This year marks a decade since the original descriptions of the JAK2V617F mutation in myeloproliferative neoplasms.1–4 In the intervening years we have witnessed this finding influencing diagnostic processes, prognostic determination and more recently therapeutics. Whilst some perhaps naively anticipated that the benefits of JAK2V617F as a therapeutic target would parallel those of BCR/ABL inhibitors we have learnt surprising information and delivered substantial benefits to patients from the results to date. Albert Einstein is credited with the quote “Any fool can know. The point is to understand… It’s not that I am so smart. But I stay with the questions much longer” So what have we learnt from “staying longer” regarding the molecular pathogenesis of myeloproliferative neoplasms and the benefits and drawbacks of JAK inhibition?
Our understanding of the molecular pathogenesis of myeloproliferative neoplasms has moved forward at a substantial pace and a decade on we have a more detailed view of the degree of molecular complexity of these disorders. Several novel mutations have been identified, of which the CALR mutations are probably the most important given their prevalence, specificity to the myeloproliferative neoplasms and apparent impact upon disease behaviors.5–8 In addition, we have begun to understand, particularly in myelofibrosis, that the individual mutational profile may have an impact on prognosis.8 A few weeks ago an elegant paper was published, delineating for the first time how the order in which certain mutations occur affects the disease phenotype but also response to therapy. Ortmann and colleagues showed that in patients with polycythemia vera and both TET2 and JAK2 mutations that the disease in patients in whom the JAK2 mutation occurred first had a more proliferative phenotype but was also more responsive in vitro to the JAK1/2 inhibitor ruxolitinib.9 These findings from a group also “staying with the question for longer” influence our thinking on how multiple disease phenotypes can occur but the relevance to therapeutics is also striking and has yet to be explored.
In terms of targeted therapies the JAK1/2 inhibitor ruxolitinib was the first to be applied in the field of myeloproliferative neoplasms and specifically initially to patients with myelofibrosis. Early data with this agent revealed striking responses in terms of symptom improvement and spleen volume reduction.10 These benefits were the basis for the phase III studies known as the COMFORT trials which led ultimately to the approval of ruxolitinib for the treatment of myelofibrosis by the Food and Drug Administration in 2011 and the European Medicines Agency in 2012.11,12 The primary endpoints of these trials were at 24 and 48 weeks, so longer term data are clearly vital to assess the impact and safety of such a novel therapy.
In this edition of Haematologica, Verstovsek and colleagues report the 3-year efficacy and safety of ruxolitinib therapy in patients from the COMFORT-I study.13 Three-year follow-up data from the companion COMFORT-II study were also published recently.14 Verstovsek et al. demonstrated that the probability of maintaining a spleen response was 0.53 at 144 weeks and that the median spleen volume reduction at 144 weeks was 34% for the 50% of patients who were originally assigned to ruxolitinib and who remained on the drug. Overall quality of life benefits persisted without new emergent side effects or worsening of anticipated effects of JAK inhibition, namely anemia and thrombocytopenia. Perhaps more importantly Verstovsek et al. also presented a sophisticated and well-argued exploratory analysis of the survival benefits of JAK inhibition with ruxolitinib using two different methods. These analyses are necessary as both the phase III studies COMFORT-I and COMFORT-II have, for ethical reasons, permitted patients in the control arms to cross over to active therapy. The first analysis utilizes the rank-preserving structural failure time method which demonstrates that cross over from placebo to ruxolitinib may have led to an underestimation of the survival benefit from ruxolitinib. Consistent data were obtained from the second evaluation performed using the generalized Gamma distribution showing greater probabilities of death in the placebo group than in the ruxolitinib group. In order to dissect these methods in detail you will have to turn to the Online Supplementary Material for this paper; however, what emerges is a consistent demonstration of survival benefit for patients with myelofibrosis who received ruxolitinib in the COMFORT-I study, which was also reported in the COMFORT-II study in which the hazard ratio for survival advantage of ruxolitinib therapy versus control management was 0.48 (95% confidence interval 0.28–0.85, P=0.009).14
