Abstract
Cells have evolved a highly integrated network of mechanisms to coordinate cellular survival/death, proliferation, differentiation, and repair with metabolic states. It is, therefore, not surprising that proteins with canonical roles in cell death/survival also modulate nutrient and energy metabolism and vice versa. The finding that many BCL-2 (B cell lymphoma 2) proteins reside at mitochondria or can translocate to this organelle has long motivated investigation into their involvement in normal mitochondrial physiology and metabolism. These endeavors have led to the discovery of homeostatic roles for BCL-2 proteins beyond apoptosis. Here, we predominantly focus on recent findings that link select BCL-2 proteins to carbon substrate utilization at the level of mitochondrial fuel choice, electron transport, and metabolite import independent of their cell death regulatory function.
Keywords: BCL-2 proteins, mitochondria, OXPHOS, glucose, fatty acids, metabolism
The BCL-2 family proteins: A brief overview
Known for their canonical function as regulators of the intrinsic or mitochondrial pathway of apoptosis, the BCL-2 family proteins have been the subject of intense biochemical, structural and genetic studies. Below, we provide a brief highlight of molecular features that endow these proteins with exquisite control over cellular life/death decisions before turning our attention to the main focus of this review, namely their physiologic roles in nutrient and energy metabolism. For in depth discussion of cell death regulatory functions of BCL-2 family proteins, we refer the reader to several excellent recent reviews [1-3].
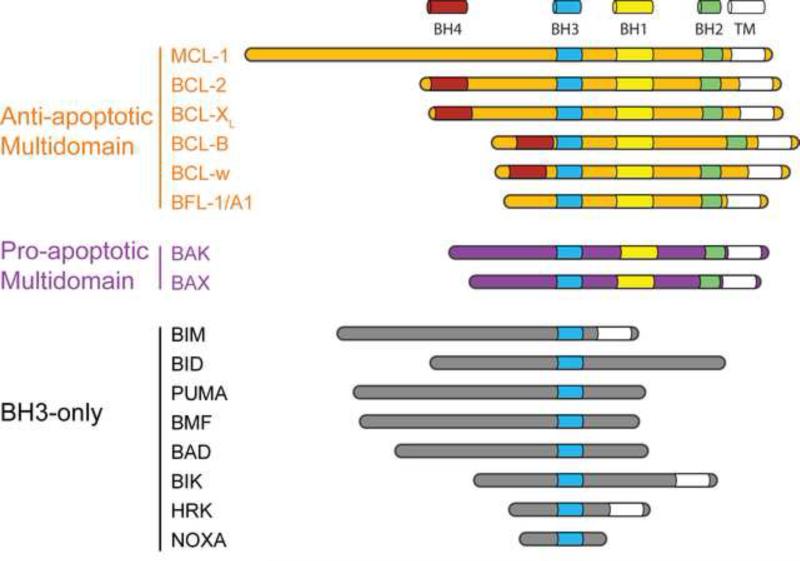
The BCL-2 family consists of anti- and pro-apoptotic proteins that share sequence homology within conserved α-helical regions known as BCL-2 homology (BH) domains. All anti-apoptotic members, such as BCL-2 and BCL-XL, and a subset of pro-apoptotic family members, such as BAX (BCL-2 associated X protein) and BAK (BCL-2 antagonist killer), are “multi-domain” proteins that contain 3-4 BH domains (Figure 1). The “BH3-only” pro-apoptotic molecules, including BAD, BID, BIM, NOXA, BIK, HRK and PUMA show sequence homology only within a single BH domain, BH3, which is an amphipathic α-helix also known as the minimal death domain required for binding to “multi-domain” BCL-2 family members [2-5] (Figure 1). In addition to BH domains, several BCL-2 family members possess a transmembrane domain and can localize to sub-cellular membranes, including the mitochondrial outer membrane and endoplasmic reticulum (ER). Other BCL-2 proteins devoid of a transmembrane domain can tether to mitochondria through interaction with other proteins, including mitochondrial resident BCL-2 family members.
Figure 1. Structural and functional classification of BCL-2 proteins.
Classifications of BCL-2 proteins according to conserved BCL-2 homology (BH) domains. C-terminal hydrophobic sequences function to target and/or anchor select BCL-2 family proteins to intracellular membranes. TM, transmembrane domain.
The capacity of different BCL-2 proteins to form highly selective interactions is integral to their regulation of apoptosis. These interactions ultimately control mitochondrial intramembranous oligomerization of BAX/BAK and the attendant mitochondrial outer membrane permeabilization (MOMP), leading to cytochrome c release. BAX/BAK are requisite gateway to mitochondrial apoptosis in that their combined deletion prevents release of cytochrome c and produces resistance to all death stimuli that activate the intrinsic pathway of apoptosis [6]. BH3-only proteins are cell death initiators whose pro-apoptotic activity is latent unless activated by transcriptional or post-translational mechanisms in a tissue-restricted and signal-specific manner [2, 4, 5]. They act upstream of BAX/BAK and serve as sentinels for distinct damage signals, thereby increasing the range of inputs for stress signals such as DNA damage, growth factor withdrawal, proteotoxic stress and hypoxia. The pro-apoptotic activity of BH3-only proteins is associated with exposure of the hydrophobic face of their amphipathic BH3 helix, enabling it to interact with the hydrophobic groove of multi-domain anti- and pro-apoptotic family members.
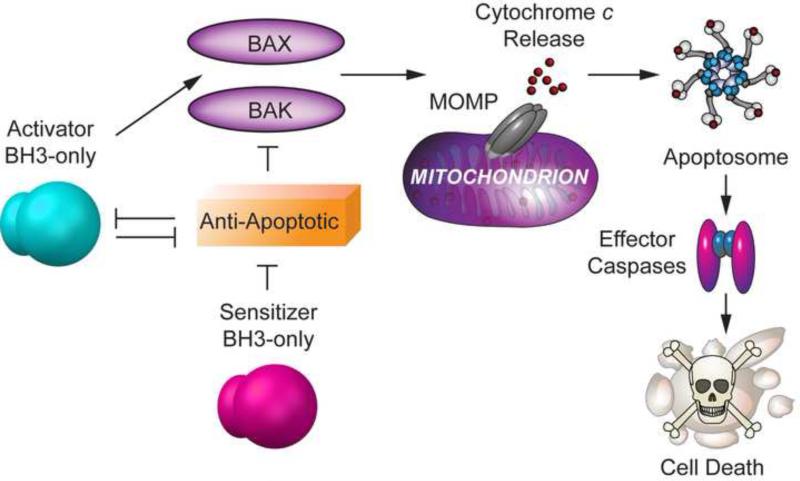
Interactions among different BCL-2 proteins are governed by cytosolic and membrane conformers of select family members that are regulated at multiple levels, including binding affinities and association with membrane lipids [1, 7]. As such, understanding the precise mechanisms controlling MOMP has been a formidable challenge in the field. While these mechanisms have been intensely debated, some consensus appears to be emerging [1-3]. BH3-only pro-apoptotic molecules fall in two functional categories. BH3-only proteins such as BAD and NOXA referred to as “sensitizers” or “de-repressors” bind to and inhibit anti-apoptotic partners, while others such as BIM, BID and PUMA known as “activators” can directly bind and induce oligomerization of BAX/BAK leading to MOMP (Figure 2). Activator BH3-only proteins can also bind and neutralize anti-apoptotic BCL-2 proteins. Anti-apoptotic proteins inhibit apoptosis by binding activator BH3-only proteins and by preventing BAX/BAK oligomerization [8] (Figure. 2). A proposed mechanism for the latter is that membrane inserted conformers of anti-apoptotic proteins such as BCL-2, which are defective in oligomerization, may bind membrane-embedded BAX/BAK, preventing their subsequent oligomerization [9]. Tonic activation of BH3-only molecules in response to a given stress signal can eventually overcome the neutralizing capacity of anti-apoptotic members. Sensitizer BH3-only proteins engage anti-apoptotic molecules, allowing activator BH3-only proteins to activate BAX/BAK. Furthermore, membrane activated conformers of BAX/BAK can subsequently activate other latent BAX/BAK molecules through an auto-activation mechanism, amplifying the signal to trigger MOMP [10, 11]. In addition, increasing evidence indicates that BAX/BAK oligomerization can be modulated by mitochondrial membrane remodeling through altered balance of mitochondrial fission and fusion events, as well as by other mitochondrial outer membrane proteins [12-14].
