Abstract
Introduction
Pain present 6 months following root canal treatment (RCT) may be either of odontogenic or nonodontogenic origin. This is importance because treatments and prognoses are different; therefore the aim of this study was to provide specific diagnoses of patients reporting pain 6 months after receiving initial orthograde RCT.
Methods
We enrolled patients from the Midwest region of an existing prospective observational study of pain after RCT. Pain at 6 months was defined as ≥1 day of pain and average pain intensity of at least 1/10 over the preceding month. An Endodontist and an Orofacial Pain practitioner independently performed clinical evaluations, which included periapical and cone-beam CT radiographs, to determine diagnoses.
Results
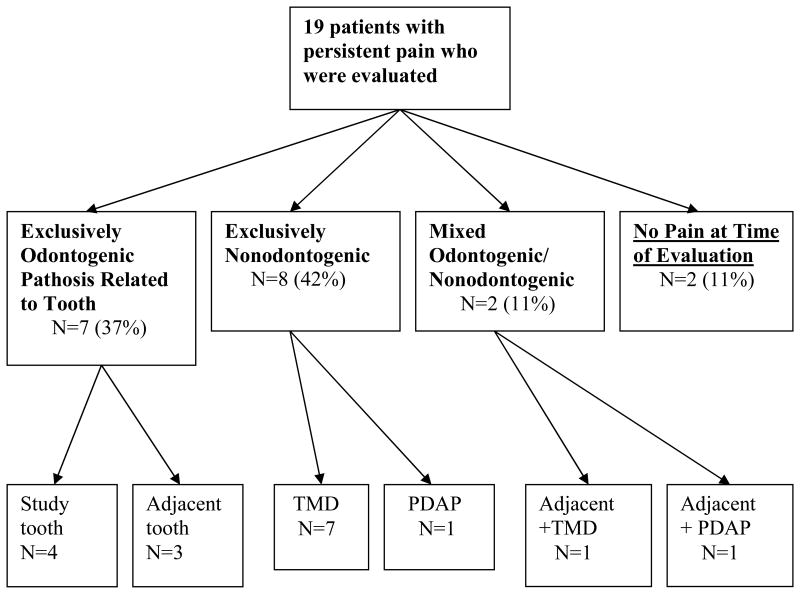
Thirty-eight out of the 354 eligible patients in the geographic area (11%) met the pain criteria, with 19 (50%) consenting to be clinically evaluated. As the sole reason for pain, 7 patients (37%) were given odontogenic diagnoses (4 involving the RCT tooth, 3 involving an adjacent tooth). Eight patients (42%) were given nonodontogenic pain diagnoses (7 from referred temporomandibular disorder (TMD) pain, 1 from persistent dentoalveolar pain disorder (PDAP)). Two patients (11%) had both odontogenic and nonodontogenic diagnoses, while 2 (11%) no longer fit the pain criteria at the time of the clinical evaluation.
Conclusion
Patients reporting “tooth” pain 6 months following RCT had a nonodontogenic pain diagnosis accounting for some of this pain, with TMD being the most frequent nonodonotgenic diagnosis. Dentists should have the necessary knowledge to differentiate between these diagnoses to adequately manage their patients.
Keywords: Root Canal Therapy, Tooth, Pain, Chronic, Temporomandibular Disorders, Diagnosis
Introduction
Approximately 20 million Americans receive root canal therapy (RCT) each year (1). Persistent pain after RCT is known to occur and is not an uncommon event, being estimated by meta-analysis to be 5.4% (2) and by prospective observation to be 10.0% at 6 months following RCT (3). Taxonomy of diagnoses underlying persistent pain after RCT can be broadly classified as either odontogenic (4) or nonodonogentic (5) in etiology. A previous meta-analysis found that 56% of all patients with pain present 6 months or more following RCT had a nonodontogenic etiology for this pain (6). This suggests that with the 10% occurrence rate of persistent pain, with half from nonodonotogenic etiology, that approximately 1 million Americans experience “tooth” pain and would not benefit from dental interventions, such as endodontic retreatment or tooth extraction.
While odontogenic sources for such pain are well described, information about nonodontogenic sources primarily resides in cross-sectional case-reports (7) and case-series (8, 9). While these reports provide information about the clinical features necessary to diagnose patients, they do not further our understanding of the prevalence of these conditions in dental clinic populations. Some studies have reported on the frequency of pain consistent with the diagnosis of Persistent Dentoalveolar Pain disorder (PDAP) following RCT (10, 11), as well as assessing the different diagnoses (12), no study has followed a cohort of patients of RCT patients with persistent pain to determine the various diagnoses underlying this pain. Furthermore, the retrospective nature of the systematic review that estimated about 50% of patients had nonodontogenic pain (6) allows only for a dichotomous outcome but does not provide information about the types of nonodontogenic diagnoses.
