Abstract
Bacteria sense and respond to numerous environmental signals through two-component signaling pathways. Typically, a given stimulus will activate a sensor histidine kinase to autophosphorylate and then phosphotransfer to a cognate response regulator, which can mount an appropriate response. Although these signaling pathways often appear to be simple switches, they can also orchestrate surprisingly sophisticated and complex responses. These temporal dynamics arise from several key regulatory features, including the bifunctionality of histidine kinases as well as positive and negative feedback loops. Two-component signaling pathways are also dynamic on evolutionary time-scales, expanding dramatically in many species through gene duplication and divergence. Here, we review recent work probing the temporal and evolutionary dynamics of two-component signaling systems.
Introduction
Bacteria frequently sense and respond to changes in their environments through two-component signal transduction systems. The canonical pathway involves a sensor histidine kinase that autophosphorylates in the presence of a stimulus, and then transfers its phosphoryl group to a cognate response regulator. Once phosphorylated, response regulators are typically active, initiating cellular responses appropriate for the stimulus or environmental change that initially activated the system, often by changing gene expression [1]. Given the wide diversity of environmental signals that most bacteria must detect, individual species typically encode dozens to hundreds of unique two-component systems, each responding to a different stimulus and activating a different cellular response [2-5].
Although two-component pathways are often depicted as static entities that simply switch ON and OFF, recent work has demonstrated that these signaling pathways are sophisticated information-processing devices that exhibit complex dynamics, on both short and long timescales [2,6]. Here, we review recent work probing the regulatory features that govern the observed temporal dynamics of two-component signal transduction, and we discuss studies of the evolutionary dynamics that are beginning to reveal how these signaling proteins expand and diversify in bacteria.
Temporal Dynamics of Two-Component Systems
Although bacterial signaling pathways typically involve fewer components than their eukaryotic counterparts, they still possess features that enable them to regulate downstream targets with a high degree of sophistication and control. One such feature is the bifunctionality of histidine kinases. Early studies demonstrated that, in addition to phosphorylating their cognate response regulators when a stimulus is present, most histidine kinases also act as phosphatases for the same response regulators in the absence of stimulus [7-10]. Thus, the phosphorylation level of a response regulator is ultimately controlled by the balance of these two activities. If the rate of either the kinase or phosphatase reaction is altered, the steady-state level of active response regulator will change accordingly [7,11]. The phosphatase activity of histidine kinases also helps to prevent cross-talk between different pathways, suppressing the activation of a response regulator by a non-cognate histidine kinase or by promiscuous phosphodonors such as acetyl-phosphate [12-14]. Recent work has further proposed that the bifunctionality of histidine kinases may serve to suppress latent bistability in two-component pathways [15]. In Escherichia coli, a variant PhoQ kinase engineered to selectively lack phosphatase activity led to bistability in the levels of its phosphorylated partner, PhoP, in individual cells, with some cells switching to and staying in the ON state, even once the stimulus was removed, an effect that severely undermined fitness.
Other features of two-component pathways that yield complex temporal dynamics are positive and negative feedback loops. Using reporter genes activated by a given response regulator or direct measurements of mRNAs from target genes, several groups have observed “impulse” responses, or partial adaptation [11,16-18]. Instead of increasing monotonically to a new steady-state level after exposure to inducing conditions, the gene expression driven by a response regulator will first increase to a high, maximal level, and subsequently decrease to reach a steady-state level intermediate to the initial and maximal levels (Fig. 1A-B).
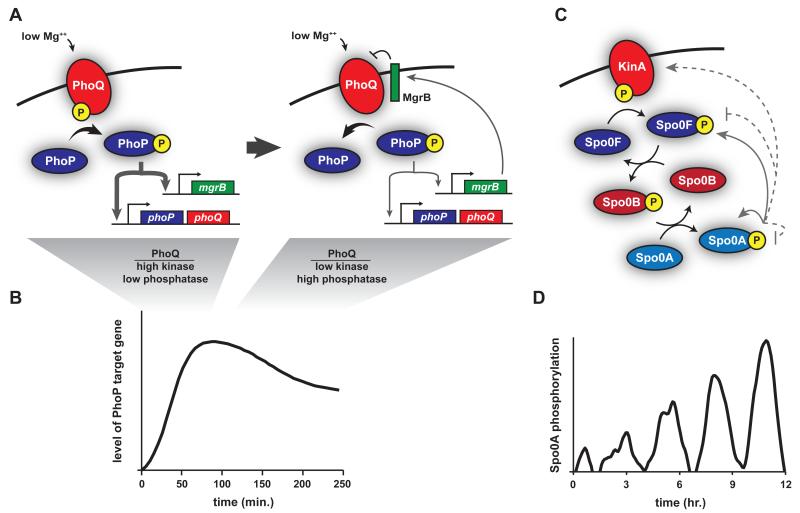
Figure 1. Temporal dynamics of two-component systems.
