Abstract
Objective
To examine the association of gene variants of uncoupling proteins (UCP)-2 and -3 with obesity and gastrointestinal (GI) traits.
Methods
In 255 overweight or obese adults, we studied the associations of gene variants in UCP-2 (−3474, rs659366) and UCP-3 (rs1626521, rs2075577, rs15763) with body weight (BW) and GI traits. Gene variants were genotyped by TaqMan® assay. We assessed the associations of genotypes with BW and GI traits (gastric emptying, gastric volume, satiety by buffet meal, satiation by nutrient drink test and GI hormones) using ANCOVA, corrected for false detection rate (FDR).
Results
We identified a novel UCP-3 gene variant, rs1626521; it was associated with BW (p=0.039), waist circumference (p=0.035), and with significantly higher postprandial gastric volume (p=0.003) and calories ingested at buffet meal (p=0.006, both significant with FDR). In a subgroup of 11 participants, rs1626521 was also associated with reduced mitochondrial bioenergetics efficiency in skeletal muscle (p=0.051). In an in vitro study in HEK293 cells, rs1626521 reduced UCP-3 protein expression (p=0.049). Associations detected between other genotypes and GI traits were non-significant with FDR.
Conclusions
A newly identified functional variant (rs1626521) in UCP-3 affects postprandial gastric functions and satiety and may contribute to weight gain and alter human mitochondrial function.
Keywords: mitochondria, gastric emptying, accommodation, volume, satiation, satiety, GLP-1, PYY
INTRODUCTION
Obesity is a complex disease, with interactions between genetic predisposition, regulation of food intake and energy expenditure, food-seeking behavior, and the environment 1, 2. Obesity has a strong association with type 2 diabetes mellitus (T2DM). The control of food intake, that is the size and frequency of meals, is a major factor in the determination of the individual’s weight 3 . Gastrointestinal functions such as gastric emptying and capacity (volume) also influence food intake 4 and may, therefore, influence body weight.
There are standardized approaches to study eating behavior in humans 5, including satiation that results in the within-meal inhibition of eating, and satiety which reflects appetite ratings, appetite-related peptides and measures of calorie intake 3. Quantitative traits facilitate the studies of the role of genetics in complex diseases 6. Thus, it is considered that endophenotype-based genetic analysis may be more likely to identify susceptibility genes compared to clinical phenotype-based approaches 7. In obesity, endophenotypes have been identified; recently, we reported that overweight and obese individuals (when compared to normal weight controls) have significant differences in gastrointestinal quantitative traits. These differences are: lower satiation manifested as higher Ensure® volume intake required to experience fullness; accelerated gastric emptying of liquids and solids [with increase of plasma glucagon-like peptide 1 (GLP-1) which may result from the accelerated gastric emptying]; increased fasting gastric volume; and decreased peak postprandial plasma peptide tyrosine-tyrosine (PYY) 4. The observation of reduced peak postprandial PYY would not be expected given the acceleration of gastric emptying, and may be responsible for the failure to inhibit intake of food given the facts that peripheral administration of PYY3–36 inhibited food intake in rodents and humans and that direct administration of PYY3–36 into the arcuate nucleus of the hypothalamus inhibited food intake in rodents 8. In addition, we noted that a higher caloric intake was required to experience satiety in those with abnormal waist circumference 4.
Uncoupling proteins (UCPs), a family of mitochondrial transporters, play a significant role in thermogenesis and energy utilization 9, 10. UCP-2 is widely expressed in human tissues including the stomach 11, 12. Subtypes of UCP mRNA and protein are regulated in a tissue- and subtype-specific fashion by leptin and food restriction 13. The orexigenic hormone ghrelin changes hypothalamic mitochondrial function through UCP-2, activating neuropeptide Y (NPY)/ Agouti-related protein (AgRP) neurons 14, triggering synaptic plasticity of pro-opiomelanocortin (POMC)-expressing neurons, and inducing food intake.
UCP-3 is highly specific for skeletal muscle and brown adipose tissue 15 and may affect the processes of adaptive thermogenesis in humans 16, and fatty acid translocation 17. However, the effect on thermogenesis is relatively minor. Recent studies suggest that the primary role of UCP-3 is in fatty acid metabolism and possibly in protecting the mitochondria against lipid-induced damage 10 by reducing mitochondrial production of reactive oxygen species (ROS) 18 and increasing the capacity to store energy as fat 19. Activation of UCP-3 is indirectly regulated by norepinephrine and is dependent upon the availability of free fatty acids [FFAs 20]. Impaired mitochondrial function in adipocytes may be linked directly to the development of metabolic diseases such as diabetes and insulin resistance 21, and mitochondrial genetic variants are associated with BMI in adults 22.
In epidemiological studies, genetic variation in UCP (as summarized in Table 1) has been associated with obesity. For example, there are several associations between genetic variations in UCP-2 and obesity, energy, and nerve functions. Since effects of ghrelin appear to be linked to UCP-2 14, and ghrelin affects gastric motor functions, satiation and appetite 23, it is relevant to assess whether genetic variations in UCP-2 alter upper gastrointestinal function.
Table 1.
Candidate genes studied
| GENE / Name |
SNP Minor allele |
MAF in locals |
Associations with Obesity | Sex | Ethnicity | Study Design |
Reference |
|---|---|---|---|---|---|---|---|
| UCP-2 | rs659366 T allele |
0.372 | Greater BMI by 0.4 or 0.5 kg/m2 in heterozygotes or homozygous minor vs. homozygous major (p=0.036) |
both | Finnish | CS single Center |
31 |
| UCP-2 | +3474 45 bp ins/del |
0.24 | Greater BMI by 2 or 3 .kg/m2 in heterozygotes or homozygous minor vs. homozygous major (p=0.005) |
both | German White |
CS single center |
40 |
| UCP-3 | rs2075577 G allele |
0.404 | Greater BMI by 0.29 or 1. 23 kg/m2 in heterozygotes or homozygotes minor vs. homozygous major (p=0.019) |
Male | Dutch, White |
CS single center |
33 |
| UCP-3 | rs15763 A allele |
0.25 | +0.38/+1.11 (BMI kg/m2 Δ of heterozygotes/ homozygotes from WT) (p= 0.033) |
Male | Finnish, White |
CS single center |
31 |
| UCP-3 | rs1626521 A allele |
0.267 | High LD (0.92) with rs15763 | Both | White | CS, single center |
24 |
CS=cross-sectional; LD= linkage disequilibrium; MAF= minor allele frequency
In a preliminary genotype-endophenotype study of 62 participants, we showed that variations in UCP-2 and UCP-3 were associated with gastrointestinal traits, especially with satiation measured in a nutrient drink test. In addition, we identified novel polymorphisms that were previously associated with obesity. These novel polymorphisms included UCP-3 rs1626521, which is in high linkage disequilibrium with UCP genes previously associated with obesity 24. The association of UCP-3 rs1626521 with obesity (e.g. BMI, waist circumference) remains unproven.
Given the interactions of UCPs with obesity, our general objective was to assess whether genetic variations in UCPs associated with obesity influence gastric functions (e.g. emptying, volume or accommodation), satiation, satiety and selected gut hormones (ghrelin, cholecystokinin, GLP-1 and PYY).
