCI.3273-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithoefer MC, Wagner MT, Mithoefer AT, Jerome L, Doblin R. The safety and efficacy of {+/—}3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: The first randomized controlled pilot study. J Psychopharmacol. 2011;25:439–452. doi: 10.1177/0269881110378371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithoefer MC, Wagner MT, Mithoefer AT, Jerome L, Martin SF, Yazar-Klosinski B, et al. Durability of improvement in post-traumatic stress disorder symptoms and absence of harmful effects or drug dependency after 3,4-methylenedioxymethamphetamine-assisted psychotherapy: A prospective long-term follow-up study. J Psychopharmacol. 2013;27:28–39. doi: 10.1177/0269881112456611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni R, Fumagalli F, Magnaghi V, Roceri M, Gennarelli M, Racagni G, et al. Modulation of fibroblast growth factor-2 by stress and corticosteroids: From developmental events to adult brain plasticity. Brain Res Brain Res Rev. 2001;37:249–258. doi: 10.1016/s0165-0173(01)00128-x. [DOI] [PubMed] [Google Scholar]
- Monti B. Histone post-translational modifications to target memory-related diseases. Curr Pharm Des. 2013;19:5065–5075. doi: 10.2174/1381612811319280005. [DOI] [PubMed] [Google Scholar]
- Morawska MM, Fendt M. The effects of muscimol and AMN082 injections into the medial prefrontal cortex on the expression and extinction of conditioned fear in mice. J Exp Biol. 2012;215:1394–1398. doi: 10.1242/jeb.068213. [DOI] [PubMed] [Google Scholar]
- Morris RW, Bouton ME. The effect of yohimbine on the extinction of conditioned fear: A role for context. Behav Neurosci. 2007;121:501–514. doi: 10.1037/0735-7044.121.3.501. [DOI] [PubMed] [Google Scholar]
- Morris MJ, Mahgoub M, Na ES, Pranav H, Monteggia LM. Loss of histone deacetylase 2 improves working memory and accelerates extinction learning. J Neurosci. 2013;33:6401–6411. doi: 10.1523/JNEUROSCI.1001-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow BA, Elsworth JD, Rasmusson AM, Roth RH. The role of mesoprefrontal dopamine neurons in the acquisition and expression of conditioned fear in the rat. Neuroscience. 1999;92:553–564. doi: 10.1016/s0306-4522(99)00014-7. [DOI] [PubMed] [Google Scholar]
- Mueller D, Bravo-Rivera C, Quirk GJ. Infralimbic D2 receptors are necessary for fear extinction and extinction-related tone responses. Biol Psychiatry. 2010;68:1055–1060. doi: 10.1016/j.biopsych.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, Cahill SP. Noradrenergic modulation of extinction learning and exposure therapy. Behav Brain Res. 2010;208:1–11. doi: 10.1016/j.bbr.2009.11.025. [DOI] [PubMed] [Google Scholar]
- Mueller D, Olivera-Figueroa LA, Pine DS, Quirk GJ. The effects of yohimbine and amphetamine on fear expression and extinction in rats. Psychopharmacology (Berl) 2009;204:599–606. doi: 10.1007/s00213-009-1491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, Porter JT, Quirk GJ. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci. 2008;28:369–375. doi: 10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muigg P, Hetzenauer A, Hauer G, Hauschild M, Gaburro S, Frank E, et al. Impaired extinction of learned fear in rats selectively bred for high anxiety-evidence of altered neuronal processing in prefrontal-amygdala pathways. Eur J Neurosci. 2008;28:2299–2309. doi: 10.1111/j.1460-9568.2008.06511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Carlezon WA, Jr, Davis M. Glutamate receptors in extinction and extinction-based therapies for psychiatric illness. Neuropsychopharmacology. 2011;36:274–293. doi: 10.1038/npp.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Nader K, LeDoux JE. Inhibition of the mesoamygdala dopaminergic pathway impairs the retrieval of conditioned fear associations. Behav Neurosci. 1999;113:891–901. doi: 10.1037//0735-7044.113.5.891. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Nave AM, Tolin DF, Stevens MC. Exposure therapy, D-cycloserine, and functional magnetic resonance imaging in patients with snake phobia: A randomized pilot study. J Clin Psychiatry. 2012;73:1179–1186. doi: 10.4088/JCP.11m07564. [DOI] [PubMed] [Google Scholar]
- Nelson ED, Kavalali ET, Monteggia LM. Activity-dependent suppression of miniature neurotransmission through the regulation of DNA methylation. J Neurosci. 2008;28:395–406. doi: 10.1523/JNEUROSCI.3796-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: Implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35:649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Normandin MD, Pietrzak RH, Piomelli D, Zheng MQ, Gujarro-Anton A, et al. Elevated brain cannabinoid CB1 receptor availability in posttraumatic stress disorder: A positron emission tomography study. Mol Psychiatry. 2013;18:1034–1040. doi: 10.1038/mp.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninan I, Bath KG, Dagar K, Perez-Castro R, Plummer MR, Lee FS, et al. The BDNF Val66Met polymorphism impairs NMDA receptor-dependent synaptic plasticity in the hippocampus. J Neurosci. 2010;30:8866–8870. doi: 10.1523/JNEUROSCI.1405-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya EM, Martynhak BJ, Zanoveli JM, Correia D, da Cunha C, Andreatini R. Spironolactone and low-dose dexamethasone enhance extinction of contextual fear conditioning. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1229–1235. doi: 10.1016/j.pnpbp.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Niyuhire F, Varvel SA, Thorpe AJ, Stokes RJ, Wiley JL, Lichtman AH. The disruptive effects of the CB1 receptor antagonist rimonabant on extinction learning in mice are task-specific. Psychopharmacology (Berl) 2007;191:223–231. doi: 10.1007/s00213-006-0650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63:1118–1126. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Nott A, Watson PM, Robinson JD, Crepaldi L, Riccio A. S-Nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature. 2008;455:411–415. doi: 10.1038/nature07238. [DOI] [PubMed] [Google Scholar]
- Numakawa T, Yokomaku D, Kiyosue K, Adachi N, Matsumoto T, Numakawa Y, et al. Basic fibroblast growth factor evokes a rapid glutamate release through activation of the MAPK pathway in cultured cortical neurons. J Biol Chem. 2002;277:28861–28869. doi: 10.1074/jbc.M202927200. [DOI] [PubMed] [Google Scholar]
