Abstract
Rationale
Although vitamin D is widely used to promote skeletal health, definitive data on benefits and risks of supplemental vitamin D alone on bone are lacking. Results from large, randomized controlled trials in the general population are sparse. Data on the effects of supplemental omega-3 fatty acids (FAs) on bone are also limited.
Design
The VITamin D and OmegA-3 TriaL (VITAL) is a double-blind, placebo-controlled trial assessing the role of vitamin D3 (2000 IU/d) and omega-3 FA (1 g/d) supplements in reducing risks of cancer and cardiovascular disease among U.S. men aged ≥50 and women aged ≥55. To comprehensively test effects of supplemental vitamin D and/or omega-3 FAs on skeletal health, the VITAL: Effects on Fractures ancillary study is determining the effects of these supplements on incident fractures among 25,875 participants enrolled in the parent trial. Study investigators adjudicate fractures through detailed review of medical records and radiological images (hip and femur). In a complementary ancillary, VITAL: Effects on Structure and Architecture is determining the effects of supplemental vitamin D and/or omega-3 FAs on bone with detailed phenotyping during in-person visits. Comprehensive assessments of bone density, turnover, structure/architecture, body composition, and physical performance are being performed at baseline and 2 years post-randomization.
Conclusion
Results from these studies will clarify the relationship between supplemental vitamin D and/or omega-3 FAs on bone health outcomes, and inform clinical care and public health guidelines on the use of supplemental vitamin D for the primary prevention of fractures in women and men.
Keywords: Vitamin D, fractures, bone turnover, omega-3 fatty acids, bone mineral density, trabecular bone score
1. Background/Aims
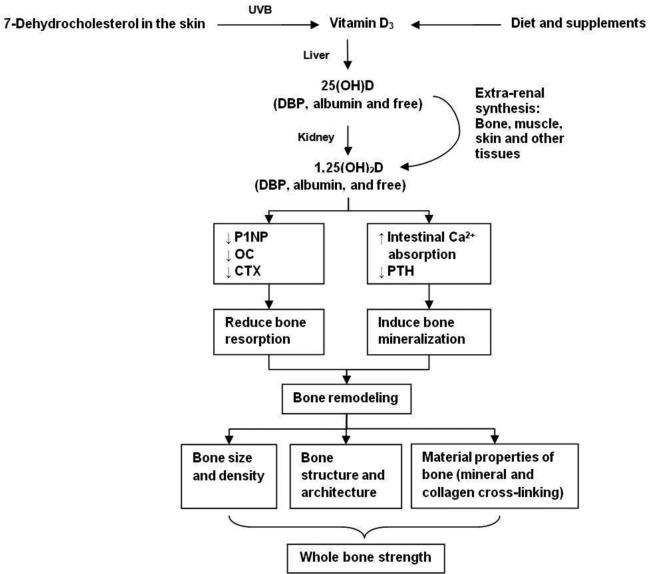
Osteoporosis and vitamin D deficiency are major public health problems in the United States. According to most recent analyses of the National Health and Nutrition Examination Surveys (NHANES) databases, 53.6 million Americans have osteoporosis and/or low bone mass, increasing their risk for fragility fractures, and a third of Americans are vitamin D deficient (defined as a 25-hydroxivitamin D [25(OH)D] level of <20 ng/mL).1,2 Older adults, blacks, obese individuals, and those with hip fractures are especially at risk for low vitamin D levels.3-12 Osteoporosis, characterized by loss of bone mass (bone mineral density [BMD]), alterations in bone structure, and a net increase in bone resorption relative to bone formation, leads to skeletal fragility and increased fracture risk. Despite the widespread use of vitamin D supplements to promote skeletal health,13 data on whether supplemental vitamin D alone is effective in the primary prevention of fractures are conflicting. Although some observational studies and randomized controlled trials (RCTs) show that supplemental vitamin D protects against fractures and has beneficial effects on bone health measures,14-17 (Fig.1) other clinical trials and meta-analyses show inconsistent results.18-33 Most trials that have tested effects of supplemental vitamin D on fracture risk have used a combined intervention of calcium with vitamin D. Data from large, placebo-controlled, RCTs testing the effects of supplemental vitamin D alone on fractures and bone health outcomes are extremely limited and inconclusive.18, 20, 34 Evidence is also insufficient to determine the effect of higher doses of supplemental vitamin D, which may be required to produce 25(OH)D levels needed to benefit bone. Some meta-analyses suggest that higher attained 25(OH)D levels and daily, supplemental vitamin D at higher doses may be required to reduce fracture risk.21, 24, 29 In order to separate the effects of calcium and vitamin D, and determine whether supplemental vitamin D alone benefits bone health and fracture outcomes, large, placebo-controlled, RCTs are needed. Given the aging of America, the age-related risk of fragility fractures, and the prevalence of low vitamin D levels, there is a clinical and public health need to determine in large RCTs whether daily, high-dose supplemental vitamin D is effective in the primary prevention of fractures.
Figure 1.
Mechanisms through which high dose vitamin D vs. placebo may benefit bone health
Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are omega-3 fatty acids (ω-3 FAs) found in fish and widely used in fish-oil supplements. Increased use of supplemental ω-3 FAs has prompted concerns about the clinical effects on skeletal health. Data from experimental studies and a limited number of clinical trials suggest potential benefits of ω-3 FAs on BMD, but results are inconclusive.35-46 An observational study in the Women's Health Initiative of women with hip fractures and controls showed those with the highest EPA levels had 54% lower relative risk for hip fractures.47 Other trials have reported that high intake of fish rich in EPA+DHA is associated with preservation of femoral neck BMD relative to low intakes.48 While these data are promising, results from trials testing supplemental ω-3 FA and bone loss are sparse. Results from large RCTs will help to determine the effects of ω-3 FA supplements on skeletal health.
2. Material and methods
2.1 Overview of study design
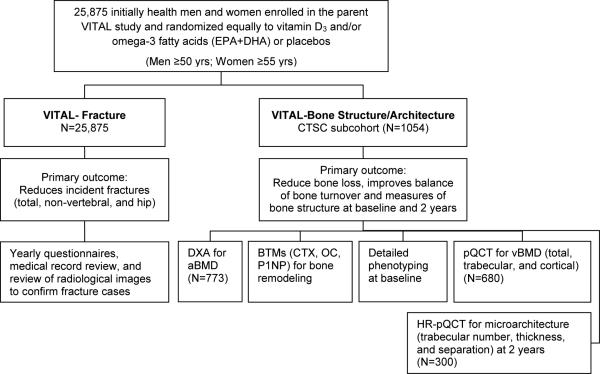
VITAL-Bone Health, composed of two ancillary studies VITAL-Fracture and VITAL-Bone Structure/Architecture, builds on the strengths of the parent VITamin D and OmegA-3 TriaL (VITAL). VITAL is a large, 2×2 factorial, double-blind, placebo-controlled trial testing the benefits and risks of vitamin D3 (cholecalciferol, 2000 IU/d) and omega-3 fatty acid (1 g/d; EPA+DHA) supplementation on cancer and cardiovascular disease. In the large overall VITAL cohort (N=25,875), randomized equally to vitamin D3 and/or omega-3 fatty acids (or placebo), we are conducting VITAL-Fracture, an ancillary fracture study to determine the safety and efficacy of high-dose, daily supplemental vitamin D in the primary prevention of incident fractures (total, hip, and non-vertebral). Incident fracture outcomes are being determined through a combination of methods including questionnaires, medical record review, and review of radiological images (hip, femur, pelvic fractures). Levels of serum 25(OH)D, calcium, and PTH will also be measured in 16,954 fasting blood samples taken at baseline. A second ancillary study VITAL-Bone Structure/Architecture will be conducted among a sub-cohort of VITAL participants (N=773) using detailed, in-person phenotyping and bone assessments to test the effects of supplemental vitamin D and/or omega-3 fatty acids on bone structure and architecture.
