Abstract
Objective
This study compared the unique and combined effects of evidence-based treatments for ADHD —stimulant medication and behavior modification—on children’s rates of reinforcement for deviant peer behavior (RDPB).
Method
Using a within-subjects design, 222 elementary school-age children attending a summer treatment program, including 151 children with ADHD (127 male), with and without comorbid conduct problems, and 71 control children (57 male), received varying combinations of behavior modification (no, low-intensity, and high-intensity) and methylphenidate (placebo, 0.15 mg/kg, 0.30 mg/kg, and 0.60 mg/kg). RDPB was measured through direct observation and compared across all behavior modification and medication conditions.
Results
Children with ADHD reinforced the deviant behavior of their peers at a significantly higher rate than control children in the absence of either intervention. However, that difference largely disappeared in the presence of both behavior modification and medication. Both low and high-intensity behavior modification, as well as medium (0.30 mg/kg) and high (0.60 mg/kg) doses of methylphenidate, significantly reduced the rate of ADHD children’s RDPB to levels similar to the control group.
Conclusions
Results indicate that although untreated children with ADHD do engage in RDPB at a greater rate than their non-ADHD peers, existing evidence-based interventions can substantially decrease the presence of RDPB, thereby limiting potential iatrogenic effects in group-based treatment settings.
Public Health Significance Statement
This study found that children with behavior disorders reinforced their peers' deviant behaviors much more than typically developing children. However, behavior modification and medication treatments both reduced reinforcement rates, indicating that peer contagion can easily be managed in group-based treatments for children
Keywords: Treatment, peer interactions, deviancy training, ADHD
Effects of behavior modification and stimulant medication on peer reinforcement of deviancy in children with attention deficit hyperactivity disorder
Decades of developmental and clinical research have demonstrated that deviant peer interactions play a prominent role in the development of antisocial behavior (e.g., Dodge, Dishion, & Lansford, 2006). Children and adolescents who associate with antisocial peers are at an increased risk for a variety of negative long-term outcomes, including increased substance use (Dishion & Skaggs, 2000; Thornberry & Krohn, 1997), violent offenses (Elliot & Menard, 1996), and early high-risk sexual behavior (Dishion, 2000). Affiliating with antisocial peers is a significantly stronger predictor of later deviant behavior than many family, school, and community variables (Elliot & Menard, 1996).
What might account for the negative long-term impact of affiliating with antisocial peers? Dishion and colleagues theorize that interacting with antisocial peers exposes individuals to an iatrogenic peer contagion process known as deviancy training, whereby peers shape an individual’s behavior by reinforcing (e.g., encouraging through laughing, prompting, mimicry) both deviant talk (e.g., recounting past deviant acts, proposing future acts) and behavior (see Dishion, McCord, & Poulin, 1999; Dishion & Tipsord, 2011). Several observational studies of peer interactions have supported this theory by finding significant associations between peer reinforcement of deviant behaviors and later expressions of antisocial behavior. For example, Dishion and colleagues observed early adolescent males discussing activities and solving problems with their best friends, finding that peer reinforcement of deviant talk predicted subsequent increases in substance use and disruptive behavior in later adolescence (Dishion & Andrews, 1995; Dishion, Capaldi, Spracklen, & Li, 1995). Likewise, other research has revealed that preschoolers who received peer reinforcement for their deviant talk and aggressive behavior later exhibited increased aggressive behavior (Patterson, Littman, & Bricker, 1967) and covert forms of antisocial behavior (Snyder et al., 2005). To date, only one study has reported on the relationship between peer reinforcement and deviant behavior in elementary school age children. Snyder and colleagues (2010) found that peer reinforcement and modeling of deviant talk and play (the authors’ proxy for deviancy training) was associated with multi-setting antisocial behavior in elementary school age children. While all of these studies demonstrate a clear association between peer reinforcement and increased deviant behavior, the limited work on elementary school-age children is surprising. Further examination of peer reinforcement for deviant behavior is vital in this population, as elementary school is commonly a time when children are referred for mental health services, most often for behavior problems (Thompson & Bhrolchain, 2011).
Deviancy Training in Treatment Settings
Deviancy training of antisocial behavior by peers also has been observed in several treatment studies, most notably in studies that aggregated adolescents for treatment and subsequently reported negative (iatrogenic) effects. Post-hoc examinations of the 1940’s Cambridge-Sommerville youth program indicated that children who attended summer camps with other deviant youth were more likely to experience negative long-term treatment effects than their matched pairs who did not attend the camps (McCord, 2003). Feldman's St. Louis study (1992) reported increased deviant behavior in at-risk teenagers who were randomly assigned to treatment groups entirely comprised of antisocial peers, as compared to those randomly assigned to groups minimally comprised of anti social peers. The Oregon Youth Study randomly assigned antisocial adolescents to receive a cognitive–behavioral intervention delivered in a group format or individually; teens who participated in groups finished the study with higher rates of smoking and other delinquent behaviors relative to those who received treatment individually (Dishion & Andrews, 1995). Even more troubling, these iatrogenic group treatment effects persisted at the 2-year and 3-year follow-up assessments (Dishion et al., 1999; Poulin, Dishion, & Burraston, 2001).
Collectively, these studies seem to suggest that group-based treatments for antisocial behavior may produce unintended negative effects, in part, because participants engage in deviancy training by socially reinforcing one another’s deviant (negative or antisocial) verbal and physical behavior during treatment. If consistently observed across studies, such evidence would strongly argue against the aggregation of antisocial youths for treatment. However, not all evidence has supported this conclusion. Most noteworthy in this regard is that two independently conducted meta-analyses examined the effects of group-based treatments for antisocial behavior in adolescents, and found no evidence of an overall negative effect of group treatment (Lipsey, 2006; Weiss et al., 2005). Both reviews concluded that deviancy training is generally not a problem in group-based treatments for antisocial teens. This discrepancy between the results of individual studies that found evidence of deviancy training in group treatment settings and results of meta-analyses that found little evidence of widespread effects across group treatment studies highlights a clear need for further research. The present study sought to address this need, by observing children with and without attention-deficit/hyperactivity disorder (ADHD) in a group treatment setting, and by examining effects of empirically supported treatments on the deviancy training-related behaviors they exhibited.
Deviancy Training in Children with ADHD
Given that at least some research suggests there is an elevated risk for peer contagion in treatments that aggregate deviant individuals, it is surprising that the effects of deviancy training remain largely unexamined in clinically diagnosed populations; children with ADHD, in particular, may be especially susceptible to the influence of deviant peers. First, children and adolescents with impulsivity and self-control deficits–both characteristic of ADHD –are theorized to be more vulnerable to deviancy training (Hirschi, 2004). Consistent with this assertion, several studies of children and adolescents have found that impulsivity and self-control deficits are significantly associated with increased vulnerability to deviant peer influence (Goodnight, Bates, Newman, Dodge, & Pettit, 2006; Snyder et al., 2010; Tripp & Alsop, 1999; Wills & Dishion, 2004). Furthermore, anywhere from 50% to 80% of children with ADHD are socially rejected by their peers (Hoza et al., 2005; Pelham & Bender, 1982), and there is evidence of a link between peer rejection and increased susceptibility to deviant peer influence (Dishion, Nelson, Winter, & Bullock, 2004). In one study of school-age children, researchers found that children who were socially rejected by their classmates were more easily influenced by deviancy training than were socially accepted children (Snyder et al., 2010). Taken together, these findings suggest that children with ADHD may be at high risk of being negatively influenced by peer deviancy training in group treatment settings.
Deviancy training may be even more problematic among children with ADHD who also present with co-occurring conduct problems. It is well established that ADHD and conduct problems (CP), namely conduct disorder (CD) and oppositional defiant disorder (ODD), are distinguishable conditions (Hinshaw, 1987), yet at least 50% of children with ADHD also meet criteria for CP, and the same or higher percent of children with CP meet criteria for ADHD (Lahey, Miller, Gordon, & Riley, 1999). Importantly, there is strong and consistent evidence that children with both ADHD and CP (ADHD-CP) differ from children with ADHD without CP (ADHD-only), often qualitatively so (see Waschbusch, 2002 for a review). Despite these differences, both children with ADHD-CP and children with ADHD-only are at risk for exhibiting elevated rates of antisocial behaviors. For example, a recent follow-up study of delinquency outcomes associated with ADHD reported that individuals diagnosed with ADHD and CD in childhood had the worst outcomes on multiple aspects of delinquency as young adults (Sibley et al., 2011). However, the delinquency outcomes of those diagnosed with ADHD and ODD and of those diagnosed with ADHD-only were also significant, with both groups displaying an earlier onset, greater variety, and greater severity of delinquency relative to controls. Although these studies suggest that children with ADHD with and without comorbid CP may be at risk for heightened rates of deviancy training, researchers have yet to directly examine this possibility.