- Nutt DJ. The role of the opioid system in alcohol dependence. J Psychopharmacol. 2014;28:8–22. doi: 10.1177/0269881113504017. [DOI] [PubMed] [Google Scholar]
- Oehrberg S, Christiansen PE, Behnke K, Borup AL, Severin B, Soegaard J, et al. Paroxetine in the treatment of panic disorder. A randomised, double-blind, placebo-controlled study. Br J Psychiatry. 1995;167:374–379. doi: 10.1192/bjp.167.3.374. [DOI] [PubMed] [Google Scholar]
- Ogden KK, Khatri A, Traynelis SF, Heldt SA. Potentiation of GluN2C/D NMDA receptor subtypes in the amygdala facilitates the retention of fear and extinction learning in mice. Neuropsychopharmacology. 2014;39:625–637. doi: 10.1038/npp.2013.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlow MJ, Moosmann B. Phenothiazine: The seven lives of pharmacology's first lead structure. Drug Discov Today. 2011;16:119–131. doi: 10.1016/j.drudis.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Okano M, Xie S, Li E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat Genet. 1998;19:219–220. doi: 10.1038/890. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev. 2012;36:1773–1802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto MW, Hong JJ, Safren SA. Benzodiazepine discontinuation difficulties in panic disorder: Conceptual model and outcome for cognitive-behavior therapy. Curr Pharm Des. 2002;8:75–80. doi: 10.2174/1381612023396726. [DOI] [PubMed] [Google Scholar]
- Otto MW, McHugh R, Kantak KM. Combined pharmacotherapy and cognitive-behavioral therapy for anxiety disorders: Medication effects, glucocorticoids, and attenuated treatment outcomes. Clin Psychol Sci Pract. 2010a;12:72–86. doi: 10.1111/j.1468-2850.2010.01198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto MW, McHugh RK, Simon NM, Farach FJ, Worthington JJ, Pollack MH. Efficacy of CBT for benzodiazepine discontinuation in patients with panic disorder: Further evaluation. Behav Res Ther. 2010b;48:720–727. doi: 10.1016/j.brat.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto MW, Tolin DF, Simon NM, Pearlson GD, Basden S, Meunier SA, et al. Efficacy of d-cycloserine for enhancing response to cognitive-behavior therapy for panic disorder. Biol Psychiatry. 2010c;67:365–370. doi: 10.1016/j.biopsych.2009.07.036. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Morgan WN, Plott SJ, Nemeroff CB. Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther. 1997;283:1305–1322. [PubMed] [Google Scholar]
- Pace-Schott EF, Verga PW, Bennett TS, Spencer RM. Sleep promotes consolidation and generalization of extinction learning in simulated exposure therapy for spider fear. J Psychiatr Res. 2012;46:1036–1044. doi: 10.1016/j.jpsychires.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona FA, Bitencourt RM, Takahashi RN. Short- and long-term effects of cannabinoids on the extinction of contextual fear memory in rats. Neurobiol Learn Mem. 2008;90:290–293. doi: 10.1016/j.nlm.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Pamplona FA, Prediger RD, Pandolfo P, Takahashi RN. The cannabinoid receptor agonist WIN 55,212-2 facilitates the extinction of contextual fear memory and spatial memory in rats. Psychopharmacology (Berl) 2006;188:641–649. doi: 10.1007/s00213-006-0514-0. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Ascher P, Neyton J. High-affinity zinc inhibition of NMDA NR1-NR2A receptors. J Neurosci. 1997;17:5711–5725. doi: 10.1523/JNEUROSCI.17-15-05711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- Papadopoulos A, Chandramohan Y, Collins A, Droste SK, Nutt DJ, Reul JM. GABAergic control of novelty stress-responsive epigenetic and gene expression mechanisms in the rat dentate gyrus. Eur Neuropsychopharmacol. 2011;21:316–324. doi: 10.1016/j.euroneuro.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Pape HC, Jungling K, Seidenbecher T, Lesting J, Reinscheid RK. Neuropeptide S: A transmitter system in the brain regulating fear and anxiety. Neuropharmacology. 2010;58:29–34. doi: 10.1016/j.neuropharm.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SM, Williams CL. Contribution of serotonin type 3 receptors in the successful extinction of cued or contextual fear conditioned responses: Interactions with GABAergic signaling. Rev Neurosci. 2012;23:555–569. doi: 10.1515/revneuro-2012-0052. [DOI] [PubMed] [Google Scholar]
- Parker RM, Herzog H. Regional distribution of Y-receptor subtype mRNAs in rat brain. Eur J Neurosci. 1999;11:1431–1448. doi: 10.1046/j.1460-9568.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- Parnas AS, Weber M, Richardson R. Effects of multiple exposures to D-cycloserine on extinction of conditioned fear in rats. Neurobiol Learn Mem. 2005;83:224–231. doi: 10.1016/j.nlm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Helmstetter FJ. Regulation of extinction-related plasticity by opioid receptors in the ventrolateral periaqueductal gray matter. Front Behav Neurosci. 2010:4. doi: 10.3389/fnbeh.2010.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RG, Ressler KJ. Implications of memory modulation for post-traumatic stress and fear disorders. Nat Neurosci. 2013;16:146–153. doi: 10.1038/nn.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes. Oxford University Press; 1927. [Google Scholar]
- Pearce JM, Bouton ME. Theories of associative learning in animals. Annu Rev Psychol. 2001;52:111–139. doi: 10.1146/annurev.psych.52.1.111. [DOI] [PubMed] [Google Scholar]
- Pereira ME, Rosat R, Huang CH, Godoy MG, Izquierdo I. Inhibition by diazepam of the effect of additional training and of extinction on the retention of shuttle avoidance behavior in rats. Behav Neurosci. 1989;103:202–205. doi: 10.1037//0735-7044.103.1.202. [DOI] [PubMed] [Google Scholar]
- Perez de la Mora M, Gallegos-Cari A, Crespo-Ramirez M, Marcellino D, Hansson AC, Fuxe K. Distribution of dopamine D(2)-like receptors in the rat amygdala and their role in the modulation of unconditioned fear and anxiety. Neuroscience. 2012;201:252–266. doi: 10.1016/j.neuroscience.2011.10.045. [DOI] [PubMed] [Google Scholar]
- Perkins ND, Felzien LK, Betts JC, Leung K, Beach DH, Nabel GJ. Regulation of NF-kappaB by cyclin-dependent kinases associated with the p300 coactivator. Science. 1997;275:523–527. doi: 10.1126/science.275.5299.523. [DOI] [PubMed] [Google Scholar]
- Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of fear extinction with hippocampal-infralimbic BDNF. Science. 2010;328:1288–1290. doi: 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer UJ, Fendt M. Prefrontal dopamine D4 receptors are involved in encoding fear extinction. Neuroreport. 2006;17:847–850. doi: 10.1097/01.wnr.0000220142.29413.6f. [DOI] [PubMed] [Google Scholar]
- Pinard CR, Muller JF, Mascagni F, McDonald AJ. Dopaminergic innervation of interneurons in the rat basolateral amygdala. Neuroscience. 2008;157:850–863. doi: 10.1016/j.neuroscience.2008.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Rasmusson AM. Ganaxolone improves behavioral deficits in a mouse model of post-traumatic stress disorder. Front Cell Neurosci. 2014;8:256. doi: 10.3389/fncel.2014.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A, Sesack SR. Ultrastructural analysis of prefrontal cortical inputs to the rat amygdala: Spatial relationships to presumed dopamine axons and D1 and D2 receptors. Brain Struct Funct. 2008;213:159–175. doi: 10.1007/s00429-008-0180-6. [DOI] [PubMed] [Google Scholar]
- Plendl W, Wotjak CT. Dissociation of within- and between-session extinction of conditioned fear. J Neurosci. 2010;30:4990–4998. doi: 10.1523/JNEUROSCI.6038-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnusamy R, Nissim HA, Barad M. Systemic blockade of D2-like dopamine receptors facilitates extinction of conditioned fear in mice. Learn Mem. 2005;12:399–406. doi: 10.1101/lm.96605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: The impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova D, Agustsdottir A, Lindholm J, Mazulis U, Akamine Y, Castren E, et al. Combination of fluoxetine and extinction treatments forms a unique synaptic protein profile that correlates with long-term fear reduction in adult mice. Eur Neuropsychopharmacol. 2014;24:1162–1174. doi: 10.1016/j.euroneuro.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Powers MB, Smits JA, Otto MW, Sanders C, Emmelkamp PM. Facilitation of fear extinction in phobic participants with a novel cognitive enhancer: A randomized placebo controlled trial of yohimbine augmentation. J Anxiety Disord. 2009;23:350–356. doi: 10.1016/j.janxdis.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Psotta L, Lessmann V, Endres T. Impaired fear extinction learning in adult heterozygous BDNF knock-out mice. Neurobiol Learn Mem. 2013;103:34–38. doi: 10.1016/j.nlm.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Quartermain D, Mower J, Rafferty MF, Herting RL, Lanthorn TH. Acute but not chronic activation of the NMDA-coupled glycine receptor with D-cycloserine facilitates learning and retention. Eur J Pharmacol. 1994;257:7–12. doi: 10.1016/0014-2999(94)90687-4. [DOI] [PubMed] [Google Scholar]
- Quirarte GL, Roozendaal B, McGaugh JL. Glucocorticoid enhancement of memory storage involves noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci U S A. 1997;94:14048–14053. doi: 10.1073/pnas.94.25.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirin M, Kuhl J, Dusing R. Oxytocin buffers cortisol responses to stress in individuals with impaired emotion regulation abilities. Psychoneuroendocrinology. 2011;36:898–904. doi: 10.1016/j.psyneuen.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Sripada CS, Abelson JL, Liberzon I, Milad MR, et al. Cannabinoid facilitation of fear extinction memory recall in humans. Neuropharmacology. 2013;64:396–402. doi: 10.1016/j.neuropharm.2012.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Railey AM, Micheli TL, Wanschura PB, Flinn JM. Alterations in fear response and spatial memory in pre- and post-natal zinc supplemented rats: Remediation by copper. Physiol Behav. 2010;100:95–100. doi: 10.1016/j.physbeh.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Rainnie DG. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. J Neurophysiol. 1999;82:69–85. doi: 10.1152/jn.1999.82.1.69. [DOI] [PubMed] [Google Scholar]
- Reich CG, Mohammadi MH, Alger BE. Endocannabinoid modulation of fear responses: Learning and state-dependent performance effects. J Psychopharmacol. 2008;22:769–777. doi: 10.1177/0269881107083999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Li X, Zhang X, Li M, Wang Y, Ma Y. The effects of intra-hippocampal microinfusion of D-cycloserine on fear extinction, and the expression of NMDA receptor subunit NR2B and neurogenesis in the hippocampus in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44:257–264. doi: 10.1016/j.pnpbp.2013.02.017. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, et al. Cognitive enhancers as adjuncts to psychotherapy: Use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Reul JM. Making memories of stressful events: A journey along epigenetic, gene transcription, and signaling pathways. Front Psychiatry. 2014;5:5. doi: 10.3389/fpsyt.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey CD, Lipps J, Shansky RM. Dopamine D1 receptor activation rescues extinction impairments in low-estrogen female rats and induces cortical layer-specific activation changes in prefrontal-amygdala circuits. Neuropsychopharmacology. 2014;39:1282–1289. doi: 10.1038/npp.2013.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riaza Bermudo-Soriano C, Perez-Rodriguez MM, Vaquero-Lorenzo C, Baca-Garcia E. New perspectives in glutamate and anxiety. Pharmacol Biochem Behav. 2012;100:752–774. doi: 10.1016/j.pbb.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Riebe CJ, Pamplona FA, Kamprath K, Wotjak CT. Fear relief-toward a new conceptual frame work and what endocannabinoids gotta do with it. Neuroscience. 2012;204:159–185. doi: 10.1016/j.neuroscience.2011.11.057. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Bauer EP, Farb CR, Schafe GE, LeDoux JE. The group I metabotropic glutamate receptor mGluR5 is required for fear memory formation and long-term potentiation in the lateral amygdala. J Neurosci. 2002;22:5219–5229. doi: 10.1523/JNEUROSCI.22-12-05219.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues H, Figueira I, Goncalves R, Mendlowicz M, Macedo T, Ventura P. CBT for pharmacotherapy non-remitters-A systematic review of a next-step strategy. J Affect Disord. 2011;129:219–228. doi: 10.1016/j.jad.2010.08.025. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Romaguera J, Sotres-Bayon F, Mueller D, Quirk GJ. Systemic propranolol acts centrally to reduce conditioned fear in rats without impairing extinction. Biol Psychiatry. 2009;65:887–892. doi: 10.1016/j.biopsych.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan MT, Leon KS, Perez DL, Kandel ER. Distinct neural signatures for safety and danger in the amygdala and striatum of the mouse. Neuron. 2005;46:309–320. doi: 10.1016/j.neuron.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Rojas JC, Bruchey AK, Gonzalez-Lima F. Neurometabolic mechanisms for memory enhancement and neuroprotection of methylene blue. Prog Neurobiol. 2012;96:32–45. doi: 10.1016/j.pneurobio.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Quirarte GL, McGaugh JL. Glucocorticoids interact with the basolateral amygdala beta-adrenoceptor-cAMP/cAMP/PKA system in influencing memory consolidation. Eur J Neurosci. 2002;15:553–560. doi: 10.1046/j.0953-816x.2001.01876.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Schelling G, McGaugh JL. Corticotropin-releasing factor in the basolateral amygdala enhances memory consolidation via an interaction with the beta-adrenoceptor-cAMP pathway: Dependence on glucocorticoid receptor activation. J Neurosci. 2008;28:6642–6651. doi: 10.1523/JNEUROSCI.1336-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Vidal LE, Do-Monte FH, Sotres-Bayon F, Quirk GJ. Hippocampal-prefrontal BDNF and memory for fear extinction. Neuropsychopharmacology. 2014;39:2161–2169. doi: 10.1038/npp.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe MK, Craske MG. Effects of an expanding-spaced vs massed exposure schedule on fear reduction and return of fear. Behav Res Ther. 1998a;36:701–717. doi: 10.1016/s0005-7967(97)10016-x. [DOI] [PubMed] [Google Scholar]
- Rowe MK, Craske MG. Effects of varied-stimulus exposure training on fear reduction and return of fear. Behav Res Ther. 1998b;36:719–734. doi: 10.1016/s0005-7967(97)10017-1. [DOI] [PubMed] [Google Scholar]
- Rudenko A, Dawlaty MM, Seo J, Cheng AW, Meng J, Le T, et al. Tet1 is critical for neuronal activity-regulated gene expression and memory extinction. Neuron. 2013;79:1109–1122. doi: 10.1016/j.neuron.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthenburg AJ, Li H, Patel DJ, Allis CD. Multivalent engagement of chromatin modifications by linked binding modules. Nat Rev Mol Cell Biol. 2007;8:983–994. doi: 10.1038/nrm2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Matsumoto M, Yanagawa Y, Hiraide S, Inoue S, Kubo Y, et al. Facilitation of fear extinction by the 5-HT(1A) receptor agonist tandospirone: Possible involvement of dopaminergic modulation. Synapse. 2013;67:161–170. doi: 10.1002/syn.21621. [DOI] [PubMed] [Google Scholar]
- Salchner P, Singewald N. 5-HT receptor subtypes involved in the anxiogenic-like action and associated Fos response of acute fluoxetine treatment in rats. Psychopharmacology (Berl) 2006;185:282–288. doi: 10.1007/s00213-005-0247-5. [DOI] [PubMed] [Google Scholar]
- Sangha S, Narayanan RT, Bergado-Acosta JR, Stork O, Seidenbecher T, Pape HC. Deficiency of the 65 kDa isoform of glutamic acid decarboxylase impairs extinction of cued but not contextual fear memory. J Neurosci. 2009;29:15713–15720. doi: 10.1523/JNEUROSCI.2620-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana N, Bortolozzi A, Serrats J, Mengod G, Artigas F. Expression of serotonin1A and serotonin2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2004;14:1100–1109. doi: 10.1093/cercor/bhh070. [DOI] [PubMed] [Google Scholar]
- Santini E, Muller RU, Quirk GJ. Consolidation of extinction learning involves transfer from NMDA-independent to NMDA-dependent memory. J Neurosci. 2001;21:9009–9017. doi: 10.1523/JNEUROSCI.21-22-09009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheeringa MS, Weems CF. Randomized placebo-controlled D-cycloserine with cognitive behavior therapy for pediatric posttraumatic stress. J Child Adolesc Psychopharmacol. 2014;24:69–77. doi: 10.1089/cap.2013.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Cain CK, Curley NG, Schwartz JS, Stern SA, Ledoux JE, et al. Evidence for recovery of fear following immediate extinction in rats and humans. Learn Mem. 2008;15:394–402. doi: 10.1101/lm.909208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Raio CM, Phelps EA. Extinction training during the reconsolidation window prevents recovery of fear. J Vis Exp. 2012:e3893. doi: 10.3791/3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneier FR, Neria Y, Pavlicova M, Hembree E, Suh EJ, Amsel L, et al. Combined prolonged exposure therapy and paroxetine for PTSD related to the World Trade Center attack: A randomized controlled trial. Am J Psychiatry. 2012;169:80–88. doi: 10.1176/appi.ajp.2011.11020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl UI, Goh G, Stölting G, de Oliveira RC, Choi M, Overton JD, et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet. 2013;45:1050–1054. doi: 10.1038/ng.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Nader K, Pruessner JC. Reconsolidation of human memory: Brain mechanisms and clinical relevance. Biol Psychiatry. 2014;76:274–280. doi: 10.1016/j.biopsych.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Schwarzer C. 30 years of dynorphins—New insights on their functions in neuro-psychiatric diseases. Pharmacol Ther. 2009;123:353–370. doi: 10.1016/j.pharmthera.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn V, Wolff SB, Herry C, Grenier F, Ehrlich I, Grundemann J, et al. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron. 2014;81:428–437. doi: 10.1016/j.neuron.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Sepulveda-Orengo MT, Lopez AV, Soler-Cedeno O, Porter JT. Fear extinction induces mGluR5-mediated synaptic and intrinsic plasticity in infralimbic neurons. J Neurosci. 2013;33:7184–7193. doi: 10.1523/JNEUROSCI.5198-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DM, Power KG, Simpson RJ, Swanson V, Anstee JA. Global measures of outcome in a controlled comparison of pharmacological and psychological treatment of panic disorder and agoraphobia in primary care. Br J Gen Pract. 1997;47:150–155. [PMC free article] [PubMed] [Google Scholar]