2.2. Aims
The primary aim of VITAL-Fracture is to test in a randomized, placebo-controlled trial whether supplemental vitamin D3 and/or omega-3 fatty acids reduces the risk of incident fractures (total, non-vertebral, and hip). In the VITAL-Bone Structure/Architecture, the primary aims are to test whether supplemental vitamin D3 and/or omega-3 fatty acids (1) has beneficial effects on areal bone density (aBMD) at the spine, hip, and total body and (2) reduces bone turnover biomarkers (BTMs) (as assessed by serum c-telopeptide [CTX], osteocalcin [OC], and amino-terminal propeptide of type 1 collagen [P1NP]).The secondary aims will test whether the study intervention improves structure and architecture at the distal radius and tibia. Additional analyses will determine whether the effect of supplemental vitamin D3 on bone structure and architecture varies according to baseline levels of serum 25(OH)D, race/skin pigmentation, body composition, and body mass index (BMI). Parallel assessments of the effects of omega-3 fatty acids (EPA+DHA) on the same aims will also be performed.
2.3. Sponsors
These ancillary studies, entitled VITAL: Effects on Fractures and VITAL: Effects on Structure and Architecture, are supported by the National Institute of Arthritis and Musculoskeletal and Skin Disease (NIAMS). The studies were approved by the Partners Human Research Committees, the Institutional Review Board of Brigham and Women's Hospital (BWH). They are registered with clinicaltrials.gov (NCT01747447 and NCT01704859, respectively). The parent trial has received IND approval from the FDA and has been registered at clinicaltrials.gov (NCT01169259). A study website for the parent trial is maintained at www.vitalstudy.org.
2.4. Summary of eligibility, recruitment, and enrollment
As a primary prevention trial, VITAL participants were selected across the U.S. for age only. Women aged 55 years or older and men aged 50 years and older were eligible for enrollment if they had no prior history of cancer or cardiovascular disease at baseline. Safety exclusions included allergy to fish, renal failure, history of hypercalcemia, hypo- or hyperparathyroidism, severe liver disease, granulomatous diseases, or other serious illnesses. Prior to enrollment, all participants signed a detailed informed consent form and were required to complete a 3-month, placebo run-in to demonstrate good pill-taking compliance (defined as taking ≥2/3 of the study pills). With a final randomized population of 25,875 participants, VITAL surpassed its original recruitment goal of 20,000 and achieved its goal of enrolling 5,108 African Americans (25% of study population).49 Study enrollment began in November 2011 and closed in March 2014.
A subcohort of 1,054 VITAL participants from the New England region was established for detailed, in-person assessments at Harvard Catalyst, the NIH-sponsored Clinical and Translational Science Center (CTSC) in Boston. Those enrolled met the same eligibility criteria as participants in the parent trial. Participants in the VITAL CTSC cohort were eligible for the VITAL-Structure/Architecture ancillary study if they did not have current use or prior history of bisphosphonates or bone-active medications within the last two years. Enrollment in the ancillary study (N=773) exceeded the projected goal of 600 participants.
2.5. Interventions
Interventions tested in VITAL are vitamin D3 (cholecalciferol, 2000 IU/d) and active fish oil (Omacor® fish oil, 1 g/d, EPA to DHA ratio of 1:1) and matching inert placebos. Participants are permitted up to 800 IU/day of additional vitamin D supplementation and intakes of 1200 mg/d of elemental calcium from all sources in accordance with safety limits indicated by the Institute of Medicine (IOM).50 Participants also agreed not to consume non-study fish-oil supplements during the trial. The doses of vitamin D3 and omega-3 fatty acids tested in VITAL were chosen after comprehensive review of the available literature to achieve optimal efficacy and safety.
2.6. Blood collection
Fasting blood samples in the parent VITAL study were collected during run-in from a subset of approximately 16,954 participants. Among this subset, follow-up samples are being obtained at years 1-4 from a randomly selected group of approximately 6,000 participants. Blood samples will confirm intervention compliance and determine whether treatment effects are modified by baseline and achieved blood levels.
Fasting bloods were also collected at baseline CTSC visits and will be repeated at follow-up visits. Levels of BTMs of resorption (CTX) and formation (OC and P1NP) will be measured in VITAL-Bone Structure/Architecture using serum specimens. These biomarkers will determine the relationship between BTM and changes in bone mineral density, structure, and micro-architecture.
2.7. Study Assessments
To test the effects of daily, supplemental vitamin D and/or omega-3 fatty acids on fracture risk and bone health measures, a comprehensive list of variables is being obtained (Table 1). Variables assessed include demographic information, anthropomorphic measures, and risk factors for osteoporosis. In addition, a food frequency questionnaire (FFQ) is being used a for detailed evaluation of nutrient intake and dietary and non-dietary sources of supplemental vitamin D, marine omega-3 fatty acids, and calcium.
Table 1.
Study Activities, Measures, and Outcomes
| Follow-up Cycle | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pre-enrollment | Enrollment | 6 MO | YR1 | YR2 | YR3 | YR4 | YR5 | |
| Eligibility screening | • | |||||||
| Waist / hip, height, weight, BMI | • | • | • | |||||
| Bone health measures | ||||||||
| DXA for bone density and body composition | • | • | ||||||
| Bone turnover biomarkers | • | • | ||||||
| pQCT | • | • | ||||||
| HR-pQCT | • | |||||||
| Physical performance measures | • | • | ||||||
| Pill compliance | • | • | • | • | • | • | • | |
| Risk factors | ||||||||
| Alcohol consumption | • | • | ||||||
| Physical activity questionnaires | • | • | • | • | ||||
| Fracture after age 50 | • | • | ||||||
| Parent and family history of fracture | • | • | • | |||||
| Sun exposure / skin pigmentation | • | |||||||
| Menopausal, premature menopause | • | |||||||
| Diet and dietary supplements | ||||||||
| Food frequency questionnaire with detailed calcium intake | • | • | • | |||||
| Fish oil / omega-3 fatty acid supplements | • | • | • | • | • | • | • | |
| Vitamin D / single supplements, multivitamins, or calcium supplements | • | • | • | • | • | • | • | |
| Medications | ||||||||
| Bisphosphonates or other osteoporosis medications | • | • | • | • | • | • | ||
| Female hormone use | • | • | • | • | • | • | ||
| Corticosteroids | • | • | • | • | • | • | ||
| Other (e.g. aromatase inhibitors, anti-depressants, calcitriols, diabetic medication, diuretics etc.) | • | • | • | • | • | • | ||
| Diagnoses / medical history | ||||||||
| Fracture | • | • | • | • | • | • | • | |
| Malabsorption or inflammatory bowel disease | • | • | • | • | • | • | ||
| Osteoporosis or low bone mass (osteopenia) | • | • | ||||||
| Hypo- or hyperparathyroidism | • | • | • | • | • | • | • | |
| Other (e.g., thyroid disorders, hypercalcemia, or other conditions) | • | • | • | • | • | • | • | |
In the VITAL-Bone Structure/Architecture subcohort, basic clinical data obtained at baseline includes medical history and physical exam (anthropometric indices and fasting bloods) and assessments of bone health, body composition, and physical performance. Identical follow-up assessments will be repeated at 2 years post-randomization. Follow-up CTSC visits began November 2014 and will be matched by month to control for seasonal variation in sun exposure.