Treatment Effects on Deviancy Training
As described earlier, several studies have shown that when deviancy training occurs in the context of group based treatments, those treatments may produce negative outcomes. Far less researched is whether certain treatments may enhance or diminish the impact of deviancy training on individuals. Hundreds of studies have demonstrated that stimulant medication and behavioral therapy, both individually and when used in combination, are effective, evidence-based interventions for the treatment of ADHD in children (Evans, Owens, & Bunford, 2013; Wolraich et al., 2011), but researchers have yet to consider possible effects of these treatments on the deviancy training processes in children with ADHD.
Why might treatments for ADHD be expected to have a significant and positive impact on deviancy training? As described earlier, impulsivity and self-control have been shown to be associated with susceptibility to deviancy training. Numerous studies have demonstrated that stimulant medication is effective for improving self-control deficits and reducing impulsivity in children with ADHD (Bedard et al., 2003; Firestone, Kelly, Goodman, & Davey, 1981; Tannock, Schachar, & Logan, 1995), suggesting that pharmacological treatments may reduce susceptibility to deviancy training in children with ADHD. Other research has shown that situational factors commonly manipulated in behavioral treatment, like increasing the level of monitoring by adults or the level of structure in the treatment setting, may moderate the power of deviancy training (Dishion & Tipsord, 2011). More directly relevant, several studies have demonstrated that behavior therapy reduces deviancy training among high-risk boys. For example, Feldman (1992) reported that a structured, behavioral intervention was the most effective intervention for high-risk boys; it produced equally positive outcomes, regardless of whether the boys received the intervention in groups comprised entirely of deviant peers or in groups comprised predominantly of non-deviant peers. In contrast, deviancy training was evident among high-risk boys who did not receive the behavioral intervention when they were paired with other boys also at risk for antisocial behavior. Collectively, these results suggest that both behavior therapy and stimulant medication are likely to be effective at reducing deviancy training among children with ADHD being treated in group settings.
Current Study
The present study aimed to address the gap in research on the effects of ADHD and treatment on the peer deviancy training process. First, we compared children with ADHD-only, ADHD-CP, and controls on a mechanism believed to power the deviancy training process: reinforcement of deviant peer behavior. We hypothesized that reinforcement of deviant peer behavior would be significantly more prevalent among children with ADHD-CP and ADHD-only than controls, and more prevalent among children with ADHD-CP than ADHD-only. Second, we explored the single and combined effects of stimulant medication and behavior therapy on the rate at which children reinforced deviant peer behavior, hypothesizing that both treatments would significantly reduce deviant peer reinforcement equivalently across ADHD-only and ADHD-CP groups.
Methods
Participants
Participants were 151 children with ADHD and 71 children without ADHD (controls). The children with ADHD were divided into those with comorbid CP (ADHD-CP; n = 125, including 107 males) and those without comorbid CP (ADHD-only; n = 26, including 20 males), where CP was defined as receiving a diagnosis of either ODD or CD. The 71 control children (including 57 males) were recruited on a 2:1 ratio to match children in the ADHD sample by age, gender, race, and socioeconomic status. Participants ranged in age from 5.0 years to 12.8 years (M=9.1, SD=2.0). Participants in the ADHD-only, ADHD-CP, and control groups did not significantly differ on age, race, gender, or maternal education (used as an indicator of socioeconomic status). Table 1 summarizes demographic and rating scale data, by group.
Table 1.
Means, Standard Deviations, and Percentages for Participant Characteristics
| Control n = 71 |
ADHD-only n = 26 |
ADHD-CP n = 125 |
||
|---|---|---|---|---|
| Demographics | M (SD) | M (SD) | M (SD) | p |
| Age in years | 8.8(1.9) | 9.6 (1.7) | 9.1 (2.1) | .201 |
| Full Scale IQa | 111 (16) | 103 (13) | 105 (15) | .032* |
| Gender (% Male)b | 76.9% | 76.9% | 85.6% | .440 |
| Raceb | .692 | |||
| Non-Hispanic White | 78.9% | 73.1% | 80.8% | — |
| African American | 15.5% | 19.2% | 11.2% | — |
| Other | 5.6% | 7.7% | 8.0% | — |
| Maternal Educationb | .564 | |||
| High school or less | 22.4% | 32.0% | 22.3% | — |
| Some college or more | 77.6% | 68.0% | 77.7% | — |
| DSM-IV Symptom Countc | ||||
| ADHD | 1.2 (2.1) | 15.8 (2.5) | 16.9 (1.6) | <.001*** |
| ODD | 0.1 (0.4) | 1.7 (1.0) | 6.5 (1.5) | <.001*** |
| CD | 0.0 (0.2) | 0.5 (0.7) | 2.1 (1.7) | <.001*** |
| DBD Rating Scale d | ||||
| Parent | ||||
| ADHD - Inattention | 0.3 (0.3) | 2.4 (0.6) | 2.2 (0.6) | <.001*** |
| ADHD - Hyp/Imp | 0.2 (0.3) | 1.9 (0.7) | 2.0 (0.6) | <.001*** |
| ODD | 0.2 (0.2) | 0.6 (0.3) | 1.5 (0.6) | <.001*** |
| CD | 0.0 (0.1) | 0.1 (0.1) | 0.3 (0.3) | <.001*** |
| Teacher | ||||
| ADHD - Inattention | 0.3 (0.4) | 2.1 (0.6) | 2.0 (0.8) | <.001*** |
| ADHD - Hyp/Imp | 0.3 (0.4) | 1.3 (0.8) | 1.8 (0.7) | <.001*** |
| ODD | 0.1 (0.2) | 0.6 (0.4) | 1.2 (0.8) | <.001*** |
| CD | 0.0 (0.0) | 0.1 (0.3) | 0.5 (0.6) | <.001*** |
Note.
IQ scores were estimated from vocabulary and block design subtests of the WISC-III-R (Wechsler, 1991);
Gender, race and maternal education are presented as percentages.
Total number of DSM-IV symptom criteria met, based on parent and teacher ratings.
Disruptive Behavior Disorders Rating Scale (Pelham et al., 1992).
p < .05,
p < .01,
p < .001.
All participants were required to have an estimated full-scale IQ of at least 80 on the Wechsler Intelligence Scale for Children-Third Edition (WISC-III-R; Wechsler, 1991; estimations were based on vocabulary and block design subtests). Participants with ADHD were required to meet Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; American Psychiatric Association, 1994) diagnostic criteria for attention deficit hyperactivity disorder, and to have no history of adverse response or non-response to methylphenidate (MPH). Following recommended guidelines for evidence-based assessment of ADHD (Pelham, Fabiano, & Massetti, 2005), clinicians made each diagnosis by considering both symptom and impairment criteria as evaluated using multiple sources of information. Specifically, diagnoses of ADHD, ODD and CD were evaluated using the NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV; Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000) administered to parents, as well as parent and teacher ratings on the Disruptive Behavior Disorders Rating Scale (DBD; Pelham, Gnagy, Greenslade, & Milich, 1992). Symptoms were considered present if they were endorsed (i.e., “pretty much” or “very much” on the DBD, or scored as present by the NIMH DISC-IV scoring algorithm) on any of these measures. Likewise, impairment was evaluated using parent and teacher ratings on the Impairment Rating Scale (IRS; Fabiano et al., 2006), with impairment considered present if it was endorsed (i.e., a rating of 3 or higher) on either measure. All clinical diagnoses were made by doctoral-level clinicians who specialized in ADHD and related behaviors.
Of the children with ADHD, 125 received comorbid diagnoses of either oppositional defiant disorder (ODD; n=83) or conduct disorder (CD; n=42). None of the control group children met DSM-IV diagnostic criteria for ADHD, ODD, or CD. An additional two children were enrolled in the study but dropped out; one child with ADHD-CP dropped out due to adverse medication effects and one child in the control group dropped out and gave no reason.
Procedure
Participants were recruited using a variety of methods, including school and mental health professional referrals, radio advertisements, and mailings. The University at Buffalo Health Sciences Institutional Review Board approved the protocol, and informed consent was obtained from parents and assent from children.