- Shibasaki M, Mizuno K, Kurokawa K, Ohkuma S. L-type voltage-dependent calcium channels facilitate acetylation of histone H3 through PKC gamma phosphorylation in mice with methamphetamine-induced place preference. J Neurochem. 2011;118:1056–1066. doi: 10.1111/j.1471-4159.2011.07387.x. [DOI] [PubMed] [Google Scholar]
- Shin RM, Higuchi M, Suhara T. Nitric oxide signaling exerts bidirectional effects on plasticity inductions in amygdala. PLoS One. 2013;8:e74668. doi: 10.1371/journal.pone.0074668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitaka Y, Matsuki N, Saito H, Katsuki H. Basic fibroblast growth factor increases functional L-type Ca2+ channels in fetal rat hippocampal neurons: Implications for neurite morphogenesis in vitro. J Neurosci. 1996;16:6476–6489. doi: 10.1523/JNEUROSCI.16-20-06476.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si W, Aluisio L, Okamura N, Clark SD, Fraser I, Sutton SW, et al. Neuropeptide S stimulates dopaminergic neurotransmission in the medial prefrontal cortex. J Neurochem. 2010;115:475–482. doi: 10.1111/j.1471-4159.2010.06947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl S, Flor PJ, Fendt M. Amygdaloid metabotropic glutamate receptor subtype 7 is involved in the acquisition of conditioned fear. Neuroreport. 2008;19:1147–1150. doi: 10.1097/WNR.0b013e328307f295. [DOI] [PubMed] [Google Scholar]
- Siegmund A, Golfels F, Finck C, Halisch A, Rath D, Plag J, et al. D-cycloserine does not improve but might slightly speed up the outcome of in-vivo exposure therapy in patients with severe agoraphobia and panic disorder in a randomized double blind clinical trial. J Psychiatr Res. 2011;45:1042–1047. doi: 10.1016/j.jpsychires.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MT. Extinction of a passive avoidance response in adrenalectomized and demedullated rats. Behav Biol. 1973;9:553–562. doi: 10.1016/s0091-6773(73)80050-1. [DOI] [PubMed] [Google Scholar]
- Silvestri AJ, Root DH. Effects of REM deprivation and an NMDA agonist on the extinction of conditioned fear. Physiol Behav. 2008;93:274–281. doi: 10.1016/j.physbeh.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Singewald N, Philippu A. Release of neurotransmitters in the locus coeruleus. Prog Neurobiol. 1998;56:237–267. doi: 10.1016/s0301-0082(98)00039-2. [DOI] [PubMed] [Google Scholar]
- Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol Psychiatry. 2003;53:275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- Sinnegger-Brauns MJ, Hetzenauer A, Huber IG, Renstrom E, Wietzorrek G, Berjukov S, et al. Isoform-specific regulation of mood behavior and pancreatic beta cell and cardiovascular function by L-type Ca 2+ channels. J Clin Invest. 2004;113:1430–1439. doi: 10.1172/JCI20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery DA, Naik RR, Yen YC, Sartori SB, Fuechsl A, Grund T, et al. Selective breeding for high anxiety introduces a synonymous SNP that increases neuropeptide S receptor activity. J Neurosci. 2014s doi: 10.1523/JNEUROSCI.4764-13.2015. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JA, Hofmann SG, Rosenfield D, DeBoer LB, Costa PT, Simon NM, et al. D-cycloserine augmentation of cognitive behavioral group therapy of social anxiety disorder: Prognostic and prescriptive variables. J Consult Clin Psychol. 2013a;81:1100–1112. doi: 10.1037/a0034120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JA, Rosenfield D, Davis ML, Julian K, Handelsman PR, Otto MW, et al. Yohimbine enhancement of exposure therapy for social anxiety disorder: A randomized controlled trial. Biol Psychiatry. 2014;75:840–846. doi: 10.1016/j.biopsych.2013.10.008. [DOI] [PubMed] [Google Scholar]
- Smits JA, Rosenfield D, Otto MW, Marques L, Davis ML, Meuret AE, et al. D-cycloserine enhancement of exposure therapy for social anxiety disorder depends on the success of exposure sessions. J Psychiatr Res. 2013b;47:1455–1461. doi: 10.1016/j.jpsychires.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JA, Rosenfield D, Otto MW, Powers MB, Hofmann SG, Telch MJ, et al. D-cycloserine enhancement of fear extinction is specific to successful exposure sessions: Evidence from the treatment of height phobia. Biol Psychiatry. 2013c;73:1054–1058. doi: 10.1016/j.biopsych.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder GL, Fienberg AA, Huganir RL, Greengard P. A dopamine/D1 receptor/ protein kinase A/dopamine- and cAMP-regulated phosphoprotein (Mr 32 kDa)/protein phosphatase-1 pathway regulates dephosphorylation of the NMDA receptor. J Neurosci. 1998;18:10297–10303. doi: 10.1523/JNEUROSCI.18-24-10297.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soravia LM, Heinrichs M, Aerni A, Maroni C, Schelling G, Ehlert U, et al. Glucocorticoids reduce phobic fear in humans. Proc Natl Acad Sci U S A. 2006;103:5585–5590. doi: 10.1073/pnas.0509184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soravia LM, Heinrichs M, Winzeler L, Fisler M, Schmitt W, Horn H, et al. Glucocorticoids enhance in vivo exposure-based therapy of spider phobia. Depress Anxiety. 2014;31:429–435. doi: 10.1002/da.22219. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Acquisition of fear extinction requires activation of NR2B–containing NMDA receptors in the lateral amygdala. Neuropsychopharmacology. 2007;32:1929–1940. doi: 10.1038/sj.npp.1301316. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Diaz-Mataix L, Bush DE, LeDoux JE. Dissociable roles for the ventromedial prefrontal cortex and amygdala in fear extinction: NR2B contribution. Cereb Cortex. 2009;19:474–482. doi: 10.1093/cercor/bhn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadaro PA, Bredy TW. Emerging role of non-coding RNA in neural plasticity, cognitive function, and neuropsychiatric disorders. Front Genet. 2012;3:132. doi: 10.3389/fgene.2012.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford JM, Raybuck JD, Ryabinin AE, Lattal KM. Increasing histone acetylation in the hippocampus-infralimbic network enhances fear extinction. Biol Psychiatry. 2012;72:25–33. doi: 10.1016/j.biopsych.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DJ, Ipser J, McAnda N. Pharmacotherapy of posttraumatic stress disorder: A review of meta-analyses and treatment guidelines. CNS Spectr. 2009;14:25–31. [PubMed] [Google Scholar]