Our hypothesis was that UCP genes (previously associated with obesity in epidemiological studies or obesity endophenotypes) are associated with specific quantitative traits of obesity. To explore this hypothesis, we a priori identified the pathophysiological mechanism(s) to be tested in association with each gene, based on the theoretically predicted mechanism targeted by that candidate gene.
Our specific aims were: first, to examine the association of the novel UCP-3 gene variant, rs1626521, with obesity phenotype; second, to examine, in overweight and obese adults, the associations of genetic variation at UCP-2 and UCP-3 with postprandial satiation, satiety, gastric emptying (GE), fasting gastric volume (GV), postprandial GV accommodation, and selected gut hormones. The selected genetic variants were based on prior literature (detailed in Table 1). Having identified significant genetic associations of these variants with obesity and quantitative gastrointestinal traits, we appraised the functional significance of the candidate gene in human tissues and in a model cell system.
METHODS AND PROCEDURES
Participants
We invited 1114 adults to participate in these studies; 288 fulfilled general criteria for screening (obese, non-bulimic, with no systemic disease that would interfere with the study aims). Among the 288 screened, we recruited 255 adults who fulfilled additional inclusion criteria (see below) and did not withdraw from participation (Appendix Figure 1). They were predominantly Caucasian overweight or obese participants as described elsewhere,24. A preliminary report of the results of the first 62 individuals who performed the study was published in a pilot study 24. The main inclusion criteria were: 18–65 years of age, residence within 150 miles from Rochester, Minnesota, no systemic disease that could affect gastrointestinal motility, and not on current treatment for other diseases or that could potentially alter gastrointestinal functions, appetite, or absorption (e.g., orlistat). Weight stability for at least three months was required at the screening visit with the physician. Permissible medications were multivitamins, birth control pills, estrogen, and thyroxine replacement, all at stable doses for at least 30 days prior to the quantitative GI studies. Women of childbearing potential had a negative pregnancy test within 48 hours before any radioisotopes were administered. Participants completed behavioral and physical activity questionnaires described elsewhere4.
Experimental Protocol
On three different days, participants presented to the Mayo Clinic Clinical Research Unit at 7:00 a.m. after an 8-hour fasting period and underwent one test on each study day: a dual-isotope (99mTc-egg and 111In-skim milk, 315kcal meal) gastric emptying scintigraphic study of a mixed solid-liquid meal 4; a nutrient drink satiation test 25; and a gastric accommodation study by means of single photon emission computed tomography (SPECT) which involves i.v. injection of pertechnetate, 99mTcO4 26..These methods are described in greater detail in the appendix. Gastric emptying and SPECT studies were performed at least 72 hours apart to avoid downscatter interference by 111In (which has a half-life of >60 hours and has two emission peaks, one of which overlaps with 99mTc) from the meal ingested during the gastric emptying study with the measurement of gastric volume by 99mTc-SPECT. Studies of gastrointestinal traits were performed in the morning after an overnight fast, with exception of the ad-libitum buffet meal which was consumed at lunch time after a standard breakfast meal ingested during the SPECT study of gastric volumes.
Gastrointestinal Hormones
The gut hormones measured by radioimmunoassay were active ghrelin, total cholecystokinin (CCK), total GLP-1 and total PYY levels, as described in the appendix 4.
Determination of Genotypes
UCP genes variants were selected from previous preliminary studies 24; a minor allele frequency of above 0.10 (or 10%) in the local population was used as a requisite for inclusion. Selected genes are described in Table 1.
DNA was extracted from whole blood as previously described 24. Genotyping of all the SNPs were performed using Taqman® SNP Genotyping Assays (Applied Biosystems Inc., Foster City, CA, catalog # C_32667060_10) in accordance with the manufacturer’s instructions (Taqman® SNP Genotyping Assays).
Mitochondrial Function Study in Human Skeletal Muscle
Through a concomitant protocol on mitochondrial function in muscle physiology based on open advertisement, all 255 participants in the GI quantitative trait study were eligible to enroll in the muscle physiology study. Eleven individuals participated in both protocols. Subjects consumed a standardized weight-maintenance diet (50% carbohydrate: 20% protein: 30% fat) for the three days preceding their in-patient study. All meals were provided by the metabolic kitchen of the Clinical Research Unit (CRU). Patients were admitted to the in-patient CRU at ~5:00 p.m. on the third day of their standardized diet and remained fasting from 7:00 p.m. At 7:00 a.m. the following morning, vastus lateralis muscle biopsy samples were obtained under local anesthesia (lidocaine, 2%) using a modified Bergstrom needle27. Approximately 100mg of muscle tissue was immediately placed in ice-cold biopsy preservation buffer (BIOPS: 10 mM Ca++-EGTA, 0.1 µM free Ca++, 20 mM imidazole, 20 mM taurine, 50 mM K-MES, 0.5 mM DTT, 6.56 mM MgCl2, 5.77 mM ATP, 15 mM phophocreatine). Mitochondria were isolated from the fresh muscle by gentle homogenization and differential centrifugation, as described in detail elsewhere 27, 28. Once isolated, mitochondria were resuspended in mitochondrial respiration buffer (MiR05: 0.5 mM EGTA, 3mM MgCl2*6H2O, 60 mM potassium K-lactobionate, 20 mM taurine, 10 mM KH2PO4, 20 mM HEPES, 110 mM Sucrose, 1 g/l fatty acid free BSA). Mitochondrial capacity and coupling efficiency were measured by high-resolution respirometry (Oxygraph O2k, Oroboros Instruments, Innsbruck, Austria) using a protocol described previously 27, 28. Mitochondrial oxidative capacity (State 3) was measured using substrates specific for respiratory chain complex I (10mM glutamate, 2mM malate), complex I and II (10mM glutamate, 2mM Malate, 10mM succinate), and complex II alone (10mM glutamate, 2mM malate, 10mM succinate, 0.5 µM rotenone) in the presence of 2.5mM ADP. Oxygen flux rates (JO2: pmol/s/ml) were calculated using Datlab Software® (Oroboros Instruments, Innsbruck, Austria) after correcting for background oxygen kinetics 27, 28. Mitochondrial efficiency was determined from the ratio of state 3 to state 4 respirations (respiratory control ratio, RCR).
Uncoupling Protein-3 Expression in vitro
The functional effect of UCP-3 rs1626521 polymorphism on gene expression was unknown. UCP-3 rs1626521 polymorphism is located in the 3’ UTR of the UCP-3 gene. NCBI Gene ID 7352 was used as the reference sequence for the 3’ un-translated regions (UTR) of UCP-3. Thus, we cloned the 3’ UTR region of UCP-3 in a luciferase encoding plasmid. Further details are provided in the appendix.
We then constructed a plasmid with each SNP variant in a CMV/chicken B-actin promoter plasmid with luciferase coding gene. Each plasmid construct was verified by DNA sequence. HEK293 cells were transfected with the UCP-3-Luc or UCP-3-Renilla plasmid containing the G allele or A allele. Twenty-four hours after the transfection, firefly and Renilla luciferase activities were quantitated using the dual luciferase reporter assay system (Promega, Madison, WI) according to the manufacturer's instructions. Luciferase was quantified using a luminometer (TD-20/20, Turner Designs, Sunnyvale, CA). Statistical analysis was done using unpaired Student’s t-test to compare the effect of G allele to A allele on luciferase expression.