2.7.1. Fracture measures
Primary tracking of fracture outcomes is being accomplished through participant self-reporting of incident fractures on annual questionnaires and review of medical records to confirm fracture outcomes. Participants who report a fracture are asked to sign a medical release for relevant hospital and physician records and to complete a questionnaire assessing basic information about the fracture. Participants are asked to return the questionnaire and medical release to study staff within 8 weeks. Non-responders receive two additional requests by mail and then a telephone request to collect study data.
The VITAL-Fracture study has established rigorous fracture adjudication procedures to ascertain fracture outcomes. Study staff blinded to the randomized treatment assignment reviews medical records to confirm or disconfirm the fracture event according to a defined protocol. Data collection includes the date of the fracture event, location of the bone(s) fractured, and level of trauma that caused the injury. Coding of location is also matched to ICD-9 codes. Level of trauma associated with the fracture is quantified on a scale (none, minor, moderate, or severe) and is determined through review of medical records as well as information provided by participants. Fractures of the hip and femur are further adjudicated through radiological image review by study investigators and a musculoskeletal radiologist. Fractures related to conditions affecting bone health such as tumors, bone cysts, Paget's disease, and osteogenesis imperfecta are further identified as pathological fractures. Fractures are also characterized as located near a prosthesis or as an atypical femur using established criteria.
2.7.2. Bone density, structure, and architecture measures
In VITAL-Bone Structure/Architecture, during CTSC visits at baseline and 2 years post-randomization, comprehensive bone assessments including BMD, body composition (fat and lean tissue according to dual X-ray absorptiometry [DXA]), and bone structure and architecture by peripheral quantitative computed tomography (pQCT) will be conducted at the Bone Density Unit at the Skeletal Health and Osteoporosis Center, BWH (Boston, MA).51 A more comprehensive list of measures obtained in VITAL-Bone Structure/Architecture is provided in Figure 2. Bone density of the spine (L1-L4), hip, and total body will be measured by DXA (Discovery W, APEX Software Version 4.2, Hologic, Bedford, MA). Using APEX 4.2 software which includes the FRAX® tool, 10-year total hip fracture risk will be calculated using bone density and clinical risk factors. Analyses will also be performed to generate Trabecular Bone Score (TBS) measures (Medimaps Group, Geneva, Switzerland). TBS, generated from textural analyses of spinal BMD scans, is associated with bone microarchitecture and is a risk factor for fractures, independent of BMD.52 At follow-up 2 years post-randomization, high resolution pQCT (HR-pQCT) scans will be performed in a subset of 300 CTSC participants with baseline pQCTs for measurements of bone structure and microarchitecture.
Figure 2.
Design of the VITAL bone health ancillary studies: VITAL-Fracture and VITAL-Structure/Architecture.
2.7.3. Body composition measures
Body composition measurements will be performed with a Hologic Discovery W, DXA machine. Adipose and lean mass will be calculated as well as android and gynoid sub-regions, and rate of change reports for fat and lean tissue. Changes in adiposity will be determined by total body fat, % total fat, VAT, fat mass index (FMI – fat mass/height2), and regional fat measures. Measures of pQCT will also be determined; new data show strong correlations between DXA measures of VAT and those assessed by QCT.53 Measurements of lean body mass will also be performed, including lean mass index (LMI – lean mass/height2), appendicular lean mass index (ALM – appendicular lean mass/ht2), ALM/BMI, and regional lean tissue.54
2.7.4. Physical performance measures
Physical performance measurements include assessment of standing balance, walking speed, chair stands, grip strength, and timed up and go. Standing balance, walking speed, and chair stands are the three tasks in the composite Short Physical Performance Battery (SPPB), a widely-used, objective measure of lower body function.55 Walking speed (ft/s) is being tested with a 6-meter walking course. Grip strength testing will be performed using the JAMAR Plus+ Digital Hand Dynamometer (Sammons Preston Rolyan, Bolingbrook, IL, USA). Variability in the use of the dynamometer has been minimized by extensive training and regular observations during the study period.
2.7.5. Biochemical measures
Baseline and follow-up blood levels of 25(OH)D, EPA+DHA, calcium, and PTH will be performed on baseline and follow-up samples. Measurements of serum total 25(OH)D will be conducted using two assays: Atherotech Diagnostics Abbott ARCHITECT chemiluminescent microparticle immunoassay (CMIA) and Quest Diagnostics liquid chromatography-tandem mass spectrometry (LC-MS/MS). The relationship between calcium, total 25(OH) D, and PTH levels will also be examined.56-59 Assays of BTMs (CTX, OC, and P1NP) will be performed: serum CTX levels will be measured using an e601 chemiluminescent immunoassay (Serum Elecsys CrossLaps®, Roche Diagnostics, Indianapolis, IN); serum OC concentrations will be determined by ELISA (Meso Scale Discovery, Rockville, MD); and serum P1NP levels will be determined by radioimmunoassay (Orion Diagnostica, Espoo, Finland). Baseline and follow-up samples will be run in the same assays.
2.8. Data management
For the VITAL-Bone Health studies, the computerized data management and security systems were designed for effective follow-up. Only study personnel who communicate with participants are able to access necessary information. In VITAL-Bone Structure/Architecture, all data collected at the Bone Density Unit, HR-pQCT site, and CTSC are transferred to the Division of Preventive Medicine for review, verification, and data checks to maximize accuracy. Annually, the independent Data and Safety Monitoring Board (DSMB) reviews all data on study endpoints and adverse events for the VITAL trial, including these two bone substudies.49
2.9. Analysis plan
In these bone health substudies, the first analysis will compare baseline characteristics by randomized treatment assignment to ensure that balance during randomization was established between the treatment groups. We will assess risk factors for fractures, including age, gender, race/ethnicity, and baseline vitamin D, omega-3 fatty acids, calcium levels, baseline distributions of fractures, physical activity level, use of a walking device, and the use of bone active medications.
With the 2×2 factorial design of the parent trial, the primary analysis will be an intent-to-treat analysis. The primary aim of these bone health substudies is to compare the main effects of intention-to-treat with vitamin D and with fish oil on the fracture outcomes. We will use the Cox proportional hazards model to allow for variable follow-up lengths60 and will estimate the cause-specific hazard ratio for fracture incidence for each intervention using indicators for treatment exposure. Stratification factors, including the second intervention, age, and gender will be controlled and adjustments will be made for any imbalances by treatment group in baseline risk factors, such as age, smoking, alcohol, BMI, physical activity, and calcium intake. In addition, we will conduct an analysis among compliers only, and censor participants if they stop taking the study intervention. Beyond the primary analyses, we will further assess the effect modification by omega-3 fatty acids, by baseline risk factors, and by time. We will also be assessing fractures according to the level of trauma. We will explore interactions between the vitamin D and/or EPA+DHA interventions and with the baseline self-reported dietary intake of vitamin D, of calcium, and of EPA+DHA as well as with gender, race/skin pigmentation (for vitamin D3), and BMI (for vitamin D3).
Analyses of treatment effects in the clinic-based VITAL-Bone Structure/Architecture substudy will also use the intent-to-treat principle; adherence adjustment and other analyses may be performed as secondary analyses. Changes in several measures over time for each treatment will be examined. Our interests are (1) small increases or reduced loss of aBMD in spine, hip, and total body as assessed by DXA, (2) reduced bone turnover as assessed by BTMs, (3) improved total, trabecular, and cortical volumetric BMD (vBMD) assessed by pQCT at baseline and 2 years post-randomization, (4) micro-architecture at the distal radius and tibia as assessed by HR-pQCT at 2 years post-randomization, and (5) reductions in FM or FMI or changes in total and regional FM and/or lean tissue. We will use linear regression to analyze normal or nearly normal data, and employ transformations if the distribution is non-normal, otherwise nonparametric methods, such as the Wilcoxon rank sum test will be used to compare randomized groups. Mean changes over time will also be compared in the 4 groups. To improve power, we will also condition on baseline measures using regression analyses [analysis of covariance (ANCOVA)]. The primary analysis will be of the main effect of vitamin D on changes in measures of bone health, adjusting for any effects of omega-3 FAs at baseline and during the trial. We will use regression analysis to adjust for any imbalance in key variables at baseline. Beyond the primary analysis, we will explore interactions between interventions and baseline and achieved 25(OH)D levels. In addition, we will evaluate effect modification by gender, race/skin pigmentation, season, and BMI, an important potential modifier of the treatment effect.