Therefore, to inform and improved clinical decision-making, it is necessary to recognize the differing diagnoses accounting for the symptom of pain in RCT patients. This study aimed to provide specific diagnoses of patients reporting pain 6 months after receiving initial orthograde RCT. Also, as a secondary aim, we reported the patient charateristics, as well as their clinical signs, symptoms, and imaging findings to describe how such patients may present to their dentist for evaluation.
Methods
Existing Parent Observational Cohort of RCT Patients
This study originated from a large-scale prospective longitudinal cohort study following patients that received RCT from dentists enrolled in the National Dental Practice-Based Research Network (13, 14). Sixty-two practitioner investigators in 5 geographic regions: Alabama/Mississippi, Florida/Georgia, Minnesota, Permanente Dental Associates in Oregon/Washington, and Scandinavia (Denmark and Sweden) were trained regarding the standardized study protocol. Enrollment and baseline data collection occurred over 6 months with follow up at 6 month after RCT. Patients and dentists completed questionnaires before and immediately after treatment visits. Patients also completed questionnaires at 1 week, 3 months, and 6 months after RCT. For more details of this parent study, see the publication of the Study Methods (15). Ethics approval was garnered from each institution involved in the parent study, as well as the nested study (University of Minnesota).
Selection criteria of the parent study
Inclusion criteria included: patients aged 19 to 70 years and patients with a permanent tooth requiring initial orthograde RCT. Exclusion criteria included; iatrogenic pulpal exposure (cases with carious exposure are included), previously enrolled in the parent study (each patient could only contribute 1 tooth to the study), previous endodontic treatment (previous treatment would make it unclear whether pain was associated with the prior treatment or attempt at treatment), obvious cognitive impairments (e.g., previous stroke with communication deficits, dementia or mental disability), the inability to read, understand, or complete the baseline patient questionnaire, and the anticipated inability to provide 6-month follow-up information.
Primary outcome measure of parent study
In the parent study, all enrolled patients were asked to complete a follow-up patient survey at 6 months following the obturation of the RCT treated tooth. The primary outcome measure of persistent pain at 6 months was defined by 2 questions: “How many days in the past month have you had pain in the area that was treated with a root canal?” and “In the past month, on the average, how intense was your tooth pain rated on a 0 to 10 scale where 0 is ‘no pain’ and 10 is ‘pain as bad as could be’?”. A positive response (≥1) to both questions was the criteria for persistent pain in this parent study. Patients that did not meet these criteria were defined as non-cases, which included patients providing discordant responses (e.g., patients that reported ≥1 having pain for more than one day in the past month but did not report ≥1 pain level).
Eligibility criteria and enrollment for nested study
Patients meeting the criteria for persistent pain in the parent study were eligible to enter this study. For feasibility reasons, namely local proximity, only patients within the Midwest region (Minnesota) of the network were considered for inclusion in this study so that patients could travel for evaluations to be held in one central location. Therefore, eligible patients were treated by one of the region's 33 dentists (7 endodontists, 26 general dentists) who had practices mostly in the Twin Cities area, but also in out state Minnesota and western Wisconsin.
Study setting and data collection protocol
This study was conducted in the Oral Health Clinical Research Center at the University of Minnesota School of Dentistry, Minneapolis, Minnesota. Patients were independently evaluated by a board certified Endodontist (ASL) and a board certified Orofacial Pain practitioner (DRN). Each practitioner performed a complete history and clinical examination independently, following accepted practices in each discipline, and reviewed the periapical and cone-beam CT (cbCT) radiographs that were obtained on all patients. Odontogenic diagnoses followed diagnostic criteria and terminology established for periapical/periradicular disease (16-18), while those for nonodontogenic diagnoses followed the orofacial pain criteria outlined in a current textbook (5). More specifically, the orofacial pain diagnoses were derived following the criteria for temporomandibular disorders (TMD) (19), neurovascular disorders (20), neuralgias (20), and PDAP (21). The final diagnoses were derived by consensus discussion between the two evaluators using all available data collected.
Data management and statistical analyses
The data were recorded on paper forms and entered in the database (Excel version 14.3.2 for Mac, Microsoft, Seattle, WA) with single entry and verification by another individual. Statistical analyses were performed using the same software (means and t-tests to describe continuous variables, proportions and chi-square tests for categorical variables) with associated 95% confidence intervals.
Results
The parent research study enrolled 390 patients in the Midwest region at baseline, and 354 (91%) returned data at 6 months, which comprised the study sample for this nested study. Of those 354 patients, 38 (11%, 95% CI: 8-14%) met criteria for pain at 6 months following RCT and were considered eligible cases, which was slightly higher than 10.0% observed over the entire (“parent”) study population (3). Only subjects that had consented to be contacted were invited to participate in this study. Nineteen of the 38 patients meeting persistent pain criteria (50%) agreed to participate in the nested study and were evaluated at the University of Minnesota. The average time from completion of the 6-month questionnaire to when the clinical evaluations were performed was 65 days (SD=41). The 19 patients not evaluated were found to be similar to those who were evaluated (Table 1).