The survival benefits associated with ruxolitinb therapy are intriguing and have often been attributed to improvements in patients’ performance status. What has also become clear is that ruxolitinib will generate benefits for patients regardless of whether they do or do not have the JAK2 mutation and, as shown in a sub-analysis of COMFORT-2, will benefit myelofibrosis patients with a so-called high molecular risk profile (i.e. having at least one of the following mutations – EZH2, ASXL-1, IDH1/2, SRSF2).15 A combined analysis from both COMFORT studies has also been reported, linking a spleen volume reduction of at least 10% by 24 weeks with a survival benefit.16 The story is, however, likely to be much more complex than this: we described a patient from our own clinic who achieved a near complete remission with ruxolitinib therapy and two further patients have now been reported with a similar response.17,18 Our patient continued to have minor histological abnormalities, a very low level JAK2 clone and a karyotypic abnormality. These patients are in the minority but point to the possibility of changing the disease course even more dramatically perhaps in patients with a selected as yet undiscovered molecular signature which is also likely to include order and size of different clones.
Histological responses were also reported from COMFORT-2 but the data from COMFORT-1 are not currently available. Such responses may be linked to a further effect of ruxolitinib therapy: that is, immune modulation. This may be an important facet of improvement in the disease but initially has been manifest as increased risk of infections. Most of these infections are commonly bacterial (chest or urinary tract infections) or viral (such as shingles) but rare atypical infections have been reported (reviewed by Galli et al.19). In this regard the updated data from COMFORT-1 are important as there is no increase in the rate of infection and no atypical infections were observed over time in this cohort of patients, suggesting that this is not an increasing risk over time and underlining that rare and atypical infections, such as have been reported, are precisely that – rare and atypical.13 Nonetheless in clinical practice it is important to be aware of them. There is also an increasing view that myeloproliferative neoplasms, and particularly myelofibrosis, are diseases with a significant inflammatory component;20 this argues that the immune dysregulation documented with ruxolitinib, with its effects on B, T and NK subsets, may be important.21–25 At least one of these reports linked these changes with clinical response.25 Beyond its utility in myelofibrosis ruxolitinib has been reported to demonstrate benefits and is being assessed in a range of inflammatory conditions such as psoriasis, rheumatoid arthritis and alopecia totalis.26–28 Importantly, benefits for treatment of acute graft-versus-host disease have also been reported.29
Last year the Food and Drug Administration approved the use of ruxolitinib for the treatment of a different group of patients with myeloproliferative neoplasms – patients with polycythemia vera who are intolerant or resistant to hydroxycarbamide. This approval followed the RESPONSE study that demonstrated benefits in blood count control, avoidance of phlebotomy, reduction in spleen size and symptom improvement.30 It will be interesting to study the benefits of such therapy in detail for these patients who are likely to have less complex disease at a molecular and cellular level. For patients with polycythemia vera, even those resistant to hydroxycarbamide, a single therapy is likely to deliver benefits although these need to be more clearly addressed in terms of impact on thrombosis and transformation risk.