Figure 2. BCL-2 family interactions and regulation of BAX/BAK oligomerization.
BAX/BAK activation is directly triggered by activator BH3-only proteins (BIM, BID and PUMA) and is inhibited by anti-apoptotic BCL-2 family members. Sensitizer BH3-only proteins do not activate BAX and BAK directly, but lower the threshold for apoptosis by binding anti-apoptotic members and releasing activators to trigger BAX and BAK oligomerization. Anti-apoptotic BCL-2 proteins inhibit activator BH3-only proteins and BAX/BAK oligomerization.
The expanding functional networks of BCL-2 proteins beyond regulation of cell death and survival
The ability of different BCL-2 proteins to form highly selective interactions is integral to their function. The structural details of these interactions, their dynamics, and their modulation in or at the mitochondrial outer membrane have revealed important mechanistic insights into regulation of apoptosis [1-3, 15, 16]. As mentioned above, these interactions ultimately control MOMP, leading to the release of cytochrome c. Biochemical studies have also expanded the network of BCL-2 protein interactions to a number of non-BCL-2 protein partners that either modulate the cell death regulatory capacity of BCL-2 proteins [12-14], or endow them with homeostatic functions beyond and independent of apoptosis [17-19]. These functions include, but are not limited to, mitochondrial energy and nutrient metabolism, calcium homeostasis, mitochondrial morphology, cell cycle checkpoints, and DNA damage response. Some of these functions were uncovered during investigation of apoptotic mechanisms and others were discovered in the process of examining BCL-2 proteins in healthy cells in the absence of apoptotic stimuli. How these additional physiologic roles are related or distinct from the cell death modulatory function of BCL-2 proteins is an active area of research. Within this context, identification and characterization of novel binding partners and post translational modifications of individual BCL-2 proteins will continue to provide valuable insights (Box 1). In addition, how these alternative functions are shared/redundant or specialized among individual BCL-2 family members continues to be an evolving area of research.
BCL-2 proteins and carbon substrate utilization
The capacity of cells to sense nutrients and metabolize a variety of carbon substrates (glucose, amino acids, ketone bodies, and fatty acids) not only fulfills specific anabolic and catabolic needs but can modulate cell fate and function [20, 21]. Emerging evidence also indicates that cellular preference for a specific fuel substrate as well as the capacity to switch from utilization of one substrate to another can profoundly influence adaptive responses to stress [21]. Moreover, the metabolic byproducts of nutrient metabolism such as acetyl-CoA, glucose-6-phosphate, glucosamine, succinate, fumarate, malonyl-CoA, and S-adenosyl methionine can serve as signaling messengers capable of altering organelle cross-talk, post-translational modification of proteins, chromatin states, and gene expression [22, 23]. Below, we focus on recent findings that have implicated select BCL-2 family proteins in regulation of carbon substrate utilization as well as fuel switching, and briefly review the experimental observations as to whether and how these metabolic roles are connected to, or independent of their cell death regulatory function.
BAD and BNIP3 in endocrine tissues: A tale of sugar and fat
The first evidence for the involvement of a BCL-2 family member in systemic glucose homeostasis emerged from the studies of the BH3-only protein BAD and its direct interaction with glucokinase (GK, hexokinase IV)- the maturity onset diabetes of the young type 2 (MODY2) gene product [24-27]. GK is a key component of the mammalian glucose sensing machinery with tissue-specific roles in insulin secretion by pancreatic islet β-cells, glucose utilization and storage in hepatocytes, as well as central glucose sensing [28]. Compared to other hexokinase isoforms, unique kinetic properties such as lack of product inhibition by glucose-6-phosphate, a high Km (low affinity) for glucose, and positive substrate cooperativity, render the GK-catalyzed reaction essentially a substrate-driven process particularly well-suited for regulation of blood glucose levels. The notion that the BAD-GK partnership is physiologically relevant for glucose sensing and systemic glucose homeostasis is consistent with several metabolic alterations that are phenocopied in vivo and in vitro upon ablation or depletion of each protein in β-cells and liver, including reduced glycolysis and mitochondrial handling of glucose, fasting hyperglycemia, loss of glucose responsiveness of insulin secretion in β-cells, impaired glucose tolerance, and hepatic insulin resistance [24-26].
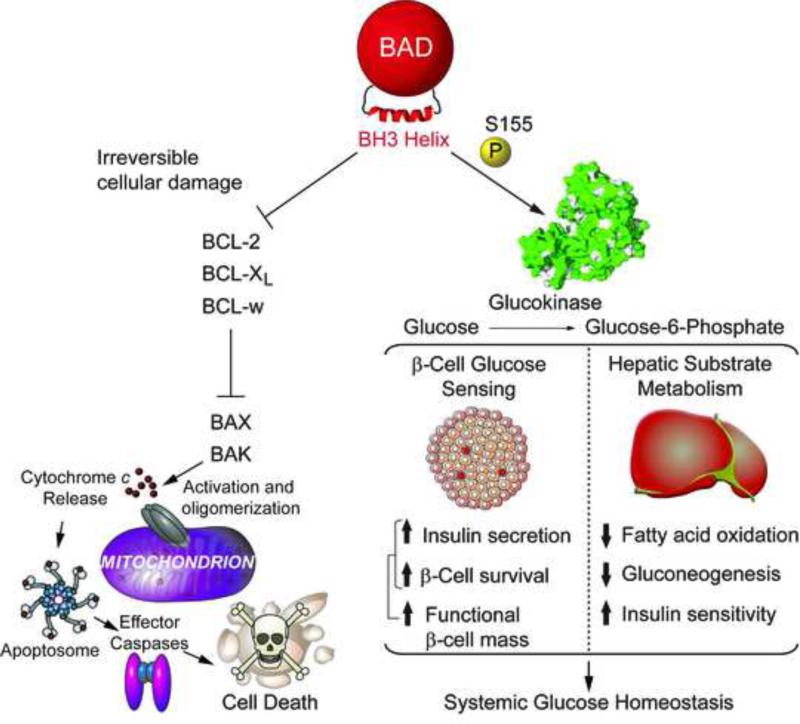
Activation of GK by BAD is dependent on phosphorylation of a conserved serine residue within the BAD BH3 domain, Ser155 in mouse BAD corresponding to Ser118 in the human sequence. Depending on the cell type and cellular context, several kinases and signalling pathways can regulate BAD phosphorylation, including RSK/PKA, AKT, and p70S6K [reviewed in 29]. These either target Ser155 phosphorylation directly or phosphorylate Ser136 upstream of the BH3 domain, which in turn primes Ser155 phosphorylation. Phosphorylation of Ser155 acts as a molecular switch that neutralizes BAD's apoptotic function by preventing its interaction with pro-survival BCL-2, BCL-XL and BCL-w proteins, and simultaneously triggers its ability to activate GK [25-27, 29] (Figure 3). At the molecular and structural level, GK activation by BAD is mediated by direct binding of the phospho-BAD BH3 helix near the active site of GK, whereby the Vmax of the enzyme is increased through a non-allosteric mechanism without changes in GK affinity for glucose [27]. Importantly, BAD phosphorylation is sensitive to glucose, nutritional (fed and fasted) states, and insulin [25, 26, 30], suggesting that the functional crosstalk between BAD and GK may serve as a homeostatic sensor of the nutrient and hormonal milieu in β-cells and hepatocytes. When the BAD BH3 domain is dephosphorylated, BAD can no longer activate GK but can bind and inhibit the pro-survival molecules BCL-2, BCL-XL, and BCL-w [reviewed in 29] (Figure 3). BAD dephosphorylation per se is not sufficient to trigger apoptosis but plays a permissive role and sensitizes cells to apoptosis when sufficient cellular damage has accumulated. As described below, in the absence of apoptotic stimuli or cellular damage, dephosporylated BAD has other effects on carbon substrate metabolism.
Figure 3. The BAD-GK axis and systemic glucose homeostasis.