Statistical Analysis
All data are presented as means ± SEM, medians ± IQR, or percentages, as noted. We determined a priori the specific UCP genes to be examined for association with each gastrointestinal quantitative trait. Thus, based on the reported mechanisms of action of UCP, we determined that UCP genes have a main effect on satiety; we corrected the results for false detection rate resulting from multiple comparisons based on the actual number of genes analyzed in relation to each specific quantitative trait. The univariate associations of genotype with subject characteristics and response measures (e.g., gastric emptying t½ values) were assessed using Fisher’s exact test (e.g., association with categorical variables like gender), ANOVA, or a nonparametric (rank sum or Kruskal-Wallis) test as warranted, based on categorical or continuous data and normality of distribution.
Association of the rs1626521UCP-3 SNP with the quantitative traits was also assessed using a dominant genetic model (that pools the minor allele homozygote with the heterozygote genotype and compares two groups, e.g., GG vs. Ga and aa) using a chi-square test, 2-sample t-test or rank sum test as warranted.
Statistical Power
A sample size assessment for detecting clinically relevant associations for each genotype was examined by estimating the differences between two groups (i.e., assuming a dominant genetic model) that could be detected given the observed variation in the measured responses and the number of subjects that were anticipated in each genotype group, based on our prior studies that estimated the minor allele frequency in the same community 24. The differences between genotype groups that could be detected with approximately 80% power (two-sided α level of 0.05) using a two-sample t-test [assuming the listed pooled standard deviation (SD)] 24, 29 are shown in Appendix Table 1. The table illustrates the effect sizes demonstrable based on different sizes of groups for each genotype; this is necessary because the minor allele frequencies vary for the different genotypes studied (Table 2). Except for gastric emptying (two endpoints), each physiologic response corresponds to a distinct null hypothesis (e.g., association of genotype of interest with nutrient drink test maximum tolerated volume, i.e., satiation).
Table 2.
Minor allele frequencies of selected genes in current study conducted on local population (n=255).
| GENE | SNP | MAF | f(p) | f(q) | q2 | N |
|---|---|---|---|---|---|---|
| UCP-2 | rs659366 | T allele | 0.705 | 0.295 | 0.087 | 255 |
| UCP-2 | +3474 ins/del | ins allele | 0.601 | 0.399 | 0.159 | 255 |
| UCP-3 | rs2075577 | G allele | 0.571 | 0.429 | 0.184 | 255 |
| UCP-3 | rs15763 | A allele | 0.705 | 0.295 | 0.087 | 255 |
| UCP-3 | rs1626521 | A allele | 0.577 | 0.423 | 0.179 | 255 |
The analyses used the SAS® Statistical Package (Version 9.3, SAS Institute, Cary, NC).
These studies were approved by the Mayo Clinic Institutional Review Board, and written informed consent was received from participants prior to inclusion in the studies.
RESULTS
Demographics and Quantitative Traits in Obese and Overweight Participants
Demographics characteristics and quantitative traits of the cohort studied are outlined in Table 3. We recruited 255 overweight and obese participants: 178 females, 77 males, with a mean (±SEM) age of 37.4±0.7 years, BMI 33±0.3kg/m2, and waist circumference 101.1±0.73cm. We observed the expected differences in body weight, BMI, waist circumference, hip circumference, systolic and diastolic blood pressure, heart rate in the obese compared to the overweight groups. Additionally, obese individuals had a larger fasting gastric volume and lower fasting plasma ghrelin levels when compared to overweight individuals (Table 3).
Table 3.
Demographics of study participants (data shown are mean±SEM).
| Total | Overweight | Obese | p value | |
|---|---|---|---|---|
| Participants (n) | 255 | 85 | 170 | |
| Females, % | 69.8 | 60 | 74.7 | 0.02 † |
| Race (Caucasian, %) | 92.3 | 91.8 | 92.4 | 1.0 † |
| Age, years | 37.2±0.7 | 37.8±1.4 | 36.9±0.9 | 0.71 |
| Anthropometrics, Cardiovascular, Fasting Glucose and Physical Activity | ||||
| Body Weight, kg | 94.3±1.0 | 80.3±1.1 | 101.3±1.1 | |
| BMI, kg/m2 | 33.1±0.3 | 27.8±0.1 | 35.7±0.3 | |
| Waist Circumference, cm | 101.2±0.8 | 91.8±1.0 | 105.8±0.8 | |
| Hip Circumference, cm | 116.1±0.7 | 107.0±0.6 | 120.6±0.7 | |
| Systolic BP, mmHg | 129.9±0.9 | 127.7±1.7 | 130.9±1.0 | 0.050 |
| Diastolic BP, mmHg | 78.3±0.7 | 76.5±1.2 | 79.2±0.8 | 0.034 |
| Heart Rate, beats/min | 71.4±0.8 | 67.5±1.5 | 73.4±0.9 | <0.001 |
| Fasting Glucose, mg/dl | 100.3±1.1 | 97.6±1.4 | 101.7±1.5 | 0.044 |
| Exercise 1 (%) | 68.0 | 80.5 | 61.8 | 0.004 † |
| Quantitative gastrointestinal traits | ||||
| Satiation Volume To Fullness, ml | 713±20 | 690±29 | 725±26 | 0.82 |
| Satiation Maximum Tolerated Volume, ml | 1278±26 | 1270±40 | 1282±34 | 0.85 |
| Satiation Symptom VAS, mm (scale 0–400) | 172.9±4.2 | 171.9±7.2 | 173.3±5.2 | 0.93 |
| Gastric Emptying Solids T1/2, min | 99.5±1.7 | 96.6±2.8 | 100.8±2.1 | 0.28 |
| Gastric Emptying Liquids T1/2, min | 19.1±0.7 | 18.1±0.8 | 19.6±1.0 | 0.70 |
| Satiety ad-libitum intake at Buffet meal, kcal | 963.6±18.3 | 948.1±31.9 | 975.6±22.9 | 0.49 |
| Fasting Gastric Volume, ml | 272.2±4.6 | 261.1±7.1 | 277.8±5.9 | 0.049 |
| Postprandial Gastric Volume, ml | 754.5±7.8 | 743.0±13 | 760.3±9.6 | 0.31# |
| Ghrelin fasting (pg/ml) | 74.9±3.6 | 84.7±7.4 | 70.0±3.9 | 0.060 |
| Ghrelin peak, pg/ml | 67.1±4.2 | 76.4±7.3 | 62.6±5.1 | 0.10 |
| CCK peak (pmol/L) | 9.0±0.4 | 8.2±0.6 | 9.4±0.5 | 0.10 |
| GLP-1 peak (pM) | 18.1±0.7 | 17.2±0.9 | 18.6±0.9 | 0.86 |
| PYY peak (pg/ml) | 161.5±4.7 | 155.6±6.0 | 164.4±6.4 | 0.67 |
VAS-agg=visual analog score of the aggregate of 4 symptoms (nausea, bloating, fullness, pain)
= 2-sample t-test;
= Fisher’s Exact test; all other analyses were by rank sum test
Associations of UCP-3 rs1626521 with Body Weight, BMI, Waist Circumference and Physical Activity
In the dominant genetic model analysis, UCP-3 rs1626521 homozygous GG genotype is associated with higher body weight (mean Δ 5.1kg, p=0.039) and increased waist circumference (Δ 3.31cm, p=0.035). Differences in BMI (mean Δ 1.2kg/m2, p=0.085) and physical activity (p=0.063) did not reach statistical significance when compared with the combined GA/AA groups (Table 4).
