Abstract
Tissue engineering is increasingly based on recapitulating human physiology, through integration of biological principles into engineering designs. In spite of all progress in engineering functional human tissues, we are just beginning to develop effective methods for establishing blood perfusion and controlling the inflammatory factors following implantation into the host. Functional vasculature largely determines tissue survival and function in vivo. The inflammatory response is a major regulator of vascularization and overall functionality of engineered tissues, through the activity of different types of macrophages and the cytokines they secrete. We discuss cell-scaffold-bioreactor systems for harnessing the inflammatory response for enhanced tissue vascularization and healing. To this end, inert scaffolds that have been considered for many decades a “gold standard” in regenerative medicine are beginning to be replaced by a new generation of “smart” tissue engineering systems designed to actively mediate tissue survival and function.
Key terms: Scaffold, vascularization, inflammatory response, tissue engineering, healing
INTRODUCTION
Tissue engineering is becoming increasingly successful in more authentically representing the actual environmental milieu of development, regeneration and disease, and in providing real-time insights into cellular and morphogenic events. For regenerative medicine, the key requirements include tissue size and shape (customized to the patient and defect being treated), internal architecture (with gradients of properties and interfaces), controllable degradation and remodeling (ideally at the rate matching tissue growth), and – most importantly – tissue function (tissue-specific: structural, mechanical, metabolic, biosynthetic). A personalized approach based on the use of the patients’ own cells and provisions for the specifics of the defect and systemic factors would be highly beneficial to regenerative medicine. For drug testing, functional tissues designed to combine high biological fidelity with use in high-throughput platforms and real-time measurement of physiological responses would be transformative to modeling of disease.
Because living cells respond to the entire context of their environment - in vivo and in vitro, under normal and pathological conditions - the biological foundation of our bioengineering designs that is often described as the “biomimetic paradigm” is critical for unlocking the full biological potential of the cells. The four main groups of key factors: (i) regulatory molecules (oxygen, nutrients, cytokines), (ii) other cells (3D context, cell-cell contacts, autocrine and paracrine signals), (iii) extracellular matrix (immobilized and released factors, structure, topology, stiffness), and (iv) physical factors (flow shear, compression, stretch, electrical signals) act in concert, with changes in space and time, in synergistic and competing fashion 87. Biomimetic environments can be engineered to provide these factors by using scaffolds (providing a structural and logistic template for cell differentiation and functional assembly) and bioreactors (providing environmental control, molecular and physical signaling). Together, the biological principles and engineering tools help recapitulate the regulatory milieu of the native environment associated with a specific tissue or organ, and orchestrate the assembly of the ‘collections of cells’ into phenotypically defined tissues that take over the function of the target tissue (Figure 1).
Figure 1. Biomaterial scaffold.
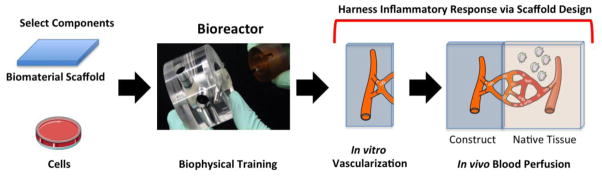
Schematic showing the steps towards an engineered construct where the scaffold is designed to harness the host tissue response and maximize the angiogenic response. The first step is to select the cell source and the biomaterial of interest. These two components are then assembled and trained in a bioreactor by applying tissue appropriate biophysical forces. At this stage, a vasculature can be formed and perfused in vitro. This “artificial vasculature” can then be connected to the host tissue vasculature by harnessing the inflammatory response at the site of implantation. Artwork provided by Servier Medical Arts.
Historically, even the most advanced approaches have been largely focused on engineering young and healthy tissues for implantation, with little attention paid to the subsequent survival of the implanted tissue, connection to the blood supply, and interaction with the inflammatory environment of the host. Although it is recognized that rapid establishment of vasculature perfused with blood largely determines survival of implanted tissues, we still lack effective methods for vascularizing engineered tissues. The inflammatory environment associated with wound healing at the implant site is not effectively incorporated into our tissue regeneration strategies. Pathological conditions such as myocardial infarction or osteoarthritis are associated with the specific time profiles of multiple inflammatory factors. These considerations are important, due to the growing evidence that the macrophages and the cytokines they secrete serve as major regulators of tissue vascularization and survival, both under healthy and pathological conditions.
At this time, inert scaffolds that have been considered for many decades a “gold standard” in regenerative medicine are being replaced by a new generation of “smart” scaffold-bioreactor systems designed to actively mediate tissue survival and function. We discuss here some strategies for mobilizing and harnessing the inflammatory response of the host towards enhanced tissue survival and functional integration.
HOST RESPONSES TO IMPLANTED TISSUE CONSTRUCTS
Any implantable device – from acellular biomaterials to living tissue constructs - intended to replace a missing or damaged tissue and drive regeneration will have to interact with native cells, immunological and inflammatory responses. The surgical implantation causes additional injury that can elicit or exacerbate the current host immune response. Therefore it is important to understand how the components of an engineered construct (synthetic and biological; molecular; structural; mechanical) interact with the local and systemic milieu at the site of implantation. Once these interactions are better understood, they could be taken into consideration during the design process, towards regenerating human tissues in the most effective way. Ideally, the goal would be not only to overcome any adverse effects of the host response, but also to harness the specific host cells and cytokines they secrete towards enhancing tissue regeneration. The use of bioreactor testing platforms that mimic in vitro the important aspects of the in vivo host tissue response 32,33 are critical for maximizing the chances of success.
Soon after implantation of a biomaterial or engineered tissue, there is an initial surge of peripheral blood mononuclear cells (PBMCs) that leave the circulation via extravasation, and home to the site of injury guided by distress signals released by local cells. Neutrophils are among the initial cells to be recruited, and are followed by a wave of monocytes that invade the site of injury where they differentiate into macrophages 30. Together with resident macrophages, recruited macrophages are responsible for resorbing necrotic tissue, removing foreign materials, and to a significant extent for the initiation of angiogenesis and extracellular matrix deposition, although these latter roles are still being defined. The inflammatory response is part of the overall host tissue response and can be acute if resolved quickly, or chronic if resolution is not achieved over a short time. The presence of macrophages marks the escalation of the inflammatory response and represents an important cellular event during wound healing that can either result in fibrous encapsulation of the engineered construct or graft integration 58,70.
Traditionally, there is a distinction between classically activated and alternatively activated macrophages 60. Classically activated macrophages (M1) have been associated with the production of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α. In the context of implantable devices, M1s are responsible for the initial removal of cellular debris and to some extent for degradation of scaffolding material. In mice, the pro-inflammatory response can last for up to 7 days 86, and is followed by gradual shift towards a more pro- healing environment, which is in turn characterized by the presence of alternatively polarized macrophages (M2). M2s express their own cytokine patterns, and are associated with immune regulation, matrix deposition, remodeling, and graft acceptance 18,47,55,61.