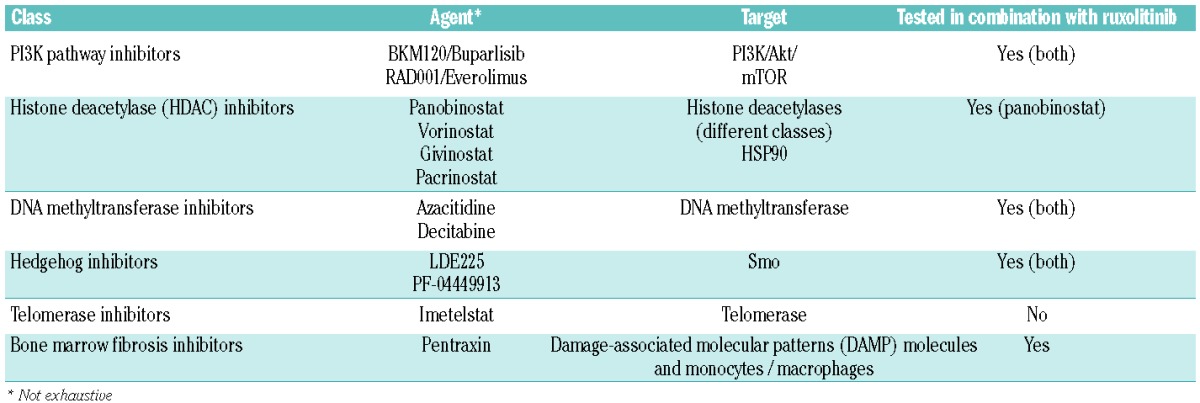
But for myelofibrosis although ruxolitinib therapy has made an impact we have a considerable amount of work still to do with a large number of unanswered questions remaining. For example, might a more specific JAK2 or indeed mutation-specific JAK2V617F inhibitor deliver more benefit for patients? Two agents, pacritinib, assessed in PERSIST-1 (NCT01773187) and PERSIST-2 (NCT02055781), and momelotinib, for which the relevant phase III studies are SIMPLIFY-1 (NCT01969838) and SMPLIFY-2 (NCT02101268), are of interest. However, apart from these agents if our ultimate goal is to achieve a cure for myelofibrosis we will probably, as for most hematologic malignancies, need to utilize a combination strategy. A number of studies investigating combinations are underway (Table 1); however, one of the next most important steps in this field will be to agree appropriate assessment tools. Spleen volume has become a standard so, is improvement a greater number of patients achieving 35% reduction or is better response defined by higher overall mean/median response? If we find an agent or combination that delivers molecular responses how would this be defined? If we continue to use quality of life improvement as a target, a likely risk is that side effects from additional therapies may mask such improvements.
Table 1.
Novel agents for the management of myeloproliferative neoplasms.
Once again we are at the stage of needing to make a stepped improvement with a second agent such as pacritinib, momelotinib, or the telomerase inhibitor imetelstat or push the boundaries of clinical and scientific research with combinations and novel endpoints. It seems we need to continue to follow the advice of Albert Einstein but, as Verstovek et al. demonstrate, many patients with myelofibrosis are currently already deriving major benefits from ruxolitinib therapy.
Footnotes
Financial and other disclosures provided by the author using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are available with the full text of this paper at www.haematologica.org.
References
- 1.James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–1148. [DOI] [PubMed] [Google Scholar]
- 2.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–397. [DOI] [PubMed] [Google Scholar]
- 3.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–1061. [DOI] [PubMed] [Google Scholar]
- 4.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–1790. [DOI] [PubMed] [Google Scholar]
- 5.Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369(25):2379–2390. [DOI] [PubMed] [Google Scholar]
- 6.Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369(25):2391–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rotunno G, Mannarelli C, Guglielmelli P, et al. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood. 2014;123(10):1552–1555. [DOI] [PubMed] [Google Scholar]
- 8.Tefferi A, Guglielmelli P, Lasho TL, et al. CALR and ASXL1 mutationsbased molecular prognostication in primary myelofibrosis: an international study of 570 patients. Leukemia. 2014;28(7):1494–500. [DOI] [PubMed] [Google Scholar]
- 9.Ortmann CA, Kent DG, Nangalia J, et al. Effect of mutation order on myeloproliferative neoplasms. N Engl J Med. 2015;372(7):601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363(12):1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787–798. [DOI] [PubMed] [Google Scholar]