Table 4.
Univariate associations of UCP-3 rs1626521 with demographics and GI quantitative traits.
| Data show mean ± SE | Genotype | p value | |||
|---|---|---|---|---|---|
| General genetic model |
Dominant genetic model |
||||
| Genotype | AA | AG | GG | ||
| Participants, n | 54 | 110 | 91 | ||
| Demographics | |||||
| Gender, female, % | 72 | 67 | 71 | 0.74† | 0.78† |
| Body weight, kg | 92.2±2.0 | 92.9±1.6 | 97.1±1.8 | 0.12 | 0.039 |
| Height, cm | 167.6±1.0 | 168.8±0.9 | 169.3±1.0 | 0.67 | 0.51 |
| BMI, kg/m2 | 32.9±0.7 | 32.6±0.5 | 33.9±0.6 | 0.22 | 0.085 |
| Waist Circumference, cm | 100.2±1.6 | 99.8±1.1# | 103.3±1.3 | 0.052 | 0.035 |
| Hip Circumference, cm | 115.6±1.5 | 115.5±1.0 | 117.1±1.1 | 0.50 | 0.24 |
| Systolic BP, mmHg | 127.2±1.9 | 130.1±1.4 | 131.1±1.5 | 0.38 | 0.29 |
| Diastolic BP, mmHg | 76.2±1.4 | 79.0±1.1 | 78.6±1.2 | 0.27 | 0.70 |
| Fasting Blood glucose, mg/dl | 99.2±1.7 | 99.4±1.2 | 102.0±2.6 | 0.89 | 0.63 |
| Exercise 1, % | 67.3 | 74.5 | 60.7 | 0.12† | 0.063† |
| Body Image (scale 9–45) | 28.4±1.0 | 28.7±0.6# | 26.7±0.6 | 0.04 | 0.016 |
| Quantitative gastrointestinal traits | |||||
| Satiation Volume To Fullness, ml | 675±47 | 746±31 | 696±32 | 0.15 | 0.83 |
| Satiation Maximum Tolerated Volume, ml | 1184±57 | 1358±43 | 1233±36 | 0.049 | 0.36 |
| Satiation Symptom VAS, mm (scale 0–400) | 164.8±8.3 | 181.4±6.6 | 167.1±7.1 | 0.24 | 0.25 |
| Gastric Emptying Liquids T1/2, min | 17.4±1.1 | 20.1±1.0 | 18.8±1.4 | 0.033 | 0.25 |
| Gastric Emptying Solids T1/2, min | 97.1±3.5 | 99.1±2.1 | 101.2±3.4 | 0.86 | 0.91 |
| Satiety ad-libitum intake at Buffet meal, kcal | 879.6±37.7 | 1035±30.2# | 924.8±26.2 | 0.006* | 0.18 |
| Satiety Carbs, gr | 116.3±5.2 | 136.4±4.1# | 122.9±3.6 | 0.011* | 0.23 |
| Satiety Protein, gr | 49.7±2.1 | 59.2±1.7# | 52.1±1.6 | 0.002* | 0.088 |
| Satiety Fat, gr | 23.4±1.1 | 27.1±0.9 | 24.5±0.8 | 0.044 | 0.30 |
| Fasting Gastric Volume, ml | 267.5±7.8 | 262.8±6.3# | 286.9±9.2 | 0.058 | 0.023 |
| Postprandial Gastric Volume, ml | 710.2±14.8# | 754.6±11.5 | 781.9±13.6 | 0.0029* | 0.009 |
| Gastric accom, ratio | 2.7±0.1# | 3.0±0.1 | 2.9±0.1 | 0.032 | 0.55 |
| GV delta, ml | 442.8±12.1# | 491.8±9.8 | 496.2±9.6 | 0.0025* | 0.065 |
| Gastric Acommodation | 98.6±2.4 | 107.1±2.0 | 103.1±2.3 | 0.032 | 0.55 |
| Ghrelin fasting, pg/ml | 83.5±5.8 | 75.3±5.7 | 69.0±6.6 | 0.014 | 0.0017 |
| Ghrelin peak, pg/ml | 68.6±9.3 | 64.9±6.5 | 65.9±7.3 | 0.95 | 0.11 |
| CCK peak, pmol/L | 9.8±1.0 | 8.9±0.5 | 8.6±0.5 | 0.91 | 0.78 |
| GLP-1 peak, pM | 17.5±1.9 | 18.5±0.9 | 18.1±1.0 | 0.17 | 0.38 |
| PYY peak, pg/ml | 161.7±10.3 | 165.2±7.5 | 156.6±7.4 | 0.99 | 0.87 |
p value <0.05 when compared to GG genotype on a Dunnett’s test or Dunn test in nonparametric comparisons;
significant with adjustment for FDR.
Chi-square test
Associations of UCP-3 rs1626521 with GI Quantitative Traits (corrected for false detection)
UCP-3 rs1626521 was associated with less satiety [that is, higher caloric intake (p=0.006)], with higher proportions of carbohydrate and protein calories ingested (p=0.01 and 0.002, respectively) during the ad-libitum buffet meal. UCP-3 rs1626521 was also associated with larger postprandial gastric volume and accommodation [measured as postprandial volume and change in gastric volume from fasting (ΔGV) respectively; all p<0.003]. These associations with total calorie intake and postprandial gastric volumes were significant when corrected for multiple comparisons (p<0.05 after FDR) (Table 4).
Univariate Associations of UCP-3 rs1626521 with Other GI Quantitative Traits
There were also univariate associations of this SNP with increased maximal tolerated volume (p=0.049, suggesting reduced satiation), accelerated gastric emptying of liquids [GE t1/2 (p=0.033)], and lower fasting plasma ghrelin (p=0.014). There were no nominal associations with age, height, hip circumference, blood pressure, postprandial satiation symptoms, GE of solids, postprandial plasma ghrelin, CCK, GLP-1 and PYY levels (Table 4).
Univariate Associations of Other UCP Gene Variants with GI Quantitative Traits
The main findings are summarized in Table 5. There were no associations with UCP-2 rs659366 and GI quantitative traits.
Table 5.