Although most reports refer to M1 and M2 macrophage subtypes, there is growing evidence that macrophages are found in a spectrum of differentiation stages that dynamically adjust to the inflammatory environments 60 (Figure 2). For example, Mantovani has proposed the M2a, M2b, and M2c subtypes of M2 macrophages. M2a subtype refers to the alternatively activated macrophages activated by IL-4 or IL-13. M2b subtype is activated by immune complexes, agonists of Toll-like receptors (TLRs), or IL-1R. M2c subtype refers to macrophages activated by IL-10 or glucocorticoid hormones 53. Each of these macrophage subtypes participates in the host tissue responses during wound healing, tumor resistance, immune-regulation, matrix deposition and tissue remodeling 53. Since macrophages play unique roles in tissue regeneration, it is of great interest to study their contributions to the host response and to tissue remodeling following implantation of engineered constructs.
Figure 2. Differentiation and polarization of macrophages.
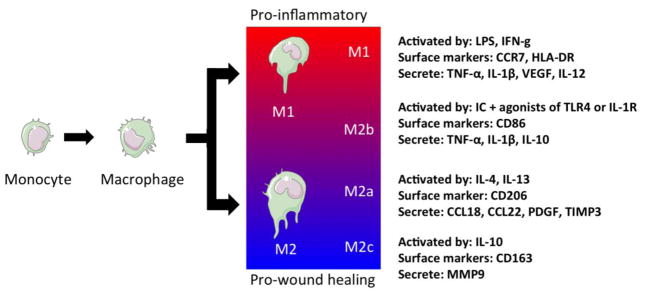
Monocytes that are recruited to the site of injury differentiate into macrophages via local signals. These macrophages polarize into a spectrum of macrophage subtypes ranging from pro-inflammatory (M1) to wound healing phenotypes (M2c). Artwork provided by Servier Medical Arts.
INFLAMMATORY RESPONSES TO SCAFFOLDS CAN BE STUDIED IN BENCH TOP PLATFORMS
Traditionally immunosuppressed, immunocompromised, or humanized animal models have served as testing platforms for engineered tissue constructs. Although animal models can help recapitulate the complexity of the host tissue response in its entirety, they often remain limited by species mismatch and cost 91.
These limitations motivated the interest in developing bench top testing platforms for modeling important aspects of the host tissue response in vitro for various types of cells and studies of important cellular events. The in vivo situation can be mimicked by using purified cytokines 32 and/or specific cell types (e.g. neutrophils, monocytes and polarized macrophages) 33. Purified cytokines are readily available through commercial sources and the control over their concentrations and purity makes them suitable to study specific target molecules. For more complex systems, white blood cells obtained from peripheral human blood 66 or the established cell lines (HL-60 and THP-1) can be used 10. However, although studies suggest that in vitro behavior of monocytes, macrophages, and dendritic cells can be used as an initial screen of inflammation, in reality the in vivo environment is a complex system of multiple cell types, and results should always be validated in vivo.
To test cell-cell and cell-biomaterials interactions, inflammatory cells can be co-cultured with the engineered tissue constructs to study paracrine and autocrine signaling, using trans-well systems to physically separate the engineered construct from the blood cells, thus avoiding donor-mismatch activation 21. Alternatively, a completely autologous cell-culture system can be developed through the use of induced pluripotent stem cells and blood donations from a single donor.
By creating in vitro testing platforms, engineered constructs can be optimized for testing in animal models, thus reducing cost and improving efficiency. For example, cardiac patches can be tested within the M1 dominated pro-inflammatory environments initially found after myocardial infarction, or by the M2 dominated stages of the myocardial remodeling process 33. Such systems can be readily established by exposing the tissue constructs to M1 and M2 macrophages derived from peripheral blood monocytes. This approach can help identify environments that are most conducive to construct engraftment and survival, and to understand the effects of injury environment on engineered constructs.
MACROPHAGES CAN ENHANCE ANGIOGENESIS AND VASCULOGENESIS
Macrophages play a crucial role in fetal development and organogenesis by regulating vascular reorganization, refinement, and anastomosis 27. Macrophage depletion during tissue development or regeneration can result in reduced endothelial cell migration, reduced numbers and sizes of blood vessels, and vascular abnormalities 5,27,28,37,46,49,69. Moreover, accumulation of macrophages is a hallmark of arteriogenesis following injury 8, and the exogenous administration of macrophages was shown to promote vascularization 38,39. Macrophage-derived metalloproteases regulate arteriogenesis during wound healing and ischemia reperfusion 4. Macrophages infiltrated into Matrigel plugs implanted subcutaneously, creating branched tunnels that become rapidly populated by endothelial progenitor cells that attach and form endothelium 3. Macrophages can even assume endothelial properties, such as expression of Tie2 and VE-cadherin 4,22, and circulating endothelial progenitor cells have been postulated to be “macrophages in disguise” 57,94.
Notably, under certain conditions, macrophages can also inhibit angiogenesis 9,75,77. Controversy still exists over which of the two phenotypes - M1 or M2 – is in fact angiogenic 45. Vascularization is generally attributed to the M2s 52, in part because of the similarities between M2s and tumor-associated macrophages, which associate themselves with the blood vessels in growing tumors 54. However, it is not entirely clear which types of macrophages promote and support vascularization of implanted biomaterials and engineered tissue constructs.
Recent studies have shown that implantation in mice of M2 macrophages that were polarized ex vivo and loaded into biomaterials resulted in increased vascularization over that achieved by M1 macrophages 41. M2 macrophages also promoted angiogenesis in a chick chorioallantoic membrane model relative to M1 macrophages 92. On the other hand, M1 macrophages secrete vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), IL-8, and other pro-angiogenic factors 28,68,75,89. M1-derived VEGF has been shown to be critical for the initiation of sprouting in early phases of wound repair 89. M1 macrophages are known to play a role in pro-inflammatory diseases characterized by excessive angiogenesis, such as rheumatoid arthritis, macular degeneration, and intervertebral disc degeneration 19,24,34,40,64,80. Signals from M1s (provided by conditioning culture medium) enhanced tube formation in vitro more than signals from M2s, as evidenced by increased sprouting of endothelial cells in Matrigel 75. While many studies support the notion that M2s contribute to vascularization of tissue engineering scaffolds 12,16,29,51,93, some assert that M1s in fact correlate with higher levels of vascularization, both in vitro and in vivo 14,83,84. Recent studies show that M2 macrophages facilitate anastomosis both directly [27, 62] and indirectly [72, 74].
THERE IS A NEED FOR SEQUENTIAL RECRUITMENT OF MACROPHAGE SUBTYPES
The apparent contradictions about which macrophage subtypes are angiogenic could be reconciled in part by the results of recent studies showing that (i) both M1s and M2s contribute to angiogenesis, but in distinctly different ways 62,75,85, and that (ii) the temporal aspects largely determine the resulting effects 75. These findings are perhaps unsurprising considering that normal wound healing depends on sequential activation of M1 and M2 macrophages, but the implications for biomaterial design are far-reaching.