- Stein MB, Ron Norton G, Walker JR, Chartier MJ, Graham R. Do selective serotonin re-uptake inhibitors enhance the efficacy of very brief cognitive behavioral therapy for panic disorder? A pilot study. Psychiatry Res. 2000;94:191–200. doi: 10.1016/s0165-1781(00)00154-2. [DOI] [PubMed] [Google Scholar]
- Storch EA, Merlo LJ, Bengtson M, Murphy TK, Lewis MH, Yang MC, et al. D-cycloserine does not enhance exposure-response prevention therapy in obsessive-compulsive disorder. Int Clin Psychopharmacol. 2007;22:230–237. doi: 10.1097/YIC.0b013e32819f8480. [DOI] [PubMed] [Google Scholar]
- Storch EA, Murphy TK, Goodman WK, Geffken GR, Lewin AB, Henin A, et al. A preliminary study of D-cycloserine augmentation of cognitive-behavioral therapy in pediatric obsessive-compulsive disorder. Biol Psychiatry. 2010;68:1073–1076. doi: 10.1016/j.biopsych.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storsve AB, McNally GP, Richardson R. US habituation, like CS extinction, produces a decrement in conditioned fear responding that is NMDA dependent and subject to renewal and reinstatement. Neurobiol Learn Mem. 2010;93:463–471. doi: 10.1016/j.nlm.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Striessnig J, Koschak A, Sinnegger-Brauns MJ, Hetzenauer A, Nguyen NK, Busquet P, et al. Role of voltage-gated L-type Ca2+ channel isoforms for brain function. Biochem Soc Trans. 2006;34:903–909. doi: 10.1042/BST0340903. [DOI] [PubMed] [Google Scholar]
- Striessnig J, Pinggera A, Kaur G, Bock G, Tuluc P. L-type Ca channels in heart and brain. Wiley Interdiscip Rev Membr Transp Signal. 2014;3:15–38. doi: 10.1002/wmts.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann GE, LeDoux JE. GABAergic antagonists block the inhibitory effects of serotonin in the lateral amygdala: A mechanism for modulation of sensory inputs related to fear conditioning. J Neurosci. 1999;19:RC8. doi: 10.1523/JNEUROSCI.19-11-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung JY, Shin SW, Ahn YS, Chung KC. Basic fibroblast growth factor-induced activation of novel CREB kinase during the differentiation of immortalized hippocampal cells. J Biol Chem. 2001;276:13858–13866. doi: 10.1074/jbc.M010610200. [DOI] [PubMed] [Google Scholar]
- Suris A, North C, Adinoff B, Powell CM, Greene R. Effects of exogenous glucocorticoid on combat-related PTSD symptoms. Ann Clin Psychiatry. 2010;22:274–279. [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Experience-dependent epigenetic modifications in the central nervous system. Biol Psychiatry. 2009;65:191–197. doi: 10.1016/j.biopsych.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney FF, O'Leary OF, Cryan JF. GABAB receptor ligands do not modify conditioned fear responses in BALB/c mice. Behav Brain Res. 2013;256:151–156. doi: 10.1016/j.bbr.2013.07.035. [DOI] [PubMed] [Google Scholar]
- Szapiro G, Vianna MR, McGaugh JL, Medina JH, Izquierdo I. The role of NMDA glutamate receptors, PKA, MAPK, and CAMKII in the hippocampus in extinction of conditioned fear. Hippocampus. 2003;13:53–58. doi: 10.1002/hipo.10043. [DOI] [PubMed] [Google Scholar]
- Szczytkowski-Thomson JL, Lebonville CL, Lysle DT. Morphine prevents the development of stress-enhanced fear learning. Pharmacol Biochem Behav. 2013;103:672–677. doi: 10.1016/j.pbb.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A, Tamano H. Cognitive decline due to excess synaptic Zn(2+) signaling in the hippocampus. Front Aging Neurosci. 2014;6:26. doi: 10.3389/fnagi.2014.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, et al. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Tart CD, Handelsman PR, Deboer LB, Rosenfield D, Pollack MH, Hofmann SG, et al. Augmentation of exposure therapy with post-session administration of D-cycloserine. J Psychiatr Res. 2013;47:168–174. doi: 10.1016/j.jpsychires.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasan RO, Bukovac A, Peterschmitt YN, Sartori SB, Landgraf R, Singewald N, et al. Altered GABA transmission in a mouse model of increased trait anxiety. Neuroscience. 2011;183:71–80. doi: 10.1016/j.neuroscience.2011.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telch MJ, Bruchey AK, Rosenfield D, Cobb AR, Smits J, Pahl S, et al. Effects of post-session administration of methylene blue on fear extinction and contextual memory in adults with claustrophobia. Am J Psychiatry. 2014;171:1091–1098. doi: 10.1176/appi.ajp.2014.13101407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terlau H, Seifert W. Fibroblast growth factor enhances long-term potentiation in the hippocampal slice. Eur J Neurosci. 1990;2:973–977. doi: 10.1111/j.1460-9568.1990.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Terzian AL, Drago F, Wotjak CT, Micale V. The dopamine and cannabinoid interaction in the modulation of emotions and cognition: Assessing the role of cannabinoid CB1 receptor in neurons expressing dopamine D1 receptors. Front Behav Neurosci. 2011;5:49. doi: 10.3389/fnbeh.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BM, Baratta MV, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. Activation of the infralimbic cortex in a fear context enhances extinction learning. Learn Mem. 2010;17:591–599. doi: 10.1101/lm.1920810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian F, Hu XZ, Wu X, Jiang H, Pan H, Marini AM, et al. Dynamic chromatin remodeling events in hippocampal neurons are associated with NMDA receptor-mediated activation of Bdnf gene promoter 1. J Neurochem. 2009;109:1375–1388. doi: 10.1111/j.1471-4159.2009.06058.x. [DOI] [PubMed] [Google Scholar]
- Tian F, Marini AM, Lipsky RH. NMDA receptor activation induces differential epigenetic modification of Bdnf promoters in hippocampal neurons. Amino Acids. 2010;38:1067–1074. doi: 10.1007/s00726-009-0315-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomilenko RA, Dubrovina NI. Effects of activation and blockade of NMDA receptors on the extinction of a conditioned passive avoidance response in mice with different levels of anxiety. Neurosci Behav Physiol. 2007;37:509–515. doi: 10.1007/s11055-007-0044-1. [DOI] [PubMed] [Google Scholar]