This investigation took place within a study designed to examine the single and combined effects of different doses of behavior modification (none, NBM; low-intensity, LBM; and high-intensity, HBM) and pharmacological (placebo, .15 mg/kg/dose, .30 mg/kg/dose, and .60 mg/kg/dose of MPH three times daily [t.i.d]) interventions on children with ADHD who were attending a 9-week Summer Treatment Program (STP) between 2002 and 2004 (see Fabiano et al., 2007; Pelham et al., 2014). Typically developing children participated in the control group (but did not receive stimulant medication treatment) solely for research purposes. Children were grouped by age, with approximately 4 control children and 8 children with ADHD per group. Each group had, on average 1.5 (SD 1.34) children with ADHD-only and 6.9 (SD 1.59) children with ADHD-CP; groups did not differ significantly on the number of children with ADHD-only versus ADHD-CP χ2 (1, 34) = 26.54, p = .82. Each group was supervised in recreational settings by five counselors, who were undergraduate and graduate students in relevant fields, and in classroom settings by a teacher and an aide.
Behavior modification dose varied in 3-week (Monday – Friday) sessions, with the order of conditions counterbalanced across groups. The HBM condition implemented all standard STP components, including a behavior-contingent reward and response cost point system, praise and social reinforcement, daily and weekly rein forcers, time-out procedures, individualized programs, daily social-skills training sessions, and daily report cards (Pelham, Greiner, & Gnagy, 1997). The LBM condition differed from the HBM condition in that children continued to receive feedback for their behavior but did not earn or lose points (e.g., “You lose 20 points for teasing a peer,” in the HBM condition versus “That’s teasing,” in the LBM condition). Parents provided weekly rather than daily rewards for daily report cards and social skills training sessions were conducted weekly rather than daily. Additionally, the LBM condition employed fixed-length sit-outs rather than the behaviorally contingent, escalating/deescalating time-outs used in the HBM condition. The NBM condition was designed to mimic a typical summer camp setting; the daily schedule and content of the activities remained the same, but none of the behavioral contingencies or other treatment components used in the HBM and LBM conditions were implemented. In all conditions, children who exhibited dangerous or severely disruptive behavior were suspended from camp activities for anywhere from an hour to the rest of the day, depending on their in-suspension behavior. All participants received behavior modification.
Medication assessment was a double-blind, within-subject evaluation of placebo and .15 mg/kg/dose, .30 mg/kg/dose, and .60 mg/kg/dose doses of immediate-release MPH. Children with ADHD (control children received neither medication nor placebo) received medication on a t.i.d. schedule, at 7:45 a.m., 11:45 a.m., and 3:45 p.m. Medication was administered on site by study staff to ensure that children received medication at the correct time and that children swallowed pills as intended. Drug conditions were randomized so that children received each medication dose at least once per week within each 3-week behavioral condition. Previous studies indicate less variability between days when children receive higher-dose MPH (e.g., Carlson, Pelham, Milich, & Dixon, 1992; Pelham et al., 1993), therefore participants received 3 days of the .60 mg/kg/dose and 4 days of all other doses. Children, their parents, and all clinical staff members were blind to medication condition; staff was instructed not to discuss ADHD diagnostic status with participants or parents, and all group members (including controls) entered the medication station and drank a cup of water to maintain staff blindness to medication. The on-site physician could unblind medication conditions in the event of adverse effects.
Staff members received intensive training in all procedures, including memorizing operational definitions of all point system behaviors verbatim, as well as daily supervision from senior staff members. Weekly observations were conducted to evaluate adherence to the treatment protocol (treatment integrity) and consistency of implementing the point system (treatment reliability; see Pelham et al., 2014). Treatment integrity data were summarized from 457 observations of recreational activities during which the dependent measurement was recorded. Counselors followed prescribed procedures in all conditions (e.g., reviewed 95% of scheduled topics in group discussions in all three conditions). For procedures that differed by condition, fidelity observations demonstrated differences in counselor behavior. For example, social reinforcement was designed to be used liberally in HBM and LBM and used less frequently in NBM. Observer ratings for effective use of social reinforcement averaged 2.6 and 2.4 on a 7-point scale ranging from 1 (superior) to 7 (inferior) in HBM and LBM, respectively, versus 4.1 on the same scale in NBM. Overall behavior management was rated at 2.8, 2.6, and 4 for the three conditions, reflecting worse ratings for the NBM condition when counselors were instructed to withhold interventions. Likewise, reliability of the point system behaviors was high, with inter-observer correlations averaging .87 across measures.
Dependent Measure
Reinforcement for deviant peer behavior (RDPB) is the frequency at which a child reinforced their peers’ negative behavior. RDPB was operationally defined as anytime a child reacted in a positive manner to another child’s rule breaking or otherwise negative behavior. Eligible reactions included: laughing, smiling, copying a behavior within 60 seconds, speaking highly of someone, cheering, clapping, or any other behavior, verbal or nonverbal, that would typically be defined as encouraging or reinforcing, and was seen or heard by the child exhibiting deviant behavior. Counselors observed and recorded any instances of RDPB, like all other coded behaviors, as they delivered each behavior modification condition. However, children never received feedback or direct consequences for RDPB in any of the behavioral conditions, which may have reduced their awareness of RDPB relative to other coded behaviors. It was measured in real time during all recreational activities (e.g., when children were participating in organized soccer, softball, or basketball games or practice). Reliability observations were collected across groups and days, coding approximately 20% of all activities. Correlations between counselors and reliability observers ranged from 0.57 (stealing) to 0.98 (intentional aggression), with an average of 0.86 across negative behaviors; mean differences ranged from 0.05 to 2.65 behaviors observed.
Analytic Plan
Overview
Data were examined using two sets of analyses. The first set of analyses examined RDPB differences between controls, children with ADHD-only, and children with ADHD-CP on days during which children with ADHD did not receive any medication. The second set of exploratory analyses examined the main effects of medication and behavior modification, as well as the interaction between the two, on RDPB exhibited by children in the ADHD-only and ADHD-CP groups. Because the primary outcome variable, RDPB, was measured as a daily count variable, and because children were nested within groups for treatment, two-level negative binomial regressions were used for both sets of analyses. Negative binomial regression is a preferred method of analysis for count data, as data transformations are no longer recommended (Coxe, West, & Aiken, 2009; Gardner, Mulvey, & Shaw, 1995). Negative binomial regression was chosen over Poisson regression because the sample variance of RDPB exceeded the sample mean. Observations were not independent due to multiple observations from each participant. Data were modeled as repeated measures using a compound symmetry variance structure; this multilevel modeling approach assumes a constant variance for repeated measures within a participant as well as a constant variance between participants. Models were tested using the more conservative Satterthwaite adjustment to approximate error terms and degrees of freedom (Satterthwaite, 1946) and to account for the unbalanced sample sizes of the groups. Significant effects were examined using Holm’s Modified Bonferroni procedures to control for family-wise Type I error (Holm, 1979).
Analyses of missing data patterns were assessed and results were consistent with the assumption of data missing completely at random1. Missing data were minimal for most variables (i.e., less than 5.7% of the cases). Missing values were imputed using an expectation maximization algorithm, which has been shown to reduce bias due to missing data (Catellier et al., 2005; Peugh & Enders, 2005).
ADHD-only vs. ADHD-CP vs. control analyses
To examine RDPB differences between the groups, a two-level negative binomial regression was performed using the generalized linear mixed models function in SPSS 19.0 (IBM Corp., 2010). For these analyses, only placebo days from all behavior modification conditions were used for children with ADHD-only and ADHD-CP, as control children did not receive medication. Level 1 included the fixed effects of diagnosis (ADHD-only v. ADHD-CP v. control), behavior modification intensity (NBM, LBM, HBM), and the interaction of the two. Participant IQ was also included as a covariate at Level 1 (only in this set of analyses) to account for significant between group differences in intelligence. Level 2 accounted for the nesting of children in treatment groups (including the level 2 intercept and error), using a random effects model with a variance components covariance matrix. Pair wise follow-up contrasts were used to detect differences between control, ADHD-only, and ADHD-CP children across behavior modification conditions.
Combined treatment analyses
The second set of analyses examined RDPB counts in the subsample of children with ADHD-only and ADHD-CP as a function of medication and behavior modification using a parallel analytic approach. Control children were not included in these analyses because they did not receive placebo or active medication. In addition to factors employed in the previous model, namely diagnosis and behavior modification intensity, medication dosage (placebo, 0.15 mg/kg, 0.3 mg/kg, and 0.6 mg/kg) was added as a Level 1 factor, along with the two- and three-way interactions of these factors. Pair wise follow-up contrasts were used to detect differences in treatment response for ADHD-only and ADHD-CP participants among increasing doses of medication and behavior modification.