Differential effects of M1s and M2s on scaffold vascularization
We recently showed that M1s stimulated sprout formation by endothelial cells relative to M2s in a Matrigel assay (Figure 3a). M2 macrophages promoted anastomosis of M1-induced sprouting vessels in a longer term assay in which endothelial cells were cultured on fibrin gels in media conditioned by either M1 or M2 macrophages, or in M1- and then M2-conditioned media (Figure 3b) 75. This work, in combination with other studies, suggests that M1 macrophages initiate angiogenesis and M2 macrophages stabilize growing blood vessels (Figure 3c). Interestingly, the sequential delivery of VEGF and platelet-derived growth factor-BB (PDGF-BB), factors that are secreted by M1 and M2 macrophages respectively, enhanced vascularization of scaffolds in numerous studies 76. However, it is worth noting that in our study, M1 macrophages stimulated endothelial sprout formation at the same level as non-activated “M0” macrophages, suggesting that M2 polarization also causes inhibition of the natural stimulatory behavior of macrophages.
Figure 3. Contributions of M1 and M2 macrophages to angiogenesis.
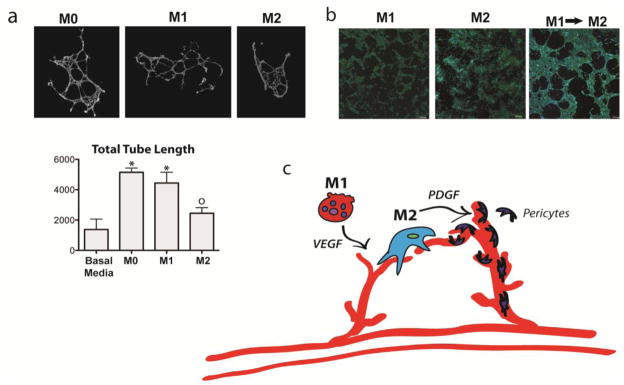
(a) M1 but not M2 macrophages increase sprouting of endothelial cells on Matrigel (* indicates p<0.05 and o indicates p>0.05 compared to control, one-way ANOVA and Tukey’s post-hoc analysis). Networks were stained with Live/Dead kit (Invitrogen), imaged at 10x magnification with an Olympus IX81 fluorescent microscope, and analyzed with ImageJ’s Angiogenesis Analyzer macro. (b) Endothelial cells organized into a loosely connected network on fibrin gel when the media is changed from M1-conditioned media to M2-conditioned media, which did not occur when endothelial cells were cultured uniformly in either M1- or M2-conditioned media. Endothelial cells were stained with DAPI and phalloidin tagged with Alexafluor-488 and imaged at 10x with an Olympus Fluoview FV1000 confocal microscope. (c) Proposed model of macrophage control over angiogenesis, in which M1 macrophages initiate sprouting via VEGF and M2 macrophages promote vessel stabilization via guiding anastomosis and possibly recruiting pericytes via PDGF. (Figure modified with permission from 75). Artwork provided by Servier Medical Arts.
M2-secreted PDGF-BB is known to recruit pericytes to stabilize blood vessels, suggesting that M2 macrophages may act directly on pericytes, although this has not been investigated in detail. One study did show that M1 macrophages can recruit muscle progenitor cells via high mobility group box 1, and that IL10-polarized M2s (M2c) recruit the same cells via matrix metalloprotease-9 (MMP9) 48.
Taken together, these results suggest that both M1 and M2 macrophages contribute to vascularization, but in different ways. To get further insights, we modified porous collagen scaffolds to elicit a range of macrophage responses 75. In a murine subcutaneous implantation model, only scaffolds surrounded by both M1s and M2s were vascularized after 10 days in vivo. Scaffolds surrounded primarily by M2s were encapsulated in fibrous tissue with no cell infiltration, while scaffolds infiltrated by M1s degraded to a fraction of their initial size. Interestingly, a study of subcutaneously implanted porous synthetic hydrogels based on poly(hydroxyethyl methacrylate) (pHEMA) also found that infiltrating macrophages were primarily M1 while those forming the fibrous capsule were primarily M279. However, in contrast to our study that showed that an association between a mixed M1/M2 population and vascularization, this study found that vascularization within pores was accompanied by an almost exclusively M1 population. Pore size was also found to play an important role in macrophage polarization, vascularization, and formation of the fibrous capsule, highlighting the complexities of comparing the interactions between macrophages and blood vessel growth in scaffolds with different properties.
In another study in which four different acellular dermal matrices were implanted around the epigastric vessels of rats, a mixed M1/M2 population was associated with constructive remodeling, as compared with scaffolds eliciting a primarily M1 or M2 response 1. Similarly, in a rat model of limb revascularization following femoral ligation, both M1s and M2s were associated with growing blood vessels 85. Suppression of M1s via injection of the anti-inflammatory drug dexamethasone caused decreased revascularization in ischemic tissue in mice, as measured by Laser Doppler imaging 85. In contrast, stimulating M2s by injection of IL4/IL13 or IL10 increased revascularization 85.
While there is evidence that M1s initiate vascularization 89, the role of M2s is less well understood. Macrophages similar to tumor-associated macrophages and not necessarily M2s were shown to directly mediate anastomosis of endothelial tip cells to complete the process of VEGF-induced sprout formation in a developing zebrafish 28. In mammalian retina, macrophages were localized at the branching points of developing vascular networks, directly facilitating anastomosis, and this behavior was mediated through Notch signaling 63. However, we recently showed that conditioned medium from M2s was sufficient to induce anastomosis, indicating that secreted factors also play a role, in addition to direct cell-to-cell contact 75.
Temporal aspects of immunomodulation
Differential effects of M1s and M2s suggest that the complementary functions of these two types of polarized macrophages in vascularization may be attributed, at least in part, to their temporal distribution. Based on what is known at present, the M1s act at early stages to initiate angiogenesis, whereas M2s act later to promote anastomosis and stabilization of the newly formed blood vessels 75.
Animal models helped establish that during the normal healing M1s appear immediately (within 1–5 days following injury), and are followed by M2s (within 7–10 days following injury) 7,85,86. We recently showed that exposing endothelial cells first to the signals derived from M1s and then to the signals from M2s (by using culture medium conditioned with one or other type of macrophages) resulted in the in vitro formation of blood vessel networks in fibrin gels 75. These findings are consistent with the vascular sprout formation at early stages of tissue repair that is induced by macrophage-derived VEGF 89. Similarly, in a mouse model of skeletal muscle injury, depletion of macrophages at early stages of repair via CD11b-diptheria toxin completely prevented muscle regeneration, while depletion at later stages just decreased the diameter of regenerating muscle fibers 7. Taken together, these studies stress the importance of the temporal presence of both the M1s and M2s for successful vascularization of regenerating tissues.
If the M1-to-M2 transition is disrupted, leaving behind M1s as the dominant phenotype over prolonged time, the wound suffers from chronic inflammation and impaired healing 43. Thus, the balance of macrophage phenotypes largely determines the course and outcome of repair, and the ratio of the numbers of M1s to M2s has been used as a predictor of wound healing in animal models of atherosclerotic lesions 42, inflammatory renal disease 88, and biomaterial implantation 15,16.
