Abstract
Objective
We examined rapid response among obese patients with binge-eating disorder (BED) in a randomized clinical trial testing anti-obesity medication and self-help cognitive-behavioral therapy (shCBT), alone and in combination, in primary-care settings.
Method
104 obese patients with BED were randomly assigned to one of four treatments: sibutramine, placebo, shCBT+sibutramine, or shCBT+placebo. Treatments were delivered by generalist primary-care physicians and the medications were given double-blind. Independent assessments were performed by trained and monitored doctoral research-clinicians monthly throughout treatment, post-treatment (4 months), and at 6- and 12-month follow-ups (i.e., 16 months after randomization). Rapid response, defined as ≥65% reduction in binge-eating by the fourth treatment week, was used to predict outcomes.
Results
Rapid response characterized 47% of patients. Rapid response was unrelated to demographic and baseline clinical characteristics. Rapid response was significantly associated prospectively with remission from binge eating at post-treatment (51% versus 9% for non-rapid responders), 6-month (53% vs 23.6%), and 12-month (46.9% vs 23.6%) follow-ups. Mixed effects model analyses revealed rapid response was significantly associated with greater decreases in binge-eating, eating-disorder psychopathology, depression, and percent weight loss.
Discussion
Our findings, based on a diverse obese patient group receiving medication and self-help CBT treatments for BED in primary care settings, indicate that patients who have a rapid response achieve good clinical outcomes through 12-month follow-ups after ending treatments. Rapid response represents a strong prognostic indicator of clinically meaningful outcomes even in low intensity medication and self-help interventions. Rapid response has important clinical implications for stepped-care treatment models for BED.
Clinical Trial Registration
clinicaltrials.gov: NCT00537810
Keywords: binge eating disorder, obesity, eating disorders, primary care, weight loss, self-help, medication
Binge-eating disorder (BED), a formal eating-disorder diagnosis in DSM-5 (APA, 2013), is defined by recurrent binge eating, marked distress about binge eating, and the absence of extreme weight compensatory behaviors. BED is prevalent and is associated strongly with obesity and biopsychosicial problems (APA, 2013). Although some psychological and medication treatments have varying levels of effectiveness for BED, many patients fail to achieve remission from binge-eating and most fail to achieve significant weight loss (Reas & Grilo, 2014). Finding reliable predictors of treatment response could inform treatment prescriptions but this has been challenging (Grilo, Masheb, & Crosby, 2012).
Rapid response (i.e., substantial improvements in symptoms during the early weeks of treatment) has been found to significantly predict treatment outcomes across diverse psychiatric problems, including medication and CBT treatments for depression (Taylor, Freemantle, Geddes, & Bhagwagar, 2006; Hardy et al., 2005) and bulimia nervosa (Sysko et al., 2010; Wilson et al., 2002). In a series of four studies, Grilo et al. (Grilo, Masheb, & Wilson, 2006; Grilo & Masheb, 2007; Grilo, White, Wilson, Gueorguieva, & Masheb, 2012; Masheb & Grilo, 2007) extended the rapid response findings to BED in several ways. First, the definition of rapid response was informed empirically using receiver operating characteristic (ROC) curves. These methods yielded “reliable” findings across studies that 65%–70% reductions in binge-eating by the fourth treatment week optimally predicted remission. Second, rapid response predicted significantly greater reductions in eating-disorder pathology in all four studies and greater weight loss in three studies (Grilo et al., 2006; Grilo & Masheb, 2007; Grilo et al., 2012). Third, rapid response was unrelated to nearly all baseline characteristics in the four studies suggesting rapid responders are not just “easy” patients nor do they show individual differences in demographic or clinical severity. Fourth, rapid response had varied prognostic significance across different treatments for BED (Grilo et al., 2006; 2012). Finally, the longer-term prognostic significance of rapid response to treatment for BED was established in the one study with follow-up (Grilo et al., 2012).
Further research on rapid response is needed to establish longer-term significance and to extend findings to additional interventions (e.g., scalable treatments such as “self-help” CBT (shCBT) (Wilson & Zandberg, 2012)) and to broader health care settings with more diverse patient groups. One study with depression found that “sudden gains” with CBT had less predictive significance in routine clinical settings than in specialist settings (Hardy et al., 2005). Members of minority groups with BED receive most of their health care from primary care (Marques et al., 2011) and it is uncertain whether “effective” treatments delivered by specialists are as effective when delivered by generalists. The present study examined rapid response among patients with BED participating in a treatment study in primary care settings (serving racially and ethnically diverse persons) testing anti-obesity medication (sibutramine (Wilfley et al., 2008)) and shCBT, alone and in combination. We examine whether rapid response was related to patient characteristics and to outcomes through 12-months of follow-up after completing treatments.