(A) PhoQ-PhoP is a canonical two-component system that is activated by low extracellular Mg++, changes in pH, and certain antimicrobial peptides. Upon sensing a stimulus, PhoQ is predominantly in the kinase state, driving PhoP phosphorylation and the increased expression of PhoP target genes, including phoPQ and mgrB (left). As MgrB accumulates, it helps drive a switch of PhoQ from the kinase to the phosphatase state. Eventually PhoQ is predominantly a phosphatase, limiting expression of PhoP-dependent genes (right). (B) This negative feedback loop mediated by MgrB likely accounts for the partial adaptation in pathway output. (C) Sporulation in B. subtilis is initiated by a four step phosphorelay. KinA (shown) or KinB/C/D/E first autophosphorylate; a phosphoryl group is then transferred to the response regulator Spo0F, then to the histidine phosphotransferase Spo0B, and finally to Spo0A (black arrows). Phosphorylated Spo0A directly promotes expression of itself and Spo0F (solid grey arrows), and indirectly promotes the production of KinA (dashed grey arrow). Phosphorylated Spo0A also indirectly drives dephosphorylation of itself and Spo0F (dashed grey lines). (D) Somehow these feedback loops produce pulses of phosphorylated Spo0A in sporulation-inducing conditions. Each pulse exhibits higher levels until a threshold level is reached to initiate sporulation.
The molecular basis of partial adaptation in two-component systems is unclear, but likely involves feedback loops. The Salmonella PhoQ-PhoP system, which responds to low extracellular magnesium and antimicrobial peptides, involves a positive feedback loop wherein phosphorylated PhoP positively autoregulates phoPQ transcription. One early study suggested that eliminating this positive feedback loop, by constitutively expressing phoP, diminished adaptation, or impluse-like behavior [16]. However, mathematical modeling has indicated that negative feedback is ultimately required for an impulse response of two-component systems [19] with subsequent work suggesting two potential sources of such feedback. One model posits that the PhoQ kinase retains ADP in its catalytic domain after autophosphorylation, promoting the phosphatase state of PhoQ and driving a decrease in phosphorylated PhoP [20]. Whether the ADP to ATP exchange rate for PhoQ is low enough in vivo that ADP would be retained for long periods of time is unclear; however, at least in vitro, histidine kinases likely undergo multiple rounds of autophosphorylation and phosphotransfer, implying high rates of nucleotide exchange. Another potential source of negative feedback for the PhoQ-PhoP system was revealed with the discovery of the small membrane protein MgrB [21]. Transcription of mgrB is directly stimulated by phosphorylated PhoP, and MgrB interacts with PhoQ in the membrane to reduce output from the PhoQ-PhoP system. However, a direct test of whether MgrB is responsible for the partial adaptation of PhoP-activated genes remains to be done. PhoQ also interacts with another small protein, SafA, although it is activates PhoQ autophosphorylation only under certain conditions in response to a different two-component pathway and thus does not form a feedback loop [22].
How positive feedback loops influence the steady-state output of two-component pathways is still unresolved, and may differ between individual systems. For the PhoQ-PhoP system, strains with and without positive autoregulation have similar steady-state outputs over a large range of input stimuli, even though a strain lacking positive autoregulation has lower total levels of PhoP than the wild type [16,23]. By contrast, for the E. coli PhoR-PhoB pathway, autoregulation plays a critical role in tuning both total PhoB levels and pathway output [24]. In phosphate-replete conditions, PhoB levels are low. As cells are depleted of inorganic phosphate, PhoR activates PhoB, which drives increased expression of PhoR and PhoB, leading to higher PhoB levels and activation of the full Pho regulon. Strikingly, the level of PhoB achieved through positive autoregulation is close to optimal. Forcing higher overall levels of PhoB does not significantly increase the amount of phosphorylated PhoB, and leads to the selection of mutations that restore a lower, optimal level of PhoB [24].
Even more complex temporal dynamics have been described for so-called phosphorelays. Although the majority of two-component pathways involve a single histidine kinase and its cognate response regulator, phosphorelays involve additional components: the phosphoryl group from a kinase is first transferred to one response regulator, then to a histidine phosphotransferase, and finally to a terminal response regulator. One such phosphorelay regulates the initiation of sporulation in Bacillus subtilus and involves five different kinases, each of which can phosphorylate the response regulator Spo0F. The phosphotransferase Spo0B then shuttles phosphoryl groups from Spo0F to Spo0A, the master regulator of sporulation initiation (Fig. 1C) [25]. Strikingly, upon shift to nutrient-limited conditions, Spo0A phosphorylation levels exhibit a series of pulses over the course of several cell cycles until sporulation is ultimately initiated (Fig. 1D) [26-28]. Each pulse becomes successively larger in amplitude, likely until a threshold of phosphorylated Spo0A is reached, which then allows commitment to sporulation. Which regulatory features of the sporulation pathway drive these pulses is unclear. There are several different feedback loops involving Spo0A and various phosphorelay components, but eliminating individual feedback loops did not disrupt pulsatile behavior.