Results
ADHD-only vs. ADHD-CP vs. control analyses
The first set of analyses revealed significant main effects of Diagnosis F (2, 4, 662) = 13.42, p < .001 and Behavior Modification F (2, 4, 662) = 52.64, p < .001 on RDPB. The covariate (participant IQ) was not significant F (1, 4, 662) = 1.57, p = .211. These main effects were qualified by a significant Diagnosis × Behavior Modification interaction, F (4, 4, 662) = 4.62, p = .001. Examination of means (see Table 2) and follow-up tests showed that, in the NBM condition, the ADHD-CP group had significantly higher RDPB than the control group (p < .001) but not the ADHD-only group, which did not differ from one another. Groups did not differ in the LBM condition. In the HBM condition, the ADHD-CP group again displayed significantly greater RDPB than the control group (p < .01), whereas ADHD-only children did not differ significantly from either group.
Table 2.
Daily RDPB means, standard deviations, and effect sizes.
| Control | ADHD- only |
ADHD-CP | Control vs. ADHD- only |
Control vs. ADHD- CP |
ADHD- only vs. ADHD- CP |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment Condition |
Mean | SD | Mean | SD | Mean | SD | Cohen’s d |
Cohen’s d |
Cohen’s d |
|
| NBM | Placebo | 1.10 | 3.12 | 1.55 | 3.63 | 3.77 | 9.46 | −0.13 | −0.38 | −0.31 |
| LowRx | — | — | 1.07 | 1.72 | 2.93 | 7.92 | — | — | −0.32 | |
| MedRx | — | — | 0.49 | 0.91 | 2.91 | 10.62 | — | — | −0.32 | |
| HighRx | — | — | 0.55 | 2.05 | 1.61 | 4.08 | — | — | −0.33 | |
| LBM | Placebo | 0.54 | 2.60 | 0.41 | 0.89 | 1.00 | 2.52 | 0.07 | −0.18 | −0.31 |
| LowRx | — | — | 0.45 | 0.91 | 0.79 | 2.41 | — | — | −0.19 | |
| MedRx | — | — | 0.42 | 0.96 | 0.61 | 2.69 | — | — | −0.09 | |
| HighRx | — | — | 0.12 | 0.43 | 0.36 | 1.33 | — | — | −0.24 | |
| HBM | Placebo | 0.18 | 1.25 | 0.68 | 1.55 | 0.53 | 1.52 | −0.36 | −0.25 | 0.10 |
| LowRx | — | — | 0.11 | 0.38 | 0.50 | 1.87 | — | — | −0.29 | |
| MedRx | — | — | 0.21 | 0.82 | 0.27 | 1.09 | — | — | −0.06 | |
| HighRx | — | — | 0.17 | 0.53 | 0.20 | 0.84 | — | — | −0.04 | |
Note. Effect sizes for Controls comparisons are Cohen’s d computed as: (Control M—ADHD M)/Pooled SD, within each behavioral condition. NBM = no behavior modification, LBM = low-intensity behavior modification, HBM = high-intensity behavior modification, LowRx = 0.15 mg/kg MPH, MedRx = 0.30 mg/kg MPH, HighRx = 0.60 mg/kg MPH.
Combined treatment analyses
The second set of analyses revealed significant main effects of Behavior Modification, F (2, 246) = 26.00, p < .001, and Medication F (3, 246) = 15.76, p < .001, but not Diagnosis F (1, 246) = 4.03, p = .061. There were also several significant two-way interactions, including a Behavior Modification × Medication interaction F (6, 246) =2.63, p = .019, and a Behavior Modification × Diagnosis interaction F (2, 246) = 3.31, p = .039, but not a Medication × Diagnosis interaction F (3, 246) = 1.21, p = .308. These effects were qualified by a significant Behavior Modification × Medication × Diagnosis interaction F (6, 246) = 6.73, p < .001. Follow-up comparisons were performed two ways. First, we examined effects of medication at each level of behavior modification, and then we examined the effects behavior modification at each level of medication. Follow-ups were computed separately for ADHD-only and ADHD-CP groups and family-wise Holm’s modified Bonferroni corrections were applied.
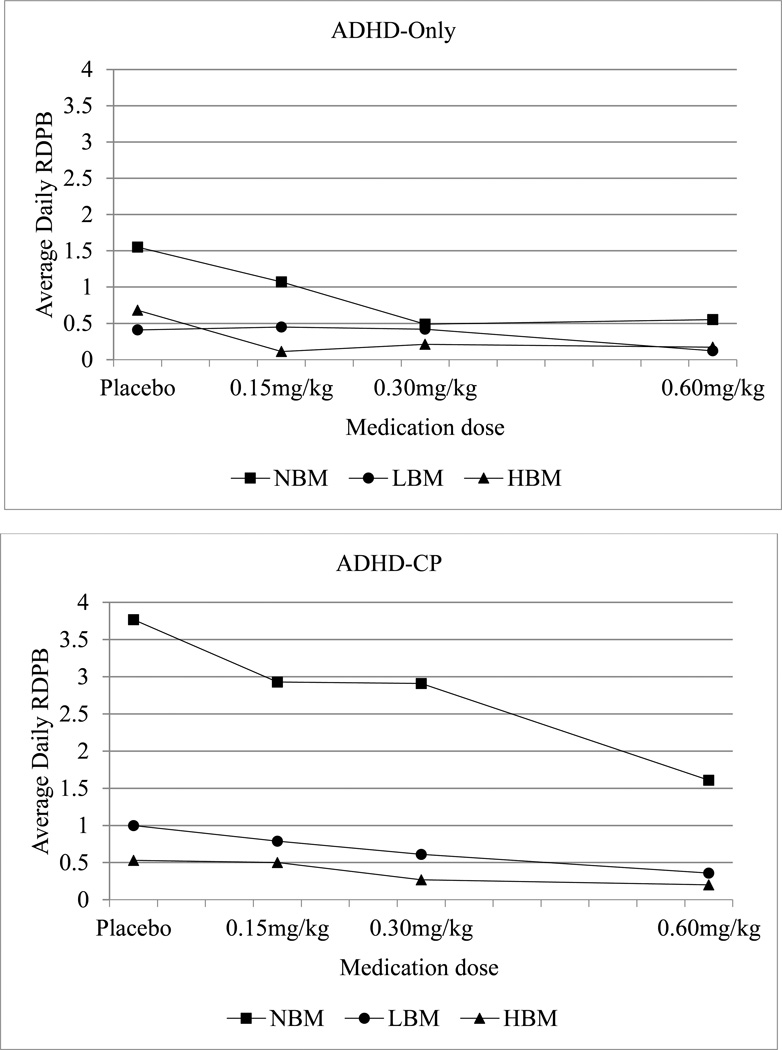
Means for children with ADHD-only are shown in Table 2 and Figure 1a, and follow-up contrasts are shown in Table 3. First, medication effects at each level of behavior modification were examined. In the NBM condition, rates of RDPB differed across all doses of medication in expected directions – higher doses of medication resulted in lower rates of RDPB, except in placebo versus low dose and medium versus high dose, which did not differ. In the LBM condition, high dose medication resulted insignificantly lower rates of RDPB as compared to medium dose medication and placebo; no other doses differed from each other. In the HBM condition, none of the medication doses differed from each other. Next, behavior modification effects at each level of medication were examined. In the placebo condition, LBM and HBM resulted in significantly lower RDPB versus the NBM condition, but did not differ from each other. All levels of behavior modification differed in the low dose medication condition. None of the behavior modification levels differed in the medium dose or high dose medication conditions after the Holm’s modified Bonferroni corrections were applied.
Figure 1.
Daily averages of children’s reinforcement of deviant peer behavior among treatment conditions and intensities for (a) children with ADHD-only and (b) children with ADHD-CP. RDPB = reinforcement of deviant peer behavior; NBM = no behavior modification; LBM = low-intensity behavior modification; HBM = high-intensity behavior modification; LowRx = 0.15 mg/kg MPH; MedRx = 0.30 mg/kg MPH; HighRx = 0.60 mg/kg MPH.
Table 3.