Methods
Participants
Participants were 104 obese patients with BED in a randomized double-blind, placebo-controlled trial in primary care testing shCBT and sibutramine, alone and in combination (Grilo et al., 2014)1. Participants had a mean age of 43.9 years (SD = 11.2), mean BMI of 38.3 (SD = 5.6), 70.2% (N=73) were female; 45.2% (N=47) were Caucasian, 34.6% (N=36) African-American, 13.5% (N=14) Hispanic-American, and 6.7% (N=7) from “other groups.” Participants provided written informed consent and the study had IRB approval.
Assessments and Repeated Measures
Diagnostic and repeated assessment procedures were performed by trained and monitored doctoral-level research-clinicians2. BED and DSM-IV-TR psychiatric diagnoses were based on the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I/P; First, Spitzer, Gibbon, & Williams, 1996). Eating Disorder Examination Interview (EDE; Fairburn & Cooper 1993) was given at baseline, post-treatment, and 6- and 12-month follow-ups. The EDE, a semi-structured interview, assesses the frequency of objective bulimic episodes (OBE; i.e., binge-eating) and yields a total global score reflecting severity. The EDE interview has good validity and test-retest reliability in BED (Grilo et al., 2004)3. Eating Disorder Examination-Questionnaire (EDE-Q; Fairburn & Beglin, 1994), the self-report EDE version, was given at baseline, monthly during treatment, and post-treatment and follow-ups. The EDE-Q obtained change data during treatment and was used to determine rapid response. The EDE-Q converges adequately with the EDE and has good test-retest reliability in BED (Reas, Grilo, & Masheb, 2006). Beck Depression Inventory (BDI; Beck & Steer, 1987) is a well-established (Beck, Steer, & Garbin, 1998) self-report measure of depression symptoms. The BDI was administered at baseline, bi-monthly during treatment, and post-treatment and follow-ups. Weight and height were measured at baseline and weight was measured monthly during treatment and at post-treatment and follow-ups using a large capacity digital scale. BMI was calculated from these measurements.
Randomization to Treatments and Maintaining Treatment Blindness
Randomization4 was to one of four treatments following a balanced 2-by-2 factorial design for 16 weeks: (1) sibutramine (15mg/day); (2) placebo; (3) shCBT plus sibutramine (15mg/day); or shCBT plus placebo. Double-blind medication status was not broken until after post-treatment when participants were notified; however, procedures maintained the blind for investigators and evaluators until after all participants completed all 12-month follow-ups. Assessments were performed independently by doctoral evaluators at our research clinic who were blinded to both the medication status and to whether participants received the shCBT.
Treatment Conditions: Self-Help Cognitive-Behavioral Therapy (shCBT)
Half the patients were randomized to shCBT and were given Overcoming Binge Eating (Fairburn, 1995), a self-help book used in numerous controlled trials (Wilson & Zandberg, 2012). Primary care physicians, without training in mental health or BED, instructed patients to read the book and focus on the self-help program in Part II. Medication (Sibutramine or Placebo). Patients were randomized to receive either sibutramine or placebo in matching capsules. Sibutramine was given at a 15 mg per day fixed-dose (Wilfley et al., 2008). Physicians provided medication and educated patients about sibutramine, including possible effects on eating/weight and potential side-effects, and instructed patients to contact them if they had concerns or side effects. These minimal contact procedures reflect medication management in “real-world” primary care clinics.
Statistical Analyses
Participants classified with and without rapid response5 were compared on demographic and baseline clinical variables with chi-square analyses for categorical variables and ANOVAs for continuous measures. Analyses examining outcomes by rapid response status were performed for all randomized participants using complementary approaches for binge-eating. First, “remission” from binge-eating (zero binges (OBEs) during past month based on EDE interview) was defined separately at post-treatment and 6- and 12-month follow-ups. For treatment dropouts and any missing data, failure to remit was imputed. Binge-eating remission rates between rapid and non-rapid responders were compared using chi-square analyses. Second, mixed models (SAS PROC MIXED), using all available data without imputation, compared rapid and non-rapid-responders on two measures of binge-eating frequency: EDE interview (baseline, post-treatment, and 6- and 12-month follow-ups) and EDE-Q (baseline, monthly during treatment, post-treatment, and 6- and 12-month follow-ups). Mixed models compared rapid and non-rapid responders on continuous measures of eating-disorder pathology (EDE and EDE-Q global scores), depression (BDI scores monthly during treatment, post-treatment, and 6- and 12-month follow-ups), and percent weight loss (monthly during treatment, post-treatment, and 6- and 12-month follow-ups). For each of these variables, a mixed-model was fitted with rapid response status (rapid, non-rapid), shCBT (yes, no), medication (sibutramine, placebo), session (available assessments throughout study and follow-ups), and all possible interactions. Distributions of data were examined and transformations applied as necessary (binge-eating frequency data were log-transformed). For each model, different variance-covariance structures were evaluated and the best-fitting structure was selected based on the Schwartz Bayesian criterion (BIC).