BAD modulates glucose metabolism in β-cells and liver by directly activating glucokinase (GK). This requires phosphorylation of the BAD BH3 domain on serine 155 (aa enumeration based on the murine sequence of BAD). In the β-cell, phosphorylated BAD enhances glucose-stimulation of insulin release through increased GK activity and mitochondrial oxidation of glucose. In addition, phospho-BAD imparts cell autonomous protective effects in β-cells that is GK-dependent. Simultaneous enhancement of insulin secretion and β-cell survival by phospho-BAD leads to improved functional β-cell mass. In the liver, GK activation by phospho-BAD increases glucose utilization, represses fatty acid oxidation and reduces gluconeogenesis. Hepatic BAD phosphorylation is a target of insulin and is required for insulin suppression of gluconeogenesis (hepatic insulin sensitivity). Accordingly, when BAD is dephosphorylated, glycolysis is diminished, FAO is disinhibited, and gluconeogenesis is increased. Separate from these effects, dephosphorylated BAD can also sensitize cells to apoptosis under conditions of cellular damage. This is mediated by BH3 domain-dependent binding and neutralization of the pro-survival BCL-2, BCL-XL and BCL-w proteins by BAD. These interactions are blocked when S155 is phosphorylated. Dephosphorylated BAD per se is not sufficient to trigger apoptosis but plays a permissive role under conditions of irreversible cellular damage. In the absence of apoptotic stimuli, when cellular survival/death decisions are not at play, dephosphorylated BAD promotes FAO.
Mitochondria play an important role in insulin secretion by β-cells through the production of “coupling factors” such as ATP/ADP and NAD(P)H that couple fuel (glucose and amino acids) oxidation to the release of insulin granules [31]. GK activity in β-cells is tightly linked to their capacity to oxidize glucose and release insulin in a glucose dose responsive manner (glucose sensing) [28]. Studies in islets derived from Bad −/− and Bad S155A knockin mice, and in human donor islets indicate that the phospho-BAD BH3 helix is necessary and sufficient for proper mitochondrial oxidation of glucose and the attendant increase in insulin secretion, but does not affect insulin release in response to amino acids or other secretagogues that donate carbons to the tricarboxylic acid (TCA) cycle independent of GK activity [25, 27, 32]. This selective defect in glucose-stimulated insulin secretion rather than a broad deficiency in insulin release is in agreement with GK as the metabolic target of BAD phosphorylation, and is further in line with the observation that mutations in BAD do not produce global defects in mitochondrial substrate metabolism and oxidative phosphorylation (OXPHOS), which would otherwise lead to a general defect in insulin secretion in response to glucose and non-glucose secretagogues.
Glucose metabolism in β-cells not only triggers insulin secretion but can also affect β-cell mass by promoting β-cell proliferation and survival as well as preventing their de-differentiation [32-36]. Recent findings indicate that genetic and pharmacologic approaches to mimic BAD phosphorylation within its BH3 helix augment functional β-cell mass through β-cell autonomous protective effects [32]. The benefits of phospho-BAD in β-cells include protection from apoptosis induced by inflammatory, oxidative and ER stress stimuli, as well as increased glucose handling and insulin secretory capacities (Figure 3). Phospho-BAD BH3 mimicry also promotes long term survival of β-cells, as evident from the superior engraftment of donor islets treated with stapled peptides modeled after the phospho-BAD BH3 helix and transplanted into diabetic mice [32]. The dual effects of phospho-BAD on glucose-stimulated insulin release and β-cell protection from stress-induced death translate into improved functional β-cell mass in vivo (Figure 3). At the mechanistic level, comparison of phospho-BH3 BAD variants indicated that the effect of BAD phosphorylation on β-cell survival is linked to its capacity to activate GK and cannot be solely explained by the lack of its inhibitory effects on pro-survival BCL-2 proteins. Consistent with these observations, the protective effect of phospho-BAD is abrogated following molecular depletion of GK [32]. The precise branch-points of glucose metabolism downstream of the BAD-GK axis that may promote β-cell survival are not known. Glucose-derived metabolites can feed a number of pathways that will change the pool of NAPDH, glutathione, nucleotides and lipid intermediates, significantly affecting the antioxidant, DNA repair, and biosynthetic capacity of β-cells that may be relevant to β-cell protection in this context. In addition, changes in ATP and Ca2+ flux following mitochondrial metabolism of glucose-derived pyruvate may provide bioenergetic benefits that can be β-cell protective.
While GK in β-cells is essential for proper glucose sensing and insulin release, its activity in the liver plays a central role in setting the balance between hepatic glucose utilization, production and storage in the fed and fasted states. In the fed state, hepatic glycolysis is increased and excess glucose is used for glycogen synthesis and lipogenesis. During long-term fasting, glucose is produced de novo (gluconeogenesis) in the liver from lactate, pyruvate, glycerol and amino acids such as alanine that are supplied by the muscle. This ensures that the blood glucose supply to the brain and other tissues is not interrupted during fasting. Gluconeogenesis and glycolysis are competing pathways that are reciprocally regulated by hormonal signals, including insulin and glucagon, as well as hepatic carbon flux [37]. Importantly, these processes are heavily influenced by mitochondria through fatty acid oxidation (FAO), TCA cycle and electron transport chain (ETC) activity, which supply the gluconeogenic pathway with metabolic precursors, reducing equivalents and ATP [38]. Findings in both liver and cultured hepatocytes in which BAD or GK are manipulated indicate that loss of GK or BAD, or inhibition of BAD phosphorylation is associated with programmatic changes in hepatic metabolism beyond reduced glycolysis. When BAD is phosphorylated and GK is active, FAO and gluconeogenesis are actively inhibited (Figure 3). When BAD is dephosphorylated or ablated, glycolysis is diminished, FAO is disinhibited, and pyruvate is preferentially partitioned to gluconeogenesis in a cell-autonomous manner (Figure 3). In this setting, increased FAO supports elevated gluconeogenesis [26]. Importantly, these observations highlight the metabolic consequences of BAD phosphorylation status in a context where cells have not been stressed with apoptotic stimuli and cellular life/death decisions are not at play. The reciprocal effects of BAD on hepatic utilization of glucose and fatty acids are GK-dependent in that when GK is molecularly depleted, the phospho-mimic variant of BAD can no longer increase hepatic glucose utilization or inhibit FAO [26]. How the reciprocal utilization of glucose and fatty acids is controlled by the hepatic BAD-GK axis at the metabolite level awaits additional studies. It is possible that BAD phosphorylation state may help mitochondria distinguish the glycolytic versus gluconeogenic source of pyruvate (pyruvate derived from gluconeogenic amino acids that are supplied by the muscle during fasting), thereby modulating its metabolic fate. It is also possible that BAD phosphorylation controls the level of a “switch” metabolite that actively represses FAO.
Exaggerated gluconeogenesis in the absence of BAD or when BAD phosphorylation is blocked is refractory to insulin action (hepatic insulin resistance) [26]. This is consistent with the observation that BAD phosphorylation, which can downregulate gluconeogenesis through GK activation, is triggered by insulin, and suggests that BAD may serve as a downstream effector of insulin in suppression of hepatic glucose production. The physiologic relevance of phospho-BAD in the context of systemic glucose metabolism is further evident from the observations that hepatic expression of BAD S155D phospho-mimic variant in high fat-fed mice or leptin resistant obese (ob/ob) diabetic mice downregulates hepatic gluconeogenesis, allays fasting hyperglycemia, and significantly improves glucose homeostasis [26].
Another BH3-only protein, BNIP3, can also influence hepatic adaptation to fed and fasted states. Hepatic BNIP3 expression is increased during fasting, and Bnip3-deficient mice show increased lipogenesis and reduced FAO, which is consistent with excessive hepatic lipid accumulation (hepatic steatosis) and a failure to augment hepatic glucose production during fasting [39]. This hepatic phenotype is opposite of Bad −/− liver and hepatocytes. The molecular mediators of BNIP3-dependent changes in mitochondrial FAO have not been elucidated. In light of published reports on the role of BNIP3 in mitophagy and clearance of suboptimal or damaged mitochondria [40, 41], it is possible that the reduced hepatic FAO in BNIP3-deficient mice is linked to diminished mitochondrial function [39]. This scenario would be consistent with increasing evidence in multiple experimental systems that mitochondrial fuel handling is tightly coordinated with mitochondrial network architecture dictated by mitochondrial fission and fusion events, as well as mitochondrial life cycle through mitophagy [42, 43].