Table 1. Baseline characteristics of patients meeting pain criteria at 6 months.
| Characteristics | Number of Cases Evaluated (19) N (%) or Mean (SD) |
Number of Cases not Evaluated (19) N (%) or Mean (SD) |
p-value (chi-square or t-test) |
|---|---|---|---|
|
| |||
| Gender | |||
| Female | 16 (84) | 15 (79) | 0.676 |
|
| |||
| Age | |||
| In years | 49 (13) | 41 (14) | 0.098 |
|
| |||
| Ethnicity | |||
| Non Hispanic or Latino | 19 (100) | 18 (95) | 0.311 |
|
| |||
| Race | |||
| White | 17 (89) | 15 (79) | 0.374 |
|
| |||
| Dental Insurance | |||
| Yes | 17 (89) | 15 (79) | 0.374 |
|
| |||
| Education | |||
| College degree | 13 (68) | 12 (63) | 0.732 |
|
| |||
| Income | |||
| >$50,000/year household | 13 (68) | 8 (42) | 0.103 |
|
| |||
| Arch | |||
| Maxillary | 10 (53) | 12 (63) | 0.511 |
|
| |||
| Tooth type | |||
| Posterior | 17 (89) | 16 (84) | 0.631 |
|
| |||
| Pain intensity, “now” | |||
| 0-10/10 | 2.3 (2.5) | 3.7 (3.4) | 0.148 |
|
| |||
| Days in pain over last week | |||
| 0-7/7 | 4.9 (2.7) | 5.2 (2.6) | 0.807 |
Patient and tooth characteristics
The mean age of patients with pain was 49 (SD±13) years old (Table 1). Most patients were White (89%), non-Hispanic or Latino (100%), female (84%), with dental insurance (89%). Maxillary teeth compromised 53% of treated teeth and 89% were posterior teeth. Soft tissue assessment of all teeth was within normal limits. Of the 19 root canal treated teeth, none responded to pulp testing, had mobility, or cracks detected. Sixteen (84%) teeth were restored with permanent crowns, with the remaining teeth were posterior teeth and restored with amalgam (n=2) and composite (n=1) materials. Most of the teeth (79%) showed no signs of periodontal disease with probing pocket depths ≤3mm. Only one tooth had significant periodontal bone loss at a probing depth of 6mm.
Experts' consensus diagnoses for persistent pain
Clinical evaluation of the 19 patients revealed that 7 (37%, 95% CI: 15-59%) had exclusively odontogenic reasons for their pain symptom, 8 (42%, 95% CI: 20-64%) had exclusively nonodontogenic reasons, 2 (11%, 95% CI: 0-24%) had mixed odontogenic/nondontogenic reasons, and 2 (11%, 95% CI: 0-24%) were pain free and considered normal at the time of evaluation (Figure 1).
Figure 1. Experts' consensus diagnoses for the patients' pain.
1. Exclusively odontogenic pain group
The odontogenic pain group comprised 7 patients and all 7 had an apical diagnosis of symptomatic apical periodontitis (SAP). The pulpal diagnoses were previously treated for the 4 study teeth and 1 adjacent tooth, while the pulpal diagnoses were irreversible pulpitis for the 2 other patients who had pain associated with an adjacent tooth. In those 4 patients with RCT study teeth associated pain, the etiologies were though to be related either to a missed mesiobuccal canal (2 teeth), C-shaped distal canal (1 tooth), and delayed healing associated with systemic lupus (1 tooth).
2. Exclusively nonodontogenic pain group
The nonodontogenic pain group comprised 8 patients, 7 diagnosed with TMD and 1 diagnosed with PDAP. None of the patients were diagnosed as having trigeminal neuralgia, a neurovascular disorder (e.g., migraine headache,) or distant pathosis referring to the dentoalveolar region and presenting as “tooth” pain.
3. Mixed odontogenic/nonodontogenic pain group
This group comprised 2 patients, 1 diagnosed with TMD and irreversible pulpitis of an adjacent tooth and the other diagnosed with PDAP and SAP secondary to necrotic pulp in an adjacent tooth.
4. No pain group
This group comprised 2 patients that presented without pain at the time of evaluation and neither an odontogenic nor a nonodontogenic pain diagnosis could be made.