HARNESSING THE INFLAMMATORY RESPONSE IN TISSUE ENGINEERING
Scaffold approach
Scaffolds can be quite effective in modulation of inflammatory signals, in vivo and in vitro. When bone marrow stromal cells were seeded onto tubular scaffolds and implanted as a vascular graft in mice, the exogenous cells survived only a few days but the grafts were fully incorporated into the host vasculature through the actions of host monocytes and macrophages 67. In fact, this outcome was achieved even using hydrogel microparticles releasing monocyte chemoattractant protein-1 (MCP-1), showing that macrophages can be harnessed pharmacologically to promote regeneration. Macrophages have been shown to act directly on host progenitor cells during repair to promote formation of new tissue (e.g., muscle, bone, adipose), further highlighting ability to orchestrate regeneration and vascularization 2,4,48.
Scaffolds may be designed to control macrophage behavior. For example, pore structure has been shown to affect macrophage behavior, with resulting effects on vascularization and regeneration 14,50. Release of IL-4 from agarose scaffolds enhanced peripheral nerve repair in a critically-sized sciatic nerve gap model in rats compared to controls or scaffolds releasing IFN-γ, and higher ratios of M2 to M1 macrophages were correlated with increased numbers of regenerated axons 59. Similarly, controlled release of sphingosine 1-P (S1P) agonists from scaffolds for bone repair promoted the recruitment of macrophages, with concomitant increases in scaffold integration, vascularization, and formation of new bone in mandibular and tibial defects in rodents 11,25,65. Notably, macrophages exhibit a predominantly M1 phenotype at 3 days post implantation and transition to M2 phenotype by day 10, mimicking the natural sequence of activation 44.
Cellular approach
Adult mesenchymal stem cells (MSCs) are a favored cell source for tissue repair due to their ability to differentiate into mesenchymal tissues and their availability as an autologous cell source known to promote angiogenesis and vascular stabilization. A landmark study by Arinzeh et al. 6 showed that ceramic scaffolds seeded with allogeneic MSCs caused bone regeneration in critical sized segmental defects without a detectable immune response. In another study, intravenous infusion of MSCs following myocardial infarction in mice reduced inflammation and improved cardiac function 26. These two studies exemplify how MSCs enhance tissue remodeling by providing the appropriate signals to dampen the immune response via inhibition of T-Cells 82, and by modulating the inflammatory responses to improve healing 13,36,56.
The ability of MSCs to modulate macrophages may be beneficial for construct integration and healing, provided that they retain their immunomodulatory potential after differentiating into mature cell types. One study showed that MSC-seeded scaffolds that were cultured for 4 weeks prior to implantation were surrounded by more M1 macrophages than acellular scaffolds, suggesting that MSCs may lose some immunomodulatory activity during differentiation 50. MSCs cultured in tricalcium phosphate/hydroxyapatite scaffolds for only one day prior to subcutaneous implantation in immunocompromised mice elicited a greater inflammatory response than acellular scaffolds after 8 weeks in vivo 31. In parallel with the increase in the inflammatory response, more vascularization and bone formation was observed on the MSC-seeded scaffolds. Another study showed that ceramic scaffolds seeded with allogeneic MSCs developed sarcomas in immunocompromised mice but not in immunocompetent mice or when the cells were injected as cell suspensions 81. Overall these studies show that MSCs interact with the biomaterial, T cells, and macrophages to modulate the final host tissue response, ultimately affecting tissue formation.
By understanding these interactions, the immunoregulatory aspects of MSCs can be utilized in tissue engineering depending on the timing of implantation following injury and/or the type of inflammatory environment present at the site of injury.
Timing of implantation
Another aspect of the host tissue response that could be harnessed as part of a tissue engineering strategy is the timing at which an engineered construct is implanted. The host tissue response has dynamics that can persist from days to months. Given the changes observed during the different stages of inflammation, timing of implantation could play a significant role in vascularization of engineered constructs 33.
Engineers can take advantage of these events and design the engineered construct in ways that takes this environment into account to maximize survival and engraftment. A recent study looked at the effects of an engineered cardiac patch composed of cardiac extracellular matrix (ECM) and MSCs implanted during the acute or chronic phases of myocardial infarction, finding that patches implanted during the acute phase showed better healing and angiogenesis than patches implanted during the chronic phase 36. In addition, a larger number of MSCs migrated out of the patch and invaded the infarcted tissue when implanted during the acute phase.
Interestingly, pre-conditioning the MSCs with transforming growth factor-β (TGF-β) increased their migration toward the inflammatory response-derived cytokine stromal derived factor-1 (SDF-1), highlighting the idea that interactions between implanted cells and the inflammatory response can be mediated 36. Another example of the importance of timing of implantation or delivery is a study where TNF-α was administered to mouse bone fractures within 24 hrs of injury and showed accelerated healing 35, while prolonged inflammation is well-known to impair healing in a variety of tissues 15,16,43.
Based on these results, MSCs or injectable signals could be delivered soon after injury to maximize the recruitment of repair cells into the wound and increase angiogenesis. Implanted cells may also be preconditioned to interact more favorably with the inflammatory response. Although these principles might not apply to all cells and injury environments, it is likely that similar approaches can be used for different wound types once the interactions between repair cells and host tissue has been established.
SUMMARY AND FUTURE DIRECTIONS
The next generation of scaffold-bioreactor approaches to tissue regeneration will meet a number of emerging clinical needs, directed towards consideration of interactions between the engineered construct and its vasculature with the native cells and inflammatory response of the host environment (Figure 4).
Figure 4. Immunomodulation of engineered tissues for enhanced vascularization and healing.
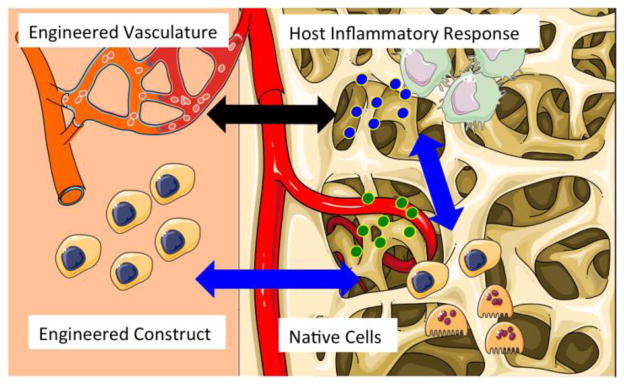
Advanced tissue engineering strategies will take into account interactions between the cells and vasculature of engineered tissue with the native cells and inflammatory response of the host environment. Artwork provided by Servier Medical Arts.
More relevant models of disease conditions
Engineered tissues are usually evaluated in healthy animals, while most patients have comorbidities that can influence the inflammatory response. For example, while stimulating inflammation may be beneficial for inducing tissue regeneration in an otherwise healthy person, it may need to be suppressed in a patient with a chronic inflammatory disease such as diabetes. It is known that macrophages in diabetic and chronic venous ulcers are stalled in the M1 state 74, so inhibiting M1 activation may be a useful strategy in treating these wounds. Implantation into chronic inflammatory environments would help develop strategies for modulating the inflammatory signals towards most stable and robust function of the engineered constructs. Design principles derived from these studies in animal models of osteoarthritis or diabetes, for example, would further extend the application of tissue engineering to clinical conditions of interest.