2.10. Statistical power
Power calculations for analysis of the time-to-event outcome in the fracture substudy are based on a 2×2 factorial trial. Rates are based on age-specific rates from women and men in the U.S.61, 62 Power is shown in Table 2 for the observed reductions in risk. We assume an average compliance of 80%, which is similar to the compliance achieved in our previous RCTs. The corresponding ‘true’ relative risk (RR) is shown. Power is calculated for a two-sided test using a log-rank analysis with a significance level of 0.05.63 We will have 80% power to detect an observed RR of 0.90 (true RR = 0.875 if 80% compliance) for any fractures, RR of 0.89 (true RR=0.86) for clinical non-vertebral fractures, and RR of 0.76 (true RR= 0.70) for hip fractures.
Table 2.
Power for the effect of a single agent on incident fracture outcomes
| Observed RRa | True RRb | Total Fracture | Non-Vertebral Fracture | Hip Fracture |
|---|---|---|---|---|
| 0.90 | 0.875 | 84.0 | 78.8 | 21.4 |
| 0.85 | 0.812 | 99.4 | 98.7 | 42.5 |
| 0.80 | 0.750 | >99.9 | >99.9 | 66.7 |
| 0.75 | 0.687 | >99.9 | >99.9 | 85.8 |
| 0.70 | 0.625 | >99.9 | >99.9 | 95.8 |
| 0.65 | 0.560 | >99.9 | >99.9 | 99.2 |
| 0.60 | 0.500 | >99.9 | >99.9 | 99.9 |
RR, rate ratio.
Observed RR = intent-to-treat RR, including noncompliant participants (compliance assumed to be 80%).
True RR = that with perfect compliance.
Power to determine the relationship between vitamin D and BMD, vBMD, and BTMs within the VITAL-Bone Structure/Architecture is based on measurements taken at baseline and 2-year follow-up among randomized participants. Power computations compare mean changes in randomized intervention groups in an intent-to-treat analysis with a two-sided test with a significance level of 0.05. Due to anticipated loss to follow-up during the trial, we assume that approximately 90% of participants will have follow-up measures, with additional calculations for 80% follow-up. To incorporate imperfect compliance, power is computed for the anticipated observed effect as well as estimated true effect under perfect compliance assuming observed compliance to study interventions is 80%.
Power was computed based on a trial of changes in BMD among 389 men and women, randomized to vitamin D with calcium, or placebo.64 In that study, the average percent change over 3 years in the placebo group was −1.09 (+/− 1.71) for total body, −0.70 (+/− 5.03) for FN, and +1.22 (+/− 4.25) for spinal BMD. Assuming the same standard deviation for change in our sub-study of 600 with 10% loss to follow-up, we would have over 80% power to detect net differences between groups of 1.22% for FN, 1.03% for lumbar spine, and 0.42% for total BMD. Assuming 20% loss to follow-up, we would have 80% power to detect differences between groups of 1.29% for FN, 1.09% for spine, and 0.44% for total BMD. In the previous trial, treatment effects of D plus calcium were 1.20, 0.90, and 1.15 % for FN, spine, and total body BMD, respectively.64 The largest decreases were seen in initial year of treatment. We thus have excellent power to detect differences between groups, especially in total BMD, even if the effect over 2 years is less than that seen over the 3 years in the prior study.
3. Discussion
The VITAL-Bone Health studies are testing the effects of daily, high-dose, supplemental vitamin D alone on fracture incidence and bone health measures. These studies have several strengths. VITAL-Fracture tests whether daily, high-dose supplemental vitamin D is effective in the primary prevention of fractures in a large, randomized cohort of 25,875 older adults, both women and men. The study cohort is geographically diverse, with representation from all 50 U.S. states. Minority participation is also high, with approximately 26% minority enrollment and an oversampling of blacks (n=5,108).49 Most RCTs to date that have tested supplemental vitamin D and fractures have predominantly included whites and data on the effects in minorities or in men are limited. VITAL-Fracture is therefore uniquely able to test the effects of high-dose, supplemental vitamin D on fractures in whites and minorities, men and women. In addition, the trial is testing an intervention of daily, high dose vitamin D alone. No calcium is included in the supplement. Currently, data from large RCTs on the effects of daily supplemental vitamin D, without calcium, on fracture outcomes are sparse and inconclusive.18, 28, 34, 65 VITAL-Bone Health is thus responsive to the “need for research protocols that examine the effects of vitamin D and calcium separately” noted by the IOM.50, 66
In the parallel CTSC component of the trial, VITAL-Bone Structure/Architecture, we are performing in-depth phenotyping and detailed bone health measures in a subcohort of participants from the New England area. While high-dose, supplemental vitamin D is widely used to support bone health, data are limited about its effects alone on bone density, structure, architecture, and turnover, which are important for bone strength. This study will elucidate the role of supplemental vitamin D in skeletal health through complementary assessments of fracture risk and bone remodeling and structure among the large VITAL cohort. Additionally, we achieve significant cost-efficiency by leveraging resources from the parent VITAL, its CTSC component, and Harvard Catalyst. These include a biorepository of baseline blood samples; validated blood assays of total 25(OH)D; measurements of PTH, calcium, and albumin; high compliance as measured with blood samples in a subset of participants; a database built from detailed questionnaires that will be a rich resource with information on medical conditions, treatments and lifestyle variables; and in-depth, in-person assessments of bone health measures from participants in the CTSC subcohort.
An additional strength is the testing the effects on fracture incidence and bone health measures of daily, supplemental omega-3 FAs. Results from nested case-control studies and observational studies such as WHI and the Framingham Osteoporosis study indicate that omega-3 FAs may have benefits on fractures.46, 47 While these data are promising, completion of the VITAL-Bone Health studies is novel as it will be the first study to test effects of supplemental ω-3 FAs EPA+DHA on fracture risk and bone health measures in a placebo-controlled trial of men and women.
The two studies discussed in this paper are ancillary studies to the VITAL trial. Ancillary studies are conducted in conjunction with a parent study, usually a larger, ongoing clinical trial. Ancillary studies enhance the scientific impact of a clinical trial by leveraging resources to address important research questions that were not included in the parent trial. For example, ancillary studies make it possible to test new hypotheses and evaluate additional clinical outcomes and biological mechanisms. Ancillary study procedures may, however, be limited by the parent trial structure. However, this is outweighed by leveraging the availability of a large, well-characterized cohort such as the VITAL study.