Univariate associations (by general genetic model) of other studied UCP-2 and UCP-3 genes variants with demographics and GI quantitative traits.
| Data show mean ± SE | General genetic model | p | ||
|---|---|---|---|---|
| Gene: | UCP2 rs659366 | |||
| Genotype | CC | CT | TT | |
| Participants (n) | 86 | 139 | 30 | |
| MTV, ml | 1266±39 | 1313±38 | 1148±66 | 0.12 |
| Fasting Ghrelin | 71.8±6.7 | 73.8 ±4.8 | 88.2±8.6 | 0.080 |
| Gene: | UCP2 −3474, 45bp ins/del | |||
| Genotype | del/del | del/ins | ins/ins | |
| Participants (n) | 115 | 114 | 26 | |
| Volume to fullness, ml | 708±27 | 748±33 | 573±45 | 0.033 |
| Fasting GV, ml | 283.8±7.2 | 263.4±6.8 | 260±10.8 | 0.045 |
| Buffet, Kcal | 919.9±25.6 | 1016±29.6 | 923.9±44.4 | 0.085 |
| Gene: | UCP3 rs2075577 | |||
| Genotype | AA | AG | GG | |
| Participants (n) | 78 | 138 | 45 | |
| Volume to fullness, ml | 681±40 | 749±28 | 664±34 | 0.14 |
| MTV, ml | 1188±45 | 1338±37 | 1257±59 | 0.028 |
| Fasting GV, ml | 266.1±8.3 | 281.2±6.4 | 256.2±10.6 | 0.14 |
| Postprandial GV, ml | 731.5±13.2 | 773.1±11.0 | 738.2±18.1 | 0.051 |
| Buffet, Kcal | 930.1±33.3 | 988.3±24.4 | 948.8±48.5 | 0.15 |
| Gene: | UCP3 rs15763 | |||
| Genotype | AA | AG | GG | |
| Participants (n) | 15 | 108 | 132 | |
| LGE T1/2, min | 16.4±1.2 | 19.8±1.0 | 18.8±1.1 | 0.23 |
| MTV, ml | 1082±98 | 1319±42 | 1266±35 | 0.071 |
| Buffet, Kcal | 811.4±42.3 | 1018±30.0 | 936.1±24.3 | 0.031 |
Bolded p values indicate univariate associations; none were significant with FDR correction
The ins/ins genotype of UCP-2 −3474, (45bp ins/del) was associated with decreased volume to fullness during the “satiation” test (p=0.033) and lower fasting gastric volume (p=0.045).
The UCP-3 (rs2075577) AA genotype was associated with decreased maximum tolerated volume during the “satiation” test (p=0.028) and decreased postprandial gastric volume (p=0.051).
The UCP-3 (rs15763) GG genotype was associated with increased caloric intake at the ad-libitum buffet meal (p=0.03).
Association of UCP-3 rs1626521 with Mitochondrial Function in Human Skeletal Muscle
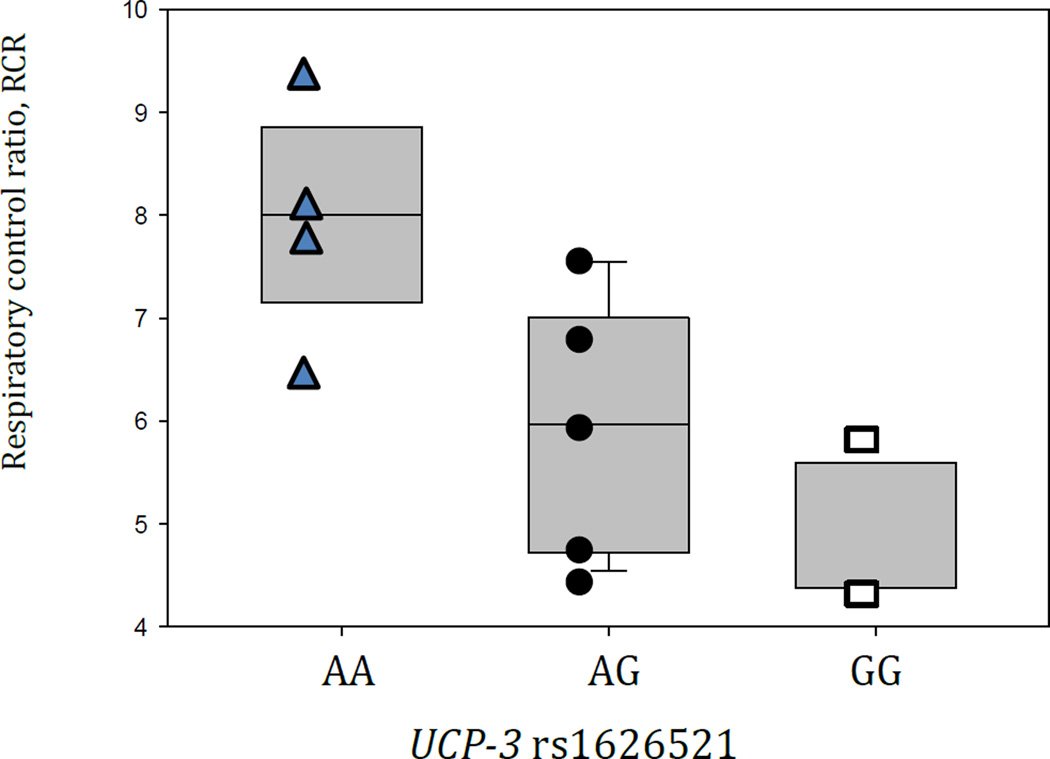
Table 6 shows subgroup demographics and other characteristics in the 11 participants in whom mitochondrial capacity and efficiency were studied in biopsies from skeletal muscle. UCP-3 rs1626521 genotype was associated overall (p=0.051 by Kruskal-Wallis test) with reduced mitochondrial bioenergetic efficiency assessed by respiratory control ratio. Thus, respiratory control ratio in the AA genotype group differed from that of AG (p=0.038) and from GG (p=0.023) genotypes (Figure 1). There was no difference in state 3 respiration across the UCP-3 rs1626521 genotypes.
Table 6.
Demographics of a subgroup of participants in a study of mitochondrial function in skeletal muscle. Note the similarities between obese and overweight, other than BMI and related characteristics.
| Total | Overweight | Obese | p value§ | |
|---|---|---|---|---|
| Participants (n) | 11 | 3 | 8 | |
| Females, % | 63.6 | 66.6 | 63 | |
| Race (Caucasian, %) | 90.9 | 100 | 87.5 | 1.0† |
| Age, years | 31.7±3.4 | 30.3±8.8 | 32.3±3.8 | 0.82 |
| Anthropometrics | ||||
| Body Weight, kg | 96.4±3.7 | 80.1±2.2 | 102.6±2.7 | |
| BMI, kg/m2 | 33.4±1.4 | 28±0.4 | 35.5±1.4 | |
| Waist Circumference, cm | 99.1±1.8 | 93.3±2 | 101.2±1.9 | |
| Hip Circumference, cm | 117.6±2.8 | 108.3±0.8 | 121.1±3 | |
| Systolic BP, mmHg | 126.3±3.2 | 126.7±9.3 | 126.1±3.2 | 0.94 |
| Diastolic BP, mmHg | 80±3.3 | 74±7.9 | 82.5±3.5 | 0.29 |
| Heart Rate, beats/min | 68.9±3.7 | 56.3±3.7 | 73.6±3.7 | 0.03 |
| Fasting Glucose, mg/dl | 95.2±2.5 | 95.5±5.5 | 97.1±2.5 | 0.78 |
| Exercise Regularly, % | 36.4 | 66.7 | 25 | 0.49 † |
| Mitochondrial function | ||||
| State 3 (CI) | 255.8±29.2 | 201±7.9 | 276.4±38 | 0.27 |
| State 3 (CI+II) | 468.3±36.2 | 392.7±29.8 | 496.6±45.3 | 0.22 |
| State 3 (CII) | 314±21.1 | 270.3±23.6 | 330.3±25.9 | 0.22 |
| State 4 | 74.7±6.8 | 67.5±6.2 | 77.4±9.1 | 0.54 |
| RCR | 6.5±0.5 | 5.9±0.7 | 6.7±0.6 | 0.51 |
Fisher’s exact test;
2-sample t-test unless otherwise noted
Figure 1.