Results
Rapid Response and Patient Characteristics
Of the 104 patients randomized, 49 (47.1%) showed a rapid response, defined as 65% or greater reduction in binge-eating by the fourth treatment week. Rapid and non-rapid responders did not differ significantly in demographic variables, psychiatric co-morbidity, binge-eating, depression, or BMI (Table 1); non-rapid responders had higher EDE global scores, accounting for only 5% of variance explained.
Table 1.
Demographic and clinical characteristics of participants with versus without rapid response.
| Rapid Response | No Rapid Response | Test Statistic | P value | Effect size | |
|---|---|---|---|---|---|
| N=49 | N=55 | ||||
| Age, mean (SD) | 43.6 (10.7) | 44.2 (11.8) | F(1,102) = 0.08 | .78 | .001 |
| Female, No (%) | 32 (65.3) | 41 (74.5) | χ2(1) = 1.06 | .30 | .101 |
| Ethnicity/Race, No (%) | χ2(3) = 3.61 | .31 | .186 | ||
| Caucasian | 21 (42.9) | 24 (43.6) | |||
| African-American | 20 (40.8) | 15 (27.3) | |||
| Hispanic-American | 5 (10.2) | 12 (21.8) | |||
| Other | 3 (6.1) | 4 (7.3) | |||
| Education, No (%) | χ2(2) = 2.79 | .25 | .164 | ||
| College | 20 (40.8) | 19 (34.5) | |||
| Some college | 20 (40.8) | 18 (32.7) | |||
| High School or less | 9 (18.4) | 18 (32.7) | |||
| DSM-IV diagnoses, lifetime, No (%) | |||||
| Mood disorders | 21 (42.9) | 27 (49.1) | χ2(1) = 0.41 | .52 | .062 |
| Anxiety disorders | 14 (28.6) | 24 (43.6) | χ2(1) = 2.54 | .11 | .156 |
| Substance use disorders | 13 (26.5) | 11 (20.0) | χ2(1) = 0.62 | .43 | .077 |
| Age onset BED, mean (SD) | 26.6 (12.7) | 25.9 (12.4) | F(1,102) = 0.07 | .79 | .001 |
| Clinical characteristics, mean (SD) | |||||
| Binge-eating episodes/month (EDE) | 17.8 (16.4) | 19.6 (16.6) | F(1,102) = 0.28 | .60 | .003 |
| Global Score (EDE) | 2.29 (0.92) | 2.70 (0.88) | F(1,102) = 5.43 | .02 | .051 |
| Body mass index | 38.4 (5.1) | 38.1 (6.0) | F(1,102) = 0.08 | .78 | .001 |
| Depression (BDI) | 12.4 (7.4) | 16.0 (11.2) | F(1,102) = 3.69 | .06 | .035 |
Note: Test statistic = chi-square for categorical variables and F value from ANOVAs for dimensional variables. P values are for two-tailed tests. SD = standard deviation. No = number. Effect size measures are phi coefficients for categorical variables and partial eta-squared for dimensional variables. BED = binge eating disorder. EDE = Eating Disorder Examination interview. BDI = Beck Depression Inventory
Rapid Response and Binge-eating Remission Outcomes
Overall, 47.1% (N=49/104) of participants had rapid response and the following overall rates of binge-eating remission were observed: 28.8% (N=30/104) at post-treatment, 37.5% (N=39/104) at 6-month follow-up, and 34.6% (N=36/104) at 12-month follow-up. Participants with rapid response were significantly more likely than non-rapid responders to achieve remission at each time point (at post-treatment (51.0% (N=25/49) vs. 9.1% (N=5/55); χ2(1)=22.20, p<0.001 (phi coefficient = 0.462)); 6-month follow-up, (53.1% (N=26/49) vs. 23.6% (N=13/55); χ2(1)=9.57, p<0.002 (phi coefficient = 0.303); and 12-month follow-up, (46.9% (N=23/49) vs. 23.6% (N=13/55); χ2(1)=6.22, p=0.01 (phi coefficient = 0.244)) and achieve sustained remission across follow-ups (42.9% (21/49) vs. 16.4% (9/55); χ2(1)=8.86, p=0.003 (phi coefficient = 0.292).