The biological function of Spo0A pulsing is also not clear yet, although the gradual increase in magnitude of the pulses may represent a time-delay mechanism. Because sporulation is a costly, irreversible decision, cells may use the time it takes to ramp up Spo0A pulses to ensure that prevailing conditions warrant a commitment to sporulation. Additionally, the gradual accumulation of phosphorylated Spo0A may help coordinate the expression of genes needed for sporulation initiation [29]. Genes with high affinity Spo0A binding sites are expressed early when active Spo0A first begins to accumulate, with lower affinity promoters expressed later as active Spo0A accumulates to higher levels [30].
It is tempting to speculate that the pulsatile dynamics of Spo0A stem in part from the four-step architecture of the phosphorelay, as such pulsing behavior has not been seen yet with canonical two-component systems. Alternatively, the multi-step phosphorelay could simply enable the integration of multiple signals by providing more points of control. The phosphorelay may also represent a noise generator [31,32], such that only some cells in a population ultimately achieve the levels of phosphorylated Spo0A needed for sporulation to initiate. Whatever the case, further studies of the B. subtilis sporulation phosphorelay promise to provide important insights into the temporal dynamics of two-component pathways.
Finally, we note that the temporal dynamics of two-component signaling pathways are often inferred from the behavior of downstream reporter genes, but this approach is inherently indirect and potentially misleading. For example, in E. coli, two different PhoB-dependent reporters yield different temporal profiles of activation, likely reflecting differential regulation by other transcription factors [33]. A more direct method for measuring response regulator phosphorylation levels in vivo involves Phos-Tag technology, in which the phosphorylated and unphosphorylated forms of a regulator can be distinguished in protein gels [33]. This approach has enabled the direct measurement of both total and phosphorylated PhoB levels during phosphate starvation in E. coli. Importantly, this study demonstrated that only ~30% of PhoB molecules are phosphorylated during the phosphate starvation response. This set point is, in part, a consequence of the higher total levels of PhoB compared to its kinase PhoR during phosphate limitation, and high regulator:kinase ratios are a common feature of two-component pathways [20,34,35].
Evolutionary Dynamics of Two-Component Systems
In addition to complex temporal dynamics, two-component signaling pathways also exhibit a striking degree of evolutionary change and plasticity. Over the course of evolution, two-component systems have grown dramatically in number within many bacterial genomes, enabling organisms to sense and respond to diverse environmental stimuli. This expansion has been driven primarily through gene duplication and the subsequent divergence of paralogous systems [36]. However, the individual mutations responsible for establishing new pathways post-duplication and the order in which these mutations must occur remain poorly defined. Recent work has begun to explore these issues, combining phylogenetic analyses, molecular genetics, biochemistry, and mathematical modeling.
A key step in the divergence of two-component genes post-duplication is the establishment of insulated pathways, as extant histidine kinases rarely phosphorylate non-cognate response regulators in vivo, even those derived from recent duplication events [12,37,38]. Direct molecular recognition is the primary mechanism by which histidine kinases correctly phosphorylate their cognate response regulators. Phosphoprofiling experiments in vitro demonstrated that histidine kinases will specifically phosphorylate, and dephosphorylate, their cognate response regulators relative to all non-cognate substrates on short, biologically relevant timescales [38]. The intrinsic ability of a histidine kinase to discriminate its cognate partner from all non-cognate partners implies the existence of specificity-determining residues in each protein. Such residues presumably must coevolve to maintain the interaction of cognate proteins. Analyses of amino-acid coevolution in large sets of cognate kinase-regulator pairs from across the bacterial kingdom have revealed a small set of putative specificity residues in each protein [39,40]. These residues were experimentally shown to dictate protein-protein interaction specificity through the rational reprogramming of two-component signaling proteins [39,41]. For example, mutating the specificity residues of the histidine kinase EnvZ to match those of RstB, produced a mutant EnvZ that specifically phosphorylated RstA, the cognate regulator of RstB, while abolishing interaction with its usual partner, OmpR.