Mitochondrial function comparing respiratory control ratio (RCR) with the UCP-3 rs1626521 genotype. Overall p-value is 0.04 by ANOVA; RCR for AA genotype differed with AG (p=0.038) and GG (p=0.023) genotypes.
Role of UCP-3 rs1626521 Polymorphism in Cellular Expression of UCP-3: in vitro Assay in HEK293 Cells
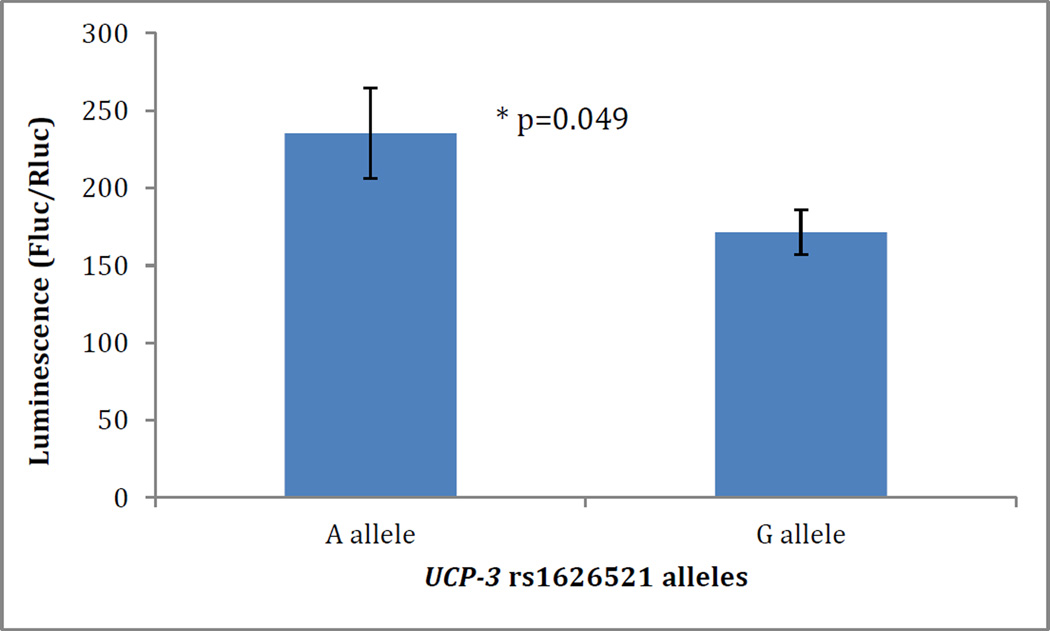
To determine the functional effects of the UCP-3 rs1626521 polymorphism, we transfected HEK-293 cells with a 3’UTR UCP-3 rs1626521 luciferase reporter gene construct encoding for either the G allele or the A allele. In luciferase activity assay, the 3’UTR UCP3-luc encoding the G allele was associated with decreased expression of luciferase by an average 27% when compared to the 3’UTR UCP3-luc encoding for the A allele (p=0.049) (Figure 2).
Figure 2.
UCP-3 rs1626521-induced luciferase expression. Hek293 were transfected with a 3’UTR UCP-3 rs1626521 luciferase reporter gene construct. Data represent mean±SE of three separate experiments. *p=0.049.
DISCUSSION
We have shown that the novel variant of UCP-3, rs1626521, is associated with body weight and waist circumference, as well as with quantitative gastrointestinal functions in obesity. The differences in body weight may be explained by alterations in both domains of energy balance: first, changes in food intake regulation as a result of the genetic variation altering gastric motor function, satiation and satiety; and second, mitochondrial efficiency. These factors may play a role in the development of obesity, insulin resistance and type 2 diabetes.
Genes that have been associated with type 2 diabetes or obesity in epidemiological studies have been preliminarily shown to alter gastric motor functions, satiation or satiety 24, 29, 30. Previously, other UCP-3 gene variants have been associated with obesity or abdominal obesity 31–33. In this paper, we report in a larger patient cohort than in our prior study 24, that UCP-3 rs1626521 is associated with body weight (an average difference of 5kg in body weight and 1.2kg/m2 in BMI) and waist circumference (an average difference of 3cm). These differences are clinically relevant and similar to the magnitude of effect of other UCP-3 genes associated with obesity in Caucasians 33. However, in addition to the observed association with body mass, our data provide insights on the mechanisms whereby UCP-3, rs1626521 predisposes to obesity, that is, by alterations in food intake, physical activity and mitochondrial efficiency. In addition, whereas the previous associations UCP-2 and UCP-3 with obesity were typically observed with the minor allele, the association reported for UCP-3 rs1626521 polymorphism was with the major GG genotype, which in the local population in the upper Midwest of the United States comprises about 33% of Caucasians. We also noted that, whereas other genetic studies in obesity typically required larger sample sizes (>1000 individuals) to demonstrate genotype-related average differences in BMI of usually less than 1kg/m2 34, our study of 255 patients identified an average BMI effect of UCP-3 rs1626521 of 1.2kg/m2. It would be of great interest, to validate our findings in other populations, including different ethnicities and a larger cohort of overweight and obese males and compare them to normal weight individuals.
In this study, we have also characterized the functional relevance and potential action of the UCP-3 rs1626521 polymorphism; our in vitro studies in transfected HEK293 cells suggested that rs1626521 is functionally significant by decreasing the expression of UCP-3 protein. This change in expression results in a change in mitochondrial function in human skeletal muscle obtained from 11 participants of the same cohort. The mitochondrial dysfunction may be the result of lipid peroxidation and damage to the mitochondrial machinery in obese people who express less UCP-3. Under normal conditions, UCP-3 is up-regulated in response to lipid overload as a protective mechanism, resulting in reduced production of reactive oxygen species.
The attenuation of this mechanism, observed here in association with the novel polymorphism UCP-3 rs1626521, may damage the mitochondria and result in decreased energy efficiency and increased lipid accumulation, which could lead to lipotoxicity and insulin resistance 35. These changes are consistent with the known effects of UCP-3 which increases fatty acid metabolism in muscle and is involved in mitochondrial protection against lipotoxicity 17, 36, 37. It is interesting to note that the over-expression of UCP-3 in mice causes fat-specific weight loss and improves insulin action 38, 39. The decreased mitochondrial efficiency in skeletal muscle may also contribute to the decreased physical activity observed in association with UCP-3 rs1626521 GG genotype.