There are also a few limitations to these studies. VITAL is testing only one dose of the intervention. To achieve the optimal balance between efficacy and safety, after comprehensive review of the available literature, a high dose of vitamin D was chosen for this primary prevention trial in the general population.31 Some clinical trials and subsequent meta-analyses have concluded that daily doses >800 IU/d of supplemental vitamin D alone are needed to promote skeletal health.25, 29 An additional limitation is that the findings will not be generalizable to younger populations. The VITAL cohort includes older adults who are at higher risk for osteoporotic fractures and vitamin D deficiency. Obese and overweight individuals are at elevated risk due to vitamin D sequestration in fat and increased volume distribution. Currently, the IOM, in accordance with a comprehensive review, recommends 600 to 800 IU/d for most of the adult population.50 However, other large organizations, such as the National Osteoporosis Foundation (NOF) and the International Osteoporosis Foundation (IOF) recommend that adults aged ≥50 may need 800 to 1000 IU/d. The Endocrine Society Task Force recommends “at least 1500 to 2000 IU/d of supplemental vitamin D,” and doses 2 to 3 times higher in obese patients.67-72 Black individuals are also at increased risk for vitamin D deficiency due to lower vitamin D intakes and also because darkly pigmented skin generates less vitamin D in response to UVB radiation.4, 73 Paradoxically, while blacks are more often characterized as vitamin D deficient according to total 25(OH)D levels, they have higher BMD and lower fracture risk as compared to whites.74 Recently, some studies have reported that, in contrast to total 25(OH)D levels, bioavailable (free and albumin-bound fraction) vitamin D and free 25(OH)D are more strongly correlated with BMD.74-76 Results from these cross-sectional, observational studies highlight gaps in our understanding of the relationship between total, bioavailable, and free 25(OH)D levels. Data from RCTs are needed to determine these relationships, their influence on fracture outcomes, and whether there is variation according to BMI and race/ethnicity.
With the increasing use of vitamin D and omega-3 fatty acid supplements, it is important to evaluate the safety of these nutritional agents and their efficacy in promoting skeletal health. The VITAL-Bone Health ancillaries are uniquely positioned to answer open questions and determine whether daily, high-dose, supplemental vitamin D prevents fractures and has beneficial effects on bone health outcomes. Results from these studies are expected to clarify the role of high-dose, supplemental vitamin D on skeletal health and inform clinical and public health guidelines regarding its use to prevent fractures and maintain bone density, structure, and strength.
Acknowledgements
The ancillary studies VITAL: Effects on Fractures and VITAL: Effects on Structure and Architecture are supported by the grants R01AR060574 and R01AR59775, respectively, from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The parent trial is supported by grant U01CA138962. Consultants to the VITAL: Effects on Fractures trial include Douglas C. Bauer, Peggy M. Cawthon, and Dennis M. Black. Members of the VITAL Data and Safety Monitoring Board include Lawrence S. Cohen, Theodore Colton, Mark A. Espeland, I. Craig Henderson, Alice H. Lichtenstein, Rebecca A. Silliman, and Nanette K. Wenger (chair), and Josephine Boyington, Cindy D. Davis, Rebecca B. Costello, Lawrence Fine, and Peter Greenwald (ex-officio members). We would also like to acknowledge Georgina Friedenberg and Joseph Walter for their roles in the VITAL-Fractures study, and Joel S. Finkelstein and Mary L. Bouxsein for their roles in the VITAL-Structure/Architecture study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was conducted with support from Harvard Catalyst -- the Harvard Clinical and Translational Science Center (NCRR and NCATS, NIH Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Meryl S. LeBoff, Dept. of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, 02115.
Amy Y. Yue, Division of Endocrinology, Diabetes and Hypertension, Department of Medicine, Brigham and Women's Hospital, Boston, MA 02115 AYUE@PARTNERS.ORG.
Trisha Copeland, Division of Preventive Medicine, Department of Medicine, Brigham and Women's Hospital, 900 Commonwealth Avenue, Boston, MA 02215 PCOPELAND2@PARTNERS.ORG.
Nancy R. Cook, Division of Preventive Medicine, Department of Medicine, Brigham and Women's Hospital, 900 Commonwealth Avenue, Boston, MA 02215 NCOOK@PARTNERS.ORG.
Julie E. Buring, Division of Preventive Medicine, Department of Medicine, Brigham and Women's Hospital, 900 Commonwealth Avenue, Boston, MA 02215 JBURING@PARTNERS.ORG.
JoAnn E. Manson, Division of Preventive Medicine, Department of Medicine, Brigham and Women's Hospital, 900 Commonwealth Avenue, Boston, MA 02215 JMANSON@PARTNERS.ORG.
References
- 1.Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, Dawson-Hughes B. The Recent Prevalence of Osteoporosis and Low Bone Mass in the United States Based on Bone Mineral Density at the Femoral Neck or Lumbar Spine. J Bone Miner Res. 2014;29(11):2520–2526. doi: 10.1002/jbmr.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, Sempos CT. Vitamin D status: United States, 2001-2006. NCHS Data Brief. 2011(59):1–8. Epub 2011/05/20. PubMed PMID: 21592422. [PubMed] [Google Scholar]
- 3.MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985;76(4):1536–8. doi: 10.1172/JCI112134. Epub 1985/10/01. doi: 10.1172/JCI112134. PubMed PMID: 2997282; PubMed Central PMCID: PMC424123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris SS. Vitamin D and African Americans. J Nutr. 2006;136(4):1126–9. doi: 10.1093/jn/136.4.1126. PubMed PMID: 16549493. [DOI] [PubMed] [Google Scholar]
- 5.Harris SS, Dawson-Hughes B. Reduced sun exposure does not explain the inverse association of 25-hydroxyvitamin D with percent body fat in older adults. J Clin Endocrinol Metab. 2007;92(8):3155–7. doi: 10.1210/jc.2007-0722. Epub 2007/05/31. doi: jc.2007-0722 [pii] 10.1210/jc.2007-0722. PubMed PMID: 17535990. [DOI] [PubMed] [Google Scholar]