Pain-related characteristics
Table 3 details the pain characteristics in relation to the different diagnoses. The majority of patients with an odontogenic reason for their persistent pain (N=4/7, 57%) reported a pain intensity of “0” at the time of their clinical evaluation for this research, with the average of this pain intensity being 0.6/10. For the most part, these patients described their pain as “well localized” with either “dull/achy” or “sharp” in quality. Also, almost half characterized their pain as “intermittent” and almost half as “constant”, with 1 patient not responding. The majority of patients with a nonodontogenic reason for their persistent pain (N=6/8, 75%) had a pain of mild-to moderate intensity with the average intensity being 1.5/10 at the time of the evaluation. “Dull” and “achy”, as well as “throbbing”, were the most used descriptions by these patients. There was a difference noted in the report of pain localization by classification, with 83% (N=5/6) of patients with an odontogenic diagnosis describing their pain as “well localized” versus only 25% (N=2/8) of patients with a nonodontogenic diagnosis using the same description.
Table 3. Physical findings related to persistent pain.
| Clinical signs | Exclusively Odontogenic (study & adjacent teeth) Number (%) |
Exclusively Nonodontogenic (TMD & PDAP) Number (%) |
Mixed (Odontogenic/Nonodontogenic) Number (%) |
Normal Number (%) |
|---|---|---|---|---|
|
| ||||
| Percussion testing, vertical | ||||
| Tender | 6/7 (86) | 5/8 (62) | 2/2 (100) | 0 |
| Nontender | 1/7 (14) | 3/8 (38) | 0 | 2/2 (100) |
|
| ||||
| Palpation testing (apical tissue, buccal to tooth) | ||||
| Tender | 1/7 (14) | 3/8 (38) | 0 | 0 |
| Nontender | 6/7 (86) | 5/8 (62) | 2/2 (100) | 2/2 (100) |
The majority of the persistent pain patients (63%) reported a history of chronic pain elsewhere in the body, including neck, shoulder, knee, ankle and pelvic pain, and one case of multiple sclerosis. Surprisingly, 75% of patients with nonodontogenic reasons for their pain, which was mainly TMD, reported no previous history of TMD diagnoses.
Physical findings related to pain
Clinical findings supporting an odontogenic diagnosis of persistent pain included responding positively to tenderness to percussion on the study tooth and/or the adjacent tooth (6/7, 86%) (Table 3). Palpation of the area buccal to the tooth apex produced tenderness in 1 patient and this patient also experienced tenderness to percussion. No maxillary-mandibular arch referral of pain was noted in our sample of patients.
Clinical findings supporting a nonodontogenic diagnosis included tenderness to palpation on the masseter, temporalis, and lateral pterygoid muscles, as well as the temporalis tendons, reproducing a component of the patient's complaints of persistent pain. These patients fit the TMD diagnosis of myofascial pain with referral. A positive response to sensory testing, such as pain to touch (i.e., allodynia), suggested the presence of nerve dysfunction and supported the diagnosis of PDAP in 2 patients. One of these patients diagnosed with PDAP reported a history of pain with exposure to cold air that started after a midface injury that occurred years prior to RCT. On the other hand, the other patient diagnosed with PDAP had no such report and therefore was believed to represent a new onset of sensory nerve dysfunction associated with dental disease and treatment.
Radiographic findings
The majority of patients with an odontogenic reason for their persistent pain demonstrated significant findings on their periapical films and cbCT scans (Table 4). In 57% of the patients the findings were evident on their periapical films, while 100% of patients had findings evident on their cbCT scans. Examples of the findings were missed canals, C-shaped canal, and overfilled/underfilled canals of either the study tooth or an adjacent tooth. On the other hand, patients with nonodontogenic reasons for their pain had fewer findings on their periapical films and cbCT scans. The radiographs of most patients (75%) with a nonodontogenic diagnosis revealed no potential etiology for persistent pain, while only 25% had periapical radiolucencies. Pre-operative radiographs were not available to determine whether there was radiographic evidence of “healing” in these patients.
Table 4. Radiographic findings in relation to diagnoses.
| PA films | CBCT scans | |||
|---|---|---|---|---|
| Normal Number (%) |
Radiolucency Number (%) |
Normal Number (%) |
*Significant Number (%) |
|
|
Odontogenic (RCT tooth, adjacent tooth) |
3/7 (43) | 4/7 (57) | 0/7 (0) | 7/7 (100) |
|
Nonodontogenic (TMD &PDAP) |
6/8 (75) | 2/8 (25) | 7/8 (89) | 1/8 (11) |
| Mixed odontogenic/Nonodontogenic | 1/2 (50) | 1/2 (50) | 1/2 (50) | 1/2 (50) |
| Normal | 1/2 (50) | 1/2 (50) | 1/2 (50) | 1/2 (50) |
Significant findings included missed canals, C-shaped canals, overfilled and/or underfilled canals.