BAD-dependent glucose-to-ketone body fuel switch and the excitable brain
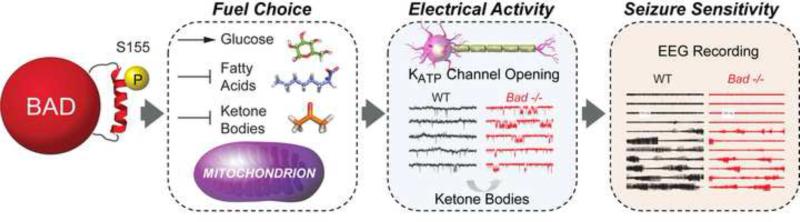
Regulation of glucose metabolism by BAD is not limited to tissues that express GK, suggesting cell type-specific mechanisms/binding partners that mediate BAD's metabolic function [44, 45]. In particular, BAD modulation of substrate metabolism in the brain is relevant for neuronal excitability and seizure sensitivity [45]. Glucose is the main source of energy in the brain, however neural cells can switch to alternative substrates such as ketone bodies under conditions of glucose limitation and dietary restriction [46, 47]. Ketone bodies and certain fatty acids can cross the blood brain barrier and provide up to 60% of energy in the brain in cases of low glucose availability [48, 49], and their metabolism is integrated through acetyl-CoA pools. In addition, ketone bodies can be synthesized locally by astrocytes [50, 51]. The capacity of mitochondria to process carbohydrates and ketone bodies can influence neuronal excitability. For example, the ketogenic diet (KD), which reduces glucose metabolism and promotes the breakdown of fatty acids to generate ketone bodies, is effective in pharmacoresistant epilepsies [52, 53]. The molecular underpinnings of this diet-induced glucose-to-ketone body fuel switch and the attendant changes in neuronal activity are not fully understood, but several mechanisms have been put forward. These include changes in energy charge, reactive oxygen species (ROS) detoxification capacity, increased synthesis of the inhibitory neurotransmitter GABA, and opening of ATP-sensitive potassium (KATP) channels [reviewed in 53]. Identification of cell-intrinsic molecular modulators that can induce a similar glucose-to-ketone body fuel switch in the brain without the complex systemic effects of dietary manipulation will greatly aid the molecular understanding of the link between metabolism and neuronal activity. Recent findings implicated BAD as such cell-intrinsic modulator of glucose versus ketone body utilization in the brain, independent of dietary treatment [45]. Specifically, BAD S155 phosphorylation in neurons and astrocytes not only stimulates mitochondrial metabolism of glucose but inhibits ketone body utilization, producing a glucose-to-ketone body fuel switch in these cells. An electrophysiologic consequence of this fuel preference is a marked increase in the open probability of the metabolically sensitive KATP channel in dentate granule neurons (DGNs) within the dentate gyrus [45] (Figure 4), an area that is believed to function as a “seizure gate” in the brain [54-56]. The link between BAD-dependent programming of neural metabolism and neuronal excitability involves reductions in electrographic and behavioral seizures in vivo [45] (Figure 4). Seizure resistance in this setting cannot be explained by changes in neural cell apoptosis following BAD modifications, but genetic evidence indicates that the opening of the KATP channel is a necessary downstream mediator of BAD's effect on neuronal excitation and seizure responses [45]. The precise mechanism of KATP channel activation by BAD-dependent metabolic changes is not fully known. Glucose deprivation or elevation of ketone body metabolism can each increase KATP channel activity [57, 58]. Additional data indicate that seizure resistance in BAD-deficient mice is likely related to changes in neural metabolism rather than systemic metabolic alterations. Consistent with this, the whole brain content of the ketone body species β-hydroxybutyrate (BHB) is significantly higher in Bad −/− mice, while its serum levels are not altered [45]. This suggests potential BAD-dependent local changes in ketone body generation and handling such as ketogenesis by astrocytes. As ketone bodies can increase the activity of the KATP channel in central neurons [57, 58], their higher content in this setting may explain, at least in part, the observed increase in KATP channel activity. In addition, the contribution of other mechanisms beyond changes in KATP channel activity downstream of BAD cannot be ruled out. Characterization of the metabolite signature of the glucose-to-ketone-body fuel switch, which is directly and acutely associated with BAD modification, is an important goal of ongoing studies. This may pinpoint the potential mechanism by which BAD alters the fuel choice in the brain, and thereby modifies neuronal excitability. In addition, given that GK is not expressed in the dentate gyrus, the potential BAD-interacting partners that may influence the glucose-toketone-body fuel switch in this setting will be highly informative.
Figure 4. BAD-dependent glucose-to-ketone body fuel switch and metabolic control of neuronal excitability.
BAD phosphorylation sets the preference for carbon substrate utilization in the brain, favoring mitochondrial oxidation of glucose as the main source of energy over alternative fuels such as ketone bodies or fatty acids. BAD dephosphorylation triggers a glucose-to-ketone body fuel switch that is associated with opening of the K+-ATP-sensitive (KATP) channels. While this has negligible impact in neuronal physiology under steady state conditions, the increase in KATP channel activity significantly buffers excessive neuronal firing under pathological conditions such as epilepsy. When BAD is ablated or S155 is dephosphorylated, increased consumption of ketone bodies over glucose in the brain and the attendant increase in KATP channel opening produce resistance to electrographic and behavioural seizures. These effects are independent of BAD's apoptotic activity. EEG, electroencephalography.
NOXA and the pentose phosphate pathway
NOXA is another BH3-only molecule that can regulate glucose metabolism through a phosphorylation-dependent mechanism [59]. Cyclin-dependent kinase 5 (CDK5) phosphorylates human NOXA on Ser13 upstream of its BH3 domain in a glucose-dependent manner, leading to re-routing of glucose-derived carbons from glycolysis to the pentose phosphate pathway (PPP) [59]. This might be especially relevant in T lymphocyte activation and the attendant increase in demand for energy and biosynthetic intermediates [60, 61]. Metabolic byproducts of PPP include ribose sugars for DNA synthesis and NADPH, which can support anti-oxidant mechanisms as well as fatty acids and cholesterol synthesis. As such, PPP activation may influence T cell proliferation as well as the synthesis of cholesterol-rich lipid rafts and T-cell receptor signaling. It is also possible that NOXA modulation of PPP may change protein glycosylation that can in turn modify T lymphocyte migration and homing. Which of these branch points are predominantly dependent on NOXA phosphorylation, is not known. Other studies reported increased expression of NOXA during T lymphocyte activation [62], which is consistent with findings that activated T cells have a glycolytic profile compared to resting or memory T cells, which display increased OXPHOS [60, 61, 63]. Similarly to BAD, phosphorylation inactivates the apoptotic function of NOXA and triggers its metabolic function [59]. Initial studies suggested that bifunctional activities of NOXA in apoptosis and glucose metabolism may be regulated through two distinct macromolecular complexes [59], the constituents of which have not been fully characterized. Interestingly, both complexes contain the long isoform of MCL-1 (MCL-1L), yet the mode of NOXA interaction with MCL-1L in these complexes appears to be distinct [59]. How these different interactions are precisely coordinated with metabolic or stress signals awaits future studies. It is also important to note that Ser13 upstream of the human NOXA sequence is not present in the mouse or rat sequence of the protein [64]. Thus, if and how mouse NOXA has similar dual roles in apoptosis and glucose metabolism is not known. In addition, compared to human NOXA, the mouse NOXA sequence contains two BH3 domains, and whether serine residues upstream of the second mouse BH3 domain may play an equivalent role as Ser13 in human NOXA has not been determined. Studies in NOXA-deficient mice have revealed a potential role for this BH3-only protein in regulating the size of distinct T cell lineages [65, 66]. However, given the differences between human and mouse NOXA, the potential contribution of a metabolic role for NOXA in this setting is not known.
