The therapy of chronic lymphocytic leukemia (CLL) is in an era of momentous change from chemotherapy towards targeted therapy. The first phase was the introduction of monoclonal antibodies, especially the anti-CD20 antibodies rituximab, ofatumumab, and obinutuzumab in combination immunochemotherapy. More recently, small molecular inhibitors have emerged which target the B-cell receptor signaling pathways (ibrutinib and idelalisib), BCL-2 (ABT-199), and immunomodulation [immunomodulatory drugs (IMiDs), lenalidomide]. In this rapidly changing landscape, what is the role of the anti-CD20 monoclonal antibody ofatumumab in CLL?
Ofatumumab in relapsed/refractory chronic lymphocytic leukemia
Ofatumumab recognizes a different epitope to rituximab that includes both the large and small extracellular domains of CD20, and has a slower dissociation rate compared to rituximab. These characteristics have suggested potentially superior activity.1,2 Single agent ofatumumab was first utilized in relapsed/refractory (R/R) CLL and demonstrated overall response rates of approximately 50%, though these mainly consisted of partial responses. As such, the United States Food and Drug Administration (FDA) granted accelerated approval for the use of ofatumumab in previously treated CLL in October 2009. In April 2010, the European Medicines Agency (EMA) recommended a conditional marketing authorization for the use of ofatumumab in fludarabine- and alemtuzumab-refractory CLL.
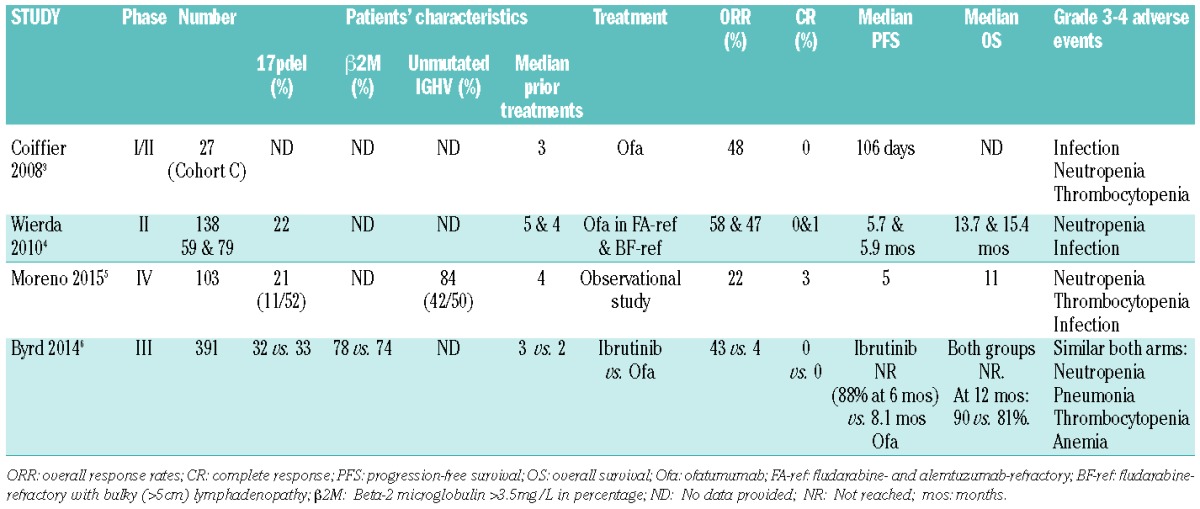
These approvals were largely based on two trials: Coiffier et al. (2008)3 and Wierda et al. (2010).4 The first trial was a phase I-II dose escalating multicenter study of ofatumumab in 33 patients with R/R CLL who had received a median of 3 prior treatment regimens. They reported an overall response rate (ORR) of 48% (13 of 27 patients) with no complete responses (CR). The median progression-free survival (PFS) was 106 days. Grade 3 or more adverse events included infection, thrombocytopenia and neutropenia. The second trial was a phase II international trial using ofatumumab in fludarabine- and alemtuzumab-refractory (FA-ref) CLL and in fludarabine-refractory CLL with bulky (>5cm) lymphadenopathy (BF-ref). At the interim analysis, there were 59 and 79 patients with a median of 5 and 4 prior treatments in the FA-ref and BF-ref groups, respectively. The ORR was 58% and 47% in the FA-ref and BF-ref groups, respectively. All responders achieved a partial response (PR) except for one in the BF-ref who attained a CR. Median PFS and overall survival (OS) were 5.7 and 13.7 months in the FA-ref group, respectively, and 5.9 and 15.4 months in the BF-ref group, respectively. The grade 3 or more adverse event profile included infection and neutropenia. One patient did develop progressive multifocal leukoencephalopathy (PML).