Our findings also suggest that individuals with UCP-3 rs1626521 GG genotype reported less frequent physical activity than the AG and AA genotypes and may reflect an intolerance to exercise due to poor energy efficiency and capacity. Prior to our study, UCP-3 had not been associated with energy intake or gastric motor functions. The strength of these associations relies on a strict statistical analysis in which the results were corrected for a false detection rate for multiple comparisons. In summary, these metabolic consequences of altered UCP-3 function may contribute to alterations in body weight. Further studies of the effects of UCP-3 mitochondrial efficiency in organs involved in food intake, satiation and satiety would be of great interest.
In conclusion, a unifying hypothesis of many pathobiological changes in obesity appraised by our current study is that UCP-3 rs1626521 may explain the association of obesity in part by decreased physical activity (possibly due to decreased mitochondrial efficiency leading to exercise intolerance) and in part by increased postprandial gastric volume and accommodation, and tolerance of a higher calorie intake at an ad-libitum meal. Overall, these data support the role of mitochondria in the regulation of energy intake, gastrointestinal function, and skeletal muscle mitochondrial efficiency in the development of obesity. UCP-3 may be an interesting target for potential interventions with pharmacological or intense physical activity.
Supplementary Material
What is already known about this subject
-
--
Gene variants of uncoupling protein (UCP)-2 (rs659366 and +3474 45 bp ins/del) and UCP-3 (rs2075577 and rs15763) have been associated with obesity.
-
--
Uncoupling proteins (UCPs), a family of mitochondrial transporters, play a significant role in thermogenesis and energy utilization.
UCP-3 is highly specific for skeletal muscle and brown adipose tissue and may affect the processes of adaptive thermogenesis in humans, and fatty acid translocation.
What this study adds
-
--
A newly identified functional variant (rs1626521) of UCP-3 is associated with body weight and waist circumference, as well as with quantitative gastrointestinal functions in obesity.
-
--
The differences in body weight may be explained by alterations in both domains of energy balance: first, changes in food intake regulation as a result of the genetic variation altering gastric motor function by increased postprandial gastric volume and accommodation, satiation and satiety by higher calorie intake at an ad-libitum meal; and second, mitochondrial efficiency resulting in decreased physical activity.
-
--
Overall, these data support the role of mitochondria in the regulation of energy intake, gastrointestinal function, and skeletal muscle mitochondrial efficiency in the development of obesity.
ACKNOWLEDGEMENTS
The authors thank Michael Ryks and Deborah Rhoten of the Endoscopy, Physiology and Imaging Core, the nursing staff, and the Immunochemistry Core Laboratory of the Mayo Clinic CCaTS for technical support and patient care. We also thank Cindy Stanislav for secretarial assistance.
Funding: This work was funded by R01-DK67071 (Dr. M. Camilleri, Principal Investigator) and supported by CCaTS #UL1-TR000135 from National Institutes of Health. This work was supported, in part, by the Genetics and Model Systems Core of the Mayo Clinic Center for Cell Signaling in Gastroenterology (P30DK084567).
Footnotes
CONFLICTS OF INTEREST
All authors have no financial or personal relationships that could present a potential conflict of interest.
Authors’ contributions:
A. Acosta: fellow co-investigator; analysis and interpretation of data; conduct of the study; drafting and writing and critical revision of the manuscript
M. Camilleri: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript; funding from NIH
A. Shin: fellow co-investigator; conduct of the study; critical revision of the manuscript
M. Vazquez-Roque: fellow co-investigator; conduct of the study; critical revision of the manuscript
J. Iturrino: fellow co-investigator; conduct of the study; critical revision of the manuscript
D. Burton: technical support; study supervision
J. O’Neill: study coordinator
D. Eckert: study coordinator
P. Carlson: technical support
A. Vella: Endocrinology, diabetes management, incretin and hormone interpretation and critical revision of manuscript
S. Nair: lead investigator on mitochondrial function and critical revision of manuscript
I. Lanza: investigator on mitochondrial function studies and critical revision of manuscript
A.R. Zinsmeister: staff statistician; study design; analysis and interpretation of data; critical revision of the manuscript
REFERENCES
- 1.Blundell JE, Cooling J. Routes to obesity: phenotypes, food choices, activity. Br J Nutrition. 2000;83(Suppl.S1):S33–S38. doi: 10.1017/s0007114500000933. [DOI] [PubMed] [Google Scholar]
- 2.Acosta A, Abu Dayyeh BK, Port JD, Camilleri M. Recent advances in clinical practice challenges and opportunities in the management of obesity. Gut. 2014;63:687–695. doi: 10.1136/gutjnl-2013-306235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blundell J, de Graaf C, Hulshof T, Jebb S, Livingstone B, Lluch A, et al. Appetite control: methodological aspects of the evaluation of foods. Obes Rev. 2010;11:251–270. doi: 10.1111/j.1467-789X.2010.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acosta A, Camilleri M, Iturrino J, O’Neill J, Eckert D, Burton D, et al. Quantitative gastrointestinal and psychological traits in obesity identify predictors of response in a clinical trial. Gastroenterology. 2014 doi: 10.1053/j.gastro.2014.11.020. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibbons C, Finlayson G, Dalton M, Caudwell P, Blundell JE. Metabolic Phenotyping Guidelines: studying eating behaviour in humans. J Endocrinol. 2014;222:G1–G12. doi: 10.1530/JOE-14-0020. [DOI] [PubMed] [Google Scholar]
- 6.Huang GH, Hsieh CC, Chen CH, Chen WJ. Statistical validation of endophenotypes using a surrogate endpoint analytic analogue. Genet Epidemiol. 2009;33:549–558. doi: 10.1002/gepi.20407. [DOI] [PubMed] [Google Scholar]
- 7.Pan WH, Lynn KS, Chen CH, Wu YL, Lin CY, Chang HY. Using endophenotypes for pathway clusters to map complex disease genes. Genet Epidemiol. 2006;30:143–154. doi: 10.1002/gepi.20136. [DOI] [PubMed] [Google Scholar]
- 8.Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, et al. Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 9.Schrauwen P, Hesselink M. UCP2 and UCP3 in muscle controlling body metabolism. J Exp Biol. 2002;205:2275–2285. doi: 10.1242/jeb.205.15.2275. [DOI] [PubMed] [Google Scholar]
- 10.Schrauwen P, Hoeks J, Hesselink MK. Putative function and physiological relevance of the mitochondrial uncoupling protein-3: involvement in fatty acid metabolism? Prog Lipid Res. 2006;45:17–41. doi: 10.1016/j.plipres.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Boss O, Samec S, Dulloo A, Seydoux J, Muzzin P, Giacobino JP. Tissue-dependent upregulation of rat uncoupling protein-2 expression in response to fasting or cold. FEBS Lett. 1997;412:111–114. doi: 10.1016/s0014-5793(97)00755-2. [DOI] [PubMed] [Google Scholar]