BCL-2 proteins and mitochondrial electron transport chain activity
Processing of carbon substrates in the TCA cycle generates reducing equivalents that feed electrons to the electron transport chain (ETC) and support OXPHOS. Failure in the ETC activity leads to bioenergetic deficiencies and excessive production of ROS [67-69]. The functional fidelity of ETC is controlled by several homeostatic mechanisms that ensure proper synthesis, assembly, and organization of ETC complexes in the inner membrane. Mechanisms that can directly or indirectly converge on regulation of ETC are complex and diverse, including dedicated chaperones, dynamic membrane architecture [70, 71], membrane lipid composition [72], and organization of supercomplexes composed of individual ETC complexes [73, 74]. Below, we highlight findings that link select BCL-2 proteins to ETC activity and OXPHOS.
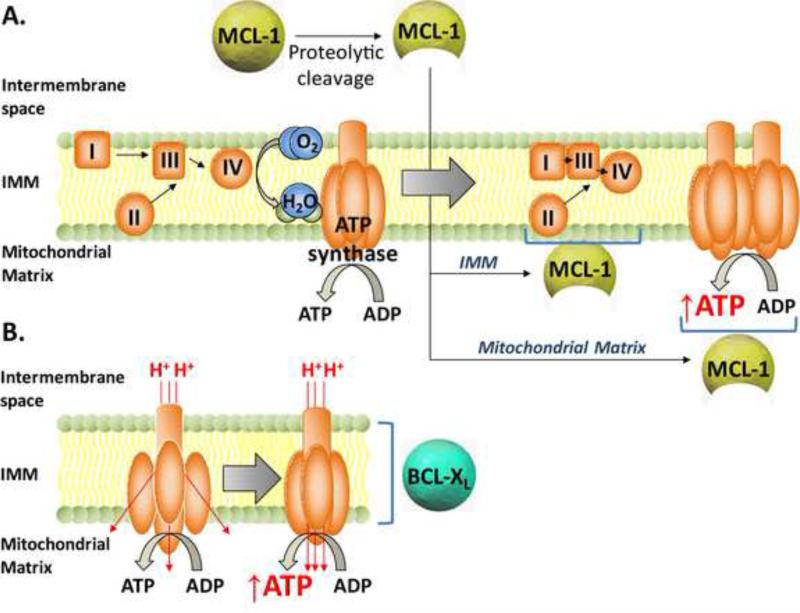
The anti-apoptotic protein MCL-1 affects mitochondrial respiration and ETC complex assembly separate and beyond its pro-survival function [75, 76]. Mcl-1 deletion results in loss of the tubular mitochondrial network and aberrations in mitochondrial ultrastructure, including abnormalities in cristae morphology [75, 76]. Genetic reconstitution studies revealed that these effects are mediated by a specific MCL-1 isoform that is produced by proteolytic processing and is selectively targeted to the mitochondrial matrix. In addition to a C terminal transmembrane domain that enables mitochondrial outer membrane localization/anchoring, MCL-1 has mitochondrial targeting and cleavage signals at the N-terminus [77]. The matrix targeted isoform is produced following N terminal processing by a matrix processing protease during MCL-1 import into mitochondria [75, 77]. However, other studies indicated that the N terminus of MCL-1 can be cleaved to produce a shorter isoform that remains at the outer mitochondrial membrane [78]. The production of the shorter membrane-bound isoform depends on an intact mitochondrial membrane potential [78], but the protease in charge has not been characterized. While the precise molecular details of MCL-1 processing to these different isoforms remain to be worked out, evidence indicates that these isoforms do not share similar functions. Specifically both the full length and N terminally cleaved membrane-bound isoforms have anti-apoptotic activity, while the matrix targeted isoform does not [75, 77]. Rather, the matrix isoform of MCL-1 contributes to the assembly of large respiratory supercomplexes comprising complexes I, III and IV, as well as the oligomerization of ATP synthase (complex V) [75] (Figure 5A). This is consistent with changes in mitochondrial respiration in the absence of MCL-1 and is further in line with recent findings that the dynamic assembly of complexes I, III and IV into supercomplexes is tightly integrated with the pattern and kinetics of electron flow through the ETC, ultimately influencing the efficiency of substrate oxidation [79]. In addition, as the assembly of respiratory complexes into supercomplexes is highly dependent on the integrity of mitochondrial cristae ultrastructure [80], MCL-1-dependent morphological changes in mitochondria are likely associated with the observed alterations in ETC activity.
Figure 5. The BCL-2 family and oxidative phosphorylation.
A. The anti-apoptotic MCL-1 protein undergoes proteolytic cleavage to produce a 36 KDa isoform that is imported into mitochondria, where it is tethered to the inner mitochondrial membrane (IMM) and exposed to the mitochondrial matrix. Within the IMM, cleaved MCL-1 helps stabilize the assembly of large respiratory supercomplexes comprised of complexes I, III and IV that have higher functional efficiency. In addition, the matrix-targeted cleaved form of MCL-1 promotes oligomerization of mitochondrial ATP-synthase and increased efficiency of ATP synthesis.
B. A small pool of BCL-XL resides in the IMM, where it interacts with the β-subunit of the ATP synthase complex. This may stabilize ATP synthase and increase the efficiency of coupling between proton gradient and ATP synthesis.
Other points of cross-talk between mitochondrial energy metabolism and BCL-2 family proteins have also been reported. For example, BCL-2 was found to stimulate mitochondrial respiration in tumor cells by increasing the cytochrome c oxidase (COX) activity of the respiratory complex IV [81]. This is proposed to be mediated by the physical interaction of BCL-2 and the COX Va subunit, leading to recruitment of the COX Vb subunit and subsequent increase in mitochondrial respiration [82]. Other studies showed that a small pool of BCL-XL resides at the mitochondrial inner membrane, interacts with the β subunit of the F1F0 ATP synthase, and augments the coupling efficiency of mitochondria [83, 84] (Figure 5B). This interaction as well as the association between BCL-2 and COX Va suggest non-canonical localization of BCL-2 and BCL-XL to mitochondrial inner membrane, the underlying mechanism of which remains to be elucidated. BCL-XL may also have other effects on OXPHOS and mitochondrial bioenergetics by lowering acetyl-CoA levels [85]. These effects have been predominantly examined within the context of anti-apoptotic/protective role of BCL-XL [85], and the potential regulation of acetyl-CoA pools by BCL-XL under settings independent of cellular life/death decisions awaits further studies.
Changes in OXPHOS can affect cellular signaling through ROS, a byproduct of ETC activity [69]. Depending on their levels and cellular context, ROS can influence cellular signaling. By affecting mitochondrial ATP and ROS production through OXPHOS, select BCL-2 family members may alter redox signaling. For example, increased recruitment of BID to mitochondria is associated with elevated mitochondrial ROS production and cycling of hematopoietic stem cells (HSCs), which can exhaust the quiescent pool of HSCs [86]. This can in turn be regulated by ATM-mediated phosphorylation of BID as well as its interaction with the mitochondrial carrier homologue 2 (MTCH2) [14, 86]. BID phosphorylation reduces its mitochondrial recruitment and the attendant ROS production. A physiologic consequence of reduced mitochondrial ROS levels in this setting is down regulation of G1 cell cycle regulators and preservation of the quiescent state in HSCs [86]. When prevented from phosphorylation, higher levels of BID are found at the mitochondria, and mitochondrial ROS levels are increased. If and how the effect of BID in this setting occurs through its interaction with MTCH2, which is required for its mitochondrial recruitment [14], and how OXPHOS is precisely regulated by the ATM-BID-MTCH2 axis awaits future studies.
BCL-2 proteins and mitochondrial import of substrates and metabolites
Metabolite traffic across the mitochondrial membranes is tightly controlled to ensure proper oxidation of substrates, as well as regulated efflux or exchange of intermediary metabolites generated in mitochondria, which are subsequently diverted to other subcellular compartments and metabolic pathways. Additionally, selective permeability of the inner mitochondrial membrane is required to maintain the proton gradient for ATP generation by the ATP synthase complex. Breakdown in regulation of mitochondrial permeability can lead to bioenergetic decline and disruption of calcium homeostasis, with a range of pathologic consequences such as cell death [87]. Below, we highlight several examples of the functional partnership between certain BCL-2 family proteins and mitochondrial permeability to metabolites and substrate import.