Pair wise Comparisons for the ADHD-only group to follow up the significant Diagnosis x Medication x Behavior Modification interaction
| Contrast Estimate |
Std. Error |
t(6, 246) | p | 95% CI | ||
|---|---|---|---|---|---|---|
| Effects of Medication at each Level of Behavior Modification | ||||||
| NBM | Placebo vs. LowRx | 0.14 | 0.18 | 0.793 | .429 | −0.21 — 0.50 |
| Placebo vs. MedRx | 0.74 | 0.30 | 2.50 | .014† | 0.15 — 1.33 | |
| Placebo vs. HighRx | 0.75 | 0.29 | 2.62 | .010† | 0.18 — 1.32 | |
| LowRx vs. MedRx | 0.60 | 0.18 | 3.43 | 004† | 0.22 — 0.98 | |
| LowRx vs. HighRx | 0.61 | 0.19 | 3.14 | .009† | 0.19 — 1.03 | |
| MedRx vs. HighRx | 0.01 | 0.15 | 0.05 | .963 | −0.29 — 0.30 | |
| LBM | Placebo vs. LowRx | −0.08 | 0.16 | −0.53 | .598 | −0.39 — 0.22 |
| Placebo vs. MedRx | −0.06 | 0.06 | −0.94 | .357 | −0.18 — 0.07 | |
| Placebo vs. HighRx | 0.23 | 0.07 | 3.40 | .002† | 0.09 — 0.37 | |
| LowRx vs. MedRx | 0.02 | 0.12 | 0.20 | .846 | −0.22 — 0.27 | |
| LowRx vs. HighRx | 0.31 | 0.16 | 2.01 | .047 | 0.00 — 0.62 | |
| MedRx vs. HighRx | 0.29 | 0.09 | 3.19 | .004† | 0.10 — 0.47 | |
| HBM | Placebo vs. LowRx | 0.498 | 0.30 | 1.66 | .100 | −0.10 — 1.09 |
| Placebo vs. MedRx | 0.37 | 0.31 | 1.19 | .235 | −0.24 — 0.98 | |
| Placebo vs. HighRx | 0.46 | 0.29 | 1.55 | .123 | −0.13 — 1.03 | |
| LowRx vs. MedRx | −0.13 | 0.09 | −1.42 | .165 | −0.31 — 0.06 | |
| LowRx vs. HighRx | −0.04 | 0.04 | −1.07 | .284 | −0.12 — 0.04 | |
| MedRx vs. HighRx | 0.09 | 0.09 | 1.01 | .321 | −0.09 — 0.26 | |
| Effects of Behavior Modification at each Level of Medication | ||||||
| Placebo | NBM vs. LBM | 0.86 | 0.35 | 2.44 | .017† | 0.16 — 1.56 |
| NBM vs. HBM | 0.61 | 0.25 | 2.42 | .017† | 0.11— 1.11 | |
| LBM vs. HBM | −0.25 | 0.27 | −0.92 | .361 | −0.79 — 0.29 | |
| LowRx | NBM vs. LBM | 0.64 | 0.24 | 2.63 | .013† | 0.14 — 1.13 |
| NBM vs. HBM | 0.97 | 0.29 | 3.37 | .005† | 0.35 — 1.58 | |
| LBM vs. HBM | 0.33 | 0.16 | 2.02 | .046† | 0.01 — 0.65 | |
| MedRx | NBM vs. LBM | 0.06 | 0.08 | 0.72 | .472 | −0.10 — 0.22 |
| NBM vs. HBM | 0.24 | 0.12 | 1.99 | .048 | 0.00 — 0.47 | |
| LBM vs. HBM | 0.18 | 0.08 | 2.30 | .023 | 0.02 — 0.33 | |
| HighRx | NBM vs. LBM | 0.34 | 0.18 | 1.87 | .063 | −0.02 — 0.70 |
| NBM vs. HBM | 0.31 | 0.16 | 2.00 | .048 | 0.00 — 0.62 | |
| LBM vs. HBM | −0.03 | 0.05 | −0.53 | .598 | −0.12 — 0.07 | |
Note. NBM = no behavior modification; LBM = low-intensity behavior modification; HBM = high-intensity behavior modification; LowRx = 0.15 mg/kg MPH; MedRx = 0.30 mg/kg MPH; HighRx = 0.60 mg/kg MPH; CI = confidence interval.
Significant with family-wise Holm’s modified Bonferroni correction employed.
Means for children with ADHD-CP are shown in Table 2 and Figure 1b, and follow-up contrasts are shown in Table 4. First, medication effects at each level of behavior modification were examined. In the NBM condition, the high dose of medication resulted in marginally significantly lower rates of RDPB as compared to the placebo and low dose medication, with medium dose medication not differing from any other dose. None of the medication levels differed in the LBM condition. In the HBM condition, both high dose and medium dose medication differed significantly from low dose medication. Next, behavior medication effects were examined at each level of medication. In the placebo condition, only HBM resulted in significantly lower RDPB than NBM. In the low, medium and high medication dose conditions, LBM and HBM resulted in significantly lower RDPB versus NBM, but did not differ from each other.
Table 4.
Pair wise Comparisons for the ADHD-CP group to follow up the significant Diagnosis x Medication x Behavior Modification interaction
| Treatment Conditions | Contrast Estimate |
Std. Error |
t(6, 246) | p | 95% CI | |
|---|---|---|---|---|---|---|
| Effects of Medication at each Level of Behavior Modification | ||||||
| NBM | Placebo vs. LowRx | 0.50 | 0.38 | 1.32 | .187 | −0.24 — 1.25 |
| Placebo vs. MedRx | 0.68 | 0.47 | 1.46 | .145 | −0.24 — 1.60 | |
| Placebo vs. HighRx | 1.32 | 0.51 | 2.57 | .012 | 0.30 — 2.35 | |
| LowRx vs. MedRx | 0.18 | 0.34 | 0.54 | .592 | −0.48 — 0.84 | |
| LowRx vs. HighRx | 0.82 | 0.32 | 2.61 | .011 | 0.20 — 1.45 | |
| MedRx vs. HighRx | 0.64 | 0.35 | 1.81 | .071 | −0.06 — 1.34 | |
| LBM | Placebo vs. LowRx | 0.14 | 0.11 | 1.38 | .168 | −0.06 — 0.35 |
| Placebo vs. MedRx | 0.31 | 0.14 | 2.15 | .033 | 0.02 — 0.59 | |
| Placebo vs. HighRx | 0.44 | 0.18 | 2.45 | .016 | 0.09 — 0.80 | |
| LowRx vs. MedRx | 0.16 | 0.12 | 1.41 | .161 | −0.06 — 0.39 | |
| LowRx vs. HighRx | 0.30 | 0.13 | 2.21 | .029 | 0.03 — 0.56 | |
| MedRx vs. HighRx | 0.14 | 0.12 | 1.13 | .261 | −0.10 — 0.37 | |
| HBM | Placebo vs. LowRx | 0.05 | 0.09 | 0.58 | .562 | −0.12 — 0.23 |
| Placebo vs. MedRx | 0.20 | 0.10 | 2.03 | .044 | 0.01 — 0.40 | |
| Placebo vs. HighRx | 0.24 | 0.12 | 2.09 | .038 | 0.01 — 0.47 | |
| LowRx vs. MedRx | 0.15 | 0.05 | 3.19 | .003† | 0.06 — 0.24 | |
| LowRx vs. HighRx | 0.19 | 0.07 | 2.68 | .009† | 0.05 — 0.34 | |
| MedRx vs. HighRx | 0.04 | 0.05 | 0.89 | .381 | −0.06 — 0.14 | |
| Effects of Behavior Modification at each Level of Medication | ||||||
| Placebo | NBM vs. LBM | 1.60 | 0.70 | 2.28 | .026 | 0.20 — 3.00 |
| NBM vs. HBM | 1.95 | 0.71 | 2.74 | .008† | 0.53 — 3.38 | |
| LBM vs. HBM | 0.35 | 0.21 | 1.66 | .100 | −0.07 — 0.77 | |
| LowRx | NBM vs. LBM | 1.24 | 0.50 | 2.50 | .015† | 0.25 — 2.24 |
| NBM vs. HBM | 1.50 | 0.51 | 2.96 | .005† | 0.48 — 2.52 | |
| LBM vs. HBM | 0.26 | 0.13 | 1.97 | .051 | −0.01 — 0.52 | |
| MedRx | NBM vs. LBM | 1.22 | 0.52 | 2.38 | .020† | 0.20 — 2.25 |
| NBM vs. HBM | 1.47 | 0.53 | 2.78 | .007† | 0.41 — 2.53 | |
| LBM vs. HBM | 0.25 | 0.13 | 1.92 | .057 | −0.07 — 0.50 | |
| HighRx | NBM vs. LBM | 0.72 | 0.30 | 2.37 | .021† | 0.11 — 1.33 |
| NBM vs. HBM | 0.87 | 0.27 | 3.20 | .003† | 0.32 — 1.43 | |
| LBM vs. HBM | 0.15 | 0.10 | 1.53 | .130 | −0.05 — 0.35 | |
Note. NBM = no behavior modification; LBM = low-intensity behavior modification; HBM = high-intensity behavior modification; LowRx = 0.15 mg/kg MPH; MedRx = 0.30 mg/kg MPH; HighRx = 0.60 mg/kg MPH; CI = confidence interval.
Significant with family-wise Holm’s modified Bonferroni correction employed.