The mapping of specificity-determining residues in two-component pathways has enabled studies of how specificity evolves post-duplication [42]. Phylogenetic and sequence analyses have revealed that specificity residues, which are relatively static in the absence of gene duplication, exhibit a burst of diversification approximately coincident with a pathway duplication event. Typically, both a kinase and regulator diversify, coevolving to retain their interaction while becoming insulated from their duplicated counterparts (Fig. 2A). Once insulated pathways are established, specificity residues are again relatively static, thereby preserving interactions between cognate partners.
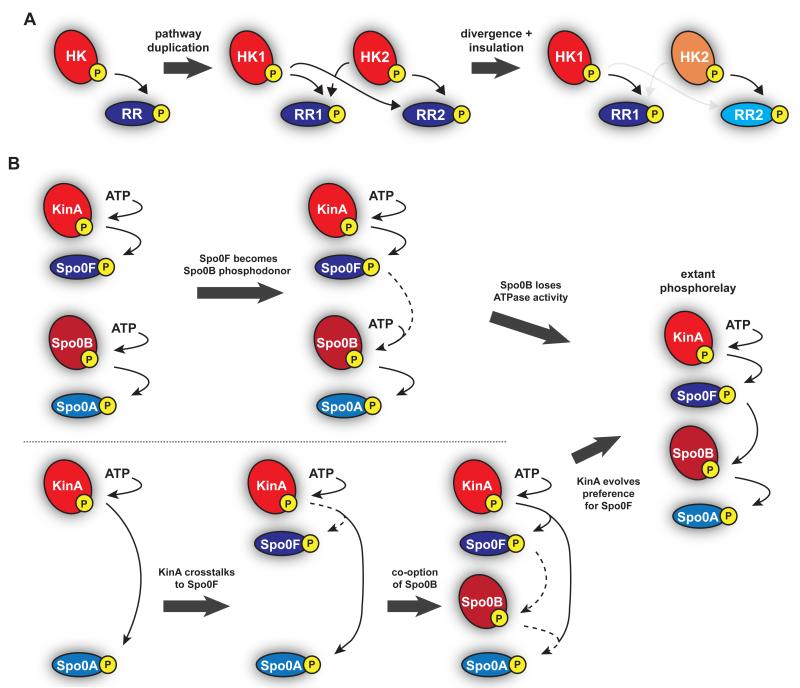
Figure 2. Evolution of two-component systems.
(a) Canonical two-component systems expand and diversify through gene duplication and subsequent divergence. After a pathway duplication, the specificity residues on the two identical systems diverge, such that each pathway continues to interact while reducing cross-talk between the two systems. (b) Two possible model for the origin of the phosphorelay driving B. subtilis sporulation. (top) Two canonical two-component pathways could have merged, with the response regulator of one pathway becoming the phosphodonor to another histidine kinase which then lost autophosphorylation activity. (bottom) KinA could have evolved to cross-talk with an existing response regulator with subsequent co-option of Spo0B as a histidine phosphotransferase and changes in KinA to prefer Spo0F over Spo0A.
This process of pathway divergence and insulation can involve changes in one or both pathways produced by a duplication event. Additionally, in some cases, insulation can require changes to yet other pathways [42]. For instance, in α-proteobacteria, the NtrB-NtrC pathway was duplicated with subsequent divergence of one copy yielding the extant NtrX-NtrY pathway. Phylogenetic analyses indicated that the emergence of NtrX-NtrY likely produced cross-talk with another, unrelated two-component pathway, the PhoR-PhoB system. Consequently, the α-proteobacterial PhoR and PhoB accumulated substitutions in their specificity residues that retained a PhoR-PhoB interaction while eliminating cross-talk between PhoR and NtrY.
Consistent with this model, restoring the ancestral specificity residues to an extant α-proteobacterial PhoR from Caulobacter crescentus led to increased cross-talk with NtrY in vitro and put Caulobacter cells at a selective disadvantage relative to the wild type in conditions that activate PhoR. Collectively, these observations demonstrated that the avoidance of cross-talk is a major selective pressure for two-component pathways, driving the diversification of specificity residues to produce insulated pathways, particularly after gene duplication events.
Although cross-talk is selected against, it is unavoidable immediately after pathway duplication, raising the question of what steps are taken, and in what order, to create a new pathway (Fig. 2A). Recent mathematical modeling has suggested that changes to specificity residues that eliminate cross-talk should occur first, followed by acquisition of new input/output functionalities, assuming cells must follow neutral or near-neutral mutational trajectories [43]. This work also highlights a major gap in our understanding of two-component pathway evolution, namely how histidine kinases gain new input functions and how response regulators gain new output functions. Domain shuffling likely plays a prominent role, but experimental studies of this process are an important future challenge.