The voltage-dependent anion channel (VDAC) in the mitochondrial outer membrane mediates metabolite and anion exchange between mitochondria and the cytosol, and together with the adenine nucleotide translocator (ANT), modulates ATP/ADP exchange between these compartments. As such, the opening state of VDAC can influence metabolic flux and the coupling of glycolysis with mitochondrial respiration [88, 89]. VDAC opening is regulated by several mechanisms, including phosphorylation and interaction with multiple protein partners, metabolites, and mitochondrial membrane lipids [90]. The association of select BCL-2 family proteins with various VDAC isoforms has led to several models as to their potential involvement in cell death/survival, which has been the subject of intense debate [reviewed in 91, 92]. Other studies suggest that the interaction of select BCL-2 family proteins and VDAC may modulate metabolic flux across the mitochondrial outer membrane and energy metabolism. For example, structural characterization of the binding site of BCL-XL on VDAC indicated shared common residues with those of NADH, suggesting NADH and BCL-XL may compete for VDAC binding [93, 94]. This parallels previous studies of NADH and BCL-XL antagonism on VDAC closure [93-95], and gives rise to testable predictions for potential physiologic effect of BCL-XL on VDAC. In particular, it has been suggested that triggering VDAC closure by glucose-derived NADH may mediate the inhibitory effects of glycolysis on mitochondrial respiration (the Crabtree effect) [96]. It is possible that by competing with NADH binding to VDAC, BCL-XL may interfere with inhibition of mitochondrial respiration by glycolysis. This possibility remains to be formally tested.
Whereas the exchange of small metabolites between mitochondria and cytosol is facilitated by VDAC and ANT, other mechanisms are in place for the import of larger molecules. For example, the import of long-chain fatty acids requires the mitochondrial outer membrane enzyme carnitine palmitoyl transferase-1 (CPT-1), which conjugates “activated” acyl-CoA with carnitine, and mediates the subsequent transfer of the acylcarnitine complex across the mitochondrial inner membrane to the matrix. Consistent with its rate-limiting role in oxidation of long-chain fatty acids, the activity of CPT-1 is tightly regulated [97]. BID may be one such regulator. Specifically, the caspase-cleaved form of BID, tBID, which is known to translocate to mitochondria to induce cell death in a variety of settings, can also inhibit CPT-1 activity [98]. This is likely through indirect effects as tBID and CPT-1 do not interact [98]. Such an indirect effect may be mediated by changes in the mitochondrial membrane composition, in particular cardiolipin [99, 100]. It is possible that tBID may distort the lipid composition and distribution of mitochondrial membrane, leading to inhibition of CPT-1, which normally relies on the presence of cardiolipin for proper function [98]. A connection between BCL-2 and CPT-1 may also exist. In particular, BCL-2 was shown to interact with CPT-1, however the functional consequences of this interaction have not been reported [101].
The above observations give rise to the question whether and how regulation of CPT-1 by tBID is related to tBID's capacity to induce apoptosis. It is possible that tBID regulation of CPT-1 influences the partitioning of fatty acids for mitochondrial oxidation versus ceramide synthesis. As such, inhibition of CPT-1 by tBID may lead to increased availability of fatty acid precursors for ceramide synthesis, which may facilitate apoptosis under certain conditions, while in other settings, it may play an important role in signaling [98, 102]. If tBID's modulation of CPT-1 is independent of its apoptotic function, this would imply the existence of a pool of BID in healthy cells that can be cleaved under physiological, non-lethal conditions.
Concluding remarks and future perspectives
Nutrient sensing and utilization pathways are not only fundamental to cell fate and function but profoundly influence adaptive responses to physiological and patho-physiological stress at the cellular and organismal level. The expanding roles of BCL-2 proteins in nutrient and energy metabolism provide insights into additional ways in which these molecules modulate cellular homeostasis beyond their well recognized role in regulation of apoptosis. The effect of certain BCL-2 family members on nutrient and energy metabolism ranges from fuel-specific modulation to more general effects on ETC activity and OXPHOS, suggesting specialized metabolic roles for select individual BCL-2 family members. This specialization may stem from specific non- BCL-2 interaction partners that may or may not involve similar protein folds or binding pockets as those utilized for interactions among BCL-2 family members. Within this context, alternative function and protein targets for the BH3 helix derived from select family members has been reported. In addition, specific post translational modifications of select BCL-2 proteins may endow these proteins specialized functions beyond regulation of apoptosis. Understanding the precise molecular mechanisms mediating the diverse roles of BCL-2 proteins in nutrient and energy metabolism and how they can be independently manipulated is critical for effective targeting of these proteins in disease settings (Box 1).
Box 1. Outstanding questions.
How is the sub-cellular and sub-mitochondrial localization of select BCL-2 proteins regulated to allow their modulation of mitochondrial substrate and energy metabolism?
How is the selective effect of distinct BCL-2 proteins on mitochondrial metabolism mediated at the molecular level? What are the interacting proteins and post translational modifications that are required for these effects?
How are the metabolic effects of distinct BCL-2 proteins integrated with tissue-specific physiology independent of their role in apoptosis?
How is the cell death regulatory function of select BCL-2 proteins distinct from or related to their effects on normal mitochondrial physiology at the molecular level?
HIGHLIGHTS.
Select BCL-2 family proteins control mitochondrial substrate and energy metabolism
Metabolic functions of BCL-2 proteins can be independent of cell death regulation
Metabolic effects of BCL-2 proteins can alter cellular and systemic physiology
Acknowledgments
The authors would like to thank Eric Smith for part of the graphics in this article. This work was supported by the U.S. NIH grants R01DK078081 and R01NS083844 (N.N.D.), Juvenile Diabetes Research Foundation grant 17-2011-595 (N.N.D.), Hjärnfonden (Swedish Brain Foundation) grant FO2014-0257 (A. G.-C.), Petrus och Augusta Hedlunds Foundation grant M-59-2014 (A. G.-C.), and Åke Wibergs Foundation grant M14-0112 (A. G.-C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chi X, et al. Regulating cell death at, on, and in membranes. Biochimica et biophysica acta. 2014;1843:2100–2113. doi: 10.1016/j.bbamcr.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Czabotar PE, et al. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nature reviews. Molecular cell biology. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 3.Moldoveanu T, et al. Many players in BCL-2 family affairs. Trends in biochemical sciences. 2014;39:101–111. doi: 10.1016/j.tibs.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 5.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nature reviews. Molecular cell biology. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 6.Wei MC, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang T, Saghatelian A. Emerging roles of lipids in BCL-2 family-regulated apoptosis. Biochimica et biophysica acta. 2013;1831:1542–1554. doi: 10.1016/j.bbalip.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Llambi F, et al. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Molecular cell. 2011;44:517–531. doi: 10.1016/j.molcel.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dlugosz PJ, et al. Bcl-2 changes conformation to inhibit Bax oligomerization. The EMBO journal. 2006;25:2287–2296. doi: 10.1038/sj.emboj.7601126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gavathiotis E, et al. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan C, et al. Auto-activation of the apoptosis protein Bax increases mitochondrial membrane permeability and is inhibited by Bcl-2. The Journal of biological chemistry. 2006;281:14764–14775. doi: 10.1074/jbc.M602374200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montessuit S, et al. Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell. 2010;142:889–901. doi: 10.1016/j.cell.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ott M, et al. Mitochondrial targeting of tBid/Bax: a role for the TOM complex? Cell death and differentiation. 2009;16:1075–1082. doi: 10.1038/cdd.2009.61. [DOI] [PubMed] [Google Scholar]