Also, biomaterial scaffolds are traditionally evaluated in small animal models, even though their immune systems and response to injury are quite different from that of humans 71. A deeper understanding of the similarities and differences between rodent and human immune systems, and between in vitro and in vivo behavior of both rodent and human tissue constructs, would allow extension of experimental findings to clinical applications. A range of microtissue platforms is being developed for testing drug toxicity 17,78 and studies of interactions between implanted cells and the inflammatory system of the host 15,16,32,33,62,70,75. These platforms are likely to be a major driver towards understanding differences between rodent and human systems, and using these insights to design tissue constructs for maximal survival and function after implantation.
In addition, the use of humanized mice engrafted with human hematopoietic stem cells, peripheral blood mononuclear cells, or fetal liver and thymus tissue may also allow more complex studies of the interactions between engineered tissues and cells of the human immune response73. These systems have already been used to develop tissue-engineered human liver as a drug testing platform20. However, components of the mouse innate immune system remain in these models and represent an impediment to faithful recapitulation of the human immune system72.
Advanced methods for analyzing data
Future studies will also require advanced methods for analyzing macrophage behavior, and a more nuanced understanding of their phenotypes. Most current studies use one or two markers to indicate macrophage phenotype without considering that hybrid phenotypes may also play significant roles in tissue healing. Macrophages exist as a spectrum of phenotypes and their response to engineered tissues have not been explored in depth. In addition, in vitro studies typically employ relatively homogenous populations of M1 and M2 macrophages, in contrast to the dynamically changing populations that are observed in vivo. Most studies consider M2a and M2c macrophage subtypes as one single general category, even though they have little in common 48,75. While it has been described that M1 and M2 macrophages appear sequentially during progression of normal wound healing, the temporal distribution of M2a and M2c macrophages has not been studied.
Contributing to our tendency to oversimplify macrophage behavior is the difficulty in characterizing macrophage phenotypes. Most studies have used the presence or absence of a single surface receptor as indicator of macrophage phenotype, but in reality all macrophage subtypes express multiple and often overlapping markers, and it is the up-regulation or down-regulation that actually marks the phenotype changes, than the presence or absence of expression 75. Thus, the intensity of expression would be a better indicator of macrophage phenotype.
New generation of scaffold-bioreactor systems
Biomaterial scaffolds are now being designed with perfusable microfluidic channels that mimic small blood vessels and provide interfacial area for transport between the vascular and parenchymal compartments 90. Other approaches are proposed for synergizing vascular development and tissue development within the same scaffold 23. Controlled-release scaffolds providing sequential presentation of pro-angiogenic and pro-maturation factors are used to enhance vascularization and integration of implanted tissues 17. These trends will likely continue, as they are tackling a fundamental problem of the whole field of tissue engineering.
The next generation of tissue engineering strategies should continue to take into consideration the inflammatory response. We recently showed that scaffold vascularization can be achieved solely through manipulation of macrophage behavior 75. One can envision that immunomodulatory factors may be released from scaffolds in a sequential fashion to recruit the M1 and M2 macrophages. Many elements are needed for developing tissue-engineering scaffolds with the capacity to actively modulate vascularization, integration and maturation of implanted engineered tissues. Harnessing the inflammatory signals of the host is emerging as a particularly effective component of these strategies.
Acknowledgments
The authors gratefully acknowledge funding from NIH (DE016525, HL076485, EB17103, EB002520, AR061988) and NYSTEM (C026721A) of the work described in this article.
Abbreviations, symbols, terminology
- M0
mature and non-polarized macrophages
- M1
macrophages polarized to the inflammatory phenotype, either by in vitro stimulation or in the in vivo environment
- M2
macrophages polarized to the anti-inflammatory phenotype, either by in vitro stimulation or in the in vivo environment
- M2a
macrophages polarized through the addition of IL-4
- M2c
macrophages polarized through the addition of IL-10
- PBMCs
peripheral blood mononuclear cells
- MSCs
mesenchymal stem cells
- IL
interleukin
- TNF-α
tumor necrosis factor-alpha
- TLRs
toll-like receptors
- VEGF
vascular endothelial growth factor
- bFGF
basic fibroblast growth factor
- PDGF-BB
platelet-derived growth factor-BB
- MMP9
matrix metalloprotease-9
- MCP-1
monocyte chemoattractant protein-1
- IFN-γ
interferon-gamma
- S1P
sphingosine-1-phosphate
- ECM
extracellular matrix
- TGF-β
transforming growth factor-β
- SDF-1
stromal-derived factor 1
References
- 1.Agrawal H, Tholpady SS, Capito AE, Drake DB, Katz AJ. Macrophage phenotypes correspond with remodeling outcomes of various acellular dermal matrices. Open Journal of Regenerative Medicine. 2012;1:51–59. [Google Scholar]
- 2.Alexander KA, Chang MK, Maylin ER, Kohler T, Muller R, Wu AC, Van Rooijen N, Sweet MJ, Hume DA, Raggatt LJ, Pettit AR. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J Bone Miner Res. 2011;26:1517–1532. doi: 10.1002/jbmr.354. [DOI] [PubMed] [Google Scholar]
- 3.Anghelina M, Krishnan P, Moldovan L, Moldovan NI. Monocytes and macrophages form branched cell columns in matrigel: implications for a role in neovascularization. Stem Cells Dev. 2004;13:665–676. doi: 10.1089/scd.2004.13.665. [DOI] [PubMed] [Google Scholar]