Discussion
This nested case series determined that a little over a third of patients reporting pain 6 months following RCT had solely an odontogentic reason for this pain and almost half had a nonodontogenic reason. The remaining patients had either both odontogenic and nonodontogenic reasons or no pain diagnoses (Figure 1).
Of all patients who were diagnosed with odontogenic reasons for “tooth” pain, only 3 patients (16%) were determined to have persistent pathosis associated with the RCT tooth, likely related to failure to remove all the diseased or necrotic pulpal tissue in the case of missed canals (i.e., missed mesiobuccal canal and C-shaped canal), or possibly extruded root canal filling/debris (22). In two-thirds of the patients with an odontogenic diagnosis, the pain was related to pathosis in an adjacent tooth. This presentation of dental-related disease in adjacent tissues should not be unexpected because factors related to the presentation of oral disease are known to have local effects, both to the site of disease and to the person experiencing the disease, such as secondary caries (23).
The most common nonodontogenic reason for “tooth” pain was TMD, which was identified in 42% of all patients with pain 6 months following RCT. The subtype of TMD related to the RCT tooth was myofascial pain with referral and involved the masseter, temporalis, and lateral pterygoid muscles, as well as the temporalis tendon. Patients' perception of their TMD symptoms as “tooth” pain can be explained as the concept of referred pain (19), with the most common referred pain source to the teeth being the masseter and the lateral pterygoid muscles (8).
Due to the lack of diagnostic information prior to RCT, this study cannot address the questions of whether the initial symptoms of pain may have been misdiagnosed as odontogenic in origin (12, 24), whether the odontogenic pathosis sensitized the somatosensory system and contributed to the initiation of TMD that was maintained while the pathosis was adequately treated (25-27), or whether the onset of TMD was more related to the provision of RCT, such as the patient's mouth being open wide for a protracted period of time (28). This is a question of considerable importance to clinicians and future research should investigate how TMD and odontogenic pain are related, especially the possible bidirectional interactions.
PDAP, which many feel has underlying dysfunction of the somatosensory system (29-32), was diagnosed in 11% of those presenting with pain 6 months following RCT. One of the 2 patients diagnosed with PDAP had long-standing symptoms consistent with neuropathic pain and is likely secondary to a prior midfacial fracture; thereby being considered a pre-existing condition. The other patient appeared to have had a new onset of this pain disorder, thus fitting the definition of an incident case of PDAP.
The presence of a mixed odontogenic/nonodontogenic pain group is important because it requires the clinician to diagnose both etiologies for the report of pain, which can be challenging.
Two patients in this study reported no pain and no objective pain-related findings, leading to the opinion that their symptoms of pain at 6 months following RCT may have resolved by the time they presented for their clinical evaluation. We speculated that these patients were experiencing odontogenic pain that was associated with a delay in healing.
Findings related to pain characteristics
Due to the small sample size it was difficult to draw conclusions regarding certain pain characteristics that can help differentiate those with odontogenic reasons versus nonodontogenic reasons. However, it is worth mentioning that patients within the nonodontogenic group that were diagnosed with TMD had no prior TMD diagnosis. This highlights the importance of performing a thorough TMD evaluation at baseline, to rule out the possibility of TMD representing the complaint of “tooth” pain and again at follow up 6 month after RCT to determine an etiology of the persistent pain complaint.
Findings related to radiographs
The fact that cbCT scans revealed more findings which were significant in rendering a diagnosis compared to the PA films demonstrates its value and is consistent with previous research (33). While this study is not longitudinal in nature, it nonetheless suggests that there is importance for using cbCT, in selected instances, to assess the integrity of RCT when pain persists 6 months after treatment. Even though cbCT imaging was helpful in confirming the absence of odontogenic findings in some patients, specifically the patients with referred TMD pain, it is important to always use clinical judgment to limit patient exposure to ionizing radiation used for diagnostic purposes (34). Furthermore, our imaging findings are in line with findings from a study investigating the value of adding cbCT imaging to periapical films for patients diagnosed with PDAP (35).
Strengths of the study
Minimized bias
The nested case series design of this study provides an added benefit because it allowed for prospective assessment of pain at 6 months, which limited previous studies that used retrospective assessment. This design minimizes bias in case selection since eligibility criteria for being a case was pre-determined and study investigators were not involved in the enrollment process. Additionally, the consensus expert driven diagnoses were reached after independent evaluations by two boarded clinicians in the two fields of interest (i.e., Enododontics, Orofacial Pain), thus rendering reliable results and minimizing bias.
Generalizability of the results to the typical endodontic patient
Original recruitment of patients in the parent study through the National Dental PBRN offered the advantage of recruiting large numbers of patients from various geographic areas and multiple practices including both general dentists and endodontists. Most endodontic studies report data from patients treated by endodontists (36) although the majority of patients receiving RCT are treated by general dentists (1).