Rapid Response and Time Course of Binge-Eating Frequency
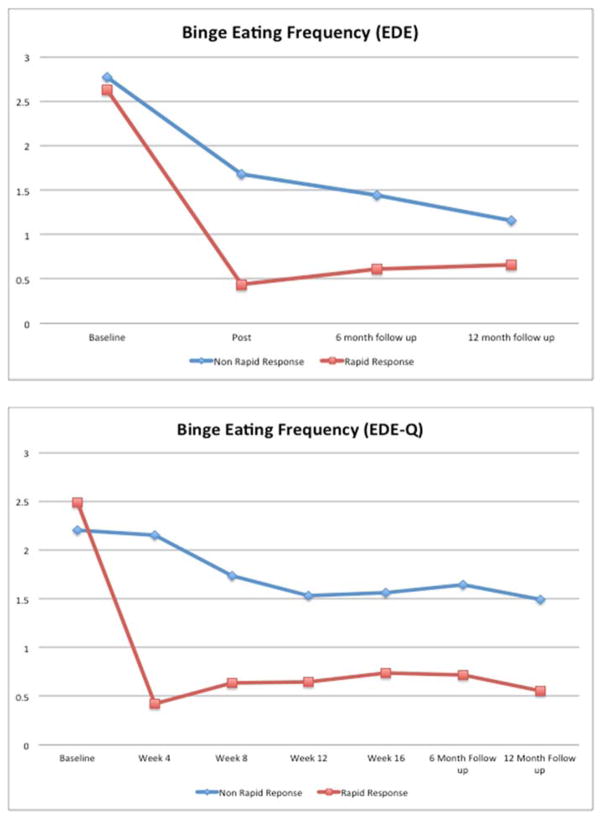
Figure 1 shows weekly frequency of binge-eating by rapid response status at major assessments based on the EDE (top Figure) and at all assessments based on the EDE-Q (bottom Figure). For EDE binge-eating frequency, mixed models revealed significant main effects for time F(3,239)=98.10, p<0.0001) and for rapid response (F(1,116)=20.77, p<0.0001) indicating significant improvements over time and significantly better outcomes for rapid responders. A significant interaction between rapid response and time (F(3,239)=9.38, p<0.0001) and a significant interaction between rapid response, medication, and session ((F(3,239)=2.92, p=0.03) were observed. Post-hoc tests revealed significant mean differences for rapid and non-rapid responders at all post-treatment time-points: post-treatment (F(1,276)=34.77, p<0.0001), 6-month follow-up (F(1,267)=15.15, p=0.0001), and 12-month follow-up (F(1,277)=4.69, p=0.03). Rapid and non-rapid responders taking sibutramine did not differ significantly at 12-month follow-up (F(1,290)=0.08, p=0.77).
Figure 1. Frequency of Binge-eating Over Time by Rapid Response Status.
Frequency of binge-eating (per week) by participants with rapid response versus without rapid response over time based on two complementary assessments. The top figure shows binge-eating frequency during the major assessment points through the 12-month follow-up. The bottom figure shows binge-eating frequency based on the Eating Disorder Examination – Questionnaire monthly during the course of treatment and at 6- and 12-month post-treatment follow-up assessments. The data shown are based on estimated marginal means (derived from mixed models analyses of log transformed binge-eating data) for all N=104 participants.
For EDE-Q binge-eating frequency data, mixed models revealed significant main effects of time F(6,391)=24.93, p<0.0001) and rapid response (F(1,128)=45.88, p<0.0001) indicating significant improvements over time and significantly better outcomes for rapid responders. A significant interaction between rapid response and time (F(6,391)=16.25, p<0.0001) was found; post-hoc tests revealed significant mean differences between rapid and non-rapid responders at all post-baseline time-points (all p<0.0001).