- Toth I, Dietz M, Peterlik D, Huber SE, Fendt M, Neumann ID, et al. Pharmacological interference with metabotropic glutamate receptor subtype 7 but not subtype 5 differentially affects within- and between-session extinction of Pavlovian conditioned fear. Neuropharmacology. 2012a;62:1619–1626. doi: 10.1016/j.neuropharm.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Toth I, Neumann ID, Slattery DA. Central administration of oxytocin receptor ligands affects cued fear extinction in rats and mice in a timepoint-dependent manner. Psychopharmacology (Berl) 2012b;223:149–158. doi: 10.1007/s00213-012-2702-4. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Corcoran KA, Jovasevic V, Radulovic J. Fear conditioning and extinction: Emotional states encoded by distinct signaling pathways. Trends Neurosci. 2012;35:145–155. doi: 10.1016/j.tins.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouche S, Sasaki JM, Tu T, Reijmers LG. Fear extinction causes target-specific remodeling of perisomatic inhibitory synapses. Neuron. 2013;80:1054–1065. doi: 10.1016/j.neuron.2013.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trugman JM, James CL, Wooten GF. D1/D2 dopamine receptor stimulation by L-dopa. A [14C]-2-deoxyglucose autoradiographic study. Brain. 1991;114(Pt 3):1429–1440. doi: 10.1093/brain/114.3.1429. [DOI] [PubMed] [Google Scholar]
- Tsai LH, Graff J. On the resilience of remote traumatic memories against exposure therapy-mediated attenuation. EMBO Rep. 2014;15:853–861. doi: 10.15252/embr.201438913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Veinante P, Freund-Mercier MJ. Distribution of oxytocin- and vasopressin-binding sites in the rat extended amygdala: A histoautoradiographic study. J Comp Neurol. 1997;383:305–325. [PubMed] [Google Scholar]
- Verma D, Tasan RO, Herzog H, Sperk G. NPY controls fear conditioning and fear extinction by combined action on Y(1) and Y(2) receptors. Br J Pharmacol. 2012;166:1461–1473. doi: 10.1111/j.1476-5381.2012.01872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervliet B. Learning and memory in conditioned fear extinction: Effects of D-cycloserine. Acta Psychol (Amst) 2008;127:601–613. doi: 10.1016/j.actpsy.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Vervliet B, Baeyens F, Van den Bergh O, Hermans D. Extinction, generalization, and return of fear: A critical review of renewal research in humans. Biol Psychol. 2013;92:51–58. doi: 10.1016/j.biopsycho.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Viviani D, Charlet A, van den Burg E, Robinet C, Hurni N, Abatis M, et al. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science. 2011;333:104–107. doi: 10.1126/science.1201043. [DOI] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltereit R, Mannhardt S, Nescholta S, Maser-Gluth C, Bartsch D. Selective and protracted effect of nifedipine on fear memory extinction correlates with induced stress response. Learn Mem. 2008;15:348–356. doi: 10.1101/lm.808608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CC, Lin HC, Chan YH, Gean PW, Yang YK, Chen PS. 5-HT1A–receptor agonist modified amygdala activity and amygdala-associated social behavior in a valproate-induced rat autism model. Int J Neuropsychopharmacol. 2013;16:2027–2039. doi: 10.1017/S1461145713000473. [DOI] [PubMed] [Google Scholar]
- Weber M, Hart J, Richardson R. Effects of D-cycloserine on extinction of learned fear to an olfactory cue. Neurobiol Learn Mem. 2007;87:476–482. doi: 10.1016/j.nlm.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Wei W, Coelho CM, Li X, Marek R, Yan S, Anderson S, et al. p300/CBP-Associated Factor Selectively Regulates the Extinction of Conditioned Fear. J Neurosci. 2012;32:11930–11941. doi: 10.1523/JNEUROSCI.0178-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner DM, Levey AI, Sunahara RK, Niznik HB, O'Dowd BF, Seeman P, et al. D1 and D2 dopamine receptor mRNA in rat brain. Proc Natl Acad Sci U S A. 1991;88:1859–1863. doi: 10.1073/pnas.88.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, et al. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci. 2007;27:684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner-Seidler A, Richardson R. Effects of D-cycloserine on extinction: Consequences of prior exposure to imipramine. Biol Psychiatry. 2007;62:1195–1197. doi: 10.1016/j.biopsych.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Wessa M, Flor H. Failure of extinction of fear responses in posttraumatic stress disorder: Evidence from second-order conditioning. Am J Psychiatry. 2007;164:1684–1692. doi: 10.1176/appi.ajp.2007.07030525. [DOI] [PubMed] [Google Scholar]
- Whittle N, Hauschild M, Lubec G, Holmes A, Singewald N. Rescue of impaired fear extinction and normalization of cortico-amygdala circuit dysfunction in a genetic mouse model by dietary zinc restriction. J Neurosci. 2010;30:13586–13596. doi: 10.1523/JNEUROSCI.0849-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle N, Schmuckermair C, Gunduz Cinar O, Hauschild M, Ferraguti F, Holmes A, et al. Deep brain stimulation, histone deacetylase inhibitors and glutamatergic drugs rescue resistance to fear extinction in a genetic mouse model. Neuropharmacology. 2013;64:414–423. doi: 10.1016/j.neuropharm.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle N, Singewald N. HDAC inhibitors as cognitive enhancers in fear, anxiety and trauma therapy: Where do we stand? Biochem Soc Trans. 2014;42:569–581. doi: 10.1042/BST20130233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S, Buhlmann U, Tolin DF, Meunier SA, Pearlson GD, Reese HE, et al. Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. Am J Psychiatry. 2008;165:335–341. doi: 10.1176/appi.ajp.2007.07050776. quiz 409. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Roth WT. Acute and delayed effects of alprazolam on flight phobics during exposure. Behav Res Ther. 1997;35:831–841. doi: 10.1016/s0005-7967(97)00033-8. [DOI] [PubMed] [Google Scholar]
- Willard SS, Koochekpour S. Glutamate, glutamate receptors, and downstream signaling pathways. Int J Biol Sci. 2013;9:948–959. doi: 10.7150/ijbs.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jonsson B, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:655–679. doi: 10.1016/j.euroneuro.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Woods AM, Bouton ME. D-cycloserine facilitates extinction but does not eliminate renewal of the conditioned emotional response. Behav Neurosci. 2006;120:1159–1162. doi: 10.1037/0735-7044.120.5.1159. [DOI] [PubMed] [Google Scholar]