Discussion
The present study was the first to evaluate the effects of clinically diagnosed ADHD, with or without comorbid CP, and evidence-based treatments for ADHD on the rate at which elementary school age children reinforce deviant peer behavior within the context of a group-based treatment program. We hypothesized that children with ADHD-only and ADHD-CP would exhibit higher rates of RDPB than control children, and that children with ADHD-CP would exhibit significantly higher rates than children with ADHD-only. This hypothesis was partially supported, with results indicating that children with ADHD-CP did reinforce their peers’ deviant behavior at a significantly higher rate than control children, but children with ADHD-only did not differ from controls. We also hypothesized that both behavior modification and medication would reduce the rate of RDPB in children with ADHD-only and ADHD-CP. The results supported this second hypothesis, indicating that both behavior modification and medication produced significant reductions in the rate at which children with ADHD-only and ADHD-CP reinforced deviant peer behavior.
With no treatment in place (neither stimulant medication nor behavior therapy), children with ADHD-CP differed significantly from controls (see Tables 2 and 4), averaging nearly 4 instances of peer reinforcement per day compared to the control children’saverage of just 1.1 instances of peer reinforcement per day. These results join a substantial body of research demonstrating that the combination of ADHD-CP is especially potent in increasing the risk for antisocial behavior. This pattern has been observed in both longitudinal studies (Moffitt, 1990; Sibley et al., 2011) and in empirical reviews (Lilienfeld & Waldman, 1990; Waschbusch, 2002) but to our knowledge the present study marks the first extension of this line of research to the study of peer deviancy training. Our findings may also support current developmental theories of conduct problems, which have implicated deviant peer interactions as one of several causal mechanisms (Moffitt, 1993, 2003). To the extent that our results may generalize from a treatment setting to children’s “real life” settings, our findings suggest that higher rates of RDPB may, in part, serve to propel children along developmental trajectories associated with conduct problems. Given that children with ADHD-only did not significantly differ from controls, and given that the majority of elementary school age children with CP also meet criteria for ADHD (e.g., Pelham et al., 1992; Szatmari, Boyle, & Offord, 1989), it may be that increased RDPB is specific to children with ADHD-CP. These conclusions are speculative because we did not examine longitudinal outcomes in this study, but if our speculations are confirmed with future studies, it would suggest that efforts to reduce the impact of peer deviancy training may be most effective by preventing children with ADHD-CP from reinforcing deviant behavior in peers while simultaneously inoculating other children from being influenced by this reinforcement.
This study was also one of the first to examine the effects of two evidence-based interventions for ADHD – stimulant medication and behavior therapy – on reinforcement of peer deviancy. The significant Diagnosis × Medication × Behavior Modification interaction (see Tables 3 and 4) suggested that there were differences in how children with ADHD-only and ADHD-CP responded to the effects of medication and behavior modification. Follow-up tests and examination of means (see Figure 1a and 1b) showed several interesting findings. First, in the absence of medication (i.e., in the placebo condition), both low and high levels of behavior modification (LBM and HBM) produced significantly lower rates of RDPB than those observed in NBM. This pattern was observed in both the ADHD-only and ADHD-CP groups. These results are consistent with previous studies (e.g., Fabiano et al., 2007; Pelham et al., 2014) in demonstrating that even modest levels of behavior therapy produce significant positive effects when used to treat ADHD, and this is true even for children with ADHD-CP who are arguably among the most behaviorally impaired. Results also support previous findings that behavioral programs may reduce the effects of deviancy training (Feldman, 1992; Kellam, Ling, Merisca, Brown, & Ialongo, 1998) and extend these findings by showing the same is true for children clinically diagnosed with behavioral disorders. Though some disagreement remains regarding the impact of deviancy training across group-based interventions for adolescents (Lipsey, 2006; Weiss et al., 2005), the results of the present study suggest that the use of structured behavioral strategies sufficiently reduces RDPB, thereby limiting opportunities for deviancy training to occur in the first place.
Second, results showed that when no behavior modification was implemented (NBM), medium and high doses of medication produced significantly lower rates of RDPB in children with ADHD-only relative to both the placebo and low dose conditions; essentially, medium/high doses of medication were effective while placebo/low doses of medication were not. The same was not true for children with ADHD-CP; no dose of medication was able to significantly reduce the rate of RDPB in the absence of behavior modification. These results are somewhat in contrast with results of many other studies which generally report that stimulant medication treatment has significant positive effects on antisocial behavior, at least in the short term, in children with ADHD-only and ADHD-CP (see Connor, Glatt, Lopez, Jackson, & Melloni, 2002; Hinshaw, 1991 for reviews). It is possible that medication alone did not show as positive an effect on RDPB among children with ADHD-CP because RDPB is not a measure of antisocial behavior, but rather a child’s reaction to a peer’s antisocial behavior. Children with ADHD-CP may reinforce deviant peer behavior simply because it’s socially rewarding to do so – they find it fun to laugh along with others’ disruptiveness – rather than due to impulsivity or other reasons. If that is the case, then medication, which is known to improve executive functions like impulsivity and self-control (Bedard et al., 2003; Firestone et al., 1981; Tannock et al., 1995), might not be expected to influence this type of peer-directed behavior.
Third, the results also provided evidence about the effects of combining stimulant medication and behavior modification. Among children with ADHD-only (see Figure 1a), the low and high dose of behavior modification – even in the absence of medication –reduced rates of RDPB as effectively as a medium or high dose of medication given in the absence of behavior therapy. Thus, relatively simple behavioral procedures were able to nearly eliminate RDPB among children with ADHD-only, negating the need for medication to address this particular behavior. The same trend was apparent for children with ADHD-CP (see Figure 1b), but the effect was much more dramatic; even the highest dose of medication alone was not as effective as the low dose of behavior modification delivered in the absence of medication. Only once behavior modification was implemented did medication begin to have an effect, on both RDPB and on other behavioral outcomes in this study (see Fabiano et al., 2007; Pelham et al., 2014). Thus, behavior modification appears to be virtually required if the aim is to reduce RDPB among ADHD-CP children, who (as discussed earlier) arguably are those who most need this behavior targeted.
This study had several limitations. The study was conducted in a carefully controlled treatment setting, so the results may not generalize to natural settings. The t.i.d. medication dosage has become increasingly rare since the advent of sustained-release preparations, though both formulations have been found to produce similar effects (Pelham et al., 2001). Though all staff, including those who worked directly with the children and collected the data, were unaware of children’s medication conditions, they were aware of the behavior modification condition. We did not collect information on the reliability of children’s diagnoses, but our diagnostic procedures were consistent with recommended guidelines for the empirically based assessment of ADHD (see Pelham et al., 2005) and are virtually identical to procedures used in many other studies. Another limitation was our inability to directly assess reliability for the RDPB variable, due to financial and logistical constraints. Gender differences could not be evaluated due to the predominance of males in the ADHD sample (86%); future studies should over sample females to see what role gender may play in rates of RDPB exhibited by children with ADHD. Though accounted for statistically (i.e., Satterthwaite, 1946), the present study had a relatively small sample of children with ADHD-only. Future researchers may wish to over sample for children with ADHD-only or consider including a CP-only group, to further clarify what ADHD and CP uniquely contribute to deviant peer interactions. Finally, this study did not evaluate immediate or long-term effects of peer reinforcement on the receiver’s behavior.
Clinical Implications
Behavior modification and stimulant medication were each able to reduce RDPB in children with ADHD-only (although medium to high doses of medication were required to produce the same effects as low doses of behavior modification), while behavior modification was necessary to reduce RDPB in children with ADHD-CP. Given that peer reinforcement is hypothesized to be a key mechanism powering the deviancy training process (Dishion & Dodge, 2006), this has an implication for interventionists treating groups of at-risk or clinically diagnosed youth; regardless of intervention content or target population, clinicians should incorporate structured behavioral strategies into the treatment process when using group-based interventions. Utilizing strategies to provide immediate consequences for displaying or reinforcing (e.g., laughing at or encouraging) negative behavior and positive consequences for doing the opposite (not being disruptive and ignoring disruptive behavior that does occur) are important and apparently necessary strategies for successfully implementing group-based treatments with youth. This is true even if the child is receiving stimulant medication treatment, and appears to be particularly important for those with ADHD-CP, who showed up to 86% reductions in RDPB in behavior modification conditions.