Rapid Response and Eating-disorder Pathology
Mixed models revealed significant main effects for time F(3,239)=29.08, p<0.0001) and rapid response (F(1,102)=22.02, p<0.0001) for EDE global score indicating significant improvements over time and significantly better outcome for rapid responders. A significant interaction between rapid response and time (F(3,229)=3.06, p=0.03) was observed. Post-hoc tests revealed significant mean differences between rapid and non-rapid responders at all post-treatment time-points: post-treatment (F(1,199)=20.74, p<0.0001), 6-month (F(1,200)=15.77, p<0.0001), and 12-month follow-up (F(1,219)=17.03, p=0.03). Mixed models on EDE-Q global score revealed the same pattern of significance: significant main effects of time F(6,427)=10.13, p<0.0001) and rapid response (F(1,120)=23.91, p<0.0001), a significant interaction between rapid response and time (F(6,427)=4.07, p=0.0006), and posthoc tests revealing significant mean differences between the rapid and non-rapid responders at all post-baseline time points throughout treatment and follow-ups (all p<.001).
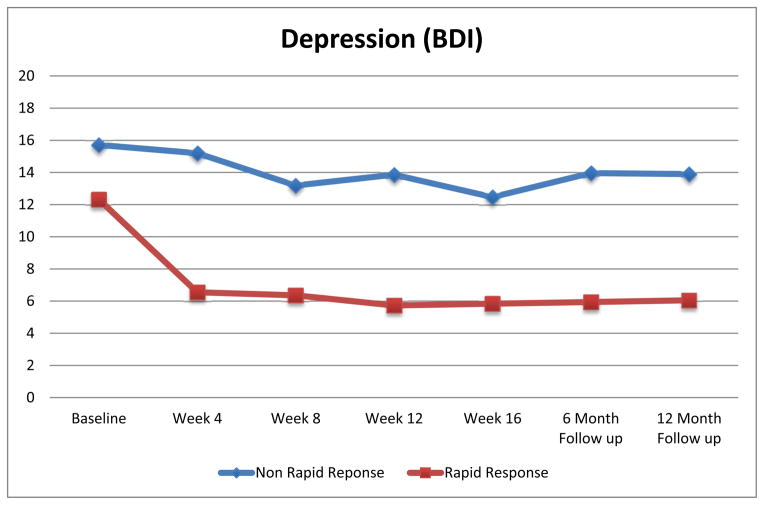
Rapid Response and Depression
Figure 2 shows depression scores by rapid response status. Mixed models revealed significant main effects of time F(6,461)=10.46, p<0.0001) and rapid response (F(1,94.9)=20.04, p<0.0001) for depression indicating significant improvements over time and better outcome for rapid responders. Significant interaction between rapid response and time (F(6,461)=3.07, p=0.01) was found; post-hoc tests revealed significant mean differences for rapid and non-rapid responders at all post-baseline time-points (all p<0.001).
Figure 2. Depression (Beck Depression Inventory (BDI) Scores) by Rapid Response Status.
Depression (Beck Depression Inventory [BDI]) scores for participants with rapid response versus without rapid response monthly during the course of treatment and at 6- and 12-month post-treatment follow-up assessments. The data shown are based on estimated marginal means (derived from mixed models analyses) for all N=104 participants.
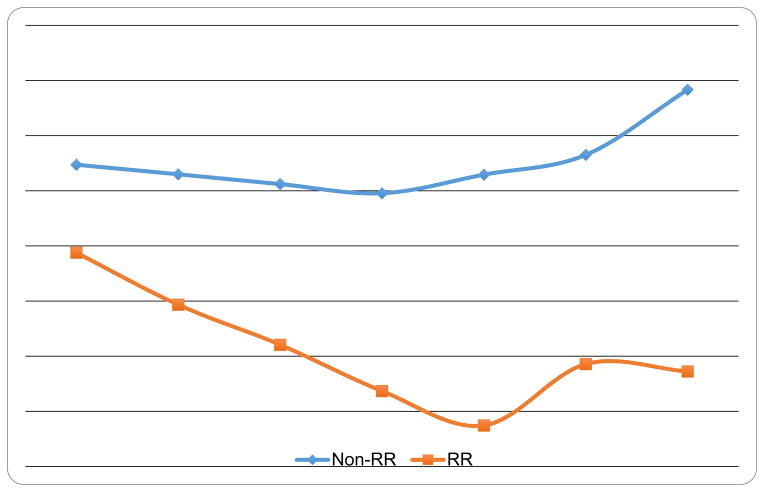
Rapid Response and Percent Weight Loss Outcomes
Figure 3 shows percent weight loss by rapid response status. Mixed models revealed significant main effects of time F(6,178)=4.58, p=0.0002) and rapid response (F(1,96.1)=14.20, p=0.0003) indicating significant weight losses over time and significantly better outcomes for rapid responders. A significant interaction between rapid response and time (F(6,178)=2.59, p=0.02) was found; post-hoc tests revealed significant mean differences between rapid and non-rapid responders on percent weight loss at all post-baseline time-points: all p<0.002 for monthly comparisons during treatment, (F(1,99.3)=18.18, p<0.0001) at post-treatment, (F(1,95.4)=5.25, p=0.024) at 6-month follow-up, and at 12-month follow-up (F(1,97.5)=5.79, p=0.018).