- 13.Verstovsek S, Mesa RA, Gotlib J, et al. Efficacy, safety, and survival with ruxolitinib in patients with myelofibrosis: results of a median 3-year follow-up of COMFORT-I. Haematologica. 2015;(4):479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cervantes F, Vannucchi AM, Kiladjian JJ, et al. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood. 2013;122(25):4047–4053. [DOI] [PubMed] [Google Scholar]
- 15.Guglielmelli P, Biamonte F, Rotunno G, et al. Impact of mutational status on outcomes in myelofibrosis patients treated with ruxolitinib in the COMFORT-II study. Blood. 2014;123(14):2157–2160. [DOI] [PubMed] [Google Scholar]
- 16.Hagop K, Kiladjian J-J, Gotlib J, et al. A pooled overall survival analysis of the COMFORT studies: 2 randomized phase 3 trials of ruxolitinib for the treatment of myelofibrosis. Blood. 2013;122(21):abstract 2820. [Google Scholar]
- 17.Wilkins BS, Radia D, Woodley C, Farhi SE, Keohane C, Harrison CN. Resolution of bone marrow fibrosis in a patient receiving JAK1/JAK2 inhibitor treatment with ruxolitinib. Haematologica. 2013;98(12):1872–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Ali HK, Hubert K, Lange T, et al. Complete clinical, histopathologic and molecular remission of primary myelofibrosis with long-term treatment with the JAK1/2 inhibitor ruxolitinib. 56th ASH Annual Meeting San Fransisco, CA, USA 6–9, 2014 Abstract 1836. [Google Scholar]
- 19.Galli S, McLornan D, Harrison C. Safety evaluation of ruxolitinib for treating myelofibrosis. Expert Opin Drug Saf. 2014;13(7):967–976. [DOI] [PubMed] [Google Scholar]
- 20.Hasselbalch HC. Chronic inflammation as a promotor of mutagenesis in essential thrombocythemia, polycythemia vera and myelofibrosis. A human inflammation model for cancer development? Leukemia Res. 2013;37(2):214–220. [DOI] [PubMed] [Google Scholar]
- 21.Massa M, Rosti V, Campanelli R, Fois G, Barosi G. Rapid and long-lasting decrease of T-regulatory cells in patients with myelofibrosis treated with ruxolitinib. Leukemia. 2014;28(2):449–451. [DOI] [PubMed] [Google Scholar]
- 22.Rudolph J, Cornez I, Brossart P, Wolf D. The JAK1/JAK2 inhibitor ruxolitinib substantially affects NK cell biology. Blood. 2013;122(21):16. [Google Scholar]
- 23.Wolschke C, Alchalby H, Ayuk F, et al. The Pan- JAK inhibitor ruxolitinib impairs T-cell activation, cytokine production and proliferation in vivo and in vitro. Blood. 2013;122(21):2001.24175353 [Google Scholar]
- 24.Campanelli R, Fois G, Poletto V, et al. Decrease of T regulatory cells in patients with myelofibrosis receiving ruxolitinib. Blood. 2013;122(21):4057. [Google Scholar]
- 25.Kordasti SY, Seidl T, Abellan PP, et al. JAK inhibition reduces CD25 high CD27+ FOXp3+ T regulatory cells and causes a silencing of T effector cells in patients with myeloproliferative neoplasms whilst promoting a TH17 phenotype. Blood. 2013;122(21):4092. [Google Scholar]
- 26.Kwatra SG, Dabade TS, Gustafson CJ, Feldman SR. JAK inhibitors in psoriasis: a promising new treatment modality. J Drugs Dermatol. 2012;11(8):913–918. [PubMed] [Google Scholar]
- 27.Ghoreschi K, Gadina M. Jakpot! New small molecules in autoimmune and inflammatory diseases. Exp Dermatol. 2014;23(1):7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nature Med. 2014;20(9):1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spoerl S, Mathew NR, Bscheider M, et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood. 2014;123(24):3832–3842. [DOI] [PubMed] [Google Scholar]
- 30.Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015;372(5):426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]