Future Directions
Deviancy training is thought to be an intermittent process within social interactions that can have an impact on long-term development. As such, researchers must examine the complex sequence of events within peer exchanges in experimental settings. Purposefully manipulating proposed mechanisms in experimental settings, like the rate of RDPB given and received, may shed further light on the potency of peer reinforcement on individual behavioral outcomes. In their meta-analysis of deviancy training, Weiss et al. (2005) argued that adolescents are already exposed to so much natural deviancy training in their chosen peer groups that any additional exposure they may face in treatment is likely inconsequential. However, the same may not be true for younger individuals whose peer interactions are typically more structured and monitored by the adults in their lives (i.e., parents and teachers). Recent efforts to expand naturalistic deviancy training research to younger populations (e.g., Snyder et al., 2005, 2010) are a step in the right direction, but more work must be done to determine what, if any, role deviancy training may play in group-based interventions for younger children. Finally, researchers need to focus more on populations with clinical levels of disruptive behavior disorders, (i.e., ADHD, ODD, and CD), to determine what role psychopathology plays in deviant peer interactions, on both the individual level and as it pertains to group composition. These individuals are arguably the most likely to become involved with deviant peers, both in their daily lives and during the course of treatment, and therefore may beat greatest risk for exposure to the process of deviancy training.
Acknowledgments
This research was funded in part by a grant from the National Institute of Mental Health (MH62946) awarded to William E. Pelham, Jr. During the preparation of this manuscript, Daniel Waschbusch was supported in part by a grant from the National Institute of Mental Health (7R34MH085796). William E. Pelham, Jr., was also supported in part by grants from the National Institute of Mental Health (MH092466, MH53554, MH065899, MH62988), the Institute of Education Sciences (R37A120169), the National Institute of Alcohol Abuse and Alcoholism (AA11873), the National Institute on Drug Abuse (DA12414, DA12986), the Institute of Education Sciences (LO30000665A, R324B06045), and the National Institute of Child Health and Human Development (HD040935).
We wish to thank the parents and children who participated in the study and the many research assistants, teachers, and clinical staff who helped conduct the study.
Footnotes
Missing data bias was assessed by computing a dummy variable reflecting the presence or absence of missing data for each variable in the model. Correlations between this dummy variable and all other variables in the model as well as an array of demographic variables were computed. No significant correlations were found (p > .05).
Portions of this manuscript were presented at the 2012 ABCT 46th Annual Convention in National Harbor, MD.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. 1994. Diagnostic and statistical manual of mental disorders (4th ed.) [Google Scholar]
- Bedard A-C, Ickowicz A, Logan GD, Hogg-Johnson S, Schachar R, Tannock R. Selective inhibition in children with attention-deficit hyperactivity disorder off and on stimulant medication. Journal of Abnormal Child Psychology. 2003;31(3):315–327. doi: 10.1023/a:1023285614844. [DOI] [PubMed] [Google Scholar]
- Carlson CL, Pelham WE, Milich R, Dixon J. Single and combined effects of methylphenidate and behavior therapy on the classroom performance of children with attention-deficit hyperactivity disorder. Journal of Abnormal Child Psychology. 1992;20(2):213–232. doi: 10.1007/BF00916549. [DOI] [PubMed] [Google Scholar]
- Catellier DJ, Hannan PJ, Murray DM, Addy CL, Conway TL, Yang S, Rice JC. Imputation of missing data when measuring physical activity by accelerometry. Medicine & Science in Sports & Exercise. 2005;37(11 Suppl):S555–S562. doi: 10.1249/01.mss.0000185651.59486.4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor DF, Glatt SJ, Lopez ID, Jackson D, Melloni RH. Psychopharmacology and aggression. I: A meta-analysis of stimulant effects on overt/covert aggression-related behaviors in ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(3) doi: 10.1097/00004583-200203000-00004. 2532-261. [DOI] [PubMed] [Google Scholar]
- Coxe S, West SG, Aiken LS. The analysis of count data: a gentle introduction to poisson regression and its alternatives. Journal of Personality Assessment. 2009;91(2):121–136. doi: 10.1080/00223890802634175. [DOI] [PubMed] [Google Scholar]
- Dishion TJ. Cross-setting consistency in early adolescent psychopathology: Deviant friendships and problem behavior sequelae. Journal of Personality. 2000;68(6):1109–1126. doi: 10.1111/1467-6494.00128. [DOI] [PubMed] [Google Scholar]
- Dishion TJ, Andrews DW. Preventing escalation in problem behaviors with high-risk young adolescents: Immediate and 1-year outcomes. Journal of Consulting and Clinical Psychology. 1995;63(4):538–548. doi: 10.1037//0022-006x.63.4.538. [DOI] [PubMed] [Google Scholar]
- Dishion TJ, Capaldi D, Spracklen KM, Li F. Peer ecology of male adolescent drug use. Development and Psychopathology. 1995;7(4):803–824. [Google Scholar]
- Dishion TJ, McCord J, Poulin F. When interventions harm: Peer groups and problem behavior. American Psychologist. 1999;54(9):755–764. doi: 10.1037//0003-066x.54.9.755. [DOI] [PubMed] [Google Scholar]
- Dishion TJ, Nelson SE, Winter CE, Bullock BM. Adolescent friendship as a dynamic system: Entropy and deviance in the etiology and course of male antisocial behavior. Journal of Abnormal Child Psychology. 2004;32(6):651–663. doi: 10.1023/b:jacp.0000047213.31812.21. [DOI] [PubMed] [Google Scholar]
- Dishion TJ, Skaggs NM. An ecological analysis of monthly “bursts” in early adolescent substance use. Applied Developmental Science. 2000;4(2):89–97. [Google Scholar]
- Dishion TJ, Tipsord JM. Peer contagion in child and adolescent social and emotional development. Annual Review of Psychology. 2011;62(1):189–214. doi: 10.1146/annurev.psych.093008.100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge KA, Dishion TJ, Lansford JE. Deviant Peer Influences in Programs for Youth: Problems and Solutions. New York: Guilford Press; 2006. p. 462. [Google Scholar]
- Elliot DS, Menard S. Delinquent friends and delinquent behavior: temporal and developmental patterns. In: Hawkins JD, editor. Delinquency and crime: Current theories. New York, NY: Cambridge University Press; 1996. pp. 28–67. [Google Scholar]
- Evans SW, Owens JS, Bunford N. Evidence-Based Psychosocial Treatments for Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. Journal of Clinical Child and Adolescent Psychology. 2013;24(4):486–502. doi: 10.1080/15374416.2013.850700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiano GA, Pelham WE, Gnagy EM, Burrows-Maclean L, Coles EK, Chacko A, Robb JA. The single and combined effects of multiple intensities of behavior modification and methylphenidate for children with attention deficit hyperactivity disorder in a classroom. School Psychology Review. 2007;36(2):195–216. [Google Scholar]
- Fabiano GA, Pelham WE, Waschbusch DA, Gnagy EM, Lahey BB, Chronis AM, Burrows-Maclean L. A practical measure of impairment: psychometric properties of the impairment rating scale in samples of children with attention deficit hyperactivity disorder and two school-based samples. Journal of Clinical Child and Adolescent Psychology. 2006;35(3):369–385. doi: 10.1207/s15374424jccp3503_3. [DOI] [PubMed] [Google Scholar]
- Feldman RA. The St. Louis experiment: Effective treatment of antisocial youths in prosocial peer groups. In: McCord J, Tremblay RE, editors. Preventing antisocial behavior: Interventions from birth through adolescence. New York: Guilford Press; 1992. pp. 233–252. [Google Scholar]
- Firestone P, Kelly MJ, Goodman JT, Davey J. Differential effects of parent training and stimulant medication with hyperactives: A progress report. Journal of the American Academy of Child Psychiatry. 1981;20(1):135–147. doi: 10.1016/s0002-7138(09)60723-8. [DOI] [PubMed] [Google Scholar]
- Gardner W, Mulvey EP, Shaw EC. Regression analyses of counts and rates: Poisson, over dispersed Poisson, and negative binomial models. Psychological Bulletin. 1995;118(3):392–404. doi: 10.1037/0033-2909.118.3.392. [DOI] [PubMed] [Google Scholar]
- Goodnight JA, Bates JE, Newman JP, Dodge KA, Pettit GS. The interactive influences of friend deviance and reward dominance on the development of externalizing behavior during middle adolescence. Journal of Abnormal Child Psychology. 2006;34(5):573–583. doi: 10.1007/s10802-006-9036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw SP. On the distinction between attentional deficits/hyperactivity and conduct problems/aggression in child psychopathology. Psychological Bulletin. 1987;101(3):443–463. [PubMed] [Google Scholar]