Some non-canonical two-component pathways, particularly those involving so-called hybrid kinases, do not face the same evolutionary pressure to maintain phosphotransfer specificity as do canonical pathways. Hybrid kinases are histidine kinases that phosphotransfer intramolecularly to a response regulator-like receiver domain. The phosphoryl group from the receiver domain can then be passed to a histidine phosphotransferase, or in some cases, enable the kinase to autophosphorylate again and phosphotransfer to a soluble response regulator. Nearly 25% of all histidine kinases are hybrids [44] and phosphotransfer profiling experiments have demonstrated that their phosphotransfer specificity is not driven by molecular recognition as their kinase domains alone often phosphorylate many soluble response regulators. Instead, phosphotransfer specificity for hybrid kinases is enforced by spatial proximity; the high effective concentration of the tethered receiver domain ensures intramolecular phosphotransfer [45,46]. Consequently, duplicated hybrid kinases are not under the same strong selective pressure to diversify their specificity residues.
Although the evolution of two-component pathways often involves the production of two insulated pathways, there are cases in which components are added to, or integrated with, an existing pathway, yielding novel pathway architectures. A primary example is the B. subtilis sporulation phosphorelay in which five separate kinases, KinA/B/C/D/E, can each initiate a four-step phosphotransfer pathway culminating in phosphorylation of Spo0A [47]. The architecture of this phosphorelay is dictated by the preference of the kinases for phosphorylating Spo0F relative to Spo0A [37]. Presumably, the B. subtilis sporulation phosphorelay evolved from an ancient, canonical two-component system, but how, at a molecular level, did this occur?
One possibility is that an ancestral sporulation pathway involved kinases that directly activated an ancestral Spo0A ortholog with subsequent integration of the two middle components (Fig. 2B, bottom). Such a scenario would have required a change in kinase specificity to prefer the new Spo0F-like regulator over Spo0A. Interestingly, there are extant species, including various Clostridia species, that lack Spo0F and Spo0B orthologs and that initiate sporulation through a more canonical two-component pathway [48]. For example, in C. botulinum and C. acetobutylicum, several histidine kinases can directly phosphorylate their respective Spo0A orthologs [49,50]. Additionally, the C. acetobutylicum kinases can phosphorylate B. subtilis Spo0A but not Spo0F, while B. subtilis KinA cannot phosphorylate Clostridium Spo0A. These observations may imply that Bacilli species gained the intermediate components Spo0F and Spo0B. However, it could also be that the phosphorelay was present in a common ancestor of the Bacilli and Clostridia with subsequent loss of the middle two pathways components in Clostridia. Additionally, we note that the Clostridia kinases are not obvious homologs of the Bacilli kinases KinA-E suggesting that Clostridia species may have lost all components except Spo0A and then co-opted other kinases to directly phosphorylate Spo0A.
An alternative possibility for the origin of the sporulation phosphorelay is that it arose from the joining of two previously independent systems (Fig. 2B, top), such that the response regulator of one pathway became a phosphodonor for the other histidine kinase, which may have then lost its ability to autophosphorylate. Notably, the histidine phosphotransferase Spo0B, like many other phosphotransferases, structurally resembles a histidine kinase, but has lost the ability to autophosphorylate [51].
There are yet other possible scenarios for how the sporulation phosphorelay arose and exactly what happened remains uncertain. More in-depth, detailed phylogenetic studies are needed, along with better characterizations of the pathways controlling Spo0A phosphorylation in various Firmicutes, the clade containing Bacilli and Clostridia. Reconstructing the origins of the phosphorelay promises to reveal the evolutionary dynamics that shape and influence all two-component pathways, including their connectivities and functions.
Conclusions
Recent work has begun to probe the complexity of two-component signaling dynamics, on short and long timescales. Although these pathways are often thought to simply switch from OFF to ON after exposure to stimulus, studies of the induction kinetics have revealed more sophisticated behavior, including single impulses and sustained pulsing. Understanding how cells exploit and benefit from these dynamics remains a challenge for the future. Recent work has also started to reveal the complexities of two-component pathway evolution, but much more work is needed to understand how, at a molecular level, these pathways have expanded to respond to such a wide range of stimuli.
Highlights.