- 6.Barrett-Connor E, Laughlin GA, Li H, Nielson CM, Wang PY, Dam TT, Cauley JA, Ensrud KE, Stefanick ML, Lau E, Hoffman AR, Orwoll ES. Osteoporotic Fractures in Men Research G. The association of concurrent vitamin D and sex hormone deficiency with bone loss and fracture risk in older men: the osteoporotic fractures in men (MrOS) study. J Bone Miner Res. 2012;27(11):2306–13. doi: 10.1002/jbmr.1697. doi: 10.1002/jbmr.1697. PubMed PMID: 22777902; PubMed Central PMCID: PMC3474871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rouzi A, Al-Sibiani S, Al-Senani N, Radaddi R, Ardawi M. Independent predictors of all osteoporosis-related fractures among healthy Saudi postmenopausal women: the CEOR Study. Bone. 2012;50(3):713–22. doi: 10.1016/j.bone.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 8.De Boer I, Levin G, Robinson-Cohen C, Biggs M, Hoofnagle A, Siscovick D, Kestenbaum B. Serum 25-hydroxyvitamin D concentration and risk for major clinical disease events in a community-based population of older adults: a cohort study. Ann Intern Med. 2012;156(9):627–34. doi: 10.1059/0003-4819-156-9-201205010-00004. PubMed Central PMCID: PMC22547472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson-Cohen C, Katz R, Hoofnagle AN, Cauley JA, Furberg CD, Robbins JA, Chen Z, Siscovick DS, de Boer IH, Kestenbaum B. Mineral metabolism markers and the long-term risk of hip fracture: the cardiovascular health study. J Clin Endocrinol Metab. 2011;96(7):2186–93. doi: 10.1210/jc.2010-2878. doi: 10.1210/jc.2010-2878. PubMed PMID: 21508146; PubMed Central PMCID: PMC3135189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cauley JA, Danielson ME, Boudreau R, Barbour KE, Horwitz MJ, Bauer DC, Ensrud KE, Manson JE, Wactawski-Wende J, Shikany JM, Jackson RD. Serum 25-hydroxyvitamin D and clinical fracture risk in a multiethnic cohort of women: The women's health initiative (WHI). Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26(10):2378–88. doi: 10.1002/jbmr.449. Epub 2011/09/29. doi: 10.1002/jbmr.449. PubMed PMID: 21710614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Looker AC, Mussolino ME. Serum 25-hydroxyvitamin D and hip fracture risk in older U.S. white adults. J Bone Miner Res. 2008;23(1):143–50. doi: 10.1359/jbmr.071003. Epub 2007/10/03. doi: 10.1359/jbmr.071003. PubMed PMID: 17907920. [DOI] [PubMed] [Google Scholar]
- 12.Cauley JA, Lacroix AZ, Wu L, Horwitz M, Danielson ME, Bauer DC, Lee JS, Jackson RD, Robbins JA, Wu C, Stanczyk FZ, LeBoff MS, Wactawski-Wende J, Sarto G, Ockene J, Cummings SR. Serum 25-hydroxyvitamin D concentrations and risk for hip fractures. Ann Intern Med. 2008;149(4):242–50. doi: 10.7326/0003-4819-149-4-200808190-00005. PubMed PMID: 18711154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bailey RL, Dodd KW, Goldman JA, Gahche JJ, Dwyer JT, Moshfegh AJ, Sempos CT, Picciano MF. Estimation of Total Usual Calcium and Vitamin D Intakes in the United States. The Journal of Nutrition. 2010;140(4):817–22. doi: 10.3945/jn.109.118539. doi: 10.3945/jn.109.118539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, Delmas PD, Meunier PJ. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327(23):1637–42. doi: 10.1056/NEJM199212033272305. PubMed PMID: 1331788. [DOI] [PubMed] [Google Scholar]
- 15.Chapuy MC, Pamphile R, Paris E, Kempf C, Schlichting M, Arnaud S, Garnero P, Meunier PJ. Combined calcium and vitamin D3 supplementation in elderly women: confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: the Decalyos II study. Osteoporos Int. 2002;13(3):257–64. doi: 10.1007/s001980200023. PubMed PMID: 11991447. [DOI] [PubMed] [Google Scholar]
- 16.Prentice RL, Pettinger MB, Jackson RD, Wactawski-Wende J, Lacroix AZ, Anderson GL, Chlebowski RT, Manson JE, Van Horn L, Vitolins MZ, Datta M, Leblanc ES, Cauley JA, Rossouw JE. Health risks and benefits from calcium and vitamin D supplementation: Women's Health Initiative clinical trial and cohort study. Osteoporos Int. 2013;24(2):567–80. doi: 10.1007/s00198-012-2224-2. doi: 10.1007/s00198-012-2224-2. PubMed PMID: 23208074; PubMed Central PMCID: PMC3557387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsen ER, Mosekilde L, Foldspang A. Vitamin D and calcium supplementation prevents osteoporotic fractures in elderly community dwelling residents: a pragmatic population-based 3-year intervention study. J Bone Miner Res. 2004;19(3):370–8. doi: 10.1359/JBMR.0301240. doi: 10.1359/JBMR.0301240. PubMed PMID: 15040824. [DOI] [PubMed] [Google Scholar]
- 18.Lips P, Graafmans W, Ooms M, Bezemer P, Bouter L. Vitamin D Supplementation and Fracture Incidence in Elderly Persons: A Randomized, Placebo-Controlled Clinical Trial. Ann Intern Med. 1996;124(4):400–6. doi: 10.7326/0003-4819-124-4-199602150-00003. [DOI] [PubMed] [Google Scholar]
- 19.Meyer HE, Smedshaug GB, Kvaavik E, Falch JA, Tverdal A, Pedersen JI. Can vitamin D supplementation reduce the risk of fracture in the elderly? A randomized controlled trial. J Bone Miner Res. 2002;17(4):709–15. doi: 10.1359/jbmr.2002.17.4.709. Epub 2002/03/29. doi: 10.1359/jbmr.2002.17.4.709. PubMed PMID: 11918228. [DOI] [PubMed] [Google Scholar]
- 20.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. Bmj. 2003;326(7387):469. doi: 10.1136/bmj.326.7387.469. PubMed PMID: 12609940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. Jama. 2005;293(18):2257–64. doi: 10.1001/jama.293.18.2257. PubMed PMID: 15886381. [DOI] [PubMed] [Google Scholar]
- 22.Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, Bassford T, Beresford SA, Black HR, Blanchette P, Bonds DE, Brunner RL, Brzyski RG, Caan B, Cauley JA, Chlebowski RT, Cummings SR, Granek I, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Johnson KC, Judd H, Kotchen JM, Kuller LH, Langer RD, Lasser NL, Limacher MC, Ludlam S, Manson JE, Margolis KL, McGowan J, Ockene JK, O'Sullivan MJ, Phillips L, Prentice RL, Sarto GE, Stefanick ML, Van Horn L, Wactawski-Wende J, Whitlock E, Anderson GL, Assaf AR, Barad D. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669–83. doi: 10.1056/NEJMoa055218. PubMed PMID: 16481635. [DOI] [PubMed] [Google Scholar]
- 23.Boonen S, Lips P, Bouillon R, Bischoff-Ferrari HA, Vanderschueren D, Haentjens P. Need for additional calcium to reduce the risk of hip fracture with vitamin d supplementation: evidence from a comparative metaanalysis of randomized controlled trials. J Clin Endocrinol Metab. 2007;92(4):1415–23. doi: 10.1210/jc.2006-1404. Epub 2007/02/01. doi: jc.2006-1404 [pii] 10.1210/jc.2006-1404. PubMed PMID: 17264183. [DOI] [PubMed] [Google Scholar]
- 24.Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet. 2007;370(9588):657–66. doi: 10.1016/S0140-6736(07)61342-7. Epub 2007/08/28. doi: S0140-6736(07)61342-7 [pii] 10.1016/S0140-6736(07)61342-7. PubMed PMID: 17720017. [DOI] [PubMed] [Google Scholar]
- 25.Bischoff-Ferrari HA, Willett WC, Wong JB, Stuck AE, Staehelin HB, Orav EJ, Thoma A, Kiel DP, Henschkowski J. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med. 2009;169(6):551–61. doi: 10.1001/archinternmed.2008.600. Epub 2009/03/25. doi: 169/6/551 [pii] 10.1001/archinternmed.2008.600 [doi]. PubMed PMID: 19307517. [DOI] [PubMed] [Google Scholar]
- 26.Avenell A, Mak JC, O'Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;4:CD000227. doi: 10.1002/14651858.CD000227.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DIPART Patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials in US and Europe. Bmj. 2010;340:b5463. doi: 10.1136/bmj.b5463. Epub 2010/01/14. doi: 10.1136/bmj.b5463 bmj.b5463 [pii]. PubMed PMID: 20068257; PubMed Central PMCID: PMC2806633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung M, Lee J, Terasawa T, Lau J, Trikalinos TA. Vitamin D with or without calcium supplementation for prevention of cancer and fractures: an updated meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155(12):827–38. doi: 10.7326/0003-4819-155-12-201112200-00005. doi: 10.7326/0003-4819-155-12-201112200-00005. PubMed PMID: 22184690. [DOI] [PubMed] [Google Scholar]