- 12.Fleury C, Neverova M, Collins S, Raimbault S, Champigny O, Levi-Meyrueis C, et al. Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat Genet. 1997;15:269–272. doi: 10.1038/ng0397-269. [DOI] [PubMed] [Google Scholar]
- 13.Sivitz WI, Fink BD, Donohoue PA. Fasting and leptin modulate adipose and muscle uncoupling protein: divergent effects between messenger ribonucleic acid and protein expression. Endocrinology. 1999;140:1511–1519. doi: 10.1210/endo.140.4.6668. [DOI] [PubMed] [Google Scholar]
- 14.Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM, et al. UCP2 mediates ghrelin's action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454:846–851. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harper JA, Stuart JA, Jekabsons MB, Roussel D, Brindle KM, Dickinson K, et al. Artifactual uncoupling by uncoupling protein 3 in yeast mitochondria at the concentrations found in mouse and rat skeletal-muscle mitochondria. Biochem J. 2002;361:49–56. doi: 10.1042/0264-6021:3610049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boss O, Samec S, Paoloni-Giacobino A, Rossier C, Dulloo A, Seydoux J, et al. Uncoupling protein-3: a new member of the mitochondrial carrier family with tissue-specific expression. FEBS Lett. 1997;408:39–42. doi: 10.1016/s0014-5793(97)00384-0. [DOI] [PubMed] [Google Scholar]
- 17.Bezaire V, Seifert EL, Harper ME. Uncoupling protein-3: clues in an ongoing mitochondrial mystery. Faseb J. 2007;21:312–324. doi: 10.1096/fj.06-6966rev. [DOI] [PubMed] [Google Scholar]
- 18.Toime LJ, Brand MD. Uncoupling protein-3 lowers reactive oxygen species production in isolated mitochondria. Free Radic Biol Med. 2010;49:606–611. doi: 10.1016/j.freeradbiomed.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saltzman E, Roberts SB. The role of energy expenditure in energy regulation: findings from a decade of research. Nutr Rev. 1995;53:209–220. doi: 10.1111/j.1753-4887.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- 20.Sprague JE, Yang X, Sommers J, Gilman TL, Mills EM. Roles of norepinephrine, free Fatty acids, thyroid status, and skeletal muscle uncoupling protein 3 expression in sympathomimetic-induced thermogenesis. J Pharmacol Exp Ther. 2007;320:274–280. doi: 10.1124/jpet.106.107755. [DOI] [PubMed] [Google Scholar]
- 21.Boudina S, Graham TE. Mitochondrial function/dysfunction in white adipose tissue. Exp Physiol. 2014;99:1168–1178. doi: 10.1113/expphysiol.2014.081414. [DOI] [PubMed] [Google Scholar]
- 22.Flaquer A, Baumbach C, Kriebel J, Meitinger T, Peters A, Waldenberger M, et al. Mitochondrial genetic variants identified to be associated with BMI in adults. PloS One. 2014;9:e105116. doi: 10.1371/journal.pone.0105116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tack J, Depoortere I, Bisschops R, Delporte C, Coulie B, Meulemans A, et al. Influence of ghrelin on interdigestive gastrointestinal motility in humans. Gut. 2006;55:327–333. doi: 10.1136/gut.2004.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papathanasopoulos A, Camilleri M, Carlson P, Vella A, Linker Nord S, Burton D, et al. A preliminary candidate genotype-intermediate phenotype study of satiation and gastric motor function in obesity. Obesity. 2010;18:1201–1211. doi: 10.1038/oby.2009.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chial H, Camilleri C, Delgado-Aros S, Burton D, Thomforde G, Ferber I, et al. A nutrient drink test to assess maximum tolerated volume and postprandial symptoms: effects of gender, body mass index and age in health. Neurogastroenterol Motil. 2002;14:249–253. doi: 10.1046/j.1365-2982.2002.00326.x. [DOI] [PubMed] [Google Scholar]
- 26.Bouras E, Delgado-Aros S, Camilleri M, Castillo EJ, Burton DD, Thomforde GM, et al. SPECT imaging of the stomach: comparison with barostat effects of sex, age, body mass index, and fundoplication Single photon emission computed tomography. Gut. 2002;51:781–786. doi: 10.1136/gut.51.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lanza IR, Zabielski P, Klaus KA, Morse DM, Heppelmann CJ, Bergen HR, 3rd, et al. Chronic caloric restriction preserves mitochondrial function in senescence without increasing mitochondrial biogenesis. Cell Metab. 2012;16:777–788. doi: 10.1016/j.cmet.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanza IR, Nair KS. Functional assessment of isolated mitochondria in vitro. Methods Enzymol. 2009;457:349–372. doi: 10.1016/S0076-6879(09)05020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Acosta A, Camilleri M, Shin A, Carlson P, Burton D, O'Neill J, et al. Association of melanocortin 4 receptor gene variation with satiation and gastric emptying in overweight and obese adults. Genes Nutr. 2014;9:384–390. doi: 10.1007/s12263-014-0384-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vazquez Roque M, Camilleri M, Clark M, TePoel DA, Jensen MD, Graszer KM, et al. Alteration of gastric functions and candidate genes associated with weight reduction in response to sibutramine. Clin Gastroenterol Hepatol. 2007;5:829–837. doi: 10.1016/j.cgh.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 31.Salopuro T, Pulkkinen L, Lindstrom J, Kolehmainen M, Tolppanen AM, Eriksson JG, et al. Variation in the UCP2 and UCP3 genes associates with abdominal obesity and serum lipids: the Finnish Diabetes Prevention Study. BMC Med Genet. 2009;10:94. doi: 10.1186/1471-2350-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brondani LA, Assmann TS, de Souza BM, Bouças AP, Canani LH, Crispim D. Meta-analysis reveals the association of common variants in the uncoupling protein (UCP) 1–3 genes with body mass index variability. PloS One. 2013:9. doi: 10.1371/journal.pone.0096411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Abeelen AF, de Krom M, Hendriks J, Grobbee DE, Adan RA, van der Schouw YT. Variations in the uncoupling protein-3 gene are associated with specific obesity phenotypes. Eur J Endocrinol. 2008;158:669–676. doi: 10.1530/EJE-07-0834. [DOI] [PubMed] [Google Scholar]
- 34.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liesa M, Shirihai OS. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013;17:491–506. doi: 10.1016/j.cmet.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nabben M, Hoeks J. Mitochondrial uncoupling protein 3 and its role in cardiac- and skeletal muscle metabolism. Physiol Behavior. 2008;94:259–269. doi: 10.1016/j.physbeh.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 37.Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2:85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Cadenas S, Echtay KS, Harper JA, Jekabsons MB, Buckingham JA, Grau E, et al. The basal proton conductance of skeletal muscle mitochondria from transgenic mice overexpressing or lacking uncoupling protein-3. J Biol Chem. 2002;277:2773–2778. doi: 10.1074/jbc.M109736200. [DOI] [PubMed] [Google Scholar]
- 39.Clapham JC, Arch JR, Chapman H, Haynes A, Lister C, Moore GB, et al. Mice overexpressing human uncoupling protein-3 in skeletal muscle are hyperphagic and lean. Nature. 2000;406:415–418. doi: 10.1038/35019082. [DOI] [PubMed] [Google Scholar]
- 40.Evans D, Minouchehr S, Hagemann G, Mann WA, Wendt D, Wolf A, et al. Frequency of and interaction between polymorphisms in the beta3-adrenergic receptor and in uncoupling proteins 1 and 2 and obesity in Germans. Int J Obes Relat Metab Disord. 2000;24:1239–1245. doi: 10.1038/sj.ijo.0801402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.