Kinase bifunctionality and feedback loops produce complex temporal dynamics
Many two-component pathways exhibit partial adaptation or pulsing behavior
Phosphotransfer specificity is determined largely by molecular recognition
Specificity residues must coevolve to insulate pathways post-duplication
How phosphorelays evolve from canonical two-component systems is unclear
Acknowledgements
This work is supported by NSF grant MCB-1408243 (M.T.L) and NIH Pre-Doctoral Training Grant T32GM007287 (M.E.S).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended readings
- 1.Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu. Rev. Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- 2.Capra EJ, Laub MT. Evolution of two-component signal transduction systems. Annu. Rev. Microbiol. 2012;66:325–347. doi: 10.1146/annurev-micro-092611-150039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galperin MY. A census of membrane-bound and intracellular signal transduction proteins in bacteria: Bacterial IQ, extroverts and introverts. BMC Microbiol. 2005;5:35. doi: 10.1186/1471-2180-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krell T, Lacal J, Busch A, Silva-Jiménez H, Guazzaroni M-E, Ramos JL. Bacterial Sensor Kinases: Diversity in the Recognition of Environmental Signals. Annu. Rev. Microbiol. 2010;64:539–559. doi: 10.1146/annurev.micro.112408.134054. [DOI] [PubMed] [Google Scholar]
- 5.Podgornaia AI, Laub MT. Determinants of specificity in two-component signal transduction. Curr. Opin. Microbiol. 2013;16:156–162. doi: 10.1016/j.mib.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Goulian M. Two-component signaling circuit structure and properties. Curr. Opin. Microbiol. 2010;13:184–189. doi: 10.1016/j.mib.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsing W, Russo FD, Bernd KK, Silhavy TJ. Mutations That Alter the Kinase and Phosphatase Activities of the Two-Component Sensor EnvZ. J. Bacteriol. 1998;180:4538–4546. doi: 10.1128/jb.180.17.4538-4546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Igo MM, Ninfa AJ, Stock JB, Silhavy TJ. Phosphorylation and dephosphorylation of a bacterial transcriptional activator by a transmembrane receptor. Genes Dev. 1989;3:1725–1734. doi: 10.1101/gad.3.11.1725. [DOI] [PubMed] [Google Scholar]
- 9.Russo FD, Silhavy TJ. The essential tension: opposed reactions in bacterial two-component regulatory systems. Trends Microbiol. 1993;1:306–310. doi: 10.1016/0966-842x(93)90007-e. [DOI] [PubMed] [Google Scholar]
- 10.Huynh TN, Noriega CE, Stewart V. Conserved mechanism for sensor phosphatase control of two-component signaling revealed in the nitrate sensor NarX. Proc. Natl. Acad. Sci. 2010;107:21140–21145. doi: 10.1073/pnas.1013081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao R, Stock AM. Probing kinase and phosphatase activities of two-component systems in vivo with concentration-dependent phosphorylation profiling. Proc. Natl. Acad. Sci. 2013;110:672–677. doi: 10.1073/pnas.1214587110. * Used Phos-Tag technology to directly measure response regulator phosphorylation levels in vivo.
- 12.Siryaporn A, Goulian M. Cross-talk suppression between the CpxA–CpxR and EnvZ–OmpR two-component systems in E. coli. Mol. Microbiol. 2008;70:494–506. doi: 10.1111/j.1365-2958.2008.06426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groban ES, Clarke EJ, Salis HM, Miller SM, Voigt CA. Kinetic Buffering of Cross Talk between Bacterial Two-Component Sensors. J. Mol. Biol. 2009;390:380–393. doi: 10.1016/j.jmb.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boll JM, Hendrixson DR. A specificity determinant for phosphorylation in a response regulator prevents in vivo cross-talk and modification by acetyl phosphate. Proc. Natl. Acad. Sci. 2011;108:20160–20165. doi: 10.1073/pnas.1113013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ram S, Goulian M. The Architecture of a Prototypical Bacterial Signaling Circuit Enables a Single Point Mutation to Confer Novel Network Properties. PLoS Genet. 2013;9:e1003706. doi: 10.1371/journal.pgen.1003706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shin D, Lee E-J, Huang H, Groisman EA. A Positive Feedback Loop Promotes Transcription Surge That Jump-Starts Salmonella Virulence Circuit. Science. 2006;314:1607–1609. doi: 10.1126/science.1134930. * Demonstrated that PhoP-dependent gene expression exhibits partial adaptation following pathway activation.
- 17.Hutchings MI, Hong H-J, Buttner MJ. The vancomycin resistance VanRS two-component signal transduction system of Streptomyces coelicolor. Mol. Microbiol. 2006;59:923–935. doi: 10.1111/j.1365-2958.2005.04953.x. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto K, Ishihama A. Transcriptional response of Escherichia coli to external zinc. J. Bacteriol. 2005;187:6333–6340. doi: 10.1128/JB.187.18.6333-6340.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ray JCJ, Igoshin OA. Adaptable functionality of transcriptional feedback in bacterial two-component systems. PLoS Comput. Biol. 2010;6:e1000676. doi: 10.1371/journal.pcbi.1000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeo W-S, Zwir I, Huang HV, Shin D, Kato A, Groisman EA. Intrinsic negative feedback governs activation surge in two-component regulatory systems. Mol. Cell. 2012;45:409–421. doi: 10.1016/j.molcel.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lippa AM, Goulian M. Feedback inhibition in the PhoQ/PhoP signaling system by a membrane peptide. PLoS Genet. 2009;5:e1000788. doi: 10.1371/journal.pgen.1000788. * Identified MgrB, a small membrane protein, that is up-regulated by PhoP and that inhibits PhoQ, potentially forming a key negative feedback loop that explains the partial adaptation of the PhoQ-PhoP system.