- 29.Bischoff-Ferrari HA, Willett WC, Orav EJ, Lips P, Meunier PJ, Lyons RA, Flicker L, Wark J, Jackson RD, Cauley JA, Meyer HE, Pfeifer M, Sanders KM, Stahelin HB, Theiler R, Dawson-Hughes B. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012;367(1):40–9. doi: 10.1056/NEJMoa1109617. Epub 2012/07/06. doi: 10.1056/NEJMoa1109617. PubMed PMID: 22762317. [DOI] [PubMed] [Google Scholar]
- 30.Moyer VA, on behalf of the USPSTF Vitamin D and Calcium Supplementation to Prevent Fractures in Adults: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2013;158(9):691–6. doi: 10.7326/0003-4819-158-9-201305070-00603. doi: 10.7326/0003-4819-158-9-201305070-00603. PubMed PMID: 23440163. [DOI] [PubMed] [Google Scholar]
- 31.Newberry S, Shekelle P, Booth MS, Liu J, Maher A, Motala A, Perry T, Shanman R, Chung M, Balk E. Vitamin D and Calcium: A Systematic Review of Health Outcome (Update). Agency for Healthcare Research and Quality (AHRC) 2014 doi: 10.23970/AHRQEPCERTA217. [DOI] [PubMed] [Google Scholar]
- 32.Reid IR, Bolland MJ, Grey A. Effects of vitamin D supplements on bone mineral density: a systematic review and meta-analysis. Lancet. 2014;383(9912):146–155. doi: 10.1016/S0140-6736(13)61647-5. [DOI] [PubMed] [Google Scholar]
- 33.Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035. doi: 10.1136/bmj.g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanders K, Stuart A, Williamson E, Simpson J, Kotowicz M, Young D, Nicholson G. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303(18):1815–22. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 35.Sun D, Krishnan A, Zaman K, Lawrence R, Bhattacharya A, Fernandes G. Dietary n-3 fatty acids decrease osteoclastogenesis and loss of bone mass in ovariectomized mice. J Bone Miner Res. 2003;18(7):1206–16. doi: 10.1359/jbmr.2003.18.7.1206. Epub 2003/07/12. PubMed PMID: 12854830. [DOI] [PubMed] [Google Scholar]
- 36.Matsushita H, Barrios JA, Shea JE, Miller SC. Dietary fish oil results in a greater bone mass and bone formation indices in aged ovariectomized rats. J Bone Miner Metab. 2008;26(3):241–7. doi: 10.1007/s00774-007-0815-3. Epub 2008/05/13. doi: 10.1007/s00774-007-0815-3 [doi]. PubMed PMID: 18470664. [DOI] [PubMed] [Google Scholar]
- 37.Bonnet N, Ferrari S. New Frontiers in Skeletal Research: Bone, Fat, and Brain Connections. Bethesda, MD: Apr 27-28, 2009. A long-term diet enriched in omega-3 fatty acids improves cortical bone structure and mechanical properties in mice. Abstract#M47. [Google Scholar]
- 38.Salari P, Rezaie A, Larijani B, Abdollahi M. A systematic review of the impact of n-3 fatty acids in bone health and osteoporosis. Med Sci Monit. 2008;14(3):RA37–44. Epub 2008/02/28. doi: 836571 [pii]. PubMed PMID: 18301367. [PubMed] [Google Scholar]
- 39.Sakaguchi K, Morita I, Murota S. Eicosapentaenoic acid inhibits bone loss due to ovariectomy in rats. Prostaglandins Leukot Essent Fatty Acids. 1994;50(2):81–4. doi: 10.1016/0952-3278(94)90151-1. Epub 1994/02/01. PubMed PMID: 8171071. [DOI] [PubMed] [Google Scholar]
- 40.Yamada Y, Fushimi H, Inoue T, Matsuyama Y, Kameyama M, Minami T, Okazaki Y, Noguchi Y, Kasama T. Effect of eicosapentaenoic acid and docosahexaenoic acid on diabetic osteopenia. Diabetes Res Clin Pract. 1995;30(1):37–42. doi: 10.1016/0168-8227(95)01139-0. Epub 1995/10/01. doi: 0168822795011390 [pii]. PubMed PMID: 8745204. [DOI] [PubMed] [Google Scholar]
- 41.Reinwald S, Li Y, Moriguchi T, Salem N, Jr., Watkins BA. Repletion with (n-3) fatty acids reverses bone structural deficits in (n-3)-deficient rats. J Nutr. 2004;134(2):388–94. doi: 10.1093/jn/134.2.388. Epub 2004/01/30. PubMed PMID: 14747677. [DOI] [PubMed] [Google Scholar]
- 42.Watkins BA, Li Y, Lippman HE, Feng S. Modulatory effect of omega-3 polyunsaturated fatty acids on osteoblast function and bone metabolism. Prostaglandins Leukot Essent Fatty Acids. 2003;68(6):387–98. doi: 10.1016/s0952-3278(03)00063-2. Epub 2003/06/12. doi: S0952327803000632 [pii]. PubMed PMID: 12798659. [DOI] [PubMed] [Google Scholar]
- 43.Watkins BA, Li Y, Lippman HE, Seifert MF. Omega-3 polyunsaturated fatty acids and skeletal health. Exp Biol Med (Maywood) 2001;226(6):485–97. doi: 10.1177/153537020122600601. Epub 2001/06/09. PubMed PMID: 11395919. [DOI] [PubMed] [Google Scholar]
- 44.Weiss LA, Barrett-Connor E, von Muhlen D. Ratio of n-6 to n-3 fatty acids and bone mineral density in older adults: the Rancho Bernardo Study. Am J Clin Nutr. 2005;81(4):934–8. doi: 10.1093/ajcn/81.4.934. Epub 2005/04/09. doi: 81/4/934 [pii]. PubMed PMID: 15817874. [DOI] [PubMed] [Google Scholar]
- 45.Van Papendrop DH, Coetzer H, Kruger MG. Biochemical profile of osteoporotic patients on essential fatty acid supplementation. Nutr Res. 1995;(15):325–34. [Google Scholar]
- 46.Farina EK, Kiel DP, Roubenoff R, Schaefer EJ, Cupples LA, Tucker KL. Dietary Intakes of Arachidonic Acid and {alpha}-Linolenic Acid Are Associated with Reduced Risk of Hip Fracture in Older Adults. The Journal of nutrition. 2011;141(6):1146–53. doi: 10.3945/jn.110.133728. Epub 2011/04/22. doi: 10.3945/jn.110.133728. PubMed PMID: 21508210; PubMed Central PMCID: PMC3095142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Orchard TS, Ing SW, Lu B, Belury MA, Johnson K, Wactawski-Wende J, Jackson RD. The association of red blood cell n-3 and n-6 fatty acids with bone mineral density and hip fracture risk in the women's health initiative. J Bone Miner Res. 2013;28(3):505–15. doi: 10.1002/jbmr.1772. Epub 2012/09/29. doi: 10.1002/jbmr.1772. PubMed PMID: 23018646; PubMed Central PMCID: PMCPMC3785326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farina EK, Kiel DP, Roubenoff R, Schaefer EJ, Cupples LA, Tucker KL. Protective effects of fish intake and interactive effects of long-chain polyunsaturated fatty acid intakes on hip bone mineral density in older adults: the Framingham Osteoporosis Study. The American journal of clinical nutrition. 2011;93(5):1142–51. doi: 10.3945/ajcn.110.005926. Epub 2011/03/04. doi: 10.3945/ajcn.110.005926. PubMed PMID: 21367955; PubMed Central PMCID: PMC3076660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manson JE, Bassuk SS, Lee IM, Cook NR, Albert MA, Gordon D, Zaharris E, Macfadyen JG, Danielson E, Lin J, Zhang SM, Buring JE. The VITamin D and OmegA-3 TriaL (VITAL): Rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2011 doi: 10.1016/j.cct.2011.09.009. Epub 2011/10/12. doi: S1551-7144(11)00245-X [pii] 10.1016/j.cct.2011.09.009. PubMed PMID: 21986389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ross AC, Taylor CL, Yaktine AL, Del Valle HB. Dietary reference intakes for calcium and vitamin D. National Academies Press; 2011. Institute of Medicine. http://www.iom.edu/vitaminD. [PubMed] [Google Scholar]