- 14.Zaltsman Y, et al. MTCH2/MIMP is a major facilitator of tBID recruitment to mitochondria. Nature cell biology. 2010;12:553–562. doi: 10.1038/ncb2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kale J, et al. Shedding light on apoptosis at subcellular membranes. Cell. 2012;151:1179–1184. doi: 10.1016/j.cell.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Walensky LD, Gavathiotis E. BAX unleashed: the biochemical transformation of an inactive cytosolic monomer into a toxic mitochondrial pore. Trends in biochemical sciences. 2011;36:642–652. doi: 10.1016/j.tibs.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonneau B, et al. Non-apoptotic roles of Bcl-2 family: the calcium connection. Biochimica et biophysica acta. 2013;1833:1755–1765. doi: 10.1016/j.bbamcr.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Danial NN, et al. Homeostatic functions of BCL-2 proteins beyond apoptosis. Advances in experimental medicine and biology. 2010;687:1–32. doi: 10.1007/978-1-4419-6706-0_1. [DOI] [PubMed] [Google Scholar]
- 19.Hardwick JM, et al. Multipolar functions of BCL-2 proteins link energetics to apoptosis. Trends in cell biology. 2012;22:318–328. doi: 10.1016/j.tcb.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green DR, et al. Cell biology. Metabolic control of cell death. Science. 2014;345:1250256. doi: 10.1126/science.1250256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanley IA, et al. Changing appetites: the adaptive advantages of fuel choice. Trends in cell biology. 2014;24:118–127. doi: 10.1016/j.tcb.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaelin WG, Jr., McKnight SL. Influence of metabolism on epigenetics and disease. Cell. 2013;153:56–69. doi: 10.1016/j.cell.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu C, Thompson CB. Metabolic regulation of epigenetics. Cell Metab. 2012;16:9–17. doi: 10.1016/j.cmet.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danial NN, et al. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature. 2003;424:952–956. doi: 10.1038/nature01825. [DOI] [PubMed] [Google Scholar]
- 25.Danial NN, et al. Dual role of proapoptotic BAD in insulin secretion and beta cell survival. Nature medicine. 2008;14:144–153. doi: 10.1038/nm1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gimenez-Cassina A, et al. Regulation of Hepatic Energy Metabolism and Gluconeogenesis by BAD. Cell Metab. 2014;19:272–284. doi: 10.1016/j.cmet.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szlyk B, et al. A phospho-BAD BH3 helix activates glucokinase by a mechanism distinct from that of allosteric activators. Nature structural & molecular biology. 2014;21:36–42. doi: 10.1038/nsmb.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matschinsky FM. Assessing the potential of glucokinase activators in diabetes therapy. Nature reviews. Drug discovery. 2009;8:399–416. doi: 10.1038/nrd2850. [DOI] [PubMed] [Google Scholar]
- 29.Danial NN. BAD: undertaker by night, candyman by day. Oncogene. 2008;27(Suppl 1):S53–70. doi: 10.1038/onc.2009.44. [DOI] [PubMed] [Google Scholar]
- 30.Liu S, et al. Insulin signaling regulates mitochondrial function in pancreatic beta-cells. PloS one. 2009;4:e7983. doi: 10.1371/journal.pone.0007983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prentki M, et al. Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 2013;18:162–185. doi: 10.1016/j.cmet.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 32.Ljubicic S, et al. Phospho-BAD BH3 Mimicry Protects beta Cells and Restores Functional beta Cell Mass in Diabetes. Cell Rep. 2015;10:497–504. doi: 10.1016/j.celrep.2014.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porat S, et al. Control of pancreatic beta cell regeneration by glucose metabolism. Cell Metab. 2011;13:440–449. doi: 10.1016/j.cmet.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salpeter SJ, et al. Glucose and aging control the quiescence period that follows pancreatic beta cell replication. Development. 2010;137:3205–3213. doi: 10.1242/dev.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z, et al. Pancreatic beta cell dedifferentiation in diabetes and redifferentiation following insulin therapy. Cell Metab. 2014;19:872–882. doi: 10.1016/j.cmet.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei P, et al. Effects of glucokinase activators GKA50 and LY2121260 on proliferation and apoptosis in pancreatic INS-1 beta cells. Diabetologia. 2009;52:2142–2150. doi: 10.1007/s00125-009-1446-0. [DOI] [PubMed] [Google Scholar]
- 37.Lin HV, Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metab. 2011;14:9–19. doi: 10.1016/j.cmet.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satapati S, et al. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. Journal of lipid research. 2012;53:1080–1092. doi: 10.1194/jlr.M023382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glick D, et al. BNip3 regulates mitochondrial function and lipid metabolism in the liver. Molecular and cellular biology. 2012;32:2570–2584. doi: 10.1128/MCB.00167-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Landes T, et al. The BH3-only Bnip3 binds to the dynamin Opa1 to promote mitochondrial fragmentation and apoptosis by distinct mechanisms. EMBO reports. 2010;11:459–465. doi: 10.1038/embor.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rikka S, et al. Bnip3 impairs mitochondrial bioenergetics and stimulates mitochondrial turnover. Cell death and differentiation. 2011;18:721–731. doi: 10.1038/cdd.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liesa M, Shirihai OS. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013;17:491–506. doi: 10.1016/j.cmet.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friedman JR, Nunnari J. Mitochondrial form and function. Nature. 2014;505:335–343. doi: 10.1038/nature12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng H, et al. Phosphorylation of Bad at Thr-201 by JNK1 promotes glycolysis through activation of phosphofructokinase-1. The Journal of biological chemistry. 2008;283:20754–20760. doi: 10.1074/jbc.M800024200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gimenez-Cassina A, et al. BAD-dependent regulation of fuel metabolism and K(ATP) channel activity confers resistance to epileptic seizures. Neuron. 2012;74:719–730. doi: 10.1016/j.neuron.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zielke HR, et al. Direct measurement of oxidative metabolism in the living brain by microdialysis: a review. Journal of neurochemistry. 2009;109(Suppl 1):24–29. doi: 10.1111/j.1471-4159.2009.05941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mergenthaler P, et al. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends in neurosciences. 2013;36:587–597. doi: 10.1016/j.tins.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeVivo DC, et al. Chronic ketosis and cerebral metabolism. Annals of neurology. 1978;3:331–337. doi: 10.1002/ana.410030410. [DOI] [PubMed] [Google Scholar]
- 49.Owen OE, et al. Brain metabolism during fasting. The Journal of clinical investigation. 1967;46:1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Auestad N, et al. Fatty acid oxidation and ketogenesis by astrocytes in primary culture. Journal of neurochemistry. 1991;56:1376–1386. doi: 10.1111/j.1471-4159.1991.tb11435.x. [DOI] [PubMed] [Google Scholar]
- 51.Blazquez C, et al. Role of carnitine palmitoyltransferase I in the control of ketogenesis in primary cultures of rat astrocytes. Journal of neurochemistry. 1998;71:1597–1606. doi: 10.1046/j.1471-4159.1998.71041597.x. [DOI] [PubMed] [Google Scholar]
- 52.Hartman AL, et al. The neuropharmacology of the ketogenic diet. Pediatric neurology. 2007;36:281–292. doi: 10.1016/j.pediatrneurol.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lutas A, Yellen G. The ketogenic diet: metabolic influences on brain excitability and epilepsy. Trends in neurosciences. 2013;36:32–40. doi: 10.1016/j.tins.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dunn-Meynell AA, et al. Distribution and phenotype of neurons containing the ATP- sensitive K+ channel in rat brain. Brain research. 1998;814:41–54. doi: 10.1016/s0006-8993(98)00956-1. [DOI] [PubMed] [Google Scholar]
- 55.Karschin C, et al. Overlapping distribution of K(ATP) channel-forming Kir6.2 subunit and the sulfonylurea receptor SUR1 in rodent brain. FEBS letters. 1997;401:59–64. doi: 10.1016/s0014-5793(96)01438-x. [DOI] [PubMed] [Google Scholar]
- 56.Zawar C, et al. Cell-type specific expression of ATP-sensitive potassium channels in the rat hippocampus. The Journal of physiology. 1999;514(Pt 2):327–341. doi: 10.1111/j.1469-7793.1999.315ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma W, et al. Ketogenic diet metabolites reduce firing in central neurons by opening K(ATP) channels. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:3618–3625. doi: 10.1523/JNEUROSCI.0132-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanner GR, et al. Single K ATP channel opening in response to action potential firing in mouse dentate granule neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:8689–8696. doi: 10.1523/JNEUROSCI.5951-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lowman XH, et al. The proapoptotic function of Noxa in human leukemia cells is regulated by the kinase Cdk5 and by glucose. Molecular cell. 2010;40:823–833. doi: 10.1016/j.molcel.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 60.MacIver NJ, et al. Metabolic regulation of T lymphocytes. Annual review of immunology. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pearce EL, et al. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342:1242454. doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alves NL, et al. The Noxa/Mcl-1 axis regulates susceptibility to apoptosis under glucose limitation in dividing T cells. Immunity. 2006;24:703–716. doi: 10.1016/j.immuni.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 63.Ghesquiere B, et al. Metabolism of stromal and immune cells in health and disease. Nature. 2014;511:167–176. doi: 10.1038/nature13312. [DOI] [PubMed] [Google Scholar]