- 22.Eguchi Y, Ishii E, Yamane M, Utsumi R. The connector SafA interacts with the multi-sensing domain of PhoQ in Escherichia coli. Mol. Microbiol. 2012;85:299–313. doi: 10.1111/j.1365-2958.2012.08114.x. [DOI] [PubMed] [Google Scholar]
- 23.Miyashiro T, Goulian M. High stimulus unmasks positive feedback in an autoregulated bacterial signaling circuit. Proc. Natl. Acad. Sci. U. S. A. 2008;105:17457–17462. doi: 10.1073/pnas.0807278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao R, Stock AM. Evolutionary tuning of protein expression levels of a positively autoregulated two-component system. PLoS Genet. 2013;9:e1003927. doi: 10.1371/journal.pgen.1003927. ** Showed that transcriptional autoregulation tunes PhoB levels such that cells minimize wasteful production of PhoB in phosphate-replete conditions while producing sufficient PhoB in phosphate-deplete conditions to respond appropriately.
- 25.Hilbert DW, Piggot PJ. Compartmentalization of Gene Expression during Bacillus subtilis Spore Formation. Microbiol. Mol. Biol. Rev. 2004;68:234–262. doi: 10.1128/MMBR.68.2.234-262.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levine JH, Fontes ME, Dworkin J, Elowitz MB. Pulsed Feedback Defers Cellular Differentiation. PLoS Biol. 2012;10:e1001252. doi: 10.1371/journal.pbio.1001252. ** Demonstrated the pulsatile behavior of phosphorylated Spo0A, the master regulator of B. subitlis sporulation initiation, and suggested that pulsing enables cells to defer differentiation.
- 27.Veening J-W, Murray H, Errington J. A mechanism for cell cycle regulation of sporulation initiation in Bacillus subtilis. Genes Dev. 2009;23:1959–1970. doi: 10.1101/gad.528209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levine JH, Elowitz MB. Polyphasic feedback enables tunable cellular timers. Curr. Biol. 2014;24:R994–R995. doi: 10.1016/j.cub.2014.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujita M, Losick R. Evidence that entry into sporulation in Bacillus subtilis is governed by a gradual increase in the level and activity of the master regulator Spo0A. Genes Dev. 2005;19:2236–2244. doi: 10.1101/gad.1335705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujita M, González-Pastor JE, Losick R. High- and Low-Threshold Genes in the Spo0A Regulon of Bacillus subtilis. J. Bacteriol. 2005;187:1357–1368. doi: 10.1128/JB.187.4.1357-1368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jong de IG, Veening J-W, Kuipers OP. Heterochronic Phosphorelay Gene Expression as a Source of Heterogeneity in Bacillus subtilis Spore Formation. J. Bacteriol. 2010;192:2053–2067. doi: 10.1128/JB.01484-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chastanet A, Vitkup D, Yuan G-C, Norman TM, Liu JS, Losick RM. Broadly heterogeneous activation of the master regulator for sporulation in Bacillus subtilis. Proc. Natl. Acad. Sci. 2010;107:8486–8491. doi: 10.1073/pnas.1002499107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kinoshita E, Kinoshita-Kikuta E, Koike T. Separation and detection of large phosphoproteins using Phos-tag SDS-PAGE. Nat. Protoc. 2009;4:1513–1521. doi: 10.1038/nprot.2009.154. [DOI] [PubMed] [Google Scholar]
- 34.Cai SJ, Inouye M. EnvZ-OmpR Interaction and Osmoregulation in Escherichia coli. J. Biol. Chem. 2002;277:24155–24161. doi: 10.1074/jbc.M110715200. [DOI] [PubMed] [Google Scholar]
- 35.Li G-W, Burkhardt D, Gross C, Weissman JS. Quantifying Absolute Protein Synthesis Rates Reveals Principles Underlying Allocation of Cellular Resources. Cell. 2014;157:624–635. doi: 10.1016/j.cell.2014.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alm E, Huang K, Arkin A. The Evolution of Two-Component Systems in Bacteria Reveals Different Strategies for Niche Adaptation. PLoS Comput Biol. 2006;2:e143. doi: 10.1371/journal.pcbi.0020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grimshaw CE, Huang S, Hanstein CG, Strauch MA, Burbulys D, Wang L, Hoch JA, Whiteley JM. Synergistic Kinetic Interactions between Components of the Phosphorelay Controlling Sporulation in Bacillus subtilis†. Biochemistry (Mosc.) 1998;37:1365–1375. doi: 10.1021/bi971917m. [DOI] [PubMed] [Google Scholar]