- 51.Rinaldi G, Wisniewski CA, Setty NG, Leboff MS. Peripheral quantitative computed tomography: optimization of reproducibility measures of bone density, geometry, and strength at the radius and tibia. J Clin Densitom. 2011;14(3):367–73. doi: 10.1016/j.jocd.2011.05.002. Epub 2011/07/05. doi: S1094-6950(11)00114-4 [pii] 10.1016/j.jocd.2011.05.002. PubMed PMID: 21723765. [DOI] [PubMed] [Google Scholar]
- 52.Hans D, Goertzen AL, Krieg MA, Leslie WD. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res. 2011;26(11):2762–9. doi: 10.1002/jbmr.499. doi: 10.1002/jbmr.499. PubMed PMID: 21887701. [DOI] [PubMed] [Google Scholar]
- 53.Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL. Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity (Silver Spring) 2012;20(5):1109–14. doi: 10.1038/oby.2011.367. Epub 2012/01/14. doi: 10.1038/oby.2011.367. PubMed PMID: 22240726; PubMed Central PMCID: PMCPMC3343346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, Ferrucci L, Guralnik JM, Fragala MS, Kenny AM, Kiel DP, Kritchevsky SB, Shardell MD, Dam TT, Vassileva MT. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69(5):547–58. doi: 10.1093/gerona/glu010. Epub 2014/04/17. doi: 10.1093/gerona/glu010. PubMed PMID: 24737557; PubMed Central PMCID: PMCPMC3991146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, Studenski S, Berkman LF, Wallace RB. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–31. doi: 10.1093/gerona/55.4.m221. Epub 2000/05/16. PubMed PMID: 10811152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.LeBoff MS, Shoback D, Brown EM, Thatcher J, Leombruno R, Beaudoin D, Henry M, Wilson R, Pallotta J, Marynick S, et al. Regulation of parathyroid hormone release and cytosolic calcium by extracellular calcium in dispersed and cultured bovine and pathological human parathyroid cells. J Clin Invest. 1985;75(1):49–57. doi: 10.1172/JCI111696. Epub 1985/01/01. doi: 10.1172/JCI111696. PubMed PMID: 3965511; PubMed Central PMCID: PMC423397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.LeBoff MS, Kohlmeier L, Hurwitz S, Franklin J, Wright J, Glowacki J. Occult vitamin D deficiency in postmenopausal US women with acute hip fracture. Jama. 1999;281(16):1505–11. doi: 10.1001/jama.281.16.1505. PubMed PMID: 10227320. [DOI] [PubMed] [Google Scholar]
- 58.Haden ST, Fuleihan GE, Angell JE, Cotran NM, LeBoff MS. Calcidiol and PTH levels in women attending an osteoporosis program. Calcif Tissue Int. 1999;64(4):275–9. doi: 10.1007/s002239900618. PubMed PMID: 10089217. [DOI] [PubMed] [Google Scholar]
- 59.Brent GA, LeBoff MS, Seely EW, Conlin PR, Brown EM. Relationship between the concentration and rate of change of calcium and serum intact parathyroid hormone levels in normal humans. J Clin Endocrinol Metab. 1988;67(5):944–50. doi: 10.1210/jcem-67-5-944. Epub 1988/11/01. PubMed PMID: 3141451. [DOI] [PubMed] [Google Scholar]
- 60.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359(9319):1761–7. doi: 10.1016/S0140-6736(02)08657-9. Epub 2002/06/07. doi: S0140-6736(02)08657-9 [pii] 10.1016/S0140-6736(02)08657-9. PubMed PMID: 12049882. [DOI] [PubMed] [Google Scholar]
- 61.Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE, Cauley J, Black D, Vogt TM. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332(12):767–73. doi: 10.1056/NEJM199503233321202. PubMed PMID: 7862179. [DOI] [PubMed] [Google Scholar]
- 62.Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569–85. doi: 10.1016/j.cct.2005.05.006. Epub 2005/08/09. doi: S1551-7144(05)00107-2 [pii] 10.1016/j.cct.2005.05.006. PubMed PMID: 16084776. [DOI] [PubMed] [Google Scholar]
- 63.Freedman LS. Tables of the number of patients required in clinical trials using the logrank test. Stat Med. 1982;1(2):121–9. doi: 10.1002/sim.4780010204. PubMed PMID: 7187087. [DOI] [PubMed] [Google Scholar]
- 64.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337(10):670–6. doi: 10.1056/NEJM199709043371003. PubMed PMID: 9278463. [DOI] [PubMed] [Google Scholar]
- 65.Pietrogrande L, Raimondo E, Fossali A, Zaolino C. Biological and pharmacological factors influencing the fracture healing. Aging Clin Exp Res. 2011;23(2 Suppl):65–8. Epub 2011/10/06. doi: 7956 [pii]. PubMed PMID: 21970928. [PubMed] [Google Scholar]
- 66.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D from the Institute of Medicine: What Clinicians Need to Know. J Clin Endocrinol Metab. 2011;96(1):53–8. doi: 10.1210/jc.2010-2704. Epub 01/2011. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dawson-Hughes B. A revised clinician's guide to the prevention and treatment of osteoporosis. J Clin Endocrinol Metab. 2008;93(7):2463–5. doi: 10.1210/jc.2008-0926. PubMed PMID: 18544615. [DOI] [PubMed] [Google Scholar]
- 68.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465–75. doi: 10.1359/jbmr.061113. PubMed PMID: 17144789. [DOI] [PubMed] [Google Scholar]
- 69.Chun RF, Adams JS, Hewison M. Back to the future: a new look at ‘old’ vitamin D. J Endocrinol. 2008;198(2):261–9. doi: 10.1677/JOE-08-0170. Epub 2008/05/23. doi: JOE-08-0170 [pii] 10.1677/JOE-08-0170. PubMed PMID: 18495944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dawson-Hughes B, Mithal A, Bonjour JP, Boonen S, Burckhardt P, Fuleihan GE, Josse RG, Lips P, Morales-Torres J, Yoshimura N. IOF position statement: vitamin D recommendations for older adults. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2010;21(7):1151–4. doi: 10.1007/s00198-010-1285-3. Epub 2010/04/28. doi: 10.1007/s00198-010-1285-3. PubMed PMID: 20422154. [DOI] [PubMed] [Google Scholar]
- 71.Cosman F, Lindsay R, LeBoff MS, de Beur SJ, Tanner B. Clinician's Guide to Prevention and Treatment of Osteoporosis. National Osteoporosis Foundation; Washington, DC: 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM, Endocrine S. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30. doi: 10.1210/jc.2011-0385. doi: 10.1210/jc.2011-0385. PubMed PMID: 21646368. [DOI] [PubMed] [Google Scholar]
- 73.Moore CE, Murphy MM, Holick MF. Vitamin D intakes by children and adults in the United States differ among ethnic groups. J Nutr. 2005;135(10):2478–85. doi: 10.1093/jn/135.10.2478. PubMed PMID: 16177216. [DOI] [PubMed] [Google Scholar]
- 74.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D, Bhan I, Karumanchi SA, Powe NR, Thadhani R. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. New England Journal of Medicine. 2013;369(21):1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Powe CE, Ricciardi C, Berg AH, Erdenesanaa D, Collerone G, Ankers E, Wenger J, Karumanchi SA, Thadhani R, Bhan I. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J Bone Miner Res. 2011;26(7):1609–16. doi: 10.1002/jbmr.387. doi: 10.1002/jbmr.387. PubMed PMID: 21416506; PubMed Central PMCID: PMC3351032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bhan I, Powe CE, Berg AH, Ankers E, Wenger JB, Karumanchi SA, Thadhani RI. Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney Int. 2012;82(1):84–9. doi: 10.1038/ki.2012.19. Epub 2012/03/09. doi: 10.1038/ki.2012.19. PubMed PMID: 22398410; PubMed Central PMCID: PMC3376220. [DOI] [PMC free article] [PubMed] [Google Scholar]