- 4.Anghelina M, Krishnan P, Moldovan L, Moldovan NI. Monocytes/macrophages cooperate with progenitor cells during neovascularization and tissue repair: conversion of cell columns into fibrovascular bundles. Am J Pathol. 2006;168:529–541. doi: 10.2353/ajpath.2006.050255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arendt LM, McCready J, Keller PJ, Baker DD, Naber SP, Seewaldt V, Kuperwasser C. Obesity Promotes Breast Cancer by CCL2-Mediated Macrophage Recruitment and Angiogenesis. Cancer Res. 2013;73:6080–6093. doi: 10.1158/0008-5472.CAN-13-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arinzeh TL, Peter SJ, Archambault MP, van den Bos C, Gordon S, Kraus K, Smith A, Kadiyala S. Allogeneic mesenchymal stem cells regenerate bone in a critical-sized canine segmental defect. J Bone Joint Surg Am. 2003;85-A:1927–1935. doi: 10.2106/00004623-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arras M, Ito WD, Scholz D, Winkler B, Schaper J, Schaper W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest. 1998;101:40–50. doi: 10.1172/JCI119877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JL, Mizel DE, Anzano M, Greenwell-Wild T, Wahl SM, Deng C, Roberts AB. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1:260–266. doi: 10.1038/12971. [DOI] [PubMed] [Google Scholar]
- 10.Auwerx J. The human leukemia cell line, THP-1: a multifacetted model for the study of monocyte-macrophage differentiation. Experientia. 1991;47:22–31. doi: 10.1007/BF02041244. [DOI] [PubMed] [Google Scholar]
- 11.Awojoodu AO, Ogle ME, Sefcik LS, Bowers DT, Martin K, Brayman KL, Lynch KR, Peirce-Cottler SM, Botchwey E. Sphingosine 1-phosphate receptor 3 regulates recruitment of anti-inflammatory monocytes to microvessels during implant arteriogenesis. Proc Natl Acad Sci U S A. 2013;110:13785–13790. doi: 10.1073/pnas.1221309110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A. 2008;14:1835–1842. doi: 10.1089/ten.tea.2007.0264. [DOI] [PubMed] [Google Scholar]
- 13.Bartunek J, Behfar A, Vanderheyden M, Wijns W, Terzic A. Mesenchymal stem cells and cardiac repair: principles and practice. Journal of cardiovascular translational research. 2008;1:115–119. doi: 10.1007/s12265-008-9021-5. [DOI] [PubMed] [Google Scholar]
- 14.Bota PC, Collie AM, Puolakkainen P, Vernon RB, Sage EH, Ratner BD, Stayton PS. Biomaterial topography alters healing in vivo and monocyte/macrophage activation in vitro. J Biomed Mater Res A. 2010;95:649–657. doi: 10.1002/jbm.a.32893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown BN, Londono R, Tottey S, Zhang L, Kukla KA, Wolf MT, Daly KA, Reing JE, Badylak SF. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 2012;8:978–987. doi: 10.1016/j.actbio.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30:1482–1491. doi: 10.1016/j.biomaterials.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brudno Y, Ennett-Shepard AB, Chen RR, Aizenberg M, Mooney DJ. Enhancing microvascular formation and vessel maturation through temporal control over multiple pro-angiogenic and pro-maturation factors. Biomaterials. 2013;34:9201–9209. doi: 10.1016/j.biomaterials.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunelli S, Rovere-Querini P. Pharmacol Res. 2008;58:117–121. doi: 10.1016/j.phrs.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Cao X, Shen D, Patel MM, Tuo J, Johnson TM, Olsen TW, Chan CC. Macrophage polarization in the maculae of age-related macular degeneration: a pilot study. Pathol Int. 2011;61:528–535. doi: 10.1111/j.1440-1827.2011.02695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen AA, Thomas DK, Ong LL, Schwartz RE, Golub TR, Bhatia SN. Humanized mice with ectopic artificial liver tissues. Proc Natl Acad Sci U S A. 2011;108:11842–11847. doi: 10.1073/pnas.1101791108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho DI, Kim MR, Jeong HY, Jeong HC, Jeong MH, Yoon SH, Kim YS, Ahn Y. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med. 2014;46:e70. doi: 10.1038/emm.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conejo-Garcia JR, Buckanovich RJ, Benencia F, Courreges MC, Rubin SC, Carroll RG, Coukos G. Vascular leukocytes contribute to tumor vascularization. Blood. 2005;105:679–681. doi: 10.1182/blood-2004-05-1906. [DOI] [PubMed] [Google Scholar]
- 23.Correia C, Grayson WL, Park M, Hutton D, Zhou B, Guo XE, Niklason L, Sousa RA, Reis RL, Vunjak-Novakovic G. In vitro model of vascularized bone: synergizing vascular development and osteogenesis. PLoS One. 2011;6:e28352. doi: 10.1371/journal.pone.0028352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costa C, Incio J, Soares R. Angiogenesis and chronic inflammation: cause or consequence? Angiogenesis. 2007;10:149–166. doi: 10.1007/s10456-007-9074-0. [DOI] [PubMed] [Google Scholar]
- 25.Das A, Segar CE, Hughley BB, Bowers DT, Botchwey EA. The promotion of mandibular defect healing by the targeting of S1P receptors and the recruitment of alternatively activated macrophages. Biomaterials. 2013;34:9853–9862. doi: 10.1016/j.biomaterials.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dayan V, Yannarelli G, Billia F, Filomeno P, Wang XH, Davies JE, Keating A. Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res Cardiol. 2011;106:1299–1310. doi: 10.1007/s00395-011-0221-9. [DOI] [PubMed] [Google Scholar]
- 27.DeFalco T, Bhattacharya I, Williams AV, Sams DM, Capel B. Yolk-sac-derived macrophages regulate fetal testis vascularization and morphogenesis. Proc Natl Acad Sci U S A. 2014;111:E2384–2393. doi: 10.1073/pnas.1400057111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116:829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fishman JM, Lowdell MW, Urbani L, Ansari T, Burns AJ, Turmaine M, North J, Sibbons P, Seifalian AM, Wood KJ, Birchall MA, De Coppi P. Immunomodulatory effect of a decellularized skeletal muscle scaffold in a discordant xenotransplantation model. Proc Natl Acad Sci U S A. 2013;110:14360–14365. doi: 10.1073/pnas.1213228110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frangogiannis NG. Pharmacol Res. 2008;58:88–111. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frescaline G, Bouderlique T, Mansoor L, Carpentier G, Baroukh B, Sineriz F, Trouillas M, Saffar JL, Courty J, Lataillade JJ, Papy-Garcia D, Albanese P. Glycosaminoglycan mimetic associated to human mesenchymal stem cell-based scaffolds inhibit ectopic bone formation, but induce angiogenesis in vivo. Tissue Eng Part A. 2013;19:1641–1653. doi: 10.1089/ten.tea.2012.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freytes DO, Kang JW, Marcos-Campos I, Vunjak-Novakovic G. Macrophages modulate the viability and growth of human mesenchymal stem cells. J Cell Biochem. 2013;114:220–229. doi: 10.1002/jcb.24357. [DOI] [PubMed] [Google Scholar]