- 38.Skerker JM, Prasol MS, Perchuk BS, Biondi EG, Laub MT. Two-Component Signal Transduction Pathways Regulating Growth and Cell Cycle Progression in a Bacterium: A System-Level Analysis. PLoS Biol. 2005;3:e334. doi: 10.1371/journal.pbio.0030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skerker JM, Perchuk BS, Siryaporn A, Lubin EA, Ashenberg O, Goulian M, Laub MT. Rewiring the specificity of two-component signal transduction systems. Cell. 2008;133:1043–1054. doi: 10.1016/j.cell.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weigt M, White RA, Szurmant H, Hoch JA, Hwa T. Identification of direct residue contacts in protein-protein interaction by message passing. Proc. Natl. Acad. Sci. U. S. A. 2009;106:67–72. doi: 10.1073/pnas.0805923106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Capra EJ, Perchuk BS, Lubin EA, Ashenberg O, Skerker JM, Laub MT. Systematic dissection and trajectory-scanning mutagenesis of the molecular interface that ensures specificity of two-component signaling pathways. PLoS Genet. 2010;6:e1001220. doi: 10.1371/journal.pgen.1001220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Capra EJ, Perchuk BS, Skerker JM, Laub MT. Adaptive mutations that prevent crosstalk enable the expansion of paralogous signaling protein families. Cell. 2012;150:222–232. doi: 10.1016/j.cell.2012.05.033. ** Showed how and why specificity residues on histidine kinases and response regulators diversify following the duplication of a two-component system to create new, insulated pathways.
- 43.Rowland MA, Deeds EJ. Crosstalk and the evolution of specificity in two-component signaling. Proc. Natl. Acad. Sci. 2014;111:5550–5555. doi: 10.1073/pnas.1317178111. * Used mathematical modeling to suggest that changes to pathway specificity likely precede the diversification of input and output domains during two-component pathway duplication and divergence.
- 44.Wuichet K, Zhulin IB. Origins and Diversification of a Complex Signal Transduction System in Prokaryotes. Sci. Signal. 2010;3:ra50–ra50. doi: 10.1126/scisignal.2000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Capra EJ, Perchuk BS, Ashenberg O, Seid CA, Snow HR, Skerker JM, Laub MT. Spatial tethering of kinases to their substrates relaxes evolutionary constraints on specificity. Mol. Microbiol. 2012;86:1393–1403. doi: 10.1111/mmi.12064. [DOI] [PubMed] [Google Scholar]
- 46.Townsend GE, Raghavan V, Zwir I, Groisman EA. Intramolecular arrangement of sensor and regulator overcomes relaxed specificity in hybrid two-component systems. Proc. Natl. Acad. Sci. 2013;110:E161–E169. doi: 10.1073/pnas.1212102110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burbulys D, Trach KA, Hoch JA. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell. 1991;64:545–552. doi: 10.1016/0092-8674(91)90238-t. [DOI] [PubMed] [Google Scholar]
- 48.Galperin MY, Mekhedov SL, Puigbo P, Smirnov S, Wolf YI, Rigden DJ. Genomic determinants of sporulation in Bacilli and Clostridia: towards the minimal set of sporulation-specific genes. Environ. Microbiol. 2012;14:2870–2890. doi: 10.1111/j.1462-2920.2012.02841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wörner K, Szurmant H, Chiang C, Hoch JA. Phosphorylation and functional analysis of the sporulation initiation factor Spo0A from Clostridium botulinum. Mol. Microbiol. 2006;59:1000–1012. doi: 10.1111/j.1365-2958.2005.04988.x. [DOI] [PubMed] [Google Scholar]
- 50.Steiner E, Dago AE, Young DI, Heap JT, Minton NP, Hoch JA, Young M. Multiple orphan histidine kinases interact directly with Spo0A to control the initiation of endospore formation in Clostridium acetobutylicum. Mol. Microbiol. 2011;80:641–654. doi: 10.1111/j.1365-2958.2011.07608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zapf J, Sen U, Madhusudan, Hoch JA, Varughese KI. A transient interaction between two phosphorelay proteins trapped in a crystal lattice reveals the mechanism of molecular recognition and phosphotransfer in signal transduction. Structure. 2000;8:851–862. doi: 10.1016/s0969-2126(00)00174-x. [DOI] [PubMed] [Google Scholar]