Limitations of the study
Even with large numbers, 11% prevalence of pain at 6 months resulted in a small sample of patients in the Midwest region from which to draw our study sample. Therefore the prevalence estimates have wide confidence intervals. This can be improved upon by increasing the original samples size, such as enrolling more patients in the parent study or having other sites in the parent network conducting the nested study protocol.
The low recruitment rate, 19/38 (50%) of those who reported pain at 6 months, was likely related to not having this nested study included within the initial consenting process of the parent study; however, those enrolled in this study were similar at baseline to those who did not participate (Table 1). It is recognized that sample sizes for many of the individual diagnoses are small, leading to wide confidence intervals that make it difficult to draw definitive and precise conclusions. However even with these limitations, this study has reduced other potential biases and addresses an important gap in knowledge. Taken together, this small project was designed to be an incremental step towards understanding persistent pain following RCT.
Finally, the lack of pre-operative diagnostic information, specifically the indication for initiation of RCT, limits the ability to assess healing following RCT. Furthermore, having pre-operative radiographs could have allowed for assessment of longitudinal changes, such as increased or decreased size of a periapical radiolucency and changes in the lamina dura.
Conclusion
We found that most patients reporting “tooth” pain 6 months following RCT had a nonodontogenic pain diagnosis accounting for some of this pain with TMD being the most frequent nonodonotgenic diagnosis. The reported pain was related to the RCT tooth in about one fifth of the patients. This suggests that patients experiencing a persistent pain following RCT should be evaluated for TMD. It also suggests that further research should investigate the relationship between odontogenic and nonodonotogenic pains.
Table 2. Patients' pain related characteristics in relation to diagnoses.
| Odontogenic (study & adjacent teeth) Number (%) |
Nonodontogenic (TMD & PDAP) Number (%) |
Mixed Odontogenic/Nonodontogenic Number (%) |
Normal Number (%) |
|
|---|---|---|---|---|
|
| ||||
| Pain intensity | ||||
| 0 | 4/7 (57) | 2/8 (25) | 0 | 2/2 (100) |
| 1-2 | 2/7 (29) | 5/8 (62) | 1/2 (50) | 0 |
| 3-4 | 1/7 (14) | 1/8 (13) | 1/2 (50) | 0 |
|
| ||||
| Pain quality | ||||
| No pain | 1/7 (17) | 1/8 (13) | 0 | 2/2 (100) |
| Dull achy | 4/7 (50) | 4/8 (50) | 1/2 (50) | 0 |
| Sharp | 2/7 (33) | 1/8 (13) | 0 | 0 |
| Throbbing | 0 | 2/8 (25) | 1/2 (50) | 0 |
|
| ||||
| *Pain localization | ||||
| No pain | 1/7 (17) | 3/8 (38) | 0 | 2/2 (100) |
| Well localized | 6/7 (83) | 2/8 (25) | 0 | 0 |
| Diffuse | 0 | 2/8 (25) | 2/2 (100) | 0 |
|
| ||||
| *Temporality of pain | ||||
| No pain | 1/7 (17) | 2/8 (25) | 0 | 0 |
| Intermittent | 3/7 (50) | 2/8 (25) | 0 | 0 |
| Constant | 3/7 (33) | 2/8 (25) | 1/2 (50) | 1/2 (33) |
| Variable | 0 | 1/8 (13) | 1/2 (50) | 2/2 (67) |
|
| ||||
| History of Orofacial pain | ||||
| - TMD | ||||
| - HA | 3/7 (43) | 2/8 (25) | 1/2 (50) | 0 |
| - Sinusitis | 4/7 (57) | 3/8 (38) | 1/2 (50) | 0 |
| 1/7 (14) | 2/8 (25) | 0 | 0 | |
|
| ||||
| **History of other chronic pain | ||||
| Yes | 5/7 (71) | 6/8 (75) | 2/2 (100) | 1/2 (50) |
1 patient with missing data for nonodontogenic group
Other overall chronic pain included: neck, shoulder, knee, ankle and pelvic pain, and one case of multiple sclerosis.
Acknowledgments
Supported by National Institutes of Health (K12-RR023247, U01-DE016746, U01-DE016747, U19-DE022516), the American Academy of Orofacial Pain, and the American Association of Endodontists Foundation. The authors declare that there are no conflicts of interest. Opinions and assertions contained herein are those of the authors and are not to be construed as necessarily representing the views of the respective organizations or the National Institutes of Health.