In this issue, Moreno and colleagues5 conducted a study on behalf of the European Research Initiative on CLL (ERIC group) in response to the conditional authorization of the drug in Europe. They report the results of a phase IV, non-interventional, observational study on single agent ofatumumab in poor-prognosis CLL. Notably, they were not able to reproduce similar ORR to that demonstrated by Coiffier et al.3 and Wierda et al.4 which raises questions over the use of ofatumumab as monotherapy in R/R CLL. One hundred and three patients with R/R CLL who had received a median of 4 prior treatment regimens were reported to have an ORR based on an intention-to-treat (ITT) of 22% (3CR, 1CR incomplete, 19PR). This is less than half that observed in the two pivotal trials upon which both FDA and EMA approval was obtained, despite consisting of patients with similar disease-risk profile. Median PFS and OS times were 5 and 11 months, respectively. These were shorter than those reported by Wierda et al. (6 and 14 months, respectively).4 The adverse event profile is comparable to that seen in the two previous trials and included infusion-related reactions, cytopenias, and infections. Two patients developed PML.
With the introduction of novel therapies, the Bruton tyrosine kinase (Btk) inhibitor, ibrutinib, was compared directly with ofatumumab in a randomized clinical trial in this setting of R/R CLL. Ibrutinib demonstrated markedly improved duration of PFS, OS and response rates when compared to ofatumumab monotherapy. Byrd et al.6 published a report last year showing their results at a median follow up of 9.4 months; the median duration of PFS was not reached in the ibrutinib group (88% at 6 months) as compared to a median PFS of 8.1 months in the ofatumumab group. OS at 12 months was 90% and 81% in the ibrutinib and ofatumumab groups, respectively. ORRs were significantly higher in the ibrutinib group (43% vs. 4%) consisting of only partial responses. Grade 3 or more adverse events included neutropenia (16% ibrutinib vs. 14% ofatumumab), anemia (5% ibrutinib vs. 8% ofatumumab), and pneumonia (7% ibrutinib vs. 5% ofatumumab). In the light of the efficacy and safety data of ibrutinib, it now has FDA and EMA approval for use in previously treated CLL. Given the efficacy seen with ibrutinib, the role of single agent ofatumumab in R/R setting now appears questionable (Table 1).
Table 1.
Clinical studies of single agent ofatumumab in relapsed/refractory CLL.
Other roles of ofatumumab in R/R CLL are being explored, particularly combination studies (bendamustine and ofatumumab;7 dexamethasone and ofatumumab;8 lenalidomide and ofatumumab9) and in maintenance studies10 which do look promising (Table 2), and these are likely to map out future treatment options in this setting.
Table 2.
Clinical studies of ofatumumab used in combinations or as maintenance in relapsed/refractory CLL.
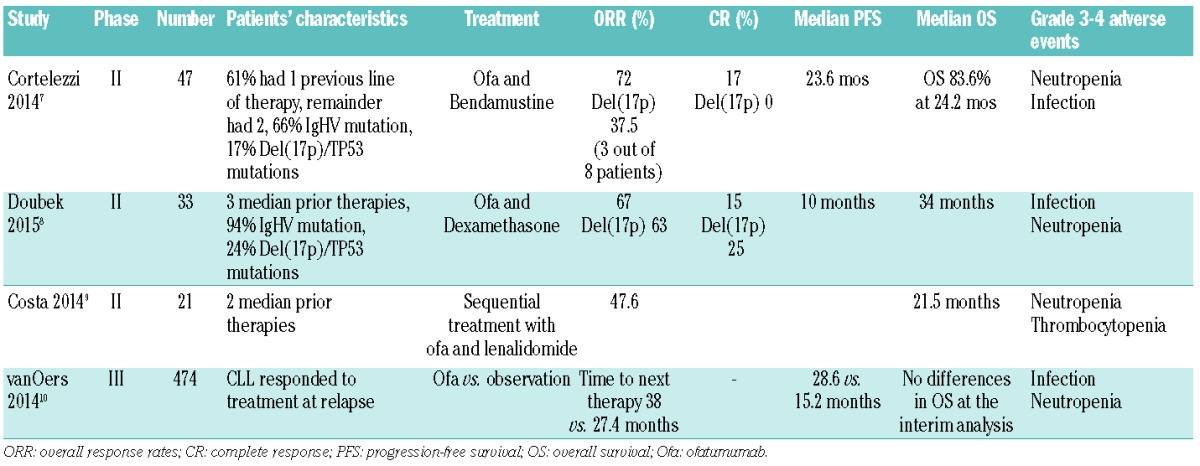
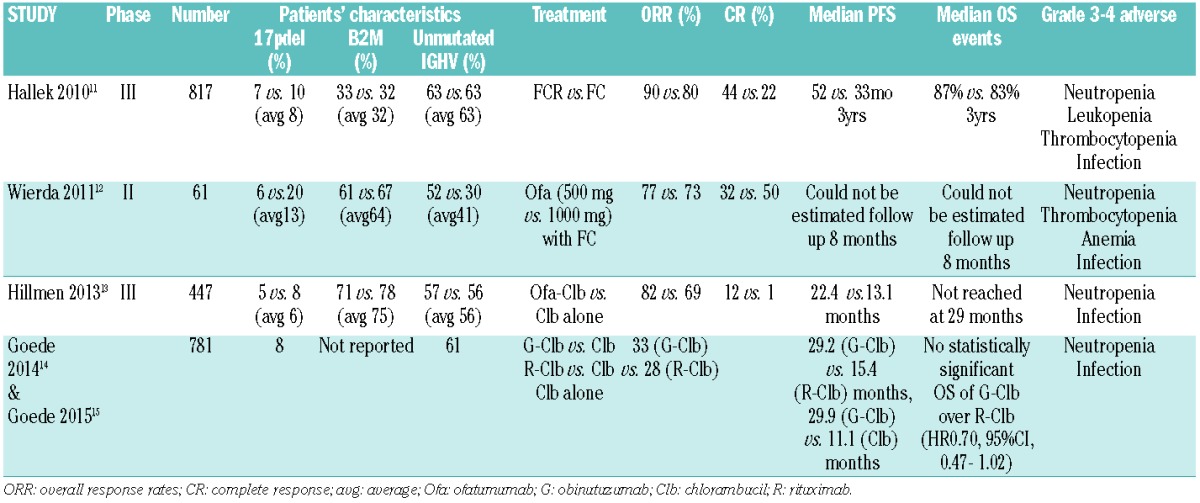
Table 3.