- Hinshaw SP. Stimulant medication and the treatment of aggression in children with attentional deficits. Journal of Clinical Child Psychology. 1991;20(3):301–312. [Google Scholar]
- Hirschi T. Self-control and crime. In: Baumeister RF, Vohs KD, editors. Handbook of self-regulation: Research, theory, and applications. New York, NY: Guilford Press; 2004. pp. 537–552. [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6(2):65–70. [Google Scholar]
- Hoza B, Gerdes AC, Mrug S, Hinshaw SP, Bukowski WM, Gold JA, Wigal T. Peer-assessed outcomes in the multimodal treatment study of children with attention deficit hyperactivity disorder. Journal of Clinical Child and Adolescent Psychology. 2005;34(1):74–86. doi: 10.1207/s15374424jccp3401_7. [DOI] [PubMed] [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY: IBM Corp; 2010. [Google Scholar]
- Kellam SG, Ling X, Merisca R, Brown CH, Ialongo NS. The effect of the level of aggression in the first grade classroom on the course and malleability of aggressive behavior into middle school. Development and Psychopathology. 1998;10(2):165–185. doi: 10.1017/s0954579498001564. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Miller TI, Gordon RA, Riley AW. Developmental epidemiology of the disruptive behavior disorders. In: Quay HC, Hogan AE, editors. Handbook of Disruptive Behavior Disorders. Boston, MA: Springer US; 1999. [Google Scholar]
- Lilienfeld SO, Waldman ID. The relation between childhood attention-deficit hyperactivity disorder and adult antisocial behavior reexamined: The problem of heterogeneity. Clinical Psychology Review. 1990;10(6):699–725. [Google Scholar]
- Lipsey MW. The Effects of Community-Based Group Treatment for Delinquency: A Meta-Analytic Search for Cross-Study Generalizations. In: Dodge KA, Dishion TJ, Lansford JE, editors. Deviant peer influences in programs for youth: Problems and solutions. New York: Guilford Press; 2006. pp. 162–184. [Google Scholar]
- McCord J. Cures That Harm: Unanticipated Outcomes of Crime Prevention Programs. The Annals of the American Academy of Political and Social Science. 2003;587(1):16–30. [Google Scholar]
- Moffitt TE. Juvenile delinquency and attention deficit disorder: boys’ developmental trajectories from age 3 to age 15. Child Development. 1990;61(3):893–910. doi: 10.1111/j.1467-8624.1990.tb02830.x. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: A developmental taxonomy. Psychological Review. 1993;100(4):674–701. [PubMed] [Google Scholar]
- Moffitt TE. Life-course-persistent and adolescence-limited antisocial behavior: A 10-year research review and a research agenda. In: Lahey BB, Moffitt TE, Caspi A, editors. Causes of conduct disorder and juvenile delinquency. New York, NY: Guilford Press; 2003. pp. 49–75. [Google Scholar]
- Patterson GR, Littman RA, Bricker W. Assertive behavior in children: A step toward a theory of aggression. Monographs of the Society for Research in Child Development. 1967;32(5):1–43. [PubMed] [Google Scholar]
- Pelham WE, Bender ME. Peer relationships in hyperactive children: Description and treatment. In: Gadow K, Bailer I, editors. Advances in Learning & Behavioral Disabilities: A Research Annual. Vol. 1. 1982. pp. 365–436. JAI. [Google Scholar]
- Pelham WE, Burrows-MacLean L, Gnagy EM, Fabiano GA, Coles EK, Wymbs BT, Waschbusch DA. A dose-ranging study of behavioral and pharmacological treatment in social settings for children with ADHD. Journal of Abnormal Child Psychology. 2014;42(6):1019–1031. doi: 10.1007/s10802-013-9843-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham WE, Carlson CL, Sams SE, Vallano G, Dixon J, Hoza B. Separate and combined effects of methylphenidate and behavior modification on boys with attention deficit-hyperactivity disorder in the classroom. Journal of Consulting and Clinical Psychology. 1993;61(3):506–515. doi: 10.1037/0022-006X.61.3.506. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Fabiano GA, Massetti GM. Evidence-based assessment of attention deficit hyperactivity disorder in children and adolescents. Journal of Clinical Child and Adolescent Psychology. 2005;34(3):449–476. doi: 10.1207/s15374424jccp3403_5. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Burrows-MacLean L, Williams A, Fabiano GA, Morrisey SM, Morse GD. Once-a-day Concert a methylphenidate versus three-times-daily methylphenidate in laboratory and natural settings. Pediatrics. 2001;107(6):E105. doi: 10.1542/peds.107.6.e105. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11389303. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Greenslade KE, Milich R. Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31(2):210–218. doi: 10.1097/00004583-199203000-00006. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Greiner AR, Gnagy EM. Summer Treatment Program Manual. Buffalo, NY: Comprehensive Treatment for Attention Deficit Disorders, Inc; 1997. [Google Scholar]
- Peugh JL, Enders CK. Using the SPSS Mixed Procedure to Fit Cross-Sectional and Longitudinal Multilevel Models. Educational and Psychological Measurement. 2005;65(5):717–741. [Google Scholar]
- Poulin F, Dishion TJ, Burraston B. 3-Year iatrogenic effects associated with aggregating high-risk adolescents in cognitive-behavioral preventive interventions. Applied Developmental Science. 2001;5(4):214–224. [Google Scholar]
- Satterthwaite FE. An Approximate Distribution of Estimates of Variance Components. Biometrics Bulletin. 1946;2(6):110–114. [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Sibley MH, Pelham WE, Molina BSG, Gnagy EM, Waschbusch DA, Biswas A, Karch KM. The delinquency outcomes of boys with ADHD with and without comorbidity. Journal of Abnormal Child Psychology. 2011;39(1):21–32. doi: 10.1007/s10802-010-9443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder J, McEachern A, Schrepferman L, Just C, Jenkins M, Roberts S, Lofgreen A. Contribution of peer deviancy training to the early development of conduct problems: Mediators and moderators. Behavior Therapy. 2010;41(3):317–328. doi: 10.1016/j.beth.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Snyder J, Schrepferman L, Oeser J, Patterson GR, Stoolmiller M, Johnson K, Snyder A. Deviancy training and association with deviant peers in young children: ocurrence and contribution to early-onset conduct problems. Development and Psychopathology. 2005;17(2):397–413. doi: 10.1017/s0954579405050194. [DOI] [PubMed] [Google Scholar]
- Szatmari P, Boyle M, Offord DR. ADDH and conduct disorder: Degree of diagnostic overlap and differences among correlates. Journal of the American Academy of Child and Adolescent Psychiatry. 1989;28(6):865–872. doi: 10.1097/00004583-198911000-00010. [DOI] [PubMed] [Google Scholar]
- Tannock R, Schachar R, Logan GD. Methylphenidate and cognitive flexibility: Dissociated dose effects in hyperactive children. Journal of Abnormal Child Psychology. 1995;23(2):235–266. doi: 10.1007/BF01447091. [DOI] [PubMed] [Google Scholar]
- Thompson E, Bhrolchain CN. The epidemiology of community paediatric outpatient referrals 2006. Child: Care, Health and Development. 2011;39(1):50–54. doi: 10.1111/j.1365-2214.2011.01348.x. [DOI] [PubMed] [Google Scholar]
- Thornberry TP, Krohn MD. Peers, drug use, and delinquency. In: Stoff DM, Breiling J, Maser JD, editors. Handbook of antisocial behavior. Hoboken, NJ: John Wiley & Sons; 1997. pp. 218–233. [Google Scholar]
- Tripp G, Alsop B. Sensitivity to reward frequency in boys with attention deficit hyperactivity disorder. Journal of Clinical Child Psychology. 1999;28(3):366–375. doi: 10.1207/S15374424jccp280309. [DOI] [PubMed] [Google Scholar]
- Waschbusch DA. A meta-analytic examination of comorbid hyperactive-impulsive-attention problems and conduct problems. Psychological Bulletin. 2002;128(1):118–150. doi: 10.1037/0033-2909.128.1.118. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Wechsler Intelligence Scale for Children - Third Edition (WISC-III) 3rd ed. San Antonio, TX: Psychological Corporation; 1991. [Google Scholar]
- Weiss B, Caron A, Ball S, Tapp J, Johnson M, Weisz JR. Iatrogenic effects of group treatment for antisocial youths. Journal of Consulting and Clinical Psychology. 2005;73(6):1036–1044. doi: 10.1037/0022-006X.73.6.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Dishion TJ. Temperament and adolescent substance use: a transactional analysis of emerging self-control. Journal of Clinical Child and Adolescent Psychology. 2004;33(1):69–81. doi: 10.1207/S15374424JCCP3301_7. [DOI] [PubMed] [Google Scholar]
- Wolraich ML, Brown L, Brown RT, DuPaul GJ, Earls M, Feldman HM, Visser S. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007–1022. doi: 10.1542/peds.2011-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]