- 33.Freytes DO, Santambrogio L, Vunjak-Novakovic G. Optimizing dynamic interactions between a cardiac patch and inflammatory host cells. Cells Tissues Organs. 2012;195:171–182. doi: 10.1159/000331392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuentes-Duculan J, Suarez-Farinas M, Zaba LC, Nograles KE, Pierson KC, Mitsui H, Pensabene CA, Kzhyshkowska J, Krueger JG, Lowes MA. A subpopulation of CD163-positive macrophages is classically activated in psoriasis. J Invest Dermatol. 2010;130:2412–2422. doi: 10.1038/jid.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glass GE, Chan JK, Freidin A, Feldmann M, Horwood NJ, Nanchahal J. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci U S A. 2011;108:1585–1590. doi: 10.1073/pnas.1018501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Godier-Furnemont AF, Martens TP, Koeckert MS, Wan L, Parks J, Arai K, Zhang G, Hudson B, Homma S, Vunjak-Novakovic G. Composite scaffold provides a cell delivery platform for cardiovascular repair. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7974–7979. doi: 10.1073/pnas.1104619108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hibino N, Yi T, Duncan DR, Rathore A, Dean E, Naito Y, Dardik A, Kyriakides T, Madri J, Pober JS, Shinoka T, Breuer CK. A critical role for macrophages in neovessel formation and the development of stenosis in tissue-engineered vascular grafts. FASEB J. 2011;25:4253–4263. doi: 10.1096/fj.11-186585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirose N, Maeda H, Yamamoto M, Hayashi Y, Lee GH, Chen L, Radhakrishnan G, Rao P, Sasaguri S. The local injection of peritoneal macrophages induces neovascularization in rat ischemic hind limb muscles. Cell Transplant. 2008;17:211–222. doi: 10.3727/000000008783906919. [DOI] [PubMed] [Google Scholar]
- 39.Hisatome T, Yasunaga Y, Yanada S, Tabata Y, Ikada Y, Ochi M. Neovascularization and bone regeneration by implantation of autologous bone marrow mononuclear cells. Biomaterials. 2005;26:4550–4556. doi: 10.1016/j.biomaterials.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 40.Hutter R, Speidl WS, Valdiviezo C, Sauter B, Corti R, Fuster V, Badimon JJ. Macrophages transmit potent proangiogenic effects of oxLDL in vitro and in vivo involving HIF-1alpha activation: a novel aspect of angiogenesis in atherosclerosis. J Cardiovasc Transl Res. 2013;6:558–569. doi: 10.1007/s12265-013-9469-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jetten N, Verbruggen S, Gijbels MJ, Post MJ, De Winther MP, Donners MM. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014;17:109–118. doi: 10.1007/s10456-013-9381-6. [DOI] [PubMed] [Google Scholar]
- 42.Khallou-Laschet J, Varthaman A, Fornasa G, Compain C, Gaston AT, Clement M, Dussiot M, Levillain O, Graff-Dubois S, Nicoletti A, Caligiuri G. Macrophage plasticity in experimental atherosclerosis. PLoS One. 2010;5:e8852. doi: 10.1371/journal.pone.0008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim YH, Furuya H, Tabata Y. Enhancement of bone regeneration by dual release of a macrophage recruitment agent and platelet-rich plasma from gelatin hydrogels. Biomaterials. 2014;35:214–224. doi: 10.1016/j.biomaterials.2013.09.103. [DOI] [PubMed] [Google Scholar]
- 45.Kitajewski J. Wnts heal by restraining angiogenesis. Blood. 2013;121:2381–2382. doi: 10.1182/blood-2013-01-479063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kubota Y, Takubo K, Shimizu T, Ohno H, Kishi K, Shibuya M, Saya H, Suda T. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J Exp Med. 2009;206:1089–1102. doi: 10.1084/jem.20081605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lambert JM, Lopez EF, Lindsey ML. Int J Cardiol. 2008;130:147–158. doi: 10.1016/j.ijcard.2008.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lolmede K, Campana L, Vezzoli M, Bosurgi L, Tonlorenzi R, Clementi E, Bianchi ME, Cossu G, Manfredi AA, Brunelli S, Rovere-Querini P. Inflammatory and alternatively activated human macrophages attract vessel-associated stem cells, relying on separate HMGB1- and MMP-9-dependent pathways. J Leukoc Biol. 2009;85:779–787. doi: 10.1189/jlb.0908579. [DOI] [PubMed] [Google Scholar]
- 49.Low-Marchelli JM, Ardi VC, Vizcarra EA, van Rooijen N, Quigley JP, Yang J. Twist1 induces CCL2 and recruits macrophages to promote angiogenesis. Cancer Res. 2013;73:662–671. doi: 10.1158/0008-5472.CAN-12-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lyons FG, Al-Munajjed AA, Kieran SM, Toner ME, Murphy CM, Duffy GP, O’Brien FJ. The healing of bony defects by cell-free collagen-based scaffolds compared to stem cell-seeded tissue engineered constructs. Biomaterials. 2010;31:9232–9243. doi: 10.1016/j.biomaterials.2010.08.056. [DOI] [PubMed] [Google Scholar]
- 51.Madden LR, Mortisen DJ, Sussman EM, Dupras SK, Fugate JA, Cuy JL, Hauch KD, Laflamme MA, Murry CE, Ratner BD. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc Natl Acad Sci U S A. 2010;107:15211–15216. doi: 10.1073/pnas.1006442107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 53.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 54.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 55.Martinez FO, Gordon S, Locati M, Mantovani A. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 56.Mathieu M, Bartunek J, El Oumeiri B, Touihri K, Hadad I, Thoma P, Metens T, da Costa AM, Mahmoudabady M, Egrise D, Blocklet D, Mazouz N, Naeije R, Heyndrickx G, McEntee K. Cell therapy with autologous bone marrow mononuclear stem cells is associated with superior cardiac recovery compared with use of nonmodified mesenchymal stem cells in a canine model of chronic myocardial infarction. The Journal of thoracic and cardiovascular surgery. 2009;138:646–653. doi: 10.1016/j.jtcvs.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 57.Medina RJ, O’Neill CL, O’Doherty TM, Knott H, Guduric-Fuchs J, Gardiner TA, Stitt AW. Myeloid angiogenic cells act as alternative M2 macrophages and modulate angiogenesis through interleukin-8. Mol Med. 2011;17:1045–1055. doi: 10.2119/molmed.2011.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mikos AG, McIntire LV, Anderson JM, Babensee JE. Host response to tissue engineered devices. Adv Drug Deliv Rev. 1998;33:111–139. doi: 10.1016/s0169-409x(98)00023-4. [DOI] [PubMed] [Google Scholar]
- 59.Mokarram N, Merchant A, Mukhatyar V, Patel G, Bellamkonda RV. Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials. 2012;33:8793–8801. doi: 10.1016/j.biomaterials.2012.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo J-L, Libby P, Weissleder R, Pittet MJ. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Outtz HH, Tattersall IW, Kofler NM, Steinbach N, Kitajewski J. Notch1 controls macrophage recruitment and Notch signaling is activated at sites of endothelial cell anastomosis during retinal angiogenesis in mice. Blood. 2011;118:3436–3439. doi: 10.1182/blood-2010-12-327015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peng B, Hao J, Hou S, Wu W, Jiang D, Fu X, Yang Y. Possible pathogenesis of painful intervertebral disc degeneration. Spine (Phila Pa 1976) 2006;31:560–566. doi: 10.1097/01.brs.0000201324.45537.46. [DOI] [PubMed] [Google Scholar]
- 65.Petrie Aronin CE, Shin SJ, Naden KB, Rios PD, Jr, Sefcik LS, Zawodny SR, Bagayoko ND, Cui Q, Khan Y, Botchwey EA. The enhancement of bone allograft incorporation by the local delivery of the sphingosine 1-phosphate receptor targeted drug FTY720. Biomaterials. 2010;31:6417–6424. doi: 10.1016/j.biomaterials.2010.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Repnik U, Knezevic M, Jeras M. Simple and cost-effective isolation of monocytes from buffy coats. Journal of immunological methods. 2003;278:283–292. doi: 10.1016/s0022-1759(03)00231-x. [DOI] [PubMed] [Google Scholar]
