One of the main mechanisms of hydrogen sulfide toxicity is thought to relate to the ability of H2S/HS− to block the activity of the mitochondrial electron transport chain, preventing the creation of a proton gradient across the mitochondrial membrane, and in turn impeding ATP regeneration in all cells (1, 2). The corollary of the impediment in ATP production is a reduction in cellular O2 utilization, leading to a reduction in peripheral O2 extraction and thus an increase in venous and tissular O2 content (and partial pressure), akin to the well documented rise in venous PO2 (and paradoxical reddish color of tissues) during cyanide poisoning-induced cellular “anoxia” (3).
Fernandes et al. (4) have recently argued that this mechanism is difficult to reconcile with the data published by Brenner et al. (5) depicting a drop, instead of an increase, in “tissular” oxyhemoglobin during sulfide intoxication, i.e. in the setting of an inhibition of oxidative phosphorylation. However, we have found that H2S intoxication dramatically decreases cardiac contractility and cardiac output (6), as soon as the concentration of free H2S/HS− reaches levels of about 3–5 microM, before signs of toxicity can be observed (6–8), leading to fatal pulseless electrical activity within minutes (9). No significant peripheral vasodilation was observed during sulfide induced circulatory failure (6). This striking and very rapid depression in cardiac contractility has been previously suggested to result from the blockade of LCa channels in cardiomyocytes (10, 11). The “poisoning” of the cardiomyocytes appears very early (6), possibly through non-ATP related mechanisms (10) at a time when the cytochrome C oxidase activity is not yet, or not dramatically, impeded in most tissues. As a consequence, a decrease in venous/peripheral O2 saturation/content is not unexpected. To clarify this matter, we have recomputed (figure), from data previously obtained in 7 sedated rats (6), the relationship between cardiac output (determined from aortic or pulmonary blood flow), V̇O2 (determined by pulmonary gas exchange), the