Footnotes
The authors deny any conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Dental Association Survey Center. 2005-06 survey of dental services rendered. Chicago: American Dental Association; 2007. [Google Scholar]
- 2.Nixdorf DR, Moana-Filho EJ, Law AS, McGuire LA, Hodges JS, John MT. Frequency of persistent tooth pain after root canal therapy: A systematic review and meta-analysis. J Endod. 2010 Feb;36(2):224–30. doi: 10.1016/j.joen.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nixdorf DR, Law AS, Lindquist K, Reams GJ, Cole E, Kanter K, et al. Frequency, impact, and predictors of persistent pain following root canal treatment: A National Dental PBRN study. doi: 10.1097/j.pain.0000000000000343. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolcott J, Rossman LE, Hasselgren G. Management of endodontic emergencies. In: Hargreaves KM, Cohen S, editors. Cohen's pathways of the pulp. 10th ed. St. Louis: Mosby Elsevier; 2011. pp. 40–8. [Google Scholar]
- 5.Mattscheck D, Law AS, Nixdorf DR. Diagnosis of non-odonogentic toothache. In: Hargreaves KM, Cohen S, editors. Cohen's Pathways of the Pulp. 10th ed. St. Louis: Mosby; 2011. pp. 49–70. [Google Scholar]
- 6.Nixdorf DR, Moana-Filho EJ, Law AS, McGuire LA, Hodges JS, John MT. Frequency of nonodontogenic pain after endodontic therapy: A systematic review and meta-analysis. J Endod. 2010 Sep;36(9):1494–8. doi: 10.1016/j.joen.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roz TM, Schiffman LE, Schlossberg S. Spontaneous dissection of the internal carotid artery manifesting as pain in an endodontically treated molar. J Am Dent Assoc. 2005;136(11):1556–9. doi: 10.14219/jada.archive.2005.0086. [DOI] [PubMed] [Google Scholar]
- 8.Wright EF. Referred craniofacial pain patterns in patients with temporomandibular disorder. J Am Dent Assoc. 2000;131(9):1307–15. doi: 10.14219/jada.archive.2000.0384. [DOI] [PubMed] [Google Scholar]
- 9.Alonso AA, Nixdorf DR. Case series of four different headache types presenting as tooth pain. J Endod. 2006 Nov;32(11):1110–3. doi: 10.1016/j.joen.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 10.Marbach JJ, Hulbrock J, Hohn C, Segal AG. Incidence of phantom tooth pain: An atypical facial neuralgia. Oral Surg Oral Med Oral Pathol. 1982 Feb;53(2):190–3. doi: 10.1016/0030-4220(82)90285-7. [DOI] [PubMed] [Google Scholar]
- 11.Polycarpou N, Ng YL, Canavan D, Moles DR, Gulabivala K. Prevalence of persistent pain after endodontic treatment and factors affecting its occurrence in cases with complete radiographic healing. Int Endod J. 2005 Mar;38(3):169–78. doi: 10.1111/j.1365-2591.2004.00923.x. [DOI] [PubMed] [Google Scholar]
- 12.Linn J, Trantor I, Teo N, Thanigaivel R, Goss AN. The differential diagnosis of toothache from other orofacial pains in clinical practice. Aust Dent J. 2007 Mar;52(1 Suppl):S100–4. doi: 10.1111/j.1834-7819.2007.tb00518.x. [DOI] [PubMed] [Google Scholar]
- 13.Gilbert GH, Williams OD, Rindal DB, Pihlstrom DJ, Benjamin PL, Wallace MC, et al. The creation and development of the Dental Practice-based Research Network. J Am Dent Assoc. 2008 Jan;139(1):74–81. doi: 10.14219/jada.archive.2008.0024. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert GH, Williams OD, Korelitz JJ, Fellows JL, Gordan VV, Makhija SK, et al. Purpose, structure, and function of the United States National Dental Practice-based Research Network. J Dent. 2013 Nov;41(11):1051–9. doi: 10.1016/j.jdent.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nixdorf DR, Law AS, Look JO, Rindal DB, Durand EU, Kang W, et al. Large-scale clinical endodontic research in the National Dental Practice-based Research Network: Study overview and methods. J Endod. 2012 Nov;38(11):1470–8. doi: 10.1016/j.joen.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutmann JL, Baumgartner JC, Gluskin AH, Hartwell GR, Walton RE. Identify and define all diagnostic terms for periapical/periradicular health and disease states. J Endod. 2009 Dec;35(12):1658–74. doi: 10.1016/j.joen.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 17.Newton CW, Hoen MM, Goodis HE, Johnson BR, McClanahan SB. Identify and determine the metrics, hierarchy, and predictive value of all the parameters and/or methods used during endodontic diagnosis. J Endod. 2009 Dec;35(12):1635–44. doi: 10.1016/j.joen.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 18.Levin LG, Law AS, Holland GR, Abbott PV, Roda RS. Identify and define all diagnostic terms for pulpal health and disease states. J Endod. 