Figure 3. Percent Weight Loss by Rapid Response Status.
Percent weight by participants with rapid response versus without rapid response monthly during the course of treatment and at 6- and 12-month post-treatment follow-up assessments. The data shown are based on estimated marginal means (derived from mixed models analyses) for all N=104 participants.
Discussion
Rapid response, defined as a 65% or greater reduction in binge eating by the fourth week of treatment, characterized 47% of patients of obese patients with BED participating in a RCT testing anti-obesity medication and shCBT in primary care. Rapid response was unrelated to patients’ demographic and baseline clinical characteristics but was significantly and robustly associated prospectively with remission from binge eating, greater decreases in binge-eating frequency, eating-disorder pathology, depression, and greater percent weight loss through 12-month follow-ups. Thus, rapid response represents a strong prognostic indicator of clinically meaningful outcomes even in low intensity medication and self-help interventions and has important clinical implications for stepped-care treatment models for BED.
The findings that roughly half of patients with BED show rapid response which, despite being unrelated to pretreatment patient factors, robustly predicts treatment outcomes extend the findings from our four previous studies with BED to generalist primary care settings. Findings provide further support for the longer-term prognostic significance of rapid response through 12-month follow-up after treatment discontinuation. The higher remission rates at 12-month follow-up for rapid than non-rapid responders (46.9% vs 23.6%) were quite similar to those reported by Grilo et al. (2012) (58.3% vs 23.1%) in a study comparing CBT and behavioral treatment delivered by specialist clinicians. These findings suggest rapid response is a reliable process prospectively associated with positive and durable outcomes through 12-months after treatment.
These findings have practical implications for stepped-care models for treating BED. Rapid response is fairly straightforward to assess and holds considerable clinical appeal (“face-validity”). Patients can be told that they will start a treatment and after four weeks they will be re-evaluated for either continuing the treatment or switching to an alternative treatment if progress is not being achieved. This basic clinical strategy would serve to make both parties in the “clinician-patient relationship” accountable and would provide a logical tool to facilitate ongoing clinical interactions. In instances of rapid response, this evaluation would serve to reinforce patient progress. In instances of non-rapid response, this evaluation could foster discussion of difficulties and potential treatment alternatives while still early in the treatment process before frustration builds and further time is spent suffering. These findings have practical implications treating BED in primary care. While the overall RCT findings (Grilo et al., 2014) suggested that these “low intensity” treatments for BED in primary care did not show long-term effectiveness relative to placebo, the present findings suggest that such treatments might still serve as initial interventions in a stepped-care approach. Rapid response occurs in a substantial subgroup of patients BED who subsequently achieve good outcomes. Patients who fail to show rapid response could be offered more intensive treatment or referral to specialized treatments.
Several potential limitations and strengths are noteworthy. Sibutramine has since been withdrawn from the market because of safety concerns. Although sibutramine is no longer available, the findings have heuristic and clinical value. Methodologically, sibutramine is a credible comparison condition. Clinically, although we do not know whether our findings would generalize to other medications, the findings are consistent with a growing list of medications for which an early rapid response represents a good prognostic sign whereas failure to respond quickly signals the need to consider other medications in studies with BED (Grilo et al., 2006), bulimia nervosa (Sysko et al., 2010), and depression (Taylor et al., 2006). Our findings may not generalize to obese patients with BED with co-existing severe medical problems. The study’s rigorous assessment protocol characterized patients and their outcomes through 12-months after finishing treatment; this is one of the few medication studies with BED with follow-up (Reas & Grilo, 2014). This study was performed in primary care settings and enrolled a diverse patient group with broad generalizability (sex, race/ethnicity, education). Our findings indicate rapid response is a robust prognostic indicator of good treatment outcomes through 16 months and has important implications for stepped-care treatment models for BED.
Public Health Significance.