- Wrubel KM, Barrett D, Shumake J, Johnson SE, Gonzalez-Lima F. Methylene blue facilitates the extinction of fear in an animal model of susceptibility to learned helplessness. Neurobiol Learn Mem. 2007;87:209–217. doi: 10.1016/j.nlm.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Wu D. Neuroprotection in experimental stroke with targeted neurotrophins. NeuroRx. 2005;2:120–128. doi: 10.1602/neurorx.2.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YL, Gall CM, Jackson VR, Civelli O, Reinscheid RK. Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J Comp Neurol. 2007;500:84–102. doi: 10.1002/cne.21159. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhu Y, Contractor A, Heinemann SF. mGluR5 has a critical role in inhibitory learning. J Neurosci. 2009;29:3676–3684. doi: 10.1523/JNEUROSCI.5716-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada D, Wada K, Sekiguchi M. Facilitating actions of an AMPA receptor potentiator upon extinction of contextually conditioned fear response in stressed mice. Neurosci Lett. 2011;488:242–246. doi: 10.1016/j.neulet.2010.11.038. [DOI] [PubMed] [Google Scholar]
- Yamada D, Zushida K, Wada K, Sekiguchi M. Pharmacological discrimination of extinction and reconsolidation of contextual fear memory by a potentiator of AMPA receptors. Neuropsychopharmacology. 2009;34:2574–2584. doi: 10.1038/npp.2009.86. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Morinobu S, Fuchikami M, Kurata A, Kozuru T, Yamawaki S. Effects of single prolonged stress and D-cycloserine on contextual fear extinction and hip-pocampal NMDA receptor expression in a rat model of PTSD. Neuropsychopharmacology. 2008;33:2108–2116. doi: 10.1038/sj.npp.1301605. [DOI] [PubMed] [Google Scholar]
- Yang YL, Chao PK, Lu KT. Systemic and intra-amygdala administration of glucocorticoid agonist and antagonist modulate extinction of conditioned fear. Neuropsychopharmacology. 2006;31:912–924. doi: 10.1038/sj.npp.1300899. [DOI] [PubMed] [Google Scholar]
- Yang YL, Chao PK, Ro LS, Wo YY, Lu KT. Glutamate NMDA receptors within the amygdala participate in the modulatory effect of glucocorticoids on extinction of conditioned fear in rats. Neuropsychopharmacology. 2007;32:1042–1051. doi: 10.1038/sj.npp.1301215. [DOI] [PubMed] [Google Scholar]
- Yang YL, Lu KT. Facilitation of conditioned fear extinction by d-cycloserine is mediated by mitogen-activated protein kinase and phosphatidylinositol 3-kinase cascades and requires de novo protein synthesis in basolateral nucleus of amygdala. Neuroscience. 2005;134:247–260. doi: 10.1016/j.neuroscience.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Yang CH, Shi HS, Zhu WL, Wu P, Sun LL, Si JJ, et al. Venlafaxine facilitates between-session extinction and prevents reinstatement of auditory-cue conditioned fear. Behav Brain Res. 2012;230:268–273. doi: 10.1016/j.bbr.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Yao YL, Yang WM, Seto E. Regulation of transcription factor YY1 by acetylation and deacetylation. Mol Cell Biol. 2001;21:5979–5991. doi: 10.1128/MCB.21.17.5979-5991.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee BK, Hauser J, Dolgov VV, Keist R, Mohler H, Rudolph U, et al. GABA receptors containing the alpha5 subunit mediate the trace effect in aversive and appetitive conditioning and extinction of conditioned fear. Eur J Neurosci. 2004;20:1928–1936. doi: 10.1111/j.1460-9568.2004.03642.x. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Risk and resilience in posttraumatic stress disorder. J Clin Psychiatry. 2004;65(Suppl 1):29–36. Review. [PubMed] [Google Scholar]
- Yehuda R, Bierer LM, Pratchett L, Malowney M. Glucocorticoid augmentation of prolonged exposure therapy: Rationale and case report. Eur J Psychotraumatol. 2010:1. doi: 10.3402/ejpt.v1i0.5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen YC, Mauch CP, Dahlhoff M, Micale V, Bunck M, Sartori SB, et al. Increased levels of conditioned fear and avoidance behavior coincide with changes in phosphorylation of the protein kinase B (AKT) within the amygdala in a mouse model of extremes in trait anxiety. Neurobiol Learn Mem. 2012;98:56–65. doi: 10.1016/j.nlm.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Yokoyama M, Suzuki E, Sato T, Maruta S, Watanabe S, Miyaoka H. Amygdalic levels of dopamine and serotonin rise upon exposure to conditioned fear stress without elevation of glutamate. Neurosci Lett. 2005;379:37–41. doi: 10.1016/j.neulet.2004.12.047. [DOI] [PubMed] [Google Scholar]
- Yu H, Wang Y, Pattwell S, Jing D, Liu T, Zhang Y, et al. Variant BDNF Val66Met polymorphism affects extinction of conditioned aversive memory. J Neurosci. 2009;29:4056–4064. doi: 10.1523/JNEUROSCI.5539-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoveli JM, Carvalho MC, Cunha JM, Brandao ML. Extracellular serotonin level in the basolateral nucleus of the amygdala and dorsal periaqueductal gray under unconditioned and conditioned fear states: An in vivo microdialysis study. Brain Res. 2009;1294:106–115. doi: 10.1016/j.brainres.2009.07.074. [DOI] [PubMed] [Google Scholar]
- Zelikowsky M, Hast TA, Bennett RZ, Merjanian M, Nocera NA, Ponnusamy R, et al. Cholinergic blockade frees fear extinction from its contextual dependency. Biol Psychiatry. 2013;73:345–352. doi: 10.1016/j.biopsych.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Asgeirsdottir HN, Cohen SJ, Munchow AH, Barrera MP, Stackman RW., Jr Stimulation of serotonin 2A receptors facilitates consolidation and extinction of fear memory in C57BL/6J mice. Neuropharmacology. 2013;64:403–413. doi: 10.1016/j.neuropharm.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JM, Maren S. NMDA receptor antagonism in the basolateral but not central amygdala blocks the extinction of Pavlovian fear conditioning in rats. Eur J Neurosci. 2010;31:1664–1670. doi: 10.1111/j.1460-9568.2010.07223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoicas I, Slattery DA, Neumann ID. Brain oxytocin in social fear conditioning and its extinction: Involvement of the lateral septum. Neuropsychopharmacology. 2012;98:56–65. doi: 10.1038/npp.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zushida K, Sakurai M, Wada K, Sekiguchi M. Facilitation of extinction learning for contextual fear memory by PEPA: A potentiator of AMPA receptors. J Neurosci. 2007;27:158–166. doi: 10.1523/JNEUROSCI.3842-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]