Clinical studies of anti-CD20 monoclonal antibodies, with emphasis on ofatumumab, in treatment-naïve chronic lymphocytic leukemia.
Ofatumumab in first-line treatment for chronic lymphocytic leukemia
The use of ofatumumab in combination with chemotherapy (fludarabine (F) and cyclophosphamide (C)) in fit, treatment-naïve CLL patients has been discouraging, represented by a lower ORR than its counterpart monoclonal anti-CD20 antibody, rituximab, in combination with the same chemotherapy (FCR: ORR 90%, CR 44%).11,12 The ORR and CR for the 500 mg and the 1000 mg ofatumumab cohorts were 77% versus 73% and 32% versus 50%, respectively. Wierda et al.12 postulate that the reduced ORR may reflect the proportion of higher-risk profiles of the patient population [13% 17p deletion and 64% Beta(β)-2-microglobulin (β2M) >3.5mg/L]. However, even though there was a lower proportion of patients with del(17p) in the FCR group (8%) compared to the O-FC group (13%), the ORR and the CR for this subgroup are not markedly different (68% and 5% for FCR vs. 63% and 13% for O-FC) with a higher proportion achieving CR in the O-FC cohort. The proportion of patients with β2M>3.5mg/L in the O-FC cohort was 2-fold that of FCR. Response to FCR treatment in the prognostic subgroup β2M was not provided,11 but with O-FC, the ORR and CR in patients with β2M>4mg/L were 68% and 29%, respectively. Interestingly, the O-FC group also had a higher rate of neutropenia when compared to that seen in FCR treated patients.
Hillmen et al.13 examined the use of ofatumumab plus chlorambucil (Clb) versus chlorambucil monotherapy in treatment-naive patients in whom fludarabine-based therapy was deemed inappropriate (due to advanced age or comorbidities). They reported promising ORR and CR of 82% and 12%, respectively, with the combination of O-Clb compared to 69% and 1% with Clb alone. The median PFS was significantly longer with the addition of ofatumumab (22.4 vs. 13.1 months). Median overall survival (OS) was not reached at a median follow up of 29 months for either group. In April 2014, FDA approved the use of ofatumumab for patients in this setting. Late in 2014, Goede et al.14,15 published data on the anti-CD20 antibodies, rituximab (R) and obinutuzumab (G) in combination with chlorambucil in a similar patient cohort that had significant morbidity or a creatinine clearance between 30 and 69 mL/min. G-Clb compared to Clb alone had a significantly longer median PFS (29.9 vs. 11.1 months). Similarly, R-Clb compared to Clb alone had a significantly longer median PFS (16.3 vs. 11.1 months). The combination of G-Clb had a longer median PFS when compared to the combination of R-Clb (29.2 vs. 15.4 months). These trials suggest that the obinutuzumab combination is superior to the ofatumumab combination, based on median PFS. A direct comparison of G-Clb and O-Clb would be required to confirm this in this group of frail patients, but this is unlikely to occur in the present environment where currently planned trials with obinutuzumab with either ibrutinib or Abt-199 are starting in this setting.