change in O2 extraction (computed as V̇O2 /cardiac output ratio), during the first minutes of H2S/HS− infusion at a rate, which is fatal within 5–6 minutes (2 mg/kg/min) (6). Such a H2S infusion produced a rapid decrease in cardiac output/O2 delivery, which was proportionally much more severe and rapid than the reduction in O2 consumption. As a result, O2 extraction rises (figure), reflecting a larger fall in the rate of O2 delivery than in the rate of cellular O2 utilization. Incidentally, a similar reduction in tissular/venous O2 saturation has also been documented during cyanide poisoning, wherein acute cardiac failure occurs (12).
Figure.
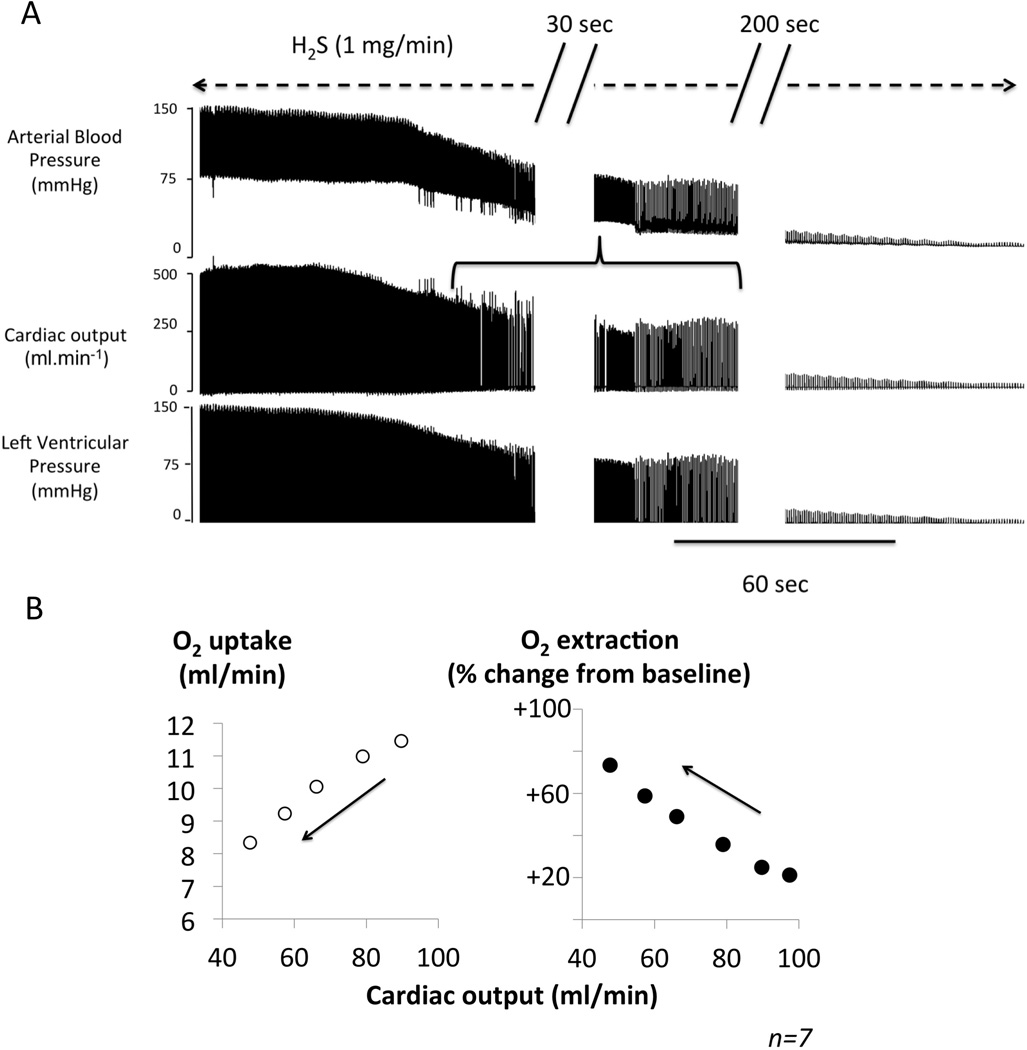
Panel A. Original recording of the changes in arterial blood pressure, cardiac output and the left ventricular pressure, during H2S infusion in a 500 g rat receiving a solution of sulfide made from NaHS (1 mg/min). There was a rapid decrease in cardiac output, arterial pressure and left ventricular pressure, which led to asystole, within 5 minutes. The horizontal bracket shows the period at which of cardiac output was determined for the computations shown in B. Panel B. V̇O2 (left panel) and the change in O2 extraction as % from baseline (Right panel) as a function of cardiac output. 10 second-averaged data obtained from 7 rats during the first minutes of an infusion of H2S at toxic levels (2 mg/min) are shown. Cardiac output dropped dramatically, while oxygen extraction increases reflecting a proportionally higher fall in O2 delivery than in O2 utilization. Note that PaO2 and thus the arterial O2 content was prevented to decrease by mechanical ventilation throughout the period of infusion.
These data support the view that a rapid cardiogenic shock leading to a profound reduction in O2 delivery to peripheral tissues is a one of the dreadful and early effects of H2S intoxication. The proper identification of this cardiogenic shock, in a clinical setting of patients exposed to mitochondrial “poisons” presenting with circulatory failure and tissue hypoxia, has crucial therapeutic implications.