- 67.Roh JD, Sawh-Martinez R, Brennan MP, Jay SM, Devine L, Rao DA, Yi T, Mirensky TL, Nalbandian A, Udelsman B, Hibino N, Shinoka T, Saltzman WM, Snyder E, Kyriakides TR, Pober JS, Breuer CK. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci U S A. 2010;107:4669–4674. doi: 10.1073/pnas.0911465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sainson RC, Johnston DA, Chu HC, Holderfield MT, Nakatsu MN, Crampton SP, Davis J, Conn E, Hughes CC. TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype. Blood. 2008;111:4997–5007. doi: 10.1182/blood-2007-08-108597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sakurai E, Anand A, Ambati BK, van Rooijen N, Ambati J. Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3578–3585. doi: 10.1167/iovs.03-0097. [DOI] [PubMed] [Google Scholar]
- 70.Sefton MV, Babensee JE, Woodhouse KA. Innate and adaptive immune responses in tissue engineering. Semin Immunol. 2008;20:83–85. doi: 10.1016/j.smim.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 71.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012;12:786–798. doi: 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 74.Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, Hainzl A, Schatz S, Qi Y, Schlecht A, Weiss JM, Wlaschek M, Sunderkotter C, Scharffetter-Kochanek K. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121:985–997. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, Vunjak-Novakovic G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35:4477–4488. doi: 10.1016/j.biomaterials.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spiller KL, Vunjak-Novakovic G. Clinical translation of controlled protein delivery systems for tissue engineering. Drug Deliv and Transl Res. 2013 Feb 21; doi: 10.1007/s13346-013-0135-1. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stefater JA, 3rd, Rao S, Bezold K, Aplin AC, Nicosia RF, Pollard JW, Ferrara N, Lang RA. Macrophage Wnt-Calcineurin-Flt1 signaling regulates mouse wound angiogenesis and repair. Blood. 2013;121:2574–2578. doi: 10.1182/blood-2012-06-434621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sung JH, Shuler ML. Microtechnology for mimicking in vivo tissue environment. Ann Biomed Eng. 2012;40:1289–1300. doi: 10.1007/s10439-011-0491-2. [DOI] [PubMed] [Google Scholar]
- 79.Sussman EM, Halpin MC, Muster J, Moon RT, Ratner BD. Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction. Ann Biomed Eng. 2014;42:1508–1516. doi: 10.1007/s10439-013-0933-0. [DOI] [PubMed] [Google Scholar]
- 80.Szekanecz Z, Koch AE. Mechanisms of Disease: angiogenesis in inflammatory diseases. Nat Clin Pract Rheumatol. 2007;3:635–643. doi: 10.1038/ncprheum0647. [DOI] [PubMed] [Google Scholar]
- 81.Tasso R, Augello A, Carida M, Postiglione F, Tibiletti MG, Bernasconi B, Astigiano S, Fais F, Truini M, Cancedda R, Pennesi G. Development of sarcomas in mice implanted with mesenchymal stem cells seeded onto bioscaffolds. Carcinogenesis. 2009;30:150–157. doi: 10.1093/carcin/bgn234. [DOI] [PubMed] [Google Scholar]
- 82.Tasso R, Pennesi G. When stem cells meet immunoregulation. Int Immunopharmacol. 2009;9:596–598. doi: 10.1016/j.intimp.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 83.Tolg C, Hamilton SR, Zalinska E, McCulloch L, Amin R, Akentieva N, Winnik F, Savani R, Bagli DJ, Luyt LG, Cowman MK, McCarthy JB, Turley EA. A RHAMM mimetic peptide blocks hyaluronan signaling and reduces inflammation and fibrogenesis in excisional skin wounds. Am J Pathol. 2012;181:1250–1270. doi: 10.1016/j.ajpath.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tous E, Weber HM, Lee MH, Koomalsingh KJ, Shuto T, Kondo N, Gorman JH, Lee D, 3rd, Gorman RC, Burdick JA. Tunable hydrogel-microsphere composites that modulate local inflammation and collagen bulking. Acta Biomater. 2012;8:3218–3227. doi: 10.1016/j.actbio.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Troidl C, Jung G, Troidl K, Hoffmann J, Mollmann H, Nef H, Schaper W, Hamm CW, Schmitz-Rixen T. The temporal and spatial distribution of macrophage subpopulations during arteriogenesis. Curr Vasc Pharmacol. 2013;11:5–12. [PubMed] [Google Scholar]
- 86.Troidl C, Mollmann H, Nef H, Masseli F, Voss S, Szardien S, Willmer M, Rolf A, Rixe J, Troidl K, Kostin S, Hamm C, Elsasser A. Classically and alternatively activated macrophages contribute to tissue remodelling after myocardial infarction. J Cell Mol Med. 2009;13:3485–3496. doi: 10.1111/j.1582-4934.2009.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vunjak-Novakovic G, Scadden DT. Biomimetic platforms for human stem cell research. Cell Stem Cell. 2011;8:252–261. doi: 10.1016/j.stem.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y, Wang YP, Zheng G, Lee VW, Ouyang L, Chang DH, Mahajan D, Coombs J, Wang YM, Alexander SI, Harris DC. Ex vivo programmed macrophages ameliorate experimental chronic inflammatory renal disease. Kidney Int. 2007;72:290–299. doi: 10.1038/sj.ki.5002275. [DOI] [PubMed] [Google Scholar]
- 89.Willenborg S, Lucas T, van Loo G, Knipper JA, Krieg T, Haase I, Brachvogel B, Hammerschmidt M, Nagy A, Ferrara N, Pasparakis M, Eming SA. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood. 2012;120:613–625. doi: 10.1182/blood-2012-01-403386. [DOI] [PubMed] [Google Scholar]
- 90.Ye X, Lu L, Kolewe ME, Park H, Larson BL, Kim ES, Freed LE. A biodegradable microvessel scaffold as a framework to enable vascular support of engineered tissues. Biomaterials. 2013;34:10007–10015. doi: 10.1016/j.biomaterials.2013.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ytrehus K. The ischemic heart--experimental models. Pharmacol Res. 2000;42:193–203. doi: 10.1006/phrs.2000.0669. [DOI] [PubMed] [Google Scholar]
- 92.Zajac E, Schweighofer B, Kupriyanova TA, Juncker-Jensen A, Minder P, Quigley JP, Deryugina EI. Angiogenic capacity of M1- and M2-polarized macrophages is determined by the levels of TIMP-1 complexed with their secreted proMMP-9. Blood. 2013;122:4054–4067. doi: 10.1182/blood-2013-05-501494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang L, Cao Z, Bai T, Carr L, Ella-Menye JR, Irvin C, Ratner BD, Jiang S. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat Biotechnol. 2013;31:553–556. doi: 10.1038/nbt.2580. [DOI] [PubMed] [Google Scholar]
- 94.Zhang SJ, Zhang H, Wei YJ, Su WJ, Liao ZK, Hou M, Zhou JY, Hu SS. Adult endothelial progenitor cells from human peripheral blood maintain monocyte/macrophage function throughout in vitro culture. Cell Res. 2006;16:577–584. doi: 10.1038/sj.cr.7310075. [DOI] [PubMed] [Google Scholar]