In summary, in the R/R CLL setting, the role of ofatumumab as monotherapy has been superseded by novel agents, and, more specifically, with ibrutinib showing substantially superior activity in a direct comparison.6 However, there may be an emerging role for ofatumumab in combination therapies and in maintenance. In the fit, treatment naïve CLL patient, FCR remains standard of care given the lower efficacy rates seen with O-FC. In the unfit, treatment-naïve CLL patient, despite having received FDA approval, the current use of ofatumumab in combination with Clb is not clear, given the demonstrated improved efficacy with the combination of obinutuzumab and Clb.
Footnotes
Financial and other disclosures provided by the author using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are available with the full text of this paper at www.haematologica.org.
References
- 1.Teeling JL, French RR, Cragg MS, et al. Characterisation of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood. 2004;104:1973–1800. [DOI] [PubMed] [Google Scholar]
- 2.Teeling JL, Marcus WJ, Wiegman LJ, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol. 2006;177:362–371. [DOI] [PubMed] [Google Scholar]
- 3.Coiffier B, Lepretre S, Pedersen LM, et al. Safety and efficacy of ofatumumab, a fully human monoclonal anti CD20 antibody, in patients with relapsed or refractory B- cell chronic lymphocytic leukemia: a phase 1–2 study. Blood. 2008;111:1094–1100. [DOI] [PubMed] [Google Scholar]
- 4.Wierda WG, Kipps TJ, Mayer J, et al. Ofatumumab as a single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreno C, Montillo M, Panayiotidis P, et al. Ofatumumab in poor-prognosis chronic lymphocytic leukemia: A phase 4, non-interventional, observational study from the European Research Initiative on CLL (ERIC). Haematologica. 2015(4):511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortelezzi A, Sciumè M, Liberati AM, et al. Bendamustine in combination with ofatumumab in relapsed or refractory chronic lymphocytic leukemia: a GIMEMA Multicenter Phase II Trial. Leukemia. 2014;28: 642–648. [DOI] [PubMed] [Google Scholar]
- 8.Doubek M, Brychtova Y, Panovska A, et al. Ofatumumab Added to Dexamethasone in Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia. Results from a Phase II Study. Am J Haematol. 2015. February 2 10.1002/ajh.23964 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Costa LJ, Fanning SR, Stephenson J, Jr, et al. Sequential Ofatumumab and Lenalidomide for the Treatment of Relapsed and Refractory Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma. Leuk Lymphoma. 2014. August 17:1–15 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.van Oers MHJ, Kuliczkowski K, Smolej L, et al. Ofatumumab (OFA) Maintenance Prolongs PFS in Relapsed CLL: Prolong Study Interim Analysis Results. Am Soc Hematology Oral Presentation. Abstract 21 December 6, 2014. [Google Scholar]
- 11.Hallek M, Fischer K, Fingerle-Rowson G, et al. International Group of Investigators, German Chronic Lymphocytic Leukaemia Study Group-CLL8 Study: Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–1174. [DOI] [PubMed] [Google Scholar]
- 12.Wierda WG, Kipps TJ, Dürig J, et al. 407 Study Investigators. Chemoimmunotherapy with O-FC in previously untreated patients with chronic lymphocytic leukemia. Blood. 2011;117: 6450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hillmen P, Robak T, Janssens A, et al. Ofatumumab + chlorambucil versus chlorambucil alone in patients with untreated chronic lymphocytic leukemia (CLL): results of the phase III study complement 1 - Am Soc Haematology Oral presentation. 2013;122:1266–1270. [Google Scholar]
- 14.Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370: 1101–10. [DOI] [PubMed] [Google Scholar]
- 15.Goede V, Fischer K, Engelke A, et al. Obinutuzumab as frontline treatment of chronic lymphocytic leukemia: Updated results of the CLL11 study. Leukemia. 2015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