Acknowledgments
This work was supported by the Counter ACT Program, National Institutes of Health Office of the Director (NIH OD), and the National Institute of Neurological Disorders and Stroke (NINDS), Grant Number 1R21NS080788-02 and 1R21NS090017-01
References
- 1.Cooper CE, Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. Journal of bioenergetics and biomembranes. 2008 Oct;40(5):533–539. doi: 10.1007/s10863-008-9166-6. PubMed PMID: 18839291. [DOI] [PubMed] [Google Scholar]
- 2.Dorman DC, Dautrebande L, Moulin FJ, McManus BE, Mahle KC, James RA, et al. Cytochrome oxidase inhibition induced by acute hydrogen sulfide inhalation: correlation with tissue sulfide concentrations in the rat brain, liver, lung, and nasal epithelium. Toxicological sciences : an official journal of the Society of Toxicology. 2002 Jan;65(1):18–25. doi: 10.1093/toxsci/65.1.18. PubMed PMID: 11752681. [DOI] [PubMed] [Google Scholar]
- 3.Johnson RP, Mellors JW. Arteriolization of venous blood gases: a clue to the diagnosis of cyanide poisoning. The Journal of emergency medicine. 1988 Sep-Oct;6(5):401–404. doi: 10.1016/0736-4679(88)90014-5. PubMed PMID: 3147294. [DOI] [PubMed] [Google Scholar]
- 4.Fernández D, Legrand M, Abujaber S, Nelson LS. Letter in response to "The Vitamin B12 analog cobinamide is an effective hydrogen sulfide antidote in a lethal rabbit model". Clinical toxicology. 2015;53(1):73. doi: 10.3109/15563650.2014.973573. [DOI] [PubMed] [Google Scholar]
- 5.Brenner M, Benavides S, Mahon SB, Lee J, Yoon D, Mukai D, et al. The vitamin B12 analog cobinamide is an effective hydrogen sulfide antidote in a lethal rabbit model. Clinical toxicology. 2014 Jun;52(5):490–497. doi: 10.3109/15563650.2014.904045. PubMed PMID: 24716792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonobe T, Haouzi P. Sulfide intoxication induced circulatory failure is mediated by a depression in cardiac contractility. Cardiovascular Toxicology. 2015 doi: 10.1007/s12012-015-9309-z. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klingerman CM, Trushin N, Prokopczyk B, Haouzi P. H2S concentrations in the arterial blood during H2S administration in relation to its toxicity and effects on breathing. American journal of physiology Regulatory, integrative and comparative physiology. 2013 Sep 15;305(6):R630–R638. doi: 10.1152/ajpregu.00218.2013. PubMed PMID: 23904109. Pubmed Central PMCID: 3763045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haouzi P, Sonobe T, Torsell-Tubbs N, Prokopczyk B, Chenuel B, Klingerman CM. In Vivo Interactions Between Cobalt or Ferric Compounds and the Pools of Sulphide in the Blood During and After H2S Poisoning. Toxicological sciences : an official journal of the Society of Toxicology. 2014 Jul 11; doi: 10.1093/toxsci/kfu140. PubMed PMID: 25015662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haouzi P, Chenuel B, Sonobe T. High-dose hydroxocobalamin administered after H2S exposure counteracts sulfide-poisoning-induced cardiac depression in sheep. Clinical toxicology. 2015 Jan;53(1):28–36. doi: 10.3109/15563650.2014.990976. PubMed PMID: 25546714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang R, Sun Y, Tsai H, Tang C, Jin H, Du J. Hydrogen sulfide inhibits L-type calcium currents depending upon the protein sulfhydryl state in rat cardiomyocytes. PloS one. 2012;7(5):e37073. doi: 10.1371/journal.pone.0037073. PubMed PMID: 22590646. Pubmed Central PMCID: 3349658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun YG, Cao YX, Wang WW, Ma SF, Yao T, Zhu YC. Hydrogen sulphide is an inhibitor of L-type calcium channels and mechanical contraction in rat cardiomyocytes. Cardiovascular research. 2008 Sep 1;79(4):632–641. doi: 10.1093/cvr/cvn140. PubMed PMID: 18524810. [DOI] [PubMed] [Google Scholar]
- 12.Pham JC, Huang DT, McGeorge FT, Rivers EP. Clarification of cyanide's effect on oxygen transport characteristics in a canine model. Emergency medicine journal : EMJ. 2007 Mar;24(3):152–156. doi: 10.1136/emj.2006.038927. PubMed PMID: 17351216. Pubmed Central PMCID: 2816935. [DOI] [PMC free article] [PubMed] [Google Scholar]