- 64.Ploner C, et al. Noxa: at the tip of the balance between life and death. Oncogene. 2008;27(Suppl 1):S84–92. doi: 10.1038/onc.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wensveen FM, et al. Pro-apoptotic protein Noxa regulates memory T cell population size and protects against lethal immunopathology. J Immunol. 2013;190:1180–1191. doi: 10.4049/jimmunol.1202304. [DOI] [PubMed] [Google Scholar]
- 66.Wensveen FM, et al. Apoptosis threshold set by Noxa and Mcl-1 after T cell activation regulates competitive selection of high-affinity clones. Immunity. 2010;32:754–765. doi: 10.1016/j.immuni.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 67.Finkel T. Signal transduction by reactive oxygen species. The Journal of cell biology. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murphy MP, et al. Unraveling the biological roles of reactive oxygen species. Cell Metab. 2011;13:361–366. doi: 10.1016/j.cmet.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Molecular cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baker MJ, et al. Quality control of mitochondrial proteostasis. Cold Spring Harbor perspectives in biology. 2011:3. doi: 10.1101/cshperspect.a007559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148:1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tatsuta T, et al. Mitochondrial lipid trafficking. Trends in cell biology. 2014;24:44–52. doi: 10.1016/j.tcb.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 73.Acin-Perez R, Enriquez JA. The function of the respiratory supercomplexes: the plasticity model. Biochimica et biophysica acta. 2014;1837:444–450. doi: 10.1016/j.bbabio.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 74.Lenaz G, Genova ML. Supramolecular organisation of the mitochondrial respiratory chain: a new challenge for the mechanism and control of oxidative phosphorylation. Advances in experimental medicine and biology. 2012;748:107–144. doi: 10.1007/978-1-4614-3573-0_5. [DOI] [PubMed] [Google Scholar]
- 75.Perciavalle RM, et al. Anti-apoptotic MCL-1 localizes to the mitochondrial matrix and couples mitochondrial fusion to respiration. Nature cell biology. 2012;14:575–583. doi: 10.1038/ncb2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang X, et al. Deletion of MCL-1 causes lethal cardiac failure and mitochondrial dysfunction. Genes & development. 2013;27:1351–1364. doi: 10.1101/gad.215855.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang CR, Yang-Yen HF. The fast-mobility isoform of mouse Mcl-1 is a mitochondrial matrix-localized protein with attenuated anti-apoptotic activity. FEBS letters. 2010;584:3323–3330. doi: 10.1016/j.febslet.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 78.Warr MR, et al. Mitochondrion-dependent N-terminal processing of outer membrane Mcl-1 protein removes an essential Mule/Lasu1 protein-binding site. The Journal of biological chemistry. 2011;286:25098–25107. doi: 10.1074/jbc.M111.218321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lapuente-Brun E, et al. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science. 2013;340:1567–1570. doi: 10.1126/science.1230381. [DOI] [PubMed] [Google Scholar]
- 80.Cogliati S, et al. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell. 2013;155:160–171. doi: 10.1016/j.cell.2013.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen ZX, Pervaiz S. Bcl-2 induces pro-oxidant state by engaging mitochondrial respiration in tumor cells. Cell death and differentiation. 2007;14:1617–1627. doi: 10.1038/sj.cdd.4402165. [DOI] [PubMed] [Google Scholar]
- 82.Chen ZX, Pervaiz S. Involvement of cytochrome c oxidase subunits Va and Vb in the regulation of cancer cell metabolism by Bcl-2. Cell death and differentiation. 2010;17:408–420. doi: 10.1038/cdd.2009.132. [DOI] [PubMed] [Google Scholar]
- 83.Alavian KN, et al. Bcl-xL regulates metabolic efficiency of neurons through interaction with the mitochondrial F1FO ATP synthase. Nature cell biology. 2011;13:1224–1233. doi: 10.1038/ncb2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen YB, et al. Bcl-xL regulates mitochondrial energetics by stabilizing the inner membrane potential. The Journal of cell biology. 2011;195:263–276. doi: 10.1083/jcb.201108059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yi CH, et al. Metabolic regulation of protein N-alpha-acetylation by Bcl-xL promotes cell survival. Cell. 2011;146:607–620. doi: 10.1016/j.cell.2011.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maryanovich M, et al. The ATM-BID pathway regulates quiescence and survival of haematopoietic stem cells. Nature cell biology. 2012;14:535–541. doi: 10.1038/ncb2468. [DOI] [PubMed] [Google Scholar]
- 87.Bernardi P. The mitochondrial permeability transition pore: a mystery solved? Frontiers in physiology. 2013;4:95. doi: 10.3389/fphys.2013.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Colombini M. VDAC structure, selectivity, and dynamics. Biochimica et biophysica acta. 2012;1818:1457–1465. doi: 10.1016/j.bbamem.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rostovtseva TK. VDAC structure, function, and regulation of mitochondrial and cellular metabolism. Biochimica et biophysica acta. 2012;1818:1437. doi: 10.1016/j.bbamem.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 90.Shoshan-Barmatz V, et al. VDAC, a multi-functional mitochondrial protein regulating cell life and death. Molecular aspects of medicine. 2010;31:227–285. doi: 10.1016/j.mam.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 91.McCommis KS, Baines CP. The role of VDAC in cell death: friend or foe? Biochimica et biophysica acta. 2012;1818:1444–1450. doi: 10.1016/j.bbamem.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rostovtseva TK, et al. On the role of VDAC in apoptosis: fact and fiction. Journal of bioenergetics and biomembranes. 2005;37:129–142. doi: 10.1007/s10863-005-6566-8. [DOI] [PubMed] [Google Scholar]
- 93.Hiller S, et al. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science. 2008;321:1206–1210. doi: 10.1126/science.1161302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Malia TJ, Wagner G. NMR structural investigation of the mitochondrial outer membrane protein VDAC and its interaction with antiapoptotic Bcl-xL. Biochemistry. 2007;46:514–525. doi: 10.1021/bi061577h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vander Heiden MG, et al. Bcl-xL promotes the open configuration of the voltage-dependent anion channel and metabolite passage through the outer mitochondrial membrane. The Journal of biological chemistry. 2001;276:19414–19419. doi: 10.1074/jbc.M101590200. [DOI] [PubMed] [Google Scholar]
- 96.Lee AC, et al. Beta-NADH decreases the permeability of the mitochondrial outer membrane to ADP by a factor of 6. The Journal of biological chemistry. 1994;269:30974–30980. [PubMed] [Google Scholar]
- 97.Bonnefont JP, et al. Carnitine palmitoyltransferases 1 and 2: biochemical, molecular and medical aspects. Molecular aspects of medicine. 2004;25:495–520. doi: 10.1016/j.mam.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 98.Giordano A, et al. tBid induces alterations of mitochondrial fatty acid oxidation flux by malonyl-CoA-independent inhibition of carnitine palmitoyltransferase-1. Cell death and differentiation. 2005;12:603–613. doi: 10.1038/sj.cdd.4401636. [DOI] [PubMed] [Google Scholar]
- 99.Schug ZT, Gottlieb E. Cardiolipin acts as a mitochondrial signalling platform to launch apoptosis. Biochimica et biophysica acta. 2009;1788:2022–2031. doi: 10.1016/j.bbamem.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 100.Raemy E, Martinou JC. Involvement of cardiolipin in tBID-induced activation of BAX during apoptosis. Chemistry and physics of lipids. 2014;179:70–74. doi: 10.1016/j.chemphyslip.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 101.Paumen MB, et al. Direct interaction of the mitochondrial membrane protein carnitine palmitoyltransferase I with Bcl-2. Biochemical and biophysical research communications. 1997;231:523–525. doi: 10.1006/bbrc.1997.6089. [DOI] [PubMed] [Google Scholar]
- 102.Paumen MB, et al. Inhibition of carnitine palmitoyltransferase I augments sphingolipid synthesis and palmitate-induced apoptosis. The Journal of biological chemistry. 1997;272:3324–3329. doi: 10.1074/jbc.272.6.3324. [DOI] [PubMed] [Google Scholar]