When treating individuals with binge eating disorder who also have excess weight in primary care, this study demonstrated the importance of an early rapid response to treatment. Individuals who responded quickly to initial treatments achieved good clinical outcomes that were well maintained for a year after finishing treatments.
Acknowledgments
This research was supported by National Institutes of Health grants R01 DK073542 and K24 DK070052 (Dr. Grilo). Drs. Grilo, White, Masheb, and Gueorguieva report no commercial or financial interests that are either relevant or discussed in this paper or which might pose a conflict of interest. Dr. Grilo reports that he serves as an advisory board member for Shire, has received consulting fees from Shire, royalties from Guilford Press and Taylor and Francis Press for academic books, honoraria for CME educational activities, honoraria from the American Psychological Association for editorial contributions, and honoraria for grand rounds presentations at universities and talks at scientific conferences.
Footnotes
Participants were recruited via flyers and referrals in primary care at a large medical health-care center for a treatment study for BED. Inclusion criteria were age 18 to 65 years, BMI ≥ 30 and < 50, and DSM-5 criteria for BED. Exclusion criteria were current use of antidepressants or any medication that influences eating/weight, severe psychiatric (schizophrenia, bipolar disorder, substance use disorder) or medical (cardiac disease, liver disease) problems, and uncontrolled hypertension, thyroid disease, or diabetes.
Assessments were successfully obtained for 84% of participants at post-treatment, for 83% of participants at the 6-month follow-up, and for 86% of participants at the 12-month follow-up. Chi-square analyses revealed no significant differences or trends in assessment rates across the different treatments at any time point (χ2(3)=2.61, p=0.46 at post-treatment; χ2(3)=1.87, p=0.60 at 6-month follow-up; and χ2(3)=2.43, p=0.49 at 12-month follow-up).
In the present study, inter-rater reliability for the EDE interview was excellent. A total of 34 taped interviews, selected randomly and representing different assessment time-points, were rated resulting in the following intra-class correlation coefficients (ICC): 0.83 for OBE episodes, 0.90 for OBE days, and 0.93 for EDE global score.
Randomization was performed by a research-pharmacist independently from the investigators using a computer-generated schedule created by a biostatistician.
Rapid response was defined, as in previous studies with BED (Grilo et al., 2006; Grilo & Masheb, 2007; Masheb & Grilo, 2007; Grilo et al., 2013; Safer & Joyce, 2011) as 65% or greater reduction in binge-eating frequency during the first month of treatment. Percent reduction in binge-eating was based on the EDE-Q given at baseline and again one month later. We chose to follow this definition from the previous studies which was determined using ROC curve analyses. We note, however, that in the present study, the ROC curve constructed with the percentage reduction from baseline to week four yielded Area Under Curve (AUC) of 0.760 (SE = 0.056); 95% confidence interval [CI] = .650 – .871, p < .001 for the null hypothesis that true area = 0.5. Inspection of this ROC curve revealed that a reduction of 65% in binge eating by the fourth week maximized sensitivity and 1-specificity (0.88 and 0.49, respectively) thus supporting our definition of rapid response in the present study. These values are strikingly similar to those of the previous studies; for example, Grilo et al (2006) reported AUC = 0.772 (95% CI = .68 – .86) for binge reduction at week four with a 65% reduction maximizing sensitivity and 1-specificity (0.70 and 0.34, respectively).
Contributor Information
Dr Carlos M. Grilo, Department of Psychiatry, Yale University School of Medicine.
Dr Marney A. White, Department of Psychiatry, Yale University School of Medicine.
Dr Robin M. Masheb, Department of Psychiatry, Yale University School of Medicine.