2009 Dec;35(12):1645–57. doi: 10.1016/j.joen.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 19.Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: Recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache. 2014 Winter;28(1):6–27. doi: 10.11607/jop.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders, 2nd edition (ICHD-II) Cephalalgia. 2004;24(Suppl 1):1–151. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 21.Nixdorf DR, Drangsholt MT, Ettlin DA, Gaul C, De Leeuw R, Svensson P, et al. Classifying orofacial pains: A new proposal of taxonomy based on ontology. J Oral Rehabil. 2012 Mar;39(3):161–9. doi: 10.1111/j.1365-2842.2011.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nair PN. On the causes of persistent apical periodontitis: A review. Int Endod J. 2006 Apr;39(4):249–81. doi: 10.1111/j.1365-2591.2006.01099.x. [DOI] [PubMed] [Google Scholar]
- 23.Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. 2007 Jan 6;369(9555):51–9. doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]
- 24.Farella M, Michelotti A, Gargano A, Cimino R, Ramaglia L. Myofascial pain syndrome misdiagnosed as odontogenic pain: A case report. Cranio. 2002 Oct;20(4):307–11. doi: 10.1080/08869634.2002.11746224. [DOI] [PubMed] [Google Scholar]
- 25.Cahana A, Forster A. Mechanisms by which acute orofacial pain becomes chronic. Rev Stomatol Chir Maxillofac. 2006 Jun;107(3):156–60. doi: 10.1016/s0035-1768(06)77011-5. [DOI] [PubMed] [Google Scholar]
- 26.Wright EF. Pulpalgia contributing to temporomandibular disorder-like pain: A literature review and case report. J Am Dent Assoc. 2008 Apr;139(4):436–40. doi: 10.14219/jada.archive.2008.0186. [DOI] [PubMed] [Google Scholar]
- 27.Wright EF, Gullickson DC. Identifying acute pulpalgia as a factor in TMD pain. J Am Dent Assoc. 1996 Jun;127(6):773–80. doi: 10.14219/jada.archive.1996.0313. [DOI] [PubMed] [Google Scholar]
- 28.Sahebi S, Moazami F, Afsa M, Nabavi Zade MR. Effect of lengthy root canal therapy sessions on temporomandibular joint and masticatory muscles. J Dent Res Dent Clin Dent Prospects. 2010 Summer;4(3):95–7. doi: 10.5681/joddd.2010.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobs R, Wu CH, Goossens K, De Laat A, Van Loven K, Antonis Y, et al. A case-control study on the psychophysical and psychological characteristics of the phantom tooth phenomenon. Clin Oral Investig. 2002;6(1):58–64. doi: 10.1007/s00784-001-0149-9. [DOI] [PubMed] [Google Scholar]
- 30.List T, Leijon G, Svensson P. Somatosensory abnormalities in atypical odontalgia: A case-control study. Pain. 2008;139(2):333–41. doi: 10.1016/j.pain.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Pigg M, Svensson P, Drangsholt M, List T. Seven-year follow-up of patients diagnosed with atypical odontalgia: A prospective study. J Orofac Pain. 2013 Spring;27(2):151–64. doi: 10.11607/jop.1033. [DOI] [PubMed] [Google Scholar]
- 32.Baad-Hansen L. Atypical odontalgia - pathophysiology and clinical management. J Oral Rehabil. 2008;35:1–11. doi: 10.1111/j.1365-2842.2007.01813.x. [DOI] [PubMed] [Google Scholar]
- 33.Estrela C, Bueno MR, Leles CR, Azevedo B, Azevedo JR. Accuracy of cone beam computed tomography and panoramic and periapical radiography for detection of apical periodontitis. J Endod. 2008 Mar;34(3):273–9. doi: 10.1016/j.joen.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 34.American Association of Endodontists; American Academy of Oral and Maxillofacial Radiology. Use of cone-beam computed tomography in endodontics Joint Position Statement of the American Association of Endodontists and the American Academy of Oral and Maxillofacial Radiology. Oral Surg Oral med Oral Pathol Endod. 2011 Feb;111(2):234–7. doi: 10.1016/j.tripleo.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Pigg M, List T, Petersson K, Lindh C, Petersson A. Diagnostic yield of conventional radiographic and cone-beam computed tomographic images in patients with atypical odontalgia. Int Endod J. 2011 Dec;44(12):1092–101. doi: 10.1111/j.1365-2591.2011.01923.x. [DOI] [PubMed] [Google Scholar]
- 36.Ng YL, Mann V, Rahbaran S, Lewsey J, Gulabivala K. Outcome of primary root canal treatment: Systematic review of the literature - part 1. Effects of study characteristics on probability of success. Int Endod J. 2007 Dec;40(12):921–39. doi: 10.1111/j.1365-2591.2007.01322.x. [DOI] [PubMed] [Google Scholar]