Dr Ralitza Gueorguieva, Department of Biostatistics, Yale University School of Public Health.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: American Psychiatric Association; 2013. (DSM-5) [Google Scholar]
- Beck AT, Steer R, Garbin M. Psychometric properties of the Beck Depression Inventory: 25 years of evaluation. Clinical Psychology Review. 1998;8:77–100. [Google Scholar]
- Beck A, Steer R. Manual for revised Beck Depression Inventory. NY: Psychol Corp; 1987. [Google Scholar]
- Fairburn C. Overcoming binge eating. New York: Guilford Press; 1995. [Google Scholar]
- Fairburn C, Cooper Z. Eating Disorder Examination. In: Fairburn C, Wilson GT, editors. Binge eating: nature, assessment, and treatment. NY: Guilford Press; 1993. pp. 317–60. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I disorders-patient Edition (SCID-I/P) New York: NYSPI; 1996. [Google Scholar]
- Grilo CM, Masheb RM. Rapid response predicts binge eating and weight loss in binge eating disorder: findings from a controlled trial of orlistat with guided self-help cognitive behavioral therapy. Behaviour Research and Therapy. 2007;45:2537–2550. doi: 10.1016/j.brat.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilo CM, Masheb RM, Crosby RD. Predictors and moderators of response to cognitive behavioral therapy and medication for the treatment of binge eating disorder. Journal of Consulting and Clinical Psychology. 2012;80:897–906. doi: 10.1037/a0027001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilo CM, Masheb RM, Lozano-Blanco C, Barry DT. Reliability of the Eating Disorder Examination in patients with binge eating disorder. International Journal of Eating Disorders. 2004;35:80–85. doi: 10.1002/eat.10238. [DOI] [PubMed] [Google Scholar]
- Grilo CM, Masheb RM, White MA, Gueorguieva R, Barnes RD, Walsh BT, Garcia R. Treatment of binge eating disorder in racially and ethnically diverse obese patients in primary care: randomized placebo-controlled trial of self-help and medication. Behaviour Research and Therapy. 2014;58:1–9. doi: 10.1016/j.brat.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilo CM, Masheb RM, Wilson GT. Rapid response to treatment for binge eating disorder. Journal of Consulting and Clinical Psychology. 2006;74:602–613. doi: 10.1037/0022-006X.74.3.602. [DOI] [PubMed] [Google Scholar]
- Grilo CM, White MA, Wilson GT, Gueorguieva R, Masheb RM. Rapid response predicts 12-month post-treatment outcomes in binge eating disorder: theoretical and clinical implications. Psychological Medicine. 2012;42:807–817. doi: 10.1017/S0033291711001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy G, Cahill J, Stiles W, Ispan C, Macaskill N, Barkham M. Sudden gains in cognitive therapy for depression. Journal Consulting Clinical Psychology. 2005;73:59–67. doi: 10.1037/0022-006X.73.1.59. [DOI] [PubMed] [Google Scholar]
- Marques L, Alegria M, Becker AE, Chen CN, Fang A, Chosak A, Diniz JB. Comparative prevalence, correlates of impairment, and service utilization for eating disorders across US ethnic groups. International Journal Eating Disorders. 2011;44:412–420. doi: 10.1002/eat.20787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masheb RM, Grilo CM. Rapid response predicts treatment outcomes in binge eating disorder. Journal of Consulting and Clinical Psychology. 2007;75:639–644. doi: 10.1037/0022-006X.75.4.639. [DOI] [PubMed] [Google Scholar]
- Reas DL, Grilo CM. Current and emerging drug treatments for binge eating disorder. Expert Opinion on Emerging Drugs. 2014;19:99–142. doi: 10.1517/14728214.2014.879291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reas D, Grilo CM, Masheb R. Reliability of the Eating Disorder Examination- Q in patients with binge eating disorder. Behaviour Research and Therapy. 2006;44:43–51. doi: 10.1016/j.brat.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Sysko R, Sha N, Wang Y, Duan N, Walsh BT. Early response to antidepressant treatment in bulimia nervosa. Psychological Medicine. 2010;40:999–1005. doi: 10.1017/S0033291709991218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M, Freemantle N, Geddes J, Bhagwagar Z. Early onset of selective serotonin reuptake inhibitor antidepressant action. Archives of General Psychiatry. 2006;63:1217–1723. doi: 10.1001/archpsyc.63.11.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilfley DE, Crow SJ, Hudson JI, Mitchell JE, Berkowitz RI, Blakesley Sibutramine BED Group. Efficacy of sibutramine for the treatment of binge eating disorder. American Journal of Psychiatry. 2008;165:51–58. doi: 10.1176/appi.ajp.2007.06121970. [DOI] [PubMed] [Google Scholar]
- Wilson GT, Fairburn CG, Agras WS, Walsh BT, Kraemer H. Cognitive-behavioral therapy for bulimia nervosa: Time course and mechanisms of change. Journal of Consulting and Clinical Psychology. 2002;70:267–274. [PubMed] [Google Scholar]
- Wilson GT, Zandberg LJ. Cognitive-behavioral guided self-help for eating disorders: effectiveness and scalability. Clinical Psychology Review. 2012;32:343–357. doi: 10.1016/j.cpr.2012.03.001. [DOI] [PubMed